Abstract
Numerous enzymes that digest carbohydrates, such as cellulases and chitinases, are present in various organisms (e.g., termites, nematodes, and so on). Recently, the presence of cellulases and chitinases has been reported in marine organisms such as urchin and bivalves, and their several roles in marine ecosystems have been proposed. In this study, we reported the presence of genes predicted to encode proteins similar to cellulases and chitinases in the genome of the coral Acropora digitifera, their gene expression patterns at various life stages, and cellulose- and chitin-degrading enzyme activities in several coral species (A. digitifera, Galaxea fascicularis, Goniastrea aspera, Montipora digitata, Pavona divaricata, Pocillopora damicornis, and Porites australiensis). Our gene expression analysis demonstrated the expressions of these cellulase- and chitinase-like genes during various life stages, including unfertilized eggs, fertilized eggs, zygotes, planula larvae, primary polyps and adults of A. digitifera. Agar plate assays confirmed cellulase and chitinase activities in the tissues extracted from adult branches of several coral species. These results suggested that corals are able to utilize cellulases and chitinases in their life histories.
Keywords: Cellulase, Coral enzymes, Enzyme activity, Gene expression, Chitinase
Introduction
Carbohydrates are substances essential for life. They are major energy sources, and monosaccharide derivatives constitute sugar chains on cell surfaces. In benthic aquatic organisms, carbohydrate levels have been suggested to reflect food shortage (Rossi et al., 2006). Reef-building corals, which are the major calcifying organisms supporting the biodiversity of coral reef ecosystems (Roberts et al., 2002), are known to secrete mucus, which is mainly composed of carbohydrates (Wild et al., 2010) and is an important primary product in coral reefs (Wild et al., 2004). Corals are thus considered to utilize carbohydrates for maintaining their life histories and also for high-degree use in material circulation in coral reef ecosystems. However, how corals use carbohydrates is not yet known.
Corals maintain holobiotic relationships with symbiotic algae (zooxanthellae) as well as with bacteria, archaea, and viruses (Rohwer et al., 2002). Recently, cellulase activity was reported in microorganisms related to corals (Sousa et al., 2016). In parallel, available genomic information on corals has greatly increased, and the whole genome sequence of Acropora digitifera was reported (Shinzato et al., 2011). Although we confirmed the presence of several genes predicted to encode enzymes that can digest carbohydrates, such as cellulases and chitinases (details are provided below) in this genome, the details of these enzymes have not yet been evaluated.
Cellulose, which consists of D-glucose units with β-1,4-linkages, serves as the main constituent of plant cell walls, and is the most abundant carbohydrate on earth (Watanabe & Tokuda, 2001). Cellulases (e.g., endo-β-1,4-glucanases (EC 3.2.14), cellobiohydrolase (EC 3.2.1.91), and β-glucosidase (EC 3.2.1.21)), which hydrolyse internal β-1,4-linkages in cellulose chains, are widely distributed in various organisms, including plants (Brummell, Lashbrook & Bennett, 1994), fungi (Tomme, Warren & Gilkes, 1995), bacteria (Tomme, Warren & Gilkes, 1995), and molluscs (Watanabe & Tokuda, 2001). Cellulases were initially identified as products of symbiotic or contaminating microorganisms in the digestive tracts of animals (Martin & Martin, 1978). However, recent studies have shown that cellulases are present as products of the hosts themselves in terrestrial animals, such as termites (Watanabe et al., 1998), nematodes (Smant et al., 1998), and beetles (Sugimura et al., 2003), and also in marine organisms such as abalones (Suzuki, Ojima & Nishita, 2003), sea urchins (Nishida et al., 2007), and bivalves (Sakamoto et al., 2007). Chitin, which consists of repeating N-acetyl-β-D-glucosamine units with β-1,4-linkages, is the main component of cuticular exoskeleton (Merzendorfer & Zimoch, 2003) and is as abundant as cellulose (Merzendorfer & Zimoch, 2003). Chitinases (EC 3.2.1.14), which randomly cleave hydrogen β-1,4-linkages in chitin and provide protection against bacterial invasion (Merzendorfer & Zimoch, 2003), is similarly widely distributed in organisms such as insects (Merzendorfer & Zimoch, 2003), hydroids (Klug et al., 1984), and fish (Matsumiya & Mochizuki, 1996; Ikeda et al., 2014; Kawashima et al., 2016). Cellulases and chitinases in corals are likely to play roles in carbohydrate utilization; however, there is virtually no information on these enzymes in corals.
This study was aimed at examining the fundamental aspects of the genes encoding enzymes that digest carbohydrates, such as cellulase and chitinase. To analyse the gene expression of these enzymes, we examined the gene expression patterns at various life stages of A. digitifera using the DNA sequences of enzymes from genomic information of this species. Additionally, the cellulase and chitinase activities were evaluated by agar plate assays using protein samples from seven dominant coral species: A. digitifera, Galaxea fascicularis, Goniastrea aspera, Montipora digitata, Pavona divaricata, Pocillopora damicornis, and Porites australiensis. Based on these results, we discussed the possible functions of cellulases and chitinases in corals and future directions for studies on genes encoding carbohydrate-catabolising enzymes in corals.
Materials and Methods
Sample collection
Gametes were collected from five A. digitifera colonies that spawned in May 2015. Then, samples at various life stages were collected and frozen at −80 °C until use. Fragments of adult corals of seven species, namely A. digitifera, G. fascicularis, G. aspera, M. digitata, P. divaricata, P. damicornis, and P. australiensis, were collected from the fringing reef of Sesoko Island, Okinawa, Japan. All samplings were conducted with permission from Okinawa Prefecture, Japan (permission No. 28-4). The collected fragments were maintained in a tank with running seawater under natural light conditions at Sesoko Station, Tropical Biosphere Research Centre, University of the Ryukyus, Okinawa, Japan, until use.
Search and design of primers for cellulase- and chitinase-likes genes in coral genome
We searched the genome database of A. digitifera (v1.1) (http://marinegenomics.oist.jp/acropora_digitifera) for cellulases and chitinases. The sequences of coral cellulase-like genes were obtained from predicted A. digitifera transcripts through BLASTP analysis (Camacho et al., 2009) (Table 1) using the sequence of Corbicula japonica cellulase (Accession No. AB264777). The sequences of A. digitifera predicted transcripts were annotated against the Swiss-Prot (http://www.uniprot.org). Based on the annotated information, we obtained chitinase-like genes of A. digitifera. PCR primers for each gene, which were designed to amplify 100–200-bp fragments and spanned the exon-intron boundaries inferred from the GTF file of A. digitifera converted from the GFF file (v1.1) using Cufflinks 2.2.1 (Trapnell et al., 2010), were designed using Primer3Plus (Untergasser et al., 2007) (Table 2). The PCR primers were designed to amplify virtually full-length cDNAs based on predicted A. digitifera transcripts (v1.0) (Table S1).
Table 1. BLAST analysis of cellulase-like genes in coral genome using sequence of Corbicula japonica cellulase (Accession No. AB264777).
| Target gene | Gene ID | Score | E-value |
|---|---|---|---|
| Cellulase-like-1 | adi_v1.03986 | 206 | 6.00E−60 |
| Cellulase-like-2 | adi_v1.23389 | 204 | 1.00E−59 |
Table 2. RT-PCR primers for cellulase- and chitinase-like genes.
GAPDH served as the internal control. Gene IDs of predicted transcripts of target genes of Acropora digitifera (v1.1) are enclosed within parentheses.
| Target gene | Forward primer (5′-3′) | Reverse primer (5′-3′) | Product size |
|---|---|---|---|
| Cellulase-like-1 (aug_v2a.03986) | CCACAGACTACCTCATTAAA | ATCGTCATATTTTCTGGTCT | 109 bp |
| Cellulase-like-2 (aug_v2a.23389) | CCACAGACTACCTCATTAAA | GAACCATCGTCATATTTTC | 114 bp |
| Chitinase-like-1 (aug_v2a.00687) | TCAGTGTGTGACAATTTTAG | TATATATCATTCGCTTGTCA | 111 bp |
| Chitinase-like-2 (aug_v2a.09189) | ATTTAACAAACATGACTTCG | CTTGTCCGTAAAAGTTAAGA | 197 bp |
| GAPDH (aug_v2a.06879) | ATTGGAAGGCTGGTTAT | CGTCTTTCACCTCTGTAGT | 154 bp |
Gene expression analysis by reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from tissue samples of A. digitifera at different life stages (unfertilized eggs, fertilized eggs, zygotes after 15 h after fertilization, zygotes after 40 h after fertilization, planula larvae, primary polyps, and adult branches) with TRIzol solution (Invitrogen), and the isolated RNA samples (900 ng) were reverse-transcribed to cDNA using the PrimeScript RT reagent kit with gDNA Eraser (Takara). RT-PCR was conducted as follows: denaturation at 94 °C for 1 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 2 min, with a final extension step at 72 °C for 5 min. The reactions were performed in a thermal cycler (Veriti; Applied Biosystems, Foster City, CA, USA) and each 10-µL reaction mixture contained 1 µL of each primer at 5 µM, 1 µL of template cDNA, 2 µL of UltraPure DNase/RNase Free-distilled water (Thermo Fisher Scientific, Waltham, MA, USA), and 5 µL of Premix Ex Taq (TaKaRa, Shiga, Japan). Then, 4 µL of each PCR product was subjected to 2% agarose gel electrophoresis to confirm amplification as a single band visualized with GelRed (Wako, Osaka, Japan) under ultraviolet light.
Agar plate assays of cellulase and chitinase activities
Branches from adult corals of seven species (A. digitifera, G. fascicularis, G. aspera, M. digitata, P. divaricata, P. damicornis, and P. australiensis) were crushed using an iron mortar and pestle on ice and homogenized using stainless steel beads in PBS buffer (pH 7.4) at 3,000 rpm for 5 s. The crushed coral samples were centrifuged at 10,000 g for 10 min at 4 °C and the supernatants were concentrated using a disposable ultrafiltration unit (type USY-1; Advantec, Dublin, CA, USA). The protein concentration in the supernatants was adjusted to 2 mg/mL, and then, the supernatants were used as enzyme solutions. Agarose plates containing 1.5% agarose, acetate buffer (pH 5.5), and 0.1% carboxymethylcellulose (CMC) (Sigma) were prepared for assessing cellulase activity in the corals. To assess chitinase activity in the corals, agarose plates containing 0.1% glycol chitin were prepared according to the method of Yamada & Imoto (1981). In brief, 5 µL of enzyme solution was deposited on the centre of each plate and the plate was incubated at 25 °C for 48 h. Then, the plates were stained with 0.1% Congo red (Wako) for 1 h and de-stained with 1 M NaCl solution.
Phylogenetic analysis
The amino acid sequences of the cellulase- and chitinase-like genes of A. digitifera were used for phylogenetic analysis. Representative protein sequences of cellulase and chitinase in other organisms were selected from GenBank. The amino acid sequences were aligned using Mafft v7.309 (Katoh & Standley, 2013). Chitinase-like-2 gene (aug_v2a.09189) was excluded from the phylogenetic analysis because of the short length of its sequence compared with the others. Then, the best-fit evolution model was selected using the Akaike Information Criterion (AIC) with ProtTest 3.4.2 (Darriba et al., 2011) and the maximum-likelihood trees were constructed by RAxML v8.2.10 (Stamatakis, 2014) with analysis of 1,000 bootstrap samples, and visualized with FigTree v1.4.3 (http://tree.bio.ed.ac.uk/software/figtree/).
Results and Discussion
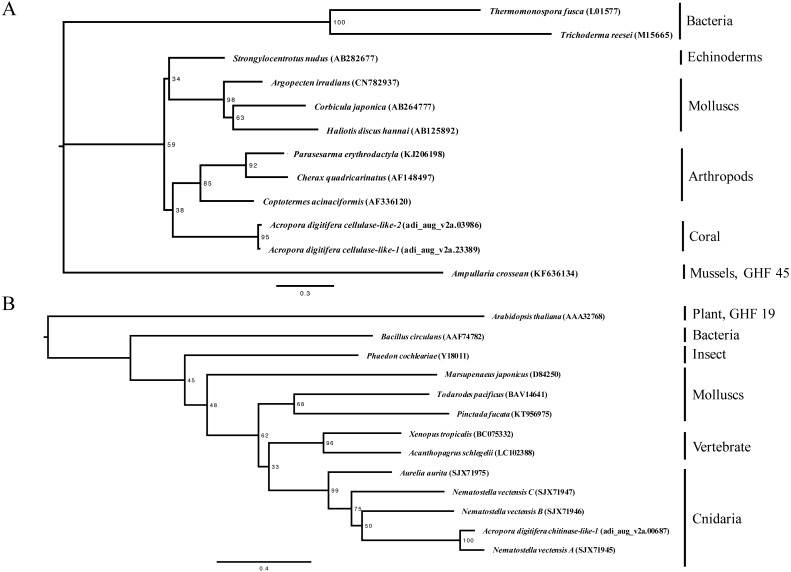
In this study, we identified two cellulase-like genes and two chitinase-like genes in the A. digitifera genome (Table 1). Phylogenetic analysis supported that these cellulase- and chitinase-like genes were classified into the animal-origin group (Fig. 1). Regarding the cellulase-like genes, two coral cellulase-like genes belonged to the GHF 9 group and were located adjacent to each other owing to high sequence similarity at the putative amino acid level. Regarding the chitinases, two types, namely, animal and plant, are known and a coral chitinase-like gene was classified into the GHF 18 group in the phylogenetic tree.
Figure 1. Maximum-likelihood trees (1,000 bootstraps samples) for (A) cellulases (the WAG + G evolution model was selected) and (B) chitinases (the WAG + G + I evolution model was selected).
Numbers at the branch points indicate the percentage of 1,000 bootstrap replicates supporting the topology. GenBank IDs are provided within parentheses. The bar represents 0.2 amino acid substitutions per site.
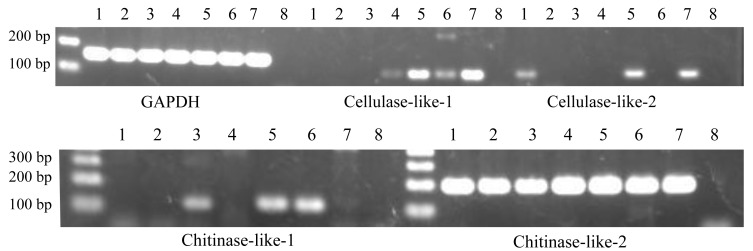
Similarly, we confirmed their gene expression at the various life stages of A. digitifera. Regarding RT-PCR with partial sequences, the cellulase-like-1 gene was expressed at four stages (40 h after fertilization, planula larvae, primary polyp, and adult branches; Fig. 2), cellulase-like-2 gene at three stages (unfertilized eggs, planula larvae, and adult branches; Fig. 2), chitinase-like-1 gene at three stages (15 h after fertilization, planula larvae, and primary polyp; Fig. 2), and chitinase-like-2 gene at all the analysed stages (Fig. 2). Regarding RT-PCR with longer sequences, the cellulase-like-1 gene was expressed in the planula larvae and adult branches (Fig. S1) and the chitinase-like-2 gene at all the analysed stages (Fig. S1); however, the expression of the cellulase-like-2 and chitinase-like-1 genes was not confirmed. These results suggested that these genes perform certain functional roles in corals and the differences in the gene expression patterns indicate that these genes may have different roles.
Figure 2. Expression patterns of cellulase- and chitinase-like genes in certain life stages of Acropora digitifera.
1, Unfertilized eggs; 2, Fertilized eggs; 3, Zygotes 15 h after fertilization; 4, Zygotes 40 h after fertilization; 5, Planula larvae; 6, Primary polyps; 7, Adult branch; 8, Negative control. GAPDH served as the internal control.
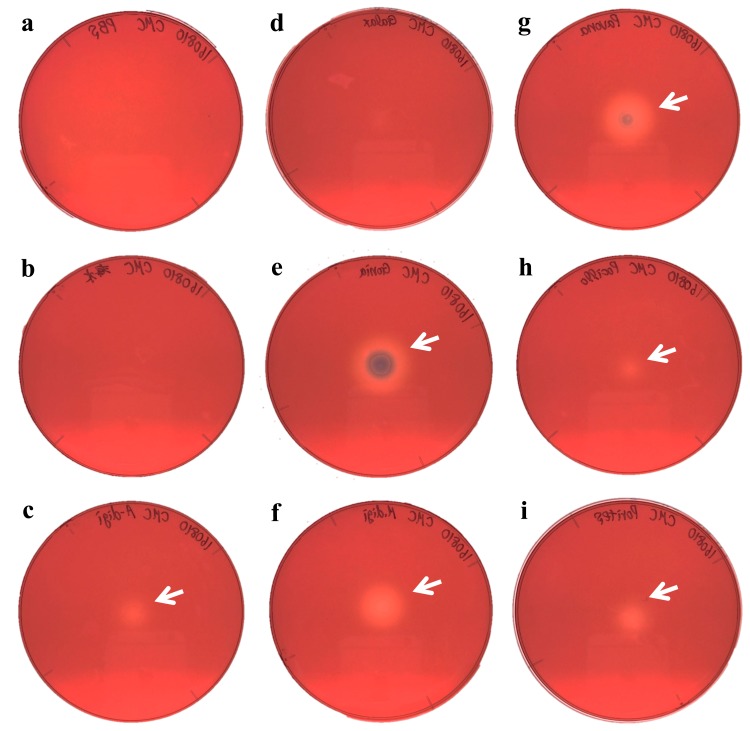
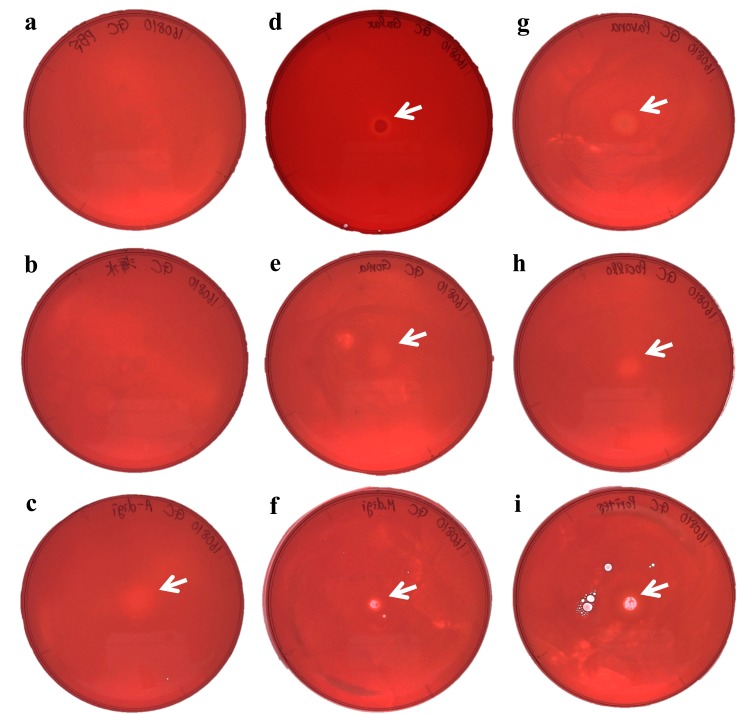
We demonstrated cellulase activity in six of seven coral species (A. digitifera, G. aspera, M. digitata, P. divaricata, P. damicornis, and P. australiensis; Fig. 3) and chitinase activity in all the seven coral species (Fig. 4), suggesting the presence of cellulases and chitinases in a wide range of coral taxa. We observed that the host coral genome contained genes predicted to encode cellulase- and chitinase-like enzymes and these genes were expressed at various life stages. Thus, the enzyme activities observed in this study may be attributed to the coral hosts. Meanwhile, corals are known to be holobionts of coral hosts, symbiotic Symbiodinium spp., and coral-related bacteria (Bourne, Morrow & Webster, 2016; Rohwer et al., 2002). The cellulase and chitinase activities observed in this study could be attributed to the bacteria surrounding the corals. Thus, to clarify the origin of the enzyme activities demonstrated in this study, we should perform further functional analyses of cellulase- and chitinase-like genes in coral genomes and DNA barcode analyses targeting bacteria may be helpful in future studies.
Figure 3. Agar plate assays for cellulase.
(A) Negative control (PBS); (B) Negative control (Sea water around coral samples); (C) Acropora digitifera; (D) Galaxea fascicularis; (E) Goniastrea aspera; (F) Montipora digitata; (G) Pavona divaricata; (H) Pocillopora damicornis; (I) Porites australiensis. White arrows indicate the site on substrate degraded by the dropped enzyme solution.
Figure 4. Agar plate assays for chitinase.
(A) Negative control (PBS); (B) Negative control (Sea water around coral samples); (C) Acropora digitifera; (D) Galaxea fascicularis; (E) Goniastrea aspera; (F) Montipora digitata; (G) Pavona divaricata; (H) Pocillopora damicornis; (I) Porites australiensis. White arrows indicate the site on substrate degraded by the dropped enzyme solution.
Corals may be able to utilize cellulases and chitinases to assimilate carbohydrates obtained by digesting plankton and detritus from the surrounding environment (Bourne, Morrow & Webster, 2016). Corals are known to be capable of heterotrophic nutrition to supplement their energy budget (Grottoli, Rodrigues & Palardy, 2006) and consumption of zooplankton, such as copepods, with chitinous exoskeletons that could be digested by chitinases. In corals, chitin is reported to be used in synthesising skeletons (Watanabe et al., 2003), suggesting the contribution of chitin to physiological functions in corals. Thus, chitinases would be indispensable for obtaining chitin to maintain corals life histories. The role of cellulase in corals may be closely related to the presence of symbiotic algae. Harii et al. (2009) reported that A. digitifera can acquire symbiotic zooxanthellae at the planula larval stage. Interestingly, our results showed that the cellulase-like genes were mainly expressed after the planula larval stage (Fig. 2, Fig. S1). The cell wall of Symbiodinium spp. was reported to be composed of a stable shell of cellulose (Markell, Trench & Iglesiasprieto, 1992). Additionally, the degradation of zooxanthellae was reported to be caused by the digestion of symbionts by the coral host (Titlyanov et al., 1996; Titlyanov et al., 1998). Thus, corals might digest zooxanthellae with cellulase to establish better symbiotic relationships. Furthermore, the degree of dependence of corals on zooxanthellae is suggested to be different among species (Grottoli, Rodrigues & Palardy, 2006), which may be related to the deficiency in cellulase activity in Galaxea fascicularis observed in this study.
There may be other possible roles for these coral cellulase- and chitinase-like enzymes. Chitinase has been shown to play a role in defense against fungal pathogens (Kramer & Muthukrishnan, 1997; Ng, 2004). Douglas et al. (2007) suggested that chitinase in sea fan corals is used in defence against chitinaceous pathogens and released into the surrounding seawater. Moreover, the mucus of the coral Acropora palmata was reported to have antibiotic properties (Ritchie, 2006). Thus, corals may utilize chitinases in defence against invading organisms. Coral reef ecosystems have been degraded by anthropogenic activities (e.g., nutrient pollution and overfishing). Under these conditions, coral reefs are expected to undergo substantial changes from healthy states to algal-dominant states (Bellwood et al., 2004). Thus, to counteract surrounding invasive algae, coral might produce cellulase-like enzymes to protect themselves from potentially invasive algae by digestion with cellulose (Tanaka et al., 2013). Further studies are required to clarify the roles of the cellulase- and chitinase-like enzymes described in this study.
Supplemental Information
Acknowledgments
We thank S Arakaki for her supports in experiment. We also thank for the staffs at Sesoko Station, Tropical Biosphere Research Center, University of the Ryukyus, Japan.
Funding Statement
This study was supported by KAKENHI (No. 15H02813). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Yuki Yoshioka conceived and designed the experiments, performed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Toshiaki Tanabe contributed reagents/materials/analysis tools, wrote the paper, reviewed drafts of the paper.
Akira Iguchi conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
All samplings were carried out after obtaining permission from Okinawa Prefecture, Japan.
Data Availability
The following information was supplied regarding data availability:
The raw code is included in the ‘Materials and Methods’ section of the manuscript.
References
- Bellwood et al. (2004).Bellwood DR, Hughes TP, Folke C, Nystrom M. Confronting the coral reef crisis. Nature. 2004;429:827–833. doi: 10.1038/nature02691. [DOI] [PubMed] [Google Scholar]
- Bourne, Morrow & Webster (2016).Bourne DG, Morrow KM, Webster NS. Insights into the coral microbiome: underpinning the health and resilience of reef ecosystems. Annual Review of Microbiology. 2016;70:317–340. doi: 10.1146/annurev-micro-102215-095440. [DOI] [PubMed] [Google Scholar]
- Brummell, Lashbrook & Bennett (1994).Brummell DA, Lashbrook CC, Bennett AB. Plant endo-1,4-β-D-glucanases. ACS Symposium Series. 1994;566:100–129. doi: 10.1021/bk-1994-0566.ch006. [DOI] [Google Scholar]
- Camacho et al. (2009).Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darriba et al. (2011).Darriba D, Taboada GL, Doallo R, Posada D. ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics. 2011;27:1164–1165. doi: 10.1093/bioinformatics/btr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas et al. (2007).Douglas NL, Mullen KM, Talmage SC, Harvell CD. Exploring the role of chitinolytic enzymes in the sea fan coral, Gorgonia ventalina. Marine Biology. 2007;150:1137–1144. doi: 10.1007/s00227-006-0444-8. [DOI] [Google Scholar]
- Grottoli, Rodrigues & Palardy (2006).Grottoli AG, Rodrigues LJ, Palardy JE. Heterotrophic plasticity and resilience in bleached corals. Nature. 2006;440:1186–1189. doi: 10.1038/nature04565. [DOI] [PubMed] [Google Scholar]
- Harii et al. (2009).Harii H, Yasuda N, Rodriguez-Lanetty M, Irie T, Hidaka M. Onset of symbiosis and distribution patterns of symbiotic dinoflagellates in the larvae of scleractinian corals. Marine Biology. 2009;156:1203–1212. doi: 10.1007/s00227-009-1162-9. [DOI] [Google Scholar]
- Ikeda et al. (2014).Ikeda M, Shirase D, Sato T, Ueda M, Hirabayashi S, Matsumiya M. Primary structure and enzymatic properties of Chitinase Isozymes Purified from the Stomach of the Marbled Rockfish Sebastiscus marmoratus. Journal of Chitin and Chitosan Science. 2014;2:106–116. doi: 10.1166/jcc.2014.1048. [DOI] [Google Scholar]
- Katoh & Standley (2013).Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima et al. (2016).Kawashima S, Ikehata H, Tada C, Ogino T, Kakizaki H, Ikeda M, Fukushima H, Matsumiya M. Stomach chitinase from Japanese sardine Sardinops melanostictus: purification, characterization, and molecular cloning of chitinase isozymes with a long linker. Marine Drugs. 2016;14:22. doi: 10.3390/md14010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug et al. (1984).Klug M, Tardent P, Smid I, Holstein T. Presence and localization of chitinase in Hydra and Podocoryne (Cnidaria, Hydrozoa) Journal of Experimental Zoology Part A. 1984;229:69–72. doi: 10.1002/jez.1402290109. [DOI] [Google Scholar]
- Kramer & Muthukrishnan (1997).Kramer KJ, Muthukrishnan S. Insect chitinases: molecular biology and potential use as biopesticides. Insect Biochem. Journal of Molecular Biology. 1997;27:887–900. doi: 10.1016/S0965-1748(97)00078-7. [DOI] [PubMed] [Google Scholar]
- Markell, Trench & Iglesiasprieto (1992).Markell DA, Trench RK, Iglesiasprieto R. Macromolecules associated with the cell walls of symbiotic dinoflagellates. Symbiosis. 1992;12:19–31. [Google Scholar]
- Martin & Martin (1978).Martin MM, Martin JS. Cellulose digestion in the midgut of the fungus-growing termite Macrotermes natalensis: the role of acquired digestive enzymes. Science. 1978;199:1453–1455. doi: 10.1126/science.199.4336.1453. [DOI] [PubMed] [Google Scholar]
- Matsumiya & Mochizuki (1996).Matsumiya M, Mochizuki A. Distribution of chitinase and β-N- acerylhexosaminidase in the organs of several fishes. Fisheries Science. 1996;62:150–151. doi: 10.2331/fishsci.62.150. [DOI] [Google Scholar]
- Merzendorfer & Zimoch (2003).Merzendorfer H, Zimoch L. Chitin metabolism in insects: structure, function and regulation of chitin synthases and chitinases. Journal of Experimental Biology. 2003;206:4393–4412. doi: 10.1242/jeb.00709. [DOI] [PubMed] [Google Scholar]
- Ng (2004).Ng TB. Antifungal proteins and peptides of leguminous and non-leguminous origins. Peptides. 2004;25:1215–1222. doi: 10.1016/j.peptides.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Nishida et al. (2007).Nishida Y, Suzuki K, Kumagai Y, Tanaka H, Inoue A, Ojima T. Isolation and primary structure of a cellulase from the Japanese sea urchin Strongylocentrotus nudus. Biochimie. 2007;89:1002–1011. doi: 10.1016/j.biochi.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Ritchie (2006).Ritchie KB. Regulation of microbial population by coral surface mucus and mucus-associated bacteria. Marine Ecology Progress Series. 2006;322:1–14. doi: 10.3354/meps322001. [DOI] [Google Scholar]
- Roberts et al. (2002).Roberts CM, McClean CJ, Veron JEN, Hawkins JP, Allen GR, McAllister DE, Mittermeier CG, Schueler FW, Spalding M, Wells F, Vynne C, Werner TB. Marine biodiversity hotspots and conservation priorities for tropical reefs. Science. 2002;295:1280–1284. doi: 10.1126/science.1067728. [DOI] [PubMed] [Google Scholar]
- Rohwer et al. (2002).Rohwer F, Seguritan V, Azam F, Knowlton N. Diversity and distribution of coral-associated bacteria. Marine Ecology Progress Series. 2002;243:1–10. doi: 10.3354/meps243001. [DOI] [Google Scholar]
- Rossi et al. (2006).Rossi S, Gili JM, Coma R, Linares C, Gori A, Vert N. Temporal variation in protein, carbohydrate, and lipid concentrations in Paramuricea clavata (Anthozoa, Octocorallia): Evidence for summer-autumn feeding constraints. Marine Biology. 2006;149:643–651. doi: 10.1007/s00227-005-0229-5. [DOI] [Google Scholar]
- Sakamoto et al. (2007).Sakamoto K, Touhata K, Yamashita M, Kasai A, Toyohara H. Cellulose digestion by common Japanese freshwater clam Corbicula japonica. Fisheries Science. 2007;73:675–683. doi: 10.1111/j.1444-2906.2007.01381.x. [DOI] [Google Scholar]
- Shinzato et al. (2011).Shinzato C, Shoguchi E, Kawashima T, Hamada M, Hisata K, Tanaka M, Fujie M, Fujiwara M, Koyanagi R, Ikuta T, Fujiyama A, Miller DJ, Satoh N. Using the Acropora digitifera genome to understand coral responses to environmental change. Nature. 2011;476:320–323. doi: 10.1038/nature10249. [DOI] [PubMed] [Google Scholar]
- Smant et al. (1998).Smant G, Stokkermans JPWG, Yan Y, De Boer JM, Baum TJ, Wang X, Hussey RS, Gommers FJ, Henrissat B, Davis EL, Helder J, Schots A, Bakker J. Endogenous cellulases in animals: isolation of β-1,4-endoglucanase genes from two species of plant-parasitic cyst nematodes. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:4906–4911. doi: 10.1073/pnas.95.9.4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa et al. (2016).Sousa FMO, Moura SR, Quinto CA, Dias JCT, Pirovani CP, Rezende RP. Functional screening for cellulolytic activity in a metagenomic fosmid library of microorganisms associated with coral. Genetics and Molecular Research. 2016;15:gmr.15048770. doi: 10.4238/gmr.15048770. [DOI] [PubMed] [Google Scholar]
- Stamatakis (2014).Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura et al. (2003).Sugimura M, Watanabe H, Lo N, Saito H. Purification, characterization, cDNA cloning and nucleotide sequencing of a cellulase from the yellow-spotted longicorn beetle, Psacothea hilaris. European Journal of Biochemistry. 2003;270:3455–3460. doi: 10.1046/j.1432-1033.2003.03735.x. [DOI] [PubMed] [Google Scholar]
- Suzuki, Ojima & Nishita (2003).Suzuki K, Ojima T, Nishita K. Purification and cDNA cloning of a cellulase from abalone Haliotis discus hannai. European Journal of Biochemistry. 2003;270:771–778. doi: 10.1046/j.1432-1033.2003.03443.x. [DOI] [PubMed] [Google Scholar]
- Tanaka et al. (2013).Tanaka Y, Iguchi A, Inoue M, Mori C, Sakai K, Suzuki A, Kawahata H, Nakamura T. Microscopic observation of symbiotic and aposymbiotic juvenile corals in nutrient-enriched seawater. Marine Pollution Bulletin. 2013;68:93–98. doi: 10.1016/j.marpolbul.2012.12.017. [DOI] [PubMed] [Google Scholar]
- Titlyanov et al. (1996).Titlyanov EA, Titlyanov TV, Leletkin VA, Tsukahara J, Van Woesik R, Yamazato K. Degradation of zooxanthellae and regulation of their density in hermatypic corals. Marine Ecology Progress Series. 1996;139:167–178. doi: 10.3354/meps139167. [DOI] [Google Scholar]
- Titlyanov et al. (1998).Titlyanov EA, Titlyanov TV, Loya Y, Yamazato K. Degradation and proliferation of zooxanthellae in planulae of the hermatypic coral Stylophora pistillata. Marine Biology. 1998;130:471–477. doi: 10.1007/s002270050267. [DOI] [Google Scholar]
- Tomme, Warren & Gilkes (1995).Tomme P, Warren RAJ, Gilkes NR. Cellulose Hydrolysis by Bacteria and Fungi. Advances in Microbial Physiology. 1995;37:1–81. doi: 10.1016/S0065-2911(08)60143-5. [DOI] [PubMed] [Google Scholar]
- Trapnell et al. (2010).Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, Van Baren MJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nature Biotechnology. 2010;28(5):511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Untergasser et al. (2007).Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts G, Leunissen KAM. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Research. 2007;35:W71–W74. doi: 10.1093/nar/gkm306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe et al. (2003).Watanabe T, Fukuda I, China K, Isa Y. Molecular analyses of protein components of the organic matrix in the exoskeleton of two scleractinian coral species. Comparative Biochemistry and Physiology–Part B: Biochemistry & Molecular Biology. 2003;136:767–774. doi: 10.1038/28527. [DOI] [PubMed] [Google Scholar]
- Watanabe et al. (1998).Watanabe H, Noda H, Tokuda G, Lo N. A cellulase gene of termite origin. Nature. 1998;394:330–331. doi: 10.1038/28527. [DOI] [PubMed] [Google Scholar]
- Watanabe & Tokuda (2001).Watanabe H, Tokuda G. Animal cellulases. Cellular and Molecular Life Sciences. 2001;58:1167–1178. doi: 10.1007/PL00000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild et al. (2004).Wild C, Huettel M, Klueter A, Kremb SG, Rasheed MYM, Jørgensen BB. Coral mucus functions as an energy carrier and particle trap in the reef ecosystem. Nature. 2004;428:66–70. doi: 10.1038/nature02344. [DOI] [PubMed] [Google Scholar]
- Wild et al. (2010).Wild C, Naumann M, Niggl W, Haas A. Carbohydrate composition of mucus released by scleractinian warm- and cold-water reef corals. Aquatic Biology. 2010;10:41–45. doi: 10.3354/ab00269. [DOI] [Google Scholar]
- Yamada & Imoto (1981).Yamada H, Imoto T. A convenient synthesis of glycolchitin, a substrate of lysozyme. Carbohydrate Research. 1981;92:160–162. doi: 10.1016/S0008-6215(00)85993-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw code is included in the ‘Materials and Methods’ section of the manuscript.