This case-control study investigates whether the incidence of dementia in the general population covaries with long-term exposure to microlevels of lithium in drinking water.
Key Points
Question
Is a higher lithium level in drinking water associated with a decreased incidence of dementia?
Findings
In this Danish nationwide, population-based, nested case-control study of 73 731 patients with dementia and 733 653 control individuals, the level of lithium exposure was lower for patients with a diagnosis of dementia than for controls. Similar patterns were found with Alzheimer disease and vascular dementia as outcomes.
Meaning
Exposure to higher long-term lithium levels in drinking water may be associated with a lower incidence of dementia.
Abstract
Importance
Results from animal and human studies suggest that lithium in therapeutic doses may improve learning and memory and modify the risk of developing dementia. Additional preliminary studies suggest that subtherapeutic levels, including microlevels of lithium, may influence human cognition.
Objective
To investigate whether the incidence of dementia in the general population covaries with long-term exposure to microlevels of lithium in drinking water.
Design, Setting, and Participants
This Danish nationwide, population-based, nested case-control study examined longitudinal, individual geographic data on municipality of residence and data from drinking water measurements combined with time-specific data from all patients aged 50 to 90 years with a hospital contact with a diagnosis of dementia from January 1, 1970, through December 31, 2013, and 10 age- and sex-matched control individuals from the Danish population. The mean lithium exposure in drinking water since 1986 was estimated for all study individuals. Data analysis was performed from January 1, 1995, through December 31, 2013.
Main Outcomes and Measures
A diagnosis of dementia in a hospital inpatient or outpatient contact. Diagnoses of Alzheimer disease and vascular dementia were secondary outcome measures. In primary analyses, distribution of lithium exposure was compared between patients with dementia and controls.
Results
A total of 73 731 patients with dementia and 733 653 controls (median age, 80.3 years; interquartile range, 74.9-84.6 years; 44 760 female [60.7%] and 28 971 male [39.3%]) were included in the study. Lithium exposure was statistically significantly different between patients with a diagnosis of dementia (median, 11.5 µg/L; interquartile range, 6.5-14.9 µg/L) and controls (median, 12.2 µg/L; interquartile range, 7.3-16.0 µg/L; P < .001). A nonlinear association was observed. Compared with individuals exposed to 2.0 to 5.0 µg/L, the incidence rate ratio (IRR) of dementia was decreased in those exposed to more than 15.0 µg/L (IRR, 0.83; 95% CI, 0.81-0.85; P < .001) and 10.1 to 15.0 µg/L (IRR, 0.98; 95% CI, 0.96-1.01; P = .17) and increased with 5.1 to 10.0 µg/L (IRR, 1.22; 95% CI, 1.19-1.25; P < .001). Similar patterns were found with Alzheimer disease and vascular dementia as outcomes.
Conclusions and Relevance
Long-term increased lithium exposure in drinking water may be associated with a lower incidence of dementia in a nonlinear way; however, confounding from other factors associated with municipality of residence cannot be excluded.
Introduction
Dementia is the leading cause of dependence and disability in the elderly population worldwide. As the mean life expectancy increases, the prevalence of dementia and associated monetary costs are expected to increase exponentially. The pathogenesis of Alzheimer disease is highly complex and likely to be multifactorial, including deregulated amyloid-β, phosphorylated τ, and glycogen synthase kinase 3, as well as inflammation, mitochondrial dysfunction, and calcium dyshomeostasis, suggesting that combination therapy or multitarget drugs might be effective. Lithium is a multitarget drug that seems to possess neuroprotective abilities by modulating a large array of intracellular cascades and pathways involved in oxidative stress, inflammation, mitochondrial dysfunction, membrane homeostasis, and inhibitory effects on glycogen synthase kinase 3. Animal studies have found that long-term lithium treatment improves learning and memory, and human observational studies suggest that continued treatment with lithium may reduce the risk of dementia among patients with bipolar disorder. In bipolar disorder, treatment with lithium in usual therapeutic daily doses (600-2400 mg) for mood stabilization increases the risk of chronic kidney disease in the elderly population. In accordance with this finding, lower doses have been used in trials investigating the effects of lithium on cognition among older individuals. A placebo-controlled, randomized 2-year trial suggested that long-term treatment with daily low-milligram doses of lithium (150-600 mg) may decrease the rate of developing Alzheimer disease and cerebrospinal fluid concentrations of phosphorylated τ among individuals with mild cognitive impairment. Furthermore, in a 15-month, placebo-controlled randomized clinical trial, a microdose of 300 µg/d of lithium stabilized cognitive impairment in patients with Alzheimer disease. We investigated whether the incidence of dementia in the general population covaries with long-term exposure to microlevels of lithium in drinking water, hypothesizing that higher long-term lithium exposure may be associated with a lower incidence of dementia.
Methods
The Registers
Data were obtained by linking Danish population–based registers using unique personal identification numbers, which are assigned to all 5.6 million persons living in Denmark. Data on municipality of residence are available at Statistics Denmark at an individual level for all individuals in Denmark from 1986 onward. Data from Statistics Denmark were linked with data on diagnoses and corresponding dates from the Danish National Patient Register (DNPR) and the Danish Psychiatric Central Research Register (DPCRR) and dates of death from the Danish Register of Causes of Death. The DPCRR contains data from 1970 onward, and the DNPR contains data from January 1, 1977, onward. The registers include data from all inpatients treated at psychiatric and somatic hospitals in Denmark. They also include data on outpatients from January 1, 1995, onward as a part of the official Danish health survey. Since January 1994, the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) has been in use in both registers. The Danish Register of Causes of Death contains data on death. The study was approved by the Danish Data Protection Agency. According to Danish law and the National Board of Health, informed consent is not necessary in register-based research. Register-based data are deidentified.
Selection of Cases
All patients with a hospital contact with a diagnosis of dementia as an inpatient or outpatient were identified in the DNPR (ICD-10 code DF F00-19 + G30.0–G30.9) and in the DPCRR (ICD-10 code DF F00-19) for the study period of January 1, 1995, through December 31, 2013. Patients in the registers with a diagnosis of dementia before entry into the study and back to January 1, 1977 (for the DNPR), and 1970 (for the DPCRR) were excluded (ICD-8 codes 290-290.19, ICD-10 codes F00-09 and G30.0–G30.9). The date of first diagnosis is referred to as the index date. Data analysis was performed from January 1, 1995, through December 31, 2013.
The validity of the diagnosis of dementia in the Danish hospital registers is high because a registered diagnosis of dementia was found to be correct, fulfilling ICD-10 and/or DSM-IV criteria for dementia in 169 of 197 (85.8%) randomly selected inpatients and outpatients from the DNPR and the DPCRR based on a systematic review and scoring system of patients’ medical journals, including history of dementia illness, cognitive test results, psychiatric evaluation findings, blood test results, electrocardiography findings, findings from computed tomography or magnetic resonance imaging of the brain, physical examination (including vital signs and neurologic examination) results, and evaluation of activities of daily living. However, with regard to dementia subtypes, the degree of agreement between the registers and the results of the validating process was low (κ = 0.36; 95% CI, 0.24-0.48).
Selection of Controls
A nested case-control study design was used. Control individuals were selected from a random sample that consisted of 1 500 000 persons in the Danish population that was registered on January 1, 1995. For each patient with dementia, we randomly sampled 10 controls from the age- (1 month) and sex-matched subpopulation under the additional condition that controls were alive and did not have dementia at the index date of the patient with dementia. Patients with dementia and controls were excluded from all analyses if no information on municipality of residence was available between 1986 and the index date.
Lithium Exposure Assessment
Drinking water samples from 151 waterworks taken from 2009 to 2010 and 2013, supplying approximately 42% of the Danish population, were used to estimate the lithium drinking water level for the entire country based on the kriging interpolation method. On the basis of the kriging map, a time-constant mean lithium level in each of the 275 municipalities in Denmark was calculated. Kriging is based on spatial autocorrelation estimates obtained by fitting a semivariogram. Different semivariograms were used to evaluate the effect of the selected spatial autocorrelation estimates on the estimated kriging map. Furthermore, inverse distance weighting was used as an alternative interpolation method. Different semivariograms used for the kriging and inverse distance weighting resulted in similar maps (details are described in the article by Knudsen et al). The Danish municipality to which the home address of a study participant (patient with dementia or control) belonged in a certain year was obtained for all years from 1986 to the index date. Thus, analyses accounted for whether individuals moved from one municipality to another, resulting in a change of exposure to lithium in drinking water. This information was used to compute the participant-specific mean level of lithium in drinking water according to the addresses of all study individuals in the study period (details are described in the article by Knudsen et al).
Statistical Analysis
Analyses were performed among individuals aged 50 to 90 years. In the primary analyses, the distribution of lithium exposure was compared between patients with dementia and controls. The association between exposure to lithium in drinking water and the incidence rate of dementia was estimated using a Cox proportional hazards regression model fitted to the nested case-control sample, providing incidence rate ratios (IRRs) of dementia with 95% CIs. In this analysis, the mean exposure to lithium in drinking water was categorized into 4 groups (2.0-5.0, 5.1-10.0, 10.1-15.0, and 15.1-27.0 µg/L). The lowest group (2.0-5.0 µg/L) was used as a reference for the IRR calculations. A sensitivity analysis was performed in which the continuous association between the mean lithium level in drinking water and the IRR of dementia was analyzed by using restricted cubic splines with 5 knots set at the quantiles of the mean lithium exposure in drinking water.
By sampling controls from the risk set at the dates of dementia diagnosis and by matching for age, we determined that the association between mean lithium exposure and dementia IRR can be interpreted as IRRs between individuals living constantly during the same age interval (since beginning of exposure ascertainment and since birth) in areas with given lithium levels in drinking water. Urbanicity (density of population) influences the risk of schizophrenia but not bipolar disorder, and it is unclear whether urbanicity influences the risk of other brain disorders, such as dementia. To investigate whether urbanicity had a confounding effect, a sensitivity analysis was conducted adjusting dynamically over time for urbanicity of place of residence (capital or capital suburb [reference], provincial city with <100 000 inhabitants, provincial town with >10 000 inhabitants, or rural areas). All analyses were repeated separately with a diagnosis of Alzheimer disease as the outcome and a diagnosis of vascular dementia as the outcome. P < .05 was considered to be statistically significant.
Results
After excluding patients with a prior diagnosis of dementia, 74 100 patients aged 50 to 90 years were identified with a diagnosis of dementia during the study period from 1995 through 2013. Municipality of residence was missing in the entire study period for 0.30% of patients and 0.49% of matched controls, leaving 73 731 patients with dementia and 733 653 controls for the analyses (median age, 80.3 years; interquartile range [IQR], 74.9-84.6 years; 44 760 female [60.7%] and 28 971 male [39.3%]). A total of 9038 participants (12.3%) were 50 to 70 years of age, 26 420 (35.8%) were 70 to 80 years of age, and 38 273 (51.9%) were 80 to 90 years of age.
The mean (SD) lithium level in drinking water was 11.6 (6.8) µg/L, ranging from 0.6 µg/L in western Denmark to 30.7 µg/L in eastern Denmark. The interpolation of the point data rendered estimated lithium levels for each of the 275 municipalities.
The distributions of the mean lithium exposure were statistically significantly different among patients with a diagnosis of dementia (median, 11.5 µg/L; IQR, 6.5-14.9 µg/L) and among controls (median, 12.2 µg/L; IQR, 7.3-16.0 µg/L; P < .001). The Table indicates that when mean lithium intake was categorized in the Cox proportional hazards regression models, the IRR of dementia was decreased among individuals exposed to 10.1 µg/L of lithium or more compared with exposure to 2.0 to 5.0 µg/L, reaching statistical significance only among individuals exposed to more than 15.0 µg/L of lithium (IRR, 0.83; 95% CI, 0.81-0.85; P < .001) compared with 2.0 to 5.0 µg/L. However, exposure to 5.1 to 10.0 µg/L of lithium was associated with an increased IRR of dementia compared with 2.0 to 5.0 µg/L (IRR, 1.22; 95% CI, 1.19-1.25; P < .001). This nonlinear pattern was unchanged when the analyses excluded data for individuals with partly missing information on municipality of residence (IRR for 5.1-10.0 µg/L, 1.23; 95% CI, 1.20-1.26; IRR for 10.1-15.0 µg/L, 0.99; 95% CI, 0.96-1.01; and IRR for 15.1-27.0 µg/L, 0.82; 95% CI, 0.80-0.85). In addition, the pattern was confirmed when the analysis was adjusted for urbanicity of residence (IRR for 5.1-10.0 µg/L, 1.18; 95% CI, 1.15-1.21; IRR for 10.1-15.0 µg/L, 1.02; 95% CI, 0.99-1.05; and IRR for 15.1-27.0 µg/L, 0.88; 95% CI, 0.85-0.91). In the latter model, there was a direct inverse association with urbanicity (with residence capital or capital suburb as the reference; IRR for provincial city, 1.03; 95% CI, 0.99-1.06; IRR for provincial town, 1.36; 95% CI, 1.33-1.40; and IRR for rural areas, 1.17; 95% CI, 1.14-1.20). Furthermore, this pattern was repeated when Alzheimer disease or vascular dementia was an outcome.
Table. Lithium Exposure and Rates of Dementia (Overall), Alzheimer Disease, and Vascular Dementia.
| Mean Lithium Exposure, µg/L | No. (%) | Incidence Rate Ratio (95% CI) | P Value | |
|---|---|---|---|---|
| Patients | Control Individuals | |||
| Dementia (Overall) | ||||
| 2.0-5.0 | 9105 (12.4) | 90 052 (12.4) | 1 [Reference] | |
| 5.1-10.0 | 19 694 (26.9) | 158 627 (21.8) | 1.22 (1.19-1.25) | <.001 |
| 10.1-15.0 | 26 471 (36.1) | 264 647 (36.3) | 0.98 (0.96-1.01) | .17 |
| 15.1-27.0 | 18 010 (24.6) | 214 947 (29.5) | 0.83 (0.81-0.85) | <.001 |
| Alzheimer Disease | ||||
| 2.0-5.0 | 3700 (12.7) | 35 485 (12.3) | 1 [Reference] | |
| 5.1-10.0 | 7974 (27.5) | 62 832 (21.8) | 1.22 (1.17-1.27) | <.001 |
| 10.1-15.0 | 10 457 (36.0) | 105 251 (36.5) | 0.95 (0.92-1.00) | .02 |
| 15.1-27.0 | 6896 (23.8) | 85 115 (29.5) | 0.78 (0.74-0.81) | <.001 |
| Vascular Dementia | ||||
| 2.0-5.0 | 1827 (12.4) | 18 328 (12.5) | 1 [Reference] | |
| 5.1-10.0 | 4019 (27.3) | 31 994 (21.9) | 1.26 (1.19-1.33) | <.001 |
| 10.1-15.0 | 5129 (34.9) | 52 788 (36.1) | 0.97 (0.92-1.03) | .34 |
| 15.1-27.0 | 3736 (25.4) | 43 181 (29.5) | 0.87 (0.81-0.91) | <.001 |
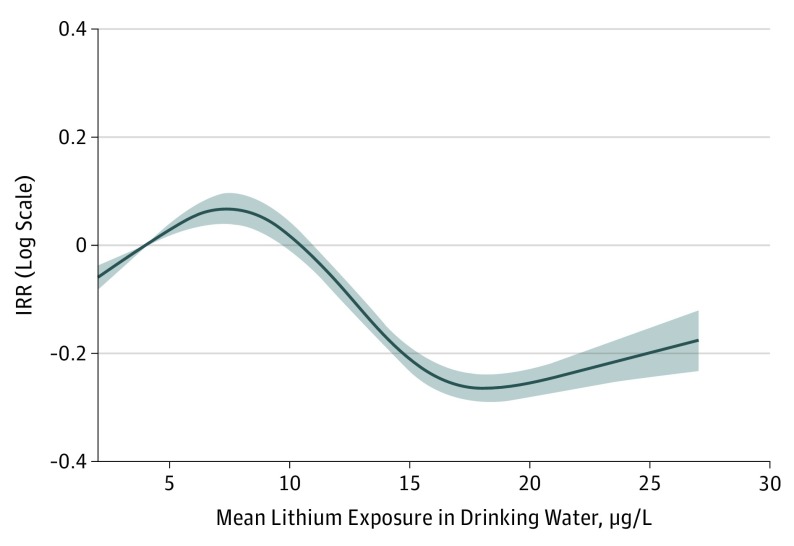
The Figure shows the restricted cubic spline analysis of the continuous association in relation to dementia overall. The reference for the IRRs was set at a mean of 4 µg/L.
Figure. Association Between Mean Lithium Exposure in Drinking Water on a Continuous Scale and the Overall Dementia Rate .
Data are the incidence rate ratios (IRRs) in relation to the mean (4 µg/L) of the lowest group on a logarithmic scale. Shaded area indicates 95% CIs.
Discussion
Overall, we confirmed the hypothesis that higher long-term lithium exposure from drinking water may be associated with a lower incidence of dementia, although the association was nonlinear (Table and Figure). This is the first study, to our knowledge, to investigate the association between lithium in drinking water and the incidence of dementia. The study has several advantages. The study was a nationwide, population-based, nested case-control study that included individualized longitudinal data on lithium exposure based on the municipality of residence of study individuals (patients with dementia or controls) during an up to 28-year exposure period from 1986 to 2013 and drinking water lithium samples from 151 waterworks sampled from 2009 to 2010 and in 2013, spatially covering all of Denmark combined with hospital data on incident dementia in an up to 19-year outcome period (1995-2013). In this way, the study took into account whether individuals moved from one municipality to another, resulting in a change of lithium exposure in drinking water. Thus, in contrast to prior ecologic studies on lithium in drinking water and the association with various outcomes, such as suicide, the present study used individualized data on lithium exposure in drinking water linked individually with a diagnosis of dementia. Furthermore, the study included all patients with incident cases of dementia that resulted in hospital contact as an outpatient or inpatient in somatic and psychiatric hospitals or wards nationwide and 10 controls per case without a hospital contact with dementia. The validity of the diagnosis of dementia in the Danish hospital registers is high, and although the hospital registers include data on diagnosis from secondary care only, the age- and sex-specific incidence of dementia is comparable to the results from the European digital elevation model (EuroDEM). In our study, the associations between long-term lithium exposure and the incidence of dementia were repeated when Alzheimer disease or vascular dementia was selected as an outcome, although the validity of dementia subtypes in the hospital registers is lower.
In addition to vegetables, drinking water is a major source of human lithium intake. The levels of lithium in groundwater and drinking water are most likely stable over time because of the chemical properties of lithium and its slow leaching into the ground. The geographic variation of lithium levels in Danish groundwater ranges from approximately 2 µg/L in some areas to nearly 30 µg/L in others. Higher levels may occur in groundwater aquifers that are in contact with marine sediments. Compared with drinking water in other regions of the world, the lithium levels observed in Danish drinking water are significantly lower and the range is generally more narrow; on a European scale, the levels are slightly higher than observed medians and means according to lithium statistics from more than 500 drinking water samples in Europe.
Limitations
We did not adjust our analyses for accessibility to health care services that vary geographically and may influence the probability of diagnosis of dementia, specifically during early stages. Nevertheless, accessibility to health care services is increased in eastern regions of Denmark, where lithium levels generally are higher, and decreased in western regions, specifically in Jutland, where lithium levels generally are lower (ie, not taking account of accessibility to health care services in the analyses may tend to underestimate the association between lithium in drinking water and incidence of dementia). However, in a sensitivity analysis, we adjusted the model for urbanicity of residence, finding a direct inverse association with increasing risk of dementia in rural areas in contrast to prior findings of increased risk of schizophrenia in urban areas. Furthermore, because all inhabitants in a given municipality are assigned the same level of lithium exposure, it cannot be excluded that other, unobserved environmental or social care factors related to individuals’ municipality of residence might have confounded the association between lithium exposure and dementia rate. Finally, it cannot be excluded that there may have been some long-term changes in lithium levels in drinking water during the study period from 1986 to 2013, although the level of lithium in groundwater and drinking water is most likely stable over time because of the chemical properties of lithium and its slow release from the soil and sediment. Accordingly, a previous comparison including data from the same waterworks 4 years apart (2009-2013) suggested that lithium levels were roughly stable over time and that there was no lithium removal or enrichment during the treatment at the waterworks.
Nonlinear dose-response associations are often found in medicine, with a gradual increase in drug response at the lower doses and gradual leveling off in response at the highest doses. Bell-shaped response curves, occasionally with multiple modes, have been observed in some of the microdose nonlithium neuroprotective literature, presumably reflecting the engagement of one neuroprotective mechanism of action followed by its loss as the dose is increased, only to engage another mechanism as the dose is further increased. Similar dose-response associations may pertain to the nonlinear findings in our study (Table and Figure).
Our findings agree with results of the 2 longer-term randomized clinical trials on lithium in subtherapeutic doses producing stabilizing effects among individuals with mild cognitive impairment treated with low doses of lithium (150-600 mg) for 2 years and patients with Alzheimer disease treated with a microdose of 300 µg of lithium for 15 months. However, 2 small short-term randomized clinical trials of adults with mild to moderate Alzheimer disease did not observe a protective effect of lithium in therapeutic doses during 16 and 10 weeks, respectively, probably because of high dropout rates associated with lithium treatment at higher levels; the findings suggest that longer-term exposure with subtherapeutic lithium doses may be necessary to reveal a protective effect.
Although studies on the effects of microdoses of lithium are controversial, observations suggest that biological effects of long-term microdoses of lithium are relevant in the pathogenesis of Alzheimer disease. For example, long-term treatment with lithium at subtherapeutic doses can modify the secretion of proinflammatory and anti-inflammatory interleukins in cocultures of cortical and hippocampal neurons with glial cells. Furthermore, long-term microdoses of lithium have compared with higher doses—a more prominent effect on membrane homeostasis (which may be disturbed in Alzheimer disease)—by activating forms of cytosolic phospholipase A2 and calcium-independent phospholipase A2 in primary cultures of cortical and hippocampal neurons. In addition, rats exposed to long-term (100 days) treatment with 125 mg/L of lithium in drinking water, which resulted in low mean (SD) blood lithium levels (0.1 [0.019] mEq/L), experienced activated brain phospholipase A2; this activation is required for memory retrieval and improved memory.
Other arguments that support the view that microlevels of lithium may affect human behavior derive from prior cross-sectional studies based on ecologic, nonindividualized data that report that levels of lithium in drinking water correlate inversely with the rate of suicide and from a preliminary study on the effects of nutritional lithium supplementation on mood. However, a newly conducted Danish study, which used individualized data as the present study did, did not confirm the protective effect of exposure to lithium in drinking water and the risk of suicide.
No brain imaging studies have been published on the effects of microlevels of lithium on brain functioning. Nevertheless, there is level 1 evidence of a positive association between lithium treatment in therapeutic doses and brain gray matter volume in multiple brain regions of relevance for Alzheimer disease, including hippocampus, amygdala, anterior cingulate, subgenual cingulate, inferior frontal gyrus, postcentral gyrus, and habenula.
Conclusions
Long-term increased exposure to lithium in drinking water may be associated with a lower incidence of dementia in a nonlinear way. However, confounding from other factors associated with municipality of residence cannot be excluded.
References
- 1.Sousa RM, Ferri CP, Acosta D, et al. Contribution of chronic diseases to disability in elderly people in countries with low and middle incomes: a 10/66 Dementia Research Group population-based survey. Lancet. 2009;374(9704):1821-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sousa RM, Ferri CP, Acosta D, et al. The contribution of chronic diseases to the prevalence of dependence among older people in Latin America, China and India: a 10/66 Dementia Research Group population-based survey. BMC Geriatr. 2010;10:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63-75.e2. [DOI] [PubMed] [Google Scholar]
- 4.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368(14):1326-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris G, Berk M. The putative use of lithium in Alzheimer’s disease. Curr Alzheimer Res. 2016;13(8):853-861. [DOI] [PubMed] [Google Scholar]
- 6.Manji HK, Moore GJ, Chen G. Clinical and preclinical evidence for the neurotrophic effects of mood stabilizers: implications for the pathophysiology and treatment of manic-depressive illness. Biol Psychiatry. 2000;48(8):740-754. [DOI] [PubMed] [Google Scholar]
- 7.Phiel CJ, Wilson CA, Lee VM, Klein PS. GSK-3α regulates production of Alzheimer’s disease amyloid-β peptides. Nature. 2003;423(6938):435-439. [DOI] [PubMed] [Google Scholar]
- 8.Caccamo A, Oddo S, Tran LX, LaFerla FM. Lithium reduces tau phosphorylation but not Aβ or working memory deficits in a transgenic model with both plaques and tangles. Am J Pathol. 2007;170(5):1669-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nocjar C, Hammonds MD, Shim SS. Chronic lithium treatment magnifies learning in rats. Neuroscience. 2007;150(4):774-788. [DOI] [PubMed] [Google Scholar]
- 10.Watase K, Gatchel JR, Sun Y, et al. Lithium therapy improves neurological function and hippocampal dendritic arborization in a spinocerebellar ataxia type 1 mouse model. PLoS Med. 2007;4(5):e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kessing LV, Søndergård L, Forman JL, Andersen PK. Lithium treatment and risk of dementia. Arch Gen Psychiatry. 2008;65(11):1331-1335. [DOI] [PubMed] [Google Scholar]
- 12.Kessing LV, Forman JL, Andersen PK. Does lithium protect against dementia? Bipolar Disord. 2010;12(1):87-94. [DOI] [PubMed] [Google Scholar]
- 13.Kessing LV, Gerds TA, Feldt-Rasmussen B, Andersen PK, Licht RW. Use of lithium and anticonvulsants and the rate of chronic kidney disease: a nationwide population-based study. JAMA Psychiatry. 2015;72(12):1182-1191. [DOI] [PubMed] [Google Scholar]
- 14.Forlenza OV, Diniz BS, Radanovic M, Santos FS, Talib LL, Gattaz WF. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry. 2011;198(5):351-356. [DOI] [PubMed] [Google Scholar]
- 15.Nunes MA, Viel TA, Buck HS. Microdose lithium treatment stabilized cognitive impairment in patients with Alzheimer’s disease. Curr Alzheimer Res. 2013;10(1):104-107. [DOI] [PubMed] [Google Scholar]
- 16.Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7)(suppl):22-25. [DOI] [PubMed] [Google Scholar]
- 17.Statistics Denmark [serial online]. 2016. http://www.dst.dk. Accessed July 11, 2017.
- 18.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7)(suppl):30-33. [DOI] [PubMed] [Google Scholar]
- 19.Munk-Jørgensen P, Mortensen PB. The Danish Psychiatric Central Register. Dan Med Bull. 1997;44(1):82-84. [PubMed] [Google Scholar]
- 20.Helweg-Larsen K. The Danish Register of Causes of Death. Scand J Public Health. 2011;39(7)(suppl):26-29. [DOI] [PubMed] [Google Scholar]
- 21.Andersen TF, Madsen M, Jørgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register: a valuable source of data for modern health sciences. Dan Med Bull. 1999;46(3):263-268. [PubMed] [Google Scholar]
- 22.World Health Organisation Klassifikation af sygdomme In: International Statistical Classification of Diseases and Related Health Problems. 10th rev. Munksgaard, Copenhagen: World Health Organisation ; 1993. [Google Scholar]
- 23.Juel K, Helweg-Larsen K. The Danish registers of causes of death. Dan Med Bull. 1999;46(4):354-357. [PubMed] [Google Scholar]
- 24.Phung TK, Andersen BB, Høgh P, Kessing LV, Mortensen PB, Waldemar G. Validity of dementia diagnoses in the Danish hospital registers. Dement Geriatr Cogn Disord. 2007;24(3):220-228. [DOI] [PubMed] [Google Scholar]
- 25.Voutchkova DD, Schullehner J, Knudsen N, et al. Exposure to selected geogenic trace elements (I, Li, and Sr) from drinking water in Denmark. Geosciences (Basel). 2015;5:45-66. [Google Scholar]
- 26.Knudsen NN, Schullehner J, Hansen B, et al. Lithium in drinking water and risk of suicide: a nation-wide individual-level cohort study with 22 years of follow-up. Int J Environ Res Public Health. 2017;14(6):627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voutchkova DD, Ernstsen V, Hansen B, Sørensen BL, Zhang C, Kristiansen SM. Assessment of spatial variation in drinking water iodine and its implications for dietary intake: a new conceptual model for Denmark. Sci Total Environ. 2014;493:432-444. [DOI] [PubMed] [Google Scholar]
- 28.Borgan L, Goldstein B, Langholz B. Methods for the analysis of sampled cohort data in the Cox proportional hazards model. Ann Stat. 1995;23(5):1749-1778. [Google Scholar]
- 29.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression and Survival Analysis. New York, NY: Springer; 2001. [Google Scholar]
- 30.van Os J, Pedersen CB, Mortensen PB. Confirmation of synergy between urbanicity and familial liability in the causation of psychosis. Am J Psychiatry. 2004;161(12):2312-2314. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen CB, Mortensen PB. Urbanicity during upbringing and bipolar affective disorders in Denmark. Bipolar Disord. 2006;8(3):242-247. [DOI] [PubMed] [Google Scholar]
- 32.Ohgami H, Terao T, Shiotsuki I, Ishii N, Iwata N. Lithium levels in drinking water and risk of suicide. Br J Psychiatry. 2009;194(5):464-465. [DOI] [PubMed] [Google Scholar]
- 33.Kapusta ND, Mossaheb N, Etzersdorfer E, et al. Lithium in drinking water and suicide mortality. Br J Psychiatry. 2011;198(5):346-350. [DOI] [PubMed] [Google Scholar]
- 34.Phung TK, Waltoft BL, Kessing LV, Mortensen PB, Waldemar G. Time trend in diagnosing dementia in secondary care. Dement Geriatr Cogn Disord. 2010;29(2):146-153. [DOI] [PubMed] [Google Scholar]
- 35.Fratiglioni L, Launer LJ, Andersen K, et al. ; Neurologic Diseases in the Elderly Research Group . Incidence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts. Neurology. 2000;54(11)(suppl 5):S10-S15. [PubMed] [Google Scholar]
- 36.Schrauzer GN. Lithium: occurrence, dietary intakes, nutritional essentiality. J Am Coll Nutr. 2002;21(1):14-21. [DOI] [PubMed] [Google Scholar]
- 37.Reimann C, Birke M. Geochemistry of European Bottled Water. Stuttgart, Germany: Borntraeger Science Publisher; 2010. [Google Scholar]
- 38.Banks D, Reimann CBM, Flem B, Filzmoser P, Frengstad B. Inorganic chemical quality of European tap-water, 1: distribution of parameters and regulatory compliance. Appl Geochem. 2015;59:200-210. [Google Scholar]
- 39.Rondeau V, Jacqmin-Gadda H, Commenges D, Helmer C, Dartigues JF. Aluminum and silica in drinking water and the risk of Alzheimer’s disease or cognitive decline: findings from 15-year follow-up of the PAQUID cohort. Am J Epidemiol. 2009;169(4):489-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Appelo CAJ, Postma D. Geochemistry, Groundwater and Pollution. 2nd ed Leiden, the Netherlands: AA Balkema Publishers; 2005. [Google Scholar]
- 41.Youdim MB, Stephenson G, Ben Shachar D. Ironing iron out in Parkinson’s disease and other neurodegenerative diseases with iron chelators: a lesson from 6-hydroxydopamine and iron chelators, desferal and VK-28. Ann N Y Acad Sci. 2004;1012:306-325. [DOI] [PubMed] [Google Scholar]
- 42.Macdonald A, Briggs K, Poppe M, Higgins A, Velayudhan L, Lovestone S. A feasibility and tolerability study of lithium in Alzheimer’s disease. Int J Geriatr Psychiatry. 2008;23(7):704-711. [DOI] [PubMed] [Google Scholar]
- 43.Hampel H, Ewers M, Bürger K, et al. Lithium trial in Alzheimer’s disease: a randomized, single-blind, placebo-controlled, multicenter 10-week study. J Clin Psychiatry. 2009;70(6):922-931. [PubMed] [Google Scholar]
- 44.De-Paula VJ, Kerr DS, Scola G, Gattaz WF, Forlenza OV. Lithium distinctly modulates the secretion of pro- and anti-inflammatory interleukins in co-cultures of neurons and glial cells at therapeutic and sub-therapeutic concentrations. Curr Alzheimer Res. 2016;13(8):848-852. [DOI] [PubMed] [Google Scholar]
- 45.De-Paula VdeJ, Kerr DS, de Carvalho MP, et al. Long-term lithium treatment increases cPLA2 and iPLA2 activity in cultured cortical and hippocampal neurons. Molecules. 2015;20(11):19878-19885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mury FB, da Silva WC, Barbosa NR, et al. Lithium activates brain phospholipase A2 and improves memory in rats: implications for Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci. 2016;266(7):607-618. [DOI] [PubMed] [Google Scholar]
- 47.Schrauzer GN, de Vroey E. Effects of nutritional lithium supplementation on mood: a placebo-controlled study with former drug users. Biol Trace Elem Res. 1994;40(1):89-101. [DOI] [PubMed] [Google Scholar]
- 48.Hajek T, Weiner MW. Neuroprotective effects of lithium in human brain? food for thought. Curr Alzheimer Res. 2016;13(8):862-872. [DOI] [PubMed] [Google Scholar]