Abstract
Importance
The etiologic factors of late-life depression are still poorly understood. Recent evidence suggests that microvascular dysfunction is associated with depression, which may have implications for prevention and treatment. However, this association has not been systematically reviewed.
Objective
To examine the associations of peripheral and cerebral microvascular dysfunction with late-life depression.
Data Sources
A systematic literature search was conducted in MEDLINE and EMBASE for and longitudinal studies published since inception to October 16, 2016, that assessed the associations between microvascular dysfunction and depression.
Study Selection
Three independent researchers performed the study selection based on consensus. Inclusion criteria were a study population 40 years of age or older, a validated method of detecting depression, and validated measures of microvascular function.
Data Extraction and Synthesis
This systematic review and meta-analysis has been registered at PROSPERO (CRD42016049158) and is reported in accordance with the PRISMA and MOOSE guidelines. Data extraction was performed by an independent researcher.
Main Outcomes and Measures
The following 5 estimates of microvascular dysfunction were considered in participants with or without depression: plasma markers of endothelial function, albuminuria, measurements of skin and muscle microcirculation, retinal arteriolar and venular diameter, and markers for cerebral small vessel disease. Data are reported as pooled odds ratios (ORs) by use of the generic inverse variance method with the use of random-effects models.
Results
A total of 712 studies were identified; 48 were included in the meta-analysis, of which 8 described longitudinal data. Data from 43 600 participants, 9203 individuals with depression, and 72 441 person-years (mean follow-up, 3.7 years) were available. Higher levels of plasma endothelial biomarkers (soluble intercellular adhesion molecule–1: OR, 1.58; 95% CI, 1.28-1.96), white matter hyperintensities (OR, 1.29; 95% CI, 1.19-1.39), cerebral microbleeds (OR, 1.18; 95% CI, 1.03-1.34), and cerebral (micro)infarctions (OR, 1.30; 95% CI, 1.21-1.39) were associated with depression. Among the studies available, no significant associations of albuminuria and retinal vessel diameters with depression were reported. Longitudinal data showed a significant association of white matter hyperintensities with incident depression (OR, 1.19; 95% CI, 1.09-1.30).
Conclusions and Relevance
This meta-analysis shows that both the peripheral and cerebral forms of microvascular dysfunction are associated with higher odds of (incident) late-life depression. This finding may have clinical implications because microvascular dysfunction might provide a potential target for the prevention and treatment of depression.
This systematic review and meta-analysis examines the associations of the peripheral and cerebral forms of microvascular dysfunction with late-life depression.
Key Points
Question
Are both the peripheral and cerebral forms of microvascular dysfunction associated with late-life depression, as suggested by the vascular depression hypothesis?
Findings
This systematic review and meta-analysis of 48 studies comprising 43 600 participants, including 9203 individuals with depression, shows that the cerebral and peripheral forms of microvascular dysfunction were associated with increased odds for (incident) late-life depression, independent of cardiovascular risk factors.
Meaning
These findings support the hypothesis that microvascular dysfunction is causally linked to late-life depression. This finding may have clinical implications because microvascular dysfunction might provide a target for the prevention and treatment of depression.
Introduction
Late-life depression is a highly prevalent and heterogeneous disease with high rates of morbidity and mortality. It is characterized by recurrent episodes: up to 50% of those who recover from a first episode of depression will experience additional episodes throughout their lifetime. Evidence suggests a cerebrovascular etiologic cause because late-life depression has been associated with vascular dementia, stroke, and white matter hyperintensities (WMHs). Moreover, a vascular etiologic cause may explain the high recurrence rate of depression, in addition to the high rate of resistance to antidepressants and/or cognitive behavioral therapy; approximately one-third of patients with depression have treatment-resistant depression.
Several studies have provided evidence that cerebral small vessel disease may play a role in the etiologic factors of late-life depression. A meta-analysis from 2014, including 19 studies and 6274 participants, showed significant cross-sectional and longitudinal associations between white matter lesions, a proxy of cerebral small vessel disease, and (incident) depression. However, multiple studies with continuous measures of WMHs were not included in this meta-analysis, and 2 large longitudinal studies became available only recently. Furthermore, the growing evidence on alternative markers of microvascular dysfunction (for instance, on biomarkers of endothelial dysfunction) was not taken into account in previous meta-analyses.
In view of these considerations, we hypothesize that microvascular dysfunction, both peripheral and cerebral, may be associated with depression. We conducted a systematic review and meta-analysis to investigate this hypothesis, both in cross-sectional and longitudinal studies.
Methods
Search Strategy
We used MEDLINE and EMBASE to conduct a systematic literature search for cross-sectional and longitudinal epidemiologic studies of humans, determining the association between markers of microvascular dysfunction and depressive symptoms and/or depressive disorder, published from inception to October 16, 2016. This study has been registered at PROSPERO (https://www.crd.york.ac.uk/PROSPERO/ [CRD42016049158]) and is reported in accordance with the PRISMA and MOOSE guidelines. We considered the following 5 estimates of microvascular dysfunction: plasma markers of endothelial function, albuminuria, measurements of skin and muscle microcirculation, retinal arteriolar and venular diameter, and markers for cerebral small vessel disease. The exact search strategy and rationale are in the eAppendix in the Supplement.
Selection Criteria and Data Extraction
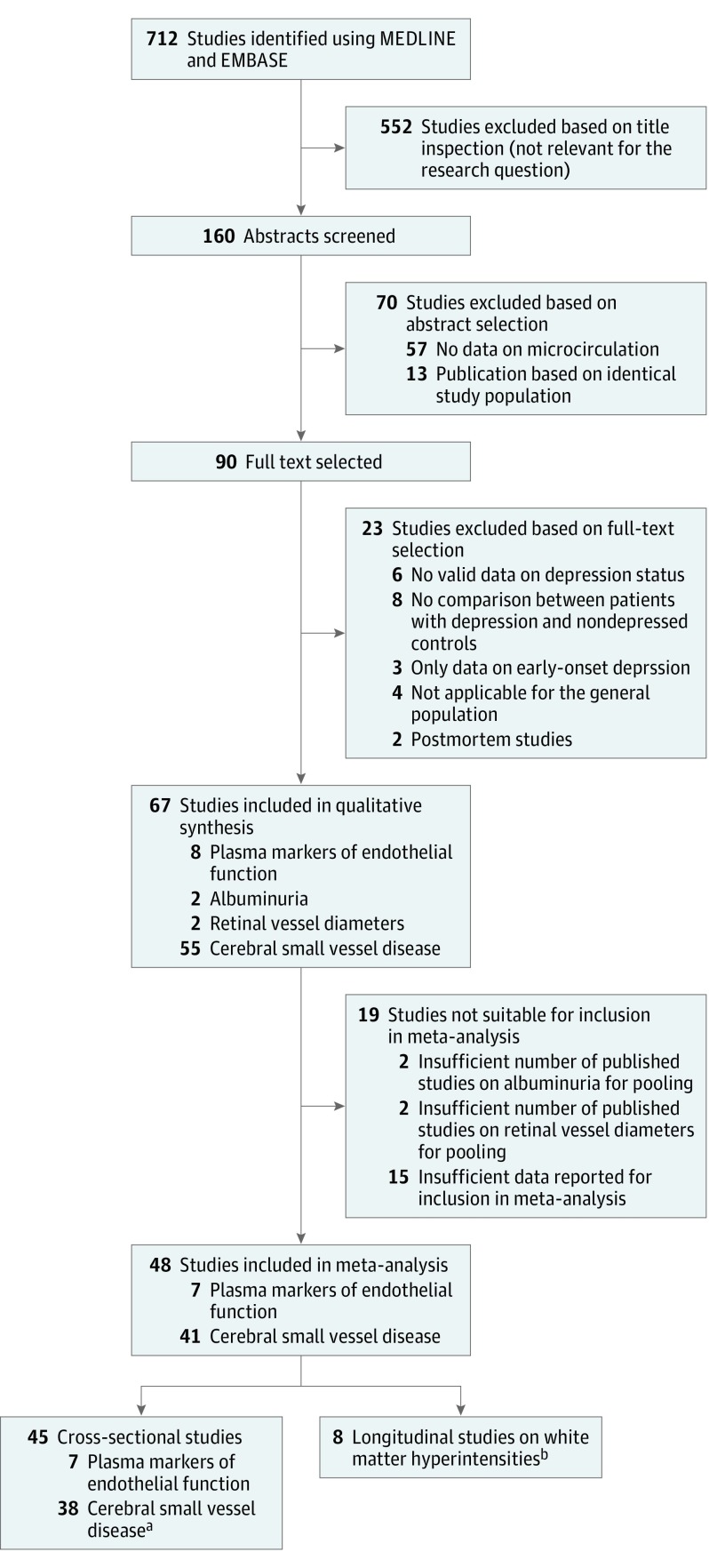
Three independent researchers performed the study selection (M.J.M.v.A., A.J.H.M.H., and M.T.S.). Population-based or case-control studies that reported on microvascular dysfunction in participants with or without depression were included. Figure 1 shows the selection procedure. Of the 67 studies included in the review, we extracted the following: baseline characteristics of the study population, study design, number of participants with or without depression, definition of microvascular dysfunction and depression (self-reported questionnaire vs diagnostic psychiatric interview), fully adjusted results including 95% CIs, SD, or range, and confounders included in the analyses. When these data were missing, the principal investigators were contacted for further information. If the principal investigator could not provide the missing data, the study was excluded. The quality of studies was assessed by use of the Newcastle-Ottawa Scale (NOS) for case-control and cohort studies (eTable 1 in the Supplement). This scale uses a 10-point grading system with a maximum score of 9 points for longitudinal studies, 6 points for cross-sectional cohort studies, and 8 points for case-control studies and assesses selection of study groups, comparability of groups, and ascertainment of exposure and outcome. We calculated the percentage of the maximum NOS score for all studies (eTable 1 in the Supplement).
Figure 1. Flowchart of Study Selection.
aIncluding 38 studies on white matter hyperintensities, 4 studies on microbleeds, and 4 studies on microinfarctions.
bFive studies provided both cross-sectional and longitudinal data.
Statistical Analysis
We performed the meta-analysis with Review Manager, version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration), by use of the generic inverse variance method with random-effects models. In studies that reported microvascular function as mean (SD) values for participants with depression compared with controls without depression, we calculated odds ratios (ORs) based on the standardized mean difference method in concordance with the Cochrane Handbook for Systematic Reviews of Interventions. If only a range of scores was reported, we estimated the SD using the formula (upper limit − lower limit)/4. We used forest plots to display the pooled ORs and 95% CIs, assessed heterogeneity using I2 statistics (values of 50%-75% indicated moderate heterogeneity, and values of >75% indicated considerable heterogeneity), and determined the risk of publication bias by visual inspection of funnel plots, the Egger test, and the trim-and-fill method. We performed subgroup and meta-regression analysis with R to explore heterogeneity, and we evaluated the methods to assess depression (diagnostic interviews vs self-reported questionnaires), study design (case-control vs cohort study), methods to assess WMHs (semiautomatic volumetry vs subjective rating scale), and study quality as assessed by the NOS score (≥60% vs <60%).
Results
Study Selection and Characteristics
We identified 712 studies, of which 90 full-text articles were assessed for eligibility. Of these, we selected 67 studies that investigated whether microvascular dysfunction was associated with depressive disorder or depressive symptoms. Of these studies, 59 had a cross-sectional design (35 case-control studies and 24 cohort studies), and 8 had a longitudinal design (8 cohort studies). In total, data from 43 600 participants, including 9203 individuals (21.1%) with depression, were included in the meta-analysis. The mean age of participants was 66 years, and 23 544 were female (54.0%). In total, 72 441 person-years were included in longitudinal analyses (mean [SD] follow-up, 3.7 [0.7] years). We found no studies that investigated the association between skin and muscle microcirculation and depressive disorder or depressive symptoms.
All studies included in the review used a dichotomous outcome measure for depression, either by use of a diagnostic interview to assess major depressive disorder or by use of a cutoff for clinically relevant depressive symptoms, including the Mini International Neuropsychiatric Interview or the Structured Clinical Interview for DSM for depressive disorder and the Centers for Epidemiological Studies Depression Scale, the Geriatric Depression Scale, the Hamilton Depression Rating Scale, the Beck Depression Inventory, and the Montgomery-Asberg Depression Rating Scale for clinically relevant depressive symptoms. Plasma samples of biomarkers (soluble intercellular adhesion molecule-1 [sICAM-1], soluble vascular cell adhesion molecule-1 [sVCAM-1], e-selectin, and von Willebrand factor [vWF]) for endothelial function were all analyzed by the use of an enzyme-linked immunosorbent assay. Albuminuria was measured by use of the albumin to creatinine ratio or 24-hour urinary albumin excretion. Retinal vessel calibers were measured by the use of stereoscopic color fundus photography. Cerebral small vessel disease was determined by magnetic resonance imaging–defined automated segmentation of WMH volume (33 studies), rating scales for WMH severity (22 studies), microbleeds (4 studies), and/or lacunar or silent infarctions (4 studies). Characteristics of all selected studies are presented in eTable 2 in the Supplement and the outcomes of the selected studies in eTable 3 in the Supplement.
Association of Endothelial Function With Depression
Eight studies investigated the cross-sectional associations between plasma markers of endothelial function and depressive symptoms (n = 6) or depressive disorder (n = 4). Most studies observed that higher levels of endothelial plasma markers, which indicate dysfunction, were associated with depression. One study described lower sVCAM-1 levels, and another study lower e-selectin levels, in participants with depression compared with controls without depression.
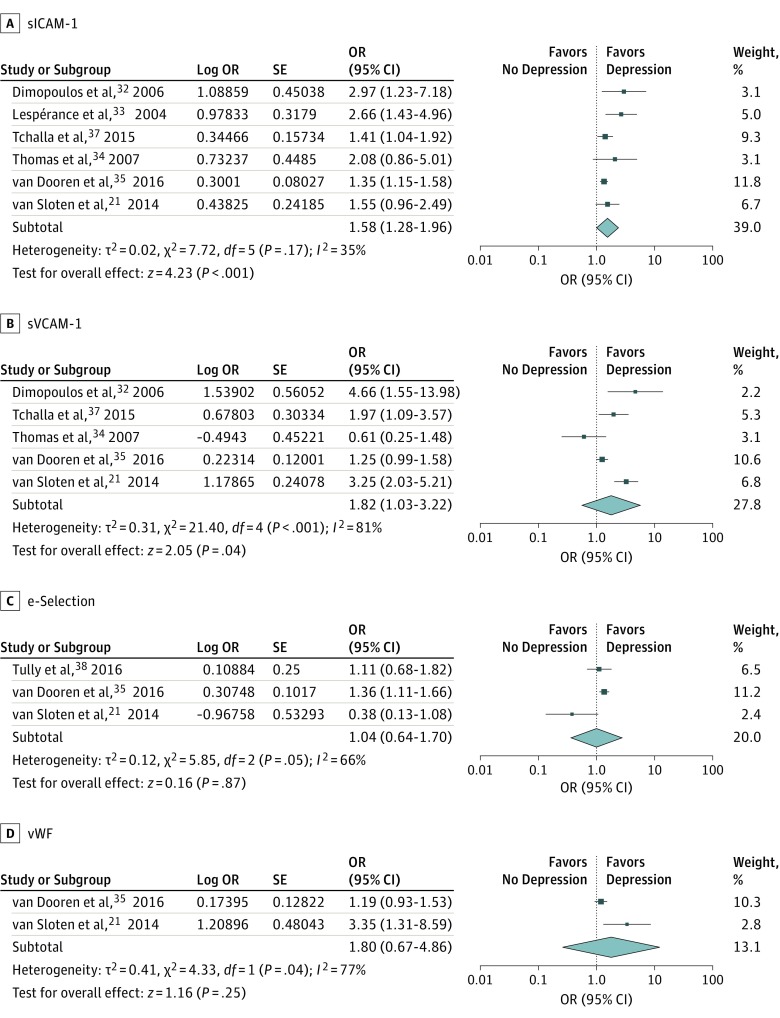
Seven studies were included in the meta-analysis on plasma markers of endothelial function (1 study provided insufficient data). We found a significant association between higher levels of plasma markers of endothelial function and depression (Figure 2; pooled OR per SD increase of sICAM-1, 1.58; 95% CI, 1.28-1.96; P < .001; I2 = 35%; pooled OR per SD increase of sVCAM-1, 1.82; 95% CI, 1.03-3.22; P < .001; I2 = 81%; pooled OR per SD increase of e-selectin, 1.04; 95% CI, 0.64-1.70; P = .87; I2 = 66%; pooled OR per SD increase of vWF, 1.80; 95% CI, 0.67-4.86; P = .25; I2 = 77%). We found no evidence of publication bias by the inspection of the funnel plots (eFigure 1 in the Supplement) or the Egger test (t = 1.569; P = .12).
Figure 2. Cross-sectional Association of Endothelial Plasma Markers With Depression.
Data are reported as pooled odds ratios (ORs) by use of the generic inverse variance method with random-effects models. sICAM-1 indicates soluble intercellular adhesion molecule-1; sVCAM-1, soluble vascular cell adhesion molecule-1; and vWF, von Willebrand factor.
Association of Albuminuria With Depression
Two studies investigated the association between albuminuria and depression. Albuminuria was not significantly associated with depression in patients with (OR, 1.29; 95% CI, 0.96-1.73) or without (OR, 1.07; 95% CI, 0.70-1.63) prior cardiovascular disease. A second study found no significant association between urinary protein (milligrams per gram) and depressive symptoms in participants with chronic kidney disease (OR, 1.07; 95% CI, 0.90-1.26).
Association of Retinal Microvascular Diameters With Depression
Two studies investigated the association between retinal arteriolar and venular diameters and depression. In a subpopulation of participants with diabetes, significant differences were found between controls (mean [SD] arteriolar diameter, 133.1 [5.5] µm; mean [SD] venular diameter, 214.2 [7.5] µm), patients with diabetes (mean [SD] arteriolar diameter, 135.7 [5.6] µm; mean [SD] venular diameter, 208.7 [7.6] µm), and patients with depression and diabetes (mean [SD] arteriolar diameter, 140.3 [5.8]; P < .01 for trend; mean [SD] venular diameter 209.9 [7.9]; P = .03 for trend). In contrast, a large longitudinal cohort study found no association between retinal arteriolar and venular diameters and incident major depressive disorder during 9 years of follow-up (hazard ratio per SD increase in arteriolar diameter, 1.01; 95% CI, 0.93-1.10; and hazard ratio per SD increase in venular diameter, 1.02; 95% CI, 0.94-1.12).
Cross-sectional Association of Cerebral Small Vessel Disease With Depression
Fifty-five studies investigated the association between cerebral small vessel disease and depression, of which 8 studies had a prospective design. Most studies focused on WMH volumes or WMH severity scores in a case-control or a population-based cohort setting. In addition to studying WMHs, 4 studies also evaluated the association of microbleeds and microinfarctions with depression. Overall, cerebral small vessel disease was associated with depression.
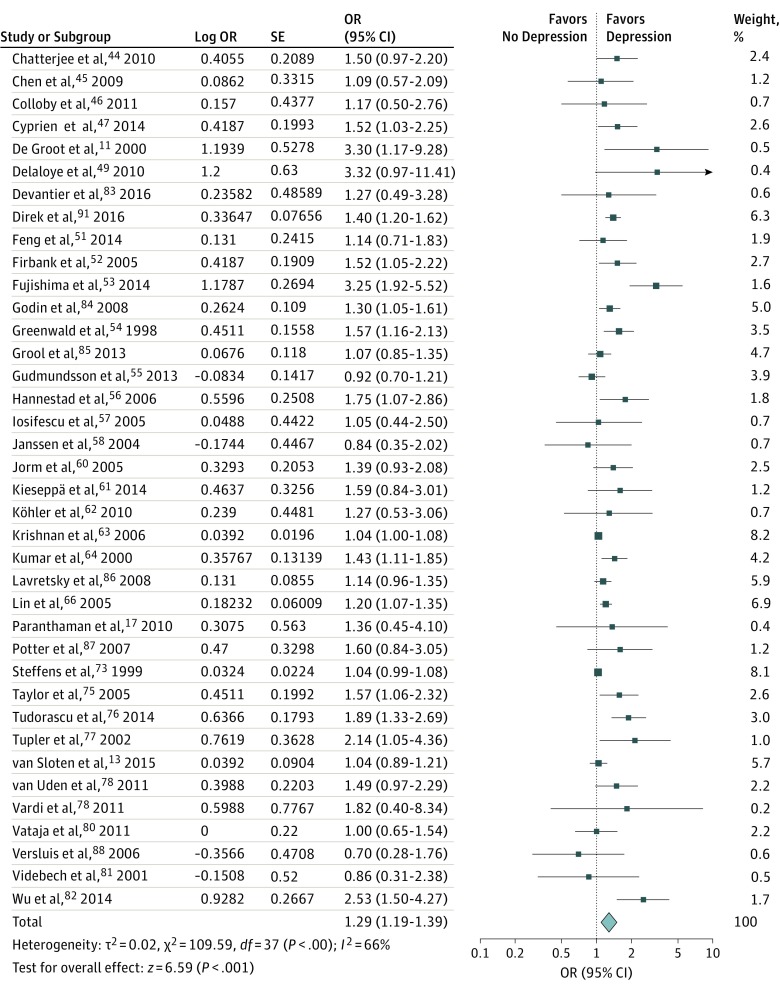
Thirty-eight studies were included in the meta-analysis on cross-sectional data (Figure 3; eFigure 2 and eFigure 3 in the Supplement). A significant association between WMHs and depression was found (pooled OR per SD, 1.29; 95% CI, 1.19-1.39; P < .001; I2 = 66%). A statistically significant association was found between a higher number of microbleeds (pooled OR, 1.18; 95% CI, 1.03-1.34; P < .05; I2 = 0%) and brain (micro)infarctions (pooled OR, 1.30; 95% CI, 1.21-1.39; P < .001; I2 = 1%) and depression. To reduce possible residual confounding by medical comorbidities, we restricted the analysis to studies that corrected for diabetes status or hypertension. The results remained statistically significant when pooling the WMH studies that corrected for diabetes status (8 studies; OR, 1.32; 95% CI, 1.15-1.52; P < .001; I2 = 46%) or hypertension (11 studies; OR, 1.18; 95% CI, 1.08-1.29; P < .001; I2 = 76%). When we restricted the analyses to the 16 studies that used a diagnostic interview to diagnose depressive disorder, the pooled OR was 1.34 per SD (95% CI, 1.19-1.51; P < .001; I2 = 24%). Twenty-two studies used questionnaires to assess depressive symptoms; pooling of these studies resulted in an OR of 1.24 per SD (95% CI, 1.14-1.35; P < .001; I2 = 69%). We further explored heterogeneity by comparing WMHs as assessed semiautomatically vs severity rating scales (OR, 1.31; 95% CI, 1.18-1.46; P < .001; I2 = 49% vs OR, 1.22; 1.10-1.34; P < .001; I2 = 66%) and case-control vs cohort studies (OR, 1.36; 95% CI, 1.22-1.52; P < .001; I2 = 37% vs OR, 1.19; 95% CI, 1.08-1.31; P < .001; I2 = 74%). We restricted analysis to 31 studies with a high methodological quality, as indicated by a NOS score of 60% or more. White matter hyperintensities were positively associated with depression (pooled OR, 1.35; 95% CI, 1.22-1.50; P < .001; I2 = 68%). Of these studies, 12 used a diagnostic interview. Restricting the analyses to these 12 studies resulted in a pooled OR of 1.33 (95% CI, 1.13-1.57; P < .001; I2 = 43%). The pooled OR for the 19 studies that used questionnaires was 1.32 (95% CI, 1.17-1.49; P < .001; I2 = 70%). In meta-regression analysis, we found a significant association between WMHs and depression (pooled OR per SD, 1.25; 95% CI, 1.05-1.49; P < .05; I2 = 51%) when we included the methods to assess depression (diagnostic interviews vs self-reported questionnaires), study design (case-control vs cohort study), and methods to assess WMHs (semiautomatic volumetry vs subjective rating scale). We found no evidence of publication bias by the inspection of the funnel plots (eFigure 1 in the Supplement), the Egger test (t = 1.569; P = .29), or the trim-and-fill (eFigure 4 in the Supplement). Owing to the limited number of studies, we could not perform subgroup analyses or valid estimations on publication bias for data on microbleeds and microinfarctions.
Figure 3. Cross-sectional Association of White Matter Hyperintensities With Depression.
Data are reported as pooled odds ratios (ORs) by use of the generic inverse variance method with random-effects models.
Longitudinal Association of Cerebral Small Vessel Disease With Depression
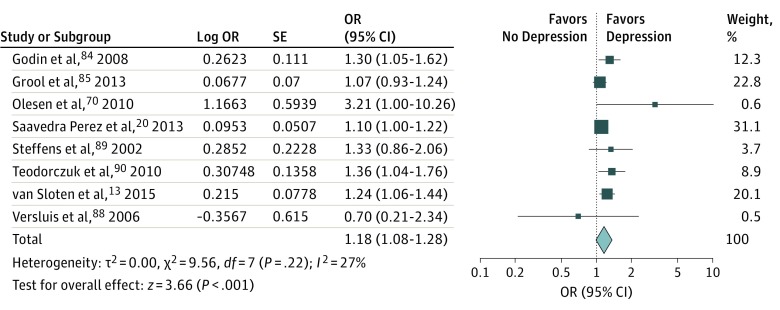
Eight studies were included in the meta-analysis of longitudinal data (Figure 4). Only data on WMHs could be pooled, because only 1 longitudinal study investigated the association of microbleeds and brain infarctions with depression. As shown in Figure 4, a statistically significant association between WMHs and the incidence of depression was found (pooled OR, 1.18; 95% CI, 1.08-1.28; P < .001; I2 = 27%) over a mean follow-up of 3.7 years. We found no evidence of publication bias based on the funnel plots (eFigure 5 in the Supplement) or the Egger test (t = 1.139; P = .30).
Figure 4. Longitudinal Association of White Matter Hyperintensities With Depression.
Data are reported as pooled odds ratios (ORs) by use of the generic inverse variance method with random-effects models. CSVD indicates cerebral small vessel disease.
Discussion
This extensive meta-analysis on 43 600 participants, including 9203 individuals with depression, shows that generalized microvascular dysfunction is associated with depression, both in cross-sectional and longitudinal settings, independent of cardiovascular risk factors. Multiple markers of microvascular dysfunction, including endothelial plasma markers and markers of cerebral small vessel disease, are cross-sectionally associated with a higher level of depressive symptoms and depressive disorder. In addition, WMHs are associated with incident depression over time. These findings are in agreement with the vascular depression hypothesis and extend this hypothesis, as peripheral microvascular dysfunction may also be associated with depression.
In this systematic review with meta-analysis, we evaluated the associations between microvascular dysfunction and depression. We included cross-sectional and longitudinal epidemiologic studies, and are the first, to our knowledge, to consider the association of multiple measures of both cerebral and peripheral microvascular dysfunction with depression. By combining the extensive evidence on WMHs with data from biomarkers of endothelial function, we aim to provide further evidence for the hypothesis that cerebral small vessel disease may originate from endothelial dysfunction. We suggest that generalized microvascular dysfunction, as can be measured throughout the body, is an important pathophysiologic factor that may contribute to the development of depression. Our results confirm and extend 2 previous meta-analyses that addressed the association between WMHs and depression by including more than 6 times the number of participants and depression cases, thus increasing statistical power. This number enabled us to overcome the major caveat of high heterogeneity, which was a major methodological issue in previous meta-analyses. The use of diagnostic interviews vs questionnaires to assess depression, and case-control vs cohort study design were found to be the sources of heterogeneity, which has important implications for future studies that investigate the pathophysiologic factors of depression. These variables, however, did not affect the observed associations, which strengthens the validity of our findings. Finally, our study focused on late-life depression, in which vascular pathologic conditions are thought to have the greatest effect, while previous meta-analyses combined early and late-life depression.
The association of microvascular dysfunction with depression can be explained by several mechanisms. First, impaired endothelial function in the cerebral microcirculation may lead to cerebral perfusion deficits, resulting in chronic ischemia in the cerebrum. Chronic ischemia could cause structural disruptions of the fiber tracts in the cerebral white matter, which are visualized as WMHs on results of magnetic resonance imaging. If the affected regions are involved in mood regulation, this may predispose the individual to the development of depression. Second, microvascular dysfunction is closely linked to and interrelated with chronic low-grade inflammation and/or oxidative stress, which may represent different pathways in the development of depression. Low-grade inflammation is known to contribute to endothelial dysfunction. In addition, the cerebral endothelium may be more vulnerable to oxidative stress, owing to a high production of reactive oxygen species in the brain as a result of the high metabolic demand. Moreover, the brain has limited antioxidant defenses, while damage related to oxidative stress has been described in psychiatric disease and may contribute to cerebral dysfunction. However, multiple studies have shown that the association between microvascular dysfunction and depression is only partly dependent on inflammation or oxidative stress, which suggests that microvascular dysfunction itself represents an independent pathway in the development of depression. Third, cardiometabolic risk factors may be involved in the association between microvascular dysfunction and depression. For instance, increased arterial stiffening may induce microvascular disease and is related to depression. Increased arterial stiffness leads to an increased pulsatile pressure load, which, owing to the low impedance of the cerebral microcirculation, can penetrate deeply into the white matter, thereby inducing microvascular dysfunction and WMHs. In addition, other cardiometabolic risk factors, such as decreased physical activity, smoking, obesity, hypertension, diabetes, and unhealthy diet, have been associated with both microvascular dysfunction and depression. However, we mainly used results that were adjusted for cardiometabolic risk factors in our meta-analysis. This finding may suggest that microvascular dysfunction represents an independent pathway in the development of depression. Fourth, acute and chronic stress can result in autonomic and hypothalamic-pituitary-adrenal axis dysregulation, which in turn can contribute to both depression and cardiovascular disease. Stress-induced elevated cortisol levels may cause cerebral atrophy, reduced neurogenesis, synaptic plasticity, and monoaminergic signaling, all of which could contribute to the development of depression.
The exact pathogenesis of WMHs is currently undetermined. Several studies have assumed that WMHs are due to ischemia; however, evidence indicates that WMHs originate from cerebral endothelial dysfunction. This evidence is supported by findings that WMHs and microinfarctions are associated with leakage of plasma fluid components, arteriolar wall infiltration, thickening of the arteriolar wall, and changes in perivascular tissue, causing disruption of the normal architecture, including damaged arteriolar smooth muscle cells and fibrin depositions. In addition, the specific anatomy of capillaries (with functional shunts and tight control of capillary flow patterns) could enable 2 distinct mechanisms to induce ischemia within the brain: limited blood supply and limited oxygen extraction due to capillary dysfunction.
Strengths and Limitations
The strengths of this study include the large number of included studies and individuals with depression, resulting in high statistical power, which allowed an extensive exploration of the cause of heterogeneity within the meta-analysis. This meta-analysis is limited by the available literature. Based on the available data, we cannot rule out the possibility of reverse causality. It is plausible to assume that the association between microvascular dysfunction and depression is bidirectional; that is, microvascular dysfunction may cause depression, and vice versa. The proposed temporality was supported by the longitudinal association for WMHs and depression, but could not be confirmed for other markers of microvascular dysfunction. Further longitudinal studies are needed to address this issue. In addition, the interrelationships among medical comorbidities, microvascular dysfunction, and depression could only partly be assessed. Therefore, an important limitation of this meta-analysis and indeed of the source studies is that we cannot exclude residual confounding by variables not considered in the source studies. However, based on our subanalyses, confounding by type 2 diabetes, hypertension, and cardiovascular risk factors is unlikely. In addition, some of the indicators of vascular dysfunction, such as albuminuria, may be less specific and may more likely reflect a general health status, which could have led to an overestimation of the association. Furthermore, most studies on plasma biomarkers of endothelial dysfunction measured multiple biomarkers; therefore, we did not calculate a pooled estimate. Nevertheless, when focusing on the pooled ORs per specific biomarker, the 95% CIs were virtually within the same range. Finally, data on the association between albuminuria, retinal diameters, and depression appeared to be scarce, while data on albuminuria were available only in study populations with disease, and therefore cannot be extrapolated to the general population.
As the cerebral microvasculature is difficult to study, there is a need to develop more advanced and powerful imaging techniques, such as 7-T magnetic resonance imaging and diffuse tensor imaging, which may provide more sensitive research tools with more detailed structural information on microvascular changes as seen in cerebral small vessel disease. Furthermore, several state-of-the-art techniques have been developed to investigate the microcirculation throughout the body, such as sublingual intravital microscopy, skin laser-Doppler flowmetry, dynamic retinal vessel analysis, and skin capillaroscopy. Large-scale studies using these new techniques are of crucial importance to unravel the association between microvascular dysfunction and depression. In addition, experimental studies are needed to demonstrate the possible causal role of microvascular dysfunction in depression. Multiple drugs, such as angiotensin-converting enzyme inhibitors and statins, as well as lifestyle interventions have been shown to improve microvascular function; it is not known, however, whether such interventions can improve brain microcirculatory function in general or WMHs specifically. Some evidence exists that angiotensin-converting enzyme inhibitors may be efficacious in the treatment of depression. However, larger randomized trials have not been performed. Statins may provide another intervention of interest in depression, although the current literature reports conflicting results. Randomized clinical trials in individuals at high risk for or with depression may provide further insight into the role of microcirculatory dysfunction in the prevention and/or treatment of depression.
Conclusions
This meta-analysis shows that generalized microvascular dysfunction is associated with higher odds of depression and that cerebral small vessel disease is associated with an increased risk for the development of depression over time. These findings support the hypothesis that microvascular dysfunction is causally linked to depression. This finding may have clinical implications, as microvascular dysfunction might provide a potential target for the prevention and treatment of depression.
eAppendix. Search Terms Used for the Systematic Review and Meta-analysis
eFigure 1. Funnel Plot of Cross-Sectional Studies on the Association Between Microvascular Dysfunction and Depression; Plasma Markers for Endothelial Function (A), WMH (B), Microbleeds (C) And Microinfarctions (D)
eFigure 2. Forest Plots With the Odds Ratios and 95% Confidence Intervals for Original Studies and the Pooled Odds Ratios for the Cross-Sectional Association Between Cerebral Microbleeds and Depression
eFigure 3. Forest Plots With the Odds Ratios and 95% Confidence Intervals for Original Studies and the Pooled Odds Ratios for the Cross-Sectional Association Between Cerebral (Micro)Infarctions and Depression
eFigure 4. Funnel Plot of Cross-Sectional Studies on the Association Between White Matter Hyperintensities and Depression After Trim-and-Fill Analysis
eFigure 5. Funnel Plot of Longitudinal Studies on the Association Between Cerebral Small Vessel Disease and Depression
eTable 1. Quality Assessment of the Included Studies by Use Of the Newcastle-Ottawa Scale (NOS)
eTable 2. Characteristics of Studies Included in the Systematic Review and Meta-Analysis
eTable 3. Reported Results of Studies Included in the Systematic Review and Meta-analysis
eReferences.
References
- 1.World Health Organisation World health statistics 2007. http://www.who.int/whosis/whostat/2007/en/. Accessed October 16, 2016.
- 2.Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586. [DOI] [PubMed] [Google Scholar]
- 3.Driscoll HC, Basinski J, Mulsant BH, et al. Late-onset major depression: clinical and treatment-response variability. Int J Geriatr Psychiatry. 2005;20(7):661-667. [DOI] [PubMed] [Google Scholar]
- 4.Hardeveld F, Spijker J, De Graaf R, et al. Recurrence of major depressive disorder across different treatment settings: results from the NESDA study. J Affect Disord. 2013;147(1-3):225-231. [DOI] [PubMed] [Google Scholar]
- 5.Hardeveld F, Spijker J, De Graaf R, Nolen WA, Beekman AT. Prevalence and predictors of recurrence of major depressive disorder in the adult population. Acta Psychiatr Scand. 2010;122(3):184-191. [DOI] [PubMed] [Google Scholar]
- 6.Trivedi MH, Rush AJ, Wisniewski SR, et al. STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40. [DOI] [PubMed] [Google Scholar]
- 7.Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54(10):915-922. [DOI] [PubMed] [Google Scholar]
- 8.Baldwin RC, O’Brien J. Vascular basis of late-onset depressive disorder. Br J Psychiatry. 2002;180:157-160. [DOI] [PubMed] [Google Scholar]
- 9.Paroni G, Seripa D, Fontana A, et al. Klotho gene and selective serotonin reuptake inhibitors: response to treatment in late-life major depressive disorder. Mol Neurobiol. 2017;54(2):1340-1351. [DOI] [PubMed] [Google Scholar]
- 10.Souery D, Papakostas GI, Trivedi MH. Treatment-resistant depression. J Clin Psychiatry. 2006;67(suppl 6):16-22. [PubMed] [Google Scholar]
- 11.de Groot JC, de Leeuw FE, Oudkerk M, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and depressive symptoms in elderly adults. Arch Gen Psychiatry. 2000;57(11):1071-1076. [DOI] [PubMed] [Google Scholar]
- 12.Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154(4):497-501. [DOI] [PubMed] [Google Scholar]
- 13.van Sloten TT, Sigurdsson S, van Buchem MA, et al. Cerebral small vessel disease and association with higher incidence of depressive symptoms in a general elderly population: the AGES-Reykjavik Study. Am J Psychiatry. 2015;172(6):570-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Direk N, Koudstaal PJ, Hofman A, Ikram MA, Hoogendijk WJ, Tiemeier H. Cerebral hemodynamics and incident depression: the Rotterdam Study. Biol Psychiatry. 2012;72(4):318-323. [DOI] [PubMed] [Google Scholar]
- 15.Bakker SL, de Leeuw FE, de Groot JC, Hofman A, Koudstaal PJ, Breteler MM. Cerebral vasomotor reactivity and cerebral white matter lesions in the elderly. Neurology. 1999;52(3):578-583. [DOI] [PubMed] [Google Scholar]
- 16.Van den Berg MD, Oldehinkel AJ, Bouhuys AL, Brilman EI, Beekman AT, Ormel J. Depression in later life: three etiologically different subgroups. J Affect Disord. 2001;65(1):19-26. [DOI] [PubMed] [Google Scholar]
- 17.Paranthaman R, Greenstein AS, Burns AS, et al. Vascular function in older adults with depressive disorder. Biol Psychiatry. 2010;68(2):133-139. [DOI] [PubMed] [Google Scholar]
- 18.Taylor WD, Kudra K, Zhao Z, Steffens DC, MacFall JR. Cingulum bundle white matter lesions influence antidepressant response in late-life depression: a pilot study. J Affect Disord. 2014;162:8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Leonards CO, Sterzer P, Ebinger M. White matter lesions and depression: a systematic review and meta-analysis. J Psychiatr Res. 2014;56:56-64. [DOI] [PubMed] [Google Scholar]
- 20.Saavedra Perez HC, Direk N, Hofman A, Vernooij MW, Tiemeier H, Ikram MA. Silent brain infarcts: a cause of depression in the elderly? Psychiatry Res. 2013;211(2):180-182. [DOI] [PubMed] [Google Scholar]
- 21.van Sloten TT, Schram MT, Adriaanse MC, et al. Endothelial dysfunction is associated with a greater depressive symptom score in a general elderly population: the Hoorn Study. Psychol Med. 2014;44(7):1403-1416. [DOI] [PubMed] [Google Scholar]
- 22.Katon WJ, Lin EH, Russo J, et al. Cardiac risk factors in patients with diabetes mellitus and major depression. J Gen Intern Med. 2004;19(12):1192-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer MJ, Xie D, Jordan N, et al. ; CRIC Study Group Investigators . Factors associated with depressive symptoms and use of antidepressant medications among participants in the Chronic Renal Insufficiency Cohort (CRIC) and Hispanic-CRIC Studies. Am J Kidney Dis. 2012;60(1):27-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed October 16, 2016.
- 26.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0. Chichester, England: The Cochrane Collaboration/Wiley; 2011. [Google Scholar]
- 27.Dinnes J, Deeks J, Kirby J, Roderick P. A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy. Health Technol Assess. 2005;9(12):1-113, iii. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455-463. [DOI] [PubMed] [Google Scholar]
- 31.R Development Core Team R: A Language and Environment for Statistical Computing. Version 3.3.1. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- 32.Dimopoulos N, Piperi C, Salonicioti A, et al. Elevation of plasma concentration of adhesion molecules in late-life depression. Int J Geriatr Psychiatry. 2006;21(10):965-971. [DOI] [PubMed] [Google Scholar]
- 33.Lespérance F, Frasure-Smith N, Théroux P, Irwin M. The association between major depression and levels of soluble intercellular adhesion molecule 1, interleukin-6, and C-reactive protein in patients with recent acute coronary syndromes. Am J Psychiatry. 2004;161(2):271-277. [DOI] [PubMed] [Google Scholar]
- 34.Thomas AJ, Morris C, Davis S, Jackson E, Harrison R, O’Brien JT. Soluble cell adhesion molecules in late-life depression. Int Psychogeriatr. 2007;19(5):914-920. [DOI] [PubMed] [Google Scholar]
- 35.van Dooren FE, Schram MT, Schalkwijk CG, et al. Associations of low grade inflammation and endothelial dysfunction with depression—the Maastricht Study. Brain Behav Immun. 2016;56:390-396. [DOI] [PubMed] [Google Scholar]
- 36.Do DP, Dowd JB, Ranjit N, House JS, Kaplan GA. Hopelessness, depression, and early markers of endothelial dysfunction in US adults. Psychosom Med. 2010;72(7):613-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tchalla AE, Wellenius GA, Sorond FA, Travison TG, Dantoine T, Lipsitz LA. Elevated circulating vascular cell adhesion molecule-1 (sVCAM-1) is associated with concurrent depressive symptoms and cerebral white matter hyperintensities in older adults. BMC Geriatr. 2015;15:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tully PJ, Baumeister H, Martin S, et al. ; Florey Adelaide Male Ageing Study . Elucidating the biological mechanisms linking depressive symptoms with type 2 diabetes in men: the longitudinal effects of inflammation, microvascular dysfunction, and testosterone. Psychosom Med. 2016;78(2):221-232. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen TT, Wong TY, Islam FM, et al. Evidence of early retinal microvascular changes in patients with type 2 diabetes and depression. Psychosom Med. 2010;72(6):535-538. [DOI] [PubMed] [Google Scholar]
- 40.Ikram MK, Luijendijk HJ, Hofman A, et al. Retinal vascular calibers and risk of late-life depression: The Rotterdam Study. Am J Geriatr Psychiatry. 2010;18(5):452-455. [DOI] [PubMed] [Google Scholar]
- 41.Aizenstein HJ, Andreescu C, Edelman KL, et al. fMRI correlates of white matter hyperintensities in late-life depression. Am J Psychiatry. 2011;168(10):1075-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Almeida JRC, Alves TCTF, Wajngarten M, et al. Late-life depression, heart failure and frontal white matter hyperintensity: a structural magnetic resonance imaging study. Braz J Med Biol Res. 2005;38(3):431-436. doi: 10.1590/S0100-879X2005000300014 [DOI] [PubMed] [Google Scholar]
- 43.Bella R, Pennisi G, Cantone M, et al. Clinical presentation and outcome of geriatric depression in subcortical ischemic vascular disease. Gerontology. 2010;56(3):298-302. [DOI] [PubMed] [Google Scholar]
- 44.Chatterjee K, Fall S, Barer D. Mood after stroke: a case control study of biochemical, neuro-imaging and socio-economic risk factors for major depression in stroke survivors. BMC Neurol. 2010;10:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y, Chen X, Mok VC, Lam WW, Wong KS, Tang WK. Poststroke depression in patients with small subcortical infarcts. Clin Neurol Neurosurg. 2009;111(3):256-260. [DOI] [PubMed] [Google Scholar]
- 46.Colloby SJ, Vasudev A, O’Brien JT, Firbank MJ, Parry SW, Thomas AJ. Relationship of orthostatic blood pressure to white matter hyperintensities and subcortical volumes in late-life depression. Br J Psychiatry. 2011;199(5):404-410. [DOI] [PubMed] [Google Scholar]
- 47.Cyprien F, Courtet P, Poulain V, et al. Corpus callosum size may predict late-life depression in women: a 10-year follow-up study. J Affect Disord. 2014;165:16-23. [DOI] [PubMed] [Google Scholar]
- 48.Dalby RB, Chakravarty MM, Ahdidan J, et al. Localization of white-matter lesions and effect of vascular risk factors in late-onset major depression. Psychol Med. 2010;40(8):1389-1399. [DOI] [PubMed] [Google Scholar]
- 49.Delaloye C, Moy G, de Bilbao F, et al. Neuroanatomical and neuropsychological features of elderly euthymic depressed patients with early- and late-onset. J Neurol Sci. 2010;299(1-2):19-23. [DOI] [PubMed] [Google Scholar]
- 50.Dotson VM, Zonderman AB, Kraut MA, Resnick SM. Temporal relationships between depressive symptoms and white matter hyperintensities in older men and women. Int J Geriatr Psychiatry. 2013;28(1):66-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feng C, Fang M, Xu Y, Hua T, Liu XY. Microbleeds in late-life depression: comparison of early- and late-onset depression. Biomed Res Int. 2014;2014:682092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Firbank MJ, O’Brien JT, Pakrasi S, et al. White matter hyperintensities and depression—preliminary results from the LADIS study. Int J Geriatr Psychiatry. 2005;20(7):674-679. [DOI] [PubMed] [Google Scholar]
- 53.Fujishima M, Maikusa N, Nakamura K, Nakatsuka M, Matsuda H, Meguro K. Mild cognitive impairment, poor episodic memory, and late-life depression are associated with cerebral cortical thinning and increased white matter hyperintensities. Front Aging Neurosci. 2014;6:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greenwald BS, Kramer-Ginsberg E, Krishnan KR, Ashtari M, Auerbach C, Patel M. Neuroanatomic localization of magnetic resonance imaging signal hyperintensities in geriatric depression. Stroke. 1998;29(3):613-617. [DOI] [PubMed] [Google Scholar]
- 55.Gudmundsson LS, Scher AI, Sigurdsson S, et al. Migraine, depression, and brain volume: the AGES-Reykjavik Study. Neurology. 2013;80(23):2138-2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hannestad J, Taylor WD, McQuoid DR, et al. White matter lesion volumes and caudate volumes in late-life depression. Int J Geriatr Psychiatry. 2006;21(12):1193-1198. [DOI] [PubMed] [Google Scholar]
- 57.Iosifescu DV, Papakostas GI, Lyoo IK, et al. Brain MRI white matter hyperintensities and one-carbon cycle metabolism in non-geriatric outpatients with major depressive disorder: part I. Psychiatry Res. 2005;140(3):291-299. [DOI] [PubMed] [Google Scholar]
- 58.Janssen J, Hulshoff Pol HE, Lampe IK, et al. Hippocampal changes and white matter lesions in early-onset depression. Biol Psychiatry. 2004;56(11):825-831. [DOI] [PubMed] [Google Scholar]
- 59.Janssen J, Hulshoff Pol HE, Schnack HG, et al. Cerebral volume measurements and subcortical white matter lesions and short-term treatment response in late life depression. Int J Geriatr Psychiatry. 2007;22(5):468-474. [DOI] [PubMed] [Google Scholar]
- 60.Jorm AF, Anstey KJ, Christensen H, et al. MRI hyperintensities and depressive symptoms in a community sample of individuals 60-64 years old. Am J Psychiatry. 2005;162(4):699-705. [DOI] [PubMed] [Google Scholar]
- 61.Kieseppä T, Mäntylä R, Tuulio-Henriksson A, et al. White matter hyperintensities and cognitive performance in adult patients with bipolar I, bipolar II, and major depressive disorders. Eur Psychiatry. 2014;29(4):226-232. doi: 10.1016/j.eurpsy.2013.08.002 [DOI] [PubMed] [Google Scholar]
- 62.Köhler S, Thomas AJ, Lloyd A, Barber R, Almeida OP, O’Brien JT. White matter hyperintensities, cortisol levels, brain atrophy and continuing cognitive deficits in late-life depression. Br J Psychiatry. 2010;196(2):143-149. [DOI] [PubMed] [Google Scholar]
- 63.Krishnan MS, O’Brien JT, Firbank MJ, et al. LADIS Group. Relationship between periventricular and deep white matter lesions and depressive symptoms in older people: The LADIS Study. Int J Geriatr Psychiatry. 2006;21(10):983-989. [DOI] [PubMed] [Google Scholar]
- 64.Kumar A, Bilker W, Jin Z, Udupa J. Atrophy and high intensity lesions: complementary neurobiological mechanisms in late-life major depression. Neuropsychopharmacology. 2000;22(3):264-274. doi: 10.1016/S0893-133X(99)00124-4 [DOI] [PubMed] [Google Scholar]
- 65.Lee SH, Payne ME, Steffens DC, et al. Subcortical lesion severity and orbitofrontal cortex volume in geriatric depression. Biol Psychiatry. 2003;54(5):529-533. [DOI] [PubMed] [Google Scholar]
- 66.Lin HF, Kuo YT, Chiang IC, Chen HM, Chen CS. Structural abnormality on brain magnetic resonance imaging in late-onset major depressive disorder. Kaohsiung J Med Sci. 2005;21(9):405-411. [DOI] [PubMed] [Google Scholar]
- 67.MacFall JR, Taylor WD, Rex DE, et al. Lobar distribution of lesion volumes in late-life depression: the Biomedical Informatics Research Network (BIRN). Neuropsychopharmacology. 2006;31(7):1500-1507. doi: 10.1038/sj.npp.1300986 [DOI] [PubMed] [Google Scholar]
- 68.Murray AD, Staff RT, McNeil CJ, et al. Depressive symptoms in late life and cerebrovascular disease: the importance of intelligence and lesion location. Depress Anxiety. 2013;30(1):77-84. [DOI] [PubMed] [Google Scholar]
- 69.Nys GM, van Zandvoort MJ, van der Worp HB, de Haan EH, de Kort PL, Kappelle LJ. Early depressive symptoms after stroke: neuropsychological correlates and lesion characteristics. J Neurol Sci. 2005;228(1):27-33. [DOI] [PubMed] [Google Scholar]
- 70.Olesen PJ, Gustafson DR, Simoni M, et al. Temporal lobe atrophy and white matter lesions are related to major depression over 5 years in the elderly. Neuropsychopharmacology. 2010;35(13):2638-2645. doi: 10.1038/npp.2010.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shimony JS, Sheline YI, D’Angelo G, et al. Diffuse microstructural abnormalities of normal-appearing white matter in late life depression: a diffusion tensor imaging study. Biol Psychiatry. 2009;66(3):245-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sheline YI, Price JL, Vaishnavi SN, et al. Regional white matter hyperintensity burden in automated segmentation distinguishes late-life depressed subjects from comparison subjects matched for vascular risk factors. Am J Psychiatry. 2008;165(4):524-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steffens DC, Helms MJ, Krishnan KR, Burke GL. Cerebrovascular disease and depression symptoms in the cardiovascular health study. Stroke. 1999;30(10):2159-2166. [DOI] [PubMed] [Google Scholar]
- 74.Tang WK, Chen YK, Lu JY, et al. White matter hyperintensities in post-stroke depression: a case control study. J Neurol Neurosurg Psychiatry. 2010;81(12):1312-1315. [DOI] [PubMed] [Google Scholar]
- 75.Taylor WD, MacFall JR, Payne ME, et al. Greater MRI lesion volumes in elderly depressed subjects than in control subjects. Psychiatry Res. 2005;139(1):1-7. [DOI] [PubMed] [Google Scholar]
- 76.Tudorascu DL, Rosano C, Venkatraman VK, et al. Multimodal MRI markers support a model of small vessel ischemia for depressive symptoms in very old adults. Psychiatry Res. 2014;224(2):73-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tupler LA, Krishnan KR, McDonald WM, Dombeck CB, D’Souza S, Steffens DC. Anatomic location and laterality of MRI signal hyperintensities in late-life depression. J Psychosom Res. 2002;53(2):665-676. [DOI] [PubMed] [Google Scholar]
- 78.van Uden IWM, van Norden AGW, de Laat KF, et al. Depressive symptoms and amygdala volume in elderly with cerebral small vessel disease: The RUN DMC Study. J Aging Res. 2011;2011:647869. doi: 10.4061/2011/647869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vardi N, Freedman N, Lester H, et al. Hyperintensities on T2-weighted images in the basal ganglia of patients with major depression: cerebral perfusion and clinical implications. Psychiatry Res. 2011;192(2):125-130. [DOI] [PubMed] [Google Scholar]
- 80.Vataja R, Pohjasvaara T, Leppävuori A, et al. Magnetic resonance imaging correlates of depression after ischemic stroke. Arch Gen Psychiatry. 2001;58(10):925-931. [DOI] [PubMed] [Google Scholar]
- 81.Videbech P, Ravnkilde B, Fiirgaard B, et al. Structural brain abnormalities in unselected in-patients with major depression. Acta Psychiatr Scand. 2001;103(4):282-286. [DOI] [PubMed] [Google Scholar]
- 82.Wu RH, Feng C, Xu Y, Hua T, Liu XY, Fang M. Late-onset depression in the absence of stroke: associated with silent brain infarctions, microbleeds and lesion locations. Int J Med Sci. 2014;11(6):587-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Devantier TA, Nørgaard BL, Poulsen MK, et al. White matter lesions, carotid and coronary atherosclerosis in late-onset depression and healthy controls. Psychosomatics. 2016;57(4):369-377. [DOI] [PubMed] [Google Scholar]
- 84.Godin O, Dufouil C, Maillard P, et al. White matter lesions as a predictor of depression in the elderly: the 3C-Dijon study. Biol Psychiatry. 2008;63(7):663-669. [DOI] [PubMed] [Google Scholar]
- 85.Grool AM, Gerritsen L, Zuithoff NP, Mali WP, van der Graaf Y, Geerlings MI. Lacunar infarcts in deep white matter are associated with higher and more fluctuating depressive symptoms during three years follow-up. Biol Psychiatry. 2013;73(2):169-176. [DOI] [PubMed] [Google Scholar]
- 86.Lavretsky H, Zheng L, Weiner MW, et al. The MRI brain correlates of depressed mood, anhedonia, apathy, and anergia in older adults with and without cognitive impairment or dementia. Int J Geriatr Psychiatry. 2008;23(10):1040-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Potter GG, Blackwell AD, McQuoid DR, et al. Prefrontal white matter lesions and prefrontal task impersistence in depressed and nondepressed elders. Neuropsychopharmacology. 2007;32(10):2135-2142. doi: 10.1038/sj.npp.1301339 [DOI] [PubMed] [Google Scholar]
- 88.Versluis CE, van der Mast RC, van Buchem MA, et al. PROSPER Study. Progression of cerebral white matter lesions is not associated with development of depressive symptoms in elderly subjects at risk of cardiovascular disease: The PROSPER Study. Int J Geriatr Psychiatry. 2006;21(4):375-381. [DOI] [PubMed] [Google Scholar]
- 89.Steffens DC, Krishnan KR, Crump C, Burke GL. Cerebrovascular disease and evolution of depressive symptoms in the cardiovascular health study. Stroke. 2002;33(6):1636-1644. [DOI] [PubMed] [Google Scholar]
- 90.Teodorczuk A, Firbank MJ, Pantoni L, et al. LADIS Group. Relationship between baseline white-matter changes and development of late-life depressive symptoms: 3-year results from the LADIS study. Psychol Med. 2010;40(4):603-610. [DOI] [PubMed] [Google Scholar]
- 91.Direk N, Perez HS, Akoudad S, et al. Markers of cerebral small vessel disease and severity of depression in the general population. Psychiatry Res. 2016;253:1-6. [DOI] [PubMed] [Google Scholar]
- 92.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28(3):652-659. [DOI] [PubMed] [Google Scholar]
- 93.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9(7):689-701. [DOI] [PubMed] [Google Scholar]
- 94.Herrmann LL, Le Masurier M, Ebmeier KP. White matter hyperintensities in late life depression: a systematic review. J Neurol Neurosurg Psychiatry. 2008;79(6):619-624. [DOI] [PubMed] [Google Scholar]
- 95.Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18(9):963-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alexopoulos GS. Vascular disease, depression, and dementia. J Am Geriatr Soc. 2003;51(8):1178-1180. [DOI] [PubMed] [Google Scholar]
- 97.Alexopoulos GS. The vascular depression hypothesis: 10 years later. Biol Psychiatry. 2006;60(12):1304-1305. [DOI] [PubMed] [Google Scholar]
- 98.Santos M, Xekardaki A, Kövari E, Gold G, Bouras C, Giannakopoulos P. Microvascular pathology in late-life depression. J Neurol Sci. 2012;322(1-2):46-49. [DOI] [PubMed] [Google Scholar]
- 99.van Dijk EJ, Prins ND, Vermeer SE, et al. C-reactive protein and cerebral small-vessel disease: The Rotterdam Scan Study. Circulation. 2005;112(6):900-905. [DOI] [PubMed] [Google Scholar]
- 100.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Banks WA. The blood-brain barrier in psychoneuroimmunology. Immunol Allergy Clin North Am. 2009;29(2):223-228. [DOI] [PubMed] [Google Scholar]
- 102.Stehouwer CD, Gall MA, Twisk JW, Knudsen E, Emeis JJ, Parving HH. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes. 2002;51(4):1157-1165. [DOI] [PubMed] [Google Scholar]
- 103.Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11(6):851-876. [DOI] [PubMed] [Google Scholar]
- 104.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44-84. [DOI] [PubMed] [Google Scholar]
- 105.Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97(6):1634-1658. [DOI] [PubMed] [Google Scholar]
- 106.Vavakova M, Durackova Z, Trebaticka J. Markers of oxidative stress and neuroprogression in depression disorder. Oxid Med Cell Longev. 2015;2015:898393. doi: 10.1155/2015/898393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hovatta I, Juhila J, Donner J. Oxidative stress in anxiety and comorbid disorders. Neurosci Res. 2010;68(4):261-275. [DOI] [PubMed] [Google Scholar]
- 108.van Sloten TT, Mitchell GF, Sigurdsson S, et al. Associations between arterial stiffness, depressive symptoms and cerebral small vessel disease: cross-sectional findings from the AGES-Reykjavik Study. J Psychiatry Neurosci. 2016;41(3):162-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol (1985). 2008;105(5):1652-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46(1):200-204. [DOI] [PubMed] [Google Scholar]
- 111.Machado MV, Vieira AB, Nascimento AR, et al. Physical exercise restores microvascular function in obese rats with metabolic syndrome. Metab Syndr Relat Disord. 2014;12(9):484-492. [DOI] [PubMed] [Google Scholar]
- 112.Rossi M, Pistelli F, Pesce M, et al. Impact of long-term exposure to cigarette smoking on skin microvascular function. Microvasc Res. 2014;93:46-51. [DOI] [PubMed] [Google Scholar]
- 113.Muris DM, Houben AJ, Kroon AA, et al. Age, waist circumference, and blood pressure are associated with skin microvascular flow motion: the Maastricht Study. J Hypertens. 2014;32(12):2439-2449. [DOI] [PubMed] [Google Scholar]
- 114.Karaca Ü, Schram MT, Houben AJ, Muris DM, Stehouwer CD. Microvascular dysfunction as a link between obesity, insulin resistance and hypertension. Diabetes Res Clin Pract. 2014;103(3):382-387. [DOI] [PubMed] [Google Scholar]
- 115.Muris DM, Houben AJ, Schram MT, Stehouwer CD. Microvascular dysfunction is associated with a higher incidence of type 2 diabetes mellitus: a systematic review and meta-analysis. Arterioscler Thromb Vasc Biol. 2012;32(12):3082-3094. [DOI] [PubMed] [Google Scholar]
- 116.Verger P, Lions C, Ventelou B. Is depression associated with health risk-related behaviour clusters in adults? Eur J Public Health. 2009;19(6):618-624. [DOI] [PubMed] [Google Scholar]
- 117.Aoqui C, Chmielewski S, Scherer E, et al. Microvascular dysfunction in the course of metabolic syndrome induced by high-fat diet. Cardiovasc Diabetol. 2014;13:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Penninx BW. Depression and cardiovascular disease: Epidemiological evidence on their linking mechanisms. Neurosci Biobehav Rev. 2017;74(pt B):277-286. [DOI] [PubMed] [Google Scholar]
- 119.de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6(6):463-475. [DOI] [PubMed] [Google Scholar]
- 120.Moody DM, Brown WR, Challa VR, Ghazi-Birry HS, Reboussin DM. Cerebral microvascular alterations in aging, leukoaraiosis, and Alzheimer’s disease. Ann N Y Acad Sci. 1997;826:103-116. [DOI] [PubMed] [Google Scholar]
- 121.González HM, Tarraf W, Whitfield K, Gallo JJ. Vascular depression prevalence and epidemiology in the United States. J Psychiatr Res. 2012;46(4):456-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12(5):483-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Østergaard L, Engedal TS, Moreton F, et al. Cerebral small vessel disease: capillary pathways to stroke and cognitive decline. J Cereb Blood Flow Metab. 2016;36(2):302-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Benjamin P, Viessmann O, MacKinnon AD, Jezzard P, Markus HS. 7 Tesla MRI in cerebral small vessel disease. Int J Stroke. 2015;10(5):659-664. [DOI] [PubMed] [Google Scholar]
- 125.Brookes RL, Herbert V, Lawrence AJ, Morris RG, Markus HS. Depression in small-vessel disease relates to white matter ultrastructural damage, not disability. Neurology. 2014;83(16):1417-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lamar M, Charlton RA, Morris RG, Markus HS. The impact of subcortical white matter disease on mood in euthymic older adults: a diffusion tensor imaging study. Am J Geriatr Psychiatry. 2010;18(7):634-642. [DOI] [PubMed] [Google Scholar]
- 127.van Uden IW, Tuladhar AM, de Laat KF, et al. White matter integrity and depressive symptoms in cerebral small vessel disease: the RUN DMC study. Am J Geriatr Psychiatry. 2015;23(5):525-535. [DOI] [PubMed] [Google Scholar]
- 128.Martens RJ, Vink H, van Oostenbrugge RJ, Staals J. Sublingual microvascular glycocalyx dimensions in lacunar stroke patients. Cerebrovasc Dis. 2013;35(5):451-454. [DOI] [PubMed] [Google Scholar]
- 129.Sörensen BM, Houben AJ, Berendschot TT, et al. Prediabetes and type 2 diabetes are associated with generalized microvascular dysfunction: the Maastricht Study. Circulation. 2016;134(18):1339-1352. [DOI] [PubMed] [Google Scholar]
- 130.Gronenschild EH, Muris DM, Schram MT, Karaca U, Stehouwer CD, Houben AJ. Semi-automatic assessment of skin capillary density: proof of principle and validation. Microvasc Res. 2013;90:192-198. [DOI] [PubMed] [Google Scholar]
- 131.Mangiacapra F, Peace AJ, Di Serafino L, et al. Intracoronary EnalaPrilat to Reduce MICROvascular Damage During Percutaneous Coronary Intervention (ProMicro) Study. J Am Coll Cardiol. 2013;61(6):615-621. [DOI] [PubMed] [Google Scholar]
- 132.Fujii K, Kawasaki D, Oka K, et al. The impact of pravastatin pre-treatment on periprocedural microcirculatory damage in patients undergoing percutaneous coronary intervention. JACC Cardiovasc Interv. 2011;4(5):513-520. [DOI] [PubMed] [Google Scholar]
- 133.Holowatz LA, Santhanam L, Webb A, Berkowitz DE, Kenney WL. Oral atorvastatin therapy restores cutaneous microvascular function by decreasing arginase activity in hypercholesterolaemic humans. J Physiol. 2011;589(pt 8):2093-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lanting SM, Johnson NA, Baker MK, Caterson ID, Chuter VH. The effect of exercise training on cutaneous microvascular reactivity: a systematic review and meta-analysis. J Sci Med Sport. 2017;20(2):170-177. [DOI] [PubMed] [Google Scholar]
- 135.Leardini-Tristao M, Borges JP, Freitas F, et al. The impact of early aerobic exercise on brain microvascular alterations induced by cerebral hypoperfusion. Brain Res. 2017;1657:43-51. [DOI] [PubMed] [Google Scholar]
- 136.Espeland MA, Erickson K, Neiberg RH, et al. Action for Health in Diabetes Brain Magnetic Resonance Imaging (Look AHEAD Brain) Ancillary Study Research Group. Brain and white matter hyperintensity volumes after 10 years of random assignment to lifestyle intervention. Diabetes Care. 2016;39(5):764-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vuckovic A, Cohen BM, Zubenko GS. The use of captopril in treatment-resistant depression: an open trial. J Clin Psychopharmacol. 1991;11(6):395-396. [DOI] [PubMed] [Google Scholar]
- 138.Celano CM, Freudenreich O, Fernandez-Robles C, Stern TA, Caro MA, Huffman JC. Depressogenic effects of medications: a review. Dialogues Clin Neurosci. 2011;13(1):109-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Germain L, Chouinard G. Captopril treatment of major depression with serial measurements of blood cortisol concentrations. Biol Psychiatry. 1989;25(4):489-493. [DOI] [PubMed] [Google Scholar]
- 140.While A, Keen L. The effects of statins on mood: a review of the literature. Eur J Cardiovasc Nurs. 2012;11(1):85-96. [DOI] [PubMed] [Google Scholar]
- 141.Santanello NC, Barber BL, Applegate WB, et al. Effect of pharmacologic lipid lowering on health-related quality of life in older persons: results from the Cholesterol Reduction in Seniors Program (CRISP) Pilot Study. J Am Geriatr Soc. 1997;45(1):8-14. [DOI] [PubMed] [Google Scholar]
- 142.Muldoon MF, Barger SD, Ryan CM, et al. Effects of lovastatin on cognitive function and psychological well-being. Am J Med. 2000;108(7):538-546. [DOI] [PubMed] [Google Scholar]
- 143.Stewart RA, Sharples KJ, North FM, Menkes DB, Baker J, Simes J; The LIPID Study Investigators . Long-term assessment of psychological well-being in a randomized placebo-controlled trial of cholesterol reduction with pravastatin. Arch Intern Med. 2000;160(20):3144-3152. [DOI] [PubMed] [Google Scholar]
- 144.Yang CC, Jick SS, Jick H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med. 2003;163(16):1926-1932. [DOI] [PubMed] [Google Scholar]
- 145.Young-Xu Y, Chan KA, Liao JK, Ravid S, Blatt CM. Long-term statin use and psychological well-being. J Am Coll Cardiol. 2003;42(4):690-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Search Terms Used for the Systematic Review and Meta-analysis
eFigure 1. Funnel Plot of Cross-Sectional Studies on the Association Between Microvascular Dysfunction and Depression; Plasma Markers for Endothelial Function (A), WMH (B), Microbleeds (C) And Microinfarctions (D)
eFigure 2. Forest Plots With the Odds Ratios and 95% Confidence Intervals for Original Studies and the Pooled Odds Ratios for the Cross-Sectional Association Between Cerebral Microbleeds and Depression
eFigure 3. Forest Plots With the Odds Ratios and 95% Confidence Intervals for Original Studies and the Pooled Odds Ratios for the Cross-Sectional Association Between Cerebral (Micro)Infarctions and Depression
eFigure 4. Funnel Plot of Cross-Sectional Studies on the Association Between White Matter Hyperintensities and Depression After Trim-and-Fill Analysis
eFigure 5. Funnel Plot of Longitudinal Studies on the Association Between Cerebral Small Vessel Disease and Depression
eTable 1. Quality Assessment of the Included Studies by Use Of the Newcastle-Ottawa Scale (NOS)
eTable 2. Characteristics of Studies Included in the Systematic Review and Meta-Analysis
eTable 3. Reported Results of Studies Included in the Systematic Review and Meta-analysis
eReferences.