Abstract
Background
Laparoscopic gastric plication (LGP) is a technique in the restrictive category of bariatric procedures that reduces the gastric volume and increases intragastric pressure. Nausea and vomiting are the most common complications after this procedure. The goal of this research is to compare the combined effect of promethazine/dexamethasone versus Metoclopramide/ dexamethasone on the prevention of nausea and vomiting after LGP.
Methods
In recovery, the patients were divided into two groups, the Metoclopramide group which was given Metoclopramide 10 mg plus dexamethasone 4 mg/8 hours intravenous for 48 hours, and the promethazine group which was given promethazine 50 mg /12 hours, intramuscular for the first 24 hours and then promethazine 25 mg/12 hours for the next 24 hours plus dexamethasone 4 mg/8 hours intravenous for 48 hours. The frequency of nausea and vomiting, number of reflux episodes, frequency of epigastric fullness, and the duration of walking around q12 hours were recorded in the first 48 hours post-operation.
Results
Eighty patients were enrolled into the study. Promethazine group were found to significantly reduce the incidence of PONV in the first 24 hours compared with the other group (41% vs. 97.5%), relative risk = 0.042 [95% CI = 0.006, 0.299]. The mean numbers of epigastric fullness and severity of epigastria pain were lower in the promethazine group (P = 0.01) and the total opioid requirement was also reduced in promethazine group (32.1 ± 2.6 VS .68.5 ± 4.6 mg). However, the patients in the promethazine group were more sedated, which caused the duration of walking q12 hours in this group to decrease.
Conclusions
In morbidly obese patients undergoing laparoscopic gastric plication, promethazine/dexametasone was more effective than Metoclopramide/dexametasone in preventing and reducing the incidence of nausea, epigastric fullness, and reflux. That combination was also more effective than Metoclopramide in reducing the severity of epigastric pain.
Keywords: Laparoscopic gastric plication, Nausea, Vomiting, Promethazine, Dexamethasone, Metoclopramide
1. Background
Bariatric surgery is considered to be an effective method for weight loss in morbidly obese individuals; however, it is not without potential complications. Laparoscopic gastric plication (LGP) is a new restrictive technique (in the arsenal of weight loss surgery techniques) that reduces the gastric volume by the plication of the greater curvature (1). Postoperative nausea and vomiting (PONV) is the most common problem that disturbs patients in the immediate post operative hours (2). The incidence of PONV according to the technique of bariatric surgery and the size of gastric lumen that remain after surgery are different which may reach 40% in some study despite a PONV prophylaxis regime following current guidelines (3).
By convention, a combination of antiemetic drugs such as metoclopramide, ondansetron, and hydrocortisone are used to manage PONV; yet somehow the incidence of PONV has not been reduced which needs further investigation of PONV prophylaxis and treatment in bariatric surgery patients.
Promethazine and dexamethasone are commonly used as antiemetic drugs which act on the central nervous system and are very effective for the prevention of PONV (4, 5). In this prospective control clinical trial, we compare the combined effect of promethazine and dexamethasone versus the combination of Metoclopramide with dexamethasone for the prevention of nausea/vomiting after LGP.
2. Methods
After being approved by the ethical board committee of anaesthesiology department of Tehran University of Medical Sciences (TUMS), this interventional randomized clinical controlled trial was conducted on 80 morbid obese patients who were candidates for LGP surgery. After explaining different aspects of the procedure, a written informed consent was obtained from all the participating patients. This study is part of a large research with IRCT number: IRCT2013052312294N4. Exclusion criteria were any contraindication to receiving dexamethasone, promethazine, and metoclopramid; uncontrolled hypertension and/or diabetes; and receiving any antiemetic drug in the preoperative 24 hours.
The whole surgery was done by a general surgeon who had completed a fellowship in advanced laparoscopic surgery. The technique of LGP used was based on the standard method in the last paper of the author (6).
All patients received anesthesia; they were induced with thiopental 4 to 5 mg/kg, fentanyl 2 µg/kg, midazolam 0.06 mg/kg, and atracurium 0.5 mg/kg. For anesthesia maintenance, a combination of isoflorane, fentanyl 1 µg/kg/h, and propofol was used. Propofol was the standard drug between two groups and in some situation we added less than 1 MAC isoflurane to decrease blood pressure during surgery.
Prior to extubation, all patients received paracetamol 1 g intravenous. The patients were then randomly allocated by using a computer-generated table into two following groups in the recovery room, the metoclopramide group which was given metoclopramide 10 mg plus dexamethasone 4 mg/8 hours intravenous for 48 hours, and the promethazine group which was given promethazine 50 mg/12 hours, IM for the first 24 hours and then promethazine 25 mg/12 hours for the next 24 hours plus dexamethasone 4 mg/8 hours intravenous for 48 hours. This type of surgery PONV usually began several hours after the operation, therefore randomization of patients was performed immediately after surgery in the recovery room.
The study was double-blinded and the anesthesiologist responsible for the postoperative patient evaluations was not involved in drug administration. The evaluations were performed q12 hours for the first 48 hours following the surgery.
The primary endpoints were the incidence of nausea and vomiting, reflux episodes, and epigastric fullness, and also the duration of walking q12hrs in the first 48 hours after surgery. The patients’ nausea score was evaluated as (1 = no nausea; 2 = mild; 3 = moderate; 4 = severe nausea). Epigastric pain was evaluated at the same time by using a visual analogue scale (VAS) (0 = no pain and 10 = worst possible pain). Postoperative pain score and the total analgesic requirement for the first 48hrs were recorded. Meperidine 50 mg IM was used as rescue analgesia in both groups when pain score exceeded 2. For patients in whom nausea and vomiting were not tolerable, ondansetron 4mg intravenous was used as rescue treatment.
Deep vein thrombosis is a severe and fatal complication in bariatric surgeries. Early ambulation after surgery is a preventable approach that reduces the incidence of DVT; therefore we measured the duration of walking after surgery.
The sample size was determined prospectively with 40 patients in each group. In regard to the high incidence of PONV after laparoscopic gastric plication, a 50% reduction in the incidence of PONV at any time within the first 48hrs of treatment could be determined with a statistical power of 80% (β = 0.85).
Statistical analysis was performed using SPSS version 14. Student’s t-test was used to compare the continuous variables between the groups. Repeated measure test (General Linear Model), Fisher’s exact test, and chi-2 were used to compare the incidence and the severity of nausea, vomiting, and pain at multiple time points. A P value < 0.05 was considered statistically significant. Data are presented as mean [standard deviation (SD)], numbers, or percentages.
3. Results
The enrollment flow chart of the patients in this study is displayed in Figure 1. A total of 80 morbid obese patients were enrolled in this study from 1 June, 2015 to 30 January, 2016 with similar demographic characteristics in both groups (Table 1).
Figure 1. The Enrollment Flow Chart of the Patients.
Table 1. Patients’ Characteristic Between Two Groups.
| Variable | Promethazine Group n = 40 | Metoclopramid Group (n = 40) | P Value |
|---|---|---|---|
| Age, y | 34.41 ± 12.05 | 33.58 ± 13.37 | 0.4 |
| Gender (F/M) | 33/7 | 34/6 | 0.1 |
| BMI, kg/m 2 | 40.87 ± 3.38 | 40.67 ± 3.68 | 0.3 |
| FBS, mg/dL | 100.23 ± 19.54 | 102.05 ± 21.91 | 0.1 |
| Triglyceride, mg/dL | 187.38 ± 39.59 | 197.95 ± 58.85 | 0.02 |
| Cholesterol, mg/dL | 201.51 ± 38.84 | 229.20 ± 50.82 | 0.01 |
| MAP, mmgh | 82.4 ± 5.2 | 84.6 ± 3.4 | 0.2 |
| Sleep apnea, n | 3 | 3 | 1 |
| Total pethedine Use, mg | 32.1 ± 2.6 | 68.5 ± 4.6 | 0.00 |
Abbreviations: BMI, body mass index; MAP, mean arterial pressure.
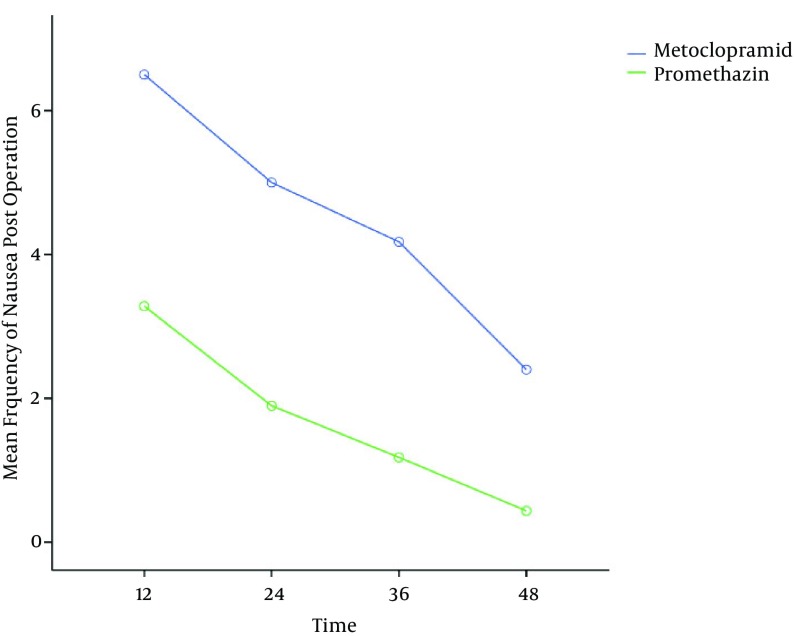
The mean number of PONV episodes in the promethazine group was significantly lower than in the metoclopramide group; however, nausea episodes were reduced in both groups (Figure 2). Promethazine group were found to significantly reduce the incidence of PONV in the first 24 hours compared with the other group (41% vs. 97.5%), relative risk = 0.042 [95% CI = 0.006, 0.299].
Figure 2. Mean Frequency of Nausea after Operation (P = 0.01).
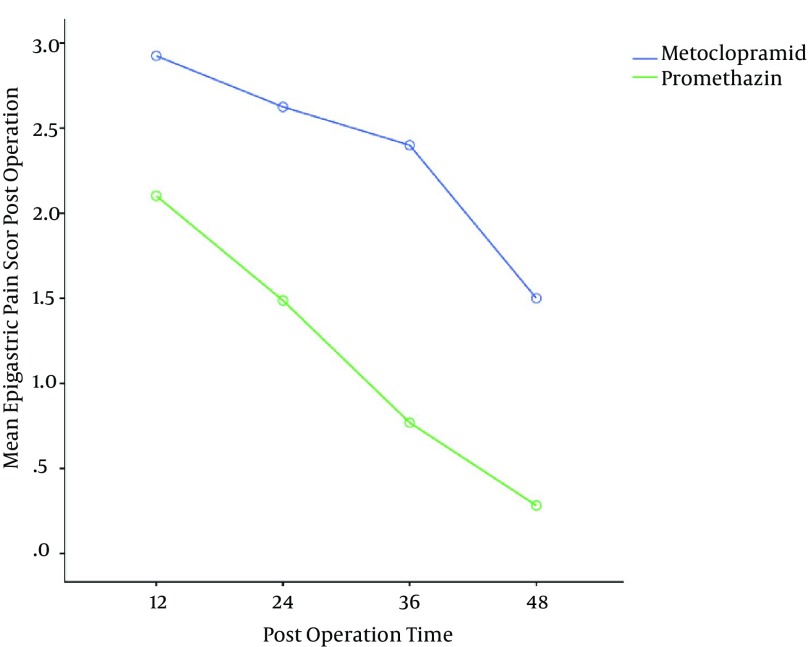
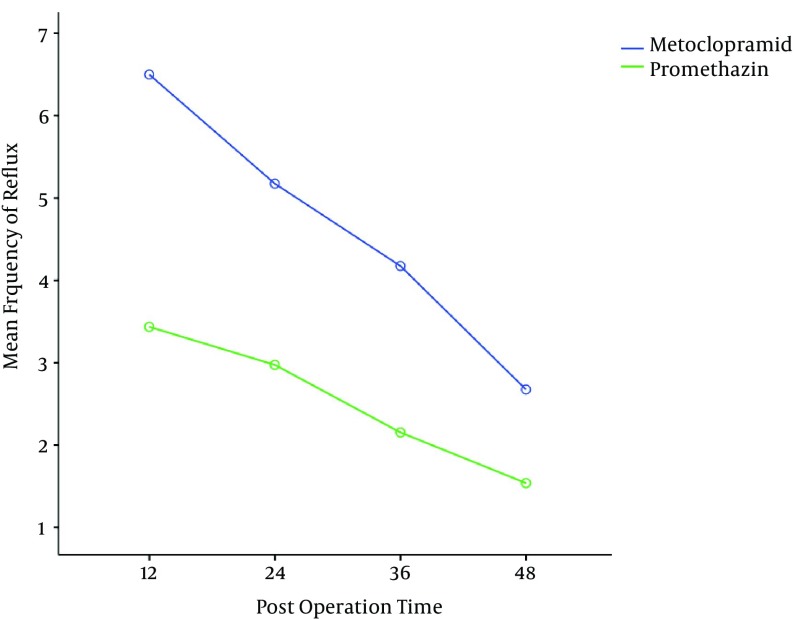
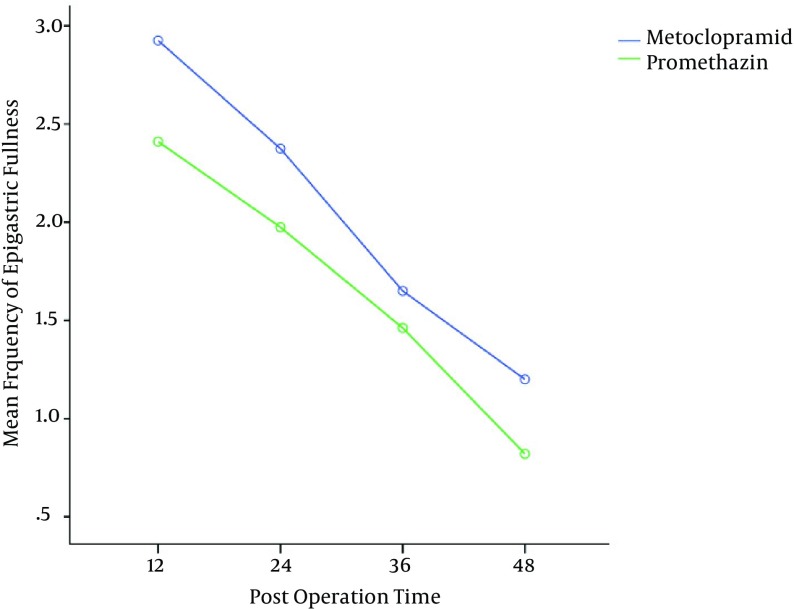
The mean severity of epigastric pain was higher in the metoclopramide group, thus these patients needed more analgesia in comparison to the other group (Figure 3). The mean numbers of episodes of reflux and epigastric fullness in the first postoperative 48hrs were lower in the promethazine group (Figures 4 and 5).
Figure 3. Mean Epigastric Pain Score Post Operation (P = 0.01).
Figure 4. Mean Frequency of Reflux After Operation (P = 0.02).
Figure 5. Mean Frequency of Epigastric Fullness After Operation (P = 0.02).
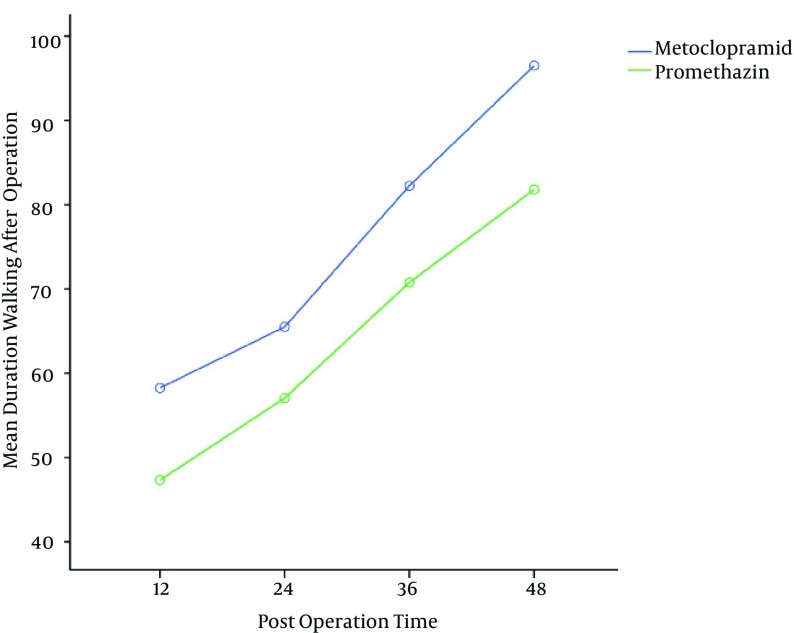
Patients in the metoclopramide group walked 10 to 12 min more than the promethazine group (P = 0.01) (Figure 6). Patients in the promethazine group were more sedated; therefore postoperative total meperidine consumption was lower in this group (Table 1).
Figure 6. Mean Duration of Walking After Operation (P = 0.01).
4. Discussion
Our results indicate a lower incidence of nausea, reflux, epigastric fullness, and also reduced epigastric pain scores when using promethazine/dexamethasone compared with metoclopramide/dexamethasone during the first postoperative days after LGP surgery.
LGP as a new surgical restrictive therapy for the treatment of morbid obesity has been shown to have acceptable results since its application 12 years ago (6). One of the most common problems with this operation is PONV. LGP, like other gastric restrictive therapies, induces increased intragastric pressure. The degree of plication and the rate of increased intragastric pressure are higher in patients who undergo LGP in comparison to patients who have underwent other gastric restriction procedures (such as sleeve gastrectomy) (6). The feeling of postoperative gastric fullness is a potential etiology for nausea in LGP cases which would be corrected after patient adaptation to the reduced stomach volume (7).
The prophylactic administration of various antiemetics, such as dopamine receptor antagonists, anticholinergics, 5-HT3 receptor antagonists, and dexamethasone has been studied extensively (8).
Because of the multifactorial etiology of PONV and involvement of several receptor systems in the development and progression of PONV, it seems clear that a combination of drugs acting at the multiple receptor systems would have greater efficacy than using a single drug for the prophylactic prevention and the treatment of PONV in high-risk subjects (9). Due to the multiple receptors involved in PONV, increasing the dose of a single class of drug will not necessarily decrease its incidence especially in patients with multiple risk factors (10).
Promethazine is a centrally acting drug with antihistamine and anticholinergic properties that are effective for the prevention of PONV (11). It also helps to reduce nervousness, restlessness, and agitation after any surgery. Co-administration of promethazine and opioids or codeine increases subjective happiness in patients (12). Deep intramuscular injections of promethazine have a 4 to 6 hour effect and have been shown to be safe in most patients. In this study, the anticholinergic effect of promethazine is the likely etiology that reduced the gastric irritability and frequency of PONV. In some current guidelines, promethazine in combination with other antiemetic was found to be more effective in reducing PONV, severity of nausea, and pain than promethazine monotherapy (13). A combination prophylactic therapy of PONV with promethazine and other drugs has been published by some authors. In a study by Etezadi et al. in morbidly obese patients undergoing laparoscopic gastric plication, prophylactic administration of dexamethasone 8mg and promethazine 50mg was effective in the first 12 hours after surgery in reducing the incidence of PONV and the severity of abdominal pain (14).
Promethazine usage might be limited by its sedative side effects. It has been suggested that the sedative effect of promethazine might be dose-dependent, but in some study, there was no difference in sedation between 6.25 mg, 12.5 mg, and 25 mg doses (15).
Dexamethasone is a well-documented anti-inflammatory drug which plays a positive role in PONV in patients undergoing chemotherapy or surgery (16). Dexamethasone potentiates euphoric effects of opioids and reduces the postoperative pain intensity and the need for rescue analgesia as compared to placebo (17).
In a study by Benevides et al. combination of dexamethasone with haloperidol and ondansetron reduced PONV, the necessity of rescue antiemetic, and opioid consumption after Laparoscopic Sleeve Gastrectomy (18). Prophylactic dexamethasone/ondansetrone has significantly reduced the incidence of PONV in patients undergoing laparoscopic cholecystectomy (19). Dexamethasone combined with other antiemetic provided better prophylaxis than single antiemetic against postoperative nausea and vomiting after laparoscopic cholecystectomy (20).
Efficacy of dexamethasone with H1 antihistamine drugs such as dimenhydrinate in the prevention of nausea and vomiting has been studied in patients undergoing rhinoplasty operations (21).
In a study by Bergese et al. there is a significant reduction in PONV when a combination of palonosetron with Dexamethasone and Promethazine is used as prophylactic therapy in patients at a high risk for developing PONV during the first 120 hours after neurosurgery (22).
One of the challengeable facts in this study is the doses of dexamethasone that seem high. The usual prophylactic dose of dexamethason for PONV is 5 to 10 mg. De Oliveira et al. in a meta-analysis showed that a 4 mg to 5 mg dose of dexamethasone seems to have similar clinical effects on the reduction of PONV as the 8 mg to 10 mg dose when dexamethasone was used as a single drug or as a combination therapy (23). However, our patients were morbidly obese and PONV usually lasted for 2 to 3 days in this procedure; therefore we continued treatment in the first 48 hours. Eventually, the underlying mechanism of dexamethasone action and its optimal dose should be further investigated.
Metoclopramide is a dopaminergic blocker with antiemetic and gastroprokinetic effects; it is commonly used to treat nausea and vomiting and to facilitate gastric emptying in people with gastroparesis. The gastroprokinetic activity of metoclopramide is mediated by muscarinic receptor activity, D2 receptor antagonist activity, and 5-HT4 receptor agonist activity (24). It increases the activity of the stomach, which may increase the incidence of PONV (25). According to the results of the study, metoclopramide could not fully decrease PONV episodes or epigastric fullness of patients after gastric placation.
A drawback to this study was that the dosage of promethazine and dexamethasone was very high in comparison to other similar studies. The high dose caused the patients in the promethazine group to be more sedated, which in turn caused their duration of walking postoperatively to be lower than the metoclopramide group. Further studies using different doses of the same medications are indicated to fully explore the range of doses truly required to prevent PONV.
4.1. Conclusions
To conclude, in patients undergoing LGP, the combination of dexamethasone and promethazine was more effective than the combination of metoclopramide and dexamethasone in the first 48 hours in reducing the incidence of PONV, epigastric fullness, reflux, and the severity of epigastric pain.
Acknowledgments
We are indebted to the research and development center of Sina hospital for their support.
Footnotes
Conflict of Interest:No conflict of interest.
Contributor Information
Farsad Imani, Email: imanifar@tums.ac.ir.
Reza Shariat Moharari, Email: moharari@tums.ac.ir.
Pejman Pourfakhr, Email: pourfakhr@razi.tums.ac.ir.
References
- 1.Darabi S, Talebpour M, Zeinoddini A, Heidari R. Laparoscopic gastric plication versus mini-gastric bypass surgery in the treatment of morbid obesity: a randomized clinical trial. Surg Obes Relat Dis. 2013;9(6):914–9. doi: 10.1016/j.soard.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Niazi M, Maleki AR, Talebpour M. Short-term outcomes of laparoscopic gastric plication in morbidly obese patients: importance of postoperative follow-up. Obes Surg. 2013;23(1):87–92. doi: 10.1007/s11695-012-0777-y. [DOI] [PubMed] [Google Scholar]
- 3.Chouillard E, Schoucair N, Alsabah S, Alkandari B, Montana L, Dejonghe B, et al. Laparoscopic Gastric Plication (LGP) as an Alternative to Laparoscopic Sleeve Gastrectomy (LSG) in Patients with Morbid Obesity: A Preliminary, Short-Term, Case-Control Study. Obes Surg. 2016;26(6):1167–72. doi: 10.1007/s11695-015-1913-2. [DOI] [PubMed] [Google Scholar]
- 4.Barrett TW, DiPersio DM, Jenkins CA, Jack M, McCoin NS, Storrow AB, et al. A randomized, placebo-controlled trial of ondansetron, metoclopramide, and promethazine in adults. Am J Emerg Med. 2011;29(3):247–55. doi: 10.1016/j.ajem.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 5.Moeini A, Rastad H, Eslami B, Manouchehrian N, Sanatkar M. The Effect of Dexamethasone on Nausea and Vomiting during Labor and Labor Pain in Parturients Undergoing Normal Vaginal Delivery. Arch Anesthesiol Crit Care. 2015;1(4):126–9. [Google Scholar]
- 6.Talebpour M, Motamedi SM, Talebpour A, Vahidi H. Twelve year experience of laparoscopic gastric plication in morbid obesity: development of the technique and patient outcomes. Ann Surg Innov Res. 2012;6(1):7. doi: 10.1186/1750-1164-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talebpour M, Amoli BS. Laparoscopic total gastric vertical plication in morbid obesity. J Laparoendosc Adv Surg Tech A. 2007;17(6):793–8. doi: 10.1089/lap.2006.0128. [DOI] [PubMed] [Google Scholar]
- 8.Halliday TA, Sundqvist J, Hultin M, Wallden J. Post-operative nausea and vomiting in bariatric surgery patients: an observational study. Acta Anaesthesiol Scand. 2017;61(5):471–9. doi: 10.1111/aas.12884. [DOI] [PubMed] [Google Scholar]
- 9.Chandrakantan A, Glass PS. Multimodal therapies for postoperative nausea and vomiting, and pain. Br J Anaesth. 2011;107 Suppl 1:i27–40. doi: 10.1093/bja/aer358. [DOI] [PubMed] [Google Scholar]
- 10.Gan TJ, Meyer T, Apfel CC, Chung F, Davis PJ, Eubanks S, et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg. 2003;97(1):62–71. doi: 10.1213/01.ane.0000068580.00245.95. table of contents. [DOI] [PubMed] [Google Scholar]
- 11.Carlisle JB, Stevenson CA. Drugs for preventing postoperative nausea and vomiting. Cochrane Database Syst Rev. 2006;(3):CD004125. doi: 10.1002/14651858.CD004125.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith HS. Combination opioid analgesics. Pain Phys. 2008;11(2):201–14. [PubMed] [Google Scholar]
- 13.Strenkoski-Nix LC, Ermer J, DeCleene S, Cevallos W, Mayer PR. Pharmacokinetics of promethazine hydrochloride after administration of rectal suppositories and oral syrup to healthy subjects. Am J Health Syst Pharm. 2000;57(16):1499–505. doi: 10.1093/ajhp/57.16.1499. [DOI] [PubMed] [Google Scholar]
- 14.Etezadi F, Omrani NG, Talebpour M, Imani F, Moharari RS, Pourfakhr P, et al. A Clinical Trial to Determine the Preventive Effective Dose of Promethazine on Postoperative Nausea and Vomiting after Laparoscopic Gastric Placation. Arch Anesthesiol Crit Care. 2017;3(1):270–2. [Google Scholar]
- 15.Habib AS, Reuveni J, Taguchi A, White WD, Gan TJ. A comparison of ondansetron with promethazine for treating postoperative nausea and vomiting in patients who received prophylaxis with ondansetron: a retrospective database analysis. Anesth Analg. 2007;104(3):548–51. doi: 10.1213/01.ane.0000252433.73485.be. [DOI] [PubMed] [Google Scholar]
- 16.Wu JI, Lo Y, Chia YY, Liu K, Fong WP, Yang LC, et al. Prevention of postoperative nausea and vomiting after intrathecal morphine for Cesarean section: a randomized comparison of dexamethasone, droperidol, and a combination. Int J Obstet Anesth. 2007;16(2):122–7. doi: 10.1016/j.ijoa.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Allen TK, Jones CA, Habib AS. Dexamethasone for the prophylaxis of postoperative nausea and vomiting associated with neuraxial morphine administration: a systematic review and meta-analysis. Anesth Analg. 2012;114(4):813–22. doi: 10.1213/ANE.0b013e318247f628. [DOI] [PubMed] [Google Scholar]
- 18.Benevides ML, Oliveira SS, de Aguilar-Nascimento JE. The combination of haloperidol, dexamethasone, and ondansetron for prevention of postoperative nausea and vomiting in laparoscopic sleeve gastrectomy: a randomized double-blind trial. Obes Surg. 2013;23(9):1389–96. doi: 10.1007/s11695-013-0923-1. [DOI] [PubMed] [Google Scholar]
- 19.Eidy M, Vafaei HR, Rajabi M, Mohammadzadeh M, Pazouki A. Effect of Ondansetron and Dexametasone on Post-Operative Nausea and Vomiting in Patients Undergoing Laparoscopic Cholecystectomy. J Minimal Invas Surg Sci. 2013;2(2):138–43. [Google Scholar]
- 20.Bisgaard T, Klarskov B, Kehlet H, Rosenberg J. Preoperative dexamethasone improves surgical outcome after laparoscopic cholecystectomy: a randomized double-blind placebo-controlled trial. Ann Surg. 2003;238(5):651–60. doi: 10.1097/01.sla.0000094390.82352.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kizilcik N, Bilgen S, Menda F, Ture H, Aydin B, Kaspar EC, et al. Comparison of Dexamethasone-Dimenhydrinate and Dexamethasone-Ondansetron in Prevention of Nausea and Vomiting in Postoperative Patients. Aesthetic Plast Surg. 2017;41(1):204–10. doi: 10.1007/s00266-016-0772-0. [DOI] [PubMed] [Google Scholar]
- 22.D. Bergese S. Studying the Effectiveness of Triple Therapy with Palonosetron, Dexamethasone and Promethazine for Prevention of Post Operative Nausea and Vomiting in High Risk Patients Undergoing Neurological Surgery and General Anesthesia. J Clin Trials. 2012;02(02) doi: 10.4172/2167-0870.1000107. [DOI] [Google Scholar]
- 23.De Oliveira GJ, Castro-Alves LJ, Ahmad S, Kendall MC, McCarthy RJ. Dexamethasone to prevent postoperative nausea and vomiting: an updated meta-analysis of randomized controlled trials. Anesth Analg. 2013;116(1):58–74. doi: 10.1213/ANE.0b013e31826f0a0a. [DOI] [PubMed] [Google Scholar]
- 24.Sweetman SC. Editor. Martindale: the complete drug reference. London: The Pharmaceutical Press; 2009. [Google Scholar]
- 25.Feldman M, Friedman LS, Brandt LJ. Sleisenger and Fordtran's Gastrointestinal and Liver Disease E-Book: Pathophysiology, Diagnosis, Management, Expert Consult Premium Edition-Enhanced Online Features. Elsevier Health Sciences; 2010. [Google Scholar]