Key Points
Question
What are the consumer preferences, characteristics, and ingredient profiles of popular moisturizers?
Findings
In this cohort study, 174 best-selling moisturizer products available from 3 major online retailers varied widely by price and marketing claims, with lotions being the most popular vehicle. Few best-selling moisturizers were free of potential skin allergens.
Meaning
When making recommendations to patients with skin conditions that benefit from over-the-counter moisturizer use, dermatologists should consider recommending widely available and affordable products with a low risk of potential allergenicity.
This cohort study examines the product performance characteristics and ingredients of best-selling moisturizers.
Abstract
Importance
Because moisturizer use is critical for the prevention and treatment of numerous dermatological conditions, patients frequently request product recommendations from dermatologists.
Objective
To determine the product performance characteristics and ingredients of best-selling moisturizers.
Design and Setting
This cohort study involved publicly available data of the top 100 best-selling whole-body moisturizing products at 3 major online retailers (Amazon, Target, and Walmart). Products marketed for use on a specific body part (eg, face, hands, eyelids) were excluded.
Main Outcomes and Measures
Pairwise comparisons of median price per ounce on the basis of marketing claims (eg, dermatologist recommended, fragrance free, hypoallergenic) and presence of ingredients represented in the North American Contact Dermatitis Group (NACDG) series were conducted using Wilcoxon rank sum tests. The effect of vehicle type (eg, ointment, lotion, cream, butter) was assessed using the Kruskal-Wallis test. Cross-reactors and botanicals for fragrances were derived from the American Contact Dermatitis Society’s Contact Allergen Management Program database.
Results
A total of 174 unique best-selling moisturizer products were identified, constituting 109 713 reviews as of August 2016. The median price per ounce was $0.59 (range, $0.10-$9.51 per ounce) with a wide range (9400%). The most popular vehicles were lotions (102 [59%]), followed by creams (22 [13%]), oils (21 [12%]), butters (14 [8%]), and ointments (3 [2%]). Only 12% (n = 21) of best-selling moisturizer products were free of NACDG allergens. The 3 most common allergens were fragrance mix (n = 87), paraben mix (n = 75), and tocopherol (n = 74). Products with the claim “dermatologist recommended” had higher median price per ounce ($0.79; interquartile range [IQR], $0.56-$1.27) than products without the claim ($0.59; IQR, $0.34-$0.92). Products with the claim “phthalate free” had higher median price per ounce ($1.38; IQR, $0.86-$1.63) than products without the claim ($0.59; IQR, $0.35-$0.91). Lotions (median, $0.49; IQR, $0.31-0.68) were statistically less expensive per ounce than butters (median, $1.20; IQR, $0.76-$1.63), creams (median, $0.80; IQR, $0.69-$1.25) and oils (median, $1.30; IQR, $0.64-$2.43). For products with a claim of “fragrance free,” 18 (45%) had at least 1 fragrance cross-reactor or botanical ingredient. Products without any ingredients in the NACDG (median, $0.83; IQR, $0.47-$1.69) were not statistically more expensive per ounce than products with 1 or more allergens (median, $0.60; IQR, $0.35-$1.06).
Conclusions and Relevance
Best-selling moisturizers vary widely by price and product characteristics. Given the lack of readily available comparison data on moisturizer efficacy, dermatologists should balance consumer preference, price, and allergenicity in their recommendations.
Introduction
Moisturizers (eg, humectants, emollients, and occlusive) improve skin barrier function, reduce transepidermal water loss, and decrease cutaneous inflammation. These products provide therapeutic benefit for numerous dermatological conditions with and without xerosis. The American Academy of Dermatology recommends moisturizers for the treatment of atopic dermatitis (AD) with level 1A evidence and describes moisturizers as a universally accepted adjuvant therapy for psoriasis. Moisturizers free of an implicated allergen or irritant are also a mainstay of both the prevention and treatment for allergic contact dermatitis (ACD) and irritant contact dermatitis. Despite the intense media attention and consumer fears surrounding systemic toxicity of cosmetics from parabens (endocrine disruptor) or formaldehyde-induced carcinogenesis, the only established health risk for moisturizers is ACD or irritant contact dermatitis.
Currently, moisturizers represent a global $2 billion market with a myriad of product choices. Patients commonly call on dermatologists to provide product recommendations to navigate these choices. However, few studies have examined the ingredient profile and characteristics of personal care products preferred by consumers using real-world data. Beyond expected clinical efficacy, dermatologists should consider features such as cosmetic elegance, which affects patient adherence. Additional considerations for moisturizer recommendations include irritancy and allergenicity, which affect safety and tolerability. A recent study found that there is a frequent mismatch between dermatologist-recommended sunscreen characteristics and consumer popularity. In the present study, we sought to determine the nature of best-selling moisturizers including ingredient profile, vehicle, price, and consumer ratings. A better understanding of consumer preferences could inform and align dermatologists’ recommendations for moisturizers to their patients.
Methods
In August 2016, 3 major online retailers (Amazon, Walmart, and Target) were separately queried for the 100 best-selling general body moisturizers, and the data were stored in an off-line database. Collectively, these retailers represent more than half of the total annual sales of the $164 billion cosmetic and personal care product market. We excluded products marketed by manufacturers for use only on a specific body part (eg, facial moisturizer, hand creams) or primarily intended for a purpose other than moisturization (eg, body wash, acne treatments, eye creams). We included moisturizers with sunscreens or sun protection factor claims. Duplicate product entries were averaged among retailers. After exclusions, 174 unique products out of 300 were included in the final analysis. The median price, consumer ratings, number of consumer reviews, available ingredient list, vehicle type, and manufacturer were determined.
All statistical analyses were performed in R, version 3.1.0. Median prices were not normally distributed. Therefore, pairwise comparisons of median price per ounce were conducted using the Wilcoxon rank sum test. The Kruskal-Wallis test was performed to assess the effect of vehicle type on median price per ounce, with post hoc comparisons conducted with the Nemenyi test. P values obtained from a priori tests were all adjusted using false-discovery rate correction for multiple comparisons. A significance level of .05 was used. Explanatory variables included a binary variable for products with 1 or more chemicals in the North American Contact Dermatitis Group (NACDG) series and 12 additional product marketing claims: “dermatologist recommended,” “approved for pediatric use,” “fragrance free,” “noncomedogenic,” “paraben free,” “nongreasy,” “organic” or “natural,” “clinically proven,” “hypoallergenic,” “phthalate free,” “formaldehyde free,” and “National Eczema Foundation approved.” Vehicles tested included butter, cream, gel, lotion, oil, ointment, spray, other (eg, serum, balm), or unknown.
A detailed analysis of the ingredient list was then conducted. We assessed the proportion of products that had 1 or more ingredients listed in the NACDG 2011 to 2012 series. For products with fragrances, we applied a strict definition of fragrance requiring the presence of the word within the ingredient declaration list. For products that were self-declared as “fragrance-free,” a secondary analysis was performed for fragrance and botanical cross-reactors based on the American Contact Dermatitis Society’s Contact Allergen Management Program database (accessed March 28, 2017). This study was deemed institutional review board exempt by Northwestern University because it was not human subjects research.
Results
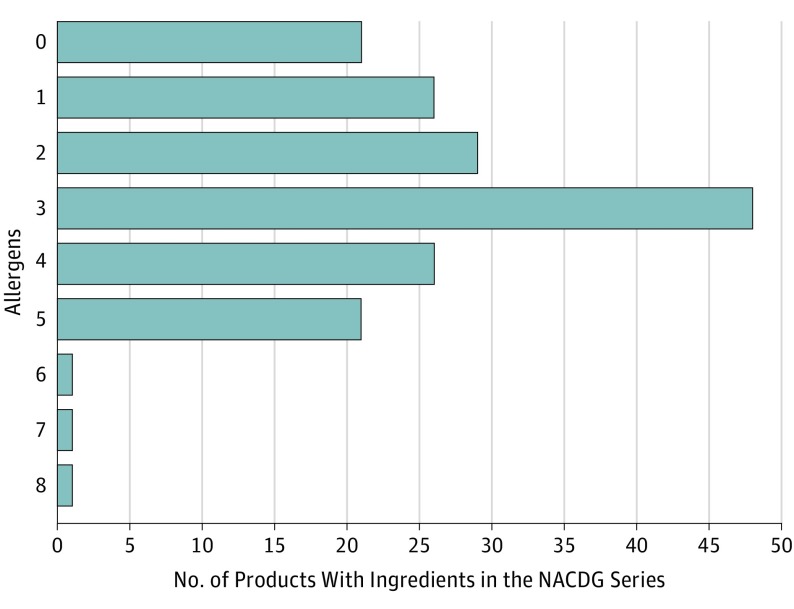
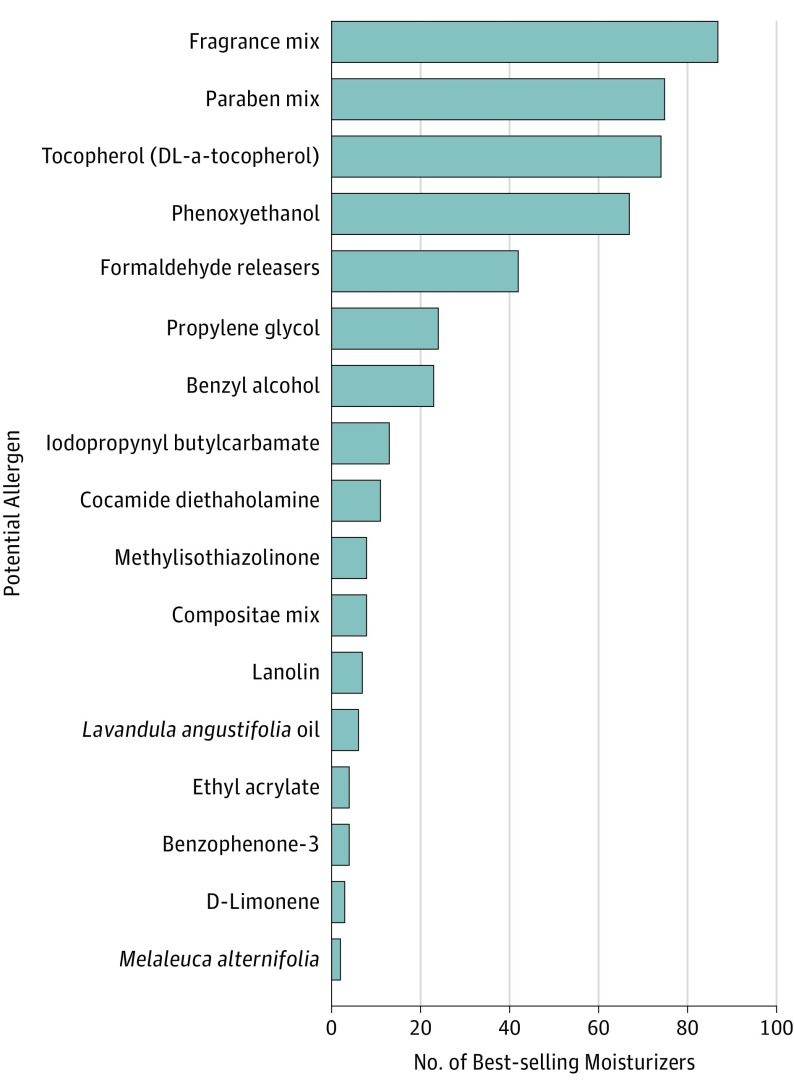
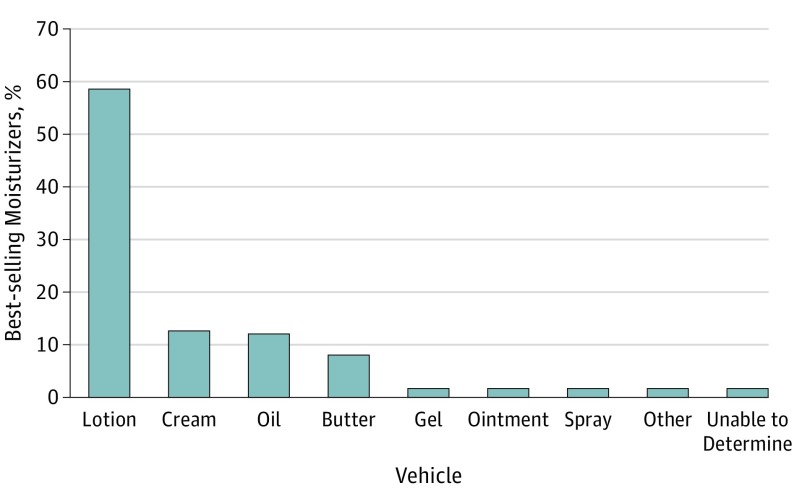
Across all 3 major retailers, there were a total of 109 713 consumer reviews for the 174 unique best-selling products used in this cohort. Best-selling products exhibited a high number of total consumer reviews and median rating. The median number of reviews was 121 (range, 0-15 916), with a median rating of 4.6 on a scale of 1 to 5 (range, 1.7-5.0). The median price was $0.59 (range, $0.10-$9.51) per ounce (to convert to price per milliliter, multiply by 29.6). Most products (120 of 174 [69%]) had additional marketing claims on their labels. The most common claims included “dermatologist recommended” (40 [23%]), “fragrance free” (40 [23%]), “nongreasy” (38 [22%]), “organic,” “natural,” or “pure” (38 [22%]), “noncomedogenic” (29 [17%]), “paraben free” (29 [17%]), “hypoallergenic” (18 [10%]), “clinically proven” (16 [9%]), “phthalate free” (15 [9%]), and National Eczema Foundation approved” (9 [5%]). For the 29 products described as “noncomedogenic,” the most common vehicle was lotions (14 [48%]), followed by creams (8 [28%]) and ointments (2 [7%]). For products claiming to be “hypoallergenic,” 15 (83%) had at least 1 ingredient in the NACDG series. For products with a “fragrance free” claim, 45% (18 of 40) had at least 1 fragrance or botanical cross-reactor ingredient. Most products (153 [88%]) had at least 1 allergen listed in the NACDG series, with 31% (n = 54), 43% (n = 75), and 13% (n = 24) having 1 to 2, 3 to 4, and 5 or more allergens, respectively (Figure 1). Overall, the 3 most common allergens in best-selling moisturizers were fragrances, parabens, and tocopherol (Figure 2). The 10 lowest-cost and most-reviewed products with and without allergens in the NACDG series were also determined (Table). The most common vehicles were lotions (102 [59%]), followed by creams (22 [13%]), oils (21 [12%]), butters (14 [8%]), and ointments (3 [2%]) (Figure 3).
Figure 1. Distribution of Allergens in the North American Contact Dermatitis Group (NACDG) Series for Best-selling Moisturizer Products.
Distribution of potential allergens in each best-selling moisturizer. Most products (121 [70%]) had 3 or fewer potential allergens. One product had 8 potential allergens, the highest in the cohort.
Figure 2. Most Common Potential Allergens in 174 Best-selling Moisturizer Products.
Common synonyms for allergens were included for each potential allergen. For example, methylisothiazolinone also includes products with methylchloroisothiazolinone or Kathon-CG in the ingredient list. Benzophenone-3 also included oxybenzone or 2-hydroxy-4-methoxy-benzophenone. Finally, Melaleuca alternifolia included the term “tea tree leaf oil.” Formaldehyde releasers were aggregated, which included DMDM hydantoin (n = 28), diazolidinyl urea (n = 13), and imidazolidinyl urea (n = 1).
Table. Best-selling Moisturizer Products Organized by Cost per Ounce and Popularitya With and Without North American Contact Dermatitis Group (NACDG) Series Ingredients.
| No. | Product Name | Reviews, No. | Median Price, $/oz | Median Ratingb | Vehicle |
|---|---|---|---|---|---|
| Top 10 Most Affordable | |||||
| 1 | Up & Up advanced therapy lotion | 5 | 0.10 | 3.8 | Lotion |
| 2 | Suave revitalizing with vitamin E body lotion | 47 | 0.12 | 4.0 | Lotion |
| 3 | Suave smoothing with cocoa butter and shea body lotion | 839 | 0.12 | 4.6 | Lotion |
| 4 | Suave advanced therapy body lotion | 757 | 0.12 | 4.7 | Lotion |
| 5 | Suave soothing with aloe body lotion | 32 | 0.12 | 4.1 | Lotion |
| 6 | Equate advanced recovery skin care lotion | 34 | 0.14 | 4.5 | Lotion |
| 7 | Equate cocoa butter conditioning body lotion | 27 | 0.14 | 4.7 | Lotion |
| 8 | Suave essentials lavender vanilla body lotion | 39 | 0.16 | 4.5 | Lotion |
| 9 | Fruit of the Earth aloe vera gel | 163 | 0.17 | 4.7 | Gel |
| 10 | Up & Up moisture rescue body lotion | 9 | 0.19 | 3.7 | Lotion |
| Top 10 Most Reviewed | |||||
| 1 | Viva Laboratories the finest organic extra virgin coconut oil | 15 916 | 0.64 | 4.7 | Oil |
| 2 | Gold Bond ultimate healing skin therapy lotion with aloe | 14 930 | 0.49 | 4.5 | Lotion |
| 3 | Better Shea Butter unrefined shea butter | 5255 | 0.90 | 4.8 | Butter |
| 4 | NOW Solutions sweet almond oil | 3719 | 0.73 | 4.7 | Oil |
| 5 | CeraVe moisturizing cream | 3461 | 0.79 | 4.7 | Cream |
| 6 | Bio-Oil: multiuse skincare oil | 3273 | 2.85 | 4.3 | Oil |
| 7 | Aveeno sheer hydration daily moisturizing lotion | 2929 | 0.58 | 4.7 | Lotion |
| 8 | Vaseline intensive care advanced repair unscented lotion | 2657 | 0.28 | 4.1 | Lotion |
| 9 | Hempz original herbal body moisturizer | 2057 | 0.85 | 4.4 | Lotion |
| 10 | Aveeno body moisture daily moisturizing lotion | 1985 | 0.88 | 4.8 | Lotion |
| Top 10 Most Affordable Without Ingredients in the NACDG Series | |||||
| 1 | Ivory raw unrefined shea butter | 785 | 0.28 | 4.3 | Butter |
| 2 | Vaseline original petroleum jelly | 98 | 0.32 | 4.8 | Ointment |
| 3 | Smellgood African shea butter | 272 | 0.37 | 4.3 | Butter |
| 4 | Home Health castor oil | 958 | 0.42 | 4.7 | Oil |
| 5 | NOW Foods apricot kernel oil | 565 | 0.46 | 4.7 | Oil |
| 6 | NOW Foods grape seed oil | 903 | 0.47 | 4.7 | Oil |
| 7 | NOW Solutions castor oil | 1969 | 0.59 | 4.7 | Oil |
| 8 | NOW Solutions sweet almond oil | 3719 | 0.73 | 4.7 | Oil |
| 9 | NOW Foods avocado oil | 837 | 0.76 | 4.7 | Oil |
| 10 | Sky Organics organic shea butter | 1295 | 0.77 | 4.7 | Butter |
| Top 10 Most Reviewed Without Ingredients in the NACDG Series | |||||
| 1 | Better Shea Butter unrefined shea butter | 5255 | 0.90 | 4.8 | Butter |
| 2 | NOW Solutions sweet almond oil | 3719 | 0.73 | 4.7 | Oil |
| 3 | NOW Solutions castor oil | 1969 | 0.59 | 4.7 | Oil |
| 4 | Aveeno eczema therapy moisturizing cream | 1780 | 1.41 | 4.8 | Cream |
| 5 | Molivera Organics ivory shea butter | 1633 | 0.87 | 4.7 | Butter |
| 6 | Sky Organics organic shea butter | 1295 | 0.77 | 4.7 | Butter |
| 7 | NOW Foods organic jojoba oil | 988 | 2.56 | 4.6 | Oil |
| 8 | Home Health castor oil | 958 | 0.42 | 4.7 | Oil |
| 9 | NOW Foods grape seed oil | 903 | 0.47 | 4.7 | Oil |
| 10 | NOW Foods avocado oil | 837 | 0.76 | 4.7 | Oil |
Popularity indicated by number of online consumer reviews.
On a scale of 1 to 5.
Figure 3. Vehicles of 174 Best-selling Moisturizers.
For best-selling moisturizers across 3 major online retailers, lotions were the most popular by a substantial margin. Ointments, a vehicle often preferred by dermatologists, represented only 2% of best-selling moisturizers.
Three significant predictors of higher price were vehicle (P < .001), the marketing claim of “phthalate free” (P < .001), and “dermatologist recommended” products (P = .04). In particular, products with the claim “dermatologist recommended” had higher median price per ounce ($0.79; interquartile range [IQR], $0.56-$1.27) than products without the claim ($0.59; IQR, $0.34-$0.92). Products with the claim “phthalate free” had higher median price per ounce ($1.38; IQR, $0.86-$1.63) than products without the claim ($0.59; IQR, $0.35-$0.91). A χ2 test of 9 vehicles showed that vehicle was a statistically significant predictor of price (χ2 = 48.2, P < .001). A post hoc analysis of vehicle showed that lotions were statistically less expensive (median price per ounce, $0.49; IQR, $0.31-0.68) compared with butters ($1.20; IQR, $0.76-$1.63; P = .005), creams ($0.80; IQR, $0.69-$1.25; P = .002), and oils ($1.30; IQR, $0.64-$2.43; P < .001). For nonlotion vehicles, there were no other significant differences in price. Products without any ingredients in the NACDG (median, $0.83; IQR, $0.47-$1.69) were not statistically more expensive per ounce than products with 1 or more allergens (median, $0.60; IQR, $0.35-$1.06). A full list of products in the cohort is presented in the eTable in the Supplement.
Discussion
The large number of available moisturizers with various formulations and ingredients creates both choice and confusion for consumers. However, the dermatological literature provides limited data on the comparative efficacy of various moisturizers. A recent Cochrane review concluded that moisturizers have beneficial effects for the treatment of AD but no reliable evidence that one moisturizer was superior to another. There are prescription moisturizers with specific ingredient ratios (eg, ceramides and free fatty acids) that are cleared by the US Food and Drug Administration (FDA) as medical devices. Whereas these products demonstrate clinical efficacy in controlled studies, they are significantly more expensive and do not demonstrate superiority against over-the-counter moisturizers in regard to clinical end points for AD. In the present study, the most expensive moisturizer was 9400% higher in price per ounce than the least expensive moisturizer. Given the substantial out-of-pocket costs of over-the-counter moisturizers for patients with AD and the lack of available comparative efficacy data, some have argued for the use of price as the differentiator.
Dermatologists are often asked to provide recommendations for patients with AD, patch-proven ACD, or patients at higher risk for the development of ACD or irritant contact dermatitis. In addition, 5% to 45% of individuals report sensitive skin, in which external exposures (eg, cosmetics, heat, wind) lead to skin reactivity including redness, irritation, itching, or pain measureable with laboratory techniques. The American Academy of Dermatology suggests that patients choose a moisturizer that is free of additives, fragrances, and perfumes to avoid common sensitizers. Overall, only 12% of products did not have ingredients present in the NACDG standard series. This finding is consistent with other studies showing the high prevalence of potential allergens in over-the-counter moisturizers and facial cosmetics. As expected, fragrances were the most common potential allergen in this cohort. For preservatives, parabens were the most commonly represented. Despite the ongoing concern for parabens as an endocrine disruptor, the best available data and the FDA indicate that at typical concentrations in cosmetics parabens are unlikely to affect human health. Parabens remain the NACDG’s preferred cosmetic preservative due to its lower irritancy and allergenicity in comparison with methylisothiazolinone or formaldehyde-releasing preservatives.
The majority of products had additional marketing claims beyond the primary purpose of skin moisturization. The 3 most common were “dermatologist recommended,” “fragrance free,” and “noncomedogenic.” Products with the label of “dermatologist recommended” had a small but statistically significant higher price per ounce. Currently, there is limited regulation or information on what constitutes a “dermatologist recommended” product. The Federal Trade Commission requires a “reasonable basis” to substantiate advertising claims.(p9) However, there is no predefined level of evidence required for this substantiation. For “dermatologist recommended” products, 95% of products had at least 1 ingredient in the NACDG series, suggesting a lack of correlation between the allergenicity of a product and the label meaning. Noncomedogenic is another claim not regulated by the FDA. The comedogenicity of a cosmetic can be determined in several ways including the use of a rabbit ear model or via a cyanoacrylate biopsy on human subjects to determine the number follicles and microcomedones per square inch. There is no universal industry standard for comedogenicity that determines the veracity of a “noncomedogenic” label. For the 29 products described as “noncomedogenic,” the most common vehicles were lotions, followed by creams and ointments. Although not all of the products in our cohort claiming to be “fragrance free” listed fragrance ingredients in the NACDG series on their labels, fragrance labeling can be misleading. Often, products labeled as “fragrance free” or “unscented” have masking agents that are fragrance allergens, cross-reactors, or botanicals with allergenic potential. A review of “fragrance free” products in our cohort showed that nearly half (45%) had a fragrance or botanical cross-reactor. For products claiming to be “hypoallergenic,” 83% had at least 1 ingredient in the NACDG series, suggesting, again, a lack of correlation between allergenicity and the label meaning. Dermatologists should remind patients that most of these claims, including “dermatologist recommended,” “noncomedogenic,” and “hypoallergenic,” are marketing tools with minimal to no regulatory oversight or substantiation. Importantly, bland products—ie, moisturizers without NACDG ingredients—were not associated with higher cost compared with other best-selling moisturizers.
The present study highlights a popular trend surrounding “natural” or “organic” products. In fact, many of the most affordable moisturizers without NACDG ingredients were natural oils and butters. Overall, these products represented 12% (21 of 174) of all best-selling moisturizers. Coconut oil and sunflower seed oil have exhibited moisturizing properties and clinical efficacy for improving xerosis in small studies. In 1 randomized clinical trial (n = 117) with AD, patients receiving coconut oil improved 85% more using the Scoring Atopic Dermatitis (SCORAD) scale compared with patients given mineral oil. Not all natural oils are effective. For instance, olive oil may actually reduce skin barrier integrity. For grape seed, avocado, and apricot oils, there is a lack of supporting data. Furthermore, labels such as “organic” or “natural” also have limited regulatory meaning in regards to personal care products. Consumers should be counseled that products with these labels do not necessarily equate to “safer” or “less” allergenic. Natural oils can also have botanicals and cross-reactors, although they did not list fragrances in their ingredient list. In fact, one of the products identified in this study was marketed as “all natural” and had the highest number of NACDG standard series ingredients (8).
As in sunscreens, factors related to the cosmetic elegance of a product, including its “feel” on application to the skin, color, scent, and skin absorption are important factors for consumers. Lotion, which is easily applied over a large surface area, was the most popular vehicle (59%) in this cohort. The higher water content in lotions likely contributes to its lower price and its greater cosmetic appeal. Lotions evaporate faster on the skin, which reduces the greasy feeling that consumers dislike. However, this property may also decrease the efficacy of lotions for patients with xerosis or AD. Only 2% of best-selling products were ointments. Even though ointments have the advantage of creating an occlusive oil film on the stratum corneum, require fewer preservatives in their preparation, and are often preferred by dermatologists, they are also more likely to feel greasy and leave a skin residue. Numerous reports have indicated that patients prefer nonointment vehicles. As a mixture of oil and water, creams (13% of cohort) might offer a middle ground for patients who find ointments too greasy. Ultimately, the American Academy of Dermatology guidelines for topical management of AD recommend that patients select a topical vehicle based on personal preference that they will use regularly, a recommendation supported by clinical studies. Patient adherence and willingness to use a moisturizer is more important than a specific formulation or vehicle.
Limitations
There are several important limitations of this study. First, the best-sellers list reflects a cross-sectional product ranking. Whereas the cohort is based on sales, the exact algorithm defining a product’s rank is proprietary without clear indication of temporality. However, these products are also frequently reviewed favorably. This provides evidence that the products in our cohort also have longitudinal and cumulative data suggesting consumer preference. Price estimates were applicable at the time of analysis and subject to change without notice. We attempted to mitigate this by averaging prices across different vendors. For our cohort, we excluded products marketed specifically for a given body part. However, this was not always clearly expressed on a product’s label or retailers’ descriptions. We also elected to use the NACDG series instead of the American Contact Dermatitis Society core series given a trend toward higher sensitivity. Given the high correlation between ingredients in the NACDG series and the American Contact Dermatitis Society core series, we suspect that results and conclusions would not be substantially different. Finally, the best-seller list represents general consumer purchases that may lack generalizability to patients with dermatological conditions purchasing moisturizers for therapeutic benefit.
Conclusions
Moisturizer use represents an effective and readily available strategy to manage many common dermatological conditions such as xerosis and AD. Our analysis of best-selling moisturizers across 3 major online retailers reveals products that differ substantially in price and characteristics. A large percentage contained at least 1 ingredient in the NACDG series, most commonly fragrances and preservatives. For patients with sensitive skin or existing skin conditions, dermatologists should consider balancing the risk of allergenicity and irritancy with affordability, availability, and consumer preferences. Given the wide number of product choices and inherent challenges in interpreting ingredient lists for consumers, dermatologists may have to provide specific product and manufacturer recommendations to guide patients toward the most appropriate moisturizer.
eTable. Best-Selling Moisturizers (n=174) from Three Major Online Retailers
References
- 1.Czarnowicki T, Malajian D, Khattri S, et al. Petrolatum: barrier repair and antimicrobial responses underlying this “inert” moisturizer. J Allergy Clin Immunol. 2016;137(4):1091-1102.e7. [DOI] [PubMed] [Google Scholar]
- 2.Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Psoriasis: recommendations for emollients. https://www.aad.org/practicecenter/quality/clinical-guidelines/psoriasis/topical-therapy/recommendations-for-emollients. Accessed August 6, 2017.
- 4.Saary J, Qureshi R, Palda V, et al. A systematic review of contact dermatitis treatment and prevention. J Am Acad Dermatol. 2005;53(5):845. [DOI] [PubMed] [Google Scholar]
- 5.Kirchhof MG, de Gannes GC. The health controversies of parabens. Skin Therapy Lett. 2013;18(2):5-7. [PubMed] [Google Scholar]
- 6.Ben-Shabat H, Tng W Beauty and the e-commerce beast. 2014. A.T. Kearney website. https://www.atkearney.com/consumer-products-retail/beauty-and-the-e-commerce-beast. Accessed August 6, 2017.
- 7.Educated consumers are demanding more in skin care. Chain Drug Rev. 2015;37(5):57. [Google Scholar]
- 8.Yokota M, Maibach HI. Moisturizer effect on irritant dermatitis: an overview. Contact Dermatitis. 2006;55(2):65-72. [DOI] [PubMed] [Google Scholar]
- 9.Xu S, Kwa M, Agarwal A, Rademaker A, Kundu RV. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152(8):920-927. [DOI] [PubMed] [Google Scholar]
- 10.Warshaw EM, Maibach HI, Taylor JS, et al. North American contact dermatitis group patch test results: 2011-2012. Dermatitis. 2015;26(1):49-59. [DOI] [PubMed] [Google Scholar]
- 11.van Zuuren EJ, Fedorowicz Z, Christensen R, Lavrijsen A, Arents BWM. Emollients and moisturisers for eczema. Cochrane Database Syst Rev. 2017;2:CD012119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller DW, Koch SB, Yentzer BA, et al. An over-the-counter moisturizer is as clinically effective as, and more cost-effective than, prescription barrier creams in the treatment of children with mild-to-moderate atopic dermatitis: a randomized, controlled trial. J Drugs Dermatol. 2011;10(5):531-537. [PubMed] [Google Scholar]
- 13.Filanovsky MG, Pootongkam S, Tamburro JE, Smith MC, Ganocy SJ, Nedorost ST. The financial and emotional impact of atopic dermatitis on children and their families. J Pediatr. 2016;169:284-290.e5. [DOI] [PubMed] [Google Scholar]
- 14.Xu S, Immaneni S, Hazen GB, Silverberg JI, Paller AS, Lio PA. Cost-effectiveness of prophylactic moisturization for atopic dermatitis. JAMA Pediatr. 2017;171(2):e163909. [DOI] [PubMed] [Google Scholar]
- 15.Misery L, Sibaud V, Merial-Kieny C, Taieb C. Sensitive skin in the American population: prevalence, clinical data, and role of the dermatologist. Int J Dermatol. 2011;50(8):961-967. [DOI] [PubMed] [Google Scholar]
- 16.Willis CM, Shaw S, De Lacharrière O, et al. Sensitive skin: an epidemiological study. Br J Dermatol. 2001;145(2):258-263. [DOI] [PubMed] [Google Scholar]
- 17.Seidenari S, Francomano M, Mantovani L. Baseline biophysical parameters in subjects with sensitive skin. Contact Dermatitis. 1998;38(6):311-315. [DOI] [PubMed] [Google Scholar]
- 18.American Academy of Dermatology’s newest guidelines for the management of atopic dermatitis focus on treatments [news release]. Schaumburg, IL: American Academy of Dermatology; May 7, 2014. https://www.aad.org/media/news-releases/american-academy-of-dermatology-s-newest-guidelines-for-the-management-of-atopic-dermatitis-focus-on-treatments. 2014. Accessed August 6, 2017.
- 19.Scheman A, Jacob S, Katta R, et al. Part 1 of a 4-part series: facial cosmetics: trends and alternatives: data from the American Contact Alternatives Group. J Clin Aesthet Dermatol. 2011;4(6):25-30. [PMC free article] [PubMed] [Google Scholar]
- 20.Zirwas MJ, Stechschulte SA. Moisturizer allergy: diagnosis and management. J Clin Aesthet Dermatol. 2008;1(4):38-44. [PMC free article] [PubMed] [Google Scholar]
- 21.US Food and Drug Administration. Parabens in cosmetics. https://www.fda.gov/cosmetics/productsingredients/ingredients/ucm128042.htm. Accessed January 3, 2017.
- 22.Lobemeier C, Tschoetschel C, Westie S, Heymann E. Hydrolysis of parabenes by extracts from differing layers of human skin. Biol Chem. 1996;377(10):647-651. [DOI] [PubMed] [Google Scholar]
- 23.DeKoven JG, Warshaw EM, Belsito DV, et al. North American Contact Dermatitis Group patch test results 2013-2014. Dermatitis. 2017;28(1):33-46. [DOI] [PubMed] [Google Scholar]
- 24.Davis JB, McNamara SH. Cosmetic Claims Substantiation. Cosmetic Science and Technology Series, vol 18 New York, NY: Marcel Dekker; 1997. [Google Scholar]
- 25.Fulton JE Jr, Pay SR, Fulton JE III. Comedogenicity of current therapeutic products, cosmetics, and ingredients in the rabbit ear. J Am Acad Dermatol. 1984;10(1):96-105. [DOI] [PubMed] [Google Scholar]
- 26.Draelos ZD, DiNardo JC. A re-evaluation of the comedogenicity concept. J Am Acad Dermatol. 2006;54(3):507-512. [DOI] [PubMed] [Google Scholar]
- 27.Agero AL, Verallo-Rowell VM. A randomized double-blind controlled trial comparing extra virgin coconut oil with mineral oil as a moisturizer for mild to moderate xerosis. Dermatitis. 2004;15(3):109-116. [DOI] [PubMed] [Google Scholar]
- 28.Danby SG, AlEnezi T, Sultan A, et al. Effect of olive and sunflower seed oil on the adult skin barrier: implications for neonatal skin care. Pediatr Dermatol. 2013;30(1):42-50. [DOI] [PubMed] [Google Scholar]
- 29.Evangelista MT, Abad-Casintahan F, Lopez-Villafuerte L. The effect of topical virgin coconut oil on SCORAD index, transepidermal water loss, and skin capacitance in mild to moderate pediatric atopic dermatitis: a randomized, double-blind, clinical trial. Int J Dermatol. 2014;53(1):100-108. [DOI] [PubMed] [Google Scholar]
- 30.Hon KL, Leung AK, Barankin B. Barrier repair therapy in atopic dermatitis: an overview. Am J Clin Dermatol. 2013;14(5):389-399. [DOI] [PubMed] [Google Scholar]
- 31.Puig L, Carrascosa JM, Belinchón I, et al. ; Panel de Expertos del Consenso Delphi sobre Tratamiento Tópico de la Psoriasis; Grupo de Psoriasis de la Academia Española de Dermatología y Venereología . Adherence and patient satisfaction with topical treatment in psoriasis, and the use, and organoleptic properties of such treatments: a Delphi study with an expert panel and members of the Psoriasis Group of the Spanish Academy of Dermatology and Venereology. Actas Dermosifiliogr. 2013;104(6):488-496. [DOI] [PubMed] [Google Scholar]
- 32.Tan X, Feldman SR, Chang J, Balkrishnan R. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9(10):1263-1271. [DOI] [PubMed] [Google Scholar]
- 33.American Academy of Dermatology Eczema friendly moisturizer: how to select. https://www.aad.org/public/diseases/eczema/eczema-resource-center/skin-care/select-moisturizer. Accessed February 2, 2017.
- 34.Shim JH, Park JH, Lee JH, Lee DY, Lee JH, Yang JM. Moisturizers are effective in the treatment of xerosis irrespectively from their particular formulation: results from a prospective, randomized, double-blind controlled trial. J Eur Acad Dermatol Venereol. 2016;30(2):276-281. [DOI] [PubMed] [Google Scholar]
- 35.Schalock PC, Dunnick CA, Nedorost S, Brod B, Warshaw E, Mowad C. American Contact Dermatitis Society core allergen series. Dermatitis. 2013;24(1):7-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Best-Selling Moisturizers (n=174) from Three Major Online Retailers