This pooled-analysis investigates the long-term safety and efficacy of new-generation drug-eluting stents vs early-generation drug-eluting stents in women with acute myocardial infarction.
Key Points
Question
What is the long-term safety and efficacy of new-generation drug-eluting stents (DES) vs early-generation DES in women presenting with acute myocardial infarction (MI)?
Findings
In this international, collaborative, patient-level pooled analysis of women in 26 randomized clinical trials of DES, new-generation DES were associated with a 3-year lower risk of death, MI or target lesion revascularization, and definite or probable stent thrombosis in women with acute MI. The absolute magnitude of these benefits appears to be greater per increase in severity across the spectrum of acute coronary syndromes.
Meaning
New-generation DES are associated with consistent and durable benefits over 3 years in women presenting with acute MI.
Abstract
Importance
Women with acute myocardial infarction (MI) undergoing mechanical reperfusion remain at increased risk of adverse cardiac events and mortality compared with their male counterparts. Whether the benefits of new-generation drug-eluting stents (DES) are preserved in women with acute MI remains unclear.
Objective
To investigate the long-term safety and efficacy of new-generation DES vs early-generation DES in women with acute MI.
Design, Setting, and Participants
Collaborative, international, individual patient-level data of women enrolled in 26 randomized clinical trials of DES were analyzed between July and December 2016. Only women presenting with an acute coronary syndrome were included. Study population was categorized according to presentation with unstable angina (UA) vs acute MI. Acute MI included non–ST-segment elevation MI (NSTEMI) or ST-segment elevation MI (STEMI).
Interventions
Randomization to early- (sirolimus- or paclitaxel-eluting stents) vs new-generation (everolimus-, zotarolimus-, or biolimus-eluting stents) DES.
Main Outcomes and Measures
Composite of death, MI or target lesion revascularization, and definite or probable stent thrombosis at 3-year follow-up.
Results
Overall, the mean age of participants was 66.8 years. Of 11 577 women included in the pooled data set, 4373 (37.8%) had an acute coronary syndrome as clinical presentation. Of these 4373 women, 2176 (49.8%) presented with an acute MI. In women with acute MI, new-generation DES were associated with lower risk of death, MI or target lesion revascularization (14.9% vs 18.4%; absolute risk difference, −3.5%; number needed to treat [NNT], 29; adjusted hazard ratio, 0.78; 95% CI, 0.61-0.99), and definite or probable stent thrombosis (1.4% vs 4.0%; absolute risk difference, −2.6%; NNT, 46; adjusted hazard ratio, 0.36; 95% CI, 0.19-0.69) without evidence of interaction for both end points compared with women without acute MI (P for interaction = .59 and P for interaction = .31, respectively). A graded absolute benefit with use of new-generation DES was observed in the transition from UA, to NSTEMI, and to STEMI (for death, MI, or target lesion revascularization: UA, −0.5% [NNT, 222]; NSTEMI, −3.1% [NNT, 33]; STEMI, −4.0% [NNT, 25] and for definite or probable ST: UA, −0.4% [NNT, 278]; NSTEMI, −2.2% [NNT, 46]; STEMI, −4.0% [NNT, 25]).
Conclusions and Relevance
New-generation DES are associated with consistent and durable benefits over 3 years in women presenting with acute MI. The magnitude of these benefits appeared to be greater per increase in severity of acute coronary syndrome.
Introduction
Women undergoing percutaneous coronary intervention (PCI) for acute myocardial infarction (MI) experience disproportionately higher rates of major adverse cardiovascular events (MACEs) and mortality compared with men. This observation may be partially explained by anatomical factors, as well as patient characteristics at the time of presentation. Women presenting with acute MI tend to be older and have greater prevalence of comorbidities than men. Additionally, women have often smaller and more tortuous coronary arteries and smaller mean luminal areas in the target lesions.
The introduction of drug-eluting stents (DES) in clinical practice has led to improved efficacy of PCI, primarily by reducing the risk of in-stent restenosis associated with bare metal stents. However, initial safety concerns arose with early-generation DES because of increased risk of platform thrombosis, especially among patients with acute coronary syndromes (ACS). New-generation DES have been demonstrated to overcome the safety issues observed with earlier-generation devices. In addition, among patients with ST-segment elevation MI (STEMI), new-generation DES have been demonstrated to be superior on both efficacy and safety compared with bare metal stents. Women have been historically underrepresented in randomized clinical trials (RCTs) investigating the safety and efficacy of cardiovascular devices. In addition, women are less likely to present with non-STEMI (NSTEMI) or STEMI compared with men. Therefore, the safety and efficacy of new-generation DES in women with acute MI remain unclear. In 2011, the US Food and Drug Administration identified sex-specific disparities in RCTs investigating medical devices. In response to these observations, under the auspices of the Society for Cardiovascular Angiography and Interventions’ Women in Innovation Initiative, the current collaborative women-specific patient-level pooled data set of RCTs of DES was created. In the present study, we sought to investigate the efficacy and safety of new-generation DES across the spectrum of ACS.
Methods
Study Population and Design
The rationale of the present patient-level pooled database list of trials, analytic strategy, and prespecified end points has been previously reported. Briefly, in response to the US Food and Drug Administration guidance document for the assessment of sex differences in clinical studies of medical devices, the Women in Innovation Initiative convened the Gender Data Forum to discuss the outcomes of DES in women. This forum led to the investigation of the efficacy and safety of DES in women by pooling individual patient–level data of female participants from all available RCTs of DES. The full list of the included RCTs (N = 26), study characteristics, and end point definitions are reported in eTable 1 and eTable 2 in the Supplement. All studies included in our analysis complied with the provisions of the Declaration of Helsinki. The institutional review board at each center approved the study protocols, and written informed consent was obtained for all participants included in each trial.
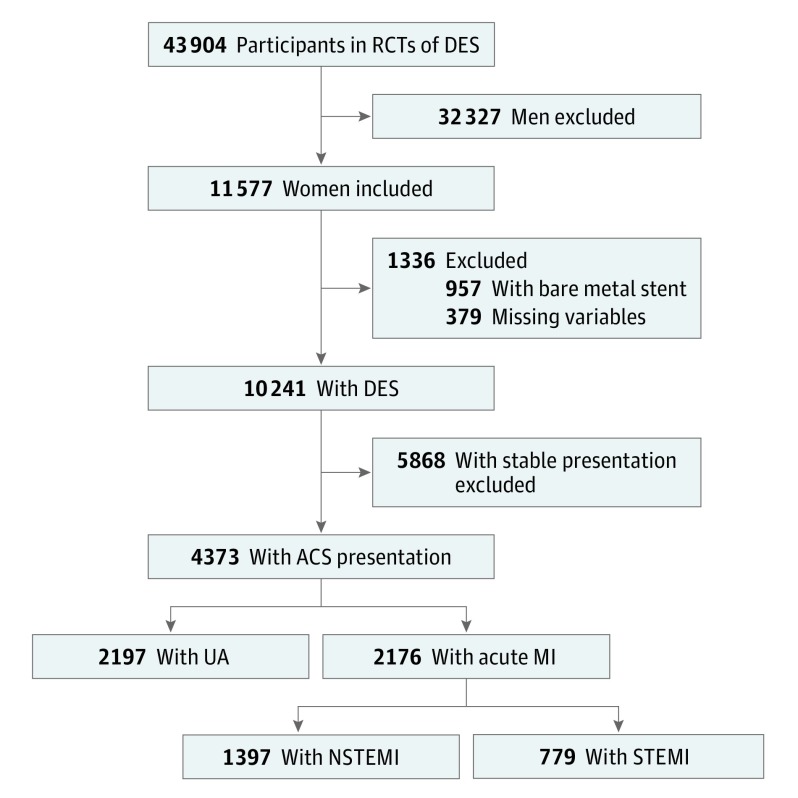
For the present analysis, the following exclusion criteria have been applied in the overall pooled data set (Figure 1): (1) women randomized to bare metal stent treatment; (2) women who presented with stable coronary artery disease; and (3) women with missing information regarding clinical presentation. Primary outcomes were first compared in the ACS population between women presenting with vs without acute MI, which included NSTEMI and STEMI. Then, outcomes were explored across the spectrum of ACS in women presenting with unstable angina (UA), NSTEMI, or STEMI.
Figure 1. Study Population Flow Diagram.
ACS indicates acute coronary syndrome; DES, drug-eluting stent; MI, myocardial infarction; NSTEMI, non–ST-segment elevation myocardial infarction; RCTs, randomized clinical trials; STEMI, ST-segment-elevation myocardial infarction; and UA, unstable angina.
Study Objectives and End Point Definitions
The objective of the present study was to evaluate the safety and efficacy of new- vs early-generation DES in women undergoing PCI. The primary end point was MACEs at 3-year follow-up. A MACE was defined as the composite of all-cause mortality, MI, and target lesion revascularization (TLR). Secondary end points included the composite of death, MI and definite or probable stent thrombosis, stent thrombosis, and TLR.
Drug-Eluting Stents
The DES used in the included RCTs were sirolimus-eluting stents, paclitaxel-eluting stents, everolimus-eluting stents, zotarolimus-eluting stents, biolimus (umirolimus)-eluting stents with biodegradable polymer coating, and sirolimus-eluting stents with biodegradable polymer coating. The DES used among trials were classified as early-generation DES (including sirolimus- and paclitaxel-eluting stents) and new-generation DES (including everolimus- and zotarolimus-eluting stents with durable polymer and biolimus- and sirolimus-eluting stents with biodegradable polymer).
Statistical Analysis
Patient-level data were aggregated and combined as 1 structured data set on a prespecified extraction sheet and analyzed with a 1-stage approach. Baseline clinical, demographic, and procedural characteristics of the study groups were reported as mean (SD) for continuous variables and as proportions for categorical variables. Continuous variables were compared with the t test. Categorical variables were compared with the χ2 test. Cumulative event rates in the study groups were calculated with the Kaplan-Meier method and compared with the log-rank test. For these analyses, the total follow-up was defined as the time from index procedure until death, last follow-up date, or 3 years, whichever came first. The association between stent generation and outcomes was assessed with the Cox proportional hazards models that included a frailty term (γ) to assess random effects across trials and account for intertrial heterogeneity. Frailties are the unmeasured factors that affect trial-specific baseline risk and are distributed as γ random variables with a mean of 1 and variance θ. The variance parameter was interpreted as a metric of heterogeneity in baseline risk between trials. The Cox proportional hazards model was further adjusted by including clinically relevant covariates (including age, body mass index, diabetes, hypertension, smoking, previous MI, previous PCI, and previous coronary artery bypass graft). The proportionality assumption was verified by means of scaled Schoenfeld residual. Multicollinearity was evaluated by means of visual inspection of the correlation matrix and by estimation of the variance inflation factor (with >10 used as a threshold to define significant multicollinearity). In the adjusted analysis evaluating the effect of stent generation on outcomes, early-generation DES was the reference category. We judged P values less than .05 to be significant, and all analyses were done with SAS, version 9.4 (SAS Institute Inc) and STATA, version 14.0 (StataCorp).
Results
Baseline Characteristics and Outcomes According to Clinical Presentation
Overall, the mean age of participants was 66.8 years. Of 11 577 women included in the pooled database, 4373 (37.8%) presented with ACS (Figure 1). Of these, 2176 (49.8%) presented with acute MI and 2197 (50.2%) with UA. Among women presenting with acute MI, 1397 (64.2%) presented with NSTEMI, and 779 (35.8%) presented with STEMI. Baseline clinical characteristics according to clinical presentation are presented in eTable 3 in the Supplement. Compared with women presenting with UA, those with NSTEMI or STEMI were less likely to have diabetes, hypertension, or hypercholesterolemia and to have history of MI or previous coronary revascularization. Angiographic and procedural data are reported in eTable 3 in the Supplement. No differences were noted in angiographic multivessel disease across groups. Women presenting with NSTEMI had greater prevalence of moderate or severe calcifications, more lesions treated, and more DES implanted. Three years’ outcomes according to clinical presentation are illustrated in the eFigure in the Supplement. Women presenting with NSTEMI or STEMI were at greater risk of death, MI or stent thrombosis, and definite or probable stent thrombosis. A trend toward greater rates of TLR was instead observed in women presenting with UA.
Effect of Stent Generation on 3-Year Clinical Outcomes
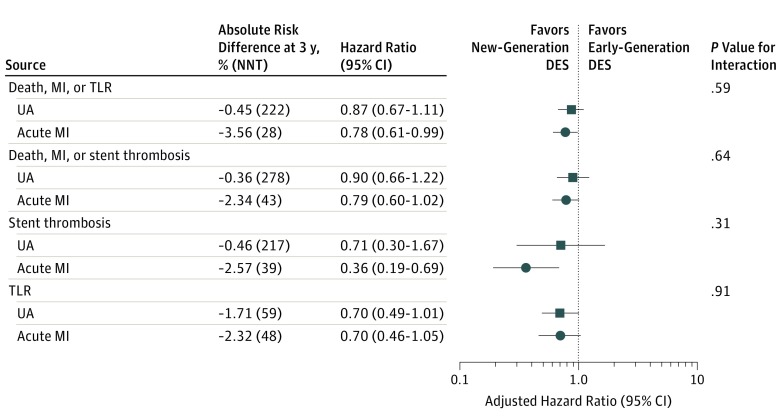
Outcomes for new- vs early-generation DES in women with or without acute MI are reported in the Table and Figure 2. In women presenting with acute MI, the use of new-generation DES was associated with a significantly lower risk of MACE (12% vs 16.6%; adjusted hazard ratio, 0.78; 95% CI, 0.61-0.99) and significantly lower risk of definite or probable stent thrombosis (1.3% vs 3.5%; adjusted hazard ratio, 0.36; 95% CI, 0.19-0.69), without evidence of interaction compared with women without acute MI (P for interaction = .59 and P for interaction = .31, respectively). The direction of the effect favoring new-generation DES was consistent across all composite and single end points (Table). Of note, the absolute benefits of new-generation DES appeared to be greater for women presenting with acute MI compared with those without acute MI (for MACEs: absolute risk difference [ARD], −3.56% [number needed to treat (NNT)], 28 vs ARD, −0.45% [NNT, 222]; and for definite or probable ST: ARD, −2.57% [NNT, 39] vs ARD, −0.46% [NNT, 217] in women with vs without acute MI, respectively).
Table. Three Years’ Outcomes in Women With and Without Acute Myocardial Infarction Treated With New- vs Early-Generation DESa.
| Outcome | Early-Generation DES | New-Generation DES | ARD, % | Adjusted HR (95% CI)b | P Value Interaction | ||
|---|---|---|---|---|---|---|---|
| No. of Events | Kaplan-Meier Estimate, % (95% CI) | No. of Events | Kaplan-Meier Estimate, % (95% CI) | ||||
| Death, Myocardial Infarction, or Target Lesion Revascularization | |||||||
| Unstable angina | 119 | 15.1 (12.8-17.8) | 168 | 14.6 (12.7-16.9) | −0.5 | 0.87 (0.67-1.11) | .59 |
| Acute myocardial infarction | 129 | 18.1 (15.4-21.3) | 168 | 14.6 (12.4-17.1) | −3.5 | 0.78 (0.61-0.99) | |
| Death | |||||||
| Unstable angina | 38 | 5.0 (3.6-6.8) | 56 | 5.5 (4.2-7.2) | 0.5 | 0.99 (0.64-1.54) | .63 |
| Acute myocardial infarction | 64 | 9.0 (7.1-11.4) | 84 | 7.9 (6.2-10.0) | −1.1 | 0.79 (0.56-1.12) | |
| Myocardial Infarction | |||||||
| Unstable angina | 44 | 5.6 (4.1-7.4) | 57 | 4.5 (3.5-5.8) | −1.1 | 0.79 (0.53-1.19) | .86 |
| Acute myocardial infarction | 49 | 7.1 (5.4-9.4) | 64 | 5.1 (3.9-6.6) | −2.0 | 0.76 (0.52-1.12) | |
| Target Lesion Revascularization | |||||||
| Unstable angina | 64 | 8.3 (6.6-10.5) | 77 | 6.6 (5.3-8.3) | −1.7 | 0.70 (0.49-1.01) | .91 |
| Acute myocardial infarction | 47 | 7.1 (5.4-9.5) | 52 | 4.8 (3.6-6.5) | −2.3 | 0.70 (0.46-1.05) | |
| Cardiac Death | |||||||
| Unstable angina | 27 | 3.6 (2.5-5.3) | 23 | 2.1 (1.4-3.2) | −1.5 | 0.57 (0.32-1.02) | .32 |
| Acute myocardial infarction | 42 | 5.9 (4.3-7.9) | 57 | 5.1 (3.8-6.8) | −0.8 | 0.75 (0.49-1.14) | |
| Death, Myocardial Infarction, or Stent Thrombosis | |||||||
| Unstable angina | 77 | 9.8 (7.9-12.1) | 107 | 9.4 (7.8-11.4) | −0.4 | 0.90 (0.66-1.22) | .64 |
| Acute myocardial infarction | 105 | 14.7 (12.3-17.6) | 139 | 11.8 (9.9-14.1) | −2.9 | 0.79 (0.60-1.02) | |
| Definite or Probable Stent Thrombosis | |||||||
| Unstable angina | 12 | 1.6 (0.9-2.8) | 14 | 1.1 (0.7-1.9) | −0.5 | 0.71 (0.30-1.67) | .31 |
| Acute myocardial infarction | 27 | 4.0 (2.7-5.8) | 18 | 1.4 (0.9-2.2) | −2.6 | 0.36 (0.19-0.69) | |
Abbreviations: ARD, absolute risk difference; DES, drug-eluting stents; HR, hazard ratio.
This study included 2197 women with unstable angina and 2176 women with acute myocardial infarction.
Adjusted HRs are generated with Cox regression analysis including trial identifier as a random effect.
Figure 2. New-Generation vs Early-Generation Drug-Eluting Stents (DES) In Women With and Without Acute Myocardial Infarction (MI).
NNT indicates number needed to treat; TLR, target lesion revascularization; and UA, unstable angina.
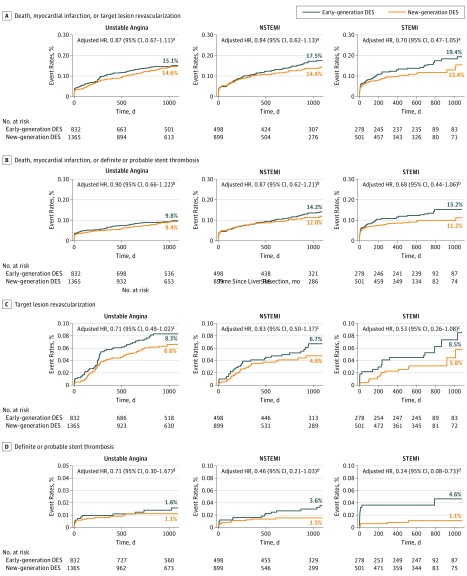
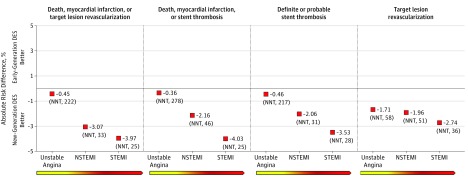
Outcomes for new- vs early-generation DES across the spectrum of ACS are presented in Figure 3 and Figure 4. The benefits over 3 years of new-generation DES were consistent across the entire spectrum of ACS for all the studied end points (Figure 3). On an absolute scale, a graded absolute benefit with use of new-generation DES was observed in the transition from UA, to NSTEMI, and to STEMI (for MACEs: ARD, −0.45% [NNT, 222], vs ARD, −3.07% [NNT, 33], vs ARD, −3.97% [NNT, 25]; for definite or probable ST: ARD, −0.46% [NNT, 278], vs ARD, −2.06% [NNT, 46], vs ARD, −3.53% [NNT, 25], respectively) (Figure 4).
Figure 3. Kaplan-Meier Curves for Women Treated With New-Generation Drug-Eluting Stents (DES) vs Early-Generation DES Across the Spectrum of Acute Coronary Syndromes.
HR indicates hazard ratio; NSTEMI, non–ST-segment elevation myocardial infarction; and STEMI, ST-segment elevation myocardial infarction.
aP for interaction = .59.
bP for interaction = .64.
cP for interaction = .91.
dP for interaction = .31.
Figure 4. Absolute Risk Differences Between New-Generation Drug-Eluting Stents (DES) vs Early-Generation DES Across the Spectrum of Acute Coronary Syndromes.
NNT indicates number needed to treat; NSTEMI, non–ST-segment elevation myocardial infarction; and STEMI, ST-segment elevation myocardial infarction.
Discussion
The main findings of this large-scale study that included patient-level data from more than 4000 women with ACS randomized to different types of currently used DES are (1) new-generation DES are associated with significantly lower risk of death, MI or TLR, and definite or probable stent thrombosis at 3 years in women presenting with acute MI and (2) the safety and efficacy of new-generation DES appeared to be greater per increase in acuity and severity of clinical presentation, with the greatest absolute benefits observed in women presenting with STEMI.
Drug-eluting stents are a technological breakthrough for the management of coronary artery disease. By reducing the rates of in-stent restenosis and the need of repeated revascularization observed with bare metal stents, introduction of DES in clinical practice improved the effectiveness of percutaneous coronary revascularization. Unfortunately, safety issues arose after the introduction of early-generation DES. Compared with bare metal stents, first-generation DES were demonstrated to be associated with higher risk of late and very late stent thrombosis, which led to widespread adoption of prolonged dual antiplatelet therapy (DAPT). Subsequent intracoronary imaging and histopathology studies elucidated some of the mechanism underlying the increased thrombotic risk, including delayed vascular healing, incomplete endothelialization, neoatherosclerosis, vascular toxicity induced by the released antiproliferative drug, localized hypersensitivity to the DES polymer, and stent malapposition due to excessive fibrin and vessel remodeling. Subsequently, new-generation DES were created. Thanks to improvements in stent architecture, platform material, polymer composition, and type and release kinetic of the antiproliferative drug, new-generation DES were demonstrated to overcome the limitations of early-generation DES and to be associated with even less thrombotic risk than bare metal stents. Currently, new-generation metallic DES represent the standard of care for patients undergoing PCI. However, large-scale studies that led to the regulatory approval of currently used DES included a small proportion of women (between 15% and 25% of the overall population in most RCTs). As a consequence, the safety and efficacy of new-generation DES in women, and particularly in high-risk subsets, remained inconclusive. In December 2011, the US Food and Drug Administration released a guidance document for the assessment of sex differences in medical device clinical studies, prompting research in this important patient population. Therefore, the current large, international, collaborative patient-level data set including female participants from the main RCTs of DES was created.
In the present study, we elucidated the long-term (3-year) safety and efficacy of new-generation DES across the spectrum of ACS in women, an underdiagnosed, undertreated, and underinvestigated patient population. We observed that compared with early-generation devices, use of new-generation DES was associated with significantly improved safety and efficacy in women with acute MI. Of note, the absolute magnitude of the benefit of new-generation DES appeared to be greater in women with acute MI on both safety and efficacy end points. Women presenting with NSTEMI or STEMI remained at greater long-term risk of thrombotic complications. Likely, as a consequence of a greater baseline risk, the antithrombotic benefits of new-generation DES appeared to be greater per increase in severity of ACS, transitioning from UA, to NSTEMI, and to STEMI. For example, for the end point of death, MI, or TLR, the NNTs for new- vs early-generation DES were 222 in women with UA compared with 25 in women with STEMI. The public health impact of such intervention is greater or comparable with that observed with statins or DAPT on similar end points and patient populations.
The clinical implications of our observation are that the benefits of new-generation DES are likely to be observed in higher-risk populations. First, this information gives reassurance regarding the performances of currently approved devices in clinical practice. Second, the enhanced antithrombotic properties of currently used DES may allow for more flexible use of DAPT for prevention of stent-related thrombotic complications. Although at least 1 year of DAPT is indicated after an ACS managed with or without PCI, DAPT may probably be discontinued with reasonable safety in women treated with new-generation DES who cannot tolerate more intense platelet inhibition or who are at high risk for bleeding. In concordance with these observations, in the Prospective Randomized Comparison of the BioFreedom Biolimus A9 Drug-Coated Stent vs the Gazelle Bare-Metal Stent in Patients at High Bleeding Risk (LEADERS FREE) Trial, in which 2466 patients were randomized to a polymer-free DES vs a bare metal stent followed by 1 month of DAPT, use of polymer-free DES was associated with greater efficacy and thrombotic safety compared with bare metal stent. Of note, the benefits of polymer-free DES were consistent between clinical presentations (ACS vs no ACS). Although further studies are needed to corroborate these data, the totality of the available evidence seems to support the significantly improved long-term performances of currently used DES in clinical practice, including high-risk subsets of patients.
Limitations
Our study has several limitations to consider. First, the data used in our analysis were derived from multiple RCTs with heterogeneous populations. Earlier trials enrolled lower-risk patients with stable presentations and simple lesions, whereas trials conducted more recently included higher-risk patients. To reduce the trial effect on outcomes, we included trial as a random effect in our adjusted analysis; however, despite the implementation of this statistical method, residual confounding can still exist. However, this remains a significant limitation that negatively impacts the strength of our conclusions. Second, variables that are known to have an important effect on outcomes following PCI, such as duration and intensity of DAPT, were not available and therefore not included in our analysis. Third, some of the trials included in our analysis were conducted more than a decade ago. Important advances in technologies used for PCI and therapies available for the management of ACS raise the question of whether the findings obtained in those trials would be replicated today. Fourth, male patients were not included in the current study, thereby limiting our findings and their interpretation exclusively to women. Fifth, this has to be considered a post-hoc analysis from RCTs not originally designed to compare early- vs new-generation DES in women with ACS. Sixth, because of a legal agreement between the device industry and investigators and because the primary objective of this collaboration was to explore outcomes of new-generation DES in women, we are not allowed to release DES-specific data from this pooled data set. Therefore, comparisons between specific types of stents could not be performed. Finally, comparisons between early- vs new-generation DES across the spectrum of ACS remains limited by the lower sample size within each subset of ACS. Notwithstanding these limitations, our analysis is based on a large, comprehensive, patient-level pooled analysis of major RCTs of DES with data monitoring and independent clinical event adjudication.
Conclusions
Compared with early-generation DES, new-generation DES are associated with consistent and durable benefits over 3 years in women presenting with acute MI. Of note, the antithrombotic benefits of new-generation DES appeared to be greater per increase severity and acuity of ACS. The results of the current large-scale analysis confirm the results of RCTs performed in predominantly male populations and consolidate new-generation DES as the standard of care for women with ACS.
eTable 1. Characteristics of Included Randomized Controlled Trials.
eTable 2. Clinical Endpoint Definitions Used Across Randomized Controlled Trials.
eTable 3. Baseline Clinical and Angiographic Characteristics.
eReferences
eFigure. Kaplan-Meier curves for death, myocardial infarction or target lesion revascularization (1A), death, myocardial infarction or stent thrombosis (1B), definite or probable stent thrombosis (1C) and target lesion revascularization (1D) in women presenting with unstable angina, non-ST elevation myocardial infarction or ST elevation myocardial infarction.
References
- 1.Pancholy SB, Shantha GP, Patel T, Cheskin LJ. Sex differences in short-term and long-term all-cause mortality among patients with ST-segment elevation myocardial infarction treated by primary percutaneous intervention: a meta-analysis. JAMA Intern Med. 2014;174(11):1822-1830. [DOI] [PubMed] [Google Scholar]
- 2.Benamer H, Tafflet M, Bataille S, et al. ; CARDIO-ARHIF Registry Investigators . Female gender is an independent predictor of in-hospital mortality after STEMI in the era of primary PCI: insights from the greater Paris area PCI Registry. EuroIntervention. 2011;6(9):1073-1079. [DOI] [PubMed] [Google Scholar]
- 3.Yu J, Mehran R, Grinfeld L, et al. Sex-based differences in bleeding and long term adverse events after percutaneous coronary intervention for acute myocardial infarction: three year results from the HORIZONS-AMI trial. Catheter Cardiovasc Interv. 2015;85(3):359-368. [DOI] [PubMed] [Google Scholar]
- 4.Jackson EA, Moscucci M, Smith DE, et al. The association of sex with outcomes among patients undergoing primary percutaneous coronary intervention for ST elevation myocardial infarction in the contemporary era: insights from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2). Am Heart J. 2011;161(1):106-112.e1. [DOI] [PubMed] [Google Scholar]
- 5.Wijnbergen I, Tijssen J, van ’t Veer M, Michels R, Pijls NH. Gender differences in long-term outcome after primary percutaneous intervention for ST-segment elevation myocardial infarction. Catheter Cardiovasc Interv. 2013;82(3):379-384. [DOI] [PubMed] [Google Scholar]
- 6.Kim SG, Apple S, Mintz GS, et al. The importance of gender on coronary artery size: in-vivo assessment by intravascular ultrasound. Clin Cardiol. 2004;27(5):291-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bangalore S, Kumar S, Fusaro M, et al. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation. 2012;125(23):2873-2891. [DOI] [PubMed] [Google Scholar]
- 8.Sabaté M, Räber L, Heg D, et al. Comparison of newer-generation drug-eluting with bare-metal stents in patients with acute ST-segment elevation myocardial infarction: a pooled analysis of the EXAMINATION (clinical Evaluation of the Xience-V stent in Acute Myocardial INfArcTION) and COMFORTABLE-AMI (Comparison of Biolimus Eluted From an Erodible Stent Coating With Bare Metal Stents in Acute ST-Elevation Myocardial Infarction) trials. JACC Cardiovasc Interv. 2014;7(1):55-63. [DOI] [PubMed] [Google Scholar]
- 9.Claessen BE, Henriques JP, Jaffer FA, Mehran R, Piek JJ, Dangas GD. Stent thrombosis: a clinical perspective. JACC Cardiovasc Interv. 2014;7(10):1081-1092. [DOI] [PubMed] [Google Scholar]
- 10.Palmerini T, Biondi-Zoccai G, Della Riva D, et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. 2012;379(9824):1393-1402. [DOI] [PubMed] [Google Scholar]
- 11.Sabaté M, Brugaletta S, Cequier A, et al. Clinical outcomes in patients with ST-segment elevation myocardial infarction treated with everolimus-eluting stents versus bare-metal stents (EXAMINATION): 5-year results of a randomised trial. Lancet. 2016;387(10016):357-366. [DOI] [PubMed] [Google Scholar]
- 12.Stefanini GG, Baber U, Windecker S, et al. Safety and efficacy of drug-eluting stents in women: a patient-level pooled analysis of randomised trials. Lancet. 2013;382(9908):1879-1888. [DOI] [PubMed] [Google Scholar]
- 13.Giustino G, Mastoris I, Baber U, et al. Correlates and impact of coronary artery calcifications in women undergoing percutaneous coronary intervention with drug-eluting stents: from the Women in Innovation and Drug-Eluting Stents (WIN-DES) collaboration. JACC Cardiovasc Interv. 2016;9(18):1890-1901. [DOI] [PubMed] [Google Scholar]
- 14.Giustino G, Baber U, Aquino M, et al. Safety and efficacy of new-generation drug-eluting stents in women undergoing complex percutaneous coronary artery revascularization: from the WIN-DES collaborative patient-level pooled analysis. JACC Cardiovasc Interv. 2016;9(7):674-684. [DOI] [PubMed] [Google Scholar]
- 15.Baber U, Giustino G, Sartori S, et al. Effect of chronic kidney disease in women undergoing percutaneous coronary intervention with drug-eluting stents: a patient-level pooled analysis of randomized controlled trials. JACC Cardiovasc Interv. 2016;9(1):28-38. [DOI] [PubMed] [Google Scholar]
- 16.Giustino G, Baber U, Salianski O, et al. Safety and efficacy of new-generation drug-eluting stents in women at high risk for atherothrombosis: from the women in innovation and drug-eluting stents collaborative patient-level pooled analysis. Circ Cardiovasc Interv. 2016;9(1):e002995. [DOI] [PubMed] [Google Scholar]
- 17.Giustino G, Baber U, Stefanini GG, et al. Impact of clinical presentation (stable angina pectoris vs unstable angina pectoris or non-ST-elevation myocardial infarction vs st-elevation myocardial infarction) on long-term outcomes in women undergoing percutaneous coronary intervention with drug-eluting stents. Am J Cardiol. 2015;116(6):845-852. [DOI] [PubMed] [Google Scholar]
- 18.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 19.Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010;56(23):1897-1907. [DOI] [PubMed] [Google Scholar]
- 20.Airoldi F, Colombo A, Morici N, et al. Incidence and predictors of drug-eluting stent thrombosis during and after discontinuation of thienopyridine treatment. Circulation. 2007;116(7):745-754. [DOI] [PubMed] [Google Scholar]
- 21.Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293(17):2126-2130. [DOI] [PubMed] [Google Scholar]
- 22.Guagliumi G, Sirbu V, Musumeci G, et al. Examination of the in vivo mechanisms of late drug-eluting stent thrombosis: findings from optical coherence tomography and intravascular ultrasound imaging. JACC Cardiovasc Interv. 2012;5(1):12-20. [DOI] [PubMed] [Google Scholar]
- 23.Nakazawa G, Otsuka F, Nakano M, et al. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J Am Coll Cardiol. 2011;57(11):1314-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakazawa G, Finn AV, Vorpahl M, Ladich ER, Kolodgie FD, Virmani R. Coronary responses and differential mechanisms of late stent thrombosis attributed to first-generation sirolimus- and paclitaxel-eluting stents. J Am Coll Cardiol. 2011;57(4):390-398. [DOI] [PubMed] [Google Scholar]
- 25.Levine GN, Bates ER, Blankenship JC, et al. ; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Society for Cardiovascular Angiography and Interventions . 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58(24):e44-e122. [DOI] [PubMed] [Google Scholar]
- 26.Mikhail GW. Coronary heart disease in women. BMJ. 2005;331(7515):467-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cannon CP, Braunwald E, McCabe CH, et al. ; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators . Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350(15):1495-1504. [DOI] [PubMed] [Google Scholar]
- 28.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels: cholesterol and recurrent events trial investigators. N Engl J Med. 1996;335(14):1001-1009. [DOI] [PubMed] [Google Scholar]
- 29.Mauri L, Kereiakes DJ, Yeh RW, et al. ; DAPT Study Investigators . Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371(23):2155-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehran R, Giustino G, Baber U. DAPT duration after DES: what is the “mandatory” duration? J Am Coll Cardiol. 2015;65(11):1103-1106. [DOI] [PubMed] [Google Scholar]
- 31.Giustino G, Baber U, Sartori S, et al. Duration of dual antiplatelet therapy after drug-eluting stent implantation: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2015;65(13):1298-1310. [DOI] [PubMed] [Google Scholar]
- 32.Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68(10):1082-1115. [DOI] [PubMed] [Google Scholar]
- 33.Baber U, Mehran R, Giustino G, et al. Coronary thrombosis and major bleeding after PCI with drug-eluting stents: risk scores from PARIS. J Am Coll Cardiol. 2016;67(19):2224-2234. [DOI] [PubMed] [Google Scholar]
- 34.Généreux P, Giustino G, Witzenbichler B, et al. Incidence, predictors, and impact of post-discharge bleeding after percutaneous coronary intervention. J Am Coll Cardiol. 2015;66(9):1036-1045. [DOI] [PubMed] [Google Scholar]
- 35.Urban P, Meredith IT, Abizaid A, et al. ; LEADERS FREE Investigators . Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med. 2015;373(21):2038-2047. [DOI] [PubMed] [Google Scholar]
- 36.Naber CK, Urban P, Ong PJ, et al. ; LEADERS FREE Investigators . Biolimus-A9 polymer-free coated stent in high bleeding risk patients with acute coronary syndrome: a Leaders Free ACS sub-study. Eur Heart J. 2017;38(13):961-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Characteristics of Included Randomized Controlled Trials.
eTable 2. Clinical Endpoint Definitions Used Across Randomized Controlled Trials.
eTable 3. Baseline Clinical and Angiographic Characteristics.
eReferences
eFigure. Kaplan-Meier curves for death, myocardial infarction or target lesion revascularization (1A), death, myocardial infarction or stent thrombosis (1B), definite or probable stent thrombosis (1C) and target lesion revascularization (1D) in women presenting with unstable angina, non-ST elevation myocardial infarction or ST elevation myocardial infarction.