Abstract
Preterm birth is the leading cause of neonatal mortality and morbidity worldwide. Spontaneous preterm birth is a complex, multifactorial condition in which cervical dysfunction plays an important role in some women. Current treatment options for cervical dysfunction include cerclage and supplemental progesterone. In addition, cervical pessary is being studied in research protocols. However, cerclage, supplemental progesterone and cervical pessary have well known limitations and there is a strong need for alternate treatment options. In this review, we discuss two novel interventions to treat cervical dysfunction: (1) injectable, silk protein-based biomaterials for cervical tissue augmentation (injectable cerclage) and (2) a patient-specific pessary. Three-dimensional computer simulation of the cervix is performed to provide a biomechanical rationale for the interventions. Further development of these novel interventions could lead to new treatment options for women with cervical dysfunction.
CLINICAL BACKGROUND
Preterm birth, defined as birth before 37 weeks of gestation, is the major contributor of neonatal mortality and morbidity worldwide.1 It can cause respiratory immaturity, intracranial hemorrhages and infections, and these conditions can result in a range of long term complications such as intellectual impairment, cerebral palsy, chronic lung disease, deafness and blindness.2 The frequency and severity of adverse outcomes of children born preterm increase with decreasing gestational age at birth.1 Preterm birth is an important complication of both singleton and multifetal pregnancies and preventing preterm birth remains a challenge in clinical obstetric care.1, 2
The worldwide incidence of preterm birth is 11.1% which varies between countries within a range of 5–13% and results in approximately 15 million children born preterm each year.3 The highest rates occur in Southeastern and South Asia where 13.4% of the children are born preterm. Approximately 1.2 million preterm births occur in high-income countries, of which more than 0.5 million occur in the United States where the estimated preterm birth rate is 11–12%.1
Although preterm birth is a complex, multifactorial condition, in some women, cervical dysfunction plays an important role.4 The composition and structure of the cervix controls its ability to remain closed during pregnancy to promote fetal development. In normal delivery, cervical effacement and dilation occurs at term. In preterm delivery, cervical effacement and dilation occurs prior to term, which can lead to a premature birth. Cervical dysfunction is detected by measuring a short cervix with transvaginal ultrasound.5–7 The risk of preterm birth is inversely proportional to the length of the cervix, with a shorter cervix conferring a higher risk.6
Various treatment strategies to prevent preterm birth in women with suspected dysfunctional cervix have been studied, including cervical cerclage, cervical pessary and progesterone.8 However, these treatment strategies are not effective in all patient populations at risk for preterm birth. There remains a strong need for alternative, effective therapies for preventing preterm birth in women with a dysfunctional cervix. In this review we discuss two novel interventions to treat cervical dysfunction that are currently being studied; injectable, silk protein-based biomaterials for cervical tissue augmentation (injectable cerclage) and a patient-specific pessary. We also demonstrate a three-dimensional computer simulation of the interventions to provide a biomechanical rationale for efficacy. These complementary interventions aim to address the pathogenesis of cervical dysfunction and to support the native, physiological properties of the cervix.
INJECTABLE BIOMATERIAL FOR CERVICAL AUGMENTATION
Cervical remodeling
In spontaneous preterm birth, the final common event is softening, shortening and dilation of the cervix, also referred to as cervical remodeling. Cervical remodeling relates to both changes in (1) the material properties of the stroma (i.e. softening) and (2) the anatomical shape of the cervix (i.e. shortening, effacement, dilation).4 There is a strong relationship between cervical remodeling and the organization and composition of the cervical extracellular matrix (ECM). The cervical ECM plays a key role in the maintenance of appropriate mechanical function of the cervix.9 Excessive cervical softening appears to be related to preterm birth.9 Preventing excessive softening, i.e. reestablishing the normal properties of the stroma, is a promising clinical target for the development of interventions that aim to prevent cervical dysfunction and preterm birth.4
Cervical cerclage
Currently, a cervical cerclage is an important treatment option for the prevention of preterm birth in women with suspected cervical dysfunction.10–13 The fact that cervical cerclage is an effective treatment for a short cervix13 suggests that cervical dysfunction is causally related to preterm birth in some women. A cervical cerclage is a surgical procedure in which a suture is placed in the stroma to provide added support for the cervix, as proposed by Shirodkar in 195514 and by McDonald in 195715. Although cerclage was efficacious in some studies,13 no efficacy was seen in other studies.16 In twins, cerclage may be associated with increased risk.17 Moreover, placing a cerclage is not without risk. Complications of cerclage include preterm premature rupture of membranes (PPROM), infection, preterm labor, suture displacement, and bleeding.18 In addition, cerclage is associated with an increased risk of cervical laceration, both in nulliparous and multiparous women.19
It is hypothesized that a cervical cerclage prevents premature cervical remodeling by providing support to the cervix. The exact mechanism, however, by which a cerclage provides support and prevents premature cervical remodeling remains unclear. In addition, a cerclage does not address excessive cervical softening, which likely relates to cerclage failure in some women. A comprehensive understanding of the complex process of cervical remodeling and the relationship between biochemical and mechanical properties of the cervix could lead to a more effective intervention to prevent spontaneous preterm birth.4
Cervical shortening – cause or consequence of preterm birth
Whether a short cervix causes preterm birth or is a consequence of a different pathophysiology is difficult to determine in individual patients. When a cerclage is not successful, it suggests either (1) the cerclage did not provide adequate support or (2) the cause was unrelated to cervical dysfunction. The most common pathophysiology associated with cervical shortening is infection and inflammation. Among women with clinically diagnosed cervical insufficiency (defined as cervical dilation ≥ 1.5 cm), the risk of intrauterine infection is 8 – 51%.20, 21 Among women with a short cervix, the levels of inflammatory cytokines (i.e. MMP-8, IL-6) are increased.22–24 In addition, the risk of adverse pregnancy outcomes are increased when cervical dysfunction and intrauterine infection/inflammation are present.20, 21, 23, 24
Although infection and inflammation may be present in cases of cervical dysfunction, it is difficult to know the natural history of ascending infection in pregnancy. It is possible that ascending infection leads to subsequent cervical shortening/insufficiency. It is also possible that infection occurs as a consequence of a short cervix. If ascending infection is a consequence of a short cervix, a therapy that prevents cervical shortening could help prevent ascending infection. A hypothesis of this research is that, in some cases, a short cervix leads to an impaired cervical barrier to infection. We advocate for the importance of a detailed study of cervical biomechanics of cervical shortening, which could reveal interventions that provide better support for the cervix compared with present therapies. Improved interventions for the cervix could not only treat cases of cervical insufficiency but also prevent ascending infection in cases where a short cervix is the cause.
Preventing cervical shortening – insights from biomechanical modeling
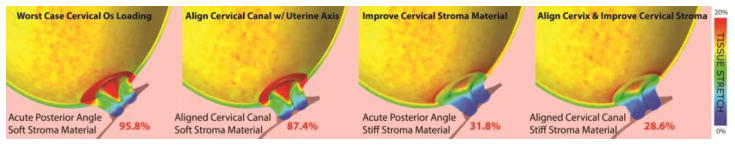
Although a short cervix plays a central role in the management of women at risk for preterm birth, a detailed understanding of the deformation mechanisms leading to a short cervix is lacking.4, 25 Insight into the key variables that influence cervical deformation can be gained from biomechanical modeling.26, 27 Studies of cervical biomechanics demonstrate that cervical shortening is a complex biomechanical problem influenced by multiple variables including anatomical geometry, cervical loading and cervical material properties.26, 27 In particular, it is known that the cervical material properties soften during the course of pregnancy28 and excessive cervical softening likely leads to preterm shortening and dilation. The central hypothesis our work is that a treatment that improves the functional performance of the cervical tissue could prevent cervical shortening and hence preterm birth. This concept is demonstrated in an initial biomechanical simulation model showing improving stromal properties reduces the amount of cervical stretching at the internal os (Figure 1). As a first attempt to test this hypothesis, we developed a prototype, injectable, silk-based gel to be used for cervical tissue augmentation.29–31
Figure 1. Computer simulation results of improving cervical stroma material and cervical angle.
The increase in stromal material stiffness and the alignment of the cervical canal with the uterine axis reduces the amount of tissue stretching at the internal os. Percentages indicate the volume ratio of the internal os that is above 8% tensile strain under intrauterine pressure of 8.67kPA. Details of the finite element model are found in Westervelt et al., 2017.48 A soft and stiff cervix are assigned collagen fiber moduli of 1.71kPa and 769kPa, respectively.
Injectable cerclage
Silk fibroin is a fibrous protein that is naturally derived and displays remarkable mechanical properties, chemical flexibility and biocompatibility.32 Purified silk protein can be processed into biodegradable gels with tunable mechanical properties, which are important features for a treatment for pregnancy.33 The physical properties of silk biomaterials can be further modified by blending with other materials to meet functional demands and can therefore suit a wide range of biomedical applications.34–36 In a recent report, Brown and colleagues studied a range of injectable silk-based materials for cervical augmentation.31 In this study, purified silk protein solutions were crosslinked by an enzyme catalyzed reaction to form elastic biomaterials, which were formulated to match the intrinsic properties of cervical tissue during pregnancy.31 From 108 different silk biomaterial formulations that were screened for mechanical properties in pregnant and non-pregnant tissue, two optimized formulations were further evaluated for biocompatibility, facile injection and in vitro degradation. In vitro degradation of these formulations was studied using concentrated protease solution, which showed tunable control of degradation rate based on the hydrogel formulation. In addition, cervical fibroblasts cultured on these biomaterials were proliferative and metabolically active. Furthermore, in vitro injection of human cervical tissue required low injection force and showed that tissue volume could be increased without significant influence on cervical stiffness. These elastic silk gels are a promising initial prototype for augmentation of cervical tissue during pregnancy.31
Preliminary animal study
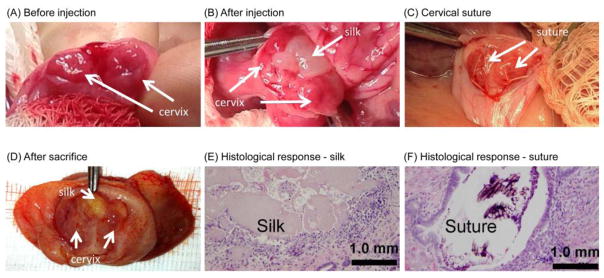
Biocompatibility and feasibility of cervical injections were studied in timed-pregnant New Zealand White rabbits, which is an accepted animal model to study biochemical changes of the cervix during pregnancy.37–39 Sterile, sonicated silk was prepared as previously described.30 Injections were performed via a midline laparotomy approach according to an IACUC approved protocol. Cervical injections were performed on gestational day 15. A laparotomy was performed and the vagina was brought to the abdomen. The wall of the vagina was incised to allow direct visualization of the two cervices. Using a 20 gauge needle, approximately 0.5 mL of sonicated silk hydrogel was injected into the cervical stroma (Figure 2A and 2B). Outcomes were compared to 4-0 Mersilene suture (Figure 2C), which was chosen as a control because it is a common non-absorbable suture used for cervical cerclage. After the cervical procedure, the vagina and abdomen were closed. Cervical treatments were well tolerated; no preterm birth was seen. Silk gel was grossly visualized within the cervical tissue both after injection and at sacrifice, which occurred at day 27 (Figure 2B and 2D). Histology revealed multifocal deposits of silk gel surrounded by a thin wall of macrophages, lymphocytes, plasma cells, eosinophils, and occasional multinucleated giant cells. A similar response was seen around the suture control (Figure 2E and 2F). The histological changes appear consistent with a mild foreign body response. These preliminary results in a limited number of animals demonstrate that cervical injections were well tolerated by pregnant rabbits. However, data regarding biodegradation or labor outcomes in vivo are currently lacking and will be assessed in future studies.
Figure 2. Cervical augmentation with silk.
(A) Two separate cervices in rabbit before injection; (B) Silk immediately after injection, gestational day 15; (C) Cervical 4-0 Mersilene suture; (D) Injected silk after sacrifice, gestational day 27; (E) Mild foreign body inflammatory response to silk biomaterial; (F) Mild foreign body inflammatory response to 4-0 Mersilene suture.
PATIENT SPECIFIC PESSARY
Arabin pessary
The cervical pessary is a flexible silicone device known since 1959 when it was used in women with recurrent miscarriage.40 The exact mechanism of the cervical pessary remains unknown, yet it has been hypothesized that the pessary relieves the pressure on the internal cervical os by changing the position of the cervical canal and distributing the weight of the uterus and fetus.41 Hence, the pessary may have the potential to prevent premature shortening and dilatation of the cervix, and premature rupture of the membranes. Another proposed mechanism is that the pessary might support the immunological barrier between chorioamnion-extraovular space and the vaginal microbiological flora.42 However, the clinical effect of a cervical pessary in the prevention of preterm birth in both singleton and multifetal pregnancies remains unclear since conflicting results have been published so far.42–46
A preliminary biomechanical computational analysis of the pessary demonstrates the device applies contact forces on the outer surface of the cervix to close the cervical canal.47 This application results in a complex and heterogeneous loading of the cervix that ultimately reduces the amount of tissue stretching at the internal os and increases the amount of tissue compression at the outer surface.47 In other words, the pessary squeezes the cervix to force closure of the canal. The squeezing action causes tissue compression, and the magnitude of tissue compression depends on the mismatch of pessary and cervix diameters. The biological and functional consequences of this change in tissue loading remain to be determined. Additionally, a biomechanical analysis of multiple cervical canal angle scenarios demonstrates that when the cervical canal is perfectly aligned with the longitudinal uterine axis the mechanical load is better distributed and the tissue stretching at the internal os is at its minimum. Conversely, if the cervical angle is moved posterior, away from the uterine axis, the amount of tissue stretching at the internal os increases with no sizable reduction of pressure on the cervix (Figure 1).48
Patient specific pessary
A patient-specific cervical pessary may prevent preterm birth by a similar mechanism to the Arabin pessary, with additional advantages. The basis of a patient-specific pessary is a custom-fit device to maternal anatomy to ensure a reduction of contact pressure on the outer cervix and a reduction of tissue stretching at the internal os by cervical canal alignment with the uterine axis (Figure 1).48 Every pregnancy can have a vastly different anatomy,49 leading to drastically different mechanical loading patterns on the lower uterine segment, fetal membranes, and internal os of the cervix.48 The Arabin pessary currently comes in 9 standard sizes, which may not be an exact fit for all patients, resulting in a pessary that is too loose, tight, short, or tall, leading to discomfort and misplacement of load support. With the acceleration of additive manufacturing processes for flexible surgical-grade materials50 and computational models of the biomechanical environment of pregnancy,48 a custom-fit pessary device that beneficially distributes the mechanical load is feasible.
As mentioned previously, the length of the cervix is proportional to one’s risk of preterm birth.6 Two recent studies investigating cervical angle correlation to PTB showed conflicting results. One study found that an extreme posterior angle is associated with PTB,51 while the other did not find any correlation.52 The discrepancy in these clinical studies highlights the fact that the mechanisms causing cervical shortening and subsequent preterm birth is multifactorial. A recent biomechanical computational study exploring cervical anatomical and stroma material properties found the largest amount of cervical tissue stretching is found for a soft cervix (i.e. a cervix that has gone through biochemical remodeling) and an acute posterior cervical canal angle (Figure 1).48 Changing the acute angle alone, such that the cervical canal aligns with the uterine longitudinal axis, gives an 8.8% relative reduction in cervical tissue stretching. Changing the stromal material properties alone, such that the cervix is made of a stiffer material, gives a 67% relative reduction in cervical tissue stretching. Changing both the cervical canal angle and the stroma material properties gives a 70% relative reduction in cervical tissue stretching. These computational results suggest a multifactorial approach may be effective to reducing the amount of cervical tissue deformation and stretching and highlight the multifactorial nature of the biomechanical environment of the cervix.
How to design a patient specific pessary
A patient-specific pessary can be designed by obtaining a patient’s anatomy via transabdominal and transvaginal ultrasound and creating a complementary device that appropriately tilts the cervix in the correct direction and fits onto the outer cervix to compress the cervical canal with minimal contact pressure. A custom-fit device requires material anatomy dimensions such as the cervical length, anterior uterocervical angle, external cervical diameter, and height of the vaginal canal. A custom pessary should have: (1) an inner diameter that matches the outer diameter of the patient, with an inner diameter lip that has a surface area reaching down the cervical length to reduce contact pressure and (2) a device height that accounts for vaginal canal dimensions and cervical canal angle such that the device can align the cervix with the uterus and the device can reach as close to the internal os as possible. The device can then be validated by running a computational simulation of the device geometry placed within a computer model of the patient’s anatomy to verify that it will truly reduce the mechanical loading on the cervix.
CONCLUSION AND FUTURE DIRECTIONS
Greater understanding of the molecular, biochemical and biomechanical function of the cervix will enhance our understanding of the physiological process of cervical function in term and preterm delivery. This multidisciplinary approach opens the field for novel interventions, such as the injectable cerclage and personalized pessary, which aim to interfere with the underlying mechanism of premature cervical softening, shortening and dilation.4 For these preventive interventions to be useful in clinical care, a clear diagnosis of cervical dysfunction/insufficiency is critical to identify patients at risk for spontaneous preterm birth. Yet, due to the lack of objective findings and criteria for the diagnosis of cervical insufficiency, the identification of women with a dysfunctional cervix remains a challenging task for care givers. This challenge reflects the complexity of preterm birth in which multiple pathways seem to be involved. Multidisciplinary collaboration involving clinicians, biologists and engineers, could promote a better understanding of the mechanisms underlying cervical dysfunction leading to preterm birth. In addition, a multidisciplinary approach to the dilemma of preterm birth could lead to novel interventions that could be used in the prevention of preterm birth.
Acknowledgments
Supported in part by the Tissue Engineering Resource Center (TERC) from the National Institute of Biomedical Imaging and Bioengineering (EB002520).
Footnotes
DISCLOSURE STATEMENTS
A patent application for a patient-specific pessary has been filed under the United States Provisional Application No. 62/303,689, filed March 4, 2016, “Devices And methods For Minimizing Likelihood Of Preterm Birth”. Inventors: Kristin M. Myers, Michael P. Fernandez, Joy Y. Vink, Ronald J. Wapner, and Andrea R. Westervelt.
A patent application for the injectable cerclage has been filed under the United States Provisional Application No. 14/345,758, filed March 19, 2014, “Methods for treatment of cervical insufficiency”. Inventors: Michael House, David Kaplan, Simona Socrate, Errol Norwitz.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blencowe H, Cousens S, Chou D, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10(Suppl 1):S2. doi: 10.1186/1742-4755-10-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–9. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 3.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–72. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 4.Myers KM, Feltovich H, Mazza E, et al. The mechanical role of the cervix in pregnancy. J Biomech. 2015;48:1511–23. doi: 10.1016/j.jbiomech.2015.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berghella V, Bega G, Tolosa JE, Berghella M. Ultrasound assessment of the cervix. Clin Obstet Gynecol. 2003;46:947–62. doi: 10.1097/00003081-200312000-00026. [DOI] [PubMed] [Google Scholar]
- 6.Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network N Engl J Med. 1996;334:567–72. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 7.Iams JD, Goldenberg RL, Mercer BM, et al. The Preterm Prediction Study: recurrence risk of spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network Am J Obstet Gynecol. 1998;178:1035–40. doi: 10.1016/s0002-9378(98)70544-7. [DOI] [PubMed] [Google Scholar]
- 8.Iams JD, Berghella V. Care for women with prior preterm birth. Am J Obstet Gynecol. 2010;203:89–100. doi: 10.1016/j.ajog.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.House M, Kaplan DL, Socrate S. Relationships between mechanical properties and extracellular matrix constituents of the cervical stroma during pregnancy. Semin Perinatol. 2009;33:300–7. doi: 10.1053/j.semperi.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alfirevic Z, Stampalija T, Roberts D, Jorgensen AL. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2012:CD008991. doi: 10.1002/14651858.CD008991.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Berghella V, Rafael TJ, Szychowski JM, Rust OA, Owen J. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth: a meta-analysis. Obstet Gynecol. 2011;117:663–71. doi: 10.1097/AOG.0b013e31820ca847. [DOI] [PubMed] [Google Scholar]
- 12.American College of O, Gynecologists. ACOG Practice Bulletin No. 142: Cerclage for the management of cervical insufficiency. Obstet Gynecol. 2014;123:372–9. doi: 10.1097/01.AOG.0000443276.68274.cc. [DOI] [PubMed] [Google Scholar]
- 13.Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201:375.e1–8. doi: 10.1016/j.ajog.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shirodkar VN. A new method of operative treatment for habitual abortions in the second trimester of pregnancy. Antiseptic. 1955;52:299–300. [Google Scholar]
- 15.McDonald IA. Suture of the cervix for inevitable miscarriage. The Journal of obstetrics and gynaecology of the British Empire. 1957;64:346–50. doi: 10.1111/j.1471-0528.1957.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 16.To MS, Alfirevic Z, Heath VC, et al. Cervical cerclage for prevention of preterm delivery in women with short cervix: randomised controlled trial. Lancet. 2004;363:1849–53. doi: 10.1016/S0140-6736(04)16351-4. [DOI] [PubMed] [Google Scholar]
- 17.Saccone G, Rust O, Althuisius S, Roman A, Berghella V. Cerclage for short cervix in twin pregnancies: systematic review and meta-analysis of randomized trials using individual patient-level data. Acta Obstet Gynecol Scand. 2015;94:352–8. doi: 10.1111/aogs.12600. [DOI] [PubMed] [Google Scholar]
- 18.Berghella V, Seibel-Seamon J. Contemporary use of cervical cerclage. Clin Obstet Gynecol. 2007;50:468–77. doi: 10.1097/GRF.0b013e31804bddfd. [DOI] [PubMed] [Google Scholar]
- 19.Landy HJ, Laughon SK, Bailit JL, et al. Characteristics associated with severe perineal and cervical lacerations during vaginal delivery. Obstet Gynecol. 2011;117:627–35. doi: 10.1097/AOG.0b013e31820afaf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SE, Romero R, Park CW, Jun JK, Yoon BH. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol. 2008;198:633.e1–8. doi: 10.1016/j.ajog.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 21.Romero R, Mazor M, Morrotti R, et al. Infection and labor. VII. Microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term. Am J Obstet Gynecol. 1992;166:129–33. doi: 10.1016/0002-9378(92)91845-2. [DOI] [PubMed] [Google Scholar]
- 22.Kiefer DG, Keeler SM, Rust OA, Wayock CP, Vintzileos AM, Hanna N. Is midtrimester short cervix a sign of intraamniotic inflammation? American Journal of Obstetrics and Gynecology. 2009:200. doi: 10.1016/j.ajog.2009.01.047. [DOI] [PubMed] [Google Scholar]
- 23.Romero R, Miranda J, Chaiworapongsa T, et al. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med. 2014:1–17. doi: 10.3109/14767058.2014.954243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaisbuch E, Hassan SS, Mazaki-Tovi S, et al. Patients with an asymptomatic short cervix (<or=15 mm) have a high rate of subclinical intraamniotic inflammation: implications for patient counseling. Am J Obstet Gynecol. 2010;202:433.e1–8. doi: 10.1016/j.ajog.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.House M, McCabe R, Socrate S. Using imaging-based, three-dimensional models of the cervix and uterus for studies of cervical changes during pregnancy. Clin Anat. 2013;26:97–104. doi: 10.1002/ca.22183. [DOI] [PubMed] [Google Scholar]
- 26.House M, Feltovich H, Hall TJ, Stack T, Patel A, Socrate S. Three-dimensional, extended field-of-view ultrasound method for estimating large strain mechanical properties of the cervix during pregnancy. Ultrason Imaging. 2012;34:1–14. doi: 10.1177/016173461203400101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez M, House M, Jambawalikar S, et al. Investigating the mechanical function of the cervix during pregnancy using finite element models derived from high-resolution 3D MRI. Comput Method Biomec. 2016;19:404–17. doi: 10.1080/10255842.2015.1033163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myers KM, Paskaleva AP, House M, Socrate S. Mechanical and biochemical properties of human cervical tissue. Acta Biomater. 2008;4:104–16. doi: 10.1016/j.actbio.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Heard AJ, Socrate S, Burke KA, Norwitz ER, Kaplan DL, House MD. Silk-based injectable biomaterial as an alternative to cervical cerclage: an in vitro study. Reprod Sci. 2013;20:929–36. doi: 10.1177/1933719112468952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Critchfield AS, McCabe R, Klebanov N, et al. Biocompatibility of a sonicated silk gel for cervical injection during pregnancy: in vivo and in vitro study. Reprod Sci. 2014;21:1266–73. doi: 10.1177/1933719114522551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown JE, Partlow BP, Berman AM, House MD, Kaplan DL. Injectable silk-based biomaterials for cervical tissue augmentation: an in vitro study. Am J Obstet Gynecol. 2016;214:118.e1–9. doi: 10.1016/j.ajog.2015.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Omenetto FG, Kaplan DL. New opportunities for an ancient material. Science. 2010;329:528–31. doi: 10.1126/science.1188936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Partlow BP, Hanna CW, Rnjak-Kovacina J, et al. Highly tunable elastomeric silk biomaterials. Adv Funct Mater. 2014;24:4615–24. doi: 10.1002/adfm.201400526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu X, Wang X, Rnjak J, Weiss AS, Kaplan DL. Biomaterials derived from silk-tropoelastin protein systems. Biomaterials. 2010;31:8121–31. doi: 10.1016/j.biomaterials.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu X, Lu Q, Sun L, et al. Biomaterials from ultrasonication-induced silk fibroin-hyaluronic acid hydrogels. Biomacromolecules. 2010;11:3178–88. doi: 10.1021/bm1010504. [DOI] [PubMed] [Google Scholar]
- 36.Vepari C, Kaplan DL. Silk as a biomaterial. Prog Polym Sci. 2007;32:991–1007. doi: 10.1016/j.progpolymsci.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belayet HM, Kanayama N, Khatun S, et al. Dehydroepiandrosterone sulphate promotes hyaluronic acid-induced cervical ripening in rabbits. Hum Reprod. 1999;14:1361–7. doi: 10.1093/humrep/14.5.1361. [DOI] [PubMed] [Google Scholar]
- 38.ElMaradny E, Kanayama N, Kobayashi H, et al. The role of hyaluronic acid as a mediator and regulator of cervical ripening. Human Reproduction. 1997;12:1080–88. doi: 10.1093/humrep/12.5.1080. [DOI] [PubMed] [Google Scholar]
- 39.Fukuda Y, Sugimura M, Suzuki K, Kanayama N. Prostaglandin E2 receptor EP4-selective antagonist inhibits lipopolysaccharide-induced cervical ripening in rabbits. Acta Obstet Gyn Scan. 2007;86:1297–302. doi: 10.1080/00016340701671788. [DOI] [PubMed] [Google Scholar]
- 40.Cross R. Treatment of habitual abortion due to cervical incompetence. Lancet. 1959;2:127. [Google Scholar]
- 41.Vitsky M. Simple treatment of the incompetent cervical os. Am J Obstet Gynecol. 1961;81:1194–7. doi: 10.1016/s0002-9378(15)33352-4. [DOI] [PubMed] [Google Scholar]
- 42.Goya M, Pratcorona L, Merced C, et al. Cervical pessary in pregnant women with a short cervix (PECEP): an open-label randomised controlled trial. Lancet. 2012;379:1800–6. doi: 10.1016/S0140-6736(12)60030-0. [DOI] [PubMed] [Google Scholar]
- 43.Goya M, de la Calle M, Pratcorona L, et al. Cervical pessary to prevent preterm birth in women with twin gestation and sonographic short cervix: a multicenter randomized controlled trial (PECEP-Twins) Am J Obstet Gynecol. 2016;214:145–52. doi: 10.1016/j.ajog.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 44.Nicolaides KH, Syngelaki A, Poon LC, et al. A Randomized Trial of a Cervical Pessary to Prevent Preterm Singleton Birth. N Engl J Med. 2016;374:1044–52. doi: 10.1056/NEJMoa1511014. [DOI] [PubMed] [Google Scholar]
- 45.Nicolaides KH, Syngelaki A, Poon LC, et al. Cervical pessary placement for prevention of preterm birth in unselected twin pregnancies: a randomized controlled trial. Am J Obstet Gynecol. 2016;214:3.e1–9. doi: 10.1016/j.ajog.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 46.Saccone G, Ciardulli A, Xodo S, et al. Cervical pessary for preventing preterm birth in twin pregnancies with short cervical length: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2017:1–8. doi: 10.1080/14767058.2016.1268595. [DOI] [PubMed] [Google Scholar]
- 47.Fernandez MWA, Vink J, House M, Nhan-Chang C, Fan M, Wapner R, Elovitz M, Myers K. Biomechanical Computer Simulation of the Arabin Cervical Pessary. Reprod Sci. 2016;23:130A. T-093 Supp 1. [Google Scholar]
- 48.Westervelt AFM, House M, Vink J, Nhan-Chang C, Wapner R, Myers K. A parameterized ultrasound-based finite element analysis of the mechanical environment of pregnancy. Journal of Biomechanical Engineering. 2017 doi: 10.1115/1.4036259. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.House M, Bhadelia RA, Myers K, Socrate S. Magnetic resonance imaging of three-dimensional cervical anatomy in the second and third trimester. Eur J Obstet Gynecol Reprod Biol. 2009;144(Suppl 1):S65–9. doi: 10.1016/j.ejogrb.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hod Lipson MK. Fabricated: The New World of 3D Printing. Wiley & sons; p. 320. [Google Scholar]
- 51.Dziadosz M, Bennett TA, Dolin C, et al. Uterocervical angle: a novel ultrasound screening tool to predict spontaneous preterm birth. Am J Obstet Gynecol. 2016;215:376.e1–7. doi: 10.1016/j.ajog.2016.03.033. [DOI] [PubMed] [Google Scholar]
- 52.Uquillas KR, Fox NS, Rebarber A, Saltzman DH, Klauser CK, Roman AS. A comparison of cervical length measurement techniques for the prediction of spontaneous preterm birth. J Matern Fetal Neonatal Med. 2017;30:50–53. doi: 10.3109/14767058.2016.1160049. [DOI] [PubMed] [Google Scholar]