Key Points
Question
Do children need to come to childhood obesity treatment with their parent for it to be effective?
Findings
In this randomized clinical trial among 150 children and their parent, results showed that parent-based treatment (parent-only, without the child) was noninferior to a family-based treatment (parent and child) on child weight loss over 24 months.
Meaning
The child does not need to come to treatment to lose weight.
Abstract
Importance
Family-based weight loss treatment (FBT) is considered the gold-standard treatment for childhood obesity and is provided to the parent and child. However, parent-based treatment (PBT), which is provided to the parent without the child, could be similarly effective and easier to disseminate.
Objective
To determine whether PBT is similarly effective as FBT on child weight loss over 24 months. Secondary aims evaluated the effect of these 2 treatments on parent weight loss, child and parent dietary intake, child and parent physical activity, parenting style, and parent feeding behaviors.
Design, Setting, and Participants
Randomized 2-arm noninferiority trial conducted at an academic medical center, University of California, San Diego, between July 2011 and July 2015. Participants included 150 overweight and obese 8- to 12-year-old children and their parents.
Interventions
Both PBT and FBT were delivered in 20 one-hour group meetings with 30-minute individualized behavioral coaching sessions over 6 months. Treatments were similar in content; the only difference was the attendance of the child.
Main Outcomes and Measures
The primary outcome measure was child weight loss (body mass index [BMI] and BMI z score) at 6, 12, and 18 months post treatment. Secondary outcomes were parent weight loss (BMI), child and parent energy intake, child and parent physical activity (moderate to vigorous physical activity minutes), parenting style, and parent feeding behaviors.
Results
One hundred fifty children (mean BMI, 26.4; mean BMI z score, 2.0; mean age, 10.4 years; 66.4% girls) and their parent (mean BMI, 31.9; mean age, 42.9 years; 87.3% women; and 31% Hispanic, 49% non-Hispanic white, and 20% other race/ethnicity) were randomly assigned to either FBT or PBT. Child weight loss after 6 months was −0.25 BMI z scores in both PBT and FBT. Intention-to-treat analysis using mixed linear models showed that PBT was noninferior to FBT on all outcomes at 6-, 12-, and 18-month follow-up with a mean difference in child weight loss of 0.001 (95% CI, −0.06 to 0.06).
Conclusions and Relevance
Parent-based treatment was as effective on child weight loss and several secondary outcomes (parent weight loss, parent and child energy intake, and parent and child physical activity). Parent-based treatment is a viable model to provide weight loss treatment to children.
Trial Registration
Clinicaltrials.gov Identifier: NCT01197443
This randomized clinical trial determines whether parent-based obesity treatment without child attendance is similarly effective as family-based treatment that includes the child on child weight loss over a period of 24 months.
Introduction
One-third of American children are overweight or obese, which is associated with significant negative health outcomes. Family-based treatment (FBT) is considered the most effective model for the treatment of children with obesity in the short term and long term. Family-based treatment is delivered to both parents and children in separate groups and includes nutrition and physical activity education and behavior therapy techniques. However, FBT is provided mainly in academic medical centers and can be challenging to attend for busy families because it requires attendance by both parent and child at specific group times.
Family-based treatment programs for parents without their child (parent-based therapy [PBT]) have favorable preliminary data. A 2013 systematic review showed that PBT programs have similar outcomes to FBT programs and are more cost-effective. However, the studies are small and underpowered, with short follow-ups. To our knowledge, no study has evaluated an appropriately powered, controlled comparison of FBT and PBT with longer follow-up.
This study reports the main outcomes of a randomized clinical trial evaluating whether PBT is noninferior to FBT on child weight outcomes at 6 months, 12 months, and 24 months. As secondary aims, we compared the 2 programs on parent weight, child and parent energy intake, and child and parent physical activity. Because FBT is grounded in changing parent behavior to assist their child, we included parenting style and parent feeding behaviors as secondary outcomes.
Methods
Study Design
The Family, Responsibility, Education, Support and Health (FRESH) study was a randomized clinial noninferiority trial that evaluated two 6-month treatments for childhood obesity: FBT, provided to parent and child, and PBT, provided to parent only, conducted between July 2011 and July 2015 in the greater San Diego, California, area. Both FBT and PBT included nutrition and physical activity recommendations, parenting skills, and behavior modification strategies. Both groups were led on the same night of the week with the same group leaders, who attended weekly supervision with the first author. The only difference between FBT and PBT was the attendance of the child. Measures were collected at baseline, 3 months, 6 months, 12 months, and 18 months. The primary outcome measure was change in child weight (body mass index [BMI] z score) during the 18-month period. Secondary outcomes included changes in child and parent energy intake and physical activity, changes in parent weight (BMI), parenting style, and parent feeding behaviors. Full design details of the trial have been reported, and the formal trial protocol can be found in Supplement 1. The institutional review boards of University of California, San Diego, and Rady Children’s Hospital, San Diego, California approved the study. Written consent and assent was obtained from parents and children, respectively.
Eligibility and Recruitment
Eligibility included a child between 8.0 and 12.9 years of age with a BMI between the 85th and 99.9th percentiles, a parent in the household with a BMI of at least 25 who could read English at a minimum of a fifth-grade level, and availability to participate in the study on designated evenings. Exclusionary criteria included a major child or parent psychiatric disorder, child diagnosis of a serious current physical disease, child with physical limitations, or a family with food restrictions. One hundred fifty children with overweight/obesity and their parent were recruited through primary care physicians, schools, listserves, local advertisements, and advertisements.
Intervention
Child-parent dyads were randomly assigned to FBT or PBT stratified by sex of the child. The treatment programs included 20 visits over 6 months, and the content was based on published trials of FBT. Parents in both FBT and PBT attended a 1-hour parent group. Children in FBT attended a 1-hour simultaneous child group. Children in PBT did not attend any treatment meetings. Parents in PBT and parents and children in FBT also attended 30-minute meetings with a behavioral coach on the same evening. See Boutelle et al for additional details on treatment components.
Outcome Measures
Assessments with child-parent dyads were conducted at baseline, 3 months (midtreatment; weight only), 6 months (posttreatment), 12 months, and 18 months. Data collection was conducted by trained staff and supervised by PhD-level psychologists. Participants received incentives for time, travel, and effort at assessments. Measures assessed were:
Anthropometry (child and parent): height and weight were measured in duplicate. The mean of the 2 values was used to calculate BMI (calculated as weight in kilograms divided by height in meters squared). For children, age-adjusted BMI percentile (BMI%) and standardized BMI (BMIz) were calculated.
Energy intake (child and parent): energy intake was assessed with three 24-hour multiple-pass dietary recalls on 3 nonconsecutive days via telephone interview. Total energy intake was calculated using the Nutrition Data Systems for Research software.
Physical activity (child and parent): physical activity was assessed using Actigraph accelerometers, model GT1M (ActiGraph Corp). A minimum of 4 of 7 days of wear time was required to be complete and accommodate error and noncompliance. All accelerometer data extraction, processing, and scoring was conducted by ActiLife software, version 6.11 (ActiGraph Corp), which provided transformed summaries aggregated across 30-second epoch lengths. Epoch-by-epoch estimates of activity were categorized into intensity-weighted summaries of physical activity using calibration thresholds previously validated for adults and children. Outcome variables were mean minutes per day of moderate and vigorous intensity physical activity.
Children’s Report of Parental Behavior Inventory (child): this 56-item measure assesses child’s perceptions of their parent’s behavior (mother and father separately) and results in 3 subscales: psychological control vs psychological autonomy, acceptance vs rejection, and firm vs lax control.
Birch Child Feeding Questionnaire (parent): this 21-item measure assessed parent’s beliefs, attitudes, and practices regarding child feeding. Three scales were used: restriction, pressure to eat, and monitoring.
Feasibility and acceptability: feasibility was assessed by number of sessions attended and overall attrition. Acceptability was assessed using questions designed by the study team specifically for this study. Parents responded to questions regarding the convenience of their assigned group, how much they liked the program, and how much they thought the program changed their family and child’s lifestyle.
Statistical Power
Power calculations focused on noninferiority tests for the primary outcome of child BMIz. All power calculations were conducted using SAS Proc Power with α = .10, corresponding to use of the upper bound of the 90% CI to test the noninferiority hypotheses (SAS Institute Inc). A sample size of 150 was used to account for dropout and achieve power greater than 0.80. See Boutelle et al for additional details.
Statistical Analysis
The primary analyses were performed for the intention-to-treat population, defined as the child-parent dyads who were allocated to either FBT or PBT. For the primary weight outcomes, we used linear mixed effects (LME) regression models of child BMIz and parent BMI assessed at 3 months, 6 months, 12 months, and 18 months.
We conducted a planned noninferiority analysis of child BMIz to determine whether a 2-tailed upper bound of the 90% CI of the treatment effect would rule out our prespecifed difference in BMIz across treatments. Noninferiority hypothesis were supported if the upper bound of the 90% CI for the main effect of treatment was less than our prespecified noninferiority margin. We set the upper bound of expected change of −0.13 to −0.17 BMIz units using the covariate-adjusted pooled standard deviation of changes in BMIz at 6 and 12 months follow-up in our previous study. We set the lower bound of expected change to suggest an effect at least half as big as we expected from the FBT treatment (50% of 0.13; 0.065). The fixed-margin approach was used and was successful if the lower limit of the 90% CI around the difference between FBT and PBT was found to greater than the margin from −0.049 to 0.051 BMIz. Superiority analyses were conducted for the other variables, using similar LME models with planned covariates; age, sex, the linear effect of time, and corresponding baseline values. Analysis of longitudinal outcomes used multiple imputed data sets (m = 50) using multivariate imputation by chained equations. We present estimated treatment effects for both maximum likelihood estimation of available data and estimates from models across 50 multiple imputed data sets.
Results
Participant Flow and Baseline Demographics
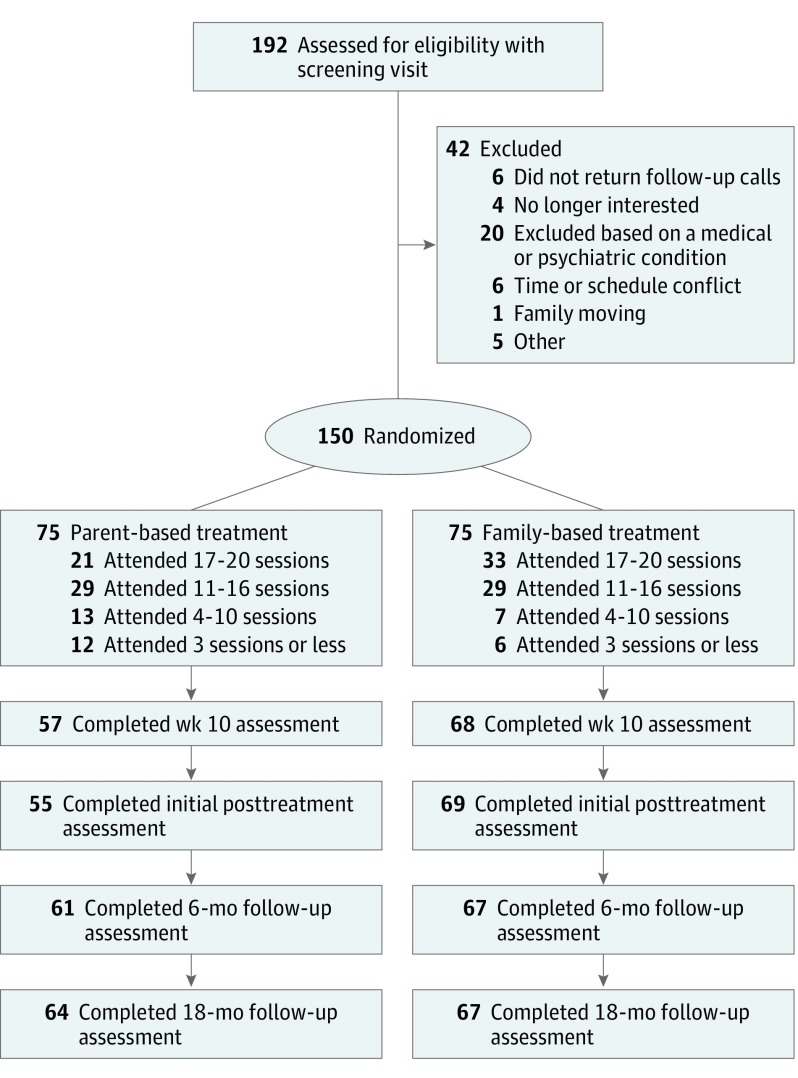
We screened by telephone 794 parent/child dyads who expressed interest, conducted assessments with 192 parent/child dyads, and enrolled 150 parent/child dyads (Figure 1). Similar baseline characteristics were observed in both PBT and FBT groups (Table 1). Of the parent/child dyads enrolled, data from 83% (n = 124), 85% (n = 128), and 87% (n = 131) were available at 6 months, 12 months, and 18 months, respectively. Logistic regression of cases not included in analyses owing to missing BMI at more than 2 assessments showed no significant differences between FBT and PBT participants (β = −1.11; SE = 0.61; P = .07). No child or parent adverse events were reported.
Figure 1. CONSORT Flow Diagram Describing Recruitment, Study Flow, and Follow-up of the Participants.
There were 794 parents who called in response to marketing, but only 192 were considered for further screening.
Table 1. Sample Characteristics.
| Characteristic | Mean (SD) | |
|---|---|---|
| PBT | FBT | |
| Child | ||
| Age | 10.43 (1.28) | 10.39 (1.27) |
| Sex, No. (%) | ||
| Boys | 25 (33.3) | 25 (33.3) |
| Girls | 50 (66.7) | 50 (66.7) |
| Race/ethnicity, No. (%) | ||
| Hispanic | 21 (28.8) | 26 (35.1) |
| Non-Hispanic other | 21 (28.8) | 15 (20.3) |
| Non-Hispanic white | 31 (42.5) | 33 (44.6) |
| Weight | ||
| BMI | 26.56 (3.52) | 26.13 (3.74) |
| BMIz | 2.02 (0.36) | 1.98 (0.32) |
| BMI % | 97.11 (2.60) | 97.02 (2.40) |
| Diet | ||
| Total calories | 1744.77 (430.08) | 1680.28 (388.10) |
| Physical activity, min/d | ||
| Moderate to vigorous | 181.16 (49.66) | 182.32 (38.72) |
| Parent | ||
| Age | 43.21 (6.65) | 42.59 (6.18) |
| Sex, No. (%) | ||
| Men | 10 (13.3) | 9 (12.0) |
| Women | 65 (86.7) | 66 (88.0) |
| Race/ethnicity, % | ||
| Hispanic | 23 (30.7) | 24 (32.0) |
| Non-Hispanic other | 16 (21.3) | 14 (18.7) |
| Non-Hispanic white | 36 (48.0) | 37 (49.3) |
| Weight | ||
| BMI | 32.11 (6.11) | 31.70 (6.53) |
| Diet | ||
| Total calories | 1725.99 (541.70) | 1685.23 (493.41) |
| Physical activity, min/d | ||
| Moderate to vigorous | 33.37 (21.50) | 31.72 (20.70) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BMIz, BMI z score; FBT, family-based weight loss treatment; PBT, parent-based treatment.
Primary Outcome: Child Weight Loss
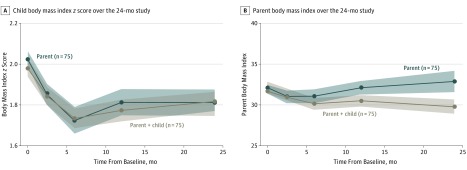
As seen in Figure 2, children in both PBT and FBT experienced similar decreases in BMIz by the end of the treatment period that was largely sustained through the 18-month assessment. Examination of unconditional models suggested the benefit of including both random intercept and slope terms (χ2 = 37.07; P < .001). The main effect of treatment group from adjusted LME models of BMIz provided an estimate and standard error of differences in the magnitude of change in child weight. The difference in BMIz observed between PBT and FBT over assessments was 0.001 (90% CI, −0.05 to 0.05; Table 2). In pooled estimation from multiple imputed data sets (m = 50), the difference in BMIz was 0.007 (90% CI, −0.04 to 0.06). This observed effect interval was greater than the noninferiority margin of −0.13 to −0.065 and thus supported noninferiority (see eTable in Supplement 2 for outcome measures by treatment arm across the 18-month period).
Figure 2. Child and Parent Weight Changes Over the 24-Month Period.
Body mass index calculated as weight in kilograms divided by height in meters squared.
Table 2. Main Effect of Treatment Group Assignment on Primary and Secondary Outcomes.
| Outcome Variable | Valuea | 95% CI | P Value |
|---|---|---|---|
| Child | |||
| BMIz | 0.001 | −0.06 to 0.06 | .96 |
| Diet, kcalb | −2.872 | −93.07 to 87.32 | .95 |
| Moderate to vigorous activity, min/db | −0.207 | −0.58 to 0.17 | .28 |
| Parent | |||
| BMI | 0.154 | −0.40 to 0.71 | .10 |
| Diet, kcalb | 13.694 | −78.37 to 105.76 | .77 |
| Moderate to vigorous activity, min/db | 0.260 | −0.05 to 0.57 | .10 |
| Overweight parent | |||
| BMI | 0.254 | −0.31 to 0.82 | .38 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BMIz, BMI z score.
Value: adjusted parameters from mixed-effects regression models reflecting differences between groups over 6-month, 12-month, and 24-month assessments.
Outcomes assessed only at 6-month and 24-month assessments. All models include planned covariates for age, sex, time, and corresponding baseline values.
Secondary Outcomes: Parent Weight Loss, Child and Parent Energy Intake, Child and Parent Physical Activity, and Parenting
As seen in Figure 2, there were no significant differences between FBT and PBT parents’ BMI across assessments (β = 0.15; SE = 0.28; P = .59). However, there was support for a small and statistically significant increase in the rate of change in BMI over time for parents in PBT relative to FBT after the 6-month time point (β = 0.02; SE = 0.01; P = .04). Mean (SD) percent weight loss for parents in FBT and PBT was −3.9% (5.3) and −5.0% (5.5) at 6 months and −1.1% (6.7) and 2.8% (13.4) by the final 18-month time, respectively. When restricted to parents who were overweight/obese on enrollment, we observed similar results, with no significant difference in BMI changes for FBT and PBT participants (β = 0.25; SE = 0.29; P = .38; see eTable in Supplement 2 for means).
Table 2 presents main effect terms comparing FBT and PBT using LME models with planned covariates for daily energy intake and minutes of moderate and vigorous intensity physical activity. For children (β = −2.87; SE = 46.01; P = .95) and parents (β = 15.30; SE = 46.99; P = .75) in either FBT or PBT, there was no significant difference in daily energy intake consumed across assessments and no significant difference in the rate of change in daily energy intake over time for children (group by time interaction). However, there was a trend for less rapid increases in energy intake in parents in FBT compared with parents in PBT following the 6-month follow-up, increasing a mean of 10.7 fewer kcal/mo during the study (β = −10.7; SE = 5.5; P = .06). No significant differences between FBT and PBT were observed in moderate and vigorous intensity physical activity among children (β = 0.98; SE = 7.61; P = .90) or parents (β = −2.87; SE = 2.81; P = .37; see eTable in Supplement 2 for means).
We also conducted comparisons between FBT and PBT using LME models with planned covariates for the Children’s Report of Parental Behavior Inventory and Child Feeding Questionnaire, using Benjamini-Hochberg methods. We did not observe significant differences between treatment groups on any of the Children’s Report of Parental Behavior Inventory and Child Feeding Questionnaire scales (data not shown). We also assessed each scale as a potential moderator in effect of treatment group on child BMIz over assessments. In separate models, baseline levels of each parenting measure were entered in primary outcome models of BMIz along with its interaction term with treatment group assignment. We did not observe any significant moderating effects of parenting variables on treatment differences in changes in BMIz (P values >.10).
Feasibility and Acceptability
Parents in PBT attended significantly fewer treatment sessions (t = −2.57; difference, 140.17; P = .01; mean [SD] PBT, 12.2 [6.33] vs FBT, 14.6 [4.98]). Parent-based treatment had greater loss of participants during the early phases of treatment, as evidenced by the 24% attrition at 3 months and 27% attrition at 6 months, compared with 12% and 8% attrition for FBT at the same times. In negative binomial regression models, there were no significant associations between parent age, racial/ethnic group, baseline weight, sex, or number of treatment sessions attended.
In terms of acceptability, parents in the PBT program rated it less convenient (somewhat/very inconvenient) compared with FBT parents (12 of 55 [21.8%] vs 6 of 68 [8.8%]) and somewhat less parents in PBT liked the program (somewhat/very much liked) compared with FBT parents (45 of 52 [86.5%] vs 65 of 68 [95.6%]). However, similar numbers of parents in PBT and parents in FBT felt that the program (somewhat/very much) helped change their family and child’s lifestyle (49 of 53 [92.5%] vs 62 of 66 [93.4%]).
Discussion
To our knowledge, this is the first large-scale clinical study to test the noninferiority of a PBT program compared with an FBT program for children with overweight/obesity over 24 months. Consistent with previous evidence, the PBT program was noninferior to FBT on child weight outcomes, child and parent energy intake, child and parent physical activity, and parenting measures at the 6-month, 12-month, and 18-month follow-ups. The PBT program was noninferior to FBT on parent weight outcomes at the 6-month follow-up; however, PBT parents gained more weight over time. Additionally, there was greater attrition and lower acceptability ratings in the PBT compared with the FBT group.
We included evaluations of parenting and parent behaviors because parents are the most important people in a child’s environment. In the process of helping their child lose weight, they serve to verbally teach their children the weight control material, model healthy behaviors, and reinforce the acquisition and maintenance of healthy eating and exercise behaviors. Noteably, there were no significant differences found between changes in parenting style and feeding behavior between the 2 groups over time. Additionally, similar numbers of parents in PBT and FBT felt that the program helped their family change their lifestyle. This trial highlights that PBT and FBT affect parenting style and feeding behavior in the same manner and that child attendance is not necessary to achieve similar outcomes.
Consistent with our previous study, there was greater retention in FBT compared with PBT at 6 months; however, these differences were somewhat attenuated by 18 months. Additionally, PBT families attended a mean of 2 fewer meetings than FBT families. Research shows that attendance is an important predictor of child weight loss and reasons for attrition range from time commitment, distance from clinic, missed school and work, appointment times, schedule, educational content, and stress. However, these reasons should apply to both PBT and FBT equally, suggesting that there is something unique about PBT that may lead to greater attrition and decreased attendance. Parent-based treatment was perceived to be less convenient by parents compared with FBT. Unfortunately, none of the families who dropped gave reasons beyond logistical issues, so we are unable to identify why more PBT families dropped in this study.
The PBT intervention has a number of strengths that should be noted. First and foremost, PBT has similar outcomes to FBT in changes in child and parent weight, nutrition, physical activity, parenting style, and parent feeding behaviors. Additionally, because only the parent’s schedule needs to be considered, there could be an added flexibility in scheduling. In PBT, a reliable and caring adult provides all the information and reinforcement to the child and can adapt the program to the child’s needs because they know the child’s learning strategies and motivators. Parent-based treatment emphasizes the role of parents as the primary agent of change, which could result in greater self-efficacy to parents regarding the treatment of their child’s weight and other child behavioral issues because the parent management skills learned can be applied to other child behaviors. However, it is also important to note that PBT places a large amount of responsibility on the parent who attends and was not as acceptable.
Family-based treatment also has strengths that should be noted. In FBT, children learn the material and are reinforced by the interventionists and other children in the group as well as by their parents at home. Learning from multiple sources could provide more durability to changes in the child’s behavior as the child transitions to adolescence and peer groups become more important. Family-based treatment also had less dropout than PBT, suggesting that it may be more acceptable to families. However, in FBT, the responsibility of learning the information is shared between the parent and child, which could also allow parents to reduce their involvement.
Limitations
Strengths of the study include the randomized design, the use of noninferiority testing, the racial/ethnic diversity of the families, the use of a validated treatment protocol, and the 24-month observation period. However, study participants were treatment-seeking volunteers with 8- to 12-year-old children whose BMI percentile was less than 99.9%, limiting the generalizability to families with children of other ages and higher weight. Additionally, this study did not include a placebo control intervention. This design was chosen because published studies show that FBT is superior to no treatment and control groups.
In considering clinical applications, there are a number of reasons why parents would prefer one model vs the other. Families may prefer FBT when parents believe that information delivered directly to the child is important in achieving weight loss. Family-based treatment may also be a preferable model for children who would benefit from social support. Parent-based treatment may be more enticing to families where the child does not want to come to treatment or scheduling does not permit time for FBT. Reasons for parent’s preference of model delivery should be explored in future research.
Conclusions
This study provides sound empirical evidence supporting a PBT model for the delivery of childhood obesity treatment. Given the high rates of obesity in children, PBT is a model that could be used to provide treatment to a greater proportion of the population.
Trial Protocol.
eTable. Child and Parent Mean Scores on Outcome Measures for Parent-Based Treatment and Family-Based Treatment at Baseline, 6-,12- and 24-Month Follow-up.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daniels SR. The consequences of childhood overweight and obesity. Future Child. 2006;16(1):47-67. [DOI] [PubMed] [Google Scholar]
- 3.Dietz WH. Health consequences of obesity in youth: childhood predictors of adult disease. Pediatrics. 1998;101(3, pt 2):518-525. [PubMed] [Google Scholar]
- 4.Dixon JB. The effect of obesity on health outcomes. Mol Cell Endocrinol. 2010;316(2):104-108. [DOI] [PubMed] [Google Scholar]
- 5.Gunnell DJ, Frankel SJ, Nanchahal K, Peters TJ, Davey Smith G. Childhood obesity and adult cardiovascular mortality: a 57-y follow-up study based on the Boyd Orr cohort. Am J Clin Nutr. 1998;67(6):1111-1118. [DOI] [PubMed] [Google Scholar]
- 6.Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes (Lond). 2011;35(7):891-898. [DOI] [PubMed] [Google Scholar]
- 7.Sorof J, Daniels S. Obesity hypertension in children: a problem of epidemic proportions. Hypertension. 2002;40(4):441-447. [DOI] [PubMed] [Google Scholar]
- 8.Key TJ, Schatzkin A, Willett WC, Allen NE, Spencer EA, Travis RC. Diet, nutrition and the prevention of cancer. Public Health Nutr. 2004;7(1A):187-200. [DOI] [PubMed] [Google Scholar]
- 9.Epstein LH, Paluch RA, Roemmich JN, Beecher MD. Family-based obesity treatment, then and now: twenty-five years of pediatric obesity treatment. Health Psychol. 2007;26(4):381-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein LH, Valoski A, Wing RR, McCurley J. Ten-year follow-up of behavioral, family-based treatment for obese children. JAMA. 1990;264(19):2519-2523. [PubMed] [Google Scholar]
- 11.Epstein LH, McCurley J, Wing RR, Valoski A. Five-year follow-up of family-based behavioral treatments for childhood obesity. J Consult Clin Psychol. 1990;58(5):661-664. [DOI] [PubMed] [Google Scholar]
- 12.Sung-Chan P, Sung YW, Zhao X, Brownson RC. Family-based models for childhood-obesity intervention: a systematic review of randomized controlled trials. Obes Rev. 2013;14(4):265-278. [DOI] [PubMed] [Google Scholar]
- 13.Epstein LH, Valoski A, Wing RR, McCurley J. Ten-year outcomes of behavioral family-based treatment for childhood obesity. Health Psychol. 1994;13(5):373-383. [DOI] [PubMed] [Google Scholar]
- 14.Boutelle KN, Cafri G, Crow SJ. Parent-only treatment for childhood obesity: a randomized controlled trial. Obesity (Silver Spring). 2011;19(3):574-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janicke DM, Sallinen BJ, Perri MG, et al. Comparison of parent-only vs family-based interventions for overweight children in underserved rural settings: outcomes from project STORY. Arch Pediatr Adolesc Med. 2008;162(12):1119-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golan M, Kaufman V, Shahar DR. Childhood obesity treatment: targeting parents exclusively v. parents and children. Br J Nutr. 2006;95(5):1008-1015.16611394 [Google Scholar]
- 17.Munsch S, Roth B, Michael T, et al. Randomized controlled comparison of two cognitive behavioral therapies for obese children: mother versus mother-child cognitive behavioral therapy. Psychother Psychosom. 2008;77(4):235-246. [DOI] [PubMed] [Google Scholar]
- 18.Jull A, Chen R. Parent-only vs. parent-child (family-focused) approaches for weight loss in obese and overweight children: a systematic review and meta-analysis. Obes Rev. 2013;14(9):761-768. [DOI] [PubMed] [Google Scholar]
- 19.Janicke DM, Sallinen BJ, Perri MG, Lutes LD, Silverstein JH, Brumback B. Comparison of program costs for parent-only and family-based interventions for pediatric obesity in medically underserved rural settings. J Rural Health. 2009;25(3):326-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boutelle KN, Braden A, Douglas JM, et al. Design of the FRESH study: a randomized controlled trial of a parent-only and parent-child family-based treatment for childhood obesity. Contemp Clin Trials. 2015;45(pt B):364-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epstein LH, Myers MD, Raynor HA, Saelens BE. Treatment of pediatric obesity. Pediatrics. 1998;101(3, pt 2):554-570. [PubMed] [Google Scholar]
- 22.Epstein LH, Roemmich JN, Raynor HA. Behavioral therapy in the treatment of pediatric obesity. Pediatr Clin North Am. 2001;48(4):981-993. [DOI] [PubMed] [Google Scholar]
- 23.Wilfley DE, Tibbs TL, Van Buren DJ, Reach KP, Walker MS, Epstein LH. Lifestyle interventions in the treatment of childhood overweight: a meta-analytic review of randomized controlled trials. Health Psychol. 2007;26(5):521-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002;(246):1-190. [PubMed] [Google Scholar]
- 25.Karvetti RL, Knuts LR. Validity of the 24-hour dietary recall. J Am Diet Assoc. 1985;85(11):1437-1442. [PubMed] [Google Scholar]
- 26.Freedson P, Bowles HR, Troiano R, Haskell W. Assessment of physical activity using wearable monitors: recommendations for monitor calibration and use in the field. Med Sci Sports Exerc. 2012;44(1)(suppl 1):S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freedson P, Pober D, Janz KF. Calibration of accelerometer output for children. Med Sci Sports Exerc. 2005;37(11)(suppl):S523-S530. [DOI] [PubMed] [Google Scholar]
- 28.Schaefer ES. Children’s reports of parental behavior: an inventory. Child Dev. 1965;36(5):413-424. [PubMed] [Google Scholar]
- 29.Birch LL, Fisher JO, Grimm-Thomas K, Markey CN, Sawyer R, Johnson SL. Confirmatory factor analysis of the Child Feeding Questionnaire: a measure of parental attitudes, beliefs and practices about child feeding and obesity proneness. Appetite. 2001;36(3):201-210. [DOI] [PubMed] [Google Scholar]
- 30.Schumi J, Wittes JT. Through the looking glass: understanding non-inferiority. Trials. 2011;12:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Buren S, Groothuis-Oudshoon K. MICE: Multivariate Imputation by Chained Equations in R. J Stat Softw. 2011;45(3):1-67. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 32.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289-300. [Google Scholar]
- 33.Theim KR, Sinton MM, Goldschmidt AB, et al. Adherence to behavioral targets and treatment attendance during a pediatric weight control trial. Obesity (Silver Spring). 2013;21(2):394-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steele MM, Steel RG, Hunter HL. Family adherence as a predictor of child outcome in an intervention for pediatric obesity: different outcomes for self-report and objective measures. Child Health Care. 2009;38(1):64-75. doi: 10.1080/02739610802615898 [DOI] [Google Scholar]
- 35.Kalarchian MA, Levine MD, Arslanian SA, et al. Family-based treatment of severe pediatric obesity: randomized, controlled trial. Pediatrics. 2009;124(4):1060-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cote MP, Byczkowski T, Kotagal U, Kirk S, Zeller M, Daniels S. Service quality and attrition: an examination of a pediatric obesity program. Int J Qual Health Care. 2004;16(2):165-173. [DOI] [PubMed] [Google Scholar]
- 37.Kitscha CE, Brunet K, Farmer A, Mager DR. Reasons for non-return to a pediatric weight management program. Can J Diet Pract Res. 2009;70(2):89-94. [DOI] [PubMed] [Google Scholar]
- 38.Barlow SE, Ohlemeyer CL. Parent reasons for nonreturn to a pediatric weight management program. Clin Pediatr (Phila). 2006;45(4):355-360. [DOI] [PubMed] [Google Scholar]
- 39.Skelton JA, Beech BM. Attrition in paediatric weight management: a review of the literature and new directions. Obes Rev. 2011;12(5):e273-e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skelton JA, Martin S, Irby MB. Satisfaction and attrition in paediatric weight management. Clin Obes. 2016;6(2):143-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Epstein LH, Wing RR, Koeske R, Valoski A. Effects of diet plus exercise on weight change in parents and children. J Consult Clin Psychol. 1984;52(3):429-437. [DOI] [PubMed] [Google Scholar]
- 42.Epstein LH, Wing RR, Penner BC, Kress MJ. Effect of diet and controlled exercise on weight loss in obese children. J Pediatr. 1985;107(3):358-361. [DOI] [PubMed] [Google Scholar]
- 43.Epstein LH, Wing RR, Steranchak L, Dickson B, Michelson J. Comparison of family-based behavior modification and nutrition education for childhood obesity. J Pediatr Psychol. 1980;5(1):25-36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
eTable. Child and Parent Mean Scores on Outcome Measures for Parent-Based Treatment and Family-Based Treatment at Baseline, 6-,12- and 24-Month Follow-up.