Short abstract
Ample evidence has demonstrated the involvement of microRNAs in Parkinson’s disease pathogenesis. miR-124-3p was reported to be able to improve neural functional recovery. However, the underlying mechanism of miR-124-3p in Parkinson’s disease progression was not well established. This study was designed to investigate the role of miR-124-3p in methyl phenyl pyridinium iodide (MPP)+-induced SH-SY5Y cells, an in vitro Parkinson’s disease model. It is observed that miR-124-3p expression was decreased, and STAT3 expression was increased in MPP+-induced SH-SY5Y cells. miR-124-3p overexpression attenuated MPP+-induced neuronal injury, displayed as increased cell viability and superoxide dismutase activities, as well as reduced cell apoptosis, Caspase-3 activity, lactate dehydrogenase activity, inflammatory factors TNF-α, and IL-1β levels and reactive oxygen species generation. Moreover, STAT3 was confirmed to be a miR-124-3p target. Restored STAT3 expression reversed miR-124-3p-induced neuroprotective effects against MPP+-mediated neuronal injury. These data demonstrated that miR-124-3p contributed to neuroprotective effects in MPP+-induced Parkinson’s disease cell model by targeting STAT3.
Impact statement
PD affects millions of people in the world, causing uncontrolled tremors. MicroRNAs, a class of endogenous single-stranded non-coding transcript with approximately 22 nucleotides, could bind to the 3″ UTR of their targets. The functional action of miR-124-3p in PD was not fully elucidated. Our study found that ectopic expression miR-124-3p attenuated MPP+-induced injury in PD model in vitro by suppressing neurotoxicity, neuronal apoptosis, neuroinflammation, and oxidative stress. Moreover, we validated that miR-124-3p could bind to STAT3 mediating the neuroprotective effect of miR-124-3p. We believe this study will be interesting for readers of this area.
Keywords: Parkinson’s disease, miR-124-3p, STAT3, MPP+
Introduction
Parkinson’s disease (PD) is the most widespread neurodegenerative disease in the central nervous system (CNS), triggering motor disturbance and cognitive impairment.1,2 To date, genetic and epigenetic factors have been demonstrated to be involved in dopamine neurons deficiency in PD.3–5 Therefore, searching strategies to abate the loss of SNpc DA neurons is in great need for the effective treatment of PD patients.
MicroRNAs, a class of endogenous single-stranded non-coding transcript with approximately 22 nucleotides, could result in mRNA degradation or translation repression via complementary with the 3″ UTR of their targets.6 Extensive evidence reveals the vital role of miRNAs in plenty of pathological and physiological processes, such as proliferation, differentiation, apoptosis, invasion, metastasis, inflammation, angiogenesis, stress resistance, and tumorigenesis.7 Also, many studies have highlighted the significance of miRNAs in neurodegenerative diseases,8 including PD.9 For example, the loss of miR-155 decreased proinflammatory responses to α-synuclein, suppressed α-synuclein, and induced neurodegeneration in vivo model of PD.10 micoRNA-205 overexpression abrogated the impairment of neurite outgrowth elicited by R1441G LRRK2.11miR-124, a miRNA abundant in brain, has been regarded to be able to facilitate neuronal differentiation.12,13 Moreover, miR-124 was found to be down-regulated in the SNpc DA neuron of MPTP-intoxicated PD mouse model, methyl phenyl pyridinium iodide (MPP+)-treated dopaminergic neurons, and MPP+-induced SH-SY5Y cells.14,15
The present study revealed that overexpression of miR-124-3p attenuated MPP+-induced injury in PD model in vitro by suppressing neurotoxicity, neuronal apoptosis, neuroinflammation, and oxidative stress. Moreover, we validated that miR-124-3p could bind to STAT3 and mediate the neuroprotective effect.
Materials and methods
Cell culture and treatments
SH-SY5Y human neuroblastoma cells were purchased from the American Type Culture Collection. Cells were cultured according to the instructions.
The shRNA plasmids were transfected into the Eca109 and TE1 cancer cell lines. The pcDNA-STAT3 and pcDNA empty vectors were obtained from Invitrogen. miR-124-3p mimics, scramble negative control (miR-NC), miR-124-3p antigomir, and antigomir control were obtained from GenePharma (Shanghai, China). All oligonucleotides or plasmids were transfected with Lipofectamine, 2000 reagent (Invitrogen). Cells were exposed to 0, 0.25, 0.5, 1, or 2 mM of MPP+ for 24 h or 1 mM MPP+ for 0 h, 6 h, 12 h, 24 h, or 48 h. The neuroprotective effects of miR-124-3p against MPP+-induced neuronal injury were examined. Cells were pretreated with miR-124-3p mimics and administrated with 1 mM MPP+ for one day. To explore the neuroprotective mechanism of miRNA, the cells were pretreated with miR-124-3p mimics or miR-124-3p mimics + pcDNA-STAT3 and then treated with 1 mM MPP+ for one day.
Quantitative real-time polymerase chain reaction
Real-time PCR was used to detect the expression of miR-124-3p and STAT3 mRNA. Total RNAs were harvested from cultured cells using Trizol reagent (Invitrogen) following the protocols of the manufacturer. For miRNA expression analysis, it was detected by TaqMan MicroRNA Assays (Applied Biosystems, Forest City, CA, USA). STAT3 mRNA expression was detected by SYBR green RT-qPCR (Applied Biosystems). The relative level of miRNA and STAT3 mRNA was determined using the 2−ΔΔCt method.16
Western blot
The assay was performed as previously described.17 Briefly, the same amount of protein was separated on a 12% SDS-PAGE gels; the gel was electrotransferred onto a PVDF membrane, and then incubated with primary antibodies against STAT3, DAT, and β-actin (Abcam, Cambridge, MA, USA). The enhanced chemiluminescence reagent (Thermo Scientific, Logan, UT, USA) was employed to determine the immunoreactive bands.
Cell viability
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was applied to determine the viability of SH-SY5Y cells. At indicated time point, the cells were incubated with 20 μL MTT (Sigma, St. Louis, Missouri, USA). Then, DMSO was used to dissolve the crystals. Finally, absorbance at 450 nm was calculated by microplate reader (Molecular Devices, CA, USA).
Apoptosis assay
Annexin V/fluorescein isothiocyanate (FITC) Apoptosis Detection kit (BD Biosciences, CA, USA) was applied to determine apoptotic rate of SH-SY5Y cells. Briefly, an amount of 3 × 105 cells was treated with Annexin V–FITC and propidium iodide and subjected to flow cytometer (BD Biosciences).
Caspase-3 activity assay
Colorimetric assay kit (Cell Signaling, MA, USA) was used to measure the activity. Briefly, SH-SY5Y cell lysates were incubated with 100 µM of enzyme-specific substrates at 37°C for 4 h. The relative activity was described as fold change by a microplate reader.
Lactate dehydrogenase release assay
The diagnostic kit (Jiancheng Bioengineering Institute, Nanjing, China) was used to assess the level of lactate dehydrogenase (LDH). Briefly, the collected supernatant was co-treated with reduced nicotinamide-adenine dinucleotide and pyruvate according to the manufacturer’s instructions. The absorbance was measured at 440 nm using microplate reader to calculate the activity of LDH.
Measurement of reactive oxygen species and superoxide dismutase production assay
ROS assay kit (Beyotime, Jiangsu, China) was applied to measure reactive oxygen species (ROS) level. Briefly, SH-SY5Y cells were cultured with dichlorofluorescein diacetate (DCF-DA) (Sigma) according to the instructions. The fluorescence intensity of oxidant-sensitive probe 2,7-DCF-DA in an Olympus BX60 microscope (Olympus Optical Co Ltd, Tokyo, Japan) at 480 nm was used to determine intracellular ROS generation. The superoxide dismutase (SOD) enzyme activities were evaluated using commercial detection kits (Cayman Chemical, MI, USA) according to the manufacture’s introduction.
ELISA
The levels of TNF-α and IL-1β were tested by specific enzyme-linked immunosorbent (ELISA) kits (Elisa biotech, Shanghai, China) referring to the directions described by the manufacture.
Luciferase reporter assays
The STAT3 3″ UTR sequences (wild-type STAT3 3″ UTR, STAT3-WT) or the mutant-binding sites (mutant STAT3 3” UTR, STAT3-MUT) were inserted into pMIR-REPORT luciferase reporter vectors (Promega, WI, USA). Subsequently, SH-SY5Y cells were co-transfected with miR-124-3p or anti-miR-124-3p and STAT3-WT or STAT3-MUT. The luciferase activities were determined by Dual-Luciferase Reporter Assay System (Promega).
Statistical analysis
All experimental data were represented as “the mean ± standard deviation.” SPSS 19.0 software (SPSS, Chicago, IL, USA) was used to evaluate the significant differences by using Student’s t-test or one-way ANOVA. The difference was statistically significant at P < 0.05. Multiple comparisons between the groups were performed using Student-Newman-Keuls (S-N-K) method.
Results
miR-124-3p expression is down-regulated and STAT3 expression is up-regulated in MPP+-induced SH-SY5Y cell PD model
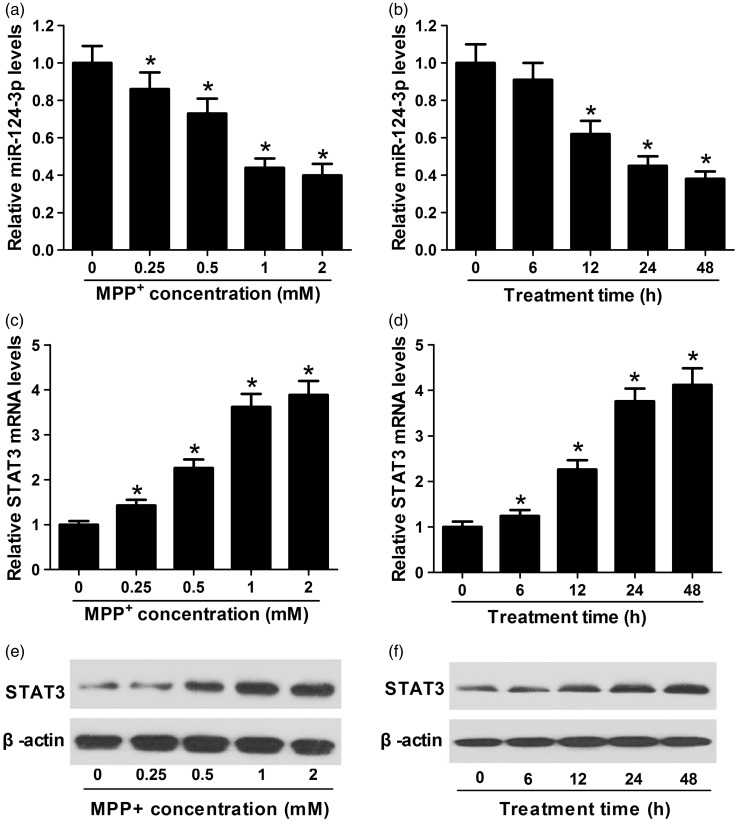
To investigate miR-124-3p and STAT3 expression levels in PD cell model, 0, 0.25, 0.5, 1, or 2 mM of MPP+ were added to SH-SY5Y cells for 24 h or 1 mM MPP+ for different times followed by qRT-PCR analysis. miR-124-3p expression (Figure 1(a) and (b)) was reduced and STAT3 mRNA level (Figure 1(c) and (d)) was increased in MPP+-treated SH-SY5Y cells in dose- and time-dependent manners. Moreover, STAT3 protein level was elevated in dose- and time-dependent manners (Figure 1(e) and (f)). All these results revealed that miR-124-3p and STAT3 may be involved in PD pathogenesis.
Figure 1.
miR-124-3p was downregulated and STAT3 was up-regulated in MPP+-induced SH-SY5Y cells. (a to d) qRT-PCR analysis revealed decreased miR-124-3p and increased STAT3 expression in MPP+-treated SH-SY5Y cells in dose- and time-dependent manners. (e and f) Western blot analysis indicated the protein level of STAT3 was increased in MPP+-treated SH-SY5Y cells in dose- and time-dependent manners. Significant differences were assessed using one-way ANOVA. Multiple comparisons between the groups were performed using S-N-K method. *P < 0.05 vs. control groups.
MPP: methyl phenyl pyridinium iodide.
miR-124-3p attenuates MPP+-induced neuronal injury
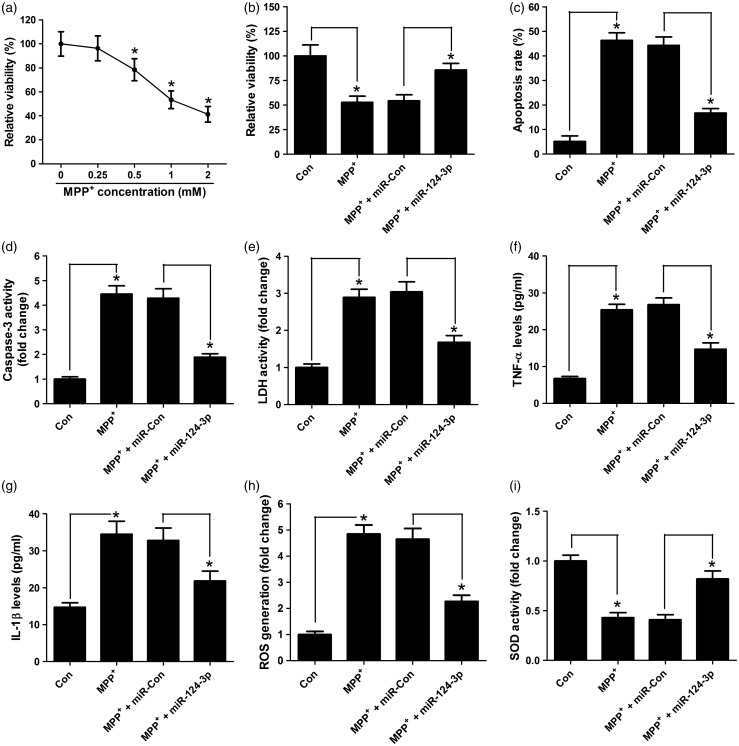
The functional effect of miR-124-3p on MPP+-induced SH-SY5Y cells was explored. Cell viability was first determined by MTT assay when treated with MPP+. As expected, MPP+ led to cytotoxicity (Figure 2(a)). Cells were treated with MPP+ or transfected with miR-124-3p before MPP+ treatment. MTT analysis displayed that miR-124-3p overexpression recovered the cell viability to 80% in SH-SY5Y cells compared with control group (Figure 2(b)). To examine whether miR-124-3p has protective effect against neuronal apoptosis, flow cytometry analysis was performed at 24 h after MPP+ treatment. The results revealed that miR-124-3p overexpression significantly alleviated MPP+-induced apoptosis from 45% to 20% (Figure 2(c)). The same effect of caspase-3 activity was also observed in Figure 2(d). In addition, miR-124-3p overexpression reversed the effect that MPP+-mediated increase in the activity of LDH from three-fold to two-fold of that in miR-con group (Figure 2(e)). Then, neuroinflammation was tested by ELISA analysis. As presented in Figure 2(f) and (g), MPP+ treatment up-regulated the expression level of inflammatory factors TNF-α and IL-1β in SH-SY5Y cells, which was weakened by miR-124-3p overexpression from 3.5-fold to 2-fold of that in miR-con group. Furthermore, ROS generation and antioxidant enzymes SOD activity were determined in SH-SY5Y cells. The results indicated that miR-124-3p pretreatment attenuated MPP+-mediated ROS generation from five-fold to three-fold in miR-con group (Figure 2(g)) and abated MPP+-induced inhibition of SOD from 1/2 to 3/4 in miR-con group (Figure 2(h)). Collectively, these data demonstrated that miR-124-3p could protect against MPP+-induced neuronal damage.
Figure 2.
miR-124-3p protects SH-SY5Y cells against MPP+-induced neuronal injury. (a) MTT assay determined the cell viability in SH-SY5Y cells treated with different concentrations of MPP+ (0, 0.25, 0.5, 1, 2 mM). SH-SY5Y cells were transfected with miR-124-3p mimics prior to 1 mM MPP+ treatment for 24 h. (b) miR-124-3p improved the viability of SH-SY5Y cells treated with MPP+. (c) The apoptosis of MPP+-treated SH-SY5Y cells was inhibited by miR-124-3p. (d) miR-124-3p reduced the Caspase-3 activity in SH-SY5Y cells treated with MPP+. (e) miR-124-3p decreased the LDH activity in SH-SY5Y cells treated with MPP+. (f and g) The level of TNF-α and IL-1β in the supernatant of MPP+-treated SH-SY5Y cells was lowered by miR-124-3p. (h) miR-124-3p prevented ROS generation in SH-SY5Y cells treated with MPP+. (i) miR-124-3p promoted the SOD activity in SH-SY5Y cells treated with MPP+. Significant differences were assessed using one-way ANOVA. Multiple comparisons between the groups were performed using S-N-K method. *P < 0.05.
MPP: methyl phenyl pyridinium iodide.
miR-124-3p directly inhibits STAT3 expression in SH-SY5Y cells
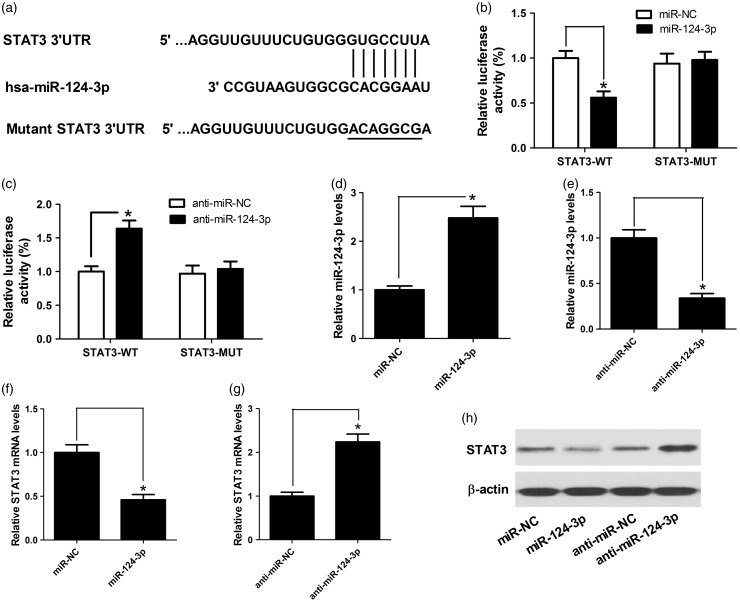
As it is well known, miRNAs exert their functional actions by inhibiting their target genes. Therefore, we searched the online target prediction software for possible miR-124-3p targets. The results revealed that there existed a predicted binding site in the 3″ UTR of STAT3 (Figure 3(a)). In order to further validate that miR-124-3p directly targeted STAT3, the assay of dual luciferase reporter was performed in SH-SY5Y cells co-transfected with STAT3-WT or STAT3-MUT reporter vectors. miR-124-3p overexpression dramatically lowered the luciferase activity of STAT3-WT vector in SH-SY5Y, oppositely, miR-124-3p inhibition remarkedly increased the luciferase activity of STAT3-WT reporter vector, while the activity of STAT3-MUT reporter vectors displayed little change (Figure 3(b) and (c)). miR-124-3p expression was elevated after miR mimics transfection and reduced by anti-miR treatment (Figure 3(d) and (f)), indicating the effectiveness of transfection. Also, as we might expect, miR-124-3p mimics inhibited STAT3 expression, while miR-124-3p inhibition elevated STAT3 level in SH-SY5Y cells at mRNA and protein level (Figure 3(f) to (h)). Taken together, it is suggested miR-124-3p could bind to STAT3.
Figure 3.
STAT3 is a functional target of miR-124-3p. (a) The putative miR-124-3p binding site in the 3′ UTR of STAT3 was shown. (b and c) The relative luciferase activity was determined by luciferase reporter assay in SH-SY5Y cells co-transfected with (miR-124-3p mimics or miR-NC) or (anti-miR-124-3p or anti-miR-NC) and wild or mutant reporter vectors (STAT3-WT or STAT3-MUT). (d and e) qRT-PCR analysis of the miR-124-3p expression in SH-SY5Y cells transfected with miR-124-3p mimics or anti-miR-124-3p. (f to h) qRT-PCR and Western blot analysis the mRNA and protein levels of STAT3 in SH-SY5Y cells transfected with miR-124-3p mimics or anti-miR-124-3p. Significant differences were assessed using Student’s t-test. *P < 0.05.
MPP: methyl phenyl pyridinium iodide.
Restoration of STAT3 expression reverses the neuroprotective effects of miR-124-3p in MPP+-induced SH-SY5Y cell PD model
To illustrate whether miR-124-3p exerted its neuroprotective action in MPP+-induced SH-SY5Y cell PD model by targeting STAT3, SH-SY5Y cells were treated with MPP+, MPP+ + miR-124-3p, or MPP+ + miR-124-3p + pcDNA-STAT3. First, STAT3 expression in pcDNA-STAT3-transfected SH-SY5Y cells was detected by Western blot analysis. STAT3 was overexpressed in SH-SY5Y cells transfected with pcDNA-STAT3, confirming the successful transfection of pcDNA-STAT3 (Figure 4(a)). Then, the restoration of STAT3 expression reversed the protective effect as evidenced by attenuating miR-124-3p-mediated increase in cell viability (Figure 4(b)) and SOD activities (Figure 4(i)), as well as by abrogating miR-124-3p-induced decrease in cell apoptosis (Figure 4(c)), Caspase-3 activity (Figure 4(d)), LDH activity (Figure 4(e)), the expression of inflammatory factors TNF-α and IL-1β (Figure 4(f) and (g)), and ROS generation (Figure 4(h)). In total, miR-124-3p exerted its neuroprotective action in MPP+-induced SH-SY5Y cell PD model by targeting STAT3.
Figure 4.
miR-124-3p attenuated MPP+-induced neuronal injury in SH-SY5Y cells by targeting STAT3. (a) The protein level of STAT3 was elevated in SH-SY5Y cells transfected with pcDNA-STAT3. SH-SY5Y cells were treated with MPP+, MPP+ + miR-124-3p or MPP+ + miR-124-3p + pcDNA-STAT3. (b) pcDNA-STAT3 reversed miR-124-3p-mediated increase in the viability of SH-SY5Y cells treated with MPP+. (c) miR-124-3p-mediated inhibition of the apoptosis of MPP+-treated SH-SY5Y cells was abolished by STAT3 overexpression. (d) STAT3 overexpression attenuated the inhibitory effect of miR-124-3p on the Caspase-3 activity in SH-SY5Y cells treated with MPP+. (e) The decreased LDH activity in SH-SY5Y cells treated with MPP+ by miR-124-3p was improved when STAT3 was overexpressed. (f and g) pcDNA-STAT3 restored the reduced supernatant level of TNF-α and IL-1β in MPP+-treated SH-SY5Y cells caused by miR-124-3p overexpression. (h) miR-124-3p prevented ROS generation in SH-SY5Y cells treated with MPP+, which was reversed by pcDNA-STAT3. (i) Restoration of STAT3 expression improved miR-124-3p-mediated suppression of the SOD activity in SH-SY5Y cells treated with MPP+. Significant differences were assessed using Student’s t-test (a) or one-way ANOVA (b to i). Multiple comparisons between the groups were performed using S-N-K method. *P < 0.05.
MPP: methyl phenyl pyridinium iodide.
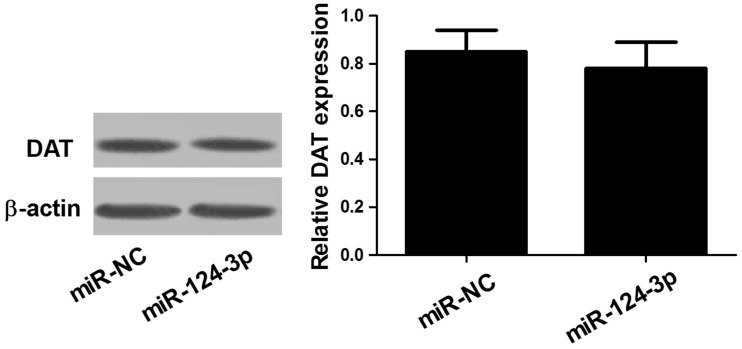
miR-124-3p has no significant effect on DAT expression
DAT could exert the protective effect through decreased cellular uptake. Therefore, the role of miR-124-3p in DAT expression was explored. The results suggested that miR-124-3p did not affect DAT expression (Figure 5), which suggested that the protective effect was mediated through other pathways rather than decreased cellular uptake of MPP+.
Figure 5.
The DAT protein level was not regulated by miR-124-3p. Western blot analysis was performed to detect the protein level of DAT in SH-SY5Y cells transfected with miR-NC or miR-124-3p.
MPP: methyl phenyl pyridinium iodide.
Discussion
In recent years, a number of studies suggested that abnormal miRNA expression is involved in neurodegenerative disorders, including PD. For example, miR-181a was remarkedly decreased in MPP+-induced PD cell model and its overexpression lowered neuron apoptosis and autophagy via suppressing MAPK/JNK pathway.18 Another study reported that enhanced expression of miR-126 contributed to PD by down-regulation of factors in IGF-1/phosphoinositide 3-kinase signaling.19 miR-34b and miR-34c have been reported to contribute to PD pathogenesis.20 All these studies demonstrated the critical role of miRNAs in PD.
Accumulating documents showed that miR-124-3p was abundant in neurons and during CNS development.21,22 Abnormal expression of miR-124 has been revealed to be implicated in many CNS disease,23 including Alzheimer’s disease,24 brain tumor,25 cerebral ischemic stroke,26 and PD.27 However, the functional mechanism of miR-124-3p is not fully illuminated. Our study constructed PD model in vitro by treating SH-SY5Y cell with MPP+. The results showed that miR-124-3p was low expressed in MPP+-induced SH-SY5Y cell. MPP+ treatment led to the increase in cell viability and SOD activity, and the reduction in cell apoptosis, Caspase-3 activity, LDH activity, the expression of inflammatory factors TNF-α and IL-1β, and ROS generation; however, the up-regulation of miR-124-3p reversed the effects. Our study showed miR-124-3p participated in various PD pathological process, including neurotoxicity, neuronal apoptosis, neuroinflammation, and oxidative stress. Consistent with our results, neuroprotective effect of miR-124-3p in PD was reported previously. For instance, miR-124-3p overexpression rescued the damage of MPTP on DA neurons by suppressing apoptosis and autophagy via AMPK/mTOR pathway.28 miR-124 decreased the loss of DA neuron by targeting Bim.15 Oxidative stress and inflammation have been disclosed to play important roles in Parkinson's disease.29
STAT3 has been demonstrated to be significant for cancer progression.30,31 Emerging evidence suggests the involvement of STAT3 in inflammation-associated injury.32,33 In this study, miR-124-3p was verified to target STAT3, in agreement with a previous documents.34 Moreover, the restoration of STAT3 expression abated the neuroprotective effects of miR-124-3p. A recent study demonstrated that nitidine inhibited microglial activation by inactivating Jak2–Stat3 pathway and improved behavioral function in PD animal models,35 coincident with our results. Also, manganese exposure resulted in the activation of JAK2–STAT3 signal pathway in microglia, causing resultant neuroinflammation and neuronal loss.36 Our study demonstrates that miR-124-3p exerts a neuroprotective action in MPP+-induced PD cell model by preventing neurotoxicity, neuronal apoptosis, neuroinflammation, and oxidative stress. More importantly, the inhibitory effect of miR-124-3p on PD pathogenesis is mediated by its functional target STAT3.
Authors’ contributions
Lijiao Geng and Wei Liu contributed equally to this work. Lijiao Geng and Yong Chen designed the experiments. Lijiao Geng and Wei Liu performed the experiments and prepared the manuscript. Yong Chen reviewed and approved the final version of the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Hernandez-Rapp J, Rainone S, Hébert SS. MicroRNAs underlying memory deficits in neurodegenerative disorders. Prog Neuropsychopharmacol Biol Psychiatry 2017; 73:79–86 [DOI] [PubMed] [Google Scholar]
- 2.Saraiva C, Paiva J, Santos T, Ferreira L, Bernardino L. MicroRNA-124 loaded nanoparticles enhance brain repair in Parkinson's disease. J Control Release 2016; 235:291–305 [DOI] [PubMed] [Google Scholar]
- 3.Sarrafchi A, Bahmani M, Shirzad H, Rafieiankopaei M. Oxidative stress and Parkinson's disease: New hopes in treatment with herbal antioxidants. Curr Pharm Des 2016; 22:238–46 [DOI] [PubMed] [Google Scholar]
- 4.Bose A, Beal MF. Mitochondrial dysfunction in Parkinson's disease. J Neurochem 2016; 139:216–31 [DOI] [PubMed] [Google Scholar]
- 5.Feng ZJ, Zhang X, Chergui K. Allosteric modulation of NMDA receptors alters neurotransmission in the striatum of a mouse model of Parkinson's disease. Exp Neurol 2014; 255:154–60 [DOI] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116:281–97 [DOI] [PubMed] [Google Scholar]
- 7.Liu HT, Gao P. The roles of microRNAs related with progression and metastasis in human cancers. Tumor Biol 2016; 37:15383–97 [DOI] [PubMed] [Google Scholar]
- 8.Basak I, Patil KS, Alves G, Larsen JP, Møller SG. microRNAs as neuroregulators, biomarkers and therapeutic agents in neurodegenerative diseases. Cell Mol Life Sci 2016; 73:811–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiu L, Zhang W, Tan EK, Zeng L. Deciphering the function and regulation of microRNAs in Alzheimer's disease and Parkinson's disease. ACS Chem Neurosci 2014; 5: 884–94 [DOI] [PubMed] [Google Scholar]
- 10.Thome AD, Harms AS, Volpicelli-Daley LA, Standaert DG. microRNA-155 Regulates alpha-synuclein-induced inflammatory responses in models of Parkinson disease. J Neurosci 2011; 36: 2383–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho HJ, Liu G, Jin SM, Parisiadou L, Xie C, Yu J, Sun L, Ma B, Ding J, Vancraenenbroeck R. MicroRNA-205 regulates the expression of Parkinson's disease-related leucine-rich repeat kinase 2 protein. Hum Mol Genet 2013; 22:608–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xue Y, Ouyang K, Huang J, Zhou Y, Ouyang H, Li H, Wang G, Wu Q, Wei C, Bi Y. Direct conversion of fibroblasts to neurons by reprogramming PTB-regulated microRNA circuits. Cell 2013; 152:82–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu JY, Chung KH, Deo M, Thompson RC, Turner DL. MicroRNA miR-124 regulates neurite outgrowth during neuronal differentiation. Exp Cell Res 2008; 314:2618–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanagaraj N, Beiping H, Dheen ST, Tay SS. Downregulation of miR-124 in MPTP-treated mouse model of Parkinson's disease and MPP iodide-treated MN9D cells modulates the expression of the calpain/cdk5 pathway proteins. Neuroscience 2014; 272:167–79 [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Ye Y, Zhu Z, Mo L, Lin C, Wang Q, Wang H, Gong X, He X, Lu G. MiR-124 regulates apoptosis and autophagy process in MPTP model of Parkinson's disease by targeting to Bim. Brain Pathol 2016; 26:167–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 2008; 3:1101–8 [DOI] [PubMed] [Google Scholar]
- 17.Shanmugam MK, Manu KA, Ong TH, Ramachandran L, Surana R, Bist P, Lim LH, Prem Kumar A, Hui KM, Sethi G. Inhibition of CXCR4/CXCL12 signaling axis by ursolic acid leads to suppression of metastasis in transgenic adenocarcinoma of mouse prostate model. Int J Cancer 2011; 129:1552–63 [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Song Y, Zhu X. MicroRNA-181a regulates apoptosis and autophagy process in Parkinson’s disease by inhibiting p38 mitogen-activated protein kinase (MAPK)/c-Jun N-Terminal Kinases (JNK) signaling pathways. Med Sci Monit 2017;23:1597–606 [DOI] [PMC free article] [PubMed]
- 19.Kim W, Lee Y, Mckenna ND, Yi M, Simunovic F, Wang Y, Kong B, Rooney RJ, Seo H, Stephens RM. miR-126 Contributes to Parkinson's disease by dysregulating the insulin-like growth factor/phosphoinositide 3-kinase signaling. Neurobiol Aging 2014; 35: 1712–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabaria S, Choi DC, Chaudhuri AD, Mouradian MM, Junn E. Inhibition of miR-34b and miR-34c enhances α-synuclein expression in Parkinson’s disease. FEBS Lett 2014; 589:319–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maiorano NA, Mallamaci A. The pro-differentiating role of miR-124: indicating the road to become a neuron. Rna Biol 2010; 7:528–33 [DOI] [PubMed] [Google Scholar]
- 22.Tapocik JD, Luu TV, Mayo CL, Wang BD, Doyle E, Lee AD, Lee NH, Elmer GI. Neuroplasticity, axonal guidance, and microRNA genes are associated with morphine self-administration behavior. Addict Biol 2013; 18:480–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang S, Luo ZM, Guo XM, Su DF, Xia L. An updated role of microRNA-124 in central nervous system disorders: a review. Front Cell Neurosci 2015; 9:193[PMC][10.3389/fncel.2015.00193] [26041995] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong Y, Wu J, Zhang D, Wan C, Yuan L. The role of miR-124 in drosophila Alzheimer's disease model by targeting delta in notch signaling pathway. Cmm 2015; 15:980–9 [DOI] [PubMed] [Google Scholar]
- 25.Deng D, Wang L, Chen Y, Li B, Xue L, Shao N, Wang Q, Xia X, Yang Y, Zhi F. MicroRNA-124-3p regulates cell proliferation, invasion, apoptosis, and bioenergetics by targeting PIM1 in astrocytoma. Cancer Sci 2016; 107:899–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Y, Gui H, Li Q, Luo ZM, Zheng MJ, Duan JL, Liu X. MicroRNA-124 protects neurons against apoptosis in cerebral ischemic stroke. CNS Neurosci Ther 2013; 19:813–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanagaraj N, Dheen TS, Peng ZF, Srinivasan, Kumar D, Tay, Samuel SW. MicroRNA-124 and its target gene are altered in the substantia nigra (SNc) of the brain of MPTP-mouse model of Parkinson's disease. J Org Chem 2004; 69:3794–800.15153011 [Google Scholar]
- 28.Gong X, Wang H, Ye Y, Shu Y, Deng Y, He X, Lu G, Zhang S. miR-124 regulates cell apoptosis and autophagy in dopaminergic neurons and protects them by regulating AMPK/mTOR pathway in Parkinson's disease. Am J Transl Res 2016; 8:2127–37 [PMC free article] [PubMed] [Google Scholar]
- 29.Phani S, Loike JD, Przedborski S. Neurodegeneration and inflammation in Parkinson's disease. Parkinsonism Relat Disord 2012; 18:S207–9 [DOI] [PubMed] [Google Scholar]
- 30.Pan Y, Wang S, Su B, Zhou F, Zhang R, Xu T, Leventaki V, Drakos E, Liu W, Claret FX. Stat3 contributes to cancer progression by regulating Jab1/Csn5 expression. Oncogene 2016; 36:1069–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren W, Sun Z, Zeng Q, Shuang H, Zhang Q, Jiang L. Aberrant expression of CUL4A is associated with IL-6/STAT3 activation in colorectal cancer progression. Arch Med Res 2016; 47:214–22 [DOI] [PubMed] [Google Scholar]
- 32.Zong S, Zeng G, YF, Peng J, Peng J, Tao Y, Li K, Zhao J. The role of IL-17 promotes spinal cord neuroinflammation via activation of the transcription factor STAT3 after spinal cord injury in the rat. Mediators Inflamm 2014;2014: 786947 [DOI] [PMC free article] [PubMed]
- 33.Shi S, Liang D, Bao M, Xie Y, Xu W, Wang L, Wang Z, Qiao Z. Gx-50 Inhibits neuroinflammation via α7 nAChR activation of the JAK2/STAT3 and PI3K/AKT pathways. J Alzheimers Dis 2016; 50:859–71 [DOI] [PubMed] [Google Scholar]
- 34.Li W, Hui H, Su J, Xin J, Zhang X, Zhang Z, Hong W. miR-124 Acts as a tumor suppressor in glioblastoma via the inhibition of signal transducer and activator of transcription 3. Immunology 1982; 47:503–106215340 [Google Scholar]
- 35.Wang B, Wang X, Yang S, Liu X, Feng D, Lu F, Zhu Y, Lu D, Tao L, Ge S. Neuroprotective effects of nitidine in Parkinson's disease models through inhibiting microglia activation: role of the Jak2–Stat3 pathway. Rsc Adv 2016; 6:71328–37 [Google Scholar]
- 36.Yin L, Dai Q, Jiang P, Zhu L, Dai H, Yao Z, Liu H, Ma X, Qu L, Jiang J. Manganese exposure facilitates microglial JAK2-STAT3 signaling and consequent secretion of TNF-a and IL-1β to promote neuronal death. Neurotoxicology 2017. [Epub ahead of print 4 April]. [DOI] [PubMed] [Google Scholar]