ABSTRACT
Klebsiella pneumoniae ST258 is a globally distributed multi-drug resistant pathogen responsible for severe invasive infections. In this study, the different virulence potential of K. pneumoniae ST258 isolates in endotoxin susceptible versus resistant animal models was shown. Furthermore, ST258 clinical isolates were found highly sensitive to the bactericidal effect of naive animal and human serum. These observations imply that LPS, released from the rapidly lysed bacteria, may contribute to the high mortality associated with ST258 bacteremia cases.
A humanized version (mAb A1102) of a previously described murine mAb specific for the conserved LPS O-antigen, was tested for endotoxin neutralization. A1102 was able to neutralize TLR-4 activation by ST258-derived LPS in vitro with an efficacy exceeding that of polymyxin B by 3 orders of magnitude. Passive immunization with A1102 afforded a significant level of protection in a galactosamine-sensitized mouse model of endotoxemia, induced by ST258-derived LPS, or upon challenge with live bacteria. Efficacy was retained using an aglycosylated IgG, as well as upon complement depletion, suggesting that Fc-independent endotoxin neutralization may be the main protective mechanism in this model, in spite of the complement-dependent bactericidal and opsonic activities additionally observed for A1102 in vitro. Furthermore, rabbits that are naturally highly susceptible to endotoxin, were also significantly protected by low doses of A1102 when challenged with an ST258 strain.
Given this unique mode of action and the high protective efficacy of this mAb, passive immunization, as prophylactic or adjunct therapeutic approach for the treatment of infections caused by ST258 isolates should be considered.
KEYWORDS: carbapenem-resistant Klebsiella, endotoxin neutralization, Klebsiella pneumoniae ST258, monoclonal antibody, protective efficacy
Introduction
Klebsiella pneumoniae ST258 is a multi-drug resistant nosocomial clone that has spread world-wide and is currently endemic in many countries.1 With the emergence of strains resistant to last resort antibiotics,2-4 treatment options have become very limited. Alternative therapeutic approaches,5 such as monoclonal antibodies,6-8 are needed, as the pipeline of novel antibiotics is dry.
Factors contributing to the success of the widely disseminated ST258 clone have not yet been fully elucidated. Several studies have shown the absence of known Klebsiella virulence traits and limited in vivo virulence of ST258 isolates.9-11 Accordingly, K. pneumoniae ST258 infections in the community are rare, and even in a nosocomial setting are restricted to patients with underlying risk factors.12-15 Therefore, the high mortality rate associated with ST258 infections may primarily originate from impaired efficacy of host defense mechanisms and antimicrobial therapy, rather than the intrinsic virulence potential of this pathogen.
Immunization approaches against Klebsiella were explored decades ago16-18 and have been revisited recently, given the urgent need for alternative therapeutic options to antibiotics. With most protein antigens being masked by bulky capsular polysaccharides and smooth LPS, these surface carbohydrate structures themselves prevail as putative antibody targets. Unfortunately, carbohydrates are not ideal antigens and moreover, show extensive variability that makes a broad spectrum immunization approach very challenging.
The genetic evolution of K. pneumoniae ST258 was recently elucidated.19,20 It was shown that ST258 represents a hybrid clone that has emerged by the genetic rearrangement of ST11- and ST422-like strains. A subsequent smaller rearrangement incorporating the capsular (cps) locus has generated 2 clades within the ST258 clone, each expressing a different capsular polysaccharide. Moreover, extensive variation within the cps locus was shown among ST258 strains by several studies9,21,22 suggesting the existence of additional capsular variants and unencapsulated strains.
Interestingly, the locus encoding the LPS O-antigen, which is located adjacent to the capsular locus, has remained essentially identical in both clades. In our previous work, we described that most ST258 strains expressed the same LPS O-antigen, a modified D-galactan-I structure that was termed D-galactan-III (gal-III).23 Conservation of this antigen implies a potential role in fitness/pathogenesis, and at the same time offers a good candidate for immunization. We previously showed that monoclonal antibodies (mAbs) raised against gal-III were able to bind to the surface of both clades of K. pneumoniae ST258, corroborating accessibility of this antigen through different capsular polysaccharides.23 In this study we assessed the potential of a humanized anti-gal-III mAb to provide protection against ST258 isolates in different animal models, and propose a unique mode of action for protection.
Methods
Bacteria and growth conditions
Klebsiella pneumoniae strains used in this study are listed in Table S1. ST258 isolates were kindly provided by C. Mammina (Italy)24,25 S. Opal (USA), M. Assous (Israel)26 and M. Gniadkowski (Poland).27 K. pneumoniae O1:K2 strain was obtained from ATCC (43816), and E. coli strains 536 and MG1655 originated from the laboratory of J. Hacker (Germany). Bacteria were inoculated routinely from chromID™ CARBA SMART plates (BioMérieux) into Luria-Bertani (LB) broth to ensure carriage of the KPC encoding plasmid. For CFU enumeration, bacteria were grown on Trypcase Soy Agar plates (BioMérieux).
Antibody expression and purification
For the A1102 mAb generation, A1102 heavy chain (HC) of human IgG1 isotype (G1m1,17) and light chain (LC) of human kappa isotype (Km3) were cloned into the pTT5 vector (Biotechnology Research Institute, National Research Council of Canada (NRC-BRI), Quebec, Canada). The F(ab’)2 of A1102 was generated by cloning the sequence encoding amino acids 1–236 of A1102 heavy chain sequence into the pTT5 vector. Aglycosylated A1102 was generated by introducing a N297Q mutation into CH2 domain of the HC of A1102 by site-directed mutagenesis using the QuickChange II XL Site-Directed Mutagenesis Kit (Agilent Technologies). The F(ab’)2 construct and LC (for A1102 F(ab’)2), or the full length HC with N297Q mutation and LC (for A1102 N297Q mAb) were co-transfected at a ratio of 1:1 into CHO-3E7 cells (NRC-BRI) using PEI MAX transfection reagent (Polysciences, Eppelheim, Germany) and grown in FreeStyle CHO expression medium (Life Technologies). Cell culture supernatants were harvested 8 d post-transfection.
A1102 and A1102 N297Q mutant mAbs expressed in CHO cells were purified from culture supernatants by Protein A affinity chromatography using HiTrap MabSelect SuRe™ resin(GE Healthcare). A1102 F(ab’)2 was purified using CaptureSelect IgG-CH1 Affinity chromatography (Life Technologies) followed by preparative size-exclusion chromatography on the Superdex 200 (GE Healthcare) to separate the A1102 F(ab’)2 from the A1102 Fab dissociation product.
Affinity and cross-reactivity measurements
Binding to the biotinylated galactan-III antigen was measured by biolayer interferometry (BLI) using a fortéBIO Octet Red instrument (Pall Life Sciences). The antigen (0.1 µg/ml) was immobilized on the streptavidin (SA) sensor (fortéBIO, Pall Life Sciences) to give sensor loadings of 0.025, 0.05 and 0.1 nm. For monovalent binding affinity, the Fab fragment of mAb A1102 was used. The association of the Fab fragment (6.25 – 200 nM) to the immobilized antigen was monitored for 600 s in solution (PBS, pH 7.2 containing 1% BSA), at 30 °C. The sensors were then immersed in the same buffer for 600 s, to monitor the dissociation of the antibody. The kinetic rate constants (kon and koff for the monovalent interaction) were determined by fitting each progress curve, corresponding to different Fab concentrations for the same sensor loading, to a 1:1 binding model (fortéBIO Analysis Software version 9.0). The dissociation constant for the monovalent interaction (Kd) was calculated as koff/kon. The dissociation equilibrium constants were also determined by steady-state analysis based on calculated equilibrium response values (fortéBIO Analysis Software version 9.0). To determine the strength of the avid binding interaction, the IgG (2 – 133 nM) was used instead of the Fab, in a set-up as described for the Fab fragment, and data were analyzed as described above, except that in this case, the apparent macroscopic dissociation constants determined (Kd values) represent avidity constants.
Cross-reactivity to another Klebsiella O-antigen (O1) was measured in a similar set-up to that described above for affinity measurements, immobilizing biotinylated O1 on SA sensors and monitoring the binding of IgG (66.7 nM) in solution (PBS, pH 7.2, containing 1% BSA) for 600 s. To determine cross-reactivity to unrelated LPS (E. coli O55), the antibody (full length IgG) was immobilized on anti-human capture (AHC) sensors and the association of LPS (30 µg/ml) in solution (PBS, pH 7.2, containing 0.1% BSA) was monitored for 600 s.
Macrophage uptake assay using RAW264.7 cells
The bacterial uptake assay was performed as described previously with minor modifications.28 Briefly, RAW 264.7 cells were cultured in DMEM supplemented with Penicillin/Streptomycin and 10% fetal bovine serum. Cells were seeded at 7.5 × 104 cells per well in a 96-well plate the day before the assay. Strain Kp31, sensitive to 100 μg/ml gentamicin was selected for the assay. Bacteria were pre-opsonized with 2.5 µg/ml of the respective antibody in the presence of 5% active or heat-inactivated naïve rabbit serum (PreClinics) for 15 minutes on ice. Macrophages were washed with PBS and infected at a multiplicity of infection (MOI) of 1, with 50 µl of the pre-opsonized bacterial mixture in antibiotic-free DMEM. After 30 minutes incubation at 37°C with gentle agitation, gentamicin was added to a final concentration of 100 µg/ml and the plate was further incubated for 30 min to completely eliminate extracellular bacteria. At 1 h post infection, cells were washed twice with PBS and lysed in 0.1% Triton X-100 in PBS for 5 minutes. Lysates were diluted in PBS and plated on TSA plates (bioMérieux) for bacterial enumeration.
LPS neutralization assay
Purified LPS or culture supernatants of mid-log cultures of selected ST258 strains grown in LB, were tested for human TLR4 activation using HEK Blue cells (InvivoGen) in the presence of antibodies, or antibody fragments, or polymyxin B according to the manufacturer's instructions. Briefly, filter sterilized culture supernatants or 1–4 ng/mL of purified LPS (LPS extraction kit, Intron) were mixed with different concentrations of the antibodies or polymyxin B and incubated in a 96-well plate for 30 min at room temperature. Afterwards, 5 × 104 hTLR4 HEK Blue cells were added to the reaction and the mixtures were incubated overnight at 37°C and 5% CO2. Absorbance at OD630 was measured and the neutralization capacity was expressed as %-inhibition of secreted alkaline phosphatase (SEAP) induction using the formula: %-inhibition = 100 – [100 × SEAP (mAb)/SEAP (LPS only control)].
Serum bactericidal assay
Serum samples originating from Blutzentrale Linz (Austria) were freshly collected from volunteers and kept as individual samples frozen at −80°C. Alternatively, individual and pooled normal human serum (NHS) samples were obtained from US Biologicals. Naïve sera were collected from 6–8 week old, female BALB/cJRj mice (Janvier, France), Wistar RjHan:WI rats (Janvier, France) as well as Crl:KBL (NZW) rabbits (PreClinics, Germany), housed under specific pathogen-free conditions.
Heat-inactivation of complement was performed at 56°C for 45 min. Where indicated, adsorption of serum was performed in 3 cycles by re-suspending a pellet of 3 × 108 CFU mid-log phase bacteria per ml of serum. Adsorbed serum was filter sterilized and kept frozen in aliquots at −80°C for subsequent serum bactericidal assays. Aliquots were thawed on ice only once and used on the same day. In the serum survival assay, 3–5 × 103 CFU of mid-log phase bacteria were incubated at 37°C in 75% serum (10, 25, and 50% serum was additionally used in pilot studies) and plated at 1 h and 2 h time-points. The recovered CFUs were correlated with the inoculum size also determined by plating.
Serum bactericidal activity mediated by antibodies was evaluated in the presence of 50% adsorbed human or rabbit serum as described previously.29
Animal experiments
Mouse experiments were performed according to Austrian Law (BGBl. I Nr. 114/2012, approved by MA58, Vienna). In all experiments, female 6–8 week old BALB/cJRj mice were used (Janvier). Protective efficacy of monoclonal antibodies was assessed by intraperitoneal injection of serially diluted (100 µg to 0.78 µg/mouse) mAbs formulated in PBS, 24 hours before challenge. Control groups received an isotype-matched (human IgG1) irrelevant control mAb at the highest dose (100 µg). Challenge was performed intravenously (i.v.) with 100 µl of bacterial suspension. Bacteria were grown to mid-log phase (OD600 of ∼0.5) in LB broth, washed with PBS and diluted to the target inoculum size. In the endotoxemia model, mice received 20 mg of D-galactosamine (GalN) intraperitoneally, while at the same time being challenged with either extracted, purified LPS (16 ng) or live bacteria (1.5–5 × 104 CFU) injected intravenously. In all cases, survival was monitored daily for up to 2 weeks.
For the depletion of complement, mice were treated (i.p.) with 1 U of cobra venom factor (Quidel, USA) one day before infection. This dose was confirmed in pilot studies to effectively inhibit complement pathways as assessed by an ELISA-based commercial kit (WIESLAB Complement system screen, Euro Diagnostica).
Blood and organ loads were determined by plating serially diluted heparinized blood samples or homogenized organ samples obtained at the indicated time-points. Triplicates were plated onto selective agar plates in all instances.
Rat and rabbit models were performed at Fidelta Ltd (Zagreb, Croatia) according to institutional approval. Rat models were performed in 9-week-old female Sprague Dawley rats (Crl:SD, Charles River, France). Rabbit studies used groups of 4 male Crl:KBL (NZW) rabbits (Charles River, France) immunized i.v. with log diluted doses of mAb A1102 or an irrelevant control mAb. The next day, animals were challenged i.v. with a minimal lethal dose of ST258 strain Kp151. Infected animals were monitored every 3 h for the first day, and then daily for up to 7 d. Statistical analyses were performed with the LogRank (Mantel-Cox) test using GraphPad Prism 5.04 Software. Differences were considered statistically significant when p < 0.05.
Results
ST258 strains are susceptible to normal human serum
A collection of clinical ST258 strains isolated in different geographical regions were tested for survival in normal human serum (NHS) samples obtained from different donors (Fig. 1 upper panels). All 18 ST258 clinical isolates tested showed high susceptibility to serum killing resulting in recovery of none or very few colonies following 2 h incubation in 75% serum. Two of the isolates (Kp30 and Kp150), were slightly less susceptible at 1 h, but were still serum sensitive based on the 2 h time-point. A similar susceptibility pattern was observed in individual 75% NHS samples obtained from a commercial source (Fig. 1 middle panels). Notably, when a commercial NHS pool was used, only 7 of the 18 tested isolates were fully sensitive, while the others survived to approximately 50 to 100% relative to the initial inoculum, with one strain (Kp150) even multiplying. Human complement is known to be unstable under certain handling conditions, therefore we measured the activity of the classical, alternative, and lectin complement pathways of all 6 human serum samples (5 individual sera and one pool) used in our bacterial survival assays. We confirmed that unlike the 5 individual serum samples, the pooled commercial sample had low complement activity (Fig. S1). To corroborate the survival differences observed at lower complement concentrations, selected K. pneumoniae ST258 strains representing the most and least susceptible isolates, were tested for survival at different (10–75%) serum concentrations (Fig. S2). As positive and negative controls, the serum resistant K. pneumoniae O1:K2 and the uropathogenic E. coli strain 536 as well as the serum susceptible rough E. coli strain MG1655 (K-12), were tested parallel. Both serum resistant control strains grew in the 2 human sera we used for these experiments, at all concentrations (including 75%). In contrast, the E. coli K-12 strain was rapidly killed even in 10% sera. Strain Kp151, representing the majority of ST258 isolates based on its high susceptibility to 75% human serum, behaved comparably to the rough E. coli strain. The less susceptible ST258 strain, Kp150, was also clearly more susceptible than the serum resistant pathogens investigated. Still, the minor difference in susceptibility observed between Kp150 and Kp151 in 75% serum (Fig. 1) increased upon dilution of serum samples (Fig. S2).
Figure 1.
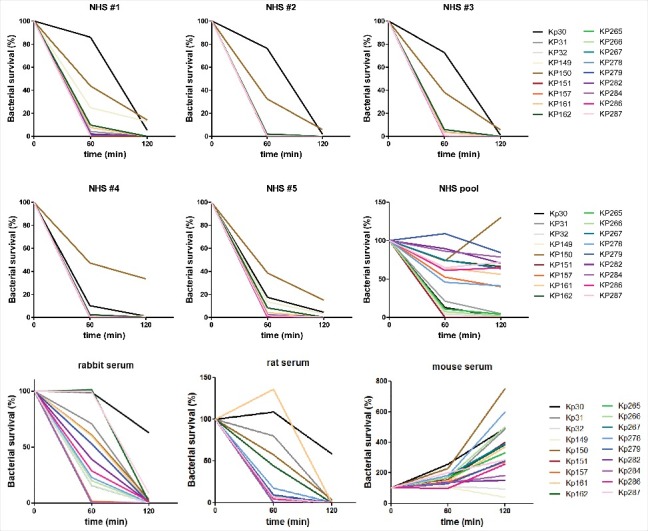
Bactericidal activity of different serum samples toward a panel of clinical ST258 isolates. 3–5 × 103 CFU of the indicated ST258 isolates were incubated at 37 °C in 75 % serum samples of different origin as indicated on the individual panels. Aliquots were plated after incubation for 1 h and 2 h respectively and the recovered CFUs were correlated with the inoculum. The detection limit was 0.1% of inoculum. NHS: normal human serum.
Survival of ST258 isolates was also investigated in sera obtained from different laboratory animal species most often used for models of bacterial infection (Fig. 1 lower panels). While ST258 isolates were sensitive to serum obtained from rabbits and rats, all strains survived (and most of them even multiplied) in mouse serum.
ST258 strains exhibit low virulence in animal models
Serial dilutions of 2 ST258 strains - Kp150 (clade 1), the least serum-sensitive strain, and Kp151 (clade 2), representative for most of the strains with high sensitivity to 75% human serum, were used to infect mice, rats and rabbits intravenously. In mice, a challenge dose as high as 108 CFU/animal (∼5 × 109 CFU/kg) did not elicit lethality irrespective of the clade type. In good agreement with this finding, the blood counts of both Klebsiella strains dropped by 3–4 logs within 30 min after i.v. infusion followed by a slower decrease over the next 2 d (Fig. S3A). The low level of bacteremia detected 48 h post-infection was due to spread from solid organs (spleen, kidney) (Fig S3B). Importantly, no advantage of the somewhat better survival of Kp151 in human serum was apparent in vivo either in terms of blood counts or in organ loads (Fig. S3).
Similarly to mice, no deaths occurred in rats with challenge doses up to 3 × 109 CFU/animal (∼1.5 × 1010 CFU/kg). In contrast, a 3 × 109 CFU/kg dose was found to be 100 % lethal in rabbits (Table S2).
Based on these data we speculated that the higher virulence of ST258 strains in rabbits may be the consequence of their higher sensitivity to endotoxin compared with mice or rats.30 To corroborate that endotoxemia induced by bacterial lysis contributes significantly to lethality in endotoxin sensitive animal models, we used a GalN mouse model, a proven model of endotoxemia.30 In GalN-sensitized mice, the minimal lethal dose was between 1.5–5 ×104 CFU/mouse (∼7.5 × 105 to 5 ×106 CFU/kg) with all ST258 strains tested (Table S2). This represents at least a 1000-fold decrease in the minimal lethal dose in GalN-sensitized vs. non-sensitized, naturally resistant mice.
Generation and characterization of a humanized anti-gal-III mAb
Discovery of murine mAbs targeting gal-III was described previously.23 The murine mAbs were humanized by CDR grafting and the mAb with the highest affinity binding to purified gal-III antigen, A1102 (human IgG1 isotype produced in CHO cells), a derivative of murine mAb 5A4,23 was used in this study.
The monovalent binding affinity of mAb A1102 for the galactan-III O-antigen was determined by measuring the affinity of its Fab fragment for the biotinylated antigen by BLI. An association rate constant of 1 × 105 M−1s−1, a dissociation rate constant of 5.4 × 10−3 s−1 (Figure S4, Table 1), and a calculated dissociation equilibrium constant of 57 nM were determined. These values are in good agreement with that obtained from the steady-state equilibrium measurement (55 nM). It is known that the density of the O-antigen on the surface of Klebsiella is relatively high, so it is likely that the antigen is presented in an avid state on the cell surface, and especially so in LPS micelles. Therefore it was also of interest to determine the strength of the avid interaction between the IgG and the polysaccharide molecules. This was performed by BLI in a ForteBio instrument, at various sensor loadings and IgG concentrations (Table 1). The macroscopic dissociation constants (Kd values) determined were 2 or more orders of magnitude lower (i.e. in the sub-nanomolar range, with relatively constant values for the same mAb concentration for the 2 lower loadings) than those for the monomeric interaction, due to a proportional increase in the dissociation rate constant. A similar effect of the avid interaction (i.e., 100-fold higher affinity in avid vs. monovalent state) was determined for other anti-carbohydrate mAbs, e.g. for the high affinity anti-Chlamydia LPS mAb, S 5.2331 although the absolute affinity was ∼10-fold lower than that of mAb A1102.
Table 1.
Equilibrium and kinetic rate constants for the binding of mAb A1102 to the biotinylated D-galactan-III antigen, determined with either the Fab fragment or the IgG in Forte-Bio in PBS, pH 7.2, containing 1% BSA at 30 °C.
| Monovalent binding (Fab) | |||||
|---|---|---|---|---|---|
| Fab (nM) | Kd (M) | kon (M−1s−1) | koff (s−1) | Steady-state Kd (M) | Sensor loading |
| 6.25 – 200 | (5.7 ± 1.6) × 10−8 | (1.0 ± 0.3) × 105 | (5.4 ± 0.9) × 10−3 | (5.5 ± 0.4) × 10−8 | 0.05 , 0.1 nm |
| Avid state binding (IgG) | |||||
| IgG (nM) |
Kd (M) |
kon (M−1s−1) |
koff (s−1) |
Response (nm) |
Sensor loading(nm) |
| 66.7 | 4.22E-10 | 1.57E+05 | 6.62E-05 | 0.6681 | 0.025 nm |
| 3.20E-10 | 1.40E+05 | 4.49E-05 | 1.2718 | 0.05 nm | |
| 1.73E-10 | 9.79E+04 | 1.70E-05 | 3.168 | 0.1 nm | |
| 33.3 | 2.18E-10 | 2.06E+05 | 4.49E-05 | 0.5749 | 0.025 nm |
| 1.66E-10 | 1.86E+05 | 3.09E-05 | 1.0688 | 0.05 nm | |
| 4.31E-11 | 1.15E+05 | 4.98E-06 | 2.658 | 0.1 nm | |
BLI measurements using Klebsiella pneumoniae O1 or E. coli O55 LPS antigens confirmed the absence of cross-reactivity to different mannan or unrelated carbohydrate structures (data not shown).
Endotoxin neutralizing activity of mAb A1102
A1102 was tested for endotoxin neutralization potency in vitro. Signaling by LPS was measured in a HEK cell-based TLR4-dependent colorimetric assay. LPS was extracted from ST258 clade 1 (Kp150), ST258 clade 2 (Kp151) or non-ST258 (PCM-27) K. pneumoniae strains expressing gal-III. Using a broad range of LPS concentrations, we determined that 1 to 4 ng/ml LPS resulted in maximal signal induction and this concentration range was subsequently used for measuring neutralization potency of A1102 (data not shown). A1102 neutralized LPS samples from all 3 strains in a dose-dependent manner with half-maximal effect (IC50) at concentrations between 0.7–2.7 nM (Fig. 2A). The potency of A1102 exceeded that of polymyxin B (an antibiotic with known endotoxin neutralization potency used as a comparator molecule) by approximately 3 orders of magnitude.
Figure 2.
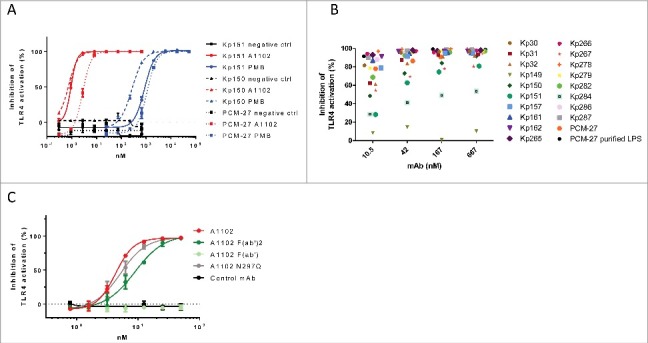
Endotoxin neutralization by a gal-III specific mAb in vitro. Endotoxin signaling through TLR4 was measured in a cell-based colorimetric assay in the presence of mAb A1102. (A) using purified LPS extracted from 3 isolates expressing gal-III (KP150, KP151, PCM-27). A1102 mAb (red curves), isotype matched control mAb (black curves), or polymyxin B (PMB, blue curves) were used at the indicated concentrations. (B) using shed LPS in supernatants of mid-log phase cultures of ST258 isolates as well as strain PCM-27. (C) Neutralizing potency of A1102 compared with its fragments as well as an aglycosylated (N297Q) version.
To confirm efficient neutralization of naturally shed LPS molecules, culture supernatants from a large panel of ST258 isolates were also tested (Fig. 2B). Dose dependent inhibition of TLR-4 signaling by A1102 was confirmed with all but one strain, Kp149, which had been shown to exhibit a rough (i.e., devoid of O-antigen) phenotype.23
Neutralization potency of the antibody was retained when using an aglycosylated mAb (N279Q), as well as F(ab’)2 fragments, however, was lost with Fab fragments (Fig. 2C).
Protective efficacy of gal-III specific mAb in vivo
The in vivo endotoxin neutralizing efficacy of mAb A1102 was assessed in a lethal model of murine endotoxemia. GalN-sensitized mice, challenged with LPS extracted from strain Kp151, were significantly protected upon prior immunization with A1102 (Fig. 3A). The protection appeared to be dose-dependent and specific, as a control IgG with irrelevant specificity given at the highest dose was not efficacious.
Figure 3.
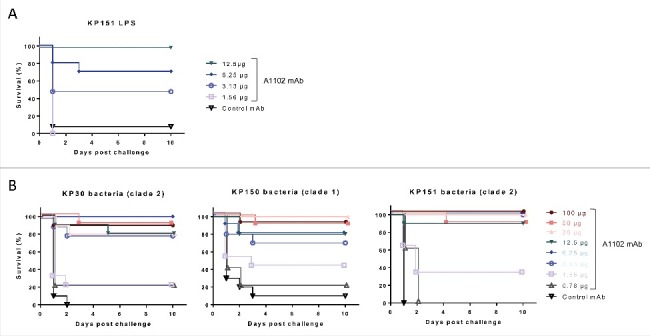
Protection in murine endotoxemia models. Protective efficacy of mAb A1102 was investigated in the GalN-sensitized murine model of endotoxemia. Groups of 5 mice were passively immunized prophylactically (i.p.) with serial dilutions of A1102 (100 µg to 0.78 µg/mouse doses) or an isotype-matched control antibody (100 µg/mouse). Mice were rendered susceptible to endotoxin by receiving an i.p. injection of GalN 24 h later and at the same time were challenged i.v. with either purified LPS, extracted from a representative ST258 strain, Kp151 (16 ng/mouse) (panel A), or with a minimal lethal dose (1.5–5 × 104 CFU/mouse) of ST258 strains (panel B). Survival was monitored daily for up to 10 d. Graphs represent the combined results from 2 independent experiments for each challenge, with a total group size of 10, except for mice receiving the 0.78 µg dose in live challenges (panel B), which was only included in one of the repeats (n = 5).
Furthermore, we performed similar mAb efficacy studies in the murine GalN model using live ST258 challenge strains with different serum susceptibility patterns (see Fig. 1) and clade types. Again, GalN-sensitized mice receiving an isotype matched irrelevant mAb rapidly succumbed to infections induced by any of the ST258 strains, while significant protection was observed by passively immunizing animals with A1102 at a dose range between 3 to 100 µg/animal (0.15 to 5 mg/kg) (p < 0.001; Log-rank test) (Fig. 3B).
Rabbits represent a host species that is comparable to humans with respect to serum bactericidal activity toward ST258 strains, as well as having high intrinsic sensitivity to endotoxin. Therefore, we tested the prophylactic efficacy of different doses of mAb A1102 in a rabbit bacteremia model. Control animals immunized with an isotype-matched control mAb succumbed to infection within 2 d post-infection, while mAb A1102 afforded a significant level of protection at all doses tested ranging from 2 µg/kg to 2000 µg/kg (Fig. 4).
Figure 4.
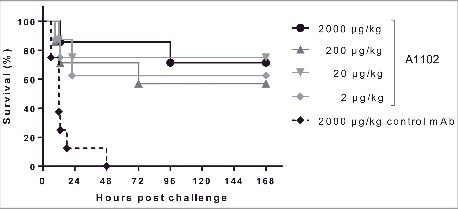
Protection in a rabbit model of bacteremia. Groups of 4 rabbits were immunized i.v. with the indicated doses of A1102 or an isotype-matched irrelevant control mAb. Next day, the animals were challenged i.v. with a lethal dose (5 × 109 CFU/kg) of ST258 strain Kp151. Infected animals were monitored every 3 h for the first day and then daily for 7 d. The graph represents combined data from 2 independent experiments (n = 8 per group in total) with similar outcome.
The gal-III specific mAb displays bactericidal activity against K. pneumoniae ST258 isolates
ST258 strains were highly sensitive to killing by human and rabbit sera. Therefore, a modified bactericidal assay was set-up using a lower percentage and adsorbed (with the respective bacterial strains) sera to investigate whether killing of K. pneumoniae ST258 strains can be enhanced in the presence of the gal-III specific antibody. We found that the A1102 mAb increased the serum bactericidal activity of both adsorbed human and rabbit sera, achieving bacterial clearance at earlier time points compared with control sera containing isotype control antibody (Fig. 5).
Figure 5.
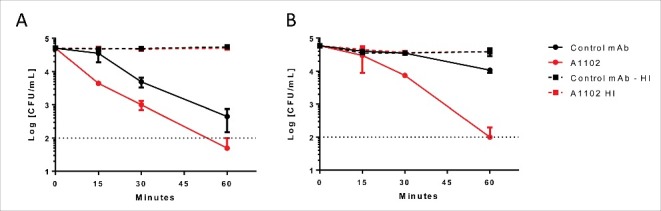
Antibody dependent bactericidal activity in human and rabbit sera. ST258 strains (A: Kp32 or B: Kp151) were incubated with 50 % adsorbed human (A) or rabbit (B) serum for up to 60 min in the presence of either A1102 mAb or an irrelevant isotype control mAb at the respective optimal antibody concentrations (A: 0.6 µg/ml; B: 2.5 µg/ml). CFUs were determined after incubation for 0, 15, 30, and 60 min. Graphs show the average survival relative to the initial inoculum +/− range from 2 individual experiments.
Opsonization of K. pneumoniae ST258 by the gal-III specific mAb
Opsonophagocytotic uptake of a gentamicin-sensitive ST258 strain by a murine macrophage cell line (RAW264.7 cells) was investigated at MOI 1 in the presence of 5% rabbit serum as complement source. Following a 30 min incubation, extracellular bacteria were killed by gentamicin, while intracellular bacteria were enumerated following cell lysis and plating. Baseline uptake of bacteria was determined in the presence of an isotype-matched control antibody. Bacterial uptake was significantly increased upon incubation in the presence of mAb A1102 (Fig. 6). Importantly, heat-inactivation of complement resulted in baseline uptake level, corroborating the need for both the specific mAb and complement for efficient opsonization.
Figure 6.
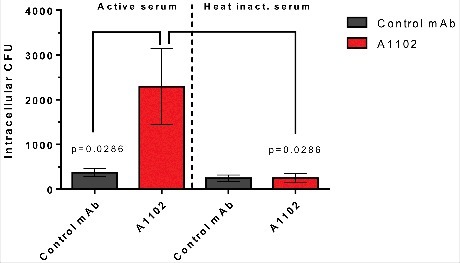
Opsonophagocytotic uptake induced by mAb A1102. RAW264.7 cells were incubated for 30 min at MOI = 1 in the presence of mAb pre-opsonized bacteria and 5% active or heat-inactivated rabbit serum. After the uptake period, gentamicin was added at 100 µg/mL to kill extracellular bacteria. Cells were further incubated for 30 min, and uptake was measured following lysis of cells and plating of surviving intracellular bacteria. Each bar shows the average uptake +/− SEM from 4 individual experiments. Statistical comparison was performed using the Mann-Whitney test.
Mode of action studies in vivo
Since multiple possible modes of action for A1102 were observed in vitro (see above), we aimed to dissect the Fc-dependent vs. –independent (presumably endotoxin neutralization) activities to the protective efficacy observed in the murine model. Efficacy of A1102 in cobra venom factor (CVF)-treated (i.e., complement depleted) mice was fully comparable with that observed in untreated mice (Fig. 7A). Additionally, we tested protection of a genetically engineered aglycosylated mutant derivative of A1102 that loses C1q-binding and is therefore unable to activate complement. As depicted on Fig. 7B, the efficacy of A1102 was unaffected by aglycosylation. Moreover, the presence of A1102 did not influence the in vivo kinetics of ST258 in the blood or organs of mice (Fig. S3A).
Figure 7.
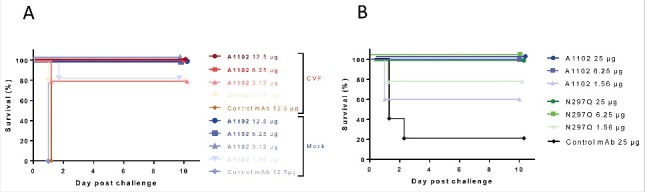
Complement independent protection by A1102. (A) Protective efficacy of A1102 was compared in cobra venom factor-treated (complement depleted) vs mock-treated mice. Groups of 5 mice each received 1 U of CVF (or buffer control) i.p. 30 h before challenge, followed by the indicated doses of A1102 (i.p., 6 h later). Challenge by Kp151 was performed intravenously 24 h after passive immunization with simultaneous GalN sensitization. (B) Efficacy of A1102 compared with its N297Q (aglycosylated) version. Groups of 5 mice were immunized with the indicated amount of antibodies. Challenge by Kp151 was performed intravenously 24 h after passive immunization with mAbs at the time of GalN sensitization. Lethality was monitored daily for 10 d.
These data support that the Fc-mediated activities of this mAb are dispensable for protective efficacy in this model.
Discussion
Therapeutic monoclonal antibodies are widely used in oncology and clinical immunology, and have only recently been re-considered for use against infectious diseases.6-8 The increasing interest in alternative anti-bacterial approaches is mainly a consequence of the low efficacy of currently available antimicrobials and the dry pipeline of novel antibiotics. Carbapenem resistant Enterobacteriaceae (CRE) are classified by the CDC as highest threat level microorganisms (Antibiotic resistance threats in the United States, 2013, www.cdc.org). The worldwide spread of CRE is, at least partly attributable to successful clonal lineages, most typically exemplified by K. pneumoniae clonal group 258. This lineage is endemic in many regions worldwide including the USA and many European countries.32,33
The bacterial factors (besides drug resistance) contributing to the successful spread of this lineage have not been fully elucidated. High susceptibility of ST258 isolates to complement-mediated killing in normal human serum has previously been reported by several groups.10,34 A recent report, however, indicated heterogeneity in the serum sensitivity pattern of an ST258 collection.9 For the design of effective anti-bacterial antibodies, it is essential to understand the behavior of the targeted bacterium in the human host, as well as in animal models. Therefore, we initially aimed to clarify the apparent contradiction in the literature. A panel of 18 ST258 isolates, collected from different continents, was tested with different human serum samples as well as in mouse, rat and rabbit (i.e., species used in animal models reported in this study) sera. It became evident that all tested K. pneumoniae strains exhibited sensitivity to high concentrations of human serum samples that retained complement activity. In contrast, at lower serum concentrations or by using serum samples with compromised complement activity (a commercial serum pool), ST258 isolates showed various survival levels. These differences were not attributable to specific IgG or IgM titers in the serum samples (data not shown) and therefore imply intrinsic differences among ST258 isolates. The different sensitivities were unrelated to the clade (i.e., CPS) type and warrant further investigation. Importantly, the in vivo survival of isolates representative for the most and least susceptible strains was not different in mice. Consequently, unless a higher prevalence of ST258 infections in patients with complement deficiency is shown, the observation of different phenotypes at low complement activity is expected to have little clinical relevance.
Similarly, high serum-sensitivity of all ST258 strains was found in freshly prepared naïve rat and rabbit serum, while mouse serum exhibited limited bactericidal activity in vitro. This latter observation is in good correlation with previous findings by several groups indicating low complement activity of mouse sera,35,36 suggesting spontaneous inactivation of some mouse complement factor(s).
In the light of the rapid bacterial lysis in human serum, the high prevalence and mortality of K. pneumoniae ST258 bacteremia cases are surprising. Serum sensitivity patterns of these isolates imply that antibiotic therapy may provide limited added benefit against the bacteria in the bloodstream and likely acts through elimination of the primary infectious loci (i.e., in solid organs). In accordance with this, no significant influence of early appropriate antibiotic treatment on in-hospital mortality due to CRKP infections was found.2 On the other hand, infections accompanied by bacteremia were shown to be associated with significantly higher mortality rates than non-bacteremic cases.2 Not surprisingly, when considering bacteremia cases only, septic shock was found to be by far the most significant independent risk factor for mortality in this and other studies.37-39 We propose that rapid complement mediated lysis in the circulation may result in the release of elevated amounts of endotoxin that contribute to the development of septic shock and consequently higher mortality.
In this study, we have characterized a humanized mAb (A1102) targeting the conserved LPS O-antigen, i.e., D-galactan-III of ST258 strains that we described recently.23 Although this mAb does not bind directly to Lipid A, which is the endotoxin portion of LPS, it exhibited potent endotoxin neutralizing activity, measured in an in vitro assay that detects TLR4 signaling. This neutralization potency was found to be superior to that of polymyxin B, a small cyclic peptide antibiotic that binds directly to Lipid A. The in vitro LPS neutralizing activity was corroborated by in vivo protection studies in a GalN sensitized murine model of endotoxemia. We showed efficacy of A1102 against challenge with extracted LPS molecules as well as with whole bacteria. While extracted LPS validated the specificity of the model for endotoxin, whole bacterial cells served as a more natural source of liberated LPS, mimicking the in vivo scenario more closely. We confirmed that the protection against live bacterial challenge in this model required neither Fc-mediated functions nor active complement. Additionally, the mAb did not influence the in vivo kinetics of bacteria corroborating that the LPS-neutralizing activity may be the sole protective mechanism. Additionally, in rabbits that are intrinsically sensitive to the LPS endotoxin, this mAb afforded significant levels of protection at very low doses (2 µg/kg) in a stringent bacteremia model.
The monoclonal antibody approach aiming for endotoxin neutralization and ultimately reduction of sepsis-related pathogenesis and mortality, has been considered previously with great expectations. However, so far all endotoxin-neutralizing antibodies have failed in the clinic.40 These failures can be attributed to the low affinity of these mAbs, their poly-reactivity when tested against irrelevant antigens, their IgM isotype or that they were non-human antibodies that specifically targeted the Lipid A or the core oligosaccharide portion of LPS. In contrast, the mAb described in this study is a humanized IgG molecule with high affinity for the O-antigen part of LPS. The affinity determined for A1102 is one of the highest measured for an anti-carbohydrate antibody, determined by similar methods,41 and similar to that reported for the anti-E. coli LPS core mAb WN1 222–5 (32 nM)42 whose complex with the polysaccharide involves extensive interactions (7 sugar moieties and 13 H-bonds)43. While Lipid A is not expected to be accessible to antibodies neither on live bacterial cells nor in released forms (buried in micelles), O-antigens are highly exposed.
On the other hand, given the variable nature of O-antigens in Enterobacteriaceae, the specificity of gal-III mAbs are restricted to K. pneumoniae ST258 strains23 and approx. 15% of other Klebsiella strains (unpublished) that express this particular O-antigen. Nevertheless, given the high medical need, such precision, narrow spectrum antimicrobials may be considered for future development. Furthermore, in comparison to broad-spectrum antibiotics, anti-bacterial monoclonal antibodies have several practical advantages, including lack of adverse effects on the microbiota and lack of intrinsic toxicity.
K. pneumoniae is an opportunistic pathogen typically infecting high-risk patients that are immuno-compromised/suppressed and/or have underlying comorbidities. In the absence of relevant clinical data, we cannot rule out that ST258 has a higher prevalence in patients with compromised complement activities. Investigation of matched pairs of serum and bacterium isolates from bacteremia cases is in progress to verify this possibility. In theory, in these patients, ST258 strains may survive in the bloodstream and therefore mAbs with bactericidal effector function would be desirable. This study shows that besides the LPS neutralizing activity, gal-III specific mAbs are able to enhance the killing of ST258 isolates in serum samples as well as to mediate opsonophagocytic uptake. Which of these modes of action is the most relevant in a clinical setting will need to be addressed by future translational studies.
The high cost of monoclonal antibody therapies compared with antibiotics has up until now been one of the limiting factors in their development as antibacterial agents. However, given the high potency of the mAb described in this study it is likely that very low doses will be required in the clinic, which could translate to a low cost therapy that could be used for a broad pre-emptive approach in ST258 colonized individuals, and during hospital outbreaks, to prevent the development of severe infections.
Supplementary Material
Disclosure of potential conflicts of Interest
All authors are employees of Arsanis Biosciences GmbH and hold shares in the company.
Funding
This work was supported by Eurostars grant E! 7563 – KLEBSICURE.
References
- [1].Livermore DM. Current epidemiology and growing resistance of gram-negative pathogens. Korean J Intern Med 2012; 27(2):128-42; PMID:22707882; http://dx.doi.org/ 10.3904/kjim.2012.27.2.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Capone A, Giannella M, Fortini D, Giordano A, Meledandri M, Ballardini M, Venditti M, Bordi E, Capozzi D, Balice MP, et al.. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin Microbiol Infect 2013; 19(1):E23-30; PMID:23137235; http://dx.doi.org/ 10.1111/1469-0691.12070 [DOI] [PubMed] [Google Scholar]
- [3].Weterings V, Zhou K, Rossen JW, van Stenis D, Thewessen E, Kluytmans J, Veenemans J. An outbreak of colistin-resistant Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae in the Netherlands (July to December 2013), with inter-institutional spread. Eur J Clin Microbiol Infect Dis 2015; 34(8):1647-55;PMID:26067658; http://dx.doi.org/ 10.1007/s10096-015-2401-2 [DOI] [PubMed] [Google Scholar]
- [4].Mammina C, Bonura C, Di Bernardo F, Aleo A, Fasciana T, Sodano C, Saporito MA, Verde MS, Tetamo R, Palma DM. Ongoing spread of colistin-resistant Klebsiella pneumoniae in different wards of an acute general hospital, Italy, June to December 2011. Euro Surveill 2012; 17(33):pii: 20248; PMID:22913977 [PubMed] [Google Scholar]
- [5].Czaplewski L, Bax R, Clokie M, Dawson M, Fairhead H, Fischetti VA, Foster S, Gilmore BF, Hancock RE, Harper D, et al.. Alternatives to antibiotics-a pipeline portfolio review. Lancet Infect Dis 2016;16(2):239-51;PMID:26795692; http://dx.doi.org/ 10.1016/S1473-3099(15)00466-1 [DOI] [PubMed] [Google Scholar]
- [6].Oleksiewicz MB, Nagy G, Nagy E. Anti-bacterial monoclonal antibodies: back to the future? Arch.Biochem Biophys 2012; 526(2):124-31; PMID:22705202; http://dx.doi.org/ 10.1016/j.abb.2012.06.001 [DOI] [PubMed] [Google Scholar]
- [7].Ter Meulen J. Monoclonal antibodies in infectious diseases: clinical pipeline in 2011. Infect Dis Clin North Am 2011; 25(4):789-802; PMID:22054756; http://dx.doi.org/ 10.1016/j.idc.2011.07.006 [DOI] [PubMed] [Google Scholar]
- [8].Casadevall A, Scharff MD. Return to the past: the case for antibody-based therapies in infectious diseases. Clin Infect Dis 1995; 21(1):150-61; PMID:7578724; http://dx.doi.org/ 10.1093/clinids/21.1.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Diago-Navarro E, Chen L, Passet V, Burack S, Ulacia-Hernando A, Kodiyanplakkal RP, Levi MH, Brisse S, Kreiswirth BN, Fries BC, et al.. Carbapenem-resistant Klebsiella pneumoniae exhibit variability in capsular polysaccharide and capsule associated virulence traits. J Infect Dis 2014; 210(5):803-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tzouvelekis LS, Miriagou V, Kotsakis SD, Spyridopoulou K, Athanasiou E, Karagouni E, Tzelepi E, Daikos GL. KPC-producing, multidrug-resistant Klebsiella pneumoniae sequence type 258 as a typical opportunistic pathogen. Antimicrob Agents Chemother 2013; 57(10):5144-6; PMID:23856769; http://dx.doi.org/ 10.1128/AAC.01052-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Siu LK, Lin JC, Gomez E, Eng R, Chiang T. Virulence and plasmid transferability of kpc Klebsiella pneumoniae at the veterans affairs healthcare system of new jersey. Microb Drug Resist 2012; 18(4):380-4; PMID:22533374;http://dx.doi.org/ 10.1089/mdr.2011.0241 [DOI] [PubMed] [Google Scholar]
- [12].Borer A, Saidel-Odes L, Eskira S, Nativ R, Riesenberg K, Livshiz-Riven I, Schlaeffer F, Sherf M, Peled N. Risk factors for developing clinical infection with carbapenem-resistant Klebsiella pneumoniae in hospital patients initially only colonized with carbapenem-resistant k pneumoniae. Am J Infect Control 2012; 40(5):421-5; PMID:21906844;http://dx.doi.org/ 10.1016/j.ajic.2011.05.022 [DOI] [PubMed] [Google Scholar]
- [13].Schechner V, Kotlovsky T, Kazma M, Mishali H, Schwartz D, Navon-Venezia S, Schwaber MJ, Carmeli Y. Asymptomatic rectal carriage of blakpc producing carbapenem-resistant enterobacteriaceae: who is prone to become clinically infected? Clin Microbiol Infect 2013; 19(5):451-6; PMID:22563800; http://dx.doi.org/ 10.1111/j.1469-0691.2012.03888.x [DOI] [PubMed] [Google Scholar]
- [14].Gasink LB, Edelstein PH, Lautenbach E, Synnestvedt M, Fishman NO. Risk factors and clinical impact of Klebsiella pneumoniae carbapenemase-producing k. pneumoniae. Infect Control Hosp Epidemiol 2009; 30(12):1180-5; PMID:19860564; http://dx.doi.org/ 10.1086/648451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 2012; 25(4):682-707; PMID:23034326; http://dx.doi.org/ 10.1128/CMR.05035-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Trautmann M, Ruhnke M, Rukavina T, Held TK, Cross AS, Marre R, Whitfield C. O-Antigen seroepidemiology of klebsiella clinical isolates and implications for immunoprophylaxis of klebsiella infections. Clin Diagn Lab Immunol 1997; 4(5):550-5; PMID:9302204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rukavina T, Tícac B, Susa M, Jendrike N, Jonjíc S, Lucin P, Marre R, Doríc M, Trautmann M. Protective effect of antilipopolysaccharide monoclonal antibody in experimental klebsiella infection. Infect Immun 1997; 65(5):1754-60; PMID:9125558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Donta ST, Peduzzi P, Cross AS, Sadoff J, Haakenson C, Cryz SJ Jr, Kauffman C, Bradley S, Gafford G, Elliston D, et al.. Immunoprophylaxis against klebsiella and pseudomonas aeruginosa infections. the federal hyperimmune immunoglobulin trial study group. J Infect Dis 1996; 174(3):537-43;PMID:8769611;http://dx.doi.org/ 10.1093/infdis/174.3.537 [DOI] [PubMed] [Google Scholar]
- [19].Chen L, Mathema B, Pitout JD, DeLeo FR, Kreiswirth BN. Epidemic Klebsiella pneumoniae st258 is a hybrid strain. MBio 2014; 5(3):e01355-14; http://dx.doi.org/ 10.1128/mBio.01355-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].DeLeo FR, Chen L, Porcella SF, Martens CA, Kobayashi SD, Porter AR, Chavda KD, Jacobs MR, Mathema B, Olsen RJ, et al.. Molecular dissection of the evolution of carbapenem-resistant multilocus sequence type 258 Klebsiella pneumoniae. Proc Natl Acad Sci U.S.A 2014; 111(13):4988-93;PMID:24639510; http://dx.doi.org/ 10.1073/pnas.1321364111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wyres KL, Gorrie C, Edwards DJ, Wertheim HF, Hsu LY, Van Kinh N, Zadoks R, Baker S, Holt KE. Extensive capsule locus variation and large-scale genomic recombination within the Klebsiella pneumoniae clonal group 258. Genome Biol Evol 2015; 7(5):1267-79; PMID:25861820; http://dx.doi.org/ 10.1093/gbe/evv062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bowers JR, Kitchel B, Driebe EM, MacCannell DR, Roe C, Lemmer D, de Man T, Rasheed JK, Engelthaler DM, Keim P, et al.. Genomic analysis of the emergence and rapid global dissemination of the clonal group 258 Klebsiella pneumoniae pandemic. PLoS One 2015; 10(7):e0133727; PMID:26196384; http://dx.doi.org/ 10.1371/journal.pone.0133727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Szijarto V, Guachalla LM, Hartl K, Varga C, Banerjee P, Stojkovic K, Kaszowska M, Nagy E, Lukasiewicz J, Nagy G. Both clades of the epidemic KPC-producing Klebsiella pneumoniae clone ST258 share a modified galactan O-antigen type. Int J Med Microbiol 2016; 306(2):89-98; PMID:26723873;http://dx.doi.org/ 10.1016/j.ijmm.2015.12.002 [DOI] [PubMed] [Google Scholar]
- [24].Geraci DM, Bonura C, Giuffrè M, Saporito L, Graziano G, Aleo A, Fasciana T, Di Bernardo F, Stampone T, Palma DM, et al.. Is the monoclonal spread of the ST258, KPC-3-producing clone being replaced in Southern Italy by the dissemination of multiple clones of carbapenem-nonsusceptible, KPC-3-producing Klebsiella pneumoniae? Clin Microbiol Infect 2015; 21(3):e15-7; PMID:25658574;http://dx.doi.org/ 10.1016/j.cmi.2014.08.022 [DOI] [PubMed] [Google Scholar]
- [25].Bonura C, Giuffrè M, Aleo A, Fasciana T, Di Bernardo F, Stampone T, Giammanco A; MDR-GN Working Group, Palma DM, Mammina C. An update of the evolving epidemic of blakpc carrying Klebsiella pneumoniae in Sicily, Italy, 2014: emergence of multiple Non-ST258 clones. PLoS One 2015; 10(7):e0132936; PMID:26177547; http://dx.doi.org/ 10.1371/journal.pone.0132936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fraenkel-Wandel Y, Raveh-Brawer D, Wiener-Well Y, Yinnon AM, Assous MV. Mortality Due to BlaKPC Klebsiella pneumoniae bacteraemia. J Antimicrob Chemother 2016; 71(4):1083-7; PMID:26661396; http://dx.doi.org/ 10.1093/jac/dkv414 [DOI] [PubMed] [Google Scholar]
- [27].Baraniak A, Grabowska A, Izdebski R, Fiett J, Herda M, Bojarska K, Żabicka D, Kania-Pudło M, Młynarczyk G, Żak-Puławska Z, et al.. Molecular characteristics of KPC-producing enterobacteriaceae at the early stage of their dissemination in Poland, 2008-2009. Antimicrob Agents Chemother 2011; 55(12):5493-9; PMID:21930889; http://dx.doi.org/ 10.1128/AAC.05118-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fodah RA, Scott JB, Tam HH, Yan P, Pfeffer TL, Bundschuh R, Warawa JM. Correlation of Klebsiella pneumoniae comparative genetic analyses with virulence profiles in a murine respiratory disease model. PLoS One 2014; 9(9):e107394; PMID:25203254; http://dx.doi.org/ 10.1371/journal.pone.0107394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Szijarto V, Guachalla LM, Visram ZC, Hartl K, Varga C, Mirkina I, Zmajkovic J, Badarau A, Zauner G, Pleban C, et al.. Bactericidal monoclonal antibodies specific to the lipopolysaccharide O antigen from multidrug-resistant escherichia coli clone ST131-O25b:H4 elicit protection in mice. Antimicrob Agents Chemother 2015; 59(6):3109-16;PMID:25779571;http://dx.doi.org/ 10.1128/AAC.04494-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Galanos C, Freudenberg MA, Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U.S.A 1979; 76(11):5939-43; PMID:293694; http://dx.doi.org/ 10.1073/pnas.76.11.5939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Muller-Loennies S, MacKenzie CR, Patenaude SI, Evans SV, Kosma P, Brade H, Brade L, Narang S. Characterization of high affinity monoclonal antibodies specific for chlamydial lipopolysaccharide. Glycobiology 2000; 10(2):121-30; PMID:10642603 [DOI] [PubMed] [Google Scholar]
- [32].Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK, et al.. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 2013; 13(9):785-96; PMID:23969216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Conte V, Monaco M, Giani T, D'Ancona F, Moro ML, Arena F, D'Andrea MM, Rossolini GM, Pantosti A, et al.. Molecular epidemiology of KPC-producing Klebsiella pneumoniae from invasive infections in Italy: increasing diversity with predominance of the ST512 clade II sublineage. J Antimicrob Chemother 2016; 71(12):3386-91; PMID:27585968 [DOI] [PubMed] [Google Scholar]
- [34].Chiang TT, Yang YS, Yeh KM, Chiu SK, Wang NC, Lin TY, Huang LY, Chang FY, Siu LK, Lin JC, et al.. quantification and comparison of virulence and characteristics of different variants of carbapenemase-producing Klebsiella pneumoniae clinical isolates from Taiwan and the United States. J Microbiol Immunol Infect 2016; 49(1):83-90; PMID:26514941 [DOI] [PubMed] [Google Scholar]
- [35].Muschel LH, Muto T. Bactericidal reaction of mouse serum. Science 1956; 123(3185):62-4; PMID:13281484 [DOI] [PubMed] [Google Scholar]
- [36].Nagy G, Dobrindt U, Hacker J, Emödy L. Oral immunization with an RfaH mutant elicits protection against salmonellosis in mice. Infect Immun 2004; 72(7):4297-301; PMID:15213179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dubrovskaya Y, Chen TY, Scipione MR, Esaian D, Phillips MS, Papadopoulos J, Mehta SA. Risk factors for treatment failure of polymyxin B monotherapy for carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother 2013; 57(11):5394-7; PMID:23959321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Papadimitriou-Olivgeris M, Marangos M, Christofidou M, Fligou F, Bartzavali C, Panteli ES, Vamvakopoulou S, Filos KS, Anastassiou ED. Risk factors for infection and predictors of mortality among patients with KPC-producing Klebsiella pneumoniae bloodstream infections in the intensive care unit. Scand J Infect Dis 2014; 46(9):642-8; PMID:25017796 [DOI] [PubMed] [Google Scholar]
- [39].Tumbarello M, Trecarichi EM, De Rosa FG, Giannella M, Giacobbe DR, Bassetti M, Losito AR, Bartoletti M, Del Bono V, Corcione S, et al.. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother 2015; 70(7):2133-43; PMID:25900159 [DOI] [PubMed] [Google Scholar]
- [40].Hurley JC. Towards clinical applications of anti-endotoxin antibodies; a Re-Appraisal of the disconnect. Toxins.(Basel) 2013; 5(12):2589-620; PMID:24351718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mackenzie CR, Muller-Loennies S. Determination of antibody affinity by surface plasmon resonance In Anticarbohydrate Antibodies, 403-29. Springer, 2012. [Google Scholar]
- [42].Muller-Loennies S, Brade L, MacKenzie CR, Di Padova FE, Brade H, et al.. Identification of a cross-reactive epitope widely present in lipopolysaccharide from enterobacteria and recognized by the cross-protective monoclonal antibody WN1 222-5. J Biol Chem 2003; 278(28):25618-27 [DOI] [PubMed] [Google Scholar]
- [43].Gomery K, Müller-Loennies S, Brooks CL, Brade L, Kosma P, Di Padova F, Brade H, Evans SV. Antibody WN1 222-5 mimics toll-like receptor 4 binding in the recognition of LPS. Proc Natl Acad Sci U.S.A 2012; 109(51):20877-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


