Abstract
Background and Aims
Studies on codon usage in monocots have focused on grasses, and observed patterns of this taxon were generalized to all monocot species. Here, non-grass monocot species were analysed to investigate the differences between grass and non-grass monocots.
Methods
First, studies of codon usage in monocots were reviewed. The current information was then extended regarding codon usage, as well as codon-pair context bias, using four completely sequenced non-grass monocot genomes (Musa acuminata, Musa balbisiana, Phoenix dactylifera and Spirodela polyrhiza) for which comparable transcriptome datasets are available. Measurements were taken regarding relative synonymous codon usage, effective number of codons, derived optimal codon and GC content and then the relationships investigated to infer the underlying evolutionary forces.
Key Results
The research identified optimal codons, rare codons and preferred codon-pair context in the non-grass monocot species studied. In contrast to the bimodal distribution of GC3 (GC content in third codon position) in grasses, non-grass monocots showed a unimodal distribution. Disproportionate use of G and C (and of A and T) in two- and four-codon amino acids detected in the analysis rules out the mutational bias hypothesis as an explanation of genomic variation in GC content. There was found to be a positive relationship between CAI (codon adaptation index; predicts the level of expression of a gene) and GC3. In addition, a strong correlation was observed between coding and genomic GC content and negative correlation of GC3 with gene length, indicating a strong impact of GC-biased gene conversion (gBGC) in shaping codon usage and nucleotide composition in non-grass monocots.
Conclusion
Optimal codons in these non-grass monocots show a preference for G/C in the third codon position. These results support the concept that codon usage and nucleotide composition in non-grass monocots are mainly driven by gBGC.
Keywords: Codon usage, codon-pair context, GC content, GC3 distribution, non-grass monocots, banana, date palm
INTRODUCTION
Codon usage bias (also known as codon bias) is the selective use of nucleotide triplets (codons) to encode specific amino acid sequences in the protein coding genes of a species. Every amino acid in a sequence can be encoded by one (in the case of methionine and tryptophan) to six different codons. The frequencies with which synonymous codons are used to encode an amino acid vary between organisms and sometimes even within the same organism (Hershberg and Petrov, 2008; Liu and Xue, 2005).
Three major hypotheses have been proposed to describe the codon usage bias in living systems: mutational bias (MB hypothesis), selection on codon usage (SCU) and GC-biased gene conversion (gBGC). To explain the codon usages that are produced by point mutations, contextual biases in the point mutation rates or biases in DNA repair, the MB hypothesis proposes that differences in mutation rates across species result in non-random variation of synonymous codon usage and an increase in GC content (Plotkin and Kudla, 2011). However, under the MB hypothesis, the increase in GC content in recombination hotspots via fixation of GC alleles at polymorphic sites cannot be explained as all classes of mutations have an equal probability of fixation (reviewed by Duret and Galtier, 2009). To explain the increase in probability of fixation of GC alleles, SCU was proposed as an alternative. The SCU hypothesis suggests natural selection as a contributor to the increase of GC content to improve translational efficiency (Akashi, 2003), arguing that synonymous mutations influence the fitness of an organism and can therefore be promoted or repressed through evolution. However, the presence of GC heterogeneity observed in pseudogenes and non-coding regions cannot be explained by SCU (Gossmann et al., 2010). Furthermore, significant fitness advantages between individuals differing in only a single base pair are unlikely to be common. This, however, is required for selection to act (Mugal et al., 2015).
After several years of debate around the MB and SCU hypotheses, a third hypothesis, gBGC, was put forward to explain codon usage and GC heterogeneity. The gBGC hypothesis suggests GC-biased gene conversion to shape codon usage bias in a manner dependent upon the local recombination rate, which favours fixation of G and C alleles over A and T at polymorphic sites. As a consequence of this association between GC and recombination, local GC content increases rapidly in genomic hotspots of recombination (Spencer, 2006) while genome-wide GC content increases rapidly in species with higher recombination rates (Figuet et al., 2014; Weber et al., 2014). Codon usage bias was recorded to be highest in areas of intermediate levels of recombination for GC-ending optimal codons (Harrison and Charlesworth, 2010). Even though the gBGC process is distinct from natural selection, it affects the probability of fixation of alleles in patterns similar to selection (reviewed by Duret and Galtier, 2009; Ratnakumar et al., 2010) and interferes with selection by promoting the fixation of deleterious alleles (Galtier et al., 2009; Necşulea et al., 2011). Hence, gBGC is considered a neutral process as it does not rely on the fitness effect of alleles of the individuals. For the last 10 years a plethora of evidence has been accumulated for gBGC as a major evolutionary force affecting codon usage and base composition in humans (reviewed by Duret and Galtier, 2009, Glémin et al., 2015), yeast (Lesecque et al., 2013) and plants (Muyle et al., 2011; Pessia et al., 2012; Wu et al., 2015; Rodgers-Melnick et al., 2016; Clément et al., 2017).
Apart from codon usage, codon context bias is also important for understanding the preferences in the sequence of a pair of codons within an organism. Codon-pair context bias is linked more to decoding accuracy than to translational speed (Moura et al., 2005). As different species have varied abundance of tRNA isoacceptors for each codon family, codon-pair context sequences impact the translational accuracy of genes (Moura et al., 2007).
Plant species are reported to show a wide diversity in terms of their gene expression, physiology and stress response in varied environmental conditions (De La Torre et al., 2015). Hence, knowledge of the codon usage and codon-pair context patterns of plants and underlying evolutionary forces will be useful to understand the molecular mechanism of environmental adaptation and biological diversity of each species.
A number of comparative analyses have examined the codon usage of genes within and between the two major groups of flowering plants, dicots and monocots (Kawabe and Miyashita, 2003; Mukhopadhyay et al., 2008; Liu, 2012; Liu et al., 2015). Still, comparatively little is known about codon usage and codon-pair context in monocot plant species, which can be sub-classified into grass monocots (Poaceae) and non-grass monocots (e.g. Orchidaceae, Musaceae, Arecaceae, Zingiberaceae and Liliaceae), as shown in Fig. 1. Furthermore, most studies on monocots focus on grass monocots and cannot explain the extent and pattern of codon usage or the forces that may be affecting codon usage in monocots in general.
Fig. 1.
The species used in the current analysis of codon usage in non-grass monocots (source of the tree; Hertweck et al., 2015).
This review outlines the methods available for the quantification of codon usage and presents the current state of knowledge of codon usage in monocots. Furthermore, we extend this information on codon usage, optimal and rare codons, GC content, GC3 (GC content in third codon position) distribution, codon-pair context patterns and shaping factors in non-grass monocot genomes by analysing four completely sequenced genomes with publicly available comparable transcriptome datasets of two major banana species, Musa acuminata and Musa balbisiana, the date palm, Phoenix dactylifera, and the small aquatic plant, duckweed, Spirodela polyrhiza. The analysis suggests that gBGC affects the nucleotide composition and codon usage in non-grass monocots. This information on optimal and rare codons, factors affecting codon usage and codon-pair context bias will facilitate further research in molecular phylogenetics and genomics, as well as in the development of varieties tolerant of various biotic and abiotic stresses among the non-grass monocots.
Tools and indices for assessing codon usage
Investigation of codon usage in an organism requires quantification of codon usage patterns within the genes and genomes of the species under study. Codon usage values can be determined based on one or more indices (described in the following sections and summarized in Table 1) for different genes within and across the species.
Table 1.
Indices for codon usage analysis
| Index | Principle | Mathematical formula | Scores | Reference |
|---|---|---|---|---|
| P2 index | Quantifies the proportion of codons that conform to the intermediate strength of the codon–anticodon interaction energy | where W = A or U, S = G or C, Y = C or U and A/C/G/U are the nucleotide composition of codon triplets | Under uniform codon usage P2 is equal to 0.5. A value more than 0.5 indicates strong codon bias and less than 0.5 indicates no codon bias | Gouy and Gautier (1982) |
| Relative synonymous codon usage (RSCU) | Calculated as the ratio of the observed frequency of a codon to the expected frequency of that codon, assuming uniform codon usage | where gij is the observed number of ith codon for the jth amino acid, which has ni kinds of synonymous codons | The synonymous codons with RSCU values of 1 indicates no codon usage bias for that amino acid and the codons are chosen equally or randomly. RSCU values above 1 indicate positive codon usage bias and RSCU values below 1 indicate negative codon usage bias | Sharp et al. (1986) |
| Effective number of codons (ENC) | Measures how far the codon usage of a gene stays from the equal usage of synonymous codons | where k (k = 2, 3, 4, and 6) is the average of k values for k-fold degenerate amino acids and can be estimated by the formula: where m is total number of codons for that amino acid and where mi is the number of occurrences of the ith codon for this amino acid | ENC values range between 20 and 61; a value of 20 indicates an extremely biased gene that uses only one codon for each amino acid, while a value of 61 indicates an unbiased gene | Ikemura (1981) |
| Chi-squared index | Calculates the divergence of the observed data from the values that would be expected under the null hypothesis of no association between observed and expected data | where oij is the number of occurrences of the jth codon for the ith amino acid, ei is the expected usage of the jth codon under conditions of equal synonymous codon usage, and fi is the degeneracy of the codons for ith amino acid | The probability (P) of codon occurrence for a particular amino acid is estimated from the chi-square distribution (upper tail) based on calculated χ2 value. If the value of P is less than 0.05, then the codon cannot occur for that particular amino acid and hence the null hypothesis cannot be accepted | Shields et al. (1988) |
| Frequency of optimal codons (Fop) | Calculated as the ratio of the frequency of optimal codons in a gene to the total number of synonymous codons based on a specific reference gene set | where Nopt = number of optimal codons and Ntot = number of synonymous codons | Fop values range between 0 and 1.0: a value of 0 indicates that there is no optimal codon, and a value of 1.0 indicates that a gene is entirely composed of optimal codons | Ikemura (1985) |
| Codon adaptation index (CAI) | Quantifies the geometric mean of the relative adaptiveness for each codon with respect to the codon usage of a reference set of highly expressed genes and is calculated based on RSCU values | where ωi is the relative adaptiveness of codon i, fij is the frequency of codon i encoding amino acid j, and L is the length of the gene | CAI values range between 0 and 1: a value of 0 indicates random codon usage and low expression level of the gene, whereas a value of 1 suggests extreme codon bias and potentially high expression level of the gene | Sharp and Li (1987) |
| Codon bias index (CBI) | Measure of codon usage bias based on the codon usage of a specific reference set of genes | where Nopt = number of optimal codons, Nran = number of optimal codons and Ntot = number of synonymous codons | CBI values range between 0 and 1: a value of 0 indicates random codon usage, whereas a value of 1 suggests extreme codon bias | Bennetzen and Hall (1982) |
Methods that use codon usage indices summarize data into useful limited variables, facilitating comparisons of codon usage among species (Shields et al., 1988). Indices are categorized into two groups: one group of codon usage indices measures the codon usage bias based on the total discrepancy between the expected codon usage (assuming no bias) and the actual codon usage, while a second group quantifies bias based on closeness to preferred codons by comparing the codon usage of a test gene to a reference set of genes. The principles for calculation and scoring are summarized in Table 1. The theories and mathematical formulas for the calculation of these indices are described in detail by Behura and Severson (2013). These indices, either alone or in combination, have been adopted in several online tools that are commonly used to measure codon usage (Table 2).
Table 2.
Online computational tools for codon analysis
| Online tools | Application | Web link |
|---|---|---|
| ACUA (Automated Codon Usage Analysis Software) | Performs statistical profiling of codon usage in high-throughput sequence data | http://www.bioinsilico.com/acua |
| ANACONDA | Performs comparative analysis of codon context patterns of genomes based on Pearson’s statistic | http://bioinformatics.ua.pt/software/anaconda/ |
| CAIcal SERVER | Runs several computations in relation to codon preference and the codon adaptation of nucleic acid sequences to host organisms | http://genomes.urv.es/CAIcal |
| Codon Adaptation Index (CAI) Calculator 2 | Measures codon bias within and across genomes | http://userpages.umbc.edu/~wug1/codon/cai/cais.php |
| Codon Explorer | A wrapper program of G-language REST/SOAP web services calculates codon usage visualizing genomic information and predicts gene expression levels from codon usage bias | http://www.g-language.org/gembassy/ |
| Codon O | Measures synonymous codon usage bias within and across genomes | http://sysbio.cvm.msstate.edu/CodonO/ |
| Codon W | Performs correspondence analysis of codon, analyses amino acid usage and measures standard indices of codon usage | http://codonw.sourceforge.net/ |
| E-CAI Calculator | Determines codon adaptation index of nucleic acid sequences | http://genomes.urv.es/CAIcal/E-CAI/ |
| GCUA (Graphical Codon Usage Analyser) | Displays codon usage frequency values or relative adaptiveness values | http://gcua.schoedl.de/ |
| GCUA (General Codon Usage Analysis) | Uses multivariate analysis to estimate the variation in codon usage amongst genes | http://bioinf.nuim.ie/gcua/ |
| Gene to codon usage | Creates a codon usage table from a DNA sequence, counting the frequency of all codons in the specified sequence | http://www.entelechon.com/2008/10/gene-to-codon-usage/ |
| JCAT (Java Codon Adaptation Tool) | Analyses nucleic acid and protein sequences by avoiding rho- independent terminators, prokaryotic ribosome binding sites and restriction sites | http://www.jcat.de/ |
| MEGA | Measures codon frequencies and using relative synonymous codon usage statistics | http://www.megasoftware.net/ |
| Optimizer | Suggests optimized codon usage of a DNA sequence for increasing expression level | http://genomes.urv.es/OPTIMIZER/ |
| Rare codon analysis | Analyses codon preference input coding sequences for use prior to heterologous expression | http://www.genscript.com/cgi-bin/tools/rare_codon_analysis |
| RCDI (Relative Codon Deoptimization Index) | Measures the codon deoptimization by comparing the codon usage of a gene with the codon usage of a reference genome | http://genomes.urv.cat/CAIcal/RCDI/ |
| RSCUNET (Relative Synonymous Codon Usage Neural Network) | Analyses codon usage variation based on a self-organizing map neural network algorithm | http://bioinf.nuigalway.ie/RescueNet |
The P2 index was one of the earliest indices developed using the principle of distance between expected and actual codon use. It predicts the proportion of preferred codons based on codon–anticodon binding strength between an mRNA and the tRNAs. The P2 index has largely been replaced by other indices in most recent publications, with use of RSCU (relative synonymous codon usage), ENC (effective number of codons) and CAI (codon adaptation index) indices being widely reported. The RSCU measures codon bias based on non-random usage of codons for a specific amino acid in a coding sequence. As the RSCU value is independent of amino acid composition, this is very useful for the comparison of genes that differ in their length and amino acid composition, and hence this index is frequently used (Mukhopadhyay et al., 2008; Sablok et al., 2011; He et al., 2016; Ning et al., 2016). ENC values are based on a principle similar to that underlying RCSU; they are also relatively independent of amino acid composition. Comparison of ENC values with GC content yields information regarding patterns in codon usage across the genome. Recent studies have used ENC to determine codon usage bias in Ginkgo biloba (He et al., 2016) and Haberlea rhodopensis (Ivanova et al., 2017). Fop (frequency of optimal codons), CAI and CBI (codon bias index) measure codon usage bias according to the codon usage of a reference set of genes, which may be composed of a gene class, such as ribosomal genes, highly expressed genes or a single gene (Table 1). As Fop, CAI and CBI indices use specific reference gene sets, they provide a measure of bias towards particular codons that appear to be translationally optimal in that species (Behura and Severson, 2013). Among these indices, use of CAI is most widely reported, for example in codon usage analysis of Oenothera (Nair et al., 2014) and G. biloba (He et al., 2016), while Fop has been applied in studies for Picea (De La Torre et al., 2015) and Medicago truncatula (Song et al., 2015). As CAI values are determined from reference sets of highly expressed genes, which may be different among species, the relative fitness values of the species will also be different. Hence, the CAI values for all genes for a particular species can be compared within the species, but cannot be compared to those for other species. There are no recent reports of codon analysis in plants using chi-squared, CBI and P2. Early methods for visualizing codon usage data involved tabulating codon usage values from a pool of different sets of genes (Greenacre, 1984). Such analyses relied heavily on the grouping of genes, and these groups were formulated based on pairwise comparisons. However, analyses that can be performed using simple tabulation are limited and, with the emergence of increasingly large sets of sequence data, it became cumbersome to tabulate the large sets of codon usage data. To address this issue in analytical approaches to codon analysis, tools that adapt multivariate analysis methods such as correspondence analysis (COA) and cluster analysis (Gouy and Gautier, 1982), either alone or in combination, have been adopted for visualizing codon usage pattern in several online tools (Table 2).
Cluster analysis is the statistical visualization method that has been most widely used to analyse codon usage in plant genomes. In this method, the frequencies of codon occurrences are calculated using codon usage indices such as RSCU and CAI. Euclidean measurements of distance between the genes and the codon frequencies are estimated and the values plotted to visually indicate the association of genes to a particular codon and their similarities with other genes (Perrière and Thioulouse, 2002). Examples of cluster analysis applied to plant species include studies of the codon usage of protein coding sequences in G. biloba (He et al., 2016), herbaceous peony (Paeonia lactiflora) (Wu et al., 2015), citrus species (Xu et al., 2013), the chloroplast genome of Oenothera (Nair et al., 2014) and the mitochondrial genomes of wheat (Triticum aestivum), maize (Zea mays), Arabidopsis thaliana, tobacco (Nicotiana tabacum), Physcomitrella patens and Marchantia polymorpha (Zhou and Li, 2009). Cluster analyses have also been used to classify and categorize codon usage in various plant genomes (Ma et al., 2015; Priya et al., 2016), but in a few cases, the method did not accurately reflect the phylogenetic relationships among plants (Nair et al., 2014; You et al., 2015). With the advent of genome-scale sequencing projects and greater computational power, improved tools are being developed to cope with the size and complexity of the data. While several sets of data are available for plant species, the lack of high-quality transcriptome data that are comparable in depth and biological context limits the value and integrity of analyses; as with any bioinformatic approach, it is critical to identify high-quality and comparable datasets for meaningful outcomes.
Codon usage patterns in monocots
Among monocots, several major food crops are members of the Poaceae (grass monocots) and have consequently been well studied, including complete genome sequencing, so it is not surprising that most of the codon usage studies in monocots have focused on this family. Campbell and Gowri (1990) were the first to illustrate the differential usage of codons in monocot species and found that the third codon nucleotide position shows bias toward A/U in dicot species and toward G/C in monocot species, concurrent with the higher GC content of monocot genomes. A bias for G/C-ending codons has also been reported for wheat, barley, maize (Kawabe and Miyashita, 2003) and rice (Wang and Hickey, 2007; Mukhopadhyay et al., 2008). The higher numbers of G/C-ending codons were proposed as supporting evidence of relatively strong mutational bias in monocot species (Kawabe and Miyashita, 2003; Wang and Hickey, 2007; Mukhopadhyay et al., 2008).
Among all plants, monocots have been reported to be the most heterogenous in terms of their GC content, which contrasts with the homogeneous GC content of the genomes of dicot species (Carels and Bernardi, 2000; Wang and Hickey, 2007; Serres-Giardi et al., 2012; Tatarinova et al., 2013). This GC heterogeneity is similar to that found in the genomes of warm-blooded vertebrates, which contain patches of GC-rich and AT-rich regions known as isochores (Duret and Galtier, 2009). These isochore structures have been reported to affect both coding and non-coding sequences of genomes, while particularly GC-rich regions have been observed to contain more genes with shorter introns (Eyre-Waker and Hurst, 2001). As GC3 is relatively independent of the protein coding potential, it is considered a marker for GC richness in a genome (Tatarinova et al., 2013). Analysis of GC3 distribution showed a tendency for genes to fall into two classes (bimodality) in grass monocots, unlike the single class of genes (unimodal distributions) in dicot genomes (Campbell and Gowri, 1990; Tatarinova et al., 2013; Clément et al., 2015). Genes in the grass monocots (rice) could be classified into the two groups based on GC3 values below or above 80 %. In the rice genome, genes with GC3 of 80 % or more showed a tendency to be mono-exonic or intron-poor and have stronger or more variable expression levels (Tatarinova et al., 2013). Based on a study of GC3 in seven grass species together with banana and oil palm, Clément et al. (2015) suggested that a bimodal distribution may be an ancestral feature of monocots that is preserved to different extents in different lineages.
To explain this notable GC heterogeneity in monocots, initially MB (Wang and Roossinck, 2006; Wang and Hickey, 2007) was proposed as a major underlying evolutionary force shaping codon use, based on the comparative analysis of Arabidopsis and rice genomes. However, mutation can hardly explain recombination-associated segregation distortion that favours GC over AT alleles and consequently high GC content in high-recombination regions, which was revealed by analyses of several varieties of rice (Oryza rufipogon, O. sativa subsp. japonica and O. sativa subsp. indica, O. barthii and O. meridionali) polymorphism datasets (Muyle et al., 2011). Alternatively, SCU was suggested as a determinant of codon usage and heterogeneous GC content in rice (Guo et al., 2007; Mukhopadhyay et al., 2008). An explanation has been put forward that GC heterogeneity could be related to the regulation of gene expression, because the local GC content is associated with the distribution of genes and chromosomal architecture (reviewed by Mugal et al., 2015). However, SCU cannot explain GC heterogeneity in non-coding regions found in rice (Muyle et al., 2011). With more genome sequences being available for monocot species, gBGC appears to be the more suitable hypothesis to explain the evolutionary forces determining overall GC heterogeneity and codon usage in monocots (Muyle et al., 2011; Serres-Giardi et al., 2012; Camiolo et al., 2015; Clément et al., 2017).
In addition to studies of codon usage in the nuclear genome, a few studies report codon usages in monocot chloroplast genomes. Codon usage bias in the chloroplast genomes of the grass monocots Brachypodium distachyon, Triticum aestivum and Hordeum vulgare showed optimal codons to mainly have A/T in the third codon position, unlike their respective codons in nuclear genomes (Sablok et al., 2011; Zhang et al., 2012). The major underlying force was proposed to be MB with the additional influence of natural selection, hydrophobicity, aromaticity and gene length.
Very little information is available on codon usage of non-grass monocots, and most of the reports relate to chloroplast genomes, rather than nuclear-encoded gene sequences, which mainly focused on preferred codon in third codon position and preferred amino acid using small datasets (Table 3). These studies cannot capture the full range of variability in this group and thus may affect the reliability of the findings for making general conclusions. So far in non-grass monocot species, a detailed study analysed codon usage and quantified the magnitude of evolutionary forces including oil palm (Elaeis guineensis) and banana (Musa acuminata) (Clément et al., 2017). The analysis showed that nucleotide composition of the non-grass monocots is far from mutation-drift equilibrium and that gBGC is a more widespread and stronger process than selection (Clément et al., 2017).
Table 3.
Summary of codons usage studies in non-grass monocots
| Parameters | Orchidaceae | Arecaceae | Arecaceae | Liliaceae | Musaceae | Amaryllidaceae | |||
|---|---|---|---|---|---|---|---|---|---|
| Species | Phalaenopsis aphrodite | Oncidium Gower Ramsey | Phoenix dactylifera | Elaeis guineensis | Colchicum autumnale | Gloriosa superba | Musa acuminata | Allium cepa | |
| Genome type | Chloroplast | Chloroplast | Chloroplast | Nuclear | Chloroplast | Chloroplast | Nuclear | Chloroplast | 43.50 % |
| GC content (%) | 37.00 | 48–50.8 | |||||||
| AT content (%) | 62.68 | ||||||||
| Codon preference at 3rd codon position | A or T | A or T | A or T | G or C | A or T | G or C | |||
| Main factor for shaping codon usage | Mutational bias | GC-biased gene conversion | GC-biased gene conversion | ||||||
| Total codons representing all the protein- coding genes | 22 950 | 22 950 | |||||||
| Most preferred stop codon | TGA | ||||||||
| Most frequent amino acids | Isoleucine | Isoleucine | Isoleucine | Isoleucine, leucine | |||||
| Least frequent amino acids | Cysteine | Cysteine | |||||||
| Reference(s) | Chang et al. (2006) | Xu et al. (2011) | Yang et al. (2010) | Jouannic et al. (2005), Nakamura et al. (2000), Low et al. (2008), Ho et al. (2007), Clément et al. (2017) | Nguyen et al. (2015) | Nguyen et al. (2015) | Clément et al. (2017) | D’Hont et al. (2012), Martin et al. (2013) | Kuhl et al. (2004) |
Motivated by the lack of comprehensive codon usage information among non-grass monocot genomes and aided by the growth of transcriptome data in publicly available databases, we investigated the patterns of codon usage within members of the non-grass monocots by analysing coding sequences (CDS) of the two major banana species, Musa acuminata (A genome) (Martin et al., 2016) and Musa balbisiana (B genome) (Davey et al., 2013), the date palm, Phoenix dactylifera (Al-Dous et al., 2011), and the small aquatic plant, duckweed, Spirodela polyrhiza (Wang et al., 2014) (Fig. 1). In addition, we used leaf transcript data for the moss bamboo, Phyllostachys heterocycla var. pubescens, as the representative grass monocot outgroup (Peng et al., 2013).
MATERIALS AND METHODS
Sequence data
To compare codon usage metrics between equivalent CDS data for each species, we selected transcriptome datasets with similar sequencing depth and from equivalent tissues (leaf was the most available across species) and for species with completed and available genome sequences. From their respective genome sequence databases, CDS of the two major banana species, Musa acuminata (A genome) (Martin et al., 2016; http://banana-genome-hub.southgreen.fr/download) and Musa balbisiana (B genome) (Davey et al., 2013; http://banana-genome-hub.southgreen.fr/organism/Musa/balbisiana), the date palm, Pho. dactylifera (Al-Dous et al., 2011; https://qatar-weill.cornell.edu/research-labs-and-programs/date-palm-research-program/date-palm-draft-sequence), and the small aquatic plant, duckweed, Spirodela polyrhiza (Wang et al., 2014; https://www.waksman.rutgers.edu/spirodela/genome), were retrieved. In addition, the CDS of the moss bamboo, Phy. heterocycla var. pubescens, was used as the representative grass monocot outgroup (Peng et al., 2013; http://202.127.18.221/bamboo/down.php). All the CDS used in the analyses were derived from young non-treated leaves. After predicting open reading frames (ORFs) using Transdecoder v3.01 (Haas and Papanicolaou, 2013), a total set of 41 390 CDS from M. acuminata, 24 250 CDS from M. balbisiana, 11 932 CDS from Pho. dactylifera, 15 151 CDS from S. polyrhiza and 27 420 CDS from P. heterocycle was obtained, which were used for further analysis using Anaconda 2 software (a software package developed by the University of Aveiro Bioinformatics research group, Portugal) (Moura et al., 2005). CDS shorter than 300 base pairs were excluded from the analysis. For each transcriptome, the sequences with more than one stop codon, lacking a start or stop codon, lacking complete reading frames and/or with undetermined nucleotides (N) were discarded by using filters in the Anaconda 2 software (Moura et al., 2005). Hypothetical and duplicated genes were identified using BLAST and were discarded from the datasets used for codon analysis.
To perform correlation analysis between GC content of CDS with genome sequences for each species, BLASTn of CDS was performed against the genomes of M. acuminata (http://banana-genome-hub.southgreen.fr/download), M. balbisiana (http://banana-genome-hub.southgreen.fr/organism/Musa/balbisiana) and Pho. dactylifera (https://qatar-weill.cornell.edu/research-labs-and-programs/date-palm-research-program/date-palm-draft-sequence). Matches with an e-value <10e-5 and at least 2 kb flanking region both 5′ and 3′ to the CDS were extracted as gene sequences, resulting in non-redundant sets of 2282 genes from M. acuminata, 9742 genes from M. balbisiana and 5701 genes from Pho. dactylifera. The GC content (%) of genes [along with untranslated region (UTR)] and of flanking regions was calculated separately.
Codon usage analyses
RSCU values were estimated using the following formula from Sharp and Li (1987) using Anaconda 2 software (Moura et al., 2005):
where gij is the observed number of the ith codon for the jth amino acid, which has ni kinds of synonymous codons.
The RSCU values obtained from the analysis are listed in conventional amino acid codon order and highlighted on a scale from green (indicating the most rarely used codons) to red (indicating the most frequently used codons). An RSCU value of 1.0 indicates no codon usage bias while values above or below 1.0 indicate codons that are utilized less or more frequently (preferred) than expected.
CAI values were obtained using the Codon W program (version 1.4.2; http://codonw.sourceforge.net). The genes falling within the top and bottom 5 % of CAI values were considered as the high and low datasets. RSCU values of these two datasets were compared using the chi-square contingency test. Those codons whose usage frequency was significantly higher (P < 0.01) in highly expressed genes compared to those genes with low levels of expression were identified as optimal codons (Sablok et al., 2011).
The nucleotide compositions of the third codon position in the full set of ORFs (A3, U3, C3 and G3) were calculated for each species using the Codon W program. The parity rule 2 (PR2) bias was detected based on AU bias [A3/(A3+U3)] as the y-axis and GC-bias [G3/(G3+C3)] as the x-axis at the third codon position of the two- and four-codon amino acids of entire CDS (Sueoka, 1995).
GC content of the genome, transcriptome and the GC frequency in the three codon positions of individual coding sequences (GC1, GC2 and GC3) were measured for all species using Anaconda 2 software (Moura et al., 2005), and GC12 was calculated as the average of GC1 and GC2.
Frequency distributions of GC3 (all CDS with start and stop codon) were plotted according to the method suggested by Carels and Bernardi (2000). Neutrality plots of GC12 against GC3 were analysed based on the method suggested by Sueoka (1988).
Effective numbers of codons (ENC) were calculated by following the formula of Wright (1990), using Anaconda 2 software (Moura et al., 2005):
where the value denotes the probability that two randomly chosen codons for an amino acid with two codons are identical.
ENC values were plotted against GC3 values. The expected ENC values were calculated using the following formula:
where S is the frequency of GC3s (Wright, 1990). To estimate the difference between the observed and the expected ENC values for all CDS, frequency distributions of (ENCexp − ENCobs)/ENCexp were plotted. A paired Student’s t-test (P < 0.001) was performed to test for significance using SPSS 16.0 (http://www.spss.com/).
COA (Greenacre, 1984) was performed with CodonW using the RSCU values to compare the intra-genomic variation of 59 informative codons, partitioned along 59 orthogonal axes with 41 degrees of freedom. Correlation analyses (Pearson correlation, two-tailed) was performed for GC content and codon usage indices with SPSS 16.0 (http://www.spss.com/).
Codon-pair context analysis
Codon-pair context quantifications of the transcriptome datasets were performed using Anaconda 2 (Moura et al., 2005). The Anaconda 2 software package uses transcriptome data to generate a frequency table consisting of codon-pair contexts using a ribosome simulation algorithm. The resulting data are interrogated via a chi-square test. The results are then transferred to a visualization module to generate a codon-pair context map.
RESULTS
Preferred, optimal and rare codons in non-grass monocots
To analyse the codon usage patterns in non-grass monocots, RSCU for M. acuminata, M. balbisiana, Pho. dactylifera, S. polyrhiza and Phy. heterocycla was calculated (Supplementary Data Table S1). M. acuminata and M. balbisiana showed an approximately equal number of preferred AT- and GC-ending codons (Table 4). By contrast, S. polyrhiza and Phy. heterocycla showed higher GC-ending codon as preferred codons. In addition, non-grass monocot species showed avoidance of XUA codons [AUA (ILE), UUA (LEU), CUA (LEU) and GUA (VAL)] in all species (Supplementary Data Table S1). This avoidance may be related to the use of UA in stop codons and/or the presence of UA-selective ribonucleases, as suggested by Beulter et al. (1989). All optimal codons for the studied species showed G/C at the third codon position (shown as ‘*’ in Table 4; Supplementary Data Table S2A–E). These codons are optimal for highly expressed genes (based on CAI) and do not reflect the corresponding tRNA pool. The most preferred stop codon for M. acuminata, M. balbisiana andPhy. heterocycla was UGA, whereas Pho. dactylifera and S. polyrhiza preferred UGA and UAG. Furthermore, rare codons (RSCU < 0.10) in non-grass monocots showed A/U at the third codon position similar to Phy. heterocycla (shown as ‘–‘ in Table 4; Supplementary Data Table S2A–E).
Table 4.
Preferred codons, optimal codons and rare codons in non-grass monocots
| Codon type | Codon 3rd base | M. acuminata | M. balbisiana | Pho. dactylifera | S. polyrhiza | Phy. heterocycla |
|---|---|---|---|---|---|---|
| RSCU > 1 | G/C/A/U | 34 | 33 | 30 | 29 | 33 |
| G/C | 16 | 17 | 12 | 26 | 21 | |
| A/U | 18 | 16 | 18 | 3 | 12 | |
| A | 6 | 6 | 6 | 1 | 4 | |
| U | 12 | 10 | 12 | 2 | 8 | |
| G | 8 | 7 | 7 | 11 | 8 | |
| C | 8 | 10 | 5 | 15 | 13 | |
| Optimal | G/C/A/U | 27 | 27 | 27 | 27 | 27 |
| G/C | 27 | 27 | 27 | 27 | 27 | |
| A/U | 0 | 0 | 0 | 0 | 1 | |
| A | 0 | 1 | 0 | 1 | 1 | |
| U | 0 | 0 | 0 | 0 | 0 | |
| G | 11 | 11 | 10 | 11 | 11 | |
| C | 16 | 16 | 16 | 16 | 16 | |
| Rare codon | G/C/A/U | 1 | 1 | 0 | 3 | 4 |
| U | 0 | 0 | 0 | 1 | 3 | |
| A | 1 | 1 | 0 | 2 | 1 |
CG dinucleotide suppression
Analysis of the XCG/XCC ratio based on RSCU values from the present analysis in non-grass monocot species showed values of 0.62 (M. acuminata), 0.64 (M. balbisiana), 0.57 (Pho. dactylifera) and 0.69 (S. polyrhiza) (Supplementary Data Table S1). This indicates moderate CG dinucleotide suppression in non-grass monocots. Compared to established non-grass species, Phy. heterocycla exhibited a higher XCG/XCC ratio, 0.82.
PR2-bias plot analysis
PR2-bias plot analysis was carried out to investigate the effect of mutation and selection pressure on codon usage bias. If only mutational bias shapes the codon usage bias, then G and C (A and U) should be used proportionally among the two- and four-fold codon amino acids. On the other hand, if natural selection dominates, it would not necessarily cause proportional use of G and C (A and U) (Wright, 1990; Sueoka, 1995). The results showed that G and C (A and U) were not used proportionally in non-grass monocot genomes (Supplementary Data Fig. S1A–D) as was also observed for Phy. heterocycla (Supplementary Data Fig. S1E), which indicates mutational force is not the major force in determining codon usage in monocots.
GC pattern in non-grass monocots
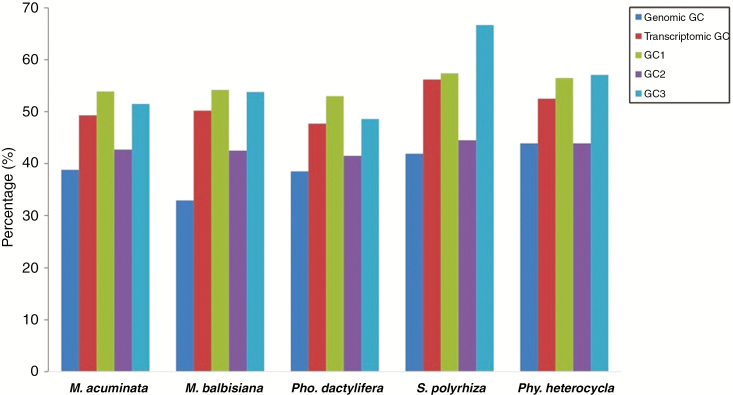
Total GC content of the genome and transcriptome and the GC (pair) content in the three codon positions of coding sequences were examined for the four non-grass species and grass monocot Phy. heterocycla (Fig. 2). A higher genomic GC content was observed in Phy. heterocycla (43.9 %) compared to the non-grass monocots. Among the non-grass monocots, S. polyrhiza showed a higher genomic GC value (41.96 %) and transcriptomic GC content (56.16 %) compared to other species. Total GC content analysis of non-grass monocots revealed a higher transcriptomic GC compared to genomic GC in all species. Furthermore, the non-grass monocot species M. acuminata, M. balbisiana and Pho. dactylifera demonstrated their highest GC contents at the first codon position, followed by GC in the third and the second codon positions (GC1 > GC3 > GC2) (Fig. 2). Analysis of the pattern of GC distribution in the grass monocot used in this study (Phy. heterocycla) showed that it shared a similar pattern for GC content in the three codon positions (GC1 > GC3 > GC2) as previously reported in grasses (Wong et al., 2002), but this was not the case for most of the non-grass monocots; three of the four non-grass monocots in our study shared a pattern of GC1 > GC3 > GC2 (Fig. 2) as previously reported for other non-grass monocots onion (Kuhl et al., 2004) and Lycoris longituba (Cui et al., 2004) as well as for the dicot Arabidopsis (Kuhl et al., 2004).
Fig. 2.
Variation of GC content in M. acuminata, M. balbisiana, Pho. dactylifera, S. polyrhiza and Phy. heterocycla
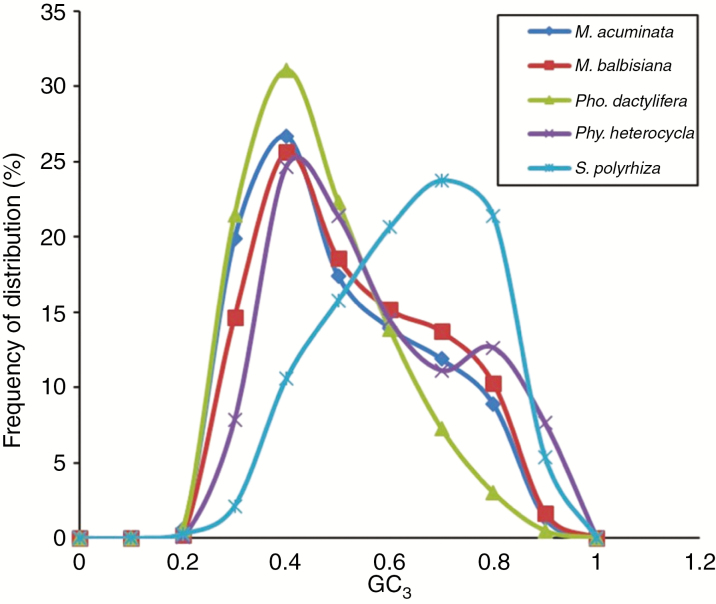
Variation of GC3 was further analysed in monocots (Fig. 3), which revealed a unimodal distribution of GC3 for all studied non-grass monocot species and bimodal distribution for the grass monocot Phy. heterocycla. Compared to M. acuminata and M. balbisiana, Pho. dactylifera and S. polyrhiza showed a clear unimodal distribution.
Fig. 3.
Distributions of GC3 content in M. acuminata, M. balbisiana, Pho. dactylifera, S. polyrhiza and Phy. heterocycla
Association between ENC and GC3 in non-grass monocots
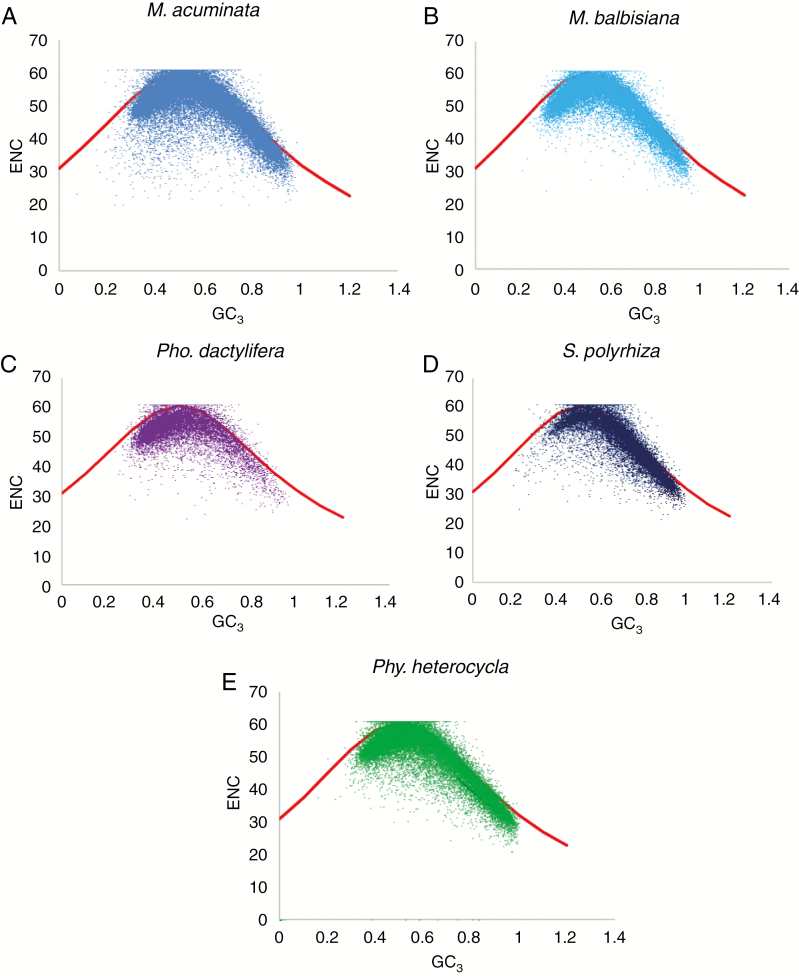
The influence of GC3 content on the codon usage of non-grass monocots was further examined by calculating the ENC (Wright, 1990) for each gene and plotting this against the GC3 composition of each individual gene (Fig. 4A–D). The CDS of non-grass monocot species showed a wide distribution of GC3 from 20 to 80 % (0.2–0.8 in Fig. 4A–D), similar to Phy. heterocycla (Fig. 4E).
Fig. 4.
Relationship between GC3 and effective numbers of codon (ENC plot) for M. acuminata, M. balbisiana, Pho. dactylifera, S. polyrhiza, and Phy. heterocycla. ENC values were plotted against GC3 values following the method of Wright (1990). The continuous curve depicts the expected curve between ENCs and GC3. (A) M. acuminata, (B) M. balbisiana, (C) Pho. dactylifera, (D) S. polyrhiza and (E) Phy. heterocycla.
Analysis of the ENC values of individual genes within each of the non-grass monocot species showed variability of bias, with values of 20.00–61.00 for M. acuminata, 23.26–61.00 for M. balbisiana, 22.38–61.00 for Pho. dactylifera and 21.78–61.00 for S. polyrhiza. This indicates the presence of genes with a high bias to no bias within non-grass monocot genomes, as was also observed for the grass monocot Phy. heterocycla (20.86–61.00). ENC values were plotted against GC3 to determine whether the difference in ENC is related to the difference in GC3 content (Fig. 4). In general, the ENC plot is a parabolic curve in which genes are represented as points and where the expected curve shows the positions of genes under no selection that are only subject to GC3 codon compositional constraint (Wright, 1990). Such comparison of the actual distribution of genes with expected distribution explains the presence of an influence of forces other than compositional constraint (Wright, 1990). The ENC plot of all the species under study showed, although some genes lay on the expected curve, large numbers of CDS with low ENC values lying below the expected curve. The presence of points far below the curve indicates these genes have additional codon usage bias that is independent of GC3s (Wright, 1990).
A significant negative correlation of GC3 with ENC values was observed for non-grass monocot species. This negative correlation indicates that genes with higher GC3 values and lower ENC values had strong codon usage bias. Liu and Xue (2005) also reported a similar negative correlation of GC3 with ENC values of CDS in four grass monocot species (rice, maize, barley and wheat). To estimate the difference between observed and expected ENC value of CDS we calculated (ENCexp − ENCobs)/ENCexp ratio (Supplementary Data Fig. S2A–E). Comparison of observed ENCs with expected ENC using paired Student’s t-tests (P < 0.001) revealed significant differences.
COA and correlations between codon usage, nucleotide composition, CAI, ENC, hydrophobicity, aromaticity and gene length in non-grass monocots
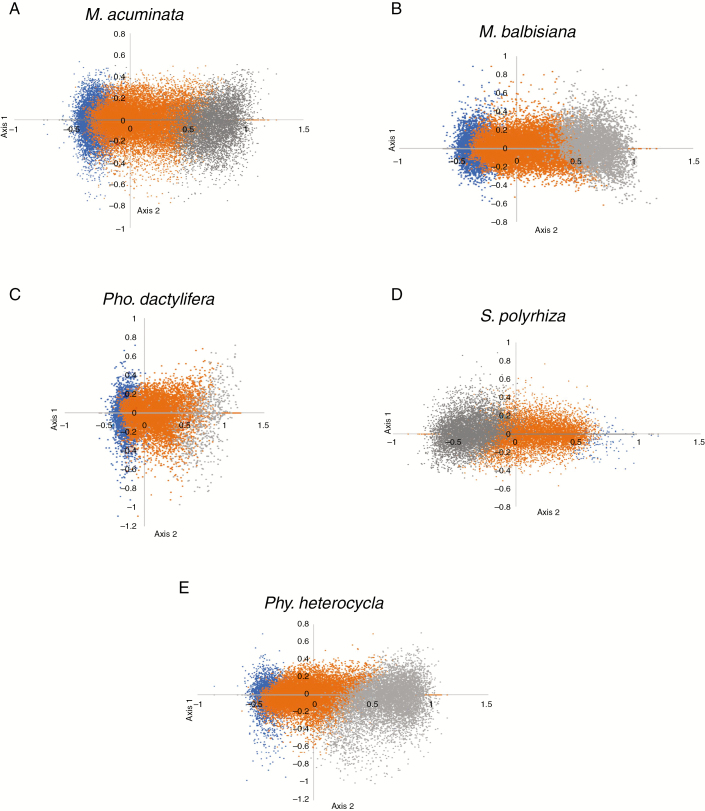
To further understand the variation in shaping codon usage bias in coding sequences of non-grass monocots, COA was performed (Fig. 5A–D). In the COA, a series of orthogonal axes were generated that reflect the trends of variation in codon usage bias based on RSCU values of the CDS (Greenacre, 1984). The distance between CDS on this plot is a reflection of their dissimilarity in RSCU with respect to the two axes. The first main dimensional coordinates, axis 1, explained 36.87 % (M. acuminata), 38.67 % (M. balbisiana), 23.28 % (Pho. dactylifera) and 36.84 % (S. polyrhiza) of overall codon usage variation in the studied non-grass monocot species. Axis 2 accounted for 4.72 % (M. acuminata), 4.53 % (M. balbisiana), 5.64 % (Pho. dactylifera) and 4.73 % (S. polyrhiza). The remaining axes 3 and 4 each represented even smaller amounts of the variance. Axis 3 showed 3.99 % (M. acuminata), 3.45 % (M. balbisiana), 4.34 % (Pho. dactylifera) and 3.66 % (S. polyrhiza) of the variation, and axis 4 3.14 % (M. acuminata), 3.19 % (M. balbisiana), 3.88 % (Pho. dactylifera) and 2.68 % (S. polyrhiza). The grass monocot (Phy. heterocycla) also showed a similar trend (axis 1: 37.99 %, axis 2: 4.67 %, axis 3: 3.50 %, axis 4: 3.09 %) (Fig. 5E). Thus, the first axis reflects the major factor that explains the differences in codon usage among studied species. To elucidate the effects of the GC content on codon usage bias, CDS with different GC contents were labelled with different colours. Genes with GC of 60 % were plotted in grey, while genes with a GC less than 45 % were plotted in blue (Fig. 5). Orange dots indicate genes with a GC content between 45 and 60 %. The plot showed a clear separation of high- and low-GC genes along the primary axis.
Fig. 5.
Correspondence analysis of coding sequences in (A) M. acuminata, (B) M. balbisiana, (C) Pho. dactylifera, (D) S. polyrhiza and (E) Phy. heterocycla. Grey: genes with GC of 60 %; blue: genes with GC < 45 %; orange: genes with GC between 45 and 60 %.
Although Fig. 5 presents a general relationship between the nucleotide content of genes and their position on the first axis of the COA, it does not give a statistical measure of this relationship. To do this, the correlation between the GC content, codon usage indices of individual CDS and their location on the primary axis of the COA was measured (Table 5). Axis 1 showed significant correlations between GC content and position on the first axis (Table 5). The gene positions on axis 1 showed strong and significant correlation with GC3 value. ENC values of M. acuminata, M. balbisiana and Pho. dactylifera showed a significant negative correlation, whereas S. polyrhiza showed a positive correlation with ENC (Table 5). In addition, correlation analysis between ENC and GC3 showed ENC values were negatively correlated with GC3 values for all species. Strong correlation between axis 1 and CAI and between GC3 and CAI was observed. A positive correlation between hydropathy and axis 1 was found for M. acuminate, M. balbisiana, Pho. dactylifera and Phy. heterocycla, but a negative correlation for S. polyrhiza. A weak negative correlation was observed for aromaticity and axis 1 for M. acuminate, M. balbisiana and Phy. heterocycla. A strong positive correlation was found between GC3 and GC content in the other two codon positions (GC1 and GC2) for all species. Gene length showed a negative correlation with axis 1 and a negative correlation with GC3 in all studied monocot species.
Table 5.
Correlation of codon usage, nucleotide composition, ENC, CAI, hydropathy, aromaticity and gene length in non-grass monocots
| Species | Parameters | Total GC | GC1 | GC2 | GC3 | ENC | CAI | Hydropathy | Aromaticity | Gene length |
|---|---|---|---|---|---|---|---|---|---|---|
| M. acuminata | Axis 1 | 0.966** | 0.606** | 0.484** | 0.988** | −0.625** | 0.973** | 0.173** | −0.006 | −0.329** |
| GC3 | 0.937** | 0.544** | 0.384** | −0.615*** | 0.973** | −0.332** | ||||
| GC12 | 0.562** | −0.323** | ||||||||
| Total GC | ||||||||||
| M. balbisiana | Axis1 | 0.970** | 0.634** | 0.527** | 0.990** | −0.673** | 0.976** | 0.173** | −0.013* | −0.302** |
| GC3 | 0.944** | 0.575** | 0.452** | −0.665** | 0.979** | −.308** | ||||
| Total GC | −0.302** | |||||||||
| GC12 | 0.604** | |||||||||
| Pho. dactylifera | Axis1 | 0.928** | 0.424** | 0.328** | .980** | −0.334** | 0.966** | 0.112** | 0.021* | −0.262** |
| GC3 | 0.892** | 0.351** | 0.235** | −0.341** | 0.960** | −0.066** | ||||
| Total GC | −0.249** | |||||||||
| GC12 | 0.3655** | |||||||||
| S. polyrhiza | Axis1 | −0.953** | −0.588** | −0.483** | −0.992** | 0.842** | 0.976** | −0.169** | 0.006 | −0.214** |
| GC3 | 0.922** | 0.534** | 0.406** | −0.840** | 0.973** | −0.213** | ||||
| Total GC | −0.218** | |||||||||
| GC12 | 0.550** | |||||||||
| Phy. heterocycla | Axis1 | 0.958** | 0.661** | 0.509** | 0.989** | −0.813** | 0.980** | 0.180** | −0.059** | −0.334** |
| GC3 | 0.931** | 0.603 ** | 0.435** | −0.808** | 0.976** | −0.332** | ||||
| Total GC | −0.353** | |||||||||
| GC12 | 0.592** |
* Correlation is significant at the 0.05 level; ** correlation is significant at the 0.01 level.
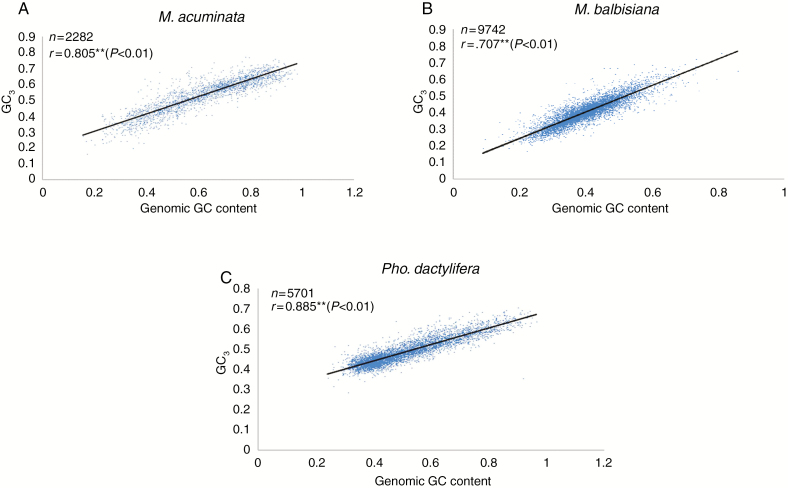
The relationship between coding sequence GC3 content and genomic GC content was further examined in three non-grass monocot species, M. acuminata, M. balbisiana and Pho. dactylifera. S. polyrhiza and Phy. heterocycla were excluded from the analysis due to a lack of suitable data. A strong and highly significant positive correlation was observed between coding GC3 content and genomic GC content (Fig. 6) for all three species. However, correlation analysis between GC3 and flanking GC content showed significant but low correlation coefficients for M. acuminata (r = 0.065, P < 0.1), M. balbisiana (r = 0.063, P < 0.1) and Pho. dactylifera (r = 0.013, P < 0.1).
Fig. 6.
Correlation analysis between GC content in third codon position and genomic GC content for (A) M. acuminata, (B) M. balbisiana and (C) Pho. dactylifera.
Variation in codon-pair context in non-grass monocots
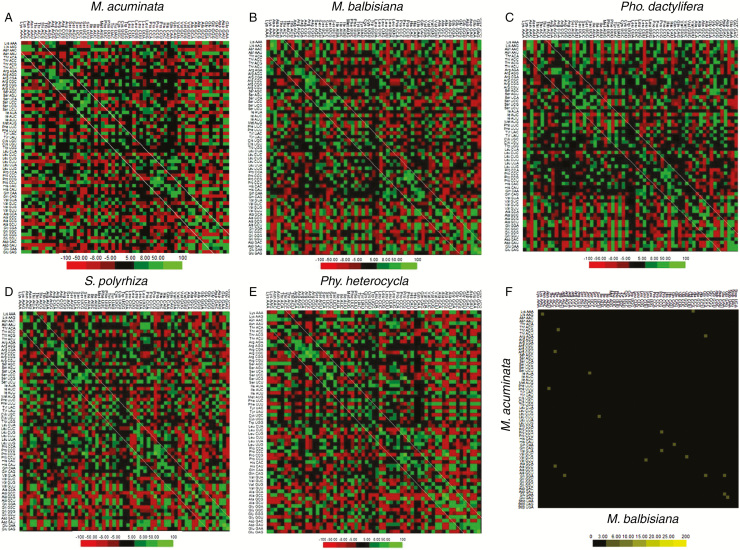
Codon-pair context maps generated for M. acuminata, M. balbisiana, Pho. dactylifera, S. polyrhiza and Phy. heterocycla transcriptomes (Fig. 7A–E) revealed variations and similarities among these non-grass monocots. The region highlighted by two parallel diagonal lines for each dataset in Fig. 7 represents the codon-pair contexts for the codons with identical or similar sequences. The codon pairs that fall within the diagonal area indicate a degree of tandem codon duplications; a characteristic of codons in eukaryotic genomes is an identical codon on their 3′ side (Moura et al., 2005). In the present monocot genome study, codon-pair context sequences GAG-GAG, AAG-AAG and GAU-GAU were observed to be the most frequently used (Supplementary Data Table S3). The homogeneous codon-pair contexts NNN-GAA, NNN-AAG and NNN-GAG were also observed to be frequently used codon-pairs for all species examined in this study (Supplementary Data Table S3).
Fig. 7.
Codon-pair context patterns for (A) M. acuminata, (B) M. balbisiana, (C) Pho. dactylifera, (D) S. polyrhiza and (E) Phy. heterocycla. Green represents preferred codon-pair contexts and red represents rejected codon-pair contexts. Values that are not statistically significant are represented in black. The regions between the two parallel diagonal lines represent the codon-pair contexts that are more frequently used compared to other contexts in the species under study. (F) Differential display map for comparative analysis of codon-pair context between M. acuminata and M. balbisiana. A colour scale based on gradation of yellow was used for the differential display. Common features are indicated in black and the differences are represented in yellow.
Condon-pair context results indicate highest similarity between M. acuminata and M. balbisiana among all of the non-grass monocot species analysed. To identify the differences in preferred codon-pair context between the two Musa species, a differential codon-pair context map (DCM) was constructed by overlapping the complete codon-pair context maps (Fig. 7F). A colour scale based on gradation of yellow was used for the differential display. Common features are indicated in black and significant differences are represented in yellow. Despite the two species being closely related, they showed significant differences.
DISCUSSION
The increase in publicly available large transcriptome datasets for non-grass monocot species has presented an opportunity for determining codon usage and codon bias in this important but so far neglected group of plants. However, the sourcing of materials for transcriptomic analysis from different tissues, which furthermore may have been exposed to different conditions, results in the creation of datasets that are not equivalent and therefore not optimal for comparison. In the present study, four non-grass monocots (M. acuminata, M. balbisiana, Pho. dactylifera, S. polyrhiza) and one grass monocot (Phy. heterocycla) were selected, for which comparable datasets were publicly available: The transcriptome datasets used in the present study were constructed from young non-treated leaves for each species and represented similar relative genome coverage, to provide as unbiased a comparison as was possible, which we believe adds value to our analysis. Whether the data were able to support each of the three current hypothesis, MB, SCU and gBGC, for the mechanisms which determine codon usage bias in non-grass monocots was also tested. Analysis was made of codon usage, optimal and rare codons, GC content, GC3 distribution, codon-pair context patterns and shaping factors in four non-grass monocots and one grass monocot genome.
To place the results in the context of previous analysis of codon usage in plants, the literature was reviewed, with a focus on monocot species. There is a consensus that GC content, which varies widely across plant genomes, is one of the most important factors in determining codon usage in a genome. The codon usage bias corresponds to the AT- or GC-rich content of a genome, while the third nucleotide position of a codon reflects genome base composition of an organism (Wu et al., 2015). The overall GC contents reported in monocot groups (55–59 %) are much higher than for dicot species (42–44 %) (Kawabe and Miyashita, 2003; Garg et al., 2011; Singh et al., 2016). GC values among non-grass monocot genomes (47–56 %; Fig. 2) were found overall to be higher than those reported for dicot species, the variation within this group probably reflecting differences in mating systems between species.
While previous reports have suggested that the high GC content of monocot genomes is a consequence of MB within monocot genomes (Wang and Roossinck, 2006; Wang and Hickey, 2007), the MB hypothesis appears not to explain nucleotide composition or codon usage of non-grass monocots. According to the MB hypothesis, high GC content is a consequence of a positive feedback loop to reduce cytosine deamination and favouring GC enrichment in monocots (Fryxell and Zuckerkandl, 2000). However, to the best of our knowledge, all mutations reported in eukaryotic species show an AT bias (Dillon et al., 2015), including rice (Muyle et al., 2011) and the non-grass monocots banana and oil palm (Clément et al., 2017). Similarly, mutations of methylated cytosine, a cause of GC enrichment in grass monocots (Ossowski et al., 2010; Alonso et al., 2015), was also found to be unlikely as methylation tends to increase the mutation bias toward AT (as methylated CpGs are usually highly mutable toward TpG) (Nachman and Crowell, 2000). Furthermore, methylation levels reported in monocots including rice and secale (Kalinka et al., 2017) are much higher compared to the dicot species poplar and Arabidopsis (Feng et al., 2010). Therefore, if MB was the major evolutionary force in shaping codon usage in monocots, one would expect monocots to have a lower GC content than dicots, which is not the case. Finally, as all classes of mutations have an equal probability of fixation, a proportionate usage between G and C and between A and U would be expected. However, analysis of the rice genome showed disproportionate usage between G and C and between A and U in the third codon position and higher probability of fixation of GC over AT bases (Muyle et al., 2011). Analysis of polymorphism data in banana and oil palm has also shown similar results (Clément et al., 2017). A similar disproportionate usage between G and C and between A and U in the third codon position in non-grass monocots was observed here (Supplementary Data Fig. S1). Hence, together with the present data, the MB hypothesis appears not to be suitable to explain nucleotide composition and codon usage in non-grass monocots. Further availability of polymorphism data on non-grass monocots will be required to completely rule out MB as the major influence in shaping nucleotide composition in non-grass monocots.
Whether SCU can better explain the data was next examined, and it was found that while some criteria can fit the model, these could also result from gBGC and that SCU is also unable to explain the GC3 heterogeneity seen in non-grass monocots. According to SCU, high GC content is the consequence of selective pressures to ensure translational efficiency of genes, which is supported by the fact that most of the monocot species have more GC-ending codons in optimal codons (Muyle et al., 2011). In the presemt data analysis, a strong correlation between GC3 we observed content and codon usage variation was observed, and between GC3 content and CAI (predicts the level of expression of a gene) values (Fig. 5A–D; Table 5). In addition, all optimal codons were found to have GC in the third codon position (Supplementary Data Table S2), which appears to be an influence of SCU (Table 5). However, the optimal codons presented in the current study show a bias for highly expressed genes (based on CAI) and do not reflect the corresponding tRNA pool (Supplementary Data Table S2). Hence, this cannot be concluded to be indicative of SCU. Beside this, our analysis on GC3 distribution between CDS of non-grass monocot species showed a remarkable GC content heterogeneity, with values ranging from 20 to 80 % (Figs 3 and 4A–D), contrary to the narrower distribution of GC3 (20–60 %) reported in dicot species (Kawabe and Miyashita, 2003; Mukhopadhyay et al., 2008) and not compatible with the SCU hypothesis. The heterogenic genome composition observed in non-grass monocots is similar to the genome organization reported in mammals (reviewed by Duret and Galtier, 2009) and the grass monocots rice, maize, barley and wheat (Kawabe and Miyashita, 2003; Tatarinova et al., 2010; Šmarda and Bureš, 2012).
Overall, we found the gBGC hypothesis, where high GC content is the result of bias of GC over AT bases during mismatch repair during meiotic recombination (reviewed by Duret and Galtier, 2009), to best explain the codon usage in non-grass monocots. Experimental evidence of gBGC has been found in the grass monocots Secale cereal, Aegilops speltoides, Triticum urartu, Triticum monococcu (Haudry et al., 2008), rice (Muyle et al., 2011) and maize (Rodgers-Melnick et al., 2016) based on recombination data of effective populations, which showed a strong correlation between recombination rate and GC content of those genomes. The heterogeneous GC3 distribution in monocots, which is incongruous with the SCU hypothesis, can easily be explained as a consequence of gBGC. An initial study on grass genomes explored the presence of a strong negative 5′–3′ GC content gradient along genes (Wong et al., 2002). This strong 5′–3′ GC decreasing pattern was also reported for both exonic and intronic regions of genes (Zhu et al., 2009) and was suggested to be a consequence of recombination hotspots around transcription start sites (Hellsten et al., 2013). Over time, this has resulted in genes which range from short mono-exonic and GC-rich to those that are longer and relatively GC-poor (Ressayre et al., 2015). Hence, grass monocots with a strong recombination and gBGC gradient clearly show a bimodal distribution, representing short and long genes (Glémin et al., 2014). By contrast, in non-grass monocots even where the gene composition is similar, a weaker gradient results in a unimodal distribution (Clément et al., 2015). Our analysis on GC3 distribution also showed a clear bimodal distribution for the grass species (Phy. heterocycla) and unimodal distribution in non-grass monocots (Fig. 3), in agreement with the gBGC hypothesis. Among non-grass monocots, there is still a lack of direct evidence of gBGC due to the unavailability of recombination data. Nevertheless, Clément et al. (2017) quantified the magnitude of gBGC based on correlative approaches between neutrality and selection indices to disentangle the processes of SCU and gBGC in several plant species, including the non-grass monocots banana and oil palm, finding a stronger intensity of gBGC in shaping nucleotide composition and codon usage in non-grass monocots. They proposed that comparisons of GC content in coding regions with introns or non-coding regions would be helpful to confirm these findings. The present data analysis showed a strong positive correlation between GC3 and genomic GC content in the three studied non-grass monocot species (Fig. 6). In addition, low but significant correlation coefficients between GC3 and flanking GC content were found, similar to that reported for other grass monocots including maize, rice, sorghum (Tatarinova et al., 2010; Glémin et al., 2014) and the non-grass monocot banana (Glémin et al., 2014) in which the GC content is mainly shaped by gBGC. These results demonstrate that the GC bias we report in non-grass monocots is not restricted to third-codon positions but affects surrounding sites as well. Furthermore, consistent with an influence of gBGC, GC-rich CDS were shorter (Table 5), similar to that reported for the grass monocots rice (Wang and Hickey, 2007, Muyle et al., 2011) and maize (Sundararajan et al., 2016).
Taken together, the analyses of non-grass monocots indicate that gBGC is the main driving force in this group of plants. Quantification of differences in the occurrence, intensity and patterns of gBGC could explain the variations at all positions across non-grass monocot genomes. Further availability of recombination data and genome data well-annotated for coding and non-coding sequences will help to elucidate the impact of gBGC in non-grass monocots.
Apart from codon usage, codon-pair context also has a significant impact on the translational selection of genes (Moura et al., 2007) due to its crucial role in suppressing mutations (Kopelowitz et al., 1992; Irwin et al., 1995). Prior to the current study, there were no reports on codon-pair contexts in monocots so here we can report that, as for other phyla, significant differences in codon-pair context patterns were found between two Musa species (M. acuminata and M. balbisiana) (Fig. 7F). The data also support the view of Moura et al. (2007) that codon-pair context maps are species-specific and can be used as a fingerprint for a specific species. Among the codon-pair contexts for the non-grass monocots, a preference was observed for homogeneous codon-pairs (Fig. 7A–D, Supplementary Data Table S3). There are no previous reports on homogeneous codon pairs other than in tea and mustard (Paul and Chakraborty, 2016) and insects (Diptera and Hymenoptera) (Behura and Severson, 2012), but this may be worth further investigation as the use of homogeneous codon pairs has been proposed as a tactic to save energy during translation: the energy required to carry out the translation of homogeneous codon-pair contexts will be lower as fewer tRNA species need to be synthesized (Moura et al., 2011). However, it has also been suggested that the continuous use of the same set of tRNAs could limit the translational speed of highly expressed proteins and narrow the amino acid variability of proteins (Irwin et al., 1995). At the current time, there are limited accessible comparable sets of gene expression data for relative analysis of tRNA and mRNA from plant genomes. The increase in plant transcriptome studies and anticipated improved databases to share assembled and annotated plant transcriptomes with associated expression data should make it feasible to provide supporting data for these hypotheses in the near future.
CONCLUSIONS
Codon usage bias is largely influenced by nucleotide composition across the genome. Knowledge about active evolutionary forces, codon usage and codon-pair context in plants is a prerequisite to deciphering the molecular mechanisms underlying survival strategies and functional adaptation of this kingdom of sessile organisms. While early studies of plant genomes led to MB and SCU being proposed as the major evolutionary forces in shaping codon usage in the plant genome, gBGC is now regarded as the prevalent and robust evolutionary process at play. Turning our attention to codon usage in non-grass monocots, our analysis of CDS based on leaf transcriptomic data for four completely sequenced transcriptomes (M. acuminata, M. balbisiana, Pho. dactylifera and S. polyrhiza) showed optimal codons of non-grass monocots have a strong preference to end in G or C and, unlike grass monocots, the non-grass monocots in our study showed a unimodal GC3 distribution. Based on our observation of a positive relationship between CAI (predicts the level of expression of a gene) and GC3 as well as a positive relationship between GC3 in codons and GC content of non-coding DNA, we conclude that codon usage and nucleotide composition in non-grass monocots are mainly influenced by gBGC. To quantify the intensity and patterns of gBGC in non-grass monocot genomes will require additional research, including comprehensive recombination and methylation maps at the gene scale. Such data can be generated either by direct sequencing of meiosis products (parents and progenies) (Yang et al., 2012) or by indirect population genomic methods (Muyle et al., 2011).
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure S1: parity rule 2-bias plot [A3/(A3+T3)] against G3/(G3+U3)] for M. acuminata, M. balbisiana, Pho. dactylifera, S. polyrhiza and Phy. heterocycla. Figure S2: frequency distribution of effective number of codons ratio for M. acuminata, M. balbisiana, Pho. dactylifera, S. polyrhiza and Phy. heterocycla. Table S1: relative synonymous codon usage values of M. acuminata, M. balbisiana, Pho. dactylifera, S. polyrhiza and Phy. heterocycla. Table S2: RSCU values of highly expressed and lowly expressed genes for M. acuminata, M. balbisiana, Pho. dactylifera, S. polyrhiza and Phy. heterocycla. Table S3: codon-pair frequency in M. acuminata, M. balbisiana, Pho. dactylifera, S. polyrhiza and Phy. heterocycla.
Supplementary Material
ACKNOWLEDGEMENTS
PM, KM and JAH are partially supported by CEBAR Research University grant (RU006-2017 and RU015-2016).
LITERATURE CITED
- Al-Dous E, George B, Al-Mahmoud ME et al. . 2011. De novo genome sequencing and comparative genomics of date palm (Phoenix dactylifera). Nature Biotechnology 29: 521–527. [DOI] [PubMed] [Google Scholar]
- Akashi H. 2003. Translational selection and yeast proteome evolution. Genetics 164: 1291–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso C, Pérez R, Bazaga P, Herrera CM. 2015. Global DNA cytosine methylation as an evolving trait: phylogenetic signal and correlated evolution with genome size in angiosperms. Frontiers in Genetics 6: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behura SK, Severson DW. 2012. Comparative analysis of codon usage bias and codon context patterns between dipteran and hymenopteran sequenced genomes. PLoS ONE 8: 43111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behura SK, Severson DW. 2013. Codon usage bias: causative factors, quantification methods and genome-wide patterns: with emphasis on insect genomes. Biological Review of Cambridge Philosophical Society 88: 49–61. [DOI] [PubMed] [Google Scholar]
- Bennetzen JL, Hall BD. 1982. Codon selection in yeast. Journal of Biological Chemistry 257: 3026–3031. [PubMed] [Google Scholar]
- Beulter E, Gelbart T, Han J, Koziol JA, Beutler B. 1989. Evolution of the genome and the genetic code: selection at the dinucleotide level by methylation and polyribonucleotide cleavage. Proceedings of the National Academy of Sciences of the United States of America 86: 192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WH, Gowri G. 1990. Codon usage in higher plants, green algae and cyanobacteria. Plant Physiology 92: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camiolo S, Melito S, Porceddu A. 2015. New insights into the interplay between codon bias determinants in plants. DNA Research 5: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carels N, Bernardi G. 2000. Two classes of genes in plants. Genetics 154: 1819–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C-C, Lin H-C, Lin I-P et al. . 2006. The chloroplast genome of Phalaenopsis aphrodite (Orchidaceae): comparative analysis of evolutionary rate with that of grasses and its phylogenetic implications. Molecular Biology and Evolution 23: 279–291. [DOI] [PubMed] [Google Scholar]
- Clément Y, Fustier MA, Nabholz B, Glémin S. 2015. The bimodal distribution of genic GC content is ancestral to monocot species. Genome Biology and Evolution 1: 336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clément Y, Sarah G, Holtz Y et al. . 2017. Evolutionary forces affecting synonymous variations in plant genomes. PLoS Genetics 13: 1006799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Zhang X, Zhou Y et al. . 2004. Identification and expression analysis of EST-based genes in the bud of Lycoris longituba. Genomics, Proteomics & Bioinformatics 2: 43–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hont A, Denoeud F, Aury JM et al. . 2012. The banana (Musa acuminata) genome and the evolution of monocotyledonous plants. Nature 488: 213–217. [DOI] [PubMed] [Google Scholar]
- Davey MW, Gudimella R, Harikrishna JA, Lee WS, Khalid N, Keulemans W. 2013. A draft Musa balbisiana genome sequence for molecular genetics in polypoid, inter- and intra-specific Musa hybrids. BMC Genomics 14: 683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duret L, Galtier N. 2009. Biased gene conversion and the evolution of mammalian genomic landscapes. Annual Review of Genomics and Human Genetics 10: 285–311. [DOI] [PubMed] [Google Scholar]
- De La Torre AR, Lin YC, Van de Peer Y, Ingvarsson PK. 2015. Genome-wide analysis reveals diverged patterns of codon bias, gene expression, and rates of sequence evolution in picea gene families. Genome Biology and Evolution 7: 1002–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon MM, Sung W, Lynch M, Cooper VS. 2015. The rate and molecular spectrum of spontaneous mutations in the GC-rich multichromosome genome of Burkholderia cenocepacia. Genetics 200: 935–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre-Walker A, Hurst LD. 2001. The evolution of isochores. Nature Reviews Genetics 2: 549–555. [DOI] [PubMed] [Google Scholar]
- Figuet E, Ballenghien M, Romiguier J, Galtier N. 2014. Biased gene conversion and GC-content evolution in the coding sequences of reptiles and vertebrates. Genome Biology and Evolution 7: 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryxell KJ, Zuckerkandl E. 2000. Cytosine deamination plays a primary role in the evolution of mammalian isochores. Molecular Biology and Evolution 17: 1371–83. [DOI] [PubMed] [Google Scholar]
- Garg R, Patel RK, Jhanwar S et al. . 2011. Gene discovery and tissue-specific transcriptome analysis in chickpea with massively parallel pyrosequencing and web resource development. Plant Physiology 156: 1661–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtier N, Duret L, Glémin S, Ranwez V. 2009. GC-biased gene conversion promotes the fixation of deleterious amino acid changes in primates. Trends in Genetics 25: 1–5. [DOI] [PubMed] [Google Scholar]
- Glémin S, Clément Y, David J, Ressayre A. 2014. GC content evolution in coding regions of angiosperm genomes: a unifying hypothesis. Trends in Genetics 30: 263–270. [DOI] [PubMed] [Google Scholar]
- Glémin S, Arndt PF, Messer PW, Petrov D, Galtier N, Duret L. 2015. Quantification of GC-biased gene conversion in the human genome. Genome Research 25: 1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenacre MJ. 1984. Theory and applications of correspondence analysis. London: Academic Press. [Google Scholar]
- Gouy M, Gautier C. 1982. Codon usage in bacteria: correlation with gene expressivity. Nucleic Acids Research 10: 7055–7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossmann TI, Song BH, Windsor AJ et al. . 2010. Genome wide analyses reveal little evidence for adaptive evolution in many plant species. Molecular Biology and Evolution 27: 1822–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Bao J, Fan L. 2007. Evidence of selectively driven codon usage in rice: implications for GC content evolution of Gramineae genes. FEBS Letters 581: 1015–1021. [DOI] [PubMed] [Google Scholar]
- Haas BJ, Papanicolaou A, Yassour M. et al. 2013. De novo transcript sequence reconstruction from RNA-Seq: reference generation and analysis with Trinity. Nature protocols 8: 1494–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RJ, Charlesworth B. 2010. Biased gene conversion affects patterns of codon usage and amino acid usage in the Saccharomyces sensu stricto group of yeasts. Molecular Biology and Evolution 28: 117–129. [DOI] [PubMed] [Google Scholar]
- Haudry A, Cenci A, Guilhaumon C et al. . 2008. Mating system and recombination affect molecular evolution in four Triticeae species. Genetics Research 90: 97–109. [DOI] [PubMed] [Google Scholar]
- He B, Dong H, Jiang C, Cao F, Tao S, Xu LA. 2016. Analysis of codon usage patterns in Ginkgo biloba reveals codon usage tendency from A/U-ending to G/C-ending. Scientific Reports 6: 35927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten U, Wright KM, Jenkins J et al. . 2013. Fine-scale variation in meiotic recombination in Mimulus inferred from population shotgun sequencing. Proceedings of the National Academy of Sciences USA 110: 19478–19482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertweck KL, Kinney MS, Stuart SA et al. . 2015. Phylogenetics, divergence times and diversification from three genomic partitions in monocots. Botanical Journal of the Linnean Society 178: 375–393. [Google Scholar]
- Hershberg R, Petrov DA. 2008. Selection on codon bias. Annual Review of Genetics 42: 287–299. [DOI] [PubMed] [Google Scholar]
- Ho C-L, Kwan Y-Y, Choi M-C et al. . 2007. Analysis and functional annotation of expressed sequence tags (ESTs) from multiple tissues of oil palm (Elaeis guineensis Jacq.). BMC Genomics 8: 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemura T. 1981. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: a proposal for a synonymous codon choice that is optimal for the E. coli system. Journal of Molecular Biology 151: 389–409. [DOI] [PubMed] [Google Scholar]
- Ikemura T. 1985. Codon usage and transfer-RNA content in unicellular and multicellular organisms. Molecular Biology and Evolution 2: 13–34. [DOI] [PubMed] [Google Scholar]
- Irwin B, Heck JD, Hatfield GW. 1995. Codon pair utilization biases influence translational elongation step times. The Journal of Biological Chemistry 270: 22801–22806. [DOI] [PubMed] [Google Scholar]
- Ivanova Z, Sablok G, Daskalova E et al. . 2017. Chloroplast genome analysis of resurrection tertiary relict Haberlea rhodopensis highlights genes important for desiccation stress response. Frontiers in Plant Science 8: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouannic S, Argout X, Lechauve F et al. . 2005. Analysis of expressed sequence tags from oil palm (Elaeis guineensis). FEBS Letters 579: 2709–2714. [DOI] [PubMed] [Google Scholar]
- Kalinka A, Achrem M, Poter P. 2017. The DNA methylation level against the background of the genome size and t-heterochromatin content in some species of the genus Secale L. PeerJ 5: 2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe A, Miyashita NT. 2003. Pattern of codon usage bias in three dicot and four monocot plant species. Genes& Genetic Systems 78: 343–352. [DOI] [PubMed] [Google Scholar]
- Kopelowitz J, Hampe C, Goldman R, Reches M, Engelberg-Kulka H. 1992. Influence of codon context on UGA suppression and read through. Journal of Molecular Biology 225: 261–269. [DOI] [PubMed] [Google Scholar]
- Kuhl JC, Cheung F, Yuan Q et al. . 2004. A unique set of 11,008 onion expressed sequence tags reveals expressed sequence and genomic differences between the monocot orders Asparagales and Poales. The Plant Cell 16: 114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesecque Y, Mouchiroud D, Duret L. 2013. GC-biased gene conversion in yeast is specifically associated with crossovers: molecular mechanisms and evolutionary significance. Molecular Biology and Evolution 30: 1409–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q. 2012. Mutational bias and translational selection shaping the codon usage pattern of tissue-specific genes in rice. PLoS ONE 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Xue Q. 2005. Comparative studies on codon usage pattern of chloroplasts and their host nuclear genes in four plant species. Journal of Genetics 84: 1. [DOI] [PubMed] [Google Scholar]
- Liu Q, Hu H, Wang H. 2015. Mutational bias is the driving force for shaping the synonymous codon usage pattern of alternatively spliced genes in rice (Oryza sativa L.). Molecular Genetics and Genomics 290: 649–660. [DOI] [PubMed] [Google Scholar]
- Low E-TL, Alias H, Boon S-H et al. . 2008. Oil palm (Elaeis guineensis Jacq.) tissue culture ESTs: Identifying genes associated with callogenesis and embryogenesis. BMC Plant Biology 8: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma QP, Li C, Wang J, Wang Y, Ding ZT. 2015. Analysis of synonymous codon usage in FAD7 genes from different plant species. Genetics and Molecular Research 14: 1414–1422. [DOI] [PubMed] [Google Scholar]
- Martin G, Baurens F-C, Cardi C, Aury J-M, D’Hont A. 2013. The complete chloroplast genome of banana (Musa acuminata, Zingiberales): insight into plastid monocotyledon evolution. PLoS ONE 8: 67350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G, Baurens F-C, Droc G et al. . 2016. Improvement of the banana ‘Musa acuminata’ reference sequence using NGS data and semi-automated bioinformatics methods. BMC Genomics 17: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura G, Pinheiro M, Silva R et al. . 2005. Comparative context analysis of codon pairs on ORFeome scale. Genome Biology 6: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura G, Pinheiro M, Arrais J et al. . 2007. Large scale comparative codon-pair context analysis unveils general rules that fine-tune evolution of mRNA primary structure. PLoS ONE 2: 847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura GR, Pinheiro M, Freitas A et al. . 2011. Species-specific codon context rules unveil non-neutrality effects of synonymous mutations. PLoS ONE 6: 26817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugal CF, Arndt PF, Holm L, Ellegren H. 2015. Evolutionary consequences of DNA methylation on the GC content in vertebrate genomes. G3: Genes, Genomes, Genetics 5: 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay P, Basak S, Ghosh TC. 2008. Differential selective constraints shaping codon usage pattern of housekeeping and tissue-specific homologous genes of rice and Arabidopsis. DNA Research 15: 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muyle A, Serres-Giardi L, Ressayre A, Escobar J, Glémin S. 2011. GC-biased gene conversion and selection affect GC content in the Oryza genus (rice). Molecular Biology and Evolution 28: 2695–2706. [DOI] [PubMed] [Google Scholar]
- Nachman MW, Crowell SL. 2000. Estimate of the mutation rate per nucleotide in humans. Genetics 156: 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair RR, Raveendran NT, Dirisala VR et al. . 2014. Mutational pressure drives evolution of synonymous codon usage in genetically distinct Oenothera plastomes. Iranian Journal of Biotechnology 12: 58–72. [Google Scholar]
- Necşulea A, Popa A, Cooper DN et al. . 2011. Meiotic recombination favors the spreading of deleterious mutations in human populations. Human Mutation 32: 198–206. [DOI] [PubMed] [Google Scholar]
- Nguyen PA, Kim JS, Kim J-H. 2015. The complete chloroplast genome of colchicine plants (Colchicum autumnale L. and Gloriosa superba L.) and its application for identifying the genus. Planta 242: 223–237. [DOI] [PubMed] [Google Scholar]
- Ning LI, Sun MH, Jiang ZS, Shu HR, Zhang SZ. 2016. Genome-wide analysis of the synonymous codon usage patterns in apple. Journal of Integrative Agriculture 15: 983–991. [Google Scholar]
- Ossowski S, Schneeberger K, Lucas-Lledó JI et al. . 2010. The rate and molecular spectrum of spontaneous mutations in Arabidopsis thaliana. Science 327: 92–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul P, Chakraborty S. 2016. Codon usage bias analysis for the coding sequences of Camellia sinensis and Brassica campestris. African Journal of Biotechnology 15: 236–251. [Google Scholar]
- Peng Z, Lu Y, Li L, Zhao Q et al. . 2013. The draft genome of the fast-growing non-timber forest species moso bamboo (Phyllostachys heterocycla). Nature Genetics 45: 456–461. [DOI] [PubMed] [Google Scholar]
- Perrière G, Thioulouse J. 2002. Use and misuse of correspondence analysis in codon usage studies. Nucleic Acids Research 30: 4548–4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessia E, Popa A, Mousset S, Rezvoy C, Duret L, Marais GA. 2012. Evidence for widespread GC-biased gene conversion in eukaryotes. Genome Biology and Evolution 4: 675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin JB, Kudla G. 2011. Synonymous but not the same: the causes and consequences of codon bias. Nature Reviews Genetics 12: 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priya R, Dass FP, Siva R. 2016. Gene expression prediction and hierarchical clustering analysis of plant CCD genes. Plant Molecular Biology Report 34: 618–627. [Google Scholar]
- Ratnakumar A, Mousset S, Glémin S et al. . 2010. Detecting positive selection within genomes: the problem of biased gene conversion. Philosophical Transactions of the Royal Society of London B: Biological Sciences 365: 2571–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressayre A, Glemin S, Montalent P, Serre-Giardi L, Dillmann C, Joets J. 2015. Introns structure patterns of variation in nucleotide composition in Arabidopsis thaliana and rice protein-coding genes. Genome Biology and Evolution 7: 2913–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers-Melnick E, Vera DL, Bass HW, Buckler ES. 2016. Open chromatin reveals the functional maize genome. Proceedings of the National Academy of Sciences USA 113: 3177–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Ming R, Yu Q. 2016. Comparative analysis of GC content variations in plant genomes. Tropical Plant Biology 1: 14. [Google Scholar]
- Sablok G, Nayak KC, Vazquez F, Tatarinova TV. 2011. Synonymous codon usage, GC3, and evolutionary patterns across plastomes of three pooid model species: emerging grass genome models for monocots. Molecular Biotechnology 49: 116–128. [DOI] [PubMed] [Google Scholar]
- Serres-Giardi L, Belkhir K, David J, Glémin S. 2012. Patterns and evolution of nucleotide landscapes in seed plants. The Plant Cell 24: 1379–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PM, Li WH. 1987. The rate of synonymous substitution in Enterobacterial genes is inversely related to codon usage bias. Molecular Biology and Evolution 4: 222–230. [DOI] [PubMed] [Google Scholar]
- Sharp PM, Tuohy TM, Mosurski KR. 1986. Codon usage in yeast: cluster analysis clearly differentiates highly and lowly expressed genes. Nucleic Acids Research 14: 5125–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields DC, Sharp PM, Higgins DG, Wright F. 1988. ‘Silent’ sites in Drosophila genes are not neutral: evidence of selection among synonymous codons. Molecular Biology and Evolution 5: 704–716. [DOI] [PubMed] [Google Scholar]
- Šmarda P, Bureš P. 2012. The variation of base composition in plant genomes. InPlant Genome Diversity Springer; Vienna: 1: 209–235. [Google Scholar]
- Song H, Wang P, Ma D, Xia H, Zhao C, Zhang Y, Zhao S. 2015. Analysis of codon usage bias in Medicago truncatula WRKY transcription factors. Journal of Agricultural Biotechnology 23: 203–212. [Google Scholar]
- Spencer CCA. 2006. Human polymorphism around recombination hotspots. Biochemical Society Transactions 34: 535–536. [DOI] [PubMed] [Google Scholar]
- Sueoka N. 1988. Directional mutation pressure and neutral molecular evolution. Proceedings of the National Academy of Sciences USA 85: 2653–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N. 1995. Intrastrand parity rules of DNA base composition and usage biases of synonymous codons. Journal of Molecular Evolution 40: 318–325. [DOI] [PubMed] [Google Scholar]
- Sundararajan A, Dukowic-Schulze S, Kwicklis M et al. . 2016. Gene evolutionary trajectories and GC patterns driven by recombination in Zea mays. Frontiers in Plant Science 7: 1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatarinova TV, Alexandrov NN, Bouck JB, Feldmann KA. 2010. GC3 biology in corn, rice, sorghum and other grasses. BMC Genomics 16: 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatarinova TV, Elhaik E, Pellegrini M. 2013. Cross-species analysis of genic GC3 content and DNA methylation patterns. Genome Biology & Evolution 5: 1443–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H-C, Hickey DA. 2007. Rapid divergence of codon usage patterns within the rice genome. BMC Evolutionary Biology 7: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Roossinck MJ. 2006. Comparative analysis of expressed sequences reveals a conserved pattern of optimal codon usage in plants. Plant Molecular Biology 61: 699–710. [DOI] [PubMed] [Google Scholar]
- Wang W, Haberer G, Gundlach H et al. . 2014. The Spirodela polyrhiza genome reveals insights into its neotenous reduction fast growth and aquatic lifestyle. Nature Communications 5: 3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber CC, Boussau B, Romiguier J, Jarvis ED, Ellegren H. 2014. Evidence for GC-biased gene conversion as a driver of between-lineage differences in avian base composition. Genome Biology 15: 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong GK, Wang J, Tao L et al. . 2002. Compositional gradients in Gramineae genes. Genome Research 12: 851–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright F. 1990. The effective number of codons used in a gene. Gene 87: 23–29. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhao D, Tao J. 2015. Analysis of codon usage patterns in herbaceous peony (Paeonia lactiflora Pall.) based on transcriptome data. Genes 6: 1125–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Cai X, Chen Q, Zhou H, Cai Y, Ben A. 2011. Factors affecting synonymous codon usage bias in chloroplast genome of Oncidium Gower Ramsey. Evolutionary Bioinformatics 7: 271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Dong J, Tong C, Gong X, Wen Q, Zhuge Q. 2013. Analysis of synonymous codon usage patterns in seven different citrus species. Evolutionary Bioinformatics 9: 215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Zhang X, Liu G et al. . 2010. The Complete chloroplast genome sequence of date palm (Phoenix dactylifera L.). PLoS ONE 5: 12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Yuan Y, Wang L et al. . 2012. Great majority of recombination events in Arabidopsis are gene conversion events. Proceedings of the National Academy of Sciences USA 109: 20992–20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You E, Wang Y, Ding ZT, Zhang XF, Pan LL, Zheng C. 2015. Codon usage bias analysis for the spermidine synthase gene from Camellia sinensis (L.) O. Kuntze. Genetics and Molecular Research 14: 7368–7376. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Nie X, Jia X et al. . 2012. Analysis of codon usage patterns of the chloroplast genomes in the Poaceae family. Australian Journal of Botany 60: 461–470. [Google Scholar]
- Zhou M, Li X. 2009. Analysis of synonymous codon usage patterns in different plant mitochondrial genomes. Molecular Biology Report 36: 2039–2046. [DOI] [PubMed] [Google Scholar]
- Zhu L, Zhang Y, Zhang W, Yang S, Chen JQ, Tian D. 2009. Patterns of exon-intron architecture variation of genes in eukaryotic genomes. BMC Genomics 10: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.