Short abstract
Alterations in gut hormone signaling are a likely contributing factor to the metabolic disturbances associated with overweight/obesity as they coordinate the timing of feeding behavior, absorption, and utilization of nutrients. These hormones are released in response to food intake, or follow a circadian or anticipatory pattern of secretion that is independent of nutrient stimulation. The aim of this study was to identify the degree to which high-fat diet-induced obesity would alter the daily rhythm of gut peptide plasma levels (glucagon-like peptide-1 [GLP-1], peptide YY [PYY], insulin or amylin [AMY]) or meal-induced levels in the middle of the light or dark cycle. Male Sprague-Dawley rats were fed a high-fat diet (OBESE) or chow (LEAN), implanted with jugular catheters, and blood samples were taken every 2 h throughout the light/dark cycle while freely feeding or after an Ensure liquid meal. We found that even when OBESE and LEAN animals ate the same kcals and have a similar pattern of food intake, there is a difference in both the levels and rhythm of plasma gut peptides. GLP-1 and PYY are higher during the light cycle in LEAN animals and AMY is higher in the OBESE group throughout the light/dark cycle. There was also a differential response of plasma gut signals after the Ensure meal, even though the composition and amount of intake of the meal were the same in both groups. These changes occur prior to the high-fat diet induced loss of glycemic control and may be a target for early intervention.
Impact statement
The aim of this study was to test if obesity would alter the daily rhythm of gut peptides or meal-induced levels in the middle of the light or dark cycle. We found that even when animals are eating the same amount (in kcal) of food that the obese animals have altered daily rhythms and meal-induced gut peptide levels. In particular, we are the first to show that obesity induces increases in peptide YY levels during the light cycle and amylin remains high throughout the light and dark cycle in obese animals. These changes occurred prior to a loss of glycemic control. Thus, the rhythm of gut peptides could be used as an early indicator of later and more serious metabolic disturbances and may be a target for early intervention.
Keywords: Gut peptides, lean, obese, rhythm, nutrient induced, rat
Introduction
Obesity affects ∼13% of the world’s population with an additional 39% categorized as overweight.1 The negative physiological impact of being overweight is seen in the alterations of the function of many organs, including changes in nutrient sensing and metabolic disturbances that contribute to the persistence of hyperphagia, weight gain, and the incidence of associated diseases (i.e. heart disease, hypertension, stroke, diabetes, and cancer2–6). Alterations in gut hormone signaling are likely a contributing factor to these obesity-related changes. Gut hormones are released from the gastrointestinal tract and accessory digestive organs to coordinate the timing of feeding behavior as well as the absorption and utilization of nutrients in the body. These hormones are typically released in response to nutrients, while a few are known to follow a circadian and/or anticipatory pattern of secretion that is independent of nutrient stimulation.7–9 The plasma levels of glucagon-like peptide 1 (GLP-1), an incretin hormone released from L cells in the small intestine, are often reduced in response to a meal in obesity.10–13 Moreover, the rhythm of GLP-1 plasma levels in response to direct intestinal delivery of nutrients during the dark cycle is attenuated in obese rats.14 The anticipatory increase in GLP-1 levels prior to mealtime is also blunted in obese animals.9 There are similar nutrient and non-nutrient induced changes in insulin levels,15–18 and many studies have identified additional obesity-related changes in other gut peptides as they relate to nutrient stimulation8 or circadian rhythms.19,20 Taken together, these obesity-related changes in gut hormone levels and the timing of the release may result in digested nutrients being shuttled inappropriately to cells of the body or in aberrant cellular metabolism, both of which could be driving factors for the negative physiological consequences of obesity. Understanding the normal nutrient induced or circadian rhythm of gut hormone release and how these are altered by obesity may help identify ways to improve nutrient utilization by altering the timing of meals or by delivering exogenous hormone analogs in treating obese individuals.
In this study, we wanted to extend the previous findings about the nutrient-induced and circadian rhythm of GLP-1 and insulin and test if there is a differential response of two additional gut peptides, peptide YY (PYY) and amylin (AMY), between obese and lean rats. It has been demonstrated that PYY has a role in inhibiting food intake and decreasing appetite,21 while AMY has been shown to be involved in glycemia, adiposity, and appetite.22 Although differences in the levels of these hormones have been identified between lean and obese individuals,23,24 a comparison of the daily rhythm of these hormones has not been investigated. Thus, we tested (1) if there is a difference in the rhythm of these gut peptides throughout the light/dark cycle, while obese and lean rats are freely feeding and (2) if there are differences in gut peptide responses to a novel palatable meal given in the middle of the light or dark cycle. For this, we fed Sprague-Dawley rats a chow or high-fat diet to induce a lean (referred to as LEAN) or obese (referred to as OBESE) phenotype and collected blood samples throughout the light/dark cycle and in response to a liquid Ensure meal in the middle of the dark or light cycle.
Methods
Animals
Two replicates of male Sprague-Dawley rats (Charles River) with an initial weight range of 275–300 g were used (n = 32). The rats were individually housed and maintained in a 12:12 h light:dark schedule (lights on at 22:00 h). Rats were acclimated to the housing conditions and fed ad libitum either standard laboratory chow (Global Diet-2018, Harlen Teklad; 3.3 kkcal/g 5% fat, 18% protein, 77% carbohydrate) or a high-fat diet (Research Diets, D12492, 5.24 kcal/g, 60% fat, 20% protein, 20% carbohydrate). Body weight was measured weekly. Tap water was always available during the experiment. All procedures were approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University.
Jugular catheterizations
After there was a significant difference in body weight between the rats on each diet (wk 5), jugular catheters were implanted in all animals. Rats were anesthetized with an intraperitoneal injection of a mixture of ketamine (100 mg/kg) and xylazine (20 mg/kg) at a dose of 1 ml/kg. The animals were shaved between the right ventral neck region and cranial to the right scapula, superficial to the right external jugular vein. The exposed skin was scrubbed with iodine followed by 70% ethanol. A 2 cm ventral cervical skin incision at the midline of the neck at the level of the clavicle was made. Using dissection tools, the right external jugular vein was separated so that a 5 mm section of the vessel was isolated. 5-0 silk sutures (Ethicon; Somerville, NJ) were loosely tied at both cranial and caudal ends of the isolated vein to maximize exposure. Using a micro-surgical scissor, an incision is made along the right external jugular vein to pass the catheter (polyethylene tubing; PE-50 Fisher Scientific). The catheter was passed into the vessel towards the heart and secured in place with the silk sutures. The cannula was tunneled under the skin and externalized through an incision made at the top of the skull. The ventral cervical skin incision was closed with 9 mm wound clips (Braintree Scientific, Inc; Braintree, MA). The exposed end of the catheter on the top of the skull was secured into place with dental acrylic cement and anchored to the skull with microscrews. Buprenorphine (0.3 mg/kg, s.c.) was used post-surgery for pain control, and animals were given liquid Ensure (Abbott; Abbott Park, Illinois) for 2 d post-surgery in addition to their chow. Patency of the catheters was maintained by infusing and withdrawing heparinized sterile saline solution two to three times per week. When blood was not being drawn, catheters were filled with a heparinized glycerol solution and the catheter capped with a cut, blunted and crimpled syringe needle.
Feeding tests
After recovery from surgery, rats were transferred and housed in AccuDiet food intake monitoring cages (13 in. by 9 in.; Accuscan Instruments, Inc, Columbus, OH). A powdered form of the standard laboratory chow (Global Diet-2018C, Harlan Teklad: 3.3 kcal/g) or high-fat diet (Research Diets, D12492P, 5.24 kcal/g) was available ad libitum throughout the feeding tests unless otherwise specified. The catheter was connected to Micro-Renathane tubing with a cut and blunted syringe needle. The tubing was run through an opening in the top of the cage. A light-weight metal spring was placed over the tubing and catheter so that it protected the tubing from twisting and damage from the animal. The Micro-Renathane tubing was secured to the ceiling of the test chamber using putty, which allowed the rats to freely move within the chamber at all times. Food intake was continuously monitored by the AccuDiet system for 22 h (for 2 h the rats had no food access so that experimenters could collect data and to prepare for the next testing cycle).
Plasma hormone assays
During wks 8 and 9, 200 µl of blood was collected at each time point (2 h intervals throughout the light/dark cycles) from all animals. On a given day, blood would be collected from only a subset of animals so that no animal gave blood more than three times a day (0.6 ml total blood), and there were two to three days in between blood collection for each animal. At the beginning of wk 10, rats were exposed to 5 ml of Ensure to prevent neophobia when given the test meal. Subsets of rats were then given a meal of liquid Ensure, 12 ml over 20 min, in the middle of the light (16:00 h) or dark cycle (04:00 h) on varied days throughout wk 10. All rats ate the entire 12 ml within the 20 min. Blood was collected immediately after the Ensure meal (16:20 h or 04:20 h) and 2 h after the meal (18:00 h or 06:00 h). Blood glucose levels were determined with a glucometer (Freestyle Lite, Abbott; Chicago, IL) using a small sample of the blood. All remaining blood was collected into EDTA-coated tubes with the addition of a DPP-IV inhibitor (DPP4-010; EMD Millipore; Billerica, MA), protease inhibitor cocktail (P8340; Sigma-Aldrich, St. Louis, MO) and Pefabloc SC (PEFBSC-RO Roche; Sigma-Aldrich) per manufacturers’ instructions. All samples were inverted several times, maintained on ice and centrifuged at 3000 r/min for 10 min within 30 min of the blood collection. Samples were then stored at −80°C until processed using the Milliplex rat metabolic magnetic bead panel protocol (RMHMAG-84K; EDM Millipore) by Johns Hopkins University School of Medicine Core Facilities personnel. Data were collected using the Luminex 200 with xPONENT software.
Data analysis
Data are presented as mean±SEM. Body weight, food intake, and plasma peptide levels were analyzed using separate two-way repeated measures ANOVA with group (LEAN vs. OBESE) as the between-subject factors and time as the within subject factor using Number Crunching Statistical Software (NCSS v 2000, Kaysville, UT). Post hoc analysis was performed using the Bonferroni correction when appropriate. Differences among groups were considered statistically significant if p < 0.05.
Results
Body weight
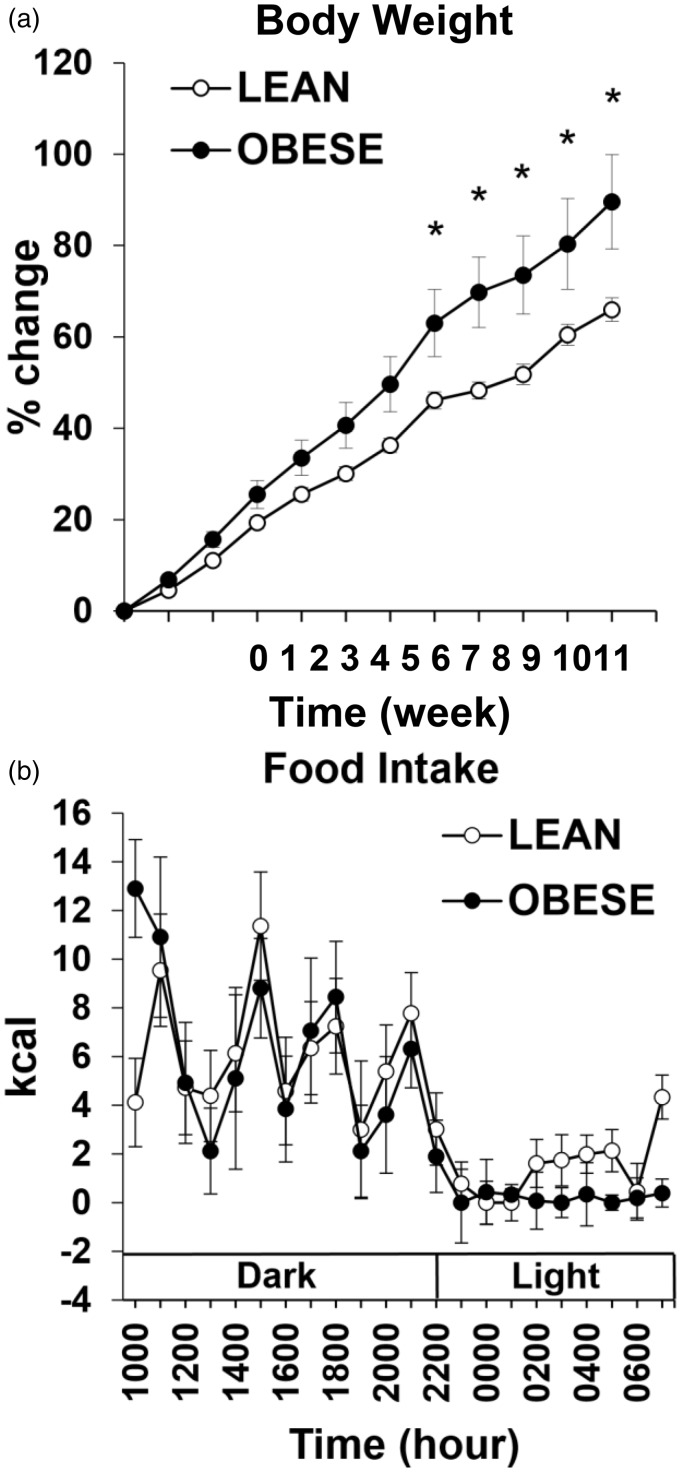
Body weight was higher in the OBESE compared with LEAN rats on wks 7–11 (p < 0.05, Figure 1(a)).
Figure 1.
Weekly percent change in body weight of both LEAN and OBESE groups over 11-week period (a). Average hourly food intake measured in kcal of LEAN and OBESE groups across wks 8 and 9 (b). • represents OBESE (high-fat fed) animals and ○ represents LEAN (chow-fed) animals. Values are mean ± SEM. *p ≤ 0.05 OBESE compared with LEAN.
Food intake
When hourly food intake (in kcals) for each animal was averaged across wks 8 and 9 for each group, there was no significant difference between OBESE and LEAN animals (Figure 1(b)). These data were collected during the same weeks that blood samples were taken for assessment of gut peptide rhythms. A higher body weight in high-fat fed rats despite equal kcal intake of rats on a control diet for multiple weeks has previously been documented.25–28
Rhythm of gut peptides and glucose
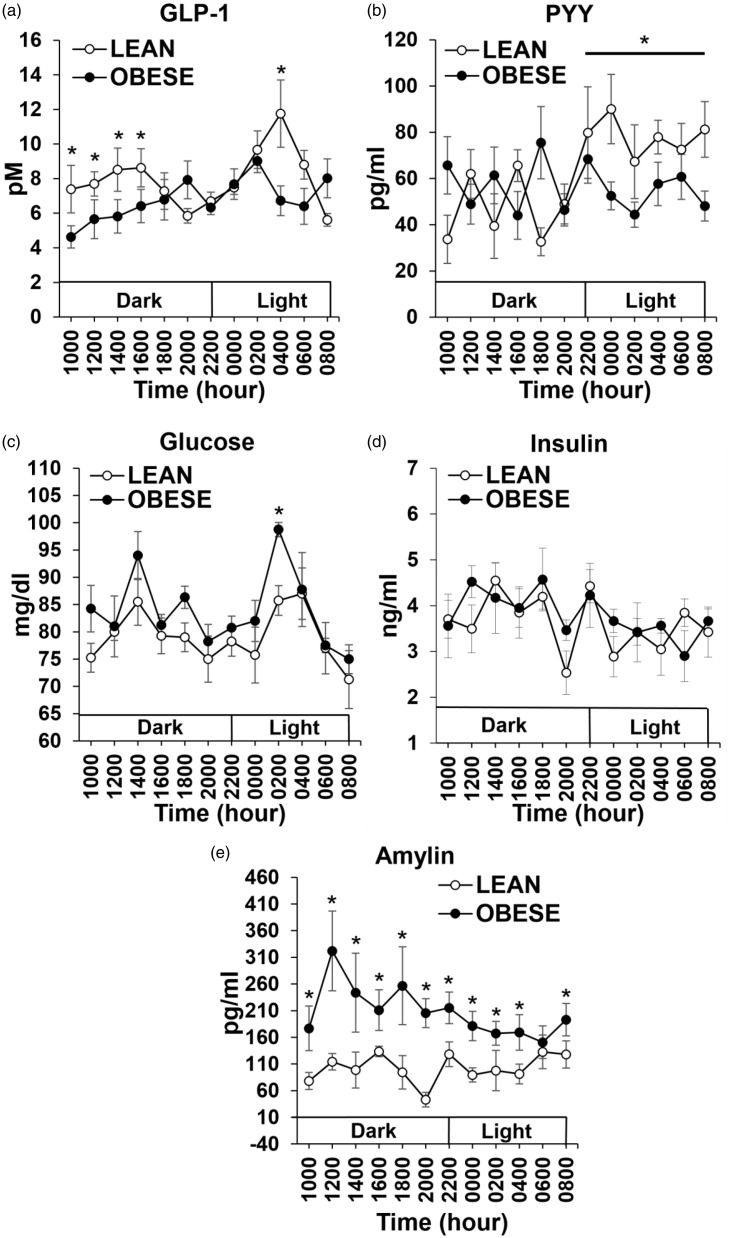
GLP-1: There was a main effect of group across time, with post hoc analysis revealing increases in GLP-1 levels in LEAN animals for the first four time points of the dark cycle when compared with OBESE animals (p < 0.05; Figure 2(a)). In addition, there was an increase in GLP-1 in the middle of the light cycle (04:00 h) in LEAN animals compared with OBESE animals (p < 0.05; Figure 2(a)). PYY: There was no significant difference in plasma PYY levels between LEAN and OBESE animals when analyzed across the entire 24 h period. When PYY levels were compared across only the light cycle, there was a significant increase in PYY levels in LEAN compared with OBESE animals (p < 0.05; Figure 2(b)). Glucose: There was a main effect of group across time, with glucose levels higher in OBESE compared with LEAN animals at 02:00 h (p < 0.05, Figure 2(c)). Insulin: There was no significant difference in insulin levels between OBESE and LEAN animals (Figure 2(d)). Amylin: There was a significant main group effect of amylin levels, with increased amylin at every time point except at 06:00 h in OBESE compared with LEAN animals (p < 0.05; Figure 2(e)).
Figure 2.
Hourly plasma hormone and glucose levels in both LEAN and OBESE groups over 24 h period; GLP-1 (a), PYY (b), glucose (c), insulin (d), and amylin (e). • represents OBESE (high-fat fed) animals and ○ represents LEAN (chow-fed) animals. Values are mean ± SEM. *p ≤ 0.05 OBESE compared with LEAN.GLP-1: glucagon-like peptide-1; PYY: peptide YY.
Meal-induced gut peptide levels
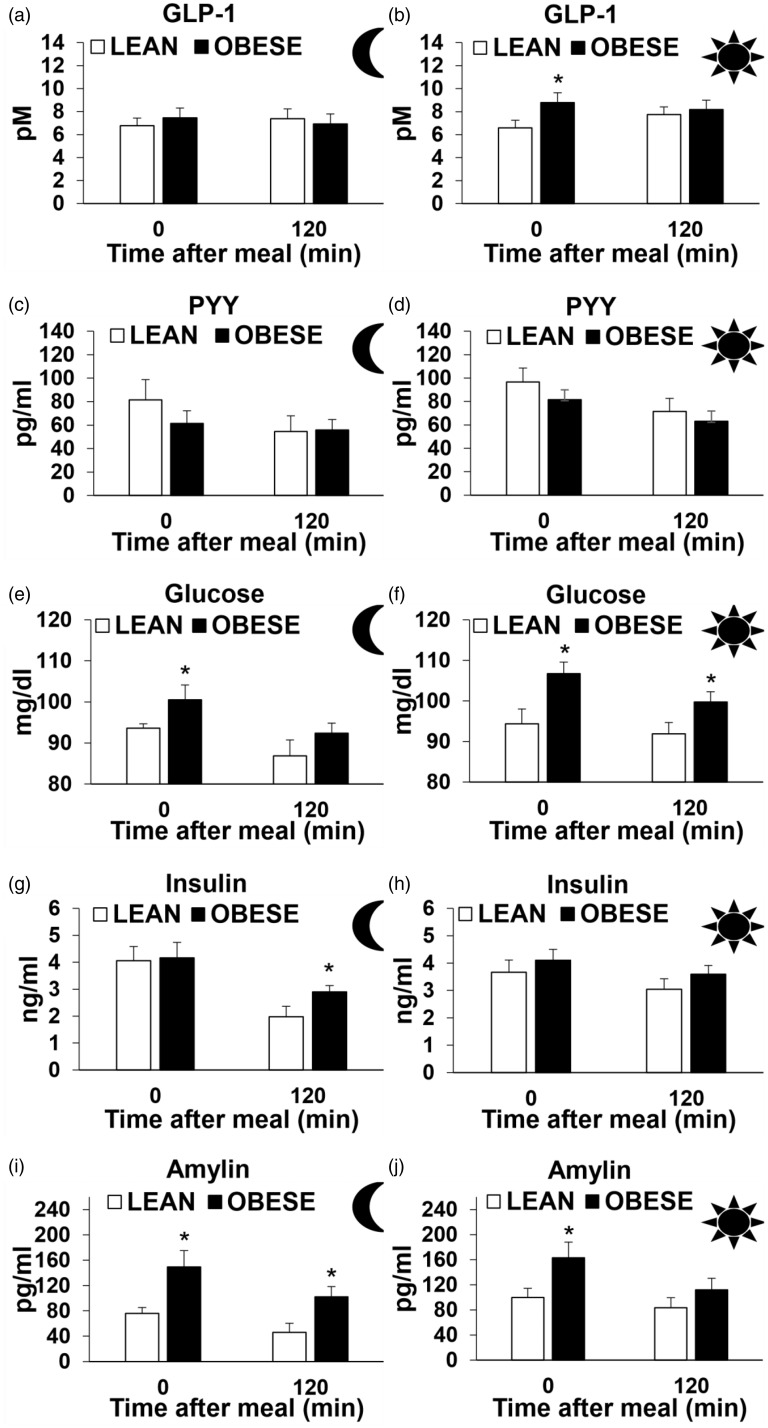
On week 10, subsets of animals received a liquid Ensure meal in the middle of the dark cycle and light cycle in both OBESE and LEAN animals, and plasma gut peptide levels were assessed immediately after and 2 h after the meal. GLP-1: GLP-1 levels were not different between the groups post-meal during the dark phase (Figure 3(a)). Immediately after the Ensure meal during the light phase, GLP-1 levels were higher in OBESE animals compared with LEAN animals, but no difference was seen 2 h post-meal (p < 0.05, Figure 3(b)). PYY: There was no difference in PYY during the light or dark phase between OBESE and LEAN animals (Figure 3 (c) and (d)). Glucose: Plasma glucose levels were higher in OBESE rats immediately after the meal in the dark phase and at both post-meal time points during the light phase when compared with LEAN animals (p < 0.05, Figure 3(e) and (f)). Insulin: OBESE rats had higher plasma insulin levels 2 h post-meal during the dark phase when compared with LEAN rats (p < 0.05, Figure 3(g) and (h)). There was no difference in the insulin levels during the light phase between the groups (Figure 3(a) and (b)). Amylin: Plasma AMY levels were higher in OBESE animals when compared with LEAN at both post-meal time points during the dark phase and immediately after the meal during the light phase (p < 0.05 Figure 3(i) and (j)).
Figure 3.
Plasma hormone levels immediately after (0 min) and 120 min after test meal in the middle of the dark period in both LEAN and OBESE groups – GLP-1 (a), PYY (c), glucose (e), insulin (g) and amylin (i). Plasma hormone levels immediately after (0 min) and 120 min after test meal in the middle of the light period in both LEAN and OBESE groups- GLP-1 (b), PYY (d), glucose (f), insulin (H) and amylin (j). Values are mean ± SEM. *p ≤ 0.05 OBESE compared with LEAN.GLP-1: glucagon-like peptide-1; PYY: peptide YY.
Discussion
The aim of this study was to test whether high-fat diet-induced obesity would alter the daily rhythm of gut peptide plasma levels or meal-induced levels in the middle of the light or dark cycle. We found that even when OBESE and LEAN animals ate the same kcals and have a similar pattern of food intake, there is a difference in both the levels and rhythm of plasma gut peptides throughout the light/dark cycle. In particular, we found that (i) GLP-1 levels were lower in OBESE animals during the first 6 h of the dark cycle and in the middle of the light cycle compared with the LEAN group (ii) PYY levels were increased during the light cycle in LEAN animals, but not in the OBESE group, and (iii) glucose and AMY levels were higher in OBESE animals throughout the light and dark cycles. We also found that when OBESE and LEAN animals were given an oral mixed meal in the middle of the light or dark cycle, there was a differential response of the plasma gut signals even though the composition and amount of intake of the meal was the same in both groups. Thus, it appears that OBESE and LEAN animals respond differently to the same amount and timing of food intake under a variety of conditions.
The difference in the levels of the gut peptides between the groups occurred when there is no clear indication of a problem with glycemic control in the OBESE animals and both groups are increasing their body weight across time. Although many rodent models of diet-induced obesity see hyperinsulinema and sustained increases in glucose across the 24-h period, we did not find this. The differential results may be due to the other studies using alternative high-fat diets29–31 or other strains of rats.32–35 In addition, the Sprague-Dawley rats used in this study eat equal kcals of the 60% high-fat diet and chow, and the growth trajectory of both groups is similar and constantly increasing throughout the entire time of the study. Another study using Sprague-Dawley rats, though, find a dissimilar growth pattern between the high-fat and chow-fed groups.36 The timing of how long the animals are eating the high-fat diet may also contribute to sustained increases in insulin and glucose levels. If the animals in the present study were maintained on the diets for a longer period of time, glycemic control may then be altered and differences in the gut peptide levels further enhanced. Nonetheless, clear differences in peptide levels are seen in response to diet-induced obesity even prior to other metabolic disturbances (as has been discussed literature37). Changes in the gut peptides could be used as an early indicator of later and more serious metabolic disturbances and may be a target for intervention.
There is no clear connection between the timing of food intake and the gut peptide levels across the 24 h period for either group. Whereas glucose and insulin levels vary at each time point, the other gut peptides show periods of sustained highs and lows. GLP-1 levels remain high throughout the dark cycle, while animals in both groups are intermittently eating. The higher GLP-1 levels in the LEAN vs. OBESE animals are consistent with previous reports.14,38 It is often thought, though, that GLP-1 is quickly degraded after release, but clearly GLP-1 remains in the systemic circulation and at levels where differences between the groups can be observed. During the light cycle when animals are not eating, there is an elevation of GLP-1 that has previously been documented.14,39 PYY levels during the dark cycle appear to mimic the pattern of food intake, with peaks and nadirs, but the pattern is flipped between the OBESE and LEAN groups. Thus, the timing of food intake may correlate with plasma PYY, but the association between meals and PYY levels was not the same between OBESE and LEAN animals. This may be explained by variations in the digestion and absorption of the high-fat versus the chow diets or related to factors associated with obesity. Higher PYY levels have been observed in lean compared with obese humans40,41 and non-human animals14,42,43 after a meal or at select times during the day or night, but sustained increases in PYY levels during the light cycle in rats has not previously been found and is not a direct meal-driven effect. High AMY levels in obese humans44 and non-human animals45 has been found, but this is the first time indicating that they remain high throughout the light/dark cycle. Taken together, the timing of food intake is clearly not the only factor dictating gut peptide levels.
The daily rhythm of physiological and behavioral events can be determined by circadian clocks. The circadian rhythm is set by the suprachiasmatic nucleus of the hypothalamus (SCN) or by intrinsic circadian clocks within the cells of certain organs. The SCN can control the timed release of gut peptides through a variety of mechanisms, including (1) the secretion of neurohormones into the circulation, (2) autonomic neural connections to the secretory cells of peripheral tissues or (3) by controlling feeding behavior and inducing nutrient, neural, and hormonal release of gut peptides. Given that animals in the OBESE and LEAN groups were eating meals at the same time of day, the timing of the meals is not dictating the rhythm of the gut peptides. The difference in the diet composition between the high-fat and chow foods may contribute to the variation in gut peptide levels in the ad libitum fed condition, but when both groups ate the same meal, 12 ml of liquid Ensure, there was still a differential gut peptide response. This suggests that the underlying mechanism for the changes in the daily rhythm or in the feeding-induced gut peptide levels may be due to obesity associated alterations in how the SCN communicates with the secretory cells of the digestive and accessory organs or how the cells are responding to exogenous signals. The idea that high-fat diet-induced obesity can alter the circadian rhythm of physiological events, independent of alterations in the light-dark cycle, is strongly supported by data (see reviews in literature46–48). We have highlighted a few of the key mechanisms by which high-fat diet-induced obesity may induce the changes in gut peptide levels in the present study.
High-fat diet-induced obesity shifts the expression of clock genes within the SCN and in peripheral oscillators.49 A shift in clock gene expression within the SCN would result in altered autonomic input to the secretory cells of the peripheral tissues. Because the parasympathetic nervous system is known to play a direct role in both β-cell and enteroendocrine peptide release,50,51 it is likely that a shift in activity would contribute to alterations in the levels of gut peptides throughout the 24 h cycle. Although the exact effect of diet-induced obesity on the timing of autonomic activity is not known, obesity is associated with a general decrease in parasympathetic and increase in sympathetic nervous system activity.52 Independent of the central circadian control, peripheral oscillators also play a role in gut peptide release. In particular, the level or timing of expression of circadian transcription factors within L cells can be directly altered by high-fat diet intake in vivo or application of palmitate, the most abundant saturated fatty acid in the circulation of obese animals, in vitro.39,53 This likely occurs because the promoter region of the proglucagon gene, the gene for GLP-1, contains an ebox domain that is known to bind circadian transcription factors.54 The molecular signaling necessary for these nutrient-induced changes have yet to be identified and the extent to which peripheral oscillators contribute to the release of the other gut peptides measured here is not clear.
Additional molecular changes in the secretory cells of the digestive and accessory organs may alter how the cells respond to nutrients or nutrient-related factors. The intestinal L cells that secrete GLP-1 and PYY and the pancreatic β-cells that secrete insulin and AMY are altered in obesity (GLP-1 and AMY are produced in other cells of the body, but the contribution of these populations to obesity-induced changes in plasma levels is negligible or unknown). As obesity develops, hyperinsulinemia and hyperamylinemia occur through overproduction in the β-cells as a result of hypernutrient stimulation, loss of insulin or amylin receptor activity, or reduced clearance of these peptides from circulation.55 As obesity persists, β-cells stop producing insulin and amylin and the cells will undergo apoptosis.55 Obesity-related changes are also seen in the L cells and include changes in cellular metabolism.56,57 These cells, though, are constantly regenerated every few days as the entire intestinal epithelium turns over. Thus, the impact of obesity may occur earlier in the proliferation and differentiation process. In fact, high-fat diet-induced obese mice show alterations in proliferation and differentiation in vivo and in intestinal epithelial stem cells isolated from obese mice in vitro.58,59 These cellular and molecular changes in the cells secreting these gut peptides would be expected to result in modifications in nutrient-sensing and processing that likely contribute to shifts in the rhythm and meal-induced levels of gut peptides between obese and lean individuals.
Authors’ contributions
All authors participated in the design, interpretation of the studies and review of the manuscript. AAM and MJD conducted the experiments, analyzed the data and wrote the manuscript.
Funding
This work was supported by National Institutes of Health Grants DK019302 (to T.H.M) and DK092126 (to M.J.D).
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384:766–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation 1983; 67:968–77 [DOI] [PubMed] [Google Scholar]
- 3.Brown CD, Higgins M, Donato KA, Rohde FC, Garrison R, Obarzanek E, Ernst ND, Horan M. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res 2000; 8:605–19 [DOI] [PubMed] [Google Scholar]
- 4.Larsson B, Bjorntorp P, Tibblin G. The health consequences of moderate obesity. Int J Obes 1981; 5:97–116 [PubMed] [Google Scholar]
- 5.Rexrode KM, Hennekens CH, Willett WC, Colditz GA, Stampfer MJ, Rich-Edwards JW, Speizer FE, Manson JE. A prospective study of body mass index, weight change, and risk of stroke in women. JAMA 1997; 277:1539–45 [DOI] [PubMed] [Google Scholar]
- 6.Ernst ND, Obarzanek E, Clark MB, Briefel RR, Brown CD, Donato K. Cardiovascular health risks related to overweight. J Am Diet Assoc 1997; 97:S47–51 [DOI] [PubMed] [Google Scholar]
- 7.Moran TH, Dailey MJ. Intestinal feedback signaling and satiety. Physiol Behav 2011; 105:77–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moran TH, Dailey MJ. Minireview: gut peptides: targets for antiobesity drug development? Endocrinology 2009; 150:2526–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dailey MJ, Stingl KC, Moran TH. Disassociation between preprandial gut peptide release and food-anticipatory activity. Endocrinology 2012; 153:132–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer-Gerspach AC, Wolnerhanssen B, Beglinger B, Nessenius F, Napitupulu M, Schulte FH, Steinert RE, Beglinger C. Gastric and intestinal satiation in obese and normal weight healthy people. Physiol Behav 2014; 129:265–71 [DOI] [PubMed] [Google Scholar]
- 11.Carr RD, Larsen MO, Jelic K, Lindgren O, Vikman J, Holst JJ, Deacon CF, Ahren B. Secretion and dipeptidyl peptidase-4-mediated metabolism of incretin hormones after a mixed meal or glucose ingestion in obese compared to lean, nondiabetic men. J Clin Endocrinol Metab 2010; 95:872–8 [DOI] [PubMed] [Google Scholar]
- 12.Adam TC, Westerterp-Plantenga MS. Glucagon-like peptide-1 release and satiety after a nutrient challenge in normal-weight and obese subjects. Br J Nutr 2005; 93:845–51 [DOI] [PubMed] [Google Scholar]
- 13.Verdich C, Toubro S, Buemann B, Lysgard Madsen J, Juul Holst J, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety – effect of obesity and weight reduction. Int J Obes Relat Metab Disord 2001; 25:1206–14 [DOI] [PubMed] [Google Scholar]
- 14.Dailey MJ, Moghadam AA, Moran TH. Nutrient-specific feeding and endocrine effects of jejunal infusions in obese animals. Am J Physiol Regul Integr Comp Physiol 2014; 306:R420–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon C, Schlienger JL, Sapin R, Imler M. Cephalic phase insulin secretion in relation to food presentation in normal and overweight subjects. Physiol Behav 1986; 36:465–9 [DOI] [PubMed] [Google Scholar]
- 16.Osuna JI, Pages I, Motino MA, Rodriguez E, Osorio C. Cephalic phase of insulin secretion in obese women. Horm Metab Res 1986; 18:473–5 [DOI] [PubMed] [Google Scholar]
- 17.Squadrito G, Cucinotta D, Frisina N, Quartarone M, Saitta A. Circadian variations in glycemia and IRI in normal subjects, young and adult diabetics and in obese subjects. Minerva Med 1979; 70:2629–36 [PubMed] [Google Scholar]
- 18.Polonsky KS. Dynamics of insulin secretion in obesity and diabetes. Int J Obes Relat Metab Disord 2000; 24(Suppl 2):S29–31 [DOI] [PubMed] [Google Scholar]
- 19.Holst JJ, Schwartz TW, Lovgreen NA, Pedersen O, Beck-Nielsen H. Diurnal profile of pancreatic polypeptide, pancreatic glucagon, gut glucagon and insulin in human morbid obesity. Int J Obes 1983; 7:529–38 [PubMed] [Google Scholar]
- 20.Yildiz BO, Suchard MA, Wong ML, McCann SM, Licinio J. Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc Natl Acad Sci USA 2004; 101:10434–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Batterham RL, Bloom SR. The gut hormone peptide YY regulates appetite. Ann N Y Acad Sci 2003; 994:162–8 [DOI] [PubMed] [Google Scholar]
- 22.Roth JD. Amylin and the regulation of appetite and adiposity: recent advances in receptor signaling, neurobiology and pharmacology. Curr Opin Endocrinol Diabetes Obes 2013; 20:8–13 [DOI] [PubMed] [Google Scholar]
- 23.Pfluger PT, Kampe J, Castaneda TR, Vahl T, D'Alessio DA, Kruthaupt T, Benoit SC, Cuntz U, Rochlitz HJ, Moehlig M, Pfeiffer AF, Koebnick C, Weickert MO, Otto B, Spranger J, Tschop MH. Effect of human body weight changes on circulating levels of peptide YY and peptide YY3-36. J Clin Endocrinol Metab 2007; 92:583–8 [DOI] [PubMed] [Google Scholar]
- 24.Enoki S, Mitsukawa T, Takemura J, Nakazato M, Aburaya J, Toshimori H, Matsukara S. Plasma islet amyloid polypeptide levels in obesity, impaired glucose tolerance and non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract 1992; 15:97–102 [DOI] [PubMed] [Google Scholar]
- 25.Oscai LB, Brown MM, Miller WC. Effect of dietary fat on food intake, growth and body composition in rats. Growth 1984; 48:415–24 [PubMed] [Google Scholar]
- 26.Blevins JE, Thompson BW, Anekonda VT, Ho JM, Graham JL, Roberts ZS, Hwang BH, Ogimoto K, Wolden-Hanson T, Nelson J, Kaiyala KJ, Havel PJ, Bales KL, Morton GJ, Schwartz MW, Baskin DG. Chronic CNS oxytocin signaling preferentially induces fat loss in high-fat diet-fed rats by enhancing satiety responses and increasing lipid utilization. Am J Physiol Regul Integr Comp Physiol 2016; 310:R640–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erdos B, Kirichenko N, Whidden M, Basgut B, Woods M, Cudykier I, Tawil R, Scarpace PJ, Tumer N. Effect of age on high-fat diet-induced hypertension. Am J Physiol Heart Circ Physiol 2011; 301:H164–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernandez LL, Grayson BE, Yadav E, Seeley RJ, Horseman ND. High fat diet alters lactation outcomes: possible involvement of inflammatory and serotonergic pathways. PLoS One 2012; 7:e32598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Axen KV, Dikeakos A, Sclafani A. High dietary fat promotes syndrome X in nonobese rats. J Nutr 2003; 133:2244–9 [DOI] [PubMed] [Google Scholar]
- 30.Harris RB, Jones WK. Physiological response of mature rats to replacement of dietary fat with a fat substitute. J Nutr 1991; 121:1109–16 [DOI] [PubMed] [Google Scholar]
- 31.Otukonyong EE, Dube MG, Torto R, Kalra PS, Kalra SP. High-fat diet-induced ultradian leptin and insulin hypersecretion are absent in obesity-resistant rats. Obes Res 2005; 13:991–9 [DOI] [PubMed] [Google Scholar]
- 32.Stern JS, Johnson PR, Batchelor BR, Zucker LM, Hirsch J. Pancreatic insulin release and peripheral tissue resistance in Zucker obese rats fed high- and low-carbohydrate diets. Am J Physiol 1975; 228:543–8 [DOI] [PubMed] [Google Scholar]
- 33.Zhou YP, Cockburn BN, Pugh W, Polonsky KS. Basal insulin hypersecretion in insulin-resistant Zucker diabetic and Zucker fatty rats: role of enhanced fuel metabolism. Metabolism 1999; 48:857–64 [DOI] [PubMed] [Google Scholar]
- 34.Greene SF, Johnson PR, Eiffert KC, Greenwoodt MR, Stern JS. The male obese Wistar diabetic fatty rat is a new model of extreme insulin resistance. Obes Res 1994; 2:432–43 [DOI] [PubMed] [Google Scholar]
- 35.Russell JC, Graham S, Hameed M. Abnormal insulin and glucose metabolism in the JCR:LA-corpulent rat. Metabolism 1994; 43:538–43 [DOI] [PubMed] [Google Scholar]
- 36.Levin BE, Sullivan AC. Glucose-induced sympathetic activation in obesity-prone and resistant rats. Int J Obes 1989; 13:235–46 [PubMed] [Google Scholar]
- 37.Parnell JA, Reimer RA. Differential secretion of satiety hormones with progression of obesity in JCR:LA-corpulent rats. Obesity 2008; 16:736–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams DL, Hyvarinen N, Lilly N, Kay K, Dossat A, Parise E, Torregrossa AM. Maintenance on a high-fat diet impairs the anorexic response to glucagon-like-peptide-1 receptor activation. Physiol Behav 2011; 103:557–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gil-Lozano M, Mingomataj EL, Wu WK, Ridout SA, Brubaker PL. Circadian secretion of the intestinal hormone GLP-1 by the rodent L cell. Diabetes 2014; 63:3674–85 [DOI] [PubMed] [Google Scholar]
- 40.Alvarez Bartolome M, Borque M, Martinez-Sarmiento J, Aparicio E, Hernandez C, Cabrerizo L, Fernandez-Represa JA. Peptide YY secretion in morbidly obese patients before and after vertical banded gastroplasty. Obes Surg 2002; 12:324–7 [DOI] [PubMed] [Google Scholar]
- 41.Guo Y, Ma L, Enriori PJ, Koska J, Franks PW, Brookshire T, Cowley MA, Salbe AD, Delparigi A, Tataranni PA. Physiological evidence for the involvement of peptide YY in the regulation of energy homeostasis in humans. Obesity 2006; 14:1562–70 [DOI] [PubMed] [Google Scholar]
- 42.Rahardjo GL, Huang XF, Tan YY, Deng C. Decreased plasma peptide YY accompanied by elevated peptide YY and Y2 receptor binding densities in the medulla oblongata of diet-induced obese mice. Endocrinology 2007; 148:4704–10 [DOI] [PubMed] [Google Scholar]
- 43.Yang N, Wang C, Xu M, Mao L, Liu L, Sun X. Interaction of dietary composition and PYY gene expression in diet-induced obesity in rats. J Huazhong Univ Sci Technol Med Sci 2005; 25:243–6 [DOI] [PubMed] [Google Scholar]
- 44.Reinehr T, de Sousa G, Niklowitz P, Roth CL. Amylin and its relation to insulin and lipids in obese children before and after weight loss. Obesity 2007; 15:2006–11 [DOI] [PubMed] [Google Scholar]
- 45.Boyle CN, Rossier MM, Lutz TA. Influence of high-fat feeding, diet-induced obesity, and hyperamylinemia on the sensitivity to acute amylin. Physiol Behav 2011; 104:20–8 [DOI] [PubMed] [Google Scholar]
- 46.Oosterman JE, Kalsbeek A, la Fleur SE, Belsham DD. Impact of nutrients on circadian rhythmicity. Am J Physiol Regul Integr Comp Physiol 2015; 308:R337–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engin A. Circadian rhythms in diet-induced obesity. Adv Exp Med Biol 2017; 960:19–52 [DOI] [PubMed] [Google Scholar]
- 48.Froy O. Circadian rhythms and obesity in mammals. ISRN Obes 2012; 2012:437198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Froy O. Metabolism and circadian rhythms – implications for obesity. Endocr Rev 2010; 31:1–24 [DOI] [PubMed] [Google Scholar]
- 50.Plamboeck A, Veedfald S, Deacon CF, Hartmann B, Vilsboll T, Knop FK, Holst JJ. The role of efferent cholinergic transmission for the insulinotropic and glucagonostatic effects of GLP-1. Am J Physiol Regul Integr Comp Physiol 2015; 309:R544–51 [DOI] [PubMed] [Google Scholar]
- 51.Lustig RH. Autonomic dysfunction of the beta-cell and the pathogenesis of obesity. Rev Endocr Metab Disord 2003; 4:23–32 [DOI] [PubMed] [Google Scholar]
- 52.Haqq AM, DeLorey DS, Sharma AM, Freemark M, Kreier F, Mackenzie ML, Richer LP. Autonomic nervous system dysfunction in obesity and Prader-Willi syndrome: current evidence and implications for future obesity therapies. Clin Obes 2011; 1:175–83 [DOI] [PubMed] [Google Scholar]
- 53.Gil-Lozano M, Wu WK, Martchenko A, Brubaker PL. High-fat diet and palmitate alter the rhythmic secretion of glucagon-like peptide-1 by the rodent L-cell. Endocrinology 2016; 157:586–99 [DOI] [PubMed] [Google Scholar]
- 54.Kieffer TJ, Hussain MA, Habener JF. Glucagon and glucagon-like peptide production and degradation. Compr Physiol 2011; Supplement 21:197–265 [Google Scholar]
- 55.Zaccardi F, Webb DR, Yates T, Davies MJ. Pathophysiology of type 1 and type 2 diabetes mellitus: a 90-year perspective. Postgrad Med J 2016; 92:63–9 [DOI] [PubMed] [Google Scholar]
- 56.Zhang H, Li J, Liang X, Luo Y, Zen K, Zhang CY. Uncoupling protein 2 negatively regulates glucose-induced glucagon-like peptide 1 secretion. J Mol Endocrinol 2012; 48:151–8 [DOI] [PubMed] [Google Scholar]
- 57.Kondo H, Minegishi Y, Komine Y, Mori T, Matsumoto I, Abe K, Tokimitsu I, Hase T, Murase T. Differential regulation of intestinal lipid metabolism-related genes in obesity-resistant A/J vs. obesity-prone C57BL/6J mice. Am J Physiol Endocrinol Metab 2006; 291:E1092–9 [DOI] [PubMed] [Google Scholar]
- 58.Mah AT, Van Landeghem L, Gavin HE, Magness ST, Lund PK. Impact of diet-induced obesity on intestinal stem cells: hyperproliferation but impaired intrinsic function that requires insulin/IGF1. Endocrinology 2014; 155:3302–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beyaz S, Mana MD, Roper J, Kedrin D, Saadatpour A, Hong SJ, Bauer-Rowe KE, Xifaras ME, Akkad A, Arias E, Pinello L, Katz Y, Shinagare S, Abu-Remaileh M, Mihaylova MM, Lamming DW, Dogum R, Guo G, Bell GW, Selig M, Nielsen GP, Gupta N, Ferrone CR, Deshpande V, Yuan GC, Orkin SH, Sabatini DM, Yilmaz OH. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 2016; 531:53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]