Abstract
The chemically simple, biologically complex eukaryotic polyamines, spermidine and spermine, are positively charged alkylamines involved in many crucial cellular processes. Along with their diamine precursor putrescine, their normally high intracellular concentrations require fine attenuation by multiple regulatory mechanisms to keep these essential molecules within strict physiologic ranges. Since the metabolism of and requirement for polyamines are frequently dysregulated in neoplastic disease, the metabolic pathway and functions of polyamines provide rational drug targets; however, these targets have been difficult to exploit for chemotherapy. It is the goal of this article to review the latest findings in the field that demonstrate the potential utility of targeting the metabolism and function of polyamines as strategies for both chemotherapy and, possibly more importantly, chemoprevention.
Polyamine function
Polyamines have been implicated in several critical cellular functions. These include nucleic acid and chromatin structure maintenance, ion channel regulation, and protein synthesis. Polyamines also serve as substrates for transglutaminase reactions and for the synthesis of an important translational regulator, hypusine [1–7]. Additionally, the natural polyamines act as free radical scavengers, thus having the capacity to protect nucleic acids from damage [8,9]. Their millimolar intracellular concentration is tightly regulated, as detailed below, although the actual amount of free intracellular polyamines is low due to their ionic association with cellular anions. An intriguing new function for the natural polyamines has been proposed related to its role in regulating the inward rectifying channel for potassium. Nakajima et al. [10] have provided data, indicating that the polyamines play an essential role in sensing weak electric fields that guide cell migration known as galvanotaxis. These novel findings implicate yet another role for polyamines in cell migration/metastasis that clearly has significance in cancer.
Polyamine metabolism
Prior to discussing the agents that have been designed to target polyamines as an antineoplastic strategy, a description of the metabolic and regulatory pathways involved in maintaining polyamine homeostasis is necessary.
Biosynthesis
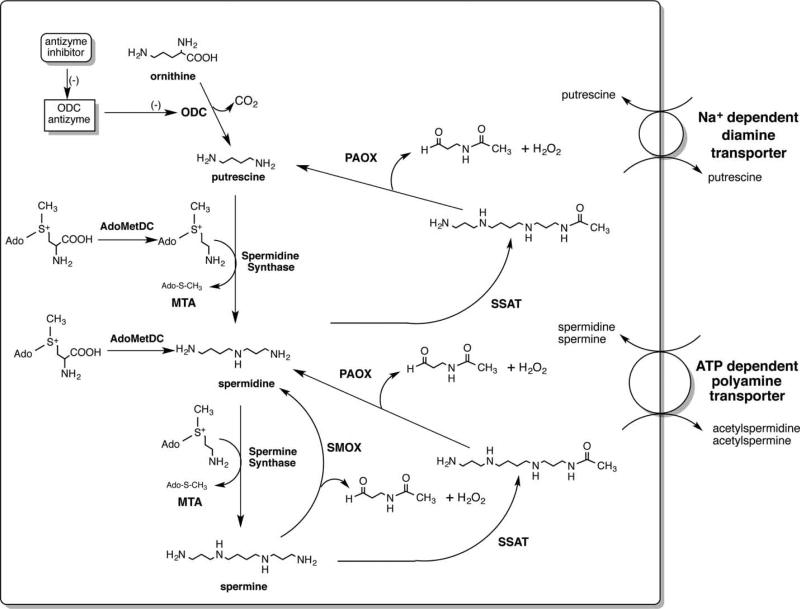
Although Leuwenhoek first described the polyamine spermine in 1678, it was the crucial discovery linking an increase in polyamine biosynthesis and intracellular polyamine concentrations with tissues induced to proliferate that prompted the initial interest in targeting polyamine biosynthesis for therapeutic benefit. In rat liver induced to proliferate by partial hepatectomy, Russell and Snyder [11] described an increase in ornithine decarboxylase (ODC, Figure 1), where Pegg and Williams-Ashman [12] demonstrated that both ODC and S-adenosylmethionine decarboxylase (AdoMetDC) were induced in androgen-stimulated rat prostate.
Figure 1. The polyamine metabolic pathway.
ODC is the first rate-limiting step in polyamine biosynthesis and produces putrescine; AdoMetDC is also rate-limiting and its activity provides the aminopropyl donor for the synthesis of both spermidine and spermine; SMOX is an inducible FAD-dependent oxidase located in both the nucleus and the cytoplasm that can produce DNA damage by its production of H2O2; PAOX is generally a constitutively expressed, peroxisomal oxidase that is rate-limited by the availability of N1-acetylated polyamines; SSAT is an inducible enzyme that is critical for the maintenance of polyamine homeostasis and is implicated in the cytotoxic activity of several polyamine analogs; MTA is produced from decarboxylated S-adenosylmethionine by the activity of both spermidine and spermine synthases.
Mammalian ODC is a pyridoxylphosphate-requiring enzyme that is active as a dimer. With an extremely short half-life (10–30 min), this rate-limiting enzyme catalyzes the decarboxylation of the urea cycle amino acid ornithine to the diamine putrescine. ODC is regulated at every level from transcription to posttranslational modulation. A novel class of regulatory proteins, ODC antizymes, bind to and inhibit monomers of ODC, targeting them for proteosomal degradation without ubiquitination [13]. In addition to the antizymes, ODC is also regulated through a noncatalytic homolog of ODC, antizyme inhibitor [14–16]. Antizyme inhibitor binds to antizyme and prevents it from binding and degrading ODC. It should also be noted that ODC was the first gene to be transcriptionally regulated by the proto-oncogene c-Myc [17,18]. The linkage of these two important growth-inducing pathways suggests possibilities in drug targeting.
The second rate-limiting step in polyamine biosynthesis is the self-processing, pyruvoyl-containing S-adenosylmethionine decarboxylase (AdoMetDC/AMD/SAMDC [19]). Mammalian AdoMetDC is also a short-lived protein that has multiple levels of regulation, including ubiquitin-dependent proteosomal degradation. AdoMetDC provides the aminopropyl donor for synthesis of the higher polyamines, spermidine and spermine (Figure 1), by catalyzing the removal of the carboxyl group of AdoMet. Subsequently, decarboxylated AdoMet is a committed intermediate for aminopropyl transfer. As a result, a portion of the AdoMet pool is no longer available for use in transmethylation reactions, in which it plays a critical role, as will be discussed below.
The balance of the mammalian polyamine biosynthetic pathway is made up of two distinct aminopropyltransferases, spermidine synthase and spermine synthesis (Figure 1) [20–22]. These two enzymes are generally rate-limited by the availability of their substrates, and the activity of each produces 5′-deoxy-5′-(methylthio) adenosine (MTA), which is a potent product inhibitor of both synthases. Importantly, the gene for MTA phosphorylase, the enzyme critical to the salvage of this product, is frequently lost in carcinogenic transformation due to its proximity to multiple tumor suppressor genes [23].
Catabolism
Mammalian polyamine catabolism represents another critical control mechanism by which polyamine homeostasis is maintained. Although there are several amine oxidases that can use polyamines as substrates, including the copper-containing serum amine oxidase and FAD-dependent monoamine oxidases, the primary intracellular polyamine-catabolizing enzymes are spermidine/spermine N1-acetyltransferase (SSAT/SAT1), N1-acetylpolyamine oxidase (PAOX/APAO/PAO), and spermine oxidase (SMOX/PAOh1/SMO) (Figure 1) [24].
The initial description of the polyamine catabolic pathway was a two-step pathway that is initiated by the acetylation of spermidine or spermine by SSAT and the subsequent oxidation by PAOX [25]. The highly inducible SSAT catalyzes the transfer of an acetyl group from acetyl-CoA to the N1 position of either spermidine or spermine. This acetylated polyamine is frequently excreted from the cell; however, the acetylpolyamines are also substrates for peroxisomal PAOX. PAOX is a flavoenzyme capable of oxidizing the acetylated polyamines to produce H2O2, 3-aceto-aminopropanal, and either spermidine or putrescine, depending on the initial substrate [26]. In most cases, PAOX is constitutively expressed and thus rate-limited by its acetylated substrate. However, some instances have been described where PAOX levels are elevated [27]. Importantly, the H2O2 produced in the peroxisome by PAOX would generally be rapidly degraded by peroxisomal catalase.
Until 2001, the two-step pathway was assumed to be the only mammalian catabolic pathway available allowing for back-conversion of the higher intracellular polyamines. However, during molecular cloning attempts to identify the human PAOX gene, a novel oxidase that catalyzed the unmodified spermine as its preferred substrate was discovered [28]. Originally termed PAOh1, SMOX is an FAD-dependent enzyme with high homology to PAOX. However, unlike PAOX, SMOX is highly inducible by many of the same stimuli as SSAT and can be rapidly induced from nearly undetectable to very high levels [29]. SMOX is found both in the cytoplasm and in the nucleus and catalyzes spermine into the aldehyde 3-aminopropanal, H2O2, and spermidine [30]. Since it is not a peroxisomal enzyme and it does produce H2O2 in close proximity to chromatin, the potential for DNA damage, as will be discussed below, is high. Also of note, it was during the discovery of SMOX that another structurally related oxidase, lysine-specific demethylase 1 (LSD1/KDM1/KDM1A), was discovered. LSD1 was the first chromatin-modifying enzyme discovered to be capable of demethylating methylated histone proteins [31].
Polyamine transport
Mammalian polyamine transport is an energy-dependent, saturable process that allows cells to accumulate polyamines from their surroundings. This process also contributes significantly to the homeostasis of intracellular polyamine content, as the human diet is rich in polyamines and the normal gut flora excretes significant quantities of polyamines into the gut lumen; extracellular polyamines from these sources can be taken up by luminal cells, resulting in increased levels of circulating polyamines. Intriguingly, although the polyamine transport system has been biochemically characterized and its substrate specificity has been thoroughly investigated, the actual molecular characterization of the mammalian polyamine transport is sorely lacking [32]. Three molecular models of polyamine transport have been proposed, each of which conforms with varying degrees to most of the biochemical evidence.
The most comprehensive model of polyamine transport has been proposed by Poulin and colleagues [33]. This model predicts a highly selective membrane permease or permeases facilitating the initial entry of the polyamines into the cell. The polyamines are then rapidly internalized into endosomes, where they can be concentrated and/or dispersed throughout the cell as needed. Data also support a second mechanism of mammalian polyamine transport proposed by Belting et al. [34]. In this model, spermine is bound to heparin sulfate moieties in glycopan-1 at the cell surface. This bound spermine is then internalized into endosomes and can subsequently be freed by a nitric oxide-mediated oxidation mechanism. Finally, Uemura et al. [35] have proposed a model in gastrointestinal tissues where caveolar endocytosis and nitric oxide production play a significant role in putrescine uptake. Additionally, their data also suggest that the solute carrier protein SLC3A2 may play roles both as an exporter of putrescine from cells and as an importer, depending on concentration gradients.
Targeting polyamine metabolism as an anticancer strategy
Inhibition of polyamine biosynthesis
Targeting ODC
Once the requirement for polyamines in cell growth was recognized, several investigators posited that targeting polyamine biosynthesis could be a rational approach for inhibiting unwanted cell proliferation such as that occurring in cancer. The most successful and widely used inhibitor of polyamine biosynthesis is 2-difluoromethylornithine (DFMO, Figure 2). DFMO was specifically designed to be an enzyme-activated irreversible inhibitor of ODC [36]. Initially, DFMO competes with ODC’s natural substrate at the active site by forming a Schiff’s base with the aldehyde moiety of the tightly bound cofactor pyridoxal phosphate. Once bound, DFMO is decarboxylated, forming a highly reactive intermediate that ultimately becomes covalently bound to ODC, resulting in permanent inactivation.
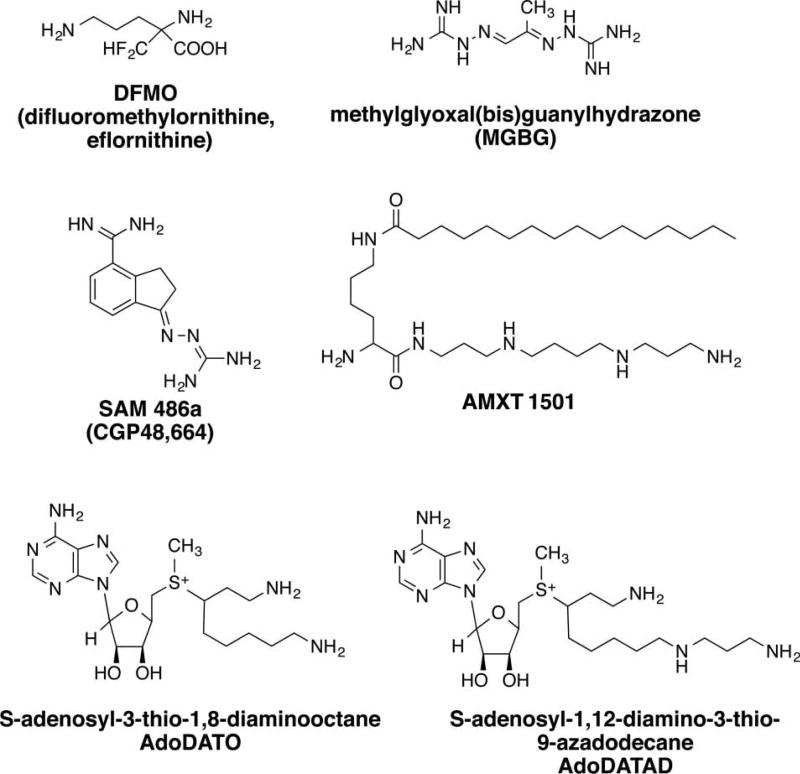
Figure 2. Inhibitors of polyamine biosynthesis and transport.
DFMO is an enzyme-activated irreversible inhibitor of ODC. MGBG is a potent, competitive inhibitor of AdoMetDC, but is also a potent mitochondrial toxin. SAM486A is a potent second-generation AdoMetDC inhibitor that does not exhibit mitochondrial toxicity. AMXT 1501 is a spermine conjugate inhibitor of polyamine transport. AdoDATO and AdoDATAD are inhibitors of the aminopropyltransferases spermidine synthase and spermine synthase, respectively.
Several investigators have used DFMO to demonstrate the requirement of polyamines for eukaryotic proliferation. Early studies elucidated several important features of DFMO treatment, the most important being that most cells and tissue types respond to DFMO-induced polyamine depletion in a cytostatic, rather than cytotoxic manner [37,38]. Treatment with DFMO typically leads to a depletion of both putrescine and spermidine, with varying effects on the spermine levels. Frequently, cell proliferation is halted prior to significant depletion of spermine. This effect is not only true for normal cells, but also tumor cells. Consequently, DFMO was viewed as a highly novel antiproliferative in that it did not result in overt cell killing, unlike most cytotoxic anticancer agents. Additionally, as further study demonstrated that polyamine metabolism and the requirement for polyamines were frequently dysregulated in cancer, the hypothesis that polyamine depletion could selectively target tumor cells was extensively tested. Early excitement with DFMO peaked when it was discovered that specific cancers responded to DFMO treatment in a cytotoxic manner, while sparing normal cells. This was first demonstrated in some promyelocytic leukemias and in many cells lines representative of small cell lung cancers [39,40].
These encouraging results in selective cancers, both in vitro and in vivo led to clinical trials with DFMO as a single agent. Although DFMO was exceedingly well tolerated, there were no significant clinical responses observed in the early trials [41–44]. More recently, a resurgence of interest in DFMO as a single agent has occurred in the treatment of neuroblastoma [45]. The molecular basis for this focus is the integral role played in neuroblastoma growth by the nexus between MYC expression, ODC transcription and cell proliferation [46–48]. Consequently, DFMO is currently in clinical trial for the treatment of refractory/high-risk neuroblastoma in children, both alone and in combination with other agents (see section below).
Targeting AdoMetDC
In addition to targeting the first rate-limiting step in polyamine biosynthesis, successful attempts have been made in targeting the second rate-limiting step, AdoMetDC. The earliest description of AdoMetDC inhibition was with the antiproliferative agent methylglyoxal bis(guanylhydrazone) (MGBG, Figure 2). MGBG, a structural analog of spermidine, was demonstrated to competitively inhibit AdoMetDC and reduce intracellular levels of both spermidine and spermine [49]. However, as the mitochondrial toxicity exhibited by MGBG treatment precedes its effects on polyamine pools, it is likely that its antiproliferative effects are a result of mitochondrial toxicity [50].
Although MGBG proved to be too toxic for further clinical development, other inhibitors of AdoMetDC were developed and tested for their ability to significantly reduce spermidine and spermine levels without significant off-target effects, including mitochondrial toxicity. One such compound, based on the structure of MGBG, is 4-amidinoidan-1-one-2′-amidinhydrazone (SAM486A/CGP48664, Figure 2) [51,52]. SAM486A has been evaluated in Phase I and II clinical trials, demonstrating significant activity against nonHodgkin’s lymphoma [53,54]. As will be discussed below, SAM486A has been used in combination with other agents as a strategy to more completely deplete polyamines for therapeutic benefit.
Inhibitors of spermidine and spermine synthase
Although no clinical trials have been performed with inhibitors of the higher polyamine synthases, two multisubstrate adduct transition state analogs have been synthesized and used as tools to define the role of each of the synthases. S-adenosyl-3-thio-1,8-diaminooctane (AdoDATO, Figure 2) was designed to specifically inhibit spermidine synthase, and S-adenosyl-1,12-diamino-3-thio-9-azadodecane (AdoDATAD, Figure 2) was designed to specifically inhibit spermine synthase [55,56]. Each compound is an efficient and highly selective inhibitor of its respective aminopropyltransferase; however, as both compounds contain primary amines, they are also substrates for SSAT and amine oxidases. Thus, their utility is limited due to rapid intracellular metabolism.
Drug combinations with DFMO
Since DFMO was demonstrated to be well tolerated, several therapies combining other agents with DFMO have been investigated. A selection of the most recent findings will be reviewed here.
One of the major limitations of cancer treatment with DFMO as a single agent is the physiologic response of cells to induce polyamine transport when biosynthesis is inhibited. As stated above, the diet and gut flora are rich sources of polyamines; consequently, the combination of DFMO with agents that block polyamine transport is currently being evaluated. Several investigators have synthesized and tested structural analogs of the polyamines that are putatively capable of binding to and/or blocking the extracellular component of the mammalian polyamine transport system, thus inhibiting the accumulation of extracellular polyamines after intracellular polyamines have been depleted by DFMO treatment. One efficient transport inhibitor, AMXT 1501 (Figure 2), has demonstrated significant synergy with DFMO in breast, prostate, and melanoma cell lines in addition to an ODC overexpression model of squamous cell carcinoma of the skin [57]. More recently, impressive results were obtained when this compound was combined with DFMO in neuroblastoma, a tumor type in which DFMO alone has shown some promise [58]. Another interesting application of what is currently being termed ‘polyamine-blocking therapy’ (PBT) is the goal of reducing T-cell immune repression, thus enhancing the antitumor immune response [59]. In that study, Burns and colleagues [59] demonstrated that in a model of breast cancer in immunocompetent mice, PBT significantly inhibits tumor proliferation in an immune-dependent manner. Equally impressively, when residual tumors are removed from animals treated with PBT, the mice become refractory to further tumor formation, demonstrating immune memory.
Phanstiel and colleagues have synthesized and evaluated compounds designed as inhibitors of polyamine transport that differ significantly from the lysyl–spermine conjugates reported by Burns and colleagues [60,61]. However, similar to AMXT 1501, the combination of these transport inhibitors with DFMO also produces significant growth inhibition in a variety of tumor cell types.
DFMO has also been used in combination with cytotoxic agents with interesting results. Levin et al. [62] reported a modest increase in survival in glioma patients treated with the combination of DFMO with a procarbazine, nitrosourea, and vincristine regimen when compared with the cytotoxic regimen alone. There are also current and recently completed clinical trials investigating the combination of DFMO with the proteosome inhibitor bortezomib in recurrent/refractory neuroblastoma (NCT02139397) and in combination with etopiside, again in recurrent/refractory neuroblastoma [63].
Recently, investigators have examined tumor necrosis factor-related apoptosis-inducing ligand in combination with DFMO and radiation in multiple brain tumor lines. Although it is too early to predict if such a combination might have clinical utility, it does underscore the continued interest in the clinical development of the well-tolerated ODC inhibitor [64].
Polyamine analogs and cancer
Several comprehensive reviews have covered the synthesis and analysis of the polyamine analogs in recent years, and the reader is directed to those reviews for a more complete coverage of the topic [65–69]. The goal here will be to highlight the rational for the development of polyamine analogs as well as a few of the most recent findings.
The original impetus for the development of polyamine analogs was based on multiple observations regarding polyamine metabolism with respect to the deficiencies encountered upon targeted inhibition of the polyamine biosynthetic enzymes. A major issue with the inhibition of either ODC or AdoMetDC was the increase in polyamine transport resulting from the depletion of intracellular polyamines. Additionally, when ODC is inhibited, AdoMetDC activity is increased, and when AdoMetDC is inhibited, ODC activity is increased. Such compensatory mechanisms thus had the potential to overcome the desired growth inhibitory effects of the inhibitors. Consequently, a desired property of the new analogs was sufficient structural similarity to the natural polyamines to enable usage of the polyamine transport system to enter the cell, thereby acting as uptake inhibitors of the natural polyamines. Additionally, the analogs should down-regulate the polyamine biosynthetic pathway through a means similar to the feedback inhibition exhibited by the natural polyamines on their own biosynthesis. Finally, the analogs should not substitute for the polyamines in their growth-sustaining functions or be subject to rapid catabolism by the polyamine catabolic enzymes. The first successful compounds fitting these criteria were the symmetrically substituted bis(alkylated)polyamines reported by Porter and colleagues [70–73]. In addition to meeting the above criteria for intended effects on polyamine metabolism, it was also discovered that one of the major mechanisms of action of the analogs was to significantly increase polyamine catabolism and its concurrent production of reactive oxygen species (ROS) in a tumor-type-specific manner [74–77]. Although this increase in ROS production was originally thought to be a consequence of increased SSAT activity followed by oxidation of the acetylated polyamines by PAOX, more recent studies have demonstrated that the polyamine analogs also efficiently induce SMOX, which appears to be the primary source of ROS, rather than PAOX [78–80]. Regardless, these findings of selective toxicity bolstered the rational for this class of compounds entering the clinical trial. Of the many compounds examined, N1,N11-bis(ethyl) norspermine (BENSpm/DENSpm, Figure 3) was evaluated in Phase I and II clinical trials. Although generally well tolerated when administered as a single daily dose, the greatest observed clinical effect was stabilization of disease [81–84].
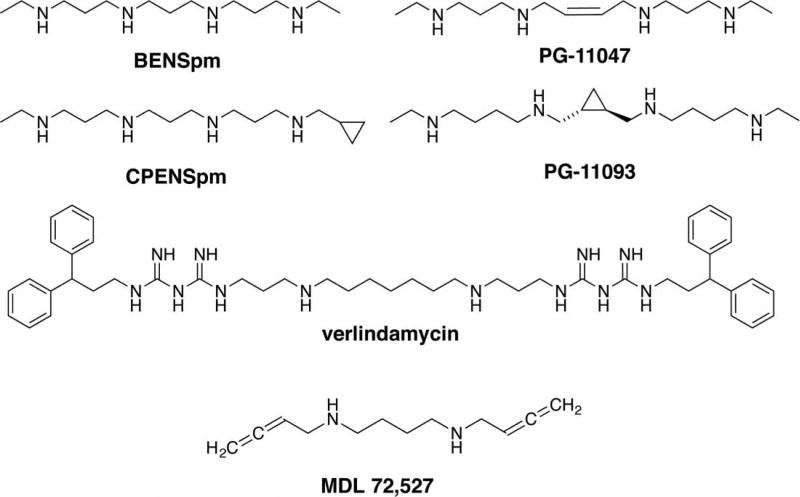
Figure 3. Polyamine analogs and inhibitors of polyamine catabolism.
BENSpm is a representative symmetrically substituted polyamine analog that down-regulates polyamine biosynthesis and up-regulates polyamine catabolism. CPENSpm is a representative unsymmetrically substituted analog that acts in a similar manner to BENSpm. PG-11047 and PG-11093 are conformationally restricted polyamine analogs that have demonstrated significant antitumor activity. Verlindamycin is a biguanide oligoamine that inhibits LSD1. MDL 72,527 inhibits both PAOX and SMOX.
In an attempt to improve upon the self-regulating paradigm, two additional classes of similar analogs were synthesized: the unsymmetrically substituted polyamine analogs typified by N1-(cyclopropylmethyl)-N11-ethlylnorspermine (CPENSpm, Figure 3) and the conformationally restricted analogs [85–89]. Both classes demonstrated some advantages over the symmetrically substituted analogs; however, only the conformationally restricted analogs PG-11093 and PG-11047 (Figure 3) have been clinically evaluated thus far. PG-11047 is well tolerated and produced promising results. However, its further development has been hampered by nonscientifically based business decisions and, unfortunately, none of the completed clinical trials have been published.
Another class of polyamine analogs, termed oligoamines, includes terminally alkylated polyamine analogs of varying propyl- and butylamine linkers [89,90]. These compounds have high affinity for chromatin and several interesting biological effects and mechanisms of action; however, none have been clinically evaluated.
The charge-depleted analogs reported by Khomutov and colleagues [91] comprise another class of biologically interesting polyamine analogs. Similarly, the biologically stable class of polyamines originally synthesized by Coward and colleagues [150], the α-methylated polyamines, has seen a resurgence in interest [92]. Although all of the classes of analogs have interesting features that have aided in a better understanding of natural polyamine regulation and function, none have thus far made a significant impact in the clinic.
Use of the polyamine structure as a targeting moiety
As both polyamine metabolism and transport are frequently dysregulated in cancers, one strategy that has been successfully applied in experimental systems involves the linkage of fluorescent/bioactive/cytotoxic moieties to a polyamine backbone with the goal of selectively facilitating transport of the agents into the cell and, in some cases, more efficiently guiding the molecules to their intended intracellular targets [93].
Annereau et al. [94] reported one recent example that combines the rationale of linking a fluorescent marker to a polyamine backbone to assess the potential transport of a cytotoxic spermine/epipodophyllotoxin conjugate. In the present study, the goal was to demonstrate the cytotoxicity of the spermine/epipodophyllotoxin conjugate in vitro and to determine the likelihood of AML sensitivity by measuring the uptake of a similar nontoxic fluorescent spermine conjugate. The results indicate that it is possible to evaluate the efficacy of leukemia cell polyamine transport and possibly use such efficiency as a surrogate for sensitivity to the cytotoxic conjugate.
Li et al. [95] have reported on a napthalimide–spermine conjugate that is selectively toxic in liver cancer cells, and Wallace, Phanstiel, and colleagues [96,97] have reported on the effectiveness of an anthracene–polyamine conjugate in leukemia cells. Another interesting polyamine conjugate recently examined in prostate cancer models was spermine functionalized with all-trans-retinoic acid [98].
As indicated above, another rational for conjugating polyamines to bioactive moieties is to not only use the polyamine transport system to enter the cell, but also to direct the agent to its intracellular molecular target. Excellent examples of this strategy have been reported by Woster and colleagues (reviewed in ref. [99]). In one such series of studies, histone deacetylases (HDACs) were efficiently targeted by conjugating nickel-chelating hydroxamic acid or benzamide moieties to a polyamine backbone [100,101]. HDACs remove activating acetylation marks from histone proteins, thus repressing active transcription. The stated goal in this case was to exploit the positive charge of the polyamine backbone to target the HDAC-inhibiting moieties to negatively charged chromatin, resulting in the increased expression of repressed genes. In each case, effective inhibition of the HDAC targets was demonstrated and increased expression of the cell cycle regulator p21 was realized, both in a dose-dependent manner. Interestingly, some HDAC homolog selectivity was observed with select conjugates. Specifically, the bifunctional HDAC6, which is thought to function as a cytoplasmic deacetylase for α-tubulin, was significantly inhibited by one the conjugates.
As part of the effort to clone and characterize the polyamine oxidases, a homologous, FAD-dependent amine oxidase, was discovered that did not have the capability to oxidize polyamines. This novel oxidase was ultimately identified as LSD1, the first verified histone demethylase [31]. On the basis of similarity in amino acid sequence, structure, and catalytic mechanisms between SMOX, PAOX, and LSD1, it was hypothesized that polyamine-like compounds that had moieties known to inhibit SMOX would also inhibit LSD1. Since LSD1 is frequently dysregulated in cancer and is responsible for the aberrant silencing of certain important tumor suppressor genes, it was hypothesized that inhibition of LSD1 would result in the re-expression of aberrantly silenced tumor suppressor genes. Ultimately, this hypothesis tested true with the polyamine analog 2d/verlindamycin (Figure 3). Therefore, the polyamine analogs provided the first proof of principle that LSD1 could be targeted as an anticancer strategy both in vitro and in vivo [102,103]. Efforts continue to improve upon this paradigm with the goal of designing more selective and potent inhibitors of LSD1 that have the added benefit of being targeting to chromatin [104–107]. Although the polyamine analogs targeting LSD1 were first into this new pharmacologic space for cancer therapy, none have yet made it into the clinical trial. However, several ongoing clinical trials are in progress with other agents that target LSD1 (NCT02177812, NCT02273102 and NCT02712905).
Targeting polyamine metabolism as a strategy for cancer prevention
ODC as a target for chemoprevention
Although recently there have been impressive successes with targeted therapy and immunotherapy in late-stage cancers, it is likely that ultimately cancer prevention will yield the most promising results. Cancer prevention can take many forms, from the reduction of exposure to known carcinogens such as cigarette smoke, radiation, and asbestos, to chemoprevention in individuals with known increased risk to specific cancers based on genetic predisposition or previous history of dysplasia or early-stage cancers.
Although there is great promise in chemoprevention, the major impediment is identifying appropriate targets and then developing agents that can be safely administered over the lifetime of an individual with no or minimal off-target effects. Since the ultimate goal is treating essentially healthy individuals, the bar for such agents is set high. Targeting polyamine metabolism has a potential advantage as a strategy for chemoprevention in that inhibition of ODC generally results in cytostasis rather than overt toxicity. Clinical trials involving DFMO and its use in the treatment of human African trypanosomiasis have demonstrated that DFMO is generally very well tolerated, even in gram doses [108,109]. Additionally, as ODC activity has been demonstrated to be necessary for the early stages of tumorigenesis, may act as an oncogene, and its haploid expression significantly impairs the development of Myc-induced lymphoma [110–114], it has naturally drawn interest as a chemoprevention target. Consequently, DFMO has been the initial mainstay of chemoprevention targeting polyamine metabolism.
Although DFMO as a potential chemopreventive agent has been used in experimental models for many years [115–119], Gerner, Meyskens, and colleagues [120,121] have been at the forefront of pushing for serious clinical evaluation of DFMO as a chemopreventative. The results from a clinical trial combining DFMO with the NSAID sulindac exhibited highly promising results in patients who had a history of resected adenomas [122]. Although the results demonstrated a highly significant reduction of recurrent adenomas in the treated group, the study suffered from the weakness that only the combination of DFMO plus sulindac was compared with the placebo arm. To overcome this potential weakness in study design, ongoing four-arm clinical trials in patients with familial adenomatous polyposis (NCT01483144) and a trial to determine whether DFMO and sulindac prevent recurrence of high-risk adenomas and second primary colorectal cancers in patients with Stage 0–III colon or rectal cancer are currently recruiting patients (NCT01349881).
In addition to gastrointestinal (GI) cancers, prostate cancer has been a proposed target for agents that interfere with polyamine metabolism as a strategy for chemoprevention [123–125]. However, these studies have not advanced as far as their counterparts in GI systems.
A recently completed Phase II study as a possible chemopreventive treatment for nonmelanoma skin cancer in sun-damaged skin combined DFMO with the NSAID diclofenac [126]. The results indicated no benefit with either agent alone or in combination. The results also indicated that inflammation was observed in the treatment groups that may have contributed to the apparent lack of benefit.
Although polyamine depletion has been thought to be the key component driving the chemoprevention effects of DFMO treatment, Witherspoon et al. [127] have proposed that in colorectal tumorigenesis, reduced levels of folate-dependent metabolites are actually responsible for the preventive effects of DFMO. With the inhibition of ODC by DFMO, AdoMetDC activity is increased, thus leading to an overutilization of S-adenosylmethionine pools, which are then regenerated through the use of tetrahydrofolate, thus depriving thymidine synthase the necessary methyl donor. Although this hypothesis requires testing in additional models, it suggests a mechanism in addition to polyamine depletion that might contribute to the antiproliferative/chemopreventive effects.
Polyamine catabolism as an emerging target for chemoprevention
ROS produced by polyamine catabolism are known to cause DNA damage and cell death. The enzymes involved in ROS production include the SSAT/PAOX, SMOX, and serum amine oxidases [24]. As stated above, one mechanism by which the polyamine analogs are thought to exert their cell type-specific cytotoxicity is through the up-regulation of polyamine catabolism, thus producing toxic bursts of ROS in tumor cells leading to cell death. However, in addition to the overt cytotoxic effects of polyamine catabolism-produced ROS, the potential that sublethal levels of ROS production could lead to DNA damage resulting in mutation or alterations of gene expression suggests a mechanism by which polyamine catabolism could play a role in the etiology of cancer.
The recognition that certain infectious and inflammatory stimuli could induce polyamine catabolism in otherwise normal tissues suggested a role for polyamine catabolism in the earliest stages of the carcinogenic process in instances of chronic or chronic episodic inflammation. The primary focus of the most recent studies has been on SMOX, which is highly induced by bacterial infection and inflammatory cytokines [128,129]. However, it should be noted that several of the same stimuli also induce SSAT. As SMOX is found in both the cytoplasm and the nucleus, the generation of H2O2 by SMOX can occur in close proximity to DNA, thus increasing the potential for damage. Additionally, since both SMOX and SSAT reduce intracellular polyamines, which are free radical scavengers, the end result is increased ROS stress in an environment with reduced protection.
The first indication that SMOX was inducible by infectious agents was observed when either GI macrophages or epithelial cells were infected with Helicobacter pylori [129,130]. Additional studies demonstrated that induction of SMOX was not limited to H. pylori, but also occurred with exposure to other infectious agents and inflammatory cytokines in the absence of immune cells [128,131]. In each case, the induction of SMOX was accompanied by increases in H2O2 production and DNA damage, each of which could be blocked by either inhibition of SMOX with a polyamine oxidase inhibitor (MDL 72,527, Figure 3) or with an siRNA directed at SMOX. Importantly, SMOX expression was shown to increase in patient samples in infectious and inflammatory conditions that are associated with cancer [132,133]. Additionally, even normal-appearing prostatic tissue of cancer patients expressed higher levels of SMOX than did prostate tissue from individuals who did not have prostate cancer [133].
To determine whether SMOX could be targeted in vivo as a strategy for chemoprevention, a mouse model of human colon cancer was investigated [134]. In the colon, altering the balance between the diverse and abundant microbiota (~1012 organisms per gram feces) and the host is a common source of inflammation. Bacteroides spp. comprise a significant proportion of colonic bacteria and include the human anaerobic pathogen enterotoxigenic Bacteroides fragilis (ETBF). ETBF represents a molecular subtype characterized by a 20-kDa metalloprotease enterotoxin (B. fragilis toxin; BFT). ETBF has been epidemiologically associated with acute diarrheal diseases in humans and livestock, inflammatory bowel disease, and colorectal cancer [135]. Exposure of intestinal epithelial cell lines to BFT results in cleavage of the cell adhesion/tumor suppressor protein E-cadherin, morphological alterations, activation of stress–response signaling pathways, induction of cytokine secretion, and increased cellular proliferation mediated by elevated expression of the c-Myc oncogene [135,136].
Wu et al. [134] studied the effect of ETBF on tumor formation in multiple intestinal neoplasia (Min) mice. Min mice develop dozens of small intestine tumors over 3–6 months due to a truncation mutation in the APC gene, a genetic defect that is also a hallmark of human colorectal cancer [137,138]. Additionally, with ETBF infection, this model develops multiple distal colon tumors that mimic the human disease. Importantly, when these animals were treated with the polyamine oxidase inhibitor MDL 72,527, the tumor incidence associated with ETBF infection was significantly reduced [131]. In addition to reduced tumor burden, cytokine expression also declined in the treated animals, suggesting a feed-forward loop where SMOX activity increases inflammation and the release of inflammatory cytokines, which in turn leads to greater increases in SMOX activity.
Recently, Sears et al. [139] demonstrated that in patient-matched colon cancer and normal-appearing tissues with or without biofilms, there was a significant increase in N1,N12-diacetylspermine in biofilm-positive samples. This increase was observed in both normal and cancer tissue from the biofilm-positive patients, when compared with patient-matched tissues from biofilm-negative patients. When patients were cleared of biofilms by antibiotic treatment, N1,N12-diacetylspermine was reduced to levels observed in the nonbiofilm patients. Although it is currently not known whether the colonic epithelium or the biofilm-forming bacteria are the source of the increased N1,N12-diacetylspermine, the results suggest that the polyamine acetylation pathway may affect both cancer development and progression. The data further suggest yet another potential chemoprevention target in the colon, should this pathway ultimately prove to be causally linked.
As stated above, another infectious agent that is closely linked to the etiology of cancer and induces SMOX is H. pylori, which is recognized by the WHO as the carcinogen responsible for the majority of gastric cancers worldwide. Consequently, since gastric cancer is the third cancer killer globally and because of the high induction of SMOX by H. pylori in model systems, the potential clinical link between H. pylori infection, SMOX induction, DNA damage, and cancer was investigated. In Nariño, Colombia, gastric cancer rates are 25-times higher in the high-risk Andean population than in the low-risk Pacific coastal population, despite the high prevalence of H. pylori infection in both populations (~90%) [140]. Therefore, to determine whether the difference in these gastric cancer rates could be explained by differences in levels of SMOX induced by clinical isolates of H. pylori from the high-risk Andean population versus the low-risk coastal population, in vitro, in vivo, and patient sample studies were performed. The studies confirmed that high-risk region clinical isolates induce SMOX, H2O2 production, and DNA damage to a greater extent than do clinical isolates from the low-risk coastal region, and this higher induction of SMOX mediates the increased risk of gastric cancer in the high-risk region [132]. Additionally, in clinical samples of infected patients, those exhibiting the highest expression of SMOX also demonstrated the greatest amount of DNA damage. Thus, the data are consistent with the hypothesis that SMOX expression is directly linked to the increased risk of gastric cancer in the Andean population.
To confirm this link, the Mongolian gerbil model of H. pylori-induced gastric cancer was used to determine whether inhibition of SMOX or perturbations in polyamine metabolism could reduce the cancer incidence in infected animals. The results of these studies indicated that either the inhibition of SMOX or the reduction of spermine levels resulted in decreased incidences of dysplasia and cancer in the infected gerbils. Consequently, both the clinical and model system results indicate that SMOX induction is tightly linked to the etiology of H. pylori-induced gastric cancer and that inhibition of SMOX activity is a rational strategy for chemoprevention of this disease.
Potential mechanisms underlying the role of SMOX in carcinogenesis
Although it is clear that H2O2 produced by SMOX is responsible for oxidative damage of DNA and that the increase in SMOX correlates with the risk of developing dysplasia/cancer in specific instances, the underlying mechanisms require further study. Obviously, the potential for ROS-mediated DNA damage leading to carcinogenic mutations is a real possibility; however, the responsible mutations may be individually difficult to find. Another possible mechanism that has supporting data is that the DNA damage resulting from SMOX induction may involve epigenetic changes leading to the silencing of important tumor suppressor genes. How might epigenetic silencing be involved? First, it has been demonstrated that damaged DNA recruits repressor complexes to the damaged site(s) to repress transcriptional machinery from transcribing damaged genes [141,142]. In some instances, these repressor complexes are retained at the damaged sites in an abnormal manner, leading to the recruitment of DNA methyltransferases that can methylate cytosines in the 5′-promoter regions of genes, resulting in permanent silencing of the associated gene. If the gene silenced is critical for growth control, the result is a cell that has lost a least one copy of a regulator of growth.
The question then becomes: does this actually happen in cancers that appear to be linked to increases in SMOX? Experiments performed in vitro clearly demonstrated that exposure to H2O2 results in recruitment of silencing complexes to the promoter region CpG islands of genes [143,144]. However, in vitro exposure to H2O2 has several potential caveats. Therefore, in addition to the in vitro model of H2O2 exposure, O’Hagan et al. [144] used the ETBF infection model previously described, in which ETBF infection is known to induce SMOX, to demonstrate that ETBF infection resulted in the rapid recruitment of repressor complexes to CpG islands. Specifically, it was found that ETBF infection increased the affinity of both SIRT1 and EZH2 for chromatin within 48 h postinfection, in a manner similar to that observed in the in vitro models. This increased affinity occurred in the distal colon, the site most relevant to ETBF colon carcinogenesis, and the region observed to produce tumors in the ETBF/Min mouse model. Importantly, after ETBF infection, local ChIP analysis revealed that the promoter CpG islands of genes that have been implicated in inflammation-associated carcinogenesis in the glutathione peroxidase 1 & 2 KO mice (Fbn1, Sez6l, and Sox17) [145] demonstrated significant recruitment of the polycomb repressor complex components EZH2 and DNMT1. Although more study will be necessary, the results suggest that the H2O2 production and DNA damage resulting from increased SMOX activity downstream from infection or inflammation could result in epigenetic silencing of important tumor suppressor genes.
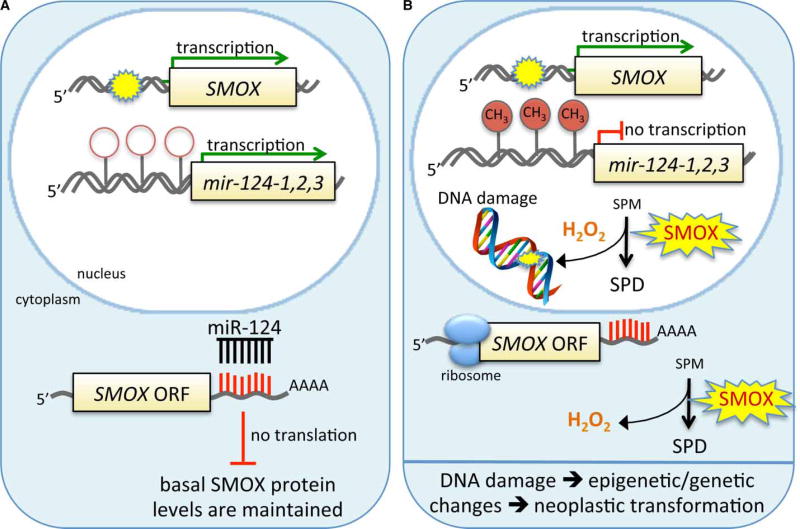
Another recent finding suggests a direct way that epigenetic factors may be involved in the link between SMOX and carcinogenesis. Murray-Stewart et al. [146] found that the tumor suppressive microRNA miR-124 negatively regulates SMOX expression through a recognition site in its 3′-UTR. This particular microRNA has three distinct loci in humans that code for the same mature miRNA, and all three loci are frequently silenced by CpG-promoter methylation in cancers. Consequently, when miR-124 expression is silenced, one mode of controlling aberrant SMOX expression is disabled. Importantly, in gastric tissues from H. pylori-infected individuals, it was found that the extent of miR-124 gene methylation correlated with the expression of SMOX. These results indicate an epigenetic mechanism by which SMOX expression can be regulated, and since epigenetic silencing is a common occurrence in cancers, it also suggests that combining specific epigenetic therapies designed to re-express aberrantly silenced genes in combination with inhibitors of SMOX may be a rational strategy for chemoprevention. Intriguingly, it also suggests a potential feed-forward loop whereby SMOX-produced H2O2 leads to increased DNA damage and epigenetic silencing of miR-124, thus leading to even greater increases in SMOX expression (Figure 4).
Figure 4. Proposed mechanism by which miR-124 regulates SMOX expression in infection/inflammation-associated carcinogenesis.
Infection or inflammatory stimuli induce SMOX transcription. Normally (
 ), miR-124 is also transcribed and modulates the translation of SMOX, thus reducing the potential for DNA damage. However, in some infection or inflammatory pathologies (B), including H. pylori-infected individuals, the CpG islands in each of the promoters of the miR-124 loci are methylated; thus, miR-124 transcription is reduced or silenced, allowing unregulated induction of SMOX that leads to a reduction of free radical-scavenging spermine and an increase in ROS production. This increases the likelihood of oxidative DNA damage, which is known to be associated with both genetic and epigenetic modifications contributing to carcinogenesis.
), miR-124 is also transcribed and modulates the translation of SMOX, thus reducing the potential for DNA damage. However, in some infection or inflammatory pathologies (B), including H. pylori-infected individuals, the CpG islands in each of the promoters of the miR-124 loci are methylated; thus, miR-124 transcription is reduced or silenced, allowing unregulated induction of SMOX that leads to a reduction of free radical-scavenging spermine and an increase in ROS production. This increases the likelihood of oxidative DNA damage, which is known to be associated with both genetic and epigenetic modifications contributing to carcinogenesis.
Although it is clear that SMOX is a rational target to be considered for chemoprevention, such a strategy is severely limited by the fact that no specific inhibitors of SMOX currently exist. Although the pan-polyamine oxidase inhibitor MDL 72,527 can be used as a pharmacologic tool, the facts that it inhibits both SMOX and PAOX and can lead to toxicity following long-term use preclude its use as a chemopreventative. The search for specific or even selective inhibitors of SMOX has been hampered by the lack of a crystal structure for the protein. However, some structural data have been inferred by its homology to other closely related FAD-dependent oxidases, and it is hoped that these data will yield promising leads to SMOX-specific inhibitors [147–149].
Conclusions
The polyamine metabolic pathway offers several opportunities for strategically targeted therapies. Although single-agent trials with inhibitors or analogs have not been successful in the treatment of existing disease, combinations of DFMO with other agents have shown impressive success, particularly in the chemoprevention arena. The advent of useful and minimally toxic polyamine transport inhibitors is a relatively new avenue being explored that may yield important results. Importantly, the validation that SMOX plays a causal role in infection/ inflammation-associated cancers offers a new and exciting chemoprevention target. Although the field of studying polyamines formally started over 300 years ago, there is still much to be learned and many potential targets to be exploited.
Abbreviations
- AdoDATAD
S-adenosyl-1,12-diamino-3-thio-9-azadodecane
- AdoDATO
S-adenosyl-3-thio-1,8-diaminooctane
- AdoMetDC
S-adenosylmethionine decarboxylase
- BENSpm
N1,N11-bis(ethyl)norspermine
- BFT
Bacteroides fragilis toxin
- CPENSpm
N1-(cyclopropylmethyl)-N11-ethylnorspermine
- DFMO
2-difluoromethylornithine
- ETBF
enterotoxigenic Bacteroides fragilis
- HDAC
histone deacetylase
- LSD1
lysine-specific demethylase 1
- Min
multiple intestinal neoplasia
- MGBG
methylglyoxal bis(guanylhydrazone)
- MTA
5′-deoxy-5′-(methylthio) adenosine
- MTAP
MTA phosphorylase
- ODC
ornithine decarboxylase
- PAOX
N1-acetylpolyamine oxidase
- PBT
polyamine-blocking therapy
- ROS
reactive oxygen species
- SAM486A
4-amidinoidan-1-one-2′-amidinhydrazone
- SMOX
spermine oxidase
- SSAT
spermidine/spermine N1-acetyltransferase
Footnotes
Competing Interests
The Authors declare that there are no competing interests associated with the manuscript.
References
- 1.Pasini A, Caldarera CM, Giordano E. Chromatin remodeling by polyamines and polyamine analogs. Amino Acids. 2014;46:595–603. doi: 10.1007/s00726-013-1550-9. [DOI] [PubMed] [Google Scholar]
- 2.Williams K. Interactions of polyamines with ion channels. Biochem. J. 1997;325(Pt 2):289–297. doi: 10.1042/bj3250289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baronas VA, Kurata HT. Inward rectifiers and their regulation by endogenous polyamines. Front. Physiol. 2014;5:325. doi: 10.3389/fphys.2014.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casero RA, Jr, Marton LJ. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat. Rev. Drug. Discov. 2007;6:373–390. doi: 10.1038/nrd2243. [DOI] [PubMed] [Google Scholar]
- 5.Pegg AE. Mammalian polyamine metabolism and function. IUBMB Life. 2009;61:880–894. doi: 10.1002/iub.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lentini A, Abbruzzese A, Caraglia M, Marra M, Beninati S. Protein-polyamine conjugation by transglutaminase in cancer cell differentiation: review article. Amino Acids. 2004;26:331–337. doi: 10.1007/s00726-004-0079-3. [DOI] [PubMed] [Google Scholar]
- 7.Park MH, Nishimura K, Zanelli CF, Valentini SR. Functional significance of eIF5A and its hypusine modification in eukaryotes. Amino Acids. 2010;38:491–500. doi: 10.1007/s00726-009-0408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ha HC, Sirisoma NS, Kuppusamy P, Zweier JL, Woster PM, Casero RA., Jr The natural polyamine spermine functions directly as a free radical scavenger. Proc. Natl Acad. Sci. USA. 1998;95:11140–11145. doi: 10.1073/pnas.95.19.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ha HC, Yager JD, Woster PA, Casero RA., Jr Structural specificity of polyamines and polyamine analogues in the protection of DNA from strand breaks induced by reactive oxygen species. Biochem. Biophys. Res. Commun. 1998;244:298–303. doi: 10.1006/bbrc.1998.8258. [DOI] [PubMed] [Google Scholar]
- 10.Nakajima K-I, Zhu K, Sun Y-H, Hegyi B, Zeng Q, Murphy CJ, et al. KCNJ15/Kir4.2 couples with polyamines to sense weak extracellular electric fields in galvanotaxis. Nat. Commun. 2015;6(8532) doi: 10.1038/ncomms9532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell DH, Snyder SH. Amine synthesis in regenerating rat liver: extremely rapid turnover of ornithine decarboxylase. Mol. Pharmacol. 1969;5:253–262. [PubMed] [Google Scholar]
- 12.Pegg AE, Williams-Ashman HG. Rapid effects of testosterone in prostatic polyamine-synthesizing enzyme systems. Biochem. J. 1968;109:32P–33P. doi: 10.1042/bj1090032pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pegg AE. Regulation of ornithine decarboxylase. J. Biol. Chem. 2006;281:14529–14532. doi: 10.1074/jbc.R500031200. [DOI] [PubMed] [Google Scholar]
- 14.Coffino P. Regulation of cellular polyamines by antizyme. Nat. Rev. Mol. Cell Biol. 2001;2:188–194. doi: 10.1038/35056508. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi S. Antizyme-dependent degradation of ornithine decarboxylase. Essays Biochem. 1995;30:37–47. [PubMed] [Google Scholar]
- 16.Kahana C. Antizyme and antizyme inhibitor, a regulatory tango. Cell. Mol. Life. Sci. 2009;66:2479–2488. doi: 10.1007/s00018-009-0033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bello-Fernandez C, Packham G, Cleveland JL. The ornithine decarboxylase gene is a transcriptional target of c-Myc. Proc. Natl Acad. Sci. USA. 1993;90:7804–7808. doi: 10.1073/pnas.90.16.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner AJ, Meyers C, Laimins LA, Hay N. c-Myc induces the expression and activity of ornithine decarboxylase. Cell. Growth Differ. 1993;4:879–883. [PubMed] [Google Scholar]
- 19.Pegg AE. S-Adenosylmethionine decarboxylase. Essays Biochem. 2009;46:25–46. doi: 10.1042/bse0460003. [DOI] [PubMed] [Google Scholar]
- 20.Wahlfors J, Alhonen L, Kauppinen L, Hyvönen T, Jänne J, Eloranta TO. Human spermidine synthase: cloning and primary structure. DNA Cell Biol. 1990;9:103–110. doi: 10.1089/dna.1990.9.103. [DOI] [PubMed] [Google Scholar]
- 21.Korhonen V-P, Halmekytö M, Kauppinen L, Myöhänen S, Wahlfors J, Keinänen T, et al. Molecular cloning of a cDNA encoding human spermine synthase. DNA Cell Biol. 1995;14:841–847. doi: 10.1089/dna.1995.14.841. [DOI] [PubMed] [Google Scholar]
- 22.Ikeguchi Y, Bewley MC, Pegg AE. Aminopropyltransferases: function, structure and genetics. J. Biochem. 2006;139:1–9. doi: 10.1093/jb/mvj019. [DOI] [PubMed] [Google Scholar]
- 23.Della Ragione F, Russo G, Oliva A, Mastropietro S, Mancini A, Borrelli A, et al. 5′-Deoxy-5′-methylthioadenosine phosphorylase and p16INK4 deficiency in multiple tumor cell lines. Oncogene. 1995;10:827–833. [PubMed] [Google Scholar]
- 24.Casero RA, Pegg AE. Polyamine catabolism and disease. Biochem. J. 2009;421:323–338. doi: 10.1042/BJ20090598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casero RA, Jr, Pegg AE. Spermidine/spermine N1-acetyltransferase—the turning point in polyamine metabolism. FASEB J. 1993;7:653–661. [PubMed] [Google Scholar]
- 26.Wu T, Yankovskaya V, McIntire WS. Cloning, sequencing, and heterologous expression of the murine peroxisomal flavoprotein, N1-acetylated polyamine oxidase. J. Biol. Chem. 2003;278:20514–20525. doi: 10.1074/jbc.M302149200. [DOI] [PubMed] [Google Scholar]
- 27.Wallace HM, Duthie J, Evans DM, Lamond S, Nicoll KM, Heys SD. Alterations in polyamine catabolic enzymes in human breast cancer tissue. Clin. Cancer Res. 2000;6:3657–3661. [PubMed] [Google Scholar]
- 28.Wang Y, Devereux W, Woster P, Stewart T, Hacker A, Casero R., Jr Cloning and characterization of a human polyamine oxidase that is inducible by polyamine analogue exposure. Cancer Res. 2001;61:5370–5373. [PubMed] [Google Scholar]
- 29.Wang Y, Hacker A, Murray-Stewart T, Fleischer JG, Woster PM, Casero RA., Jr Induction of human spermine oxidase SMO(PAOh1) is regulated at the levels of new mRNA synthesis, mRNA stabilization and newly synthesized protein. Biochem. J. 2005;386:543–547. doi: 10.1042/BJ20041084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray-Stewart T, Wang Y, Goodwin A, Hacker A, Meeker A, Casero RA., Jr Nuclear localization of human spermine oxidase isoforms —possible implications in drug response and disease etiology. FEBS J. 2008;275:2795–2806. doi: 10.1111/j.1742-4658.2008.06419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi Y, Lan F, Matson C, Mulligan P, Whetstine J, Cole PA, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 32.Poulin R, Casero RA, Soulet D. Recent advances in the molecular biology of metazoan polyamine transport. Amino Acids. 2012;42:711–723. doi: 10.1007/s00726-011-0987-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soulet D, Gagnon B, Rivest S, Audette M, Poulin R. A fluorescent probe of polyamine transport accumulates into intracellular acidic vesicles via a two-step mechanism. J. Biol. Chem. 2004;279:49355–49366. doi: 10.1074/jbc.M401287200. [DOI] [PubMed] [Google Scholar]
- 34.Belting M, Mani K, Jonsson M, Cheng F, Sandgren S, Jonsson S, et al. Glypican-1 is a vehicle for polyamine uptake in mammalian cells: a pivital role for nitrosothiol-derived nitric oxide. J. Biol. Chem. 2003;278:47181–47189. doi: 10.1074/jbc.M308325200. [DOI] [PubMed] [Google Scholar]
- 35.Uemura T, Stringer DE, Blohm-Mangone KA, Gerner EW. Polyamine transport is mediated by both endocytic and solute carrier transport mechanisms in the gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G517–G522. doi: 10.1152/ajpgi.00169.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Metcalf BW, Bey P, Danzin C, Jung MJ, Casara P, Vevert JP. Catalytic irreversible inhibition of mammalian ornithine decarboxylase (E.C4.1.1.17) by substrate and product analogues. J. Am. Chem. Soc. 1978;100:2551–2553. [Google Scholar]
- 37.Seidenfeld J. Effects of difluoromethylornithine on proliferation, polyamine content and plating efficiency of cultured human carcinoma cells. Cancer Chemother. Pharmacol. 1985;15:196–202. doi: 10.1007/BF00263885. [DOI] [PubMed] [Google Scholar]
- 38.Porter CW, Bergeron RJ. Spermidine requirement for cell proliferation in eukaryotic cells: structural specificity and quantitation. Science. 1983;219:1083–1085. doi: 10.1126/science.6823570. [DOI] [PubMed] [Google Scholar]
- 39.Luk GD, Civin CI, Weissman RM, Baylin SB. Ornithine decarboxylase: essential in proliferation but not differentiation of human promyelocytic leukemia cells. Science. 1982;216:75–77. doi: 10.1126/science.6950518. [DOI] [PubMed] [Google Scholar]
- 40.Luk GD, Goodwin G, Gazdar AF, Baylin SB. Growth-inhibitory effects of dl-alpha-difluoromethylornithine in the spectrum of human lung carcinoma cells in culture. Cancer Res. 1982;42:3070–3073. [PubMed] [Google Scholar]
- 41.Abeloff MD, Rosen ST, Luk GD, Baylin SB, Zeltzman M, Sjoerdsma A. Phase II trials of alpha-difluoromethylornithine, an inhibitor of polyamine synthesis, in advanced small cell lung cancer and colon cancer. Cancer Treat. Rep. 1986;70:843–845. [PubMed] [Google Scholar]
- 42.Abeloff MD, Slavik M, Luk GD, Griffin CA, Hermann J, Blanc O, et al. Phase I trial and pharmacokinetic studies of alpha-difluoromethylornithine—an inhibitor of polyamine biosynthesis. J. Clin. Oncol. 1984;2:124–130. doi: 10.1200/JCO.1984.2.2.124. [DOI] [PubMed] [Google Scholar]
- 43.Horn Y, Schechter PJ, Marton LJ. Phase I–II clinical trial with alpha-difluoromethylornithine—an inhibitor of polyamine biosynthesis. Eur. J. Cancer Clin. Oncol. 1987;23:1103–1107. doi: 10.1016/0277-5379(87)90141-6. [DOI] [PubMed] [Google Scholar]
- 44.Meyskens FL, Kingsley EM, Glattke T, Loescher L, Booth A. A phase II study of alpha-difluoromethylornithine (DFMO) for the treatment of metastatic melanoma. Invest. New Drugs. 1986;4:257–262. doi: 10.1007/BF00179593. [DOI] [PubMed] [Google Scholar]
- 45.Bassiri H, Benavides A, Haber M, Gilmour SK, Norris MD, Hogarty MD. Translational development of difluoromethylornithine (DFMO) for the treatment of neuroblastoma. Transl. Pediatr. 2015;4:226–238. doi: 10.3978/j.issn.2224-4336.2015.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koomoa D-L, Yco LP, Borsics T, Wallick CJ, Bachmann AS. Ornithine decarboxylase inhibition by alpha-difluoromethylornithine activates opposing signaling pathways via phosphorylation of both Akt/protein kinase B and p27Kip1 in neuroblastoma. Cancer Res. 2008;68:9825–9831. doi: 10.1158/0008-5472.CAN-08-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hogarty MD, Norris MD, Davis K, Liu X, Evageliou NF, Hayes CS, et al. ODC1 is a critical determinant of MYCN oncogenesis and a therapeutic target in neuroblastoma. Cancer Res. 2008;68:9735–9745. doi: 10.1158/0008-5472.CAN-07-6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wallick CJ, Gamper I, Thorne M, Feith DJ, Takasaki KY, Wilson SM, et al. Key role for p27Kip1, retinoblastoma protein Rb, and MYCN in polyamine inhibitor-induced G1 cell cycle arrest in MYCN-amplified human neuroblastoma cells. Oncogene. 2005;24:5606–5618. doi: 10.1038/sj.onc.1208808. [DOI] [PubMed] [Google Scholar]
- 49.Williams-Ashman HG, Schenone A. Methyl glyoxal bis(guanylhydrazone) as a potent inhibitor of mammalian and yeast S-adenosylmethionine decarboxylases. Biochem. Biophys. Res. Commun. 1972;46:288–295. doi: 10.1016/0006-291x(72)90661-4. [DOI] [PubMed] [Google Scholar]
- 50.Pleshkewych A, Kramer DL, Kelly E, Porter CW. Independence of drug action on mitochondria and polyamines in L1210 leukemia cells treated with methylglyoxal-bis(guanylhydrazone) Cancer Res. 1980;40:4533–4540. [PubMed] [Google Scholar]
- 51.Regenass U, Caravatti G, Mett H, Stanek J, Schneider P, Muller M, et al. New S-adenosylmethionine decarboxylase inhibitors with potent antitumor activity. Cancer Res. 1992;52:4712–4718. [PubMed] [Google Scholar]
- 52.Regenass U, Mett H, Stanek J, Mueller M, Kramer D, Porter CW. CGP 48664, a new S-adenosylmethionine decarboxylase inhibitor with broad spectrum antiproliferative and antitumor activity. Cancer Res. 1994;54:3210–3217. [PubMed] [Google Scholar]
- 53.Siu LL, Rowinsky EK, Hammond LA, Weiss GR, Hidalgo M, Clark GM, et al. A phase I and pharmacokinetic study of SAM486A, a novel polyamine biosynthesis inhibitor, administered on a daily-times-five every-three-week schedule in patients with advanced solid malignancies. Clin. Cancer Res. 2002;8:2157–2166. [PubMed] [Google Scholar]
- 54.Pless M, Belhadj K, Menssen HD, Kern W, Coiffier B, Wolf J, et al. Clinical efficacy, tolerability, and safety of SAM486A, a novel polyamine biosynthesis inhibitor, in patients with relapsed or refractory non-Hodgkin’s lymphoma: results from a phase II multicenter study. Clin. Cancer Res. 2004;10:1299–1305. doi: 10.1158/1078-0432.ccr-0977-03. [DOI] [PubMed] [Google Scholar]
- 55.Tang KC, Pegg AE, Coward JK. Specific and potent inhibition of spermidine synthase by the transition-state analog, S-adenosyl-3-thio-1,8-diaminooctane. Biochem. Biophys. Res. Commun. 1980;96:1371–1377. doi: 10.1016/0006-291x(80)90102-3. [DOI] [PubMed] [Google Scholar]
- 56.Woster PM, Black AY, Duff KJ, Coward JK, Pegg AE. Synthesis and biological evaluation of S-adenosyl-1,12-diamino-3-thio-9-azadodecane, a multisubstrate adduct inhibitor of spermine synthase. J. Med. Chem. 1989;32:1300–1307. doi: 10.1021/jm00126a026. [DOI] [PubMed] [Google Scholar]
- 57.Burns MR, Graminski GF, Weeks RS, Chen Y, O’Brien TG. Lipophilic lysine–spermine conjugates are potent polyamine transport inhibitors for use in combination with a polyamine biosynthesis inhibitor. J. Med. Chem. 2009;52:1983–1993. doi: 10.1021/jm801580w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Samal K, Zhao P, Kendzicky A, Yco LP, McClung H, Gerner E, et al. AMXT-1501, a novel polyamine transport inhibitor, synergizes with DFMO in inhibiting neuroblastoma cell proliferation by targeting both ornithine decarboxylase and polyamine transport. Int. J. Cancer. 2013;133:1323–1333. doi: 10.1002/ijc.28139. [DOI] [PubMed] [Google Scholar]
- 59.Hayes CS, Shicora AC, Keough MP, Snook AE, Burns MR, Gilmour SK. Polyamine-blocking therapy reverses immunosuppression in the tumor microenvironment. Cancer Immunol. Res. 2014;2:274–285. doi: 10.1158/2326-6066.CIR-13-0120-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muth A, Madan M, Archer JJ, Ocampo N, Rodriguez L, Phanstiel O. Polyamine transport inhibitors: design, synthesis, and combination therapies with difluoromethylornithine. J. Med. Chem. 2014;57:348–363. doi: 10.1021/jm401174a. [DOI] [PubMed] [Google Scholar]
- 61.Grossi M, Phanstiel O, Rippe C, Swärd K, Alajbegovic A, Albinsson S, et al. Inhibition of polyamine uptake potentiates the anti-proliferative effect of polyamine synthesis inhibition and preserves the contractile phenotype of vascular smooth muscle cells. J. Cell. Physiol. 2016;231:1334–1342. doi: 10.1002/jcp.25236. [DOI] [PubMed] [Google Scholar]
- 62.Levin VA, Uhm JH, Jaeckle KA, Choucair A, Flynn PJ, Yung WKA, et al. Phase III randomized study of postradiotherapy chemotherapy with alpha-difluoromethylornithine-procarbazine, N-(2-chloroethyl)-N′-cyclohexyl-N-nitrosurea, vincristine (DFMO-PCV) versus PCV for glioblastoma multiforme. Clin. Cancer Res. 2000;6:3878–3884. [PubMed] [Google Scholar]
- 63.Saulnier Sholler GL, Gerner EW, Bergendahl G, MacArthur RB, VanderWerff A, Ashikaga T, et al. A phase I trial of DFMO targeting polyamine addiction in patients with relapsed/refractory neuroblastoma. PLoS ONE. 2015;10:e0127246. doi: 10.1371/journal.pone.0127246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alexiou GA, Tsamis KI, Vartholomatos E, Peponi E, Tzima E, Tasiou I, et al. Combination treatment of TRAIL, DFMO and radiation for malignant glioma cells. J. Neurooncol. 2015;123:217–224. doi: 10.1007/s11060-015-1799-9. [DOI] [PubMed] [Google Scholar]
- 65.Wallace HM, Fraser AV. Polyamine analogues as anticancer drugs. Biochem. Soc. Trans. 2003;31:393–396. doi: 10.1042/bst0310393. [DOI] [PubMed] [Google Scholar]
- 66.Wallace HM, Fraser AV, Hughes A. A perspective of polyamine metabolism. Biochem. J. 2003;376:1–14. doi: 10.1042/BJ20031327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wallace HM, Niiranen K. Polyamine analogues—an update. Amino Acids. 2007;33:261–265. doi: 10.1007/s00726-007-0534-z. [DOI] [PubMed] [Google Scholar]
- 68.Casero RA, Jr, Woster PM. Recent advances in the development of polyamine analogues as antitumor agents. J. Med. Chem. 2009;52:4551–4573. doi: 10.1021/jm900187v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Phanstiel O, Archer JJ. Design of Polyamine Transport Inhibiotrs as Therapeutics. RSC Publishing; Cambridge, UK: 2012. [Google Scholar]
- 70.Porter CW, Bergeron RJ. Enzyme regulation as an approach to interference with polyamine biosynthesis—an alternative to enzyme inhibition. Adv. Enzyme Regul. 1988;27:43–55. doi: 10.1016/0065-2571(88)90009-x. [DOI] [PubMed] [Google Scholar]
- 71.Porter CW, Bergeron RJ. Regulation of polyamine biosynthetic activity by spermidine and spermine analogs—a novel antiproliferative strategy. Adv. Exp. Med. Biol. 1988;250:677–690. doi: 10.1007/978-1-4684-5637-0_60. [DOI] [PubMed] [Google Scholar]
- 72.Porter CW, Bergeron RJ, Stolowich NJ. Biological properties of N4-spermidine derivatives and their potential in anticancer chemotherapy. Cancer Res. 1982;42:4072–4078. [PubMed] [Google Scholar]
- 73.Porter CW, McManis J, Casero RA, Bergeron RJ. Relative abilities of bis(ethyl) derivatives of putrescine, spermidine, and spermine to regulate polyamine biosynthesis and inhibit L1210 leukemia cell growth. Cancer Res. 1987;47:2821–2825. [PubMed] [Google Scholar]
- 74.Bernacki RJ, Bergeron RJ, Porter CW. Antitumor activity of N,N′-bis(ethyl)spermine homologues against human MALME-3 melanoma xenografts. Cancer Res. 1992;52:2424–2430. [PubMed] [Google Scholar]
- 75.Casero RA, Jr, Celano P, Ervin SJ, Porter CW, Bergeron RJ, Libby PR. Differential induction of spermidine/spermine N1-acetyltransferase in human lung cancer cells by the bis(ethyl)polyamine analogues. Cancer Res. 1989;49:3829–3833. [PubMed] [Google Scholar]
- 76.Casero RA, Jr, Mank AR, Xiao L, Smith J, Bergeron RJ, Celano P. Steady-state messenger RNA and activity correlates with sensitivity to N1,N12-bis(ethyl)spermine in human cell lines representing the major forms of lung cancer. Cancer Res. 1992;52:5359–5363. [PubMed] [Google Scholar]
- 77.Porter CW, Ganis B, Libby PR, Bergeron RJ. Correlations between polyamine analogue-induced increases in spermidine/spermine N1-acetyltransferase activity, polyamine pool depletion, and growth inhibition in human melanoma cell lines. Cancer Res. 1991;51:3715–3720. [PubMed] [Google Scholar]
- 78.Devereux W, Wang Y, Stewart TM, Hacker A, Smith R, Frydman B, et al. Induction of the PAOh1/SMO polyamine oxidase by polyamine analogues in human lung carcinoma cells. Cancer Chemother. Pharmacol. 2003;52:383–390. doi: 10.1007/s00280-003-0662-4. [DOI] [PubMed] [Google Scholar]
- 79.Pledgie A, Huang Y, Hacker A, Zhang Z, Woster PM, Davidson NE, et al. Spermine oxidase SMO(PAOh1), not N1-acetylpolyamine oxidase PAO, is the primary source of cytotoxic H2O2 in polyamine analogue-treated human breast cancer cell lines. J. Biol. Chem. 2005;280:39843–39851. doi: 10.1074/jbc.M508177200. [DOI] [PubMed] [Google Scholar]
- 80.Pledgie-Tracy A, Billam M, Hacker A, Sobolewski MD, Woster PM, Zhang Z, et al. The role of the polyamine catabolic enzymes SSAT and SMO in the synergistic effects of standard chemotherapeutic agents with a polyamine analogue in human breast cancer cell lines. Cancer Chemother. Pharmacol. 2010;65:1067–1081. doi: 10.1007/s00280-009-1112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Creaven PJ, Perez R, Pendyala L, Meropol NJ, Loewen G, Levine E, et al. Unusual central nervous system toxicity in a phase I study of N1, N11 diethylnorspermine in patients with advanced malignancy. Invest. New Drugs. 1997;15:227–234. doi: 10.1023/a:1005827231849. [DOI] [PubMed] [Google Scholar]
- 82.Hahm HA, Ettinger DS, Bowling K, Hoker B, Chen TL, Zabelina Y, et al. Phase I study of N1, N11-diethylnorspermine in patients with non-small cell lung cancer. Clin. Cancer Res. 2002;8:684–690. [PubMed] [Google Scholar]
- 83.Streiff RR, Bender JF. Phase 1 study of N1, N11-diethylnorspermine (DENSPM) administered TID for 6 days in patients with advanced malignancies. Invest. New Drug. 2001;19:29–39. doi: 10.1023/a:1006448516938. [DOI] [PubMed] [Google Scholar]
- 84.Wolff AC, Armstrong DK, Fetting JH, Carducci MK, Riley CD, Bender JF, et al. A phase II study of the polyamine analog N1, N11-diethylnorspermine (DENSpm) daily for five days every 21 days in patients with previously treated metastatic breast cancer. Clin. Cancer Res. 2003;9:5922–5928. [PubMed] [Google Scholar]
- 85.Casero RA, Jr, Mank AR, Saab NH, Wu R, Dyer WJ, Woster PM. Growth and biochemical effects of unsymmetrically substituted polyamine analogues in human lung tumor cells 1. Cancer Chemother. Pharmacol. 1995;36:69–74. doi: 10.1007/BF00685735. [DOI] [PubMed] [Google Scholar]
- 86.Saab NH, West EE, Bieszk NC, Preuss CV, Mank AR, Casero RA, Jr, et al. Synthesis and evaluation of unsymmetrically substituted polyamine analogues as modulators of human spermidine/spermine-N1-acetyltransferase (SSAT) and as potential antitumor agents. J. Med. Chem. 1993;36:2998–3004. doi: 10.1021/jm00072a020. [DOI] [PubMed] [Google Scholar]
- 87.Hacker A, Marton LJ, Sobolewski M, Casero RA., Jr In vitro and in vivo effects of the conformationally restricted polyamine analogue CGC-11047 on small cell and non-small cell lung cancer cells. Cancer Chemother. Pharmacol. 2008;63:45–53. doi: 10.1007/s00280-008-0706-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reddy V, Valasinas A, Sarkar A, Basu H, Marton L, Frydman B. Conformationally restricted analogues of 1N, 12N-bisethylspermine: synthesis and growth inhibitory effects on human tumor cell lines. J. Med. Chem. 1998;41:4723–4732. doi: 10.1021/jm980172v. [DOI] [PubMed] [Google Scholar]
- 89.Valasinas A, Sarkar A, Reddy VK, Marton LJ, Basu HS, Frydman B. Conformationally restricted analogues of 1N,14N-bisethylhomospermine (BE-4-4-4): synthesis and growth inhibitory effects on human prostate cancer cells. J. Med. Chem. 2001;44:390–403. doi: 10.1021/jm000309t. [DOI] [PubMed] [Google Scholar]
- 90.Huang Y, Hager ER, Phillips DL, Hacker A, Frydman B, Valasinas AL, et al. Conformationally constrained polyamine analogues and oligoamines inhibit growth and induce apoptosis in human breast cancer cells. Proc. Am. Assoc. Cancer Res. 2002;43:90. <chk doi>. [Google Scholar]
- 91.Weisell J, Hyvonen MT, Alhonen L, Vepsalainen J, Keinanen TA, Khomutov AR. Charge deficient analogues of the natural polyamines. Curr. Pharm. Des. 2014;20:262–277. doi: 10.2174/13816128113199990037. [DOI] [PubMed] [Google Scholar]
- 92.Keinänen TA, Hyvönen MT, Alhonen L, Vepsäläainen J, Khomutov AR. Selective regulation of polyamine metabolism with methylated polyamine analogues. Amino Acids. 2014;46:605–620. doi: 10.1007/s00726-013-1587-9. [DOI] [PubMed] [Google Scholar]
- 93.Xie S, Wang J, Zhang Y, Wang C. Antitumor conjugates with polyamine vectors and their molecular mechanisms. Expert Opin. Drug Deliv. 2010;7:1049–1061. doi: 10.1517/17425247.2010.504205. [DOI] [PubMed] [Google Scholar]
- 94.Annereau J-P, Brel V, Dumontet C, Guminski Y, Imbert T, Broussas M, et al. A fluorescent biomarker of the polyamine transport system to select patients with AML for F14512 treatment. Leuk. Res. 2010;34:1383–1389. doi: 10.1016/j.leukres.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 95.Li M, Li Q, Zhang Y-H, Tian Z-Y, Ma H-X, Zhao J, et al. Antitumor effects and preliminary systemic toxicity of ANISpm in vivo and in vitro. Anticancer Drugs. 2013;24:32–42. doi: 10.1097/CAD.0b013e328359affd. [DOI] [PubMed] [Google Scholar]
- 96.Traquete R, Ghani RA, Phanstiel O, Wallace HM. Ant 4,4, a polyamine-anthracene conjugate, induces cell death and recovery in human promyelogenous leukemia cells (HL-60) Amino Acids. 2013;44:1193–1203. doi: 10.1007/s00726-012-1452-2. [DOI] [PubMed] [Google Scholar]
- 97.Phanstiel OT, Kaur N, Delcros JG. Structure-activity investigations of polyamine-anthracene conjugates and their uptake via the polyamine transporter. Amino Acids. 2007;33:305–313. doi: 10.1007/s00726-007-0527-y. [DOI] [PubMed] [Google Scholar]
- 98.Vourtsis D, Lamprou M, Sadikoglou E, Giannou A, Theodorakopoulou O, Sarrou E, et al. Effect of an all-trans-retinoic acid conjugate with spermine on viability of human prostate cancer and endothelial cells in vitro and angiogenesis in vivo. Eur. J. Pharmacol. 2013;698:122–130. doi: 10.1016/j.ejphar.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 99.Huang Y, Marton LJ, Woster PM, Casero RA. Polyamine analogues targeting epigenetic gene regulation. Essays Biochem. 2009;46:95–110. doi: 10.1042/bse0460007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Varghese S, Senanayake T, Murray-Stewart T, Doering K, Fraser A, Casero RA, Jr, et al. Polyaminohydroxamic acids and polyaminobenzamides as isoform selective histone deacetylase inhibitors. J. Med. Chem. 2008;51:2447–2456. doi: 10.1021/jm701384x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Varghese S, Gupta D, Baran T, Jiemjit A, Gore SD, Casero RA, Jr, et al. Alkyl-substituted polyaminohydroxamic acids: a novel class of targeted histone deacetylase inhibitors. J. Med. Chem. 2005;48:6350–6365. doi: 10.1021/jm0505009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang Y, Stewart TM, Wu Y, Baylin SB, Marton LJ, Perkins B, et al. Novel oligoamine analogues inhibit lysine-specific demethylase 1 and induce reexpression of epigenetically silenced genes. Clin. Cancer Res. 2009;15:7217–7228. doi: 10.1158/1078-0432.CCR-09-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang Y, Greene E, Murray Stewart T, Goodwin AC, Baylin SB, Woster PM, et al. Inhibition of lysine-specific demethylase 1 by polyamine analogues results in reexpression of aberrantly silenced genes. Proc. Natl Acad. Sci. USA. 2007;104:8023–8028. doi: 10.1073/pnas.0700720104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nowotarski SL, Pachaiyappan B, Holshouser SL, Kutz CJ, Li Y, Huang Y, et al. Structure-activity study for (bis)ureidopropyl-and (bis) thioureidopropyldiamine LSD1 inhibitors with 3-5-3 and 3-6-3 carbon backbone architectures. Bioorg. Med. Chem. 2015;23:1601–1612. doi: 10.1016/j.bmc.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sharma SK, Hazeldine S, Crowley ML, Hanson A, Beattie R, Varghese S, et al. Polyamine-based small molecule epigenetic modulators. Med. Chem. Commun. 2012;3:14–21. doi: 10.1039/C1MD00220A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schenk T, Chen WC, Göllner S, Howell L, Jin L, Hebestreit K, et al. Inhibition of the LSD1 (KDM1A) demethylase reactivates the all-trans-retinoic acid differentiation pathway in acute myeloid leukemia. Nat. Med. 2012;18:605–611. doi: 10.1038/nm.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sharma SK, Wu Y, Steinbergs N, Crowley ML, Hanson AS, Casero RA, et al. (Bis)urea and (Bis)thiourea inhibitors of lysine-specific demethylase 1 as epigenetic modulators. J. Med. Chem. 2010;53:5197–5212. doi: 10.1021/jm100217a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Loiseau PM, Chauffert O, Czok M. Effect of chronic trypanosomiasis on the bioavailability of alpha-difluoromethylornithine (DFMO) after oral administration: pharmacokinetics study on DFMO plasma levels in infected and noninfected mice using a high-performance liquid chromatography assay. Parasitol. Res. 1997;83:386–389. doi: 10.1007/s004360050268. [DOI] [PubMed] [Google Scholar]
- 109.McCann PP, Bitonti AJ, Bacchi CJ, Clarkson AB., Jr Use of difluoromethylornithine (DFMO, eflornithine) for late-stage African trypanosomiasis. Trans. R. Soc. Trop. Med. Hyg. 1987;81:701–702. doi: 10.1016/0035-9203(87)90465-2. [DOI] [PubMed] [Google Scholar]
- 110.Nilsson JA, Keller UB, Baudino TA, Yang C, Norton S, Old JA, et al. Targeting ornithine decarboxylase in Myc-induced lymphomagenesis prevents tumor formation. Cancer Cell. 2005;7:433–444. doi: 10.1016/j.ccr.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 111.O’Brien TG, Megosh LC, Gilliard G, Soler AP. Ornithine decarboxylase overexpression is a sufficient condition for tumor promotion in mouse skin. Cancer Res. 1997;57:2630–2637. [PubMed] [Google Scholar]
- 112.Guo Y, Zhao J, Sawicki J, Peralta Soler A, O’Brien TG. Conversion of C57Bl/6 mice from a tumor promotion-resistant to a -sensitive phenotype by enhanced ornithine decarboxylase expression. Mol. Carcinog. 1999;26:32–36. [PubMed] [Google Scholar]
- 113.Chen Y, Megosh LC, Gilmour SK, Sawicki JA, O’Brien TG. K6/ODC transgenic mice as a sensitive model for carcinogen identification. Toxicol. Lett. 2000;116:27–35. doi: 10.1016/s0378-4274(00)00196-x. [DOI] [PubMed] [Google Scholar]
- 114.Hibshoosh H, Johnson M, Weinstein IB. Effects of overexpression of ornithine decarboxylase (ODC) on growth control and oncogene-induced cell transformation. Oncogene. 1991;6:739–743. [PubMed] [Google Scholar]
- 115.Slaga TJ. Multistage skin carcinogenesis: a useful model for the study of the chemoprevention of cancer. Acta Pharmacol. Toxicol. 1984;55(Suppl. 2):107–124. doi: 10.1111/j.1600-0773.1984.tb02485.x. [DOI] [PubMed] [Google Scholar]
- 116.Malt RA, Kingsnorth AN, Lamuraglia GM, Lacaine F, Ross JS. Chemoprevention and chemotherapy by inhibition of ornithine decarboxylase activity and polyamine synthesis: colonic, pancreatic, mammary, and renal carcinomas. Adv. Enzyme Regul. 1985;24:93–102. doi: 10.1016/0065-2571(85)90071-8. [DOI] [PubMed] [Google Scholar]
- 117.Tempero MA, Nishioka K, Knott K, Zetterman RK. Chemoprevention of mouse colon tumors with difluoromethylornithine during and after carcinogen treatment. Cancer Res. 1989;49:5793–5797. [PubMed] [Google Scholar]
- 118.Reddy BS, Nayini J, Tokumo K, Rigotty J, Zang E, Kelloff G. Chemoprevention of colon carcinogenesis by concurrent administration of piroxicam, a nonsteroidal antiinflammatory drug with d,l-alpha-difluoromethylornithine, an ornithine decarboxylase inhibitor, in diet. Cancer Res. 1990;50:2562–2568. [PubMed] [Google Scholar]
- 119.Pegg AE, Shantz LM, Coleman CS. Ornithine decarboxylase as a target for chemoprevention. J. Cell Biochem. 1995;59(Suppl. 22):132–138. doi: 10.1002/jcb.240590817. [DOI] [PubMed] [Google Scholar]
- 120.Meyskens FL, Jr, Gerner EW. Development of difluoromethylornithine as a chemoprevention agent for the management of colon cancer. J. Cell Biochem. 1995;59(Suppl. 22):126–131. doi: 10.1002/jcb.240590816. [DOI] [PubMed] [Google Scholar]
- 121.Gerner EW, Meyskens FL., Jr Combination chemoprevention for colon cancer targeting polyamine synthesis and inflammation. Clin. Cancer Res. 2009;15:758–761. doi: 10.1158/1078-0432.CCR-08-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Meyskens FL, Jr, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev. Res. 2008;1:32–38. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li J, Cameron GA, Wallace HM. Decreased sensitivity to aspirin is associated with altered polyamine metabolism in human prostate cancer cells. Amino Acids. 2015;48:1003–1012. doi: 10.1007/s00726-015-2143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Meyskens FL, Jr, Simoneau AR, Gerner EW. Chemoprevention of prostate cancer with the polyamine synthesis inhibitor difluoromethylornithine. Recent Results Cancer Res. 2014;202:115–120. doi: 10.1007/978-3-642-45195-9_14. [DOI] [PubMed] [Google Scholar]
- 125.Esmat AY, Refaie FM, Shaheen MH, Said MM. Chemoprevention of prostate carcinogenesis by DFMO and/or finasteride treatment in male Wistar rats. Tumori. 2002;88:513–521. doi: 10.1177/030089160208800616. [DOI] [PubMed] [Google Scholar]
- 126.Jeter JM, Curiel-Lewandrowski C, Stratton SP, Myrdal PB, Warneke JA, Einspahr JG, et al. Phase IIB randomized study of topical difluoromethylornithine and topical diclofenac on sun-damaged skin of the forearm. Cancer Prev. Res. 2016;9:128–134. doi: 10.1158/1940-6207.CAPR-15-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Witherspoon M, Chen Q, Kopelovich L, Gross SS, Lipkin SM. Unbiased metabolite profiling indicates that a diminished thymidine pool is the underlying mechanism of colon cancer chemoprevention by alpha-difluoromethylornithine. Cancer Discov. 2013;3:1072–1081. doi: 10.1158/2159-8290.CD-12-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Babbar N, Casero RA., Jr Tumor necrosis factor-alpha increases reactive oxygen species by inducing spermine oxidase in human lung epithelial cells: a potential mechanism for inflammation-induced carcinogenesis. Cancer Res. 2006;66:11125–11130. doi: 10.1158/0008-5472.CAN-06-3174. [DOI] [PubMed] [Google Scholar]
- 129.Xu H, Chaturvedi R, Cheng Y, Bussiere FI, Asim M, Yao MD, et al. Spermine oxidation induced by Helicobacter pylori results in apoptosis and DNA damage: implications for gastric carcinogenesis. Cancer Res. 2004;64:8521–8525. doi: 10.1158/0008-5472.CAN-04-3511. [DOI] [PubMed] [Google Scholar]
- 130.Chaturvedi R, Cheng Y, Asim M, Bussiere FI, Xu H, Gobert AP, et al. Induction of polyamine oxidase 1 by Helicobacter pylori causes macrophage apoptosis by hydrogen peroxide release and mitochondrial membrane depolarization. J. Biol. Chem. 2004;279:40161–40173. doi: 10.1074/jbc.M401370200. [DOI] [PubMed] [Google Scholar]
- 131.Goodwin AC, Shields CED, Wu S, Huso DL, Wu X, Murray-Stewart TR, et al. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc. Natl Acad. Sci. USA. 2011;108:15354–15359. doi: 10.1073/pnas.1010203108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chaturvedi R, de Sablet T, Asim M, Piazuelo MB, Barry DP, Verriere TG, et al. Increased Helicobacter pylori-associated gastric cancer risk in the Andean region of Colombia is mediated by spermine oxidase. Oncogene. 2015;34:3429–3440. doi: 10.1038/onc.2014.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Goodwin AC, Jadallah S, Toubaji A, Lecksell K, Hicks JL, Kowalski J, et al. Increased spermine oxidase expression in human prostate cancer and prostatic intraepithelial neoplasia tissues. Prostate. 2008;68:766–772. doi: 10.1002/pros.20735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu S, Rhee K-J, Albesiano E, Rabizadeh S, Wu X, Yen HR, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sears CL. Enterotoxigenic Bacteroides fragilis: a rogue among symbiotes. Clin. Microbiol. Rev. 2009;22:349–369. doi: 10.1128/CMR.00053-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu S, Morin PJ, Maouyo D, Sears CL. Bacteroides fragilis enterotoxin induces c-Myc expression and cellular proliferation. Gastroenterology. 2003;124:392–400. doi: 10.1053/gast.2003.50047. [DOI] [PubMed] [Google Scholar]
- 137.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 138.Su L, Kinzler K, Vogelstein B, Preisinger A, Moser A, Luongo C, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 139.Johnson CH, Dejea CM, Edler D, Hoang LT, Santidrian AF, Felding BH, et al. Metabolism links bacterial biofilms and colon carcinogenesis. Cell. Metab. 2015;21:891–897. doi: 10.1016/j.cmet.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.de Sablet T, Piazuelo MB, Shaffer CL, Schneider BG, Asim M, Chaturvedi R, et al. Phylogeographic origin of Helicobacter pylori is a determinant of gastric cancer risk. Gut. 2011;60:1189–1195. doi: 10.1136/gut.2010.234468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Khobta A, Anderhub S, Kitsera N, Epe B. Gene silencing induced by oxidative DNA base damage: association with local decrease of histone H4 acetylation in the promoter region. Nucleic Acids Res. 2010;38:4285–4295. doi: 10.1093/nar/gkq170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Watanabe S, Ichimura T, Fujita N, Tsuruzoe S, Ohki I, Shirakawa M, et al. Methylated DNA-binding domain 1 and methylpurine-DNA glycosylase link transcriptional repression and DNA repair in chromatin. Proc. Natl Acad. Sci. USA. 2003;100:12859–12864. doi: 10.1073/pnas.2131819100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.O’Hagan HM, Mohammad HP, Baylin SB. Double strand breaks can initiate gene silencing and SIRT1-dependent onset of DNA methylation in an exogenous promoter CpG island. PLoS Genet. 2008;4:e1000155. doi: 10.1371/journal.pgen.1000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.O’Hagan HM, Wang W, Sen S, Destefano Shields C, Lee SS, Zhang YW, et al. Oxidative damage targets complexes containing DNA methyltransferases, SIRT1, and polycomb members to promoter CpG Islands. Cancer Cell. 2011;20:606–619. doi: 10.1016/j.ccr.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hahn MA, Hahn T, Lee DH, Esworthy RS, Kim B-W, Riggs AD, et al. Methylation of polycomb target genes in intestinal cancer is mediated by inflammation. Cancer Res. 2008;68:10280–10289. doi: 10.1158/0008-5472.CAN-08-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Murray-Stewart T, Sierra JC, Piazuelo MB, Mera RM, Chaturvedi R, Bravo LE, et al. Epigenetic silencing of miR-124 prevents spermine oxidase regulation: implications for Helicobacter pylori-induced gastric cancer. Oncogene. 2016 doi: 10.1038/onc.2016.91. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cervelli M, Salvi D, Polticelli F, Amendola R, Mariottini P. Structure-function relationships in the evolutionary framework of spermine oxidase. J. Mol. Evol. 2013;76:365–370. doi: 10.1007/s00239-013-9570-3. [DOI] [PubMed] [Google Scholar]
- 148.Polticelli F, Salvi D, Mariottini P, Amendola R, Cervelli M. Molecular evolution of the polyamine oxidase gene family in Metazoa. BMC Evol. Biol. 2012;12:90. doi: 10.1186/1471-2148-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tavladoraki P, Cervelli M, Antonangeli F, Minervini G, Stano P, Federico R, et al. Probing mammalian spermine oxidase enzyme-substrate complex through molecular modeling, site-directed mutagenesis and biochemical characterization. Amino Acids. 2011;40:1115–1126. doi: 10.1007/s00726-010-0735-8. [DOI] [PubMed] [Google Scholar]
- 150.Lakanen JR, Coward JK, Pegg AE. Alpha-methyl polyamines: metabolically stable spermidine and spermine mimics capable of supporting growth in cells depleted of polyamines. J. Med. Chem. 1992;35:724–734. doi: 10.1021/jm00082a013. [DOI] [PubMed] [Google Scholar]