This study examines the association of time of onset of autonomic dysfunction with disease progression and survival in Parkinson disease.
Key Points
Question
Is development of autonomic dysfunction associated with disease progression and survival in patients with Parkinson disease?
Findings
In this study of 100 patients with autopsy-confirmed Parkinson disease, earlier development of autonomic dysfunction was associated with an increase in the risk of reaching a disease milestone of 14% per year and an increase in the risk of death of 8% per year.
Meaning
Earlier autonomic dysfunction is associated with more rapid disease progression and shorter survival in patients with Parkinson disease.
Abstract
Importance
Evidence suggests that development of autonomic dysfunction (AutD) may negatively affect disease course and survival in patients with synucleinopathies. However, the few available studies on Parkinson disease (PD) have conflicting results, comprise a small number of patients, have short follow-up periods, and lack pathologic confirmation of the diagnosis.
Objective
To examine the association of time of onset of AutD with disease progression and survival in PD.
Design, Setting, and Participants
This retrospective review of clinical data from 100 consecutive patients with an autopsy-confirmed diagnosis of PD from the archives of the Queen Square Brain Bank in London, United Kingdom, from January 1, 2006, to June 3, 2016, included patients with PD regularly seen by hospital specialists (neurologists or geriatricians) in the United Kingdom throughout their disease until death. Patients with dementia before or within 1 year after onset of motor symptoms, monogenic forms of PD, comorbidities that affect autonomic function, a coexisting neuropathologic diagnosis, or insufficient clinical information were excluded.
Main Outcomes and Measures
Survival and time from diagnosis to specific disease milestones were calculated to assess disease progression. Autonomic dysfunction was defined as autonomic failure at autonomic function testing or 2 of the following symptoms: urinary symptoms, constipation, upper gastrointestinal tract dysfunction, orthostatic hypotension, sweating abnormalities, or erectile dysfunction. Multivariable Cox proportional hazards regression models on the risk of a disease milestone and death were used.
Results
A total of 100 patients (60 [60.0%] male; mean [SD] age at diagnosis, 63.9 [10.3] years; mean [SD] disease duration, 14.6 [7.7] years) were studied. Autonomic dysfunction developed in 85 patients (mean [SD] time from diagnosis, 6.7 [7.7] years) and was associated with older age at diagnosis, male sex, poor initial levodopa treatment response, and postural instability and gait difficulty motor PD subtype in linear regression analysis, but staging of α-synuclein pathologic changes was unrelated. Earlier AutD increased the risk of reaching the first milestone (hazard ratio, 0.86; 95% CI, 0.83-0.89; P < .001) and shortened survival (hazard ratio, 0.92; 95% CI, 0.88-0.96; P < .001). Older age at diagnosis and poorer levodopa treatment response were the other factors associated with shorter survival in adjusted multivariate analysis.
Conclusions and Relevance
Earlier AutD is associated with a more rapid development of disease milestones and shorter survival in patients with PD.
Introduction
Parkinson disease (PD) is clinically heterogeneous with respect to motor and nonmotor symptoms, disease course, and response to treatments. Assessment of the disease course and prognosis is crucial to provide adequate treatment, support, and counseling. Older age at diagnosis and the postural instability and gait difficulty subtype have been consistently identified as determinants of disability, whereas increasing age and dementia have been associated with shorter survival.
Autonomic dysfunction (AutD) is common in PD and includes orthostatic hypotension, constipation, bladder disturbances, and sexual dysfunction. Early AutD has been consistently associated with shorter survival in multiple system atrophy and has also been suggested as a determinant of survival in other synucleinopathies, such as dementia with Lewy bodies. Studies that assessed the prognostic association of AutD with PD are scarce, do not generally assess AutD systematically, and lack pathologic confirmation of the diagnosis. The aim of this study was to investigate whether the time from diagnosis to development of AutD has a prognostic association with disease progression and survival in a large series of patients with PD with well-documented disease progression, follow-up until death, and postmortem confirmation of the diagnosis.
Methods
Study Design
One hundred patients with a pathologic diagnosis of PD were selected from a cohort of consecutive patients from January 1, 2006, to June 3, 2016, whose brains were donated after death to the Queen Square Brain Bank for Neurological Disorders in London, United Kingdom. Patients with the following criteria were excluded: (1) diagnosis of dementia before or within 1 year after onset of motor symptoms, (2) monogenic forms of PD (eg, leucine-rich repeat kinase 2 gene [OMIM 609007], parkin, or α-synuclein), (3) coexistent neuropathologic diagnosis deemed to affect PD progression (eg, Alzheimer disease–related neurofibrillary tangle pathologic changes with Braak and Braak stage greater than IV or pathologic changes consistent with other concomitant neurodegenerative conditions), (4) comorbidities known to affect the autonomic nervous system (eg, diabetic ganglionopathies or neuropathies), and (5) insufficient information documenting autonomic symptoms and disease progression. The brain donor program was approved by a London Multi-Centre Research Ethics Committee, tissue was stored for research under a license from the Human Tissue Authority, and written informed consent was obtained from all donors. Data were pseudoanonymized for the study.
Clinical Assessment
All patients were regularly assessed throughout their illness by hospital specialists (neurologists or geriatricians) in the United Kingdom. A systematic review of the medical records was performed by a neurologist with expertise in movement disorders (E.D.P.-F.). Clinical features and disease progression data were collected. Date of onset of each feature was documented, and the time from diagnosis was calculated.
Autonomic dysfunction was defined by cardiovascular autonomic failure at autonomic function testing or documentation of any 2 of the following symptoms or signs persistent for longer than 6 months: (1) urinary urgency, increased daytime frequency, and nocturia without hesitancy as defined by the International Continence Society; (2) constipation (<3 defecations per week, having to strain to pass stools, or regular use of laxatives); (3) symptoms of upper gastrointestinal tract dysfunction, including nausea, bloating, and early satiety; (4) symptomatic or documented orthostatic hypotension defined by a greater than 20–mm Hg decrease in systolic blood pressure or a greater than 10–mm Hg decrease in diastolic blood pressure on standing; (5) sweating abnormalities; or (6) erectile dysfunction in males. These symptoms cover the principal domains of autonomic function, and they were selected because they are well documented in medical records and are clinically relevant and easily assessed in clinical settings, where availability of neurophysiologic autonomic testing may be limited. Because some of these symptoms may be multifactorial and may be influenced by other nonneurologic factors, more than one symptom with a persistent duration was required.
Six milestones were selected to define disease progression: (1) frequent falls defined by more than 2 episodes per year, (2) cognitive impairment severe enough to significantly affect tasks of daily living, (3) unintelligible speech or the offering of communication aids, (4) severe dysphagia or the offering of percutaneous endoscopic gastrostomy, (5) dependence on wheelchair for mobility, and (6) placement in residential or nursing home care. These milestones have been selected because they represent the different domains of impairment of functioning in PD, including motor progression, cognitive impairment, and global disability. They are clinically relevant and, as such, are well documented in the medical records.
Other demographic data and clinical features were also recorded, including PD motor subtype (tremor predominant, akinetic rigid, or postural instability and gait difficulty), response to initial levodopa treatment measured on a semiquantitative scale (nil to mild, moderate, good, or excellent) based on the clinical impression documented by the treating physician, maximum levodopa equivalent dose, and clinical diagnosis at time of death. Times from diagnosis to development of AutD, each autonomic symptom, first and each disease milestone, and death were calculated.
Neuropathologic Assessment
Formalin-fixed brain tissue samples were examined using routine stains supplemented with immunohistochemical analysis in representative brain regions for Aβ peptide, τ protein (AT8 antibody), TDP-43, ubiquitin, and α-synuclein according to Queen Square Brain Bank standard protocols. Systematic histologic analysis was performed by neuropathologists (T.R., J.L.H.) masked to the clinical data on AutD. Lewy pathologic changes (Lewy bodies and Lewy neurites) were assessed using a semiquantitative grading system (absent, mild, moderate, severe, or very severe). On the basis of the pattern of regional involvement, a Lewy pathologic type (brainstem, limbic, or diffuse neocortical) was assigned for each patient.
Statistical Analysis
Summary statistics are described as number (percentage) for categorical data and mean (SD) for continuous variables. Linear regression was used to perform a preliminary analysis of potential associations among the main explanatory variables. Time from diagnosis to AutD was considered as a dependent variable, and other relevant explanatory variables (eg, age, sex, levodopa treatment response, PD motor subtype, and Lewy pathologic type) were included as determinants in the model.
To assess the association of time from PD diagnosis to AutD with the risk of developing a disease milestone or the risk of death (survival), patients were divided into 2 subgroups (eg, early vs late AutD) using the median value, and Kaplan-Meier curves were plotted. Multivariable Cox proportional hazards regression models were then used to estimate the association between the main explanatory variable and the risk of developing a disease milestone or the risk of death (survival), which were considered in turn as dependent variables. Multiple statistical tests were performed with several null hypotheses being tested (rather than one single null hypothesis with an error rate modified by every test); therefore, adjustment for multiple comparisons was considered to be inappropriate. Other explanatory variables included age, sex, PD motor subtype, and response to levodopa treatment. Adjusted hazard ratios (HRs) and 95% CIs were estimated, and a null hypothesis was rejected when 95% CIs did not include the null value. Visual inspection of Kaplan-Meier curves and plots of scaled Schoenfeld residuals against time were used to assess the proportional hazards assumption. Censoring was considered to be uninformative.
Statistical significance was set at P < .05. Statistical analyses were performed using the STATA statistical software, version 12 (StataCorp).
Results
Demographic and Clinical Characteristics
A flowchart of the study is shown in eFigure 1 in the Supplement. Patients excluded with insufficient clinical information did not differ by sex, age, disease duration, levodopa treatment response, or PD motor subtype (see eFigure 1 for statistical comparisons).
A total of 100 consecutive patients with PD who fulfilled the entry criteria (60 [60.0%] male; mean [SD] age at diagnosis, 63.9 [10.3] years; mean [SD] disease duration, 14.6 [7.7] years) were included, and their demographic and clinical characteristics are summarized in Table 1. Eighty-three patients were clinically diagnosed with PD, whereas 12 were misdiagnosed with multiple system atrophy and 5 with progressive supranuclear palsy.
Table 1. Patient Demographic and Clinical Characteristics.
| Characteristic | Finding (N = 100)a |
|---|---|
| Sex | |
| Male | 60 (60.0) |
| Female | 40 (40.0) |
| Age at diagnosis, mean (SD), y | 63.9 (10.3) |
| Age at death, mean (SD), y | 78.5 (6.9) |
| Disease duration, mean (SD), y | 14.6 (7.7) |
| Clinical diagnosis | |
| PD | 83 (83.0) |
| MSA | 12 (12.0) |
| PSP | 5 (5.0) |
| PD motor subtype | |
| Tremor | 18 (18.0) |
| AR | 66 (66.0) |
| PIGD | 16 (16.0) |
| Levodopa treatment response (N = 98) | |
| Nil to mild | 4 (4.1) |
| Moderate | 8 (8.2) |
| Good | 25 (25.5) |
| Excellent | 61 (62.2) |
| Maximum levodopa equivalent dose, mean (SD), mg | 917 (446) |
| Cause of death | |
| Infection | 45 (45.0) |
| Deterioration | 36 (36.0) |
| Cardiovascular | 4 (4.0) |
| Other | 15 (15.0) |
| Lewy pathologic type (N = 99) | |
| Brainstem | 2 (2.0) |
| Limbic | 33 (33.3) |
| Neocortical | 64 (64.6) |
| First milestone | 96 (96.0) |
| Regular falls | 83 (83.0) |
| Wheelchair | 61 (61.0) |
| Speech | 12 (12.0) |
| Severe dysphagia | 13 (13.0) |
| Cognitive | 50 (50.0) |
| Care placement | 52 (52.0) |
| AutD | 85 (85.0) |
| OH | 60 (60.0) |
| Upper GI tract symptoms | 16 (16.0) |
| Constipation | 83 (83.0) |
| Urinary | 84 (84.0) |
| Erectile dysfunction (N = 60 men) | 23 (38.3) |
| Sweating | 12 (12.0) |
Abbreviations: AutD, autonomic dysfunction; AR, akinetic rigid; GI, gastrointestinal; MSA, multiple system atrophy; OH, orthostatic hypotension; PD, Parkinson disease; PIGD, postural instability and gait difficulty; PSP, progressive supranuclear palsy.
Data are presented as number (percentage) of patients unless otherwise indicated.
At least one disease milestone was reached by 96 patients (96.0%), with a mean (SD) time from diagnosis of 9.9 (6.2) years, with regular falls (83 [83.0%]; mean [SD] time from diagnosis, 10.2 [6.4] years), use of a wheelchair (61 [61.0%]; mean [SD] time from diagnosis, 11.6 [7.3] years), care placement (52 [52.0%]; mean [SD] time from diagnosis, 12.0 [7.3] years), and cognitive impairment (50 [50.0%]; mean [SD] time from diagnosis, 10.8 [7.0] years) being the most frequent milestones. Autonomic dysfunction developed in 85 patients (85.0%), with a mean (SD) time of 6.7 (7.7) years after the diagnosis of PD. Thirteen patients (13.0%) developed AutD before the diagnosis of PD. The most common AutD disturbances were urinary symptoms (84 [84.0%]; mean [SD] time from diagnosis, 5.0 [8.3] years), constipation (83 [83.0%]; mean [SD] time from diagnosis, 7.0 [7.4] years), and orthostatic hypotension (60 [60.0%]; mean [SD] time from diagnosis, 7.6 [8.8] years). Other disease milestones included speech (12 [12.0%]; mean [SD] time from diagnosis, 14.1 [7.0] years), severe dysphagia (13 [13.0%]; mean [SD] time from diagnosis, 14.0 [8.1] years), upper gastrointestinal tract symptoms (16 [16.0%]; mean [SD] time from diagnosis, 3.7 [7.3] years), sweating (12 [12.0%]; mean [SD] time from diagnosis, 5.5 [6.7] years), and erectile dysfunction in 60 men (23 [38.3%]; mean [SD] time from diagnosis, 3.0 [7.7] years).
Association Between AutD and Other Variables
Assessment of the association among explanatory variables (Table 2) revealed that patients with earlier AutD were older (regression coefficient, −0.40; 95% CI, −0.54 to −0.27; P < .001), were more likely to be male (regression coefficient, −7.13; 95% CI, −10.06 to −4.20; P < .001), had a poorer levodopa treatment response (regression coefficient, 12.19; 95% CI, 4.87 to 19.52; P = .001), had a lower maximum levodopa equivalent dose (regression coefficient, 5.56; 95% CI, 2.14 to 8.98; P = .002), and had postural instability and gait difficulty PD motor subtype (regression coefficient, −10.08; 95% CI, −15.16 to −5.00; P < .001). A clinicopathologic correlation did not reveal any significant association between AutD and limbic pathologic type (regression coefficient, 2.27; 95% CI, −9.42 to 13.96; P = .70) or diffuse neocortical pathologic subtype (regression coefficient, 2.96; 95% CI, −8.57 to 14.48; P = .61) using brainstem pathologic subtype as reference.
Table 2. Association of Time to Development of Autonomic Dysfunction (Dependent Variable) With Other Explanatory Variables Using a Linear Regression Model.
| Explanatory Variablea | Regression Coefficient (95% CI) | P Value |
|---|---|---|
| Age at diagnosis | −0.40 (−0.54 to −0.27) | <.001 |
| Male sex (vs female) | −7.13 (−10.06 to −4.20) | <.001 |
| PIGD PD subtype (vs tremor predominant) | −10.08 (−15.16 to −5.00) | <.001 |
| Excellent levodopa treatment response (vs nil to mild) | 12.19 (4.87 to 19.52) | .001 |
| Maximum levodopa equivalent dose | 5.56 (2.14 to 8.98) | .002 |
| Lewy pathologic type | ||
| Limbic (vs brainstem) | 2.27 (−9.42 to 13.96) | .70 |
| Neocortical (vs brainstem) | 2.96 (−8.57 to 14.48) | .61 |
Abbreviations: PD, Parkinson disease; PIGD, postural instability and gait difficulty.
The different forms of the main explanatory variables were considered in turn in different linear regression models (not in the same model).
Association of AutD With Disease Progression
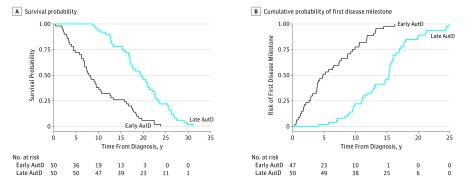
To assess progression of the disease to a certain level of disability independently of the clinical presentation, we analyzed the influence that AutD may have on the development of any (the first) disease milestone. Earlier development of AutD was associated with significantly increased risk of reaching the first disease milestone by 14% per year (HR, 0.86; 95% CI, 0.83-0.89; P < .001) (Figure and Table 3).
Figure. Kaplan-Meier Survival Curves of Survival Probability and Cumulative Probability of First Disease Milestone Among Patients With Parkinson Disease by Time to Development of Autonomic Dysfunction (AutD) (Early vs Late) .
Table 3. Cox Proportional Hazards Regression Models of Autonomic Dysfunction and Individual Autonomic Symptoms for Disease Progression and Survival.
| Outcome Variable | HR (95% CI) | P Value |
|---|---|---|
| Time to Autonomic Dysfunctiona | ||
| First milestone | 0.86 (0.83-0.89) | <.001 |
| Regular falls | 0.88 (0.84-0.92) | <.001 |
| Wheelchair | 0.93 (0.89-0.97) | .002 |
| Speech | 0.93 (0.84-1.03) | .18 |
| Severe dysphagia | 0.97 (0.88-1.07) | .57 |
| Cognitive impairment | 0.90 (0.85-0.95) | <.001 |
| Care placement | 0.91 (0.87-0.96) | .001 |
| Survival | 0.92 (0.88-0.96) | <.001 |
| Time to Autonomic Symptomsa | ||
| Orthostatic hypotension | ||
| First milestone | 0.94 (0.91-0.97) | <.001 |
| Survival | 0.92 (0.89-0.95) | <.001 |
| Upper gastrointestinal tract symptoms | ||
| First milestone | 0.93 (0.90-0.96) | <.001 |
| Survival | 0.93 (0.91-0.96) | <.001 |
| Constipation | ||
| First milestone | 0.94 (0.90-0.97) | .001 |
| Survival | 0.95 (0.90-0.98) | .005 |
| Urinary symptoms | ||
| First milestone | 0.92 (0.89-0.95) | <.001 |
| Survival | 0.94 (0.91-0.97) | <.001 |
| Erectile dysfunction | ||
| First milestone | 0.92 (0.89-0.96) | <.001 |
| Survival | 0.86 (0.82-0.89) | <.001 |
| Sweating abnormalities | ||
| First milestone | 0.88 (0.84-0.92) | <.001 |
| Survival | 0.89 (0.86-0.93) | <.001 |
Abbreviation: HR, hazard ratio.
The different forms of the main explanatory variables were considered in turn in different Cox proportional hazards regression models (not in the same model).
We also analyzed the association of AutD with the different functioning domains by estimating the risk for each milestone (Table 3 and eFigure 2 in the Supplement). Autonomic dysfunction was associated with a higher risk of falls (HR, 0.88; 95% CI, 0.84-0.92; P < .001), wheelchair dependence (HR, 0.93; 95% CI, 0.89-0.97; P = .002), cognitive impairment (HR, 0.90; 95% CI, 0.85-0.95; P < .001), and care placement (HR, 0.91; 95% CI, 0.87-0.96; P = .001). Results did not reveal an association between AutD with an increased risk of dysphagia (HR, 0.97; 95% CI, 0.88-1.07; P = .57) or speech impairment (HR, 0.93; 95% CI, 0.84-1.03; P = .18), although only a small number of patients developed these milestones.
Association of AutD With Survival
The association of time from diagnosis to AutD with survival is summarized in the Figure and Table 3. Survival analysis was adjusted by sex, age at diagnosis, PD motor subtype, and response to levodopa treatment because they have been previously reported as survival determinants and had a statistically significant association with shorter survival in the univariable analysis model in our study (see the Other Determinants of Survival subsection of the Results section). Autonomic dysfunction was associated with shorter survival, with an 8% increase of risk of death per year (HR, 0.92; 95% CI, 0.88-0.96; P < .001).
Association of Individual Autonomic Symptoms With Disease Progression and Survival
We believe that the definition of AutD reflects accurately the clinical effect of autonomic involvement in PD. To assess whether AutD was underreported by applying this definition AutD was underreported, the influence of each autonomic symptom or sign (orthostatic hypotension, urinary symptoms, constipation, upper gastrointestinal tract symptoms, sweating abnormalities, and erectile dysfunction in males) on survival and disease progression was also estimated. Earlier development of each autonomic symptom or sign was associated with a significantly higher risk of reaching a disease milestone and shorter survival (Table 3).
Other Determinants of Survival
Univariate models were used to study the influence of other clinical factors and demographic data on survival. In addition to earlier AutD development, factors associated with shorter survival included older age at diagnosis, male sex, postural instability and gait difficulty subtype, and poor levodopa treatment response. Only 3 of these variables (age at diagnosis, poor levodopa treatment response, and earlier development of AutD) were maintained in the multivariate analysis as independent determinants (Table 4).
Table 4. Multivariable Analysis of Survival Predictors.
| Variable | Crude HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value |
|---|---|---|---|---|
| Age at diagnosis, y | 1.14 (1.11-1.18) | <.001 | 1.12 (1.08-1.16) | <.001 |
| Time to AutD, y | 0.88 (0.85-0.91) | <.001 | 0.92 (0.88-0.96) | <.001 |
| Levodopa treatment response | ||||
| Good (vs nil to mild) | 0.11 (0.03-0.37) | <.001 | 0.22 (0.07-0.74) | .01 |
| Excellent (vs nil to mild) | 0.05 (0.02-0.16) | <.001 | 0.19 (0.05-0.64) | .008 |
| Male sex | 1.72 (1.14-2.61) | .01 | 1.62 (0.97-2.71) | .06 |
| PIGD subtype (vs tremor predominant) | 5.54 (2.69-11.40) | <.001 | 1.91 (0.76-4.79) | .17 |
Abbreviations: AutD, autonomic dysfunction; HR, hazard ratio; PIGD, postural instability and gait difficulty.
Discussion
The main finding of the study is that earlier development of AutD or individual autonomic abnormalities (including orthostatic hypotension, urinary symptoms, upper gastrointestinal tract symptoms, constipation, sweating abnormalities, and erectile dysfunction in males) are independent determinants of more rapid disease progression and shorter survival in patients with PD. The main strength of our study is that all included patients had a pathologically confirmed diagnosis. Our data highlight the significant proportion of patients with PD and AutD who are misdiagnosed as having multiple system atrophy, meaning that it is likely that many population studies have excluded this subgroup from the analysis.
A few studies have assessed the association of the severity of autonomic symptoms with PD, but autonomic cardiovascular tests or specific validated questionnaires have failed to reveal any association between severity of autonomic symptoms and survival in PD. Other studies have assessed the influence of the presence of several symptoms of AutD on PD progression. A recent prospective cohort study found that orthostatic hypotension was associated with shorter survival, although other symptoms and signs of AutD were not evaluated. In addition to orthostatic hypotension, Stubendorff and colleagues evaluated the presence of constipation and urinary incontinence as markers of AutD in 30 patients with dementia with Lewy bodies and PD with dementia. Orthostatic hypotension was the only symptom associated with shorter survival, although prognosis was worse in those with additional constipation or urinary incontinence. Of note, these studies with positive associations only focused on the presence or absence of symptoms and did not assess the time of onset of symptoms. In our study, analysis of individual autonomic symptoms revealed a faster progression to first milestone and shorter survival for each of them.
Studies have attempted to define new clinical subtypes of PD incorporating nonmotor symptoms (including AutD) by using different methods. These studies have found that autonomic symptoms are more common in patients without tremor-predominant subtypes of PD and that AutD might by associated with other features, such as postural instability, rapid eye movement sleep behavior disorder, and cognitive impairment, suggesting a more extensive neurodegenerative process in these patients. Patients with subtypes with prominent autonomic symptoms have worse disease progression and shorter survival. Because the analysis was performed in the context of other motor and nonmotor symptoms, no conclusion regarding the prognostic value of these autonomic symptoms in isolation can be made. Our study agrees with the view that some symptoms might cluster together because earlier AutD was associated with postural instability and gait difficulty PD motor subtypes, which were associated with a higher risk of developing cognitive disability. In our study, AutD was an independent prognostic factor despite the association with these other features, and we propose that it should be considered as a nonmotor marker of disease for the characterization of different PD subtypes.
We explored a potential association between AutD and the global distribution of α-synuclein pathologic findings in the central nervous system by using consensus criteria for the assessment of Lewy pathologic changes. These criteria provide characterization of the severity and regional involvement of Lewy pathologic changes by using a semiquantitative grading of severity in multiple areas of the brain. Our results did not reveal any association between AutD and α-synuclein pathologic findings in the central nervous system; therefore, the findings did not support the hypothesis that an underlying, more aggressive neurodegenerative process is responsible for the worse prognosis in patients with AutD. However, our study did not include any specific neuropathologic assessment of central regulatory autonomic areas or peripheral postganglionic autonomic structures known to be involved in dysautonomia in PD. Therefore, we could not assess whether the neurodegenerative process has any predilection for these autonomic structures in patients with early AutD. An alternative explanation is that the increased morbidity and mortality in patients with early AutD is a consequence of the autonomic symptoms. This explanation would have important implications in clinical practice because better therapeutic control of the symptoms could potentially improve the prognosis.
Medication to treat PD might also have a clinical effect on autonomic function. In our study, early AutD was associated with poorer levodopa treatment response, which may be a consequence of involvement of nondopaminergic structures likely to contribute to the faster progression and disability. In addition, AutD was associated with a lower maximum levodopa equivalent dose. Although these data should be interpreted with caution, it is likely that patients with early AutD were not able to tolerate the treatment because of worsening of their autonomic symptoms. However, a poor initial response may have prevented clinicians from trying higher doses of levodopa.
Our data also revealed that older age at diagnosis, male sex, PD subtype, and response to levodopa treatment were determinants of survival in univariable analysis, although identification of these determinants was not the main objective of this study. In multivariable analysis, however, only age at diagnosis, male sex, and levodopa treatment response remained significant. These features have been reported as survival determinants in PD in a previous meta-analysis.
Limitations
The retrospective nature of the study and the assessment by different professionals without clear methodologic homogeneity may potentially account for some limitations in the accuracy of the recording of autonomic symptoms. The study used strict criteria and included a large group of patients with PD with detailed and regular clinical information from hospital specialists (neurologists or geriatricians) and the family physician that allowed confident documentation of the disease process. Disease milestones were well documented and accurately describe disease progression in other parkinsonian conditions. Despite this, many cases were excluded because of insufficient clinical information. Because this exclusion may raise concerns about selection bias, we performed an analysis of the main demographic and clinical characteristics, but no significant differences between the study patients and those excluded were found. We also acknowledge that brain bank cases may be biased toward more severe or atypical clinical cases, which may limit generalization of the results, whereas studies based on a clinical diagnosis of PD alone are affected by a significant rate of incorrect diagnosis. Most of our results had a significant association between AutD and the variables, although lack of power of the study might explain the lack of association in some of the analysis, particularly in cases in which the variable developed in only a few patients, such as severe dysphagia or communication difficulties.
Conclusions
This study of patients with pathologically confirmed PD found that earlier development of autonomic symptoms or AutD is associated with shortened survival and worse progression of disease. Because the presence of autonomic symptoms in most of our patients was assessed in clinical settings, these findings have important implications, and identification of these symptoms should be part of the routine clinical assessment. The role of more aggressive treatment of autonomic symptoms in progression and survival remains unclear. Additional studies with pathologically proven PD cases and objective assessment of AutD severity are warranted to corroborate these findings.
eFigure 1. Flowchart of the Study
eFigure 2. Kaplan-Meier Failure Curves for Parkinson Disease Patients by Time to Development of Autonomic Dysfunction Groups (Early vs Late) Showing Risk of Outcome
References
- 1.Lewis SJ, Foltynie T, Blackwell AD, Robbins TW, Owen AM, Barker RA. Heterogeneity of Parkinson’s disease in the early clinical stages using a data driven approach. J Neurol Neurosurg Psychiatry. 2005;76(3):343-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marras C, Chaudhuri KR. Nonmotor features of Parkinson’s disease subtypes. Mov Disord. 2016;31(8):1095-1102. [DOI] [PubMed] [Google Scholar]
- 3.van Rooden SM, Heiser WJ, Kok JN, Verbaan D, van Hilten JJ, Marinus J. The identification of Parkinson’s disease subtypes using cluster analysis: a systematic review. Mov Disord. 2010;25(8):969-978. [DOI] [PubMed] [Google Scholar]
- 4.Post B, Merkus MP, de Haan RJ, Speelman JD Prognostic factors for the progression of Parkinson's disease: a systematic review. Mov Disord 2007;22(13):1839-1851, quiz 1988. [DOI] [PubMed]
- 5.van der Heeden JF, Marinus J, Martinez-Martin P, Rodriguez-Blazquez C, Geraedts VJ, van Hilten JJ. Postural instability and gait are associated with severity and prognosis of Parkinson disease. Neurology. 2016;86(24):2243-2250. [DOI] [PubMed] [Google Scholar]
- 6.Macleod AD, Taylor KS, Counsell CE. Mortality in Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2014;29(13):1615-1622. [DOI] [PubMed] [Google Scholar]
- 7.Asahina M, Vichayanrat E, Low DA, Iodice V, Mathias CJ. Autonomic dysfunction in parkinsonian disorders: assessment and pathophysiology. J Neurol Neurosurg Psychiatry. 2013;84(6):674-680. [DOI] [PubMed] [Google Scholar]
- 8.Tada M, Onodera O, Tada M, et al. . Early development of autonomic dysfunction may predict poor prognosis in patients with multiple system atrophy. Arch Neurol. 2007;64(2):256-260. [DOI] [PubMed] [Google Scholar]
- 9.Low PA, Reich SG, Jankovic J, et al. . Natural history of multiple system atrophy in the USA: a prospective cohort study. Lancet Neurol. 2015;14(7):710-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe H, Saito Y, Terao S, et al. . Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain. 2002;125(Pt 5):1070-1083. [DOI] [PubMed] [Google Scholar]
- 11.O’Sullivan SS, Massey LA, Williams DR, et al. . Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain. 2008;131(pt 5):1362-1372. [DOI] [PubMed] [Google Scholar]
- 12.Petrovic IN, Ling H, Asi Y, et al. . Multiple system atrophy–parkinsonism with slow progression and prolonged survival: a diagnostic catch. Mov Disord. 2012;27(9):1186-1190. [DOI] [PubMed] [Google Scholar]
- 13.Stubendorff K, Aarsland D, Minthon L, Londos E. The impact of autonomic dysfunction on survival in patients with dementia with Lewy bodies and Parkinson’s disease with dementia. PLoS One. 2012;7(10):e45451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKeith IG, Dickson DW, Lowe J, et al. ; Consortium on DLB . Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863-1872. [DOI] [PubMed] [Google Scholar]
- 15.Abrams P, Cardozo L, Fall M, et al. ; Standardisation Sub-committee of the International Continence Society . The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21(2):167-178. [DOI] [PubMed] [Google Scholar]
- 16.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25(15):2649-2653. [DOI] [PubMed] [Google Scholar]
- 17.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43-46. [PubMed] [Google Scholar]
- 18.Perneger TV. What’s wrong with Bonferroni adjustments. BMJ. 1998;316(7139):1236-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray WK, Wood BH, Walker RW. Do autonomic function tests in people with Parkinson’s disease predict survival rates at 7 years follow-up? Mov Disord. 2009;24(16):2432-2434. [DOI] [PubMed] [Google Scholar]
- 20.de Lau LM, Verbaan D, Marinus J, van Hilten JJ. Survival in Parkinson’s disease: relation with motor and non-motor features. Parkinsonism Relat Disord. 2014;20(6):613-616. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein DS, Holmes C, Sharabi Y, Wu T. Survival in synucleinopathies: a prospective cohort study. Neurology. 2015;85(18):1554-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allcock LM, Kenny RA, Burn DJ. Clinical phenotype of subjects with Parkinson’s disease and orthostatic hypotension: autonomic symptom and demographic comparison. Mov Disord. 2006;21(11):1851-1855. [DOI] [PubMed] [Google Scholar]
- 23.Müller B, Larsen JP, Wentzel-Larsen T, Skeie GO, Tysnes OB; Parkwest Study Group . Autonomic and sensory symptoms and signs in incident, untreated Parkinson’s disease: frequent but mild. Mov Disord. 2011;26(1):65-72. [DOI] [PubMed] [Google Scholar]
- 24.van Rooden SM, Colas F, Martínez-Martín P, et al. . Clinical subtypes of Parkinson’s disease. Mov Disord. 2011;26(1):51-58. [DOI] [PubMed] [Google Scholar]
- 25.Fereshtehnejad SM, Romenets SR, Anang JB, Latreille V, Gagnon JF, Postuma RB. New clinical subtypes of Parkinson disease and their longitudinal progression: a prospective cohort comparison with other phenotypes. JAMA Neurol. 2015;72(8):863-873. [DOI] [PubMed] [Google Scholar]
- 26.Anang JB, Gagnon JF, Bertrand JA, et al. . Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology. 2014;83(14):1253-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Lau LM, Verbaan D, van Rooden SM, Marinus J, van Hilten JJ. Relation of clinical subtypes in Parkinson’s disease with survival. Mov Disord. 2014;29(1):150-151. [DOI] [PubMed] [Google Scholar]
- 28.van Dijk JG, Haan J, Zwinderman K, Kremer B, van Hilten BJ, Roos RA. Autonomic nervous system dysfunction in Parkinson’s disease: relationships with age, medication, duration, and severity. J Neurol Neurosurg Psychiatry. 1993;56(10):1090-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flowchart of the Study
eFigure 2. Kaplan-Meier Failure Curves for Parkinson Disease Patients by Time to Development of Autonomic Dysfunction Groups (Early vs Late) Showing Risk of Outcome