Abstract
Purpose
Based on preclinical data suggesting that the class I selective HDAC inhibitor entinostat exerts a synergistic antitumor effect in combination with high dose interleukin 2 (IL-2) in a renal cell carcinoma model by down-regulating Foxp3 expression and function of regulatory T cells (Treg), we conducted a phase I/II clinical study with entinostat and high dose IL-2 in patients with metastatic clear cell renal cell carcinoma (ccRCC).
Experimental Design
Clear cell histology, no prior treatments, and being sufficiently fit to receive high dose IL-2 were the main eligibility criteria. The phase I portion consisted of two dose levels of entinostat (3 and 5 mg, PO every14 days) and a fixed standard dose of IL-2 (600,000 units/kg IV). Each cycle was 85 days. The primary end point was objective response rate and toxicity. Secondary end points included progression-free survival and overall survival.
Results
47 patients were enrolled. At a median follow-up of 21.9 months, the objective response rate was 37% (95% CI 22%–53%), the median progression-free survival was 13.8 months (95% CI 6.0–18.8), and the median overall survival was 65.3 months (95% CI 52.6.–65.3). The most common grade 3/4 toxicities were hypophosphatemia (16%), lymphopenia (15%), and hypocalcemia (7%), and all were transient. Decreased Treg were observed following treatment with entinostat, and lower numbers were associated with response (p=0.03).
Conclusions
This trial suggests a promising clinical activity for entinostat in combination with high dose Il-2 in ccRCC patients, and provides the first example of an epigenetic agent being rationally combined with immunotherapy.
Keywords: HDAC inhibition, immunotherapy, immunomodulation, renal cell carcinoma
Introduction
The treatment of metastatic clear cell renal cell carcinoma (ccRCC) is rapidly evolving 1,2. The use of cytokine therapies such as IL-2 and interferon-α has been progressively replaced by vascular growth factor receptor tyrosine kinase inhibitors (RTKI) such as sunitinib and pazopanib, in the first line setting. More recently, the approval of the immune checkpoint inhibitor nivolumab has introduced the use of this novel class of immunotherapy in previously treated RCC patients 3. However, there remains a critical need for improving the current standard treatments for RCC.
High dose IL-2 was approved for the treatment of RCC based on the response rate and duration of responses. 255 patients treated in 7 clinical trials at 21 institutions showed an objective response rate (ORR) of 15% 4. The recent SELECT trial (120 patients) has reported a 25% ORR by WHO criteria, a median duration of response of 20 months, and a median progression-free survival (PFS) of 4.2 months 5. This falls in a similar range with immune checkpoint inhibitor monotherapy in RCC, and until durability of checkpoint inhibitor therapy can be determined, supports a continued role for high dose IL-23. Increasing the magnitude of benefit of IL-2 therapy remains an important clinical goal.
Histone deacetylase (HDAC) inhibitors are a class of drugs targeting different enzymes that regulate the chromatin structure and the acetylation of lysine residues on the histone tails 6. Different classes of HDAC have been identified but drug development in oncology has focused on class I (HDAC 1, 2, 3, and 8) and class II (HDAC 4, 5, 6, 7, 9 and 10). Four HDAC inhibitors, one selective class I (romidepsin) and three class I/II (vorinostat, panobinostat, and belinostat) inhibitors, have been approved for the treatment of cutaneous / peripheral T cell lymphoma, and multiple myeloma. Our group has recently reported the activity of vorinostat in combination with bevacizumab in previously treated ccRCC patients 7. Entinostat is a class 1 selective oral HDAC inhibitor with anti-tumor activity in several preclinical models 8. This agent is currently in clinical development for breast cancer and other solid tumors in combination therapies 9,10,11,12. Its long 140-hour half-life allows continuous exposure with either once weekly or bi-weekly oral dosing.
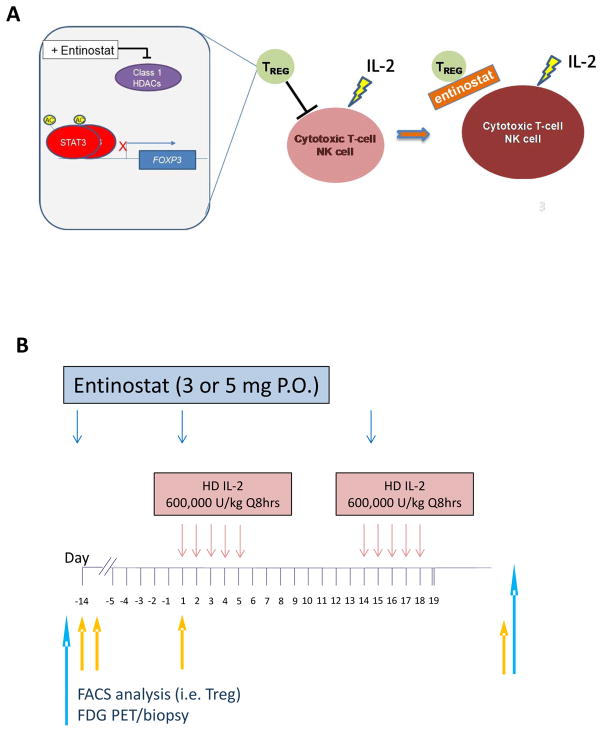
There is increasing evidence that epigenetic modulation may have immunostimulatory activity in addition to a direct antitumor effect. Our group reported that the combination of high dose IL-2 and entinostat had a synergistic antitumor effect in an immunocompetent murine model of RCC 13. The biological effect induced by low dose entinostat was associated with reduction of Foxp3 expression in Treg and impairment of their immune suppressive function without affecting T effector cells 14. Thus, based on reported clinical evidence that lower Treg numbers in the peripheral blood are associated with better outcomes in patients receiving high dose IL-215,16, we generated the hypothesis that the inhibitory effect of the selective class I HDAC inhibitor entinostat on Treg may increase the response rate and progression-free survival in patients receiving high dose IL-2 (Figure 1A) 17,18.
Figure 1. Study design.
(A) Overall hypothesis for the mechanism of action of entinostat in suppressing regulatory T cells (Treg) and expanding cytotoxic T-cells and natural killer cells (NK). (B) Clinical trial schema.
Patients and Methods
Eligibility
This was a phase 1/2 study conducted at four academic centers in the USA (Roswell Park Cancer Institute, Johns Hopkins University, Ohio State University and University of Southern California). The study was performed after approval by the institutional review board (IRB) at each participating site and was conducted in accordance to the ethical guidelines included in the Declaration of Helsinki. Eligible patients had pathological diagnosis of RCC, clear cell or predominantly clear cell that was metastatic and progressive. The patients were required to be sufficiently fit to receive high dose IL-2. Main exclusion criteria included any prior systemic therapy for metastatic ccRCC, ongoing immune suppressive therapy, and the presence of untreated brain metastases. The study was registered at ClinicalTrials.gov identifier NCT01038778. All patients provided written informed consent.
End Points
The primary objective of the Phase I portion of this study was to evaluate the safety and establish the recommended phase 2 dose of entinostat in combination with high dose IL-2. The primary objective of the Phase II portion was to evaluate the efficacy of this regimen. The primary endpoint was ORR. Secondary endpoints included PFS and overall survival, and parameters measuring immune response.
Treatment Schema
Patients were admitted to hospital units with appropriate capabilities for the administration of high dose IL-2. One cycle of treatment (85 days) consisted of 2 courses of high dose IL-2 600,000 units/kg administered intravenously every 8 hrs. on day 1–5 and day 15–19 (± 7 days) (maximum 28 doses) 4, and entinostat orally (1–2 hrs. prior to IL-2 infusion) given once every 2 weeks starting on day-14, administered before IL-2 infusion on day 1, 15, and then continuously (Figure 1B). Entinostat was provided by CTEP through a CRADA with Syndax Pharmaceuticals, Inc. Tumor response assessments were performed on week 11 (+/− 7 days) and every 12 weeks thereafter. In the event of clinical benefit (stable disease or tumor shrinkage), patients received a second cycle of therapy. Entinostat treatment continued every 2 weeks (+/− 7 days dependent on adjustments necessary for IL-2 dosing) until documented disease progression or 8 weeks following documented complete response. Patients who tolerated the combination regimen with evidence of tumor shrinkage received up to 3 cycles of high dose IL-2. Cycle 2 started on or within 2 weeks following day 85 (or last day of cycle dependent on dosing adjustment) as in cycle 1, while cycle 3 started within 2 weeks after completion of cycle 2. Tumor response assessment was performed uniformly across all patients at all institution before cycle 2 (~day 85). Patients with stable disease by RECIST V.1.1 criteria, but without evidence of tumor shrinkage after two cycles, received only entinostat until disease progression was documented. In order to assess the effect of entinostat versus the combination on proposed correlative pharmacodynamic parameters, initial treatment was with entinostat monotherapy, followed by combination with high dose IL-2. The Phase I starting dose level of entinostat was 3 mg orally every 2 weeks. The first dose level had a minimum of 3 patients treated unless the first 2 patients experienced dose-limiting toxicity(s) (DLT) before the 3rd patient was enrolled. DLTs were defined as extended grade 4 toxicity (duration of one week or more) during the first 45 days of treatment in view of the prolonged side effects induced by single agent high dose IL-2. Patients were allowed to remain on the therapy provided that they were tolerating the treatment and were progression-free. No dose de-escalation for IL-2 was allowed.
Correlative studies
Relationships between entinostat and IL-2 exposure and pharmacodynamic effects were characterized. Four aliquots of 8 ml of peripheral blood were collected for mononuclear cell fraction. Fresh samples were shipped overnight to Roswell Park Cancer Institute where they were processed and analyzed by the FACS Core facility. For activated antigen presenting cells, we used the following antibodies (BD): CD86 BB515, CD14 PE, Lin Dump FL3 PC5, HLADr PECy7, CD11c APC, CD45 APCH7, CD80 BV421, CD123 BV510. For Treg we used the following antibodies (BD and Bioscience): FOXP3 PE, CD4 PcP, CD3PC7, CD127 APC, CD45 APCH7, CD25 BV421. Expression of surface makers and intracellular protein was assessed with FACSAria or LSRII flow cytometer. Data were analyzed using Winlets software. Pre and post-treatment biopsy of accessible tumors were offered to all participating patients but were not mandatory. Formalin-fixed paraffin sections of tumor biopsies were stained for FOXP3 (Clone 236A/E7, Abcam; catalog #ab20034) and CD8 staining (Clone C8/144B, Dako; catalog# M7103). [18F]fluoro-2-deoxy-D-glucose(FDG)-PET/CT scan was performed at screening and approximately 30 days into therapy, providing nearly simultaneous acquisition of metabolic and anatomic data. FDG PET/CT studies were conducted in 22 patients who were enrolled at Roswell Park and Johns Hopkins but only 11 patients completed also the second scan.
Statistical Considerations
The reported analyses are based on a September 2, 2016 database lock. The combination treatment would have been considered unsuccessful if the response rate was 20% or less, and it would have been considered active enough to pursue further if the response rate was 40% or greater. To test this hypothesis, the fixed sample size for a single-stage study with a type I error of 10% and a type II error of 10%, based on an exact binomial test, was 36. If 11 or more of the patients experienced a response, the hypothesis that the response rate was ≤ 20% would be rejected with a target error rate of 0.10. We also planned to determine whether initial levels of specific T lymphocytes (Treg) in the peripheral blood and tumor or changes in the level of specific T lymphocytes from baseline might predict for response to this combination therapy. In this study Treg were defined as CD4+CD25hi T cells. The hypothesis was that low baseline levels of Treg would be associated with an increased probability of response and that Treg decreases from baseline would be associated with an increased probability of response. Responders and non-responders were compared using Exact Wilcoxon Rank Sum tests. Responders were compared to non-responders for 5 outcomes: C1D-14, C1D-7, C1D1, C1D-14 – C1D1 (i.e. change from C1D-14 to C1D1), C1D-7 – C1D1 (i.e. change from C1D-7 to C1D1). Since this was an exploratory analysis no adjustments were made for multiple comparisons.
Results
Patient Characteristics
Between January 2009 and December 2015, we enrolled 47 patients with ccRCC. All patients had prior nephrectomy and had either favorable or intermediate MSKCC risk factors (Table 1).
Table 1.
Baseline characteristics
| Median age, y (range) | 58 (31–68) |
| ECOG performance status 0, % | 100 |
| Prior nephrectomy, % | 100 |
| MSKCC risk factors, n (%) | |
| 0 (favorable) | 25 (53) |
| 1–2 (intermediate) | 22 (47) |
| ≥ 3 (poor) | 0 (0) |
| Metastatic sites, n | |
| Lungs | 36 |
| Lymph nodes | 26 |
| Bones | 7 |
| Liver | 6 |
Treatment Administration and Overall Safety
The phase I portion consisted of two dose levels of entinostat (3 and 5 mg) and a fixed standard dose of IL-2 (600,000 units/kg every 8 hrs.) and was enrolled according to a 3+3 design. Eleven patients were treated during the phase I portion (three patients at the 3 mg entinostat dose and eight patients at the 5 mg entinostat dose). The 5 mg dose level allowed up to 6 evaluable patients to be enrolled and two patients were not evaluable. Dose levels 1 and 2 were completed without DLTs during the first 45 days of treatment. The most common expected grade 3/4 toxicities were hypophosphatemia (attributable to entinostat) and thrombocytopenia (6 patients), as well as neutropenia and lymphopenia (2 patients) (attributable to both entinostat and IL-2). Table 2 shows the adverse events occurring during the combined phase I and phase II portion. Among all 47 patients, the most common grade 3 or 4 treatment related adverse events were hypophosphatemia (16%), decreased lymphocytes (15%), and hypocalcemia (7%). No unexpected toxicities were noted. One patient presented a rheumatoid arthritis flare. One death was reported during treatment and was deemed unrelated to study drug. The patient developed cardiac tamponade during the first cycle requiring pericardiocentesis which revealed the presence of adenocarcinoma cells from previously undiagnosed occult primary lung cancer. The median number of IL-2 doses administered was 7.5 (3–14), and twenty three patients (49%) received ≥ 1 cycle of treatment.
Table 2.
Treatment-related adverse events
| Toxicity | G3 | G4 | G5 | Total | |
|---|---|---|---|---|---|
| N | N | N | N | % | |
| Hypophosphatemia | 55 | 7 | 62 | 16.4 | |
| Decreased lymphocytes | 19 | 38 | 57 | 15.1 | |
| Hypocalcemia | 25 | 2 | 27 | 7.2 | |
| Decreased platelets | 24 | 24 | 6.4 | ||
| Hyponatremia | 20 | 20 | 5.3 | ||
| Decreased neutrophil counts | 13 | 4 | 17 | 4.5 | |
| Increased bilirubin | 15 | 1 | 16 | 4.2 | |
| Decreased white blood cells | 15 | 1 | 16 | 4.2 | |
| Hypotension | 11 | 1 | 12 | 3.2 | |
| Fever | 7 | 7 | 1.9 | ||
| Hyperglycemia | 7 | 7 | 1.9 | ||
| Hypermagnesemia | 7 | 7 | 1.9 | ||
| Leukocytosis | 7 | 7 | 1.9 | ||
| Hyperkalemia | 5 | 1 | 6 | 1.6 | |
| Fatigue | 6 | 6 | 1.6 | ||
| Diarrhea | 5 | 5 | 1.3 | ||
| Hypoalbuminemia | 5 | 5 | 1.3 | ||
| Decreased urinary output | 5 | 5 | 1.3 | ||
| Thrombotic Thromb Purpura | 4 | 4 | 1.1 | ||
| Total events | 315 | 61 | 1 | 377 | 100 |
Primary End Points
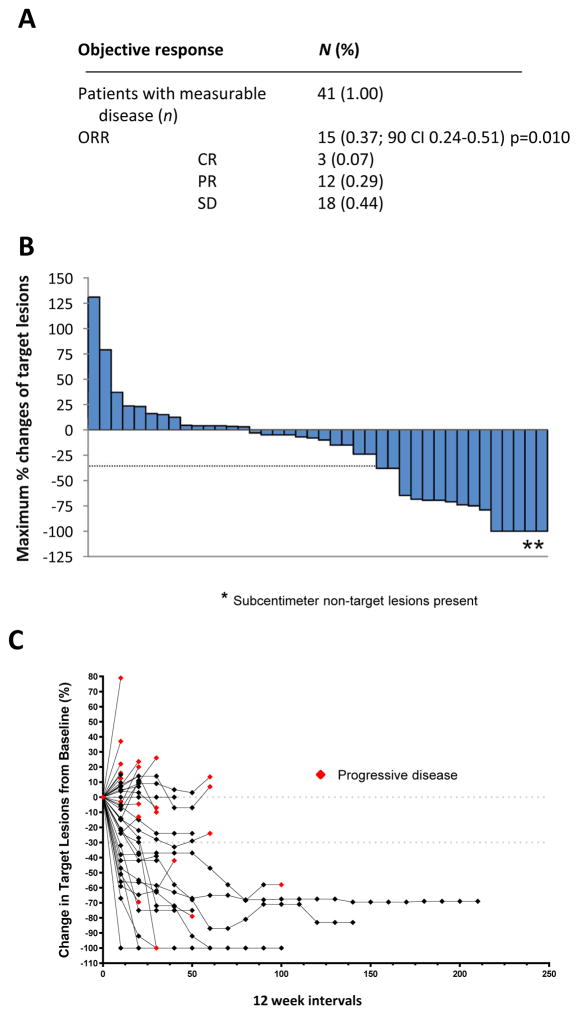
Of the 47 enrolled patients, 43 were evaluable for response. Two patients with no measurable but evaluable disease (positive FGD-PET scan only) at baseline were excluded from objective ORR analysis but were included in the progression-free and overall survival analyses. Figure 2A shows the total proportion of ORR for the 41 completers of both phase I and II. Confirmed overall response was achieved by fifteen (37%, 90% CI 24–51, p=0.010) patients including twelve partial responses (PR) and three complete responses (CR). In the phase II portion 32 patients with measurable disease were included and ten achieved an objective response (31%, 90% CI 18–47, p=0.090). Stable disease for ≥6 months was achieved by 18 patients (44%). The waterfall plot shows the depth of the clinical responses, while the spider plot highlights the duration of the responses in addition to the tumor burden reduction from baseline (Figure 2B–C). Two patients with PR achieved complete resolution of their target lesions but had persistent sub centimeter non-target lung nodules. Of note, there were two additional patients with no measurable disease but evaluable lesions who achieved resolution of FDG uptake on PET scan. These patients were not counted as objective responses. Delayed response has been observed. For example, a patient who had initial progressive disease and discontinued treatment after two cycles has subsequently achieved stable disease and did not require further therapies. After 3 years of follow up, the patient is now presenting continuous, slow reduction in number and size of the lung nodules in the absence of further treatments.
Figure 2. Clinical activity and characteristics of response.
(A) Best response in patients with measurable disease (n=41) (B) Best percentage change in target lesion tumor burden from baseline. Maximum percentage reduction in target lesion tumor burden until disease progression according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 progression. Positive change in tumor burden indicates tumor growth; negative change in tumor burden indicates tumor reduction. (C) Percentage change in target lesion tumor burden from baseline over time.
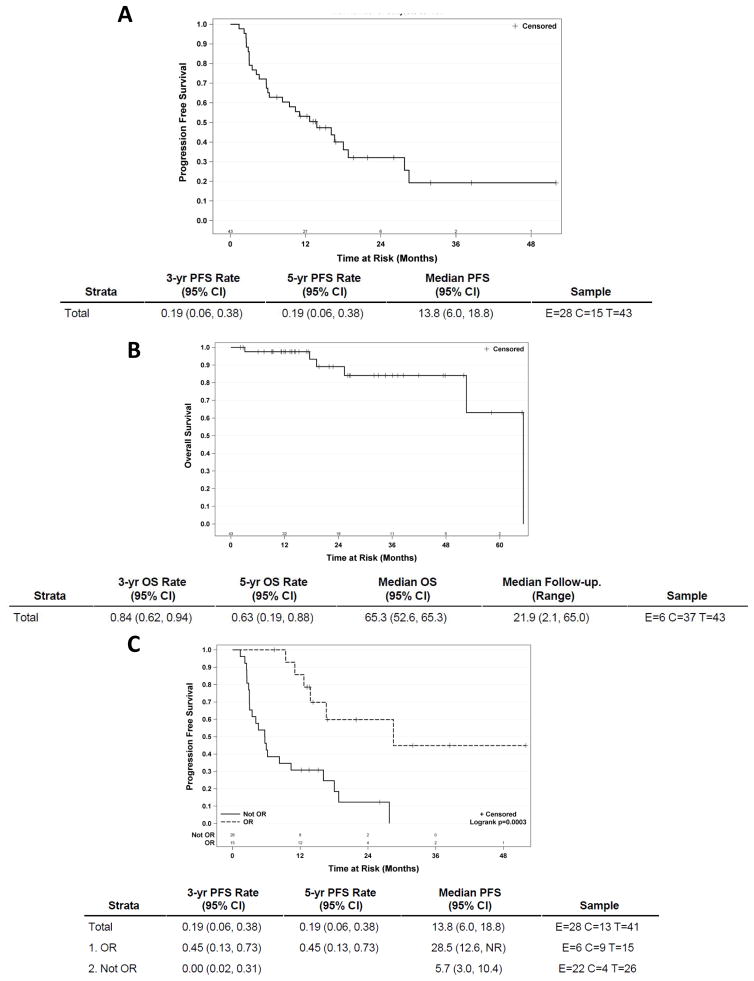
At the time of data cutoff, median follow-up was 21.9 months (95% CI 2.1–65.0). At the last follow-up, the 3-year progression-free survival was 19% (95% CI 6–38), and the median progression-free survival was 13.8 months (95% CI 6–18.8) (Figure 3A). The 3-year overall survival was 84% (95% CI 62–94), and the median overall survival was 65.3 months (95% CI 52.6–65.3) (Figure 3B). When we sub-grouped the patients between those who achieved an objective response (OR) and those who did not (not OR), the 3-year progression-free survival was 45% (95% CI 13–73) in the responders (OR) and 0% (95% CI 2–31) in the non-responders (not OR). Similarly, the median progression-free survival was 28.5 months (95% CI 12.6-NR) in the responders (OR) and 5.7 months (95% CI 3–10.4) in the non-responders (not OR) (p=0.003) (Figure 3C). Interestingly, there was no difference between the patients who achieved an objective response and those who did not in terms of the median number of IL-2 doses administered (7.8 vs 7.0).
Figure 3. Progression-free and overall survival.
Kaplan–Meier curves of progression-free survival (A), overall survival (B) and progression-free survival of responders (OR) vs non responders (not OR). E = events; C = censored; T = total.
Correlative Studies
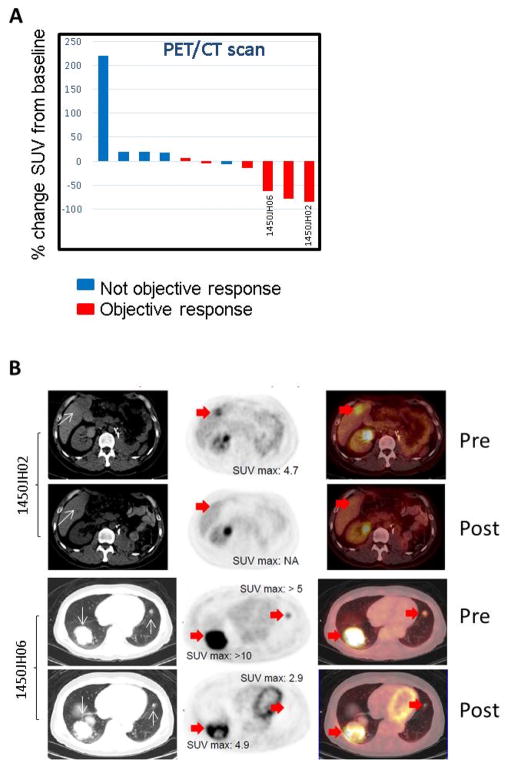
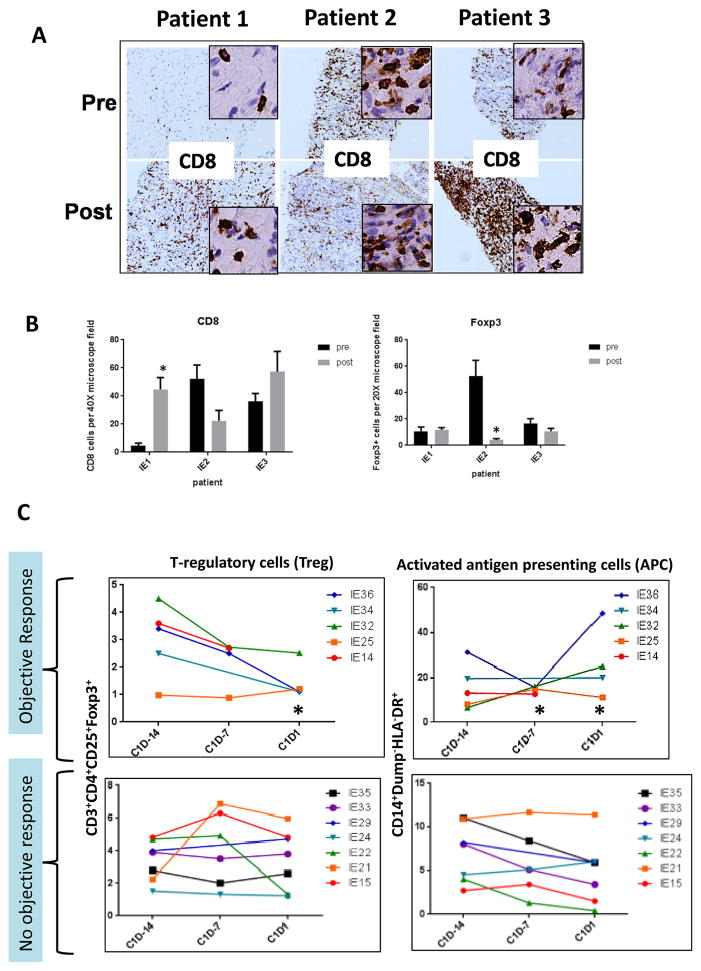
In a small number of patients we were able to perform FDG-PET/CT scans at baseline and at ~ Day 30. Following treatment with entinostat and high-dose IL-2, we observed a greater decrease in FDG uptake in the target lesions in those patients who achieved an objective response as compared to those who did not (Figure 4A–B). In 3 patients with accessible tumors we were able to perform a biopsy before starting treatment and during the first cycle at ~Day 15. The results suggest that there was a significant increase of tumor infiltrating CD8 cells in patients with either prolonged stable disease (SD) (patient 1; >15 months) or PR (patient 3) (Figure 5A–B). The biopsies also showed either stable or decreased Treg infiltration despite the administration of high dose IL-2 which was expected to increase Treg.
Figure 4. FDG-PET/CT scan response.
(A) Percentage change in target lesion of standardized uptake value (SUV) of FDG from baseline in patients with either not objective response or objective response by RECIST 1.1. (B) Representative pictures of PET/CT scan in two patients with objective response.
Figure 5. Correlative studies.
(A) Representative pictures of tumor biopsies pre and post treatment with entinostat and high dose IL-2 showing CD8+ cells tumor infiltration. (B) Quantitative analysis of tumor infiltrating CD8+ cells and Foxp3+ cells. (C) Quantitative analysis of Treg and activated antigen presenting cells pre and post treatment with entinostat. Color lines represent individual patients. * p = 0.003
We also performed flow cytometry analysis in peripheral blood mononuclear cells collected at different time points. Complete samples were available only from a portion of patients receiving treatment across the participating institutions. Our analysis has focused primarily on the “priming phase” with entinostat (cycle 1 day-14 through cycle 1 day 1) to assess the activity of entinostat alone without the potentially masking effect of high dose IL-2. Following the first dose of entinostat, we observed a statistically significant decline in peripheral Treg in 5 patients who achieved an objective response as compared to 7 patients who had progressive disease (Figure 5C). Values for responders were likely to be lower for Tregs at C1D1 (p=0.0273). Interestingly, we also observed a concomitant, statistically significant increase in circulating activated antigen presenting cells (APC). Values for responders were likely to be higher for APC at C1 D-7 (p=0.0095) and APC at C1D1 (p=0.0121). Increases in APC from C1D-14 to C1D1 were also likely to be higher for responders (p=0.0242).
Discussion
To our knowledge, this study is the first prospective clinical trial to test the immunomodulatory activity of an epigenetic agent in cancer patients receiving immunotherapy. Overall, our results, as compared to historical data with single agent high dose IL-2, suggest that the addition of the selective class I HDAC inhibitor entinostat may increase the clinical efficacy of this cytokine therapy by modulating immune suppressive cells.
The potential immunomodulatory activity of epigenetic drugs has been postulated since the beginning of their development, in view also of the sporadic tumor responses observed in patients with solid tumors, including melanoma, at doses that likely do not achieve the required micromolar concentrations for a direct antitumor effect. Our group was among the first to report the potential immunomodulatory activity of HDAC inhibitors in a preclinical model of renal cell carcinoma13. Several preclinical studies now support the hypothesis that HDAC inhibitors may synergize with immunotherapies by modulating the immune response 19,20,21. For example, HDAC inhibitors have been reported to enhance the effect of vaccine strategies 22. However, this class of agents has been described as a sort of “double-edge sword 23. On one hand there have been clinical trials that utilized pan HDAC inhibitors as adjuvant therapy to reduce graft-versus host disease in patients who underwent allogeneic bone marrow transplant by exploiting the “immunosuppressive” properties of these agents 24. On the other hand, preclinical models have shown that HDAC inhibitors have a pro-immunomodulatory activity. Intriguingly, there is preclinical evidence that HDAC inhibitors may have opposing effects as shown, for example, in modulating Treg function 25,14. Several reasons for these conflicting results may be considered. For example, the class of HDACI (I vs I/II), the dose and the schedule may be responsible for these “double-edge sword” opposing effects 17. More recently, selective HDAC inhibition focusing on HDAC3 and HDAC11 has been reported to have specific effects on immune response by regulating Treg and antigen-presenting cells, respectively 26,27. Ex vivo experiments performed on peripheral mononuclear cells have shown the potential for detrimental effects of class I/II HDAC inhibition on lymphocyte viability and function 28, confirming the challenge that the development of this class of agents presents. Further studies will be needed to elucidate the complex epigenetic regulation of the immune response and to optimally exploit the clinical benefit of HDAC inhibitors in combination with immunotherapies.
The clinical trial was designed based on the results from preclinical studies in which we observed a greater synergy between entinostat and high dose IL-2 when we treated the mice with the HDAC inhibitor first. We hypothesized that this “priming” phase of the immune response with entinostat could suppress Treg function and create a less immunosuppressive tumor microenvironment for high dose IL-2 to exert its antitumor effect. Indeed, during the two week “lead in” phase with entinostat, we observed a modulation of Treg. Despite the relatively small number of patients, we observed a statistically significant greater decrease in Treg in the patients who achieve an objective response as compared to patients who had progressive disease. This decrease in Treg during the priming phase may represent a pharmacodynamic parameter with predictive potential that warrants prospective validation in future clinical trials. This observation is clinically relevant as, to date, we do not have a validated marker to predict response to high dose IL-2. The SELECT trial attempted to define a predictive signature, but the results were not informative 5. Unfortunately, the collection of peripheral blood immune cells (i.e. Treg) was not performed in that study. Additional correlative studies on the profile of circulating immune cells and chemokines/cytokines will likely shed some light on the potential predictive values of these markers.
The treatment algorithm for ccRCC includes both anti-vascular endothelial growth factors drugs and immunotherapies. Though the use of PD-1/PDL-1 is revolutionizing the therapeutic options for the majority of solid tumors including ccRCC, the only immune checkpoint inhibitor approved to date for ccRCC is nivolumab in the second-line setting; however, the results from two phase III clinical trials involving combinations with PD-1 and PDL-1 inhibitors may lead to the approval of these drug in the first-line setting. Overall, high dose IL-2 remains an option for selected ccRCC patients who are seeking a durable response and possible cure of their disease. The acute toxicities and the logistics for the administration of this regimen represent undeniable drawbacks but the side effects are limited in time and are not chronic, unlike those observed with other therapies including potentially the immune checkpoint inhibitors. Overall, entinostat did not seem to increase the toxicities expected from high dose IL-2.
As several reports regarding the clinical benefit of sequential use of high dose IL-2 and immune checkpoint inhibitors are surfacing, it is intriguing to speculate that these two immunotherapeutic approaches may not necessarily have cross-resistance mechanisms. Our results also support the hypothesis that HDAC inhibitors may have a role in combination with other immunotherapies including PD-1/PDL-1 inhibitors as suggested by preclinical data generated in our laboratory (unpublished). Interestingly, HDAC inhibition has been shown to increase PD-L1 expression in preclinical models, including in combination with a demethylating agent 29, 30. Furthermore, there is both preclinical and clinical evidence that entinostat may affect myeloid suppressive derived cells 31, 32. Thus, over the next years, several clinical trials will test novel combinations of immunotherapies for ccRCC including checkpoint inhibitors, vaccines, adoptive T-cell therapy and T-cell agonists 33, and HDAC inhibitors may provide an additional tool to modulate the immune response more effectively.
Our study has some limitations, including the small sample size, the long time for accrual, the short follow-up, and the non-randomized design, which prevent drawing any more definitive conclusions. The accrual was initially slow because of competing studies at the participating institutions, in particular with the availability of immune checkpoint inhibitors, but it significantly picked up in the last two years, homogenously across the four sites. Despite these limitations that could have affected the outcome, the degree of clinical benefit observed with this combination exceeded the pre-specified benchmark, providing rationale to design additional studies of epigenetic priming with immunotherapy.
In conclusion, our results suggest that the combination of entinostat plus high dose IL-2 is tolerable with promising clinical activity, including higher response rate and greater median progression-free survival as compared to historical data. These findings represent the first evidence, to our knowledge, of improved benefit through immunotherapy combination with an epigenetic agent in the first-line setting treatment for ccRCC, and provide the rationale for a prospective validation of this therapeutic strategy. Based on these preliminary results, we are currently planning a multi-site, randomized Phase II study of high dose IL-2 +/1 entinostat in the same patient population.
Supplementary Material
Translational Relevance.
Based on preclinical data suggesting an immunomodulatory activity of the selective class I HDAC inhibitor entinostat and anti-tumor activity when combined with IL-2 in an animal model of renal cell cancer we conducted this clinical trial. Our results suggest that rationally designed combination strategies aimed to increase the efficacy of high dose IL-2 therapy are clinically relevant, in a selected patient population of ccRCC. This proof of principle also provides the rationale for exploration of epigenetic modulators and immunotherapies as rational combination strategies.
Acknowledgments
Grant Support
This investigator-initiated study was funded by the National Cancer Institute (R21CA137649, U01CA70095, UM1CA186717-01, P30 CA 014089).
We would like to thank all participating patients and their families, as well as the investigators and study teams, for making this trial possible. We want also thank Syndax Pharmaceuticals for providing entinostat.
Footnotes
Disclosure of Potential Conflicts of Interest
Roberto Pili is a recipient of a research grant from Syndax. All the other authors declared no financial conflicts.
Prior presentations: The Phase I/II study has been presented at the ASCO-GU annual meeting (Orlando FL 2016) and the AACR annual meeting (Philadelphia, 2016).
Authors’ Contributions
Conception and design: R. Pili, D. Hutson, M. A. Carducci
Development of methodology: R. Pili, D. I. Quinn, H. J. Hammers, P. Monk, S. George, T. B. Dorff, T. Olencki, L. Shen, D. Lamonica, R. S. Fragomeni, Z. Szabo, A. Hutson, A. Groman, S. M. Perkins, R. Piekarz, M. A. Carducci
Acquisition of data (acquired and managed patients, provided facilities, etc.): R. Pili, D. I. Quinn, H. J. Hammers, P. Monk, S. George, T. B. Dorff, T. Olencki, L. Shen, A. Orillion, D. Lamonica, R. S. Fragomeni, Z. Szabo, A. Hutson, A. Groman, S. M. Perkins, R. Piekarz, M. A. Carducci
Analysis and interpretation of data (e.g., statistical analysis, biostatistics): R. Pili, D. I. Quinn, H. J. Hammers, P. Monk, S. George, T. B. Dorff, T. Olencki, L. Shen, A. Lamonica, R. S. Fragomeni, Z. Szabo, A. Hutson, A. Groman, S. M. Perkins, R. Piekarz, M. A. Carducci
Writing, review, and/or revision of the manuscript: R. Pili, D. I. Quinn, H. J. Hammers, P. Monk, S. George, T. B. Dorff, T. Olencki, L. Shen, A. Lamonica, R. S. Fragomeni, Z. Szabo, A. Hutson, A. Groman, S. M. Perkins, R. Piekarz, M. A. Carducci
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): R. Pili, D. I. Quinn, H. J. Hammers, P. Monk, S. George, T. B. Dorff, T. Olencki, L. Shen, A. Orillion, D. Lamonica, R. S. Fragomeni, Z. Szabo, A. Hutson, A. Groman, S. M. Perkins, R. Piekarz, M. A. Carducci
Study supervision: R. Pili, R. Piekarz, M. A. Carducci
References
- 1.Albiges L, Choueiri T, Escudier B, et al. A systematic review of sequencing and combinations of systemic therapy in metastatic renal cancer. Eur Urol. 2015;67:100–10. doi: 10.1016/j.eururo.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Hutson TE, Thoreson GR, Figlin RA, et al. The Evolution of Systemic Therapy in Metastatic Renal Cell Carcinoma. Am Soc Clin Oncol Educ Book. 2016;35:113–7. doi: 10.1200/EDBK_158892. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373:1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fyfe G, Fisher RI, Rosenberg SA, et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688–96. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 5.McDermott DF, Cheng SC, Signoretti S, et al. The high-dose aldesleukin “select” trial: a trial to prospectively validate predictive models of response to treatment in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2015;21:561–8. doi: 10.1158/1078-0432.CCR-14-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newbold A, Falkenberg KJ, Prince HM, et al. How do tumor cells respond to HDAC inhibition? FEBS J. 2016;283:4032–4046. doi: 10.1111/febs.13746. [DOI] [PubMed] [Google Scholar]
- 7.Pili R, Liu G, Chintala S, et al. Combination of the histone deacetylase inhibitor vorinostat with bevacizumab in patients with clear-cell renal cell carcinoma: a multicentre, single-arm phase I/II clinical trial. Br J Cancer. 2017;116:874–883. doi: 10.1038/bjc.2017.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito A, Yamashita T, Mariko Y, et al. A synthetic inhibitor of histone deacetylase, MS-27-275, with marked in vivo antitumor activity against human tumors. Proc Natl Acad Sci U S A. 1999;96:4592–7. doi: 10.1073/pnas.96.8.4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan QC, Headlee D, Acharya M, et al. Phase I and pharmacokinetic study of MS-275, a histone deacetylase inhibitor, in patients with advanced and refractory solid tumors or lymphoma. J Clin Oncol. 2005;23:3912–22. doi: 10.1200/JCO.2005.02.188. [DOI] [PubMed] [Google Scholar]
- 10.Juergens RA, Wrangle J, Vendetti FP, et al. Combination epigenetic therapy has efficacy in patients with refractory advanced non-small cell lung cancer. Cancer Discov. 2011;1:598–607. doi: 10.1158/2159-8290.CD-11-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yardley DA, Ismail-Khan RR, Melichar B, et al. Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J Clin Oncol. 2013;31:2128–35. doi: 10.1200/JCO.2012.43.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pili R, Salumbides B, Zhao M, et al. Phase I study of the histone deacetylase inhibitor entinostat in combination with 13-cis retinoic acid in patients with solid tumours. Br J Cancer. 2012;106:77–84. doi: 10.1038/bjc.2011.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato Y, Yoshimura K, Shin T, et al. Synergistic in vivo antitumor effect of the histone deacetylase inhibitor MS-275 in combination with interleukin 2 in a murine model of renal cell carcinoma. Clin Cancer Res. 2007;13:4538–46. doi: 10.1158/1078-0432.CCR-07-0014. [DOI] [PubMed] [Google Scholar]
- 14.Shen L, Ciesielski M, Ramakrishnan S, et al. Class I histone deacetylase inhibitor entinostat suppresses regulatory T cells and enhances immunotherapies in renal and prostate cancer models. PLoS One. 2012;7:e30815. doi: 10.1371/journal.pone.0030815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cesana GC, DeRaffele G, Cohen S, et al. Characterization of CD4+CD25+ regulatory T cells in patients treated with high-dose interleukin-2 for metastatic melanoma or renal cell carcinoma. J Clin Oncol. 2006;24:1169–77. doi: 10.1200/JCO.2005.03.6830. [DOI] [PubMed] [Google Scholar]
- 16.Yao X, Ahmadzadeh M, Lu YC, et al. Levels of peripheral CD4(+)FoxP3(+) regulatory T cells are negatively associated with clinical response to adoptive immunotherapy of human cancer. Blood. 2012;119:5688–96. doi: 10.1182/blood-2011-10-386482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen L, Orillion A, Pili R. Histone deacetylase inhibitors as immunomodulators in cancer therapeutics. Epigenomics. 2016;8:415–28. doi: 10.2217/epi.15.118. [DOI] [PubMed] [Google Scholar]
- 18.Shen L, Pili R. Class I histone deacetylase inhibition is a novel mechanism to target regulatory T cells in immunotherapy. Oncoimmunology. 2012;1:948–950. doi: 10.4161/onci.20306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West AC, Mattarollo SR, Shortt J, et al. An intact immune system is required for the anticancer activities of histone deacetylase inhibitors. Cancer Res. 2013;73:7265–76. doi: 10.1158/0008-5472.CAN-13-0890. [DOI] [PubMed] [Google Scholar]
- 20.Bridle BW, Chen L, Lemay CG, et al. HDAC inhibition suppresses primary immune responses, enhances secondary immune responses, and abrogates autoimmunity during tumor immunotherapy. Mol Ther. 2013;21:887–94. doi: 10.1038/mt.2012.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hideshima T, Cottini F, Ohguchi H, et al. Rational combination treatment with histone deacetylase inhibitors and immunomodulatory drugs in multiple myeloma. Blood Cancer J. 2015;5:e312. doi: 10.1038/bcj.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vo DD, Prins RM, Begley JL, et al. Enhanced antitumor activity induced by adoptive T-cell transfer and adjunctive use of the histone deacetylase inhibitor LAQ824. Cancer Res. 2009;69:8693–9. doi: 10.1158/0008-5472.CAN-09-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroesen M, Gielen P, Brok IC, et al. HDAC inhibitors and immunotherapy; a double edged sword? Oncotarget. 2014;5:6558–72. doi: 10.18632/oncotarget.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi SW, Braun T, Chang L, et al. Vorinostat plus tacrolimus and mycophenolate to prevent graft-versus-host disease after related-donor reduced-intensity conditioning allogeneic haemopoietic stem-cell transplantation: a phase 1/2 trial. Lancet Oncol. 2014;15:87–95. doi: 10.1016/S1470-2045(13)70512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akimova T, Ge G, Golovina T, et al. Histone/protein deacetylase inhibitors increase suppressive functions of human FOXP3+ Tregs. Clin Immunol. 2010;136:348–63. doi: 10.1016/j.clim.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villagra A, Cheng F, Wang HW, et al. The histone deacetylase HDAC11 regulates the expression of interleukin 10 and immune tolerance. Nat Immunol. 2009;10:92–100. doi: 10.1038/ni.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Liu Y, Han R, et al. FOXP3(+) regulatory T cell development and function require histone/protein deacetylase 3. J Clin Invest. 2015;125:3304. doi: 10.1172/JCI77088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong DJ, Rao A, Avramis E, et al. Exposure to a histone deacetylase inhibitor has detrimental effects on human lymphocyte viability and function. Cancer Immunol Res. 2014;2:459–68. doi: 10.1158/2326-6066.CIR-13-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wrangle J, Wang W, Koch A, et al. Alterations of immune response of Non-Small Cell Lung Cancer with Azacytidine. Oncotarget. 2013;4:2067–79. doi: 10.18632/oncotarget.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woods DM, Sodre AL, Villagra A, et al. HDAC Inhibition Upregulates PD-1 Ligands in Melanoma and Augments Immunotherapy with PD-1 Blockade. Cancer Immunol Res. 2015;3:1375–85. doi: 10.1158/2326-6066.CIR-15-0077-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim K, Skora AD, Li Z, et al. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc Natl Acad Sci U S A. 2014;111:11774–9. doi: 10.1073/pnas.1410626111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomita Y, Lee MJ, Lee S, et al. The interplay of epigenetic therapy and immunity in locally recurrent or metastatic estrogen receptor-positive breast cancer: Correlative analysis of ENCORE 301, a randomized, placebo-controlled phase II trial of exemestane with or without entinostat. Oncoimmunology. 2016;5:e1219008. doi: 10.1080/2162402X.2016.1219008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carlo MI, Voss MH, Motzer RJ. Checkpoint inhibitors and other novel immunotherapies for advanced renal cell carcinoma. Nat Rev Urol. 2016;13:420–31. doi: 10.1038/nrurol.2016.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.