Abstract
Whole brain irradiation (WBI) has become an indispensible tool in the treatment of head and neck cancer, and it has greatly improved patient survival rate and total survival time. In addition, prophylactic cranial irradiation (PCI) has dramatically decreased the incidence of brain metastatic carcinoma. However, WBI may induce temporary functional deficits or even progressive, irreversible cognitive dysfunction that compromises the quality of life for survivors. Unfortunately, the exact molecular mechanisms for cognitive damage remain elusive, and no treatment or preventative measures are available for use in the clinic. In the present study, the nuclear factor of activated T cells isoform 4 (NFAT3/c4) was found to play a vital role in excitotoxic hippocampus cell apoptosis induced by radiation. Sprague–Dawley (SD) rats received 20 Gy WBI, after which we detected NFAT3/c4-mediated excitotoxicity. We found that radiation caused hippocampus excitotoxicity, resulting from overactivation of the N-methyl-D-aspartate receptor (NMDAR) and always accompanied by subsequent elevation of the intracellular calcium level and activation of calcineurin (CaN). P-NFAT3/c4 was the principal downstream target of CaN, including regulation of its nuclear translocation as well as transcriptional activities. Radiation recruited NMDAR/NFAT3/c4 activation and subsequent Bax induction in hippocampus cells. Once treated with the NFAT3/c4 inhibitor 11R-VIVIT peptide pre-irradiation, hippocampal proliferation and neuron survival (dentate gyrus cells in particular) were protected from radiation-induced injury, resulting in inhibition of the apoptosis marker Bax. Our principal aim was to illuminate the role of NFAT3/c4-mediated excitotoxicity in hippocampal apoptosis during radiation-induced brain injury. This study is the first time that radiation-induced activation of NFAT3/c4 has been recorded, and our results suggest that NFAT3/c4 may be a novel target for prevention and treatment of radiation-induced brain injury.
Keywords: NMDAR, calcineurin, NFAT3/c4, apoptosis, Bax, radiation
INTRODUCTION
Radiation therapy (RT), especially whole brain irradiation (WBI), has become an essential treatment method for primary and metastatic brain tumors. With the rapid development of modern irradiation techniques, many patients receive WBI each year, and the survival of patients has increased. However, the normal brain tissue damage that may inevitably occur is often accompanied by neurological complications, such as temporary brain functional deficits, or even progressive, irreversible cognitive dysfunction [1]. These dysfunctions seriously affect the quality of life for survivors [2]. The exact mechanisms of radiation-induced cognitive decline have not been completely characterized. It was hypothesized that injury to hippocampal proliferation and neuronal survival may be central to the pathogenesis of radiation-induced cognitive decline [3, 4].
The N-methyl-D-aspartate receptor (NMDAR) is widespread in the CNS and is associated with multiple physiological and pathological processes [5–7], especially the occurrence of excitotoxic neuronal cell death and receptor overactivation [8, 9]. The main downstream events of NMDAR overactivation are calcium influx and calcineurin (CaN) activation [10, 11]. CaN subsequently mediates NFAT3/c4 nuclear translocation and its transcriptional activities.
The transcription factor NFAT3/c4 has been shown to be active in neurogenic brain regions such as the hippocampus [12]. Previous studies have shown that the NFATc4/3-mediated pathway plays an important role in long-term changes in neuronal function [13]. Cognitive decline in Alzheimer's disease and traumatic brain injury have been associated with selective changes in CaN/NFAT signaling [14, 15]. Upon stimulation of CaN, NFAT3/c4 is dephosphorylated and then translocated from the cytosol into the nucleus, whereas it is highly phosphorylated in the cytoplasm of resting cells. The re-phosphorylation and subsequent export of NFAT3/c4 from the nucleus is mediated by several kinases, such as GSK- 3β.
In this study, our data show that the NMDAR/CaN/NFAT3/c4 pathway initiates induction of the neuronal apoptosis marker Bax and hippocampal cell apoptosis. Such results are validated by dentate gyrus (DG) cell injuries, which improved after treatment with NFAT3/c4 inhibitor 11R-VIVIT peptide pre-irradiation.
MATERIALS AND METHODS
Animals
A total of 120 male Sprague–Dawley rats (21 days old) weighing ~50–60 g were purchased from the Experimental Animal Center of Soochow University (Suzhou, China). The experimental animals were raised in temperature- and humidity-controlled conditions with a 12 h/12 h light/dark cycle (lights on at 7 AM) at 22 ± 2°C and free access to food and water ad libitum prior to the experiment. All the rules and regulations involving the care and protection of animals complied with the Soochow University Animal Care and Ethics Guidelines, which agree with national laboratory animal care standards.
The animals were divided into four groups: (i) the sham group (Sham), (ii) the sham with 11R-VIVIT peptide injection group (Sham +11R-VIVIT peptide), (iii) the irradiation group (IR), and (iv) the irradiation and 11R-VIVIT peptide injection group (IR +11R-VIVIT peptide) (n = 30 per group).
Irradiation
The rats were anesthetized with 3.6% chloral hydrate (360 mg/kg) via intraperitoneal injection. WBI treatments were administered with a single dose of 0 (control) or 20 Gy using a 4 MeV electron beam and a linear accelerator (Philips SL-18, UK) at room temperature.
Brain and body weight reduction
Six rats were randomly selected at 2 months post irradiation to be weighed and sacrificed. Their brains were collected and weighed. Brain and body weight reductions were calculated.
Drug treatment
The 11R-VIVIT peptide (RRRRR-GGG-MAGPHPVIVITGPHEE) was purchased from Sigma Genosys (Woodlands, TX). The dosage of the 11R-VIVIT peptide used for each rat was 100 μg/kg. Both the second group and the fourth group were injected intraperitoneally with 11R-VIVIT peptide for 3 days before radiation. Then, the first group of animals was sacrificed and hippocampus tissues were collected for western blot assays. The second group of animals were treated with 11R-VIVIT peptide for 3 days post irradiation and then sacrificed for immunofluorescence staining of hippocampus-proliferating cells. The third group of animals were treated with 11R-VIVIT peptide for 7 days post irradiation and were sacrificed for immunofluorescence staining of mature hippocampal neurons eight weeks after irradiation (n = 3 per group per time point).
5-Bromodeoxyuridine (BrdU) labeling and immunofluorescence staining
BrdU (Sigma, St Louis, MO, USA) at 50 mg/kg/day was administered intraperitoneally (i.p.) for 7 consecutive days (twice a day) to animals 24 h before they were sacrificed at 3 days post irradiation. The rats were perfused with cold PBS and 4% paraformaldehyde, followed by incubation in the paraformaldehyde for 24 h at 4°C. The tissue was dehydrated in 15% and 30% sucrose for 48 h. Frozen serial sections of the brains were cut (30 μm thick) through the entire hippocampus using a microtome.
The tissue slices were treated with 2 M HCl at 45°C for 30 min to denature the DNA, and then the reaction was neutralized in 0.1 M borate buffer (pH 8.5) for 10 min. The sections were incubated in buffer (10% bovine serum albumin and 0.5% Triton X-100 in PBS) to block non-specific binding for 2 h at room temperature. The slices were incubated overnight at 4°C with a primary antibody. The tissues were washed in PBS and incubated with a secondary antibody for 1.5 h, and we used an Olympus microscope equipped with a digital camera (Olympus) to analyze the sections. The total number of BrdU+ or NeuN+ cells was counted on every ninth section throughout the hippocampus (seven sections per animal). The number of positives per DG of the hippocampus was obtained by multiplying the value by 9. In addition, we had three people perform the counts independently and calculated the mean value as the ultimate result. The antibodies were purchased and diluted as follows: rat anti-body BrdU (1:500, Biolegend), NeuN (1:500, Abcam), Alexa Fluor 488 donkey anti-goat, Alexa Fluor 555 goat anti-rat, and Alexa Fluor 405 mouse anti-rabbit (1:500, Jackson ImmunoResearch, USA).
Western blot
Four groups of rat hippocampus tissues were used to extract the total protein, and NFAT3/c4 protein was extracted using a Nuclear and Cytoplasmic Extraction Kit (Cwbio, China). All the experiments were conducted on ice. Protein concentration measurements were collected using the BCA (Biomiga, China) method. The samples were incubated at 99°C for 10 min. Then, 30 μg of total protein were was separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were transferred to PVDF membranes (Millipore, Billerica, MA, USA). The membranes were blocked with 5% non-fat milk in TBST for 2 h at room temperature and were incubated overnight at 4°C with the proper primary antibodies. The antibodies were purchased and diluted as follows: rabbit anti-calcineurin (1:10 000, Abcam), rabbit anti-NMDAR (1:1000, Abcam), rabbit anti-NFAT3/c4 (1:500, Sigma), rabbit anti-GSK-3β (1:1000, Cell Signal), rabbit anti-Bax (1:1000, Cell Signal), rabbit anti- Bcl-2 (1:1000, Cell Signal), rabbit anti-GAPDH (1:1000, Goodhere), rabbit anti-Histon3 (1:500, Sigma). The membranes were then incubated with an HRP-conjugated secondary antibody (Beyotime, Nantong, China) for 1.5 h at room temperature. The membranes were immunolabeled. After washing the samples with TBST buffer, the membranes were subsequently assayed using the Thermo Scientific Pierce Femto ECL (Rockford, IL, USA).
Statistical analysis
Data were expressed as the mean ± SD and analyzed with a two-tiered Student's t test or one-way ANOVA in GraphPad Prism (version 5.0; GraphPad, La Jolla, USA). A value of P < 0.05 was considered to be statistically significant.
RESULTS
Body weight and brain weight measurements
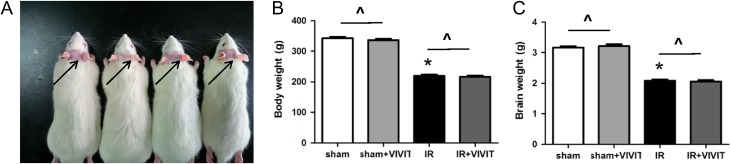
Obvious depilation was observed in the irradiated rats at 2–3 weeks post irradiation, which gradually disappeared and was absent 2 months later (Fig. 1A). Compared with the average body weight of the sham group, that of the irradiation group declined (P < 0.05, Fig. 1B), and the same trend was observed for average brain weight (P < 0.05, Fig. 1C). The 11R-VIVIT peptide had no impact on either body weight or brain weight (P > 0.05, Fig. 1B–C).
Fig. 1.
The observation of the rats’ skin reaction and the measurement of body weight and brain weight after WBI (20 Gy). We observed the representative infield depilation at 2–3 weeks post irradiation (A). Ironizing radiation induced a significant decrease in the body weight of rats, and 11R-VIVIT had no remarkable effect (B). Similarly, we observed a conspicuous decrease in the brain weight of irradiated rats, and 11R-VIVIT caused no remarkable changes with respect to this (C). Data are expressed as mean ± SD, *P < 0.05 vs sham group, #P < 0.05 vs irradiation group.
Cognitive deficits were produced after whole-brain irradiation
Our previous study demonstrated that 20 Gy of WBI resulted in cognitive damage in the surviving rats at 2 months post irradiation [16].
Whole-brain radiation activated the NMDAR/NFAT3/c4/Bax pathway and induced apoptosis
WBI significantly elevated NMDAR subunit expression (NR2A, not NR1 or NR2B) in the hippocampus at 6 h post irradiation compared with that of the sham group (P < 0.05, Fig. 2A).
Fig. 2.
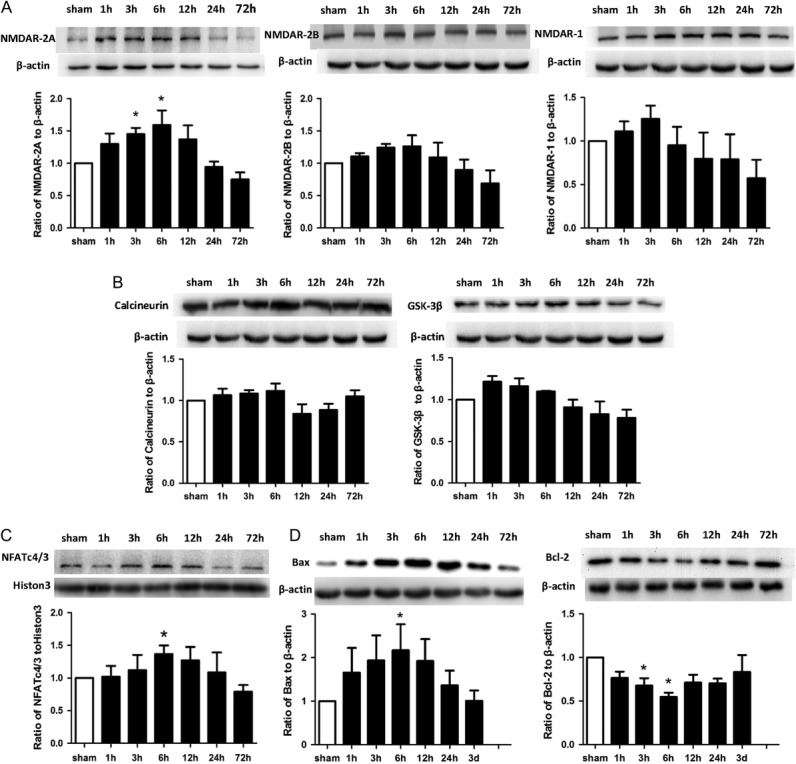
WBI activated the NMDAR/NFAT3/c4/Bax pathway and induced apoptosis. WBI significantly elevated the expression of: NMDAR subunits (NR2A, not NR1 or NR2B) (A), NFAT3/c4 in the nucleus (C), the apoptosis marker Bax (D) compared with in the sham group. In contrast, the expression of CaN and GSK-3β showed only mild enhancement compared with in the sham group (B). Data are expressed as mean ± SD, *P < 0.05 vs sham group, #P < 0.05 vs irradiation group.
The expression level of CaN and GSK-3β experienced only mild enhancement (P > 0.05, Fig. 2D), but these molecules were significantly activated, as demonstrated by the translocation of NFAT3/c4 between the nuclei and cytoplasm. With radiation stress, NFAT3/c4 rapidly accumulated in the nuclei after dephosphorylation of p-NFAT3/c4 (P < 0.05, Fig. 2B). The transcriptional activities of NFAT3/c4 promoted expression of the apoptosis marker Bax (P < 0.05, Fig. 2C).
The 11R-VIVIT peptide rescued the activation of NFAT3/c4-dependent apoptosis after radiation
Treatment with the 11R-VIVIT peptide did not have any impact on the expression of the NMDAR subunits (NR1, NR2A or NR2B) (P > 0.05, Fig. 3A). However, the 11R-VIVIT peptide prevented nuclear translocation of NFAT3/c4 and abolished its nuclear accumulation (P < 0.05, Fig. 3B). Subsequently, the expression of the apoptosis marker Bax was inhibited. (P < 0.05, Fig. 3C).
Fig. 3.
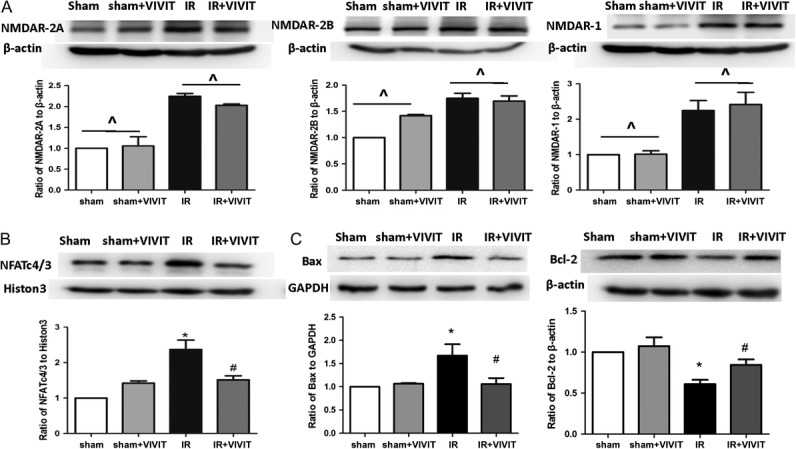
11R-VIVIT peptide relieved apoptosis induced by the activation of NFAT3/c4 after irradiation. Treatment with 11R-VIVIT peptide didn't have any impact on the expression of NMDAR subunits (NR1, NR2A or NR2B) (A). 11R-VIVIT peptide prevented nuclear translocation of NFAT3/c4 and appearance of Bax (B–C). Data are expressed as mean ± SD, *P < 0.05 vs sham group, #P < 0.05 vs irradiation group, ^P > 0.05.
The 11R-VIVIT peptide alleviated the decline in the DG proliferating cells and mature neurons
The protective effects of the 11R-VIVIT peptide on radiation-induced brain injury were assessed by analysis of BrdU proliferation cell assay and NeuN immunofluorescence staining. Our data showed that the IR+11R-VIVIT peptide promoted an increase in BrdU+ cells in the DG at 3 days post irradiation compared with the levels in the irradiated group (P < 0.05, Fig. 4A). Similarly, we found that the total number of NeuN+ mature neurons in the IR+ 11R-VIVIT peptide group increased compared with the number in the IR group at 8 weeks post irradiation (P < 0.05, Fig. 4B).
Fig. 4.
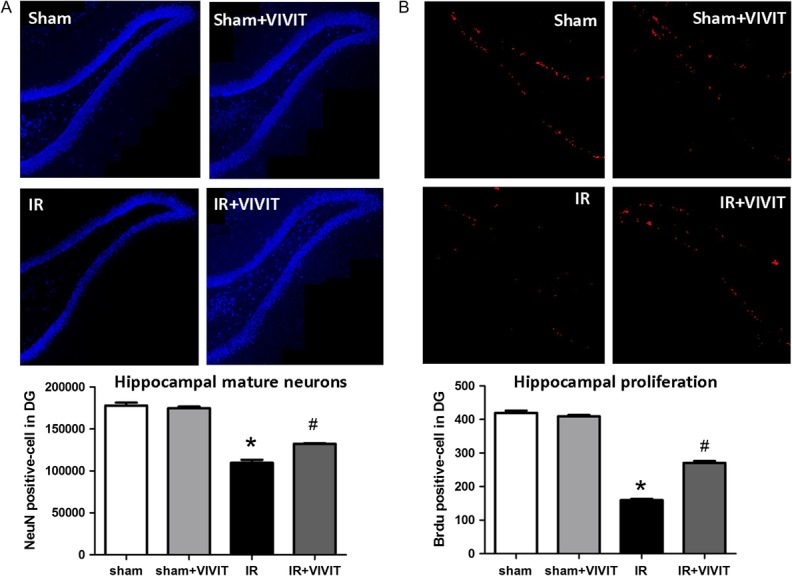
11R-VIVIT peptide alleviated the decline in the DG proliferating cells and mature neurons at 3 days and 2 months after WBI (20 Gy). Representative images indicated that 11R-VIVIT peptide significantly increased the total number of DG proliferating cells (BrdU+, red)at 3 days post irradiation (A). Similarly, 11R-VIVIT peptide increased the number of DG mature neurons (NeuN+, blue) at 2 months post irradiation (B). The original objective magnification was ×200. Data are expressed as mean ± SD, *P < 0.05 vs sham group, #P < 0.05 vs irradiation group.
DISCUSSION
In the present study, we investigated hippocampus cell apoptosis in a model of radiation-induced brain injury that was characterized by cognitive dysfunction. We also evaluated the potential mechanisms of NFAT3/c4-mediated excitotoxicity. A single-dose exposure of 20 Gy WBI was chosen to establish the model of radiation-induced brain injury in rats. We showed that: (i) WBI significantly induced hippocampus cell apoptosis and reduction of DG proliferating cells as well as mature neurons; (ii) WBI activated the NMDAR/NFAT3/c4/Bax pathway; (iii) 11R-VIVIT peptide, an NFAT3/c4 inhibitor, abolished the nuclear translocation of NFAT3/c4 and the induction of Bax; and (iv) the 11R-VIVIT peptide reversed the decline in the number of DG proliferating cells and mature neurons by protecting hippocampal proliferation and neuron survival.
An animal model for radiation-induced brain injury has not yet been clearly defined. Wong-Goodrich et al. employed a single dose of 5 Gy to produce cognitive dysfunction in C57BL/J6 male mice [17], while Conner et al. selected a total dose of 40 Gy/8 fractions to establish a model of radiation-induced brain injury with Fischer 344 rats [18]. A large single-dose exposure was more damaging than fractionated irradiation because the former lead to strong reaction in the tissue. Our preliminary work indicated that it was appropriate to apply a single-dose exposure of 20 Gy to establish the model [16], which is what we employed in this study to develop our animal model. Adolescent rats (21 days old) were selected in our study, mainly because adolescent rats are more sensitive to ionizing radiation and suffer greater damage than adult rats, so it is easier to establish a model of radiation-induced brain injury. We selected time-points of 3 days and 2 months after exposure to detect the reduction of DG proliferating cells and mature neurons, respectively. Both time-points demonstrated radiation-induced brain injury.
Previous studies have suggested that apoptosis of hippocampus cells, especially DG proliferating cells and immature neurons, plays a key role in radiation-induced cognitive damage [19, 20]. DG proliferating cells were capable of generating new neurons, astrocytes and oligodendrocytes, which are associated with brain self-recovery function [21–23]. A radiation-induced decrease in proliferating cells was accompanied by a reduction in new neurons [24, 25]. The significant decrease in mature neurons induced by radiation has been associated with hippocampal-dependent spatial learning and memory dysfunction [26, 27]. Single whole-brain irradiation (2–10 Gy) caused a sharp elevation in apoptosis in the hippocampal DG [28, 29]. Apoptosis was accompanied by a subsequent decrease in both proliferating cells and neurons [27, 30, 31]. In the present study, we found a significantly elevated expression of the apoptosis marker Bax accompanied by a steep decrease in hippocampus cells. In addition, the 11R-VIVIT peptide, an NFAT3/c4 inhibitor, inhibited the appearance of apoptosis and reversed the decline in the number of DG proliferating cells and mature neurons.
DNA damage was the most common effect of radiation injury, and DNA transcription and protein synthesis were simultaneously disrupted. Numerous potential mechanisms of radiation-induced apoptosis were present [32–34]. The radiation-induced perturbations of extracellular-signal-regulated kinase (ERK1/ERK2) and downstream signaling pathways severely affected neuronal survival [35]. The secretion of TRAIL by irradiated neural stem cells (NSCs) induced a death-signaling cascade in non-targeted NSCs [32]. Regarding these potential mechanisms, a recent NMDAR-mediated non-conventional apoptotic response to radiation has been reported, so we investigated the excitotoxic effects of overactivated NMDAR [36]. We observed that overexpression of NMDAR caused nuclear translocation and activation of the transcriptional factor NFAT3/c4, which regulates the expression of numerous proteins. Its transcriptional activities promoted the elevation of Bax. Together, our results suggested a hitherto unknown vital role of NFAT3/c4 in the study of radiation-induced brain injury.
Some limitations of this study need mentioning. First, radiation-induced apoptosis requires more extensive study to clarify the mechanisms underlying it and how it develops, including TUNEL assays and Bax immunofluorescence assays. Second, we would now like to use an NFAT3/c4 knock-out rat model to further demonstrate the important role of NFAT3/c4 in radiation-induced brain injury. Third, NFAT3/c4 acts as a powerful transcriptional factor, and the rest of its apoptotic targets need further investigation. Finally, we need to explore further the pathological changes in brain morphology and structure post irradiation with H&E staining. Exploration of the changes in synapse morphology and structure with Golgi–Cox staining is our next goal.
In summary, our present study showed for the first time that NFAT3/c4 mediated excitotoxicity in apoptosis of hippocampus cells in radiation-induced brain injury. Moreover, the results from the inhibitor 11R-VIVIT peptide supported a regulatory role for NFAT3/c4. In addition, we clarified the potential mechanisms of an NMDAR-mediated non-conventional apoptotic response to radiation. Finally, our results highlighted the importance of the NMDAR/NFAT3/c4/Bax pathway and provided evidence of its use in a potential therapeutic intervention.
FUNDING
This study was supported by the National Natural Scientific Foundation of China grants 81172128 (Dr Ye Tian), 81372411 (Dr Ye Tian), 81402517 (Dr Sheng-jun Ji) and 81472804 (Dr Liyuan Zhang); Jiangsu Province's Key Medical Department in 2011 (Dr Ye Tian); the Suzhou Science and Technology Development Program (Dr Ye Tian); the Jiangsu Provincial Special Program of Clinical Medical Science (BL2014040); the Suzhou Medical Center of Radiotherapy and Oncology (Szzxj201503); Outstanding Medical Leaders of Suzhou (Dr Ye Tian); the Practice Innovation Program for College Graduates of Jiangsu Province (Grant No. SJZZ15_0153); the Suzhou Science and Technology Project (No. SYS201651) and Jiangsu Provincial Medical Youth Talent, QNRC2016234.
REFERENCES
- 1. Verdecchia A, Baili P, Quaglia A, et al. Patient survival for all cancers combined as indicator of cancer control in Europe. Eur J Public Health 2008;18:527–32. [DOI] [PubMed] [Google Scholar]
- 2. Soffietti R, Kocher M, Abacioglu UM, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol 2013;31:65–72. [DOI] [PubMed] [Google Scholar]
- 3. Rola R, Raber J, Rizk A, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol 2004;188:316–30. [DOI] [PubMed] [Google Scholar]
- 4. Monje ML, Vogel H, Masek M, et al. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol 2007;62:515–20. [DOI] [PubMed] [Google Scholar]
- 5. Dobrek L, Thor P. Glutamate NMDA receptors in pathophysiology and pharmacotherapy of selected nervous system diseases. Postepy Hig Med Dosw 2011;65:338–46. [DOI] [PubMed] [Google Scholar]
- 6. Mony L, Kew JN, Gunthorpe MJ, et al. Allosteric modulators of NR2B-containing NMDA receptors: molecular mechanisms and therapeutic potential. Br J Pharmacol 2009;157:1301–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Suzuki Y, Goetze TA, Stroebel D, et al. Visualization of structural changes accompanying activation of N-methyl-D-aspartate (NMDA) receptors using fast-scan atomic force microscopy imaging. J Biol Chem 2013;288:778–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hynd MR, Scott HL, Dodd PR. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer's disease. Neurochem Int 2004;45:583–95. [DOI] [PubMed] [Google Scholar]
- 9. Blandini F. An update on the potential role of excitotoxicity in the pathogenesis of Parkinson's disease. Funct Neurol 2010;25:65–71. [PubMed] [Google Scholar]
- 10. Brorson JR, Marcuccilli CJ, Miller RJ. Delayed antagonism of calpain reduces excitotoxicity in cultured neurons. Stroke 1995;26:1259–66; discussion 1267. [DOI] [PubMed] [Google Scholar]
- 11. Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med 1994;330:613–22. [DOI] [PubMed] [Google Scholar]
- 12. Serrano-Perez MC, Fernandez M, Neria F, et al. NFAT transcription factors regulate survival, proliferation, migration, and differentiation of neural precursor cells. Glia 2015;63:987–1004. [DOI] [PubMed] [Google Scholar]
- 13. Benedito AB, Lehtinen M, Massol R, et al. The transcription factor NFAT3 mediates neuronal survival. J Biol Chem 2005;280:2818–25. [DOI] [PubMed] [Google Scholar]
- 14. Yan HQ, Shin SS, Ma X, et al. Differential effect of traumatic brain injury on the nuclear factor of activated T Cells C3 and C4 isoforms in the rat hippocampus. Brain Res 2014;1548:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abdul HM, Sama MA, Furman JL, et al. Cognitive decline in Alzheimer's disease is associated with selective changes in calcineurin/NFAT signaling. J Neurosci 2009;29:12957–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ji JF, Ji SJ, Sun R, et al. Forced running exercise attenuates hippocampal neurogenesis impairment and the neurocognitive deficits induced by whole-brain irradiation via the BDNF-mediated pathway. Biochem Biophys Res Commun 2014;443:646–51. [DOI] [PubMed] [Google Scholar]
- 17. Wong-Goodrich SJ, Pfau ML, Flores CT, et al. Voluntary running prevents progressive memory decline and increases adult hippocampal neurogenesis and growth factor expression after whole-brain irradiation. Cancer Res 2010;70:9329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Conner KR, Payne VS, Forbes ME, et al. Effects of the AT1 receptor antagonist L-158,809 on microglia and neurogenesis after fractionated whole-brain irradiation. Radiat Res 2010;173:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huo K, Sun Y, Li H, et al. Lithium reduced neural progenitor apoptosis in the hippocampus and ameliorated functional deficits after irradiation to the immature mouse brain. Mol Cell Neurosci 2012;51:32–42. [DOI] [PubMed] [Google Scholar]
- 20. Yang M, Kim JS, Song MS, et al. Dose–response and relative biological effectiveness of fast neutrons: induction of apoptosis and inhibition of neurogenesis in the hippocampus of adult mice. Int J Radiat Biol 2010;86:476–85. [DOI] [PubMed] [Google Scholar]
- 21. Boekhoorn K, van Dis V, Goedknegt E, et al. The microtubule destabilizing protein stathmin controls the transition from dividing neuronal precursors to postmitotic neurons during adult hippocampal neurogenesis. Dev Neurobiol 2014;74:1226–42. [DOI] [PubMed] [Google Scholar]
- 22. Sullivan JM, Sandeman DC, Benton JL, et al. Adult neurogenesis and cell cycle regulation in the crustacean olfactory pathway: from glial precursors to differentiated neurons. J Mol Histol 2007;38:527–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sohur US, Emsley JG, Mitchell BD, et al. Adult neurogenesis and cellular brain repair with neural progenitors, precursors and stem cells. Philos Trans R Soc Lond B Biol Sci 2006;361:1477–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shinohara C, Gobbel GT, Lamborn KR, et al. Apoptosis in the subependyma of young adult rats after single and fractionated doses of X-rays. Cancer Res 1997;57:2694–702. [PubMed] [Google Scholar]
- 25. Amano T, Inamura T, Wu CM, et al. Effects of single low dose irradiation on subventricular zone cells in juvenile rat brain. Neurol Res 2002;24:809–16. [DOI] [PubMed] [Google Scholar]
- 26. Monje ML, Mizumatsu S, Fike JR, et al. Irradiation induces neural precursor-cell dysfunction. Nat Med 2002;8:955–62. [DOI] [PubMed] [Google Scholar]
- 27. Raber J, Rola R, LeFevour A, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res 2004;162:39–47. [DOI] [PubMed] [Google Scholar]
- 28. Peissner W, Kocher M, Treuer H, et al. Ionizing radiation–induced apoptosis of proliferating stem cells in the dentate gyrus of the adult rat hippocampus. Brain Res Mol Brain Res 1999;71:61–8. [DOI] [PubMed] [Google Scholar]
- 29. Tada E, Parent JM, Lowenstein DH, et al. X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neuroscience 2000;99:33–41. [DOI] [PubMed] [Google Scholar]
- 30. Ferrer I, Macaya A, Blanco R, et al. Evidence of internucleosomal DNA fragmentation and identification of dying cells in X-ray–induced cell death in the developing brain. Int J Dev Neurosci 1995;13:21–8. [DOI] [PubMed] [Google Scholar]
- 31. Winocur G, Wojtowicz JM, Sekeres M, et al. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus 2006;16:296–304. [DOI] [PubMed] [Google Scholar]
- 32. Ivanov VN, Hei TK. A role for TRAIL/TRAIL-R2 in radiation-induced apoptosis and radiation-induced bystander response of human neural stem cells. Apoptosis 2014;19:399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kumar A, Ghosh S, Chandna S. Evidence for microRNA-31 dependent Bim–Bax interaction preceding mitochondrial Bax translocation during radiation-induced apoptosis. Sci Rep 2015;5:15923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ivanov VN, Hei TK. Radiation-induced glioblastoma signaling cascade regulates viability, apoptosis and differentiation of neural stem cells (NSC). Apoptosis 2014;19:1736–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Silasi G, Diaz-Heijtz R, Besplug J, et al. Selective brain responses to acute and chronic low-dose X-ray irradiation in males and females. Biochem Biophys Res Commun 2004;325:1223–35. [DOI] [PubMed] [Google Scholar]
- 36. Samari N, De Saint-Georges L, Pani G, et al. Non-conventional apoptotic response to ionising radiation mediated by N-methyl D-aspartate receptors in immature neuronal cells. Int J Mol Med 2013;31:516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]