Abstract
Background:
Fish are a source of persistent organic pollutants (POPs) in the human diet. Although species, trophic level, and means of production are typically considered in predicting fish pollutant load, and thus recommendations of consumption, capture location is usually not accounted for.
Objectives:
Yellowfin tuna (Thunnus albacares) are harvested from across the world’s oceans and are widely consumed. Here, we determined geographic variation in the overall mass, concentration, and composition of POPs in yellowfin and examined the differences in levels of several POP congeners of potential relevance to human health.
Methods:
We sampled dorsal muscle of 117 yellowfin tuna from 12 locations worldwide, and measured POP levels using combined liquid or gas chromatography and mass spectrometry according to U.S. Environmental Protection Agency standard procedures.
Results:
POP levels varied significantly among sites, more than 36-fold on a mass basis. Individual fish levels ranged from 0.16 to wet weight and lipid-normalized concentrations from . Levels of 10 congeners that interfere with the cellular defense protein P-glycoprotein, termed transporter interfering compounds (TICs), ranged from 0.05 to wet weight and from in tuna lipid. Levels of TICs, and their individual congeners, were strongly associated with the overall POP load. Risk-based analysis of several carcinogenic POPs indicated that the fish with the highest levels of these potentially harmful compounds were clustered at specific geographic locations.
Conclusions:
Capture location is an important consideration when assessing the level and risk of human exposure to POPs through ingestion of wild fish. https://doi.org/10.1289/EHP518
Introduction
Persistent organic pollutants (POPs) are hazardous environmental chemicals that bioaccumulate in animals and are routinely detected in humans (CDC 2009, 2015). Although global efforts to reduce their production and usage have resulted in corresponding declines in their environmental levels, reservoirs of these pollutants remain in the world’s oceans (Lohmann et al. 2006). Fish can accumulate high amounts of these compounds as compared with other foods (Schecter et al. 2006b, 2010a) and thus consumption of seafood is an important route of human exposure to POPs. A better understanding of the factors governing levels of POPs in fish is essential to predict and reduce exposure.
Since POPs accumulate in fat and are eliminated slowly, the species and trophic level of fish have been previously used as indicators of relative pollutant load (Connolly and Pedersen 1988; Kelly et al. 2007). However, POP levels can also vary dramatically even within a single species or at a given trophic level, limiting the utility of these predictions. For instance, recent food basket surveys have shown that canned sardines, a relatively low trophic level species (Froese and Pauly 2000), can have higher levels of hexabromocyclododecanes (HBCDDs), organochlorine pesticides (OCPs), polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs), and perfluorinated compounds (PFCs) than cod or salmon (Schecter et al. 2010c, 2010b, 2010a). Similarly, whereas it has been shown that pollutant levels also vary by species of fish, with the highest levels detected in oily fish (Knutsen et al. 2008; Smith and Gangolli 2002), differences in POP levels can also be detected within the same species based on means of production. For example, the levels of many POPs were higher in farmed versus wild salmon (Hites et al. 2004a).
A factor that may govern fish pollutant level is the patchy distribution of pollutants in the world’s oceans. For less migratory fish species that remain in single regions for most of their life, this would be reflected in pollutant variation depending on location of capture. For example, high variation in the levels of OCPs, PBDEs, and PCBs were observed in wild skipjack (Ueno et al. 2003, 2004, 2005) depending on where they were captured. However, given the limited geographic scale of these studies, it seems plausible that even greater variation in POP levels would be observed when examining fish harvested from across the world’s oceans. This geographic variation is important to address, because it has broad implications for assessment of health risks associated with POP exposure through consumption of fish.
Tuna are one of the most widely harvested fish in the world and among the top two fish species consumed in the United States, along with salmon (Loke et al. 2012). Among the tunas, yellowfin (Thunnus albacares) are the second most harvested species after skipjack (ISSF 2017). More than 1,200,000 metric tons of yellowfin are caught annually, accounting for 27% of the global tuna catch (ISSF 2017). Yellowfin are relatively large predatory fish, and most are sold as fresh or frozen fillets. Yellowfin are distributed globally, but have shorter ranges than bluefin (Block et al. 2011), and thus might be expected to reflect geographic trends in POP level variation.
Here, we measured POP levels in wild-caught yellowfin tuna from across the world’s oceans and determined their wet weight and lipid-normalized concentrations. From these data we calculated the levels of specific POP congeners that we recently demonstrated to be inhibitors of transporter proteins act as important cellular defense in humans (Nicklisch et al. 2016), termed TICs. The results of this study demonstrate the important role of geographic origin on total pollutant level of fish and reveal a close association between TICs and overall POP load.
Methods
Tuna Collection
Dorsal, white muscle filets of yellowfin tuna (Thunnus albacares) were collected from all four major yellowfin stocks, including the Atlantic Ocean, Eastern Pacific Ocean, Western Pacific Ocean, and Indian Ocean stocks (ISSF 2017). The 12 capture locations (see Figure S1) included the North Pacific Ocean (NPO), North East Pacific Ocean (NEPO), Gulf of Mexico (GOM), South East Pacific Ocean (SEPO), Northwest Atlantic (NWAO), Northeast Atlantic Ocean (NEAO), South East Atlantic Ocean (SEAO), Indian Ocean (IO), South China Sea (SCS), North China Sea (NCS), Northwest Pacific Ocean (NWPO), and the Southwest Pacific Ocean (SWPO). The target size class for sampling was 100 cm, roughly old (Kikkawa and Cushing 2002), and the sampled fish had a mean standard length of , with a range of . Only fork length measurement was available for fish from the IO. Tuna were captured either by trolling or longline. A coordinate for the capture site was recorded for each fish, accurate to within 100 km.
Pollutant Analysis
Tuna muscles were sent to AXYS (AXYS Analytical Services Ltd.) for analysis. POPs were measured according to the following U.S. Environmental Protection Agency (EPA) methods for OCPs: 608, 625, 1625, 8081, 8270; PBDEs: 1614, 625, and PCBs: 1668, 8270. Analysis of OCPs was performed on a DB-5 capillary chromatography column coupled to a high-resolution mass spectrometer (HRMS). The concentrations of all 209 PCB congeners were determined by high-resolution gas chromatography (HRGC) on an SPB-Octyl column coupled to a high-resolution mass spectrometer (HRMS). Analysis of the PBDE congeners was performed using HRGC on a DB-5HT capillary column coupled to an HRMS.
All 117 tuna from the 12 sites were screened for 29 OCPs, 11 of these sites (109 tuna) were also screened for 209 PCBs, and at eight of these sites (79 tuna) were analyzed for nine PBDEs (Tables 1 and 2; see also Figure S1). Total POP levels were defined as the sum of all OCPs, PBDEs, and PCBs measured in fish from the eight sites with full coverage. One fish from the GOM had 4.8-fold higher total POP levels than any other fish and was more than two times the interquartile range within the GOM sample group, and thus was excluded from further analysis.
Table 1.
Panel of persistent organic pollutants (POPs) screened in yellowfin tuna.
| OCPs | PBDEs | PCBs | |||||
|---|---|---|---|---|---|---|---|
| Hexachlorobenzene | PCB-1 | PCB-35 | PCB-73 | PCB-120 | PCB-158 | PCB-194 | |
| HCH, alpha | BDE-47 | PCB-2 | PCB-36 | PCB-77 | PCB-121 | PCB-159 | PCB-195 |
| HCH, beta | BDE-99 | PCB-3 | PCB-37 | PCB-78 | PCB-122 | PCB-161 | PCB-196 |
| HCH, gamma | BDE-100 | PCB-4 | PCB-38 | PCB-79 | PCB-123 | PCB-162 | |
| Heptachlor | BDE-153 | PCB-5 | PCB-39 | PCB-80 | PCB-126 | PCB-164 | |
| Aldrin | BDE-154 | PCB-6 | PCB-81 | PCB-127 | PCB-165 | PCB-201 | |
| Chlordane, oxy- | BDE-183 | PCB-7 | PCB-42 | PCB-82 | PCB-167 | PCB-202 | |
| Chlordane, gamma (trans) | BDE-209 | PCB-8 | PCB-43 | PCB-169 | PCB-203 | ||
| Chlordane, alpha (cis) | PCB-9 | PCB-84 | PCB-130 | PCB-170 | PCB-204 | ||
| Nonachlor, trans- | PCB-10 | PCB-131 | PCB-205 | ||||
| Nonachlor, cis- | PCB-11 | PCB-46 | PCB-132 | PCB-172 | PCB-206 | ||
| 2,4′-DDD | PCB-48 | PCB-133 | PCB-174 | PCB-207 | |||
| 4,4′-DDD | PCB-14 | PCB-89 | PCB-175 | PCB-208 | |||
| 2,4′-DDE | PCB-15 | PCB-176 | PCB-209 | ||||
| 4,4′-DDE | PCB-16 | PCB-52 | PCB-92 | PCB-136 | PCB-177 | ||
| 2,4′-DDT | PCB-17 | PCB-54 | PCB-137 | PCB-178 | |||
| 4,4′-DDT | PCB-55 | PCB-94 | PCB-179 | ||||
| Mirex | PCB-19 | PCB-56 | PCB-96 | PCB-141 | |||
| HCH, delta | PCB-57 | PCB-103 | PCB-142 | PCB-181 | |||
| Heptachlor epoxide | PCB-58 | PCB-104 | PCB-144 | PCB-182 | |||
| alpha-Endosulfan | PCB-22 | PCB-105 | PCB-145 | ||||
| Dieldrin | PCB-23 | PCB-60 | PCB-106 | PCB-146 | PCB-184 | ||
| Endrin | PCB-24 | PCB-186 | |||||
| beta-Endosulfan | PCB-25 | PCB-63 | PCB-109 | PCB-148 | PCB-187 | ||
| Endosulfan sulfate | PCB-64 | PCB-150 | PCB-188 | ||||
| Endrin aldehyde | PCB-27 | PCB-66 | PCB-111 | PCB-152 | PCB-189 | ||
| Endrin ketone | PCB-31 | PCB-67 | PCB-112 | PCB-190 | |||
| Methoxychlor | PCB-32 | PCB-68 | PCB-114 | PCB-155 | PCB-191 | ||
| Technical toxaphene | PCB-34 | PCB-72 | PCB-118 | PCB-192 | |||
Note: Co-eluting congeners are preceded with a plus symbol. The previously identified (Nicklisch et al. 2016) transporter interfering compounds (TICs): 4,4′-DDD, 4,4′-DDE, 4,4′-DDT, Dieldrin, Endrin, BDE-47, BDE-100, PCB-146, PCB-170, PCB-187.
Table 2.
Metadata on wild yellowfin tuna analyzed in this study.
| Site | Samples [] | Standard Length [] | Lipids [%] | OCPs [ng/g ww] | PBDEs [ng/g ww] | PCBs [ng/g ww] | Total POPs [ng/g ww] | Total POPs [] | TICs [] | TIC fraction [%] |
|---|---|---|---|---|---|---|---|---|---|---|
| North Pacific Ocean (NPO) | 10 | NA | NA | NA | NA | NA | ||||
| Northeast Pacific Ocean (NEPO) | 10 | 42 | ||||||||
| Gulf of Mexico (GOM) | 8 | 23 | ||||||||
| Southeast Pacific Ocean (SEPO) | 10 | NA | NA | NA | NA | NA | ||||
| Northwest Atlantic Ocean (NWAO) | 10 | 27 | ||||||||
| Northeast Atlantic Ocean (NEAO) | 10 | 24 | ||||||||
| Southeast Atlantic Ocean (SEAO) | 8 | NA | NA | NA | NA | NA | NA | |||
| Indian Ocean (IO) | 10 | a | 54 | |||||||
| South China Sea (SCS) | 10 | 50 | ||||||||
| North China Sea (NCS) | 10 | NA | NA | NA | NA | NA | ||||
| Northwest Pacific Ocean (NWPO) | 10 | 30 | ||||||||
| Southwest Pacific Ocean (SWPO) | 10 | 32 |
Note: Listed are the means of standard length and weight % of total lipids in tuna. The sum of the means of OCPs, PBDEs, PCBs, and total POPs for each site is shown in ng/g wet weight. In addition, the sum of the means of total POPs and the 10 transporter-inhibiting compounds (TICs) are listed as micromolar (μM) concentration in the lipid phase. The micromolar fraction of the 10 inhibitors relative to total POPs is listed in percent. Where compounds were not measured values are indicated as not available (NA).
Fork length.
In figures where individual POPs were assigned to different groups, we grouped PCBs according to their backbone chlorination into mono-, di-, tri-, tetra-, penta-, hexa-, hepta-, octa, and nonachlorinated biphenyls. The OCP congeners were grouped as follows: DDT (4,4′-DDT, 2,4′-DDT, 4,4′-DDE, 2,4′-DDE, 4,4′-DDD, 2,4′-DDD, methoxychlor); Chlordane (oxy-chlordane, , , trans-nonachlor, cis-nonachlor); endosulfan (, , endosulfan sulfate, heptachlor, heptachlor epoxide); endrin (Aldrin, dieldrin, endrin, endrin aldehyde, endrin ketone); HCH (, , , ). Hexachlorobenzene (HCB), mirex, and toxaphene were used as single compounds.
Statistics and Data Analysis
Blank correction and analyses of pollutant raw data were performed according to previous studies (Hites et al. 2004b; Schecter et al. 2006b). Briefly, where sample values were below the detection limit the values were treated as nondetectable. Most blank measurements were at or below the detection limits and thus not subtracted from samples. In runs where blank values were above detection limits, only samples with values more than twice the blank value were reported (after subtraction of the blank value). The remaining values were treated as nondetectable. The nondetectable values were assigned zero when calculating the molar concentrations of POPs in the lipid fraction of the muscle tissue, and for further statistical analysis, so as not to overestimate the level of pollutant in samples.
To relate the measured POP levels in tuna to effective concentrations of transporter inhibiting compounds (TICs) from our previous study (Nicklisch et al. 2016), we calculated the lipid-normalized levels of total POPs and the 10 TICs across sites (Table 2), assuming a lipid mass density of 1 (Young 1986). Sample tissue lipid content was determined gravimetrically (AXYS Analytical Services Ltd.) and expressed as percent of sample weight. For each normalization, we used the respective congener molecular weight derived from the National Institutes of Health (NIH) Pubchem database (https://pubchem.ncbi.nlm.nih.gov/).
To meet assumptions of normality and homogeneity of variance, all data were . For this, sample distributions were initially analyzed using JMP’s normal quantile plots and then to approximate the normal distribution. Analysis of variance (ANOVA) was then performed to test the null hypotheses of equal means between groups. A post hoc Tukey honest significant difference (HSD) test was conducted to identify significant pairwise differences between these groups. We also validated the significance of differences between medians using a Kruskal-Wallis rank sum test on the nontransformed data. Data were analyzed and plotted using JMP Pro version 12 (http://www.jmp.com) and OriginPro 2016 (http://www.originlab.com).
A principal components analysis was conducted to explore covariance among TICs (Nicklisch et al. 2016) and the total POP levels in 78 fish for which all 247 POPs were measured (see Figure S1). We performed the PCA on a covariance matrix because all our variables had the same units of measure, and their log-transformed data were normally distributed, with similar scales and ranges. Nondetects were treated as zero values. To address any potential effect of nondetects (i.e., missing values), we used the restricted maximum likelihood (REML) estimation method for calculating covariance according to JMP Pro version 12. The TIC endrin was detected in only three fish from the NEAO and was thus excluded from PCA analysis.
Risk-Based Consumption Advisory Calculations
We used the U.S. EPA’s modified approach for multiple contaminants in a single fish species to calculate consumption limits based on the carcinogenic effects of several POPs, including total PCBs, dieldrin, and toxaphene (Hites et al. 2004a; U.S. EPA 2000). Consumption limits were calculated for 108 tuna from the 11 sites (see Figure S1) where all three of these pollutant classes were measured. Current cancer slope factors (CSFs) were derived from the U.S. EPA’s Integrated Risk Information System (IRIS) database, searched through the NIH Toxnet data network (https://toxnet.nlm.nih.gov/). For PCBs, the more conservative upper-bound slope factor of 2 (mg/kg body weight)/d was chosen. Oral slope factors for dieldrin and toxaphene were 16 and 1.1 (mg/kg body weight)/d. For all monthly consumption limit calculations, we used the measured contaminant concentrations in tuna muscle in mg/kg, an acceptable risk level (ARL) of , an adult consumer body weight of 70 kg, and an average uncooked fish meal size of 227 g (8 oz).
For the nutritional recommendation thresholds, we used the Food and Drug Administration (FDA) and the U.S. EPA joint fish consumption advice (FDA 2014) to consume (12 oz) of cooked fish a week (DHHS, USDA 2015) the diet recommendation from the American Heart Association (AHA) to eat fish at least two times per week with a single serving size of (3.5 oz) of cooked fish (AHA 2015; Kris-Etherton et al. 2002). We used a conversion factor of 1.33 to convert the mass of cooked fish to a corresponding mass of uncooked fish (Minnesota Department of Health 2016). Monthly dietary intake recommendations were calculated based on an average of 4.33 weeks per month.
Results
Tuna Pollutant Levels Depend on Geographic Origin of Fish
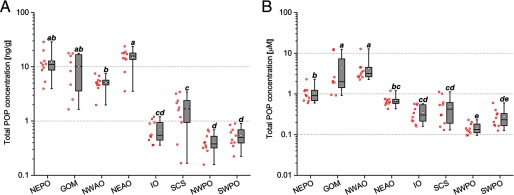
We observed large variation in pollutant levels among the tuna. On a mass basis, mean total pollutant levels varied by a factor of 36 between sites (Figure 1A) and were significantly different across sites [, , with degrees of freedom ]. In general, tuna caught from the four sites in the offshore waters of North America and Europe had total POP concentrations that were on average more than an order of magnitude higher than in fish from the four sites located in the waters of Asia and Oceania (Figure 1A; see also Figure S1). Yellowfin from the NWPO and SWPO tended to be the least contaminated fish, whereas the highest levels of POPs were observed in the NEPO, GOM, and NEAO. Given that POPs are hydrophobic and predominantly accumulate in fat, we also calculated the lipid-adjusted concentrations of total POPs for the tuna from all eight capture locations (Figure 1B). As with pollutant mass, there were significant geographic differences in total POP concentrations across all sites [, , with ].
Figure 1.
Variation of POP levels in wild yellowfin tuna (Thunnus albacares) captured at different locations across the globe. (A) Levels of total POPs across eight capture locations on a mass basis. (B) Lipid-normalized total POP concentrations across the eight sites. Whiskers represent the minimum and maximum values. Diamonds represent the mean values. The horizontal line represents the 50th percentile, and the box represents the 25th and 75th percentiles. The red hollow spheres to the left of each box plot display the individual fish values. Letters in parenthesis represent subgroups of the sample population with means that were significantly different from each other upon and using Tukey’s post hoc analysis. Note: GOM, Gulf of Mexico; IO, Indian Ocean; NEAO, Northeast Atlantic Ocean; NEPO, Northeast Pacific Ocean; NWAO, Northwest Atlantic Ocean; NWPO, Northwest Pacific Ocean; SWPO, Southwest Pacific Ocean.
Among individual fish, the mass-based levels in the most and least contaminated tuna varied by a factor of 180 (see Figure S2A). The 10 most polluted fish were caught in the NEAO, NEPO, and GOM, with total POP levels of the top 3 fish ranging from 20 to about wet weight. In contrast, the 10 least contaminated fish were all from the IO, SCS, NWPO, and SWPO and had total POP levels ranging from 0.2 to wet weight, orders of magnitude lower than the highest fish (see Figure S2A). Similarly, the lipid-normalized total POP concentrations in individual tuna varied more than 130-fold, ranging from in the NWPO to in the NWAO (see Figure S2B).
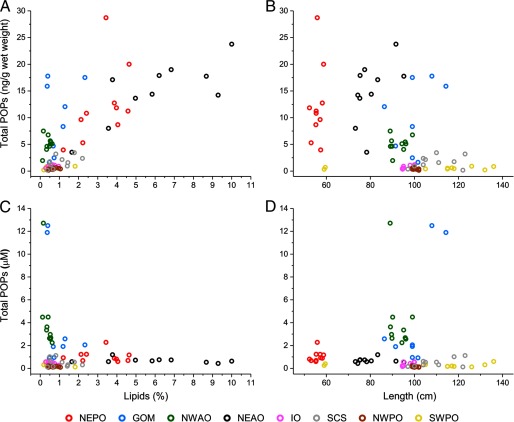
Fish with higher fat percentages generally had higher amounts of POPs on a mass basis (Figure 2A). This was most evident when comparing fish caught in the NEPO and NEAO, which showed higher total pollutant levels with an increase in the lipid content (Figure 2A). However, the correlation of pollutant mass with lipid content was modest () and was not always indicative of total POP load. For instance, fish caught in the GOM had average lipid levels of 0.93% and total POP concentrations of wet weight, which were significantly higher () than fish from the SCS with similar lipid concentrations of 1.09% with total POP levels of only wet weight (Table 2).
Figure 2.
Relationships between fish length or lipid content and the levels or concentrations of POPs. (A) POP levels, on a mass basis, of each fish versus lipid content. (B) POP levels, on a mass basis, of each individual fish against length. (C) POP concentrations of each individual fish against lipid content. (D) POP concentrations of each individual fish against length.
POP levels were also largely independent of the size of fish (; Figure 2B). Individuals from the NEPO, NEAO, and GOM showed the greatest variations in total pollutant levels while having the lowest variations in standard lengths (Figure 2B). For instance, fish caught from the NEPO had the shortest mean standard lengths of 56 cm, but total POP levels of wet weight. In contrast, fish caught in the SWPO were almost double this size, with a mean standard length of 108 cm, yet had total levels of , wet weight (Table 2).
An important observation was that when the POP levels were lipid normalized, the resulting concentrations across fish showed even greater independence from both lipid content (; Figure 2C) and length (; Figure 2D). Indeed, fish caught from the GOM and NWAO had the highest POP concentrations, despite having the lowest lipid content. For example, two fish from the GOM and one fish from the NWAO had total POP concentrations of 11.9, 12.5, and while having lipid levels of 0.36%, 0.39%, and 0.16% (Figure 2C; see also Figure S2B).
Differences in the Contribution of POP Classes to Total POP Levels
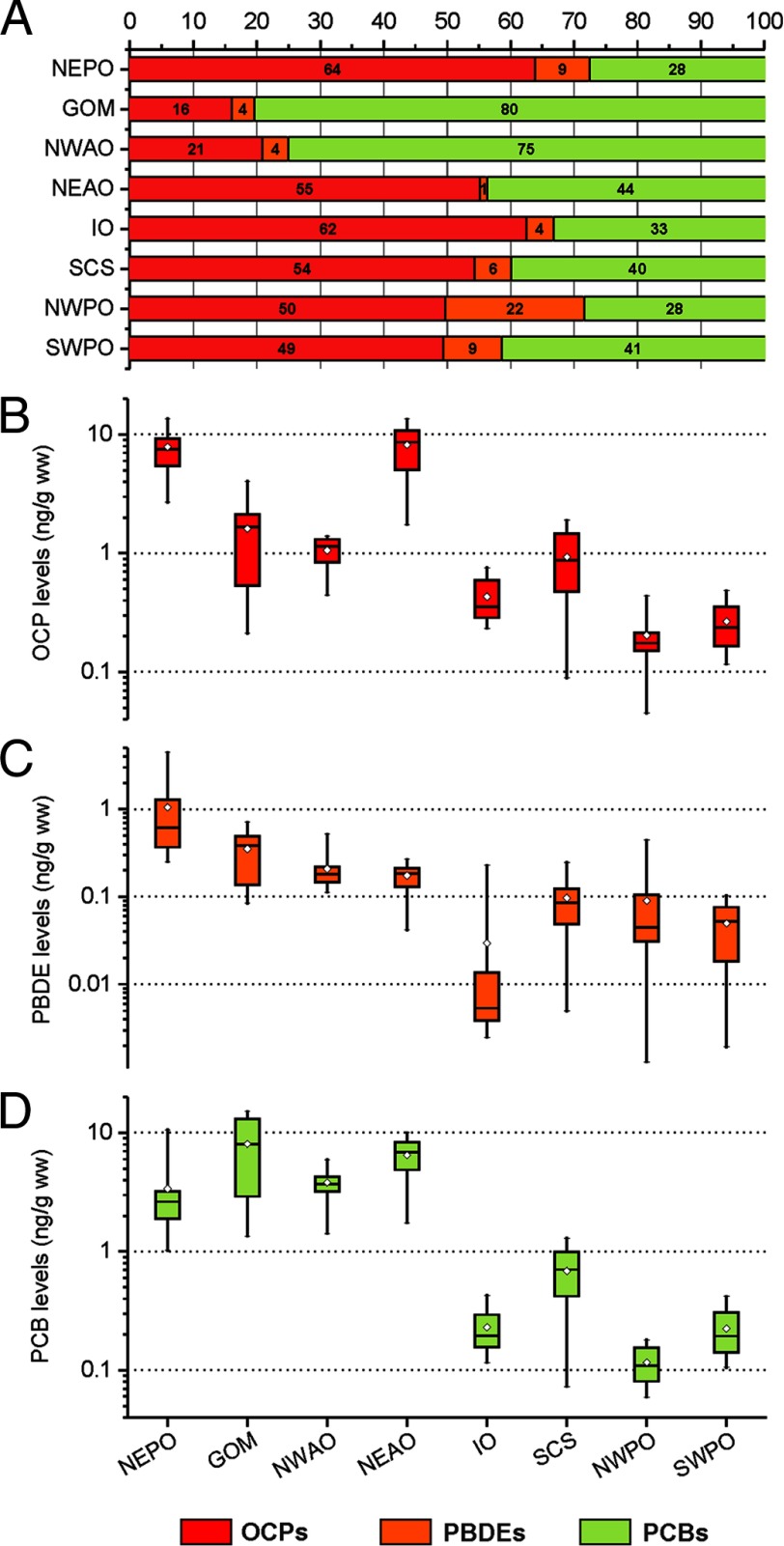
Next we examined the contributions of OCPs, PBDEs, and PCBs to the observed differences in total POP levels across the eight sites for which all three pollutant classes were measured (Table 2; see also Figure S1). With the exception of the NWAO and GOM, the relative ratios of the three POP classes were similar across sites (Figure 3A). PBDEs typically accounted for only a small fraction of the total pollutant load (). In the SWPO, NWPO, SCS, IO, NEAO, and NEPO, about 49–64% of the total POP levels were attributed to OCPs, whereas PCBs contributed (Figure 3A). However, in two of the four more polluted sites, about 75% (NWAO) and 80% (GOM) of the total POPs were PCBs. When comparing pollutant levels across sites, the geographic variation pattern observed for total POP levels was mostly conserved for PCBs and PBDEs, whereas OCP levels varied independently of the overall POP load (Figure 1A and Figure 3B–D).
Figure 3.
Geographic distributions and relative contributions of pollutant classes (OCPs, PBDEs, and PCBs) to total POP levels. Percentage contribution of OCPs, PBDEs, and PCBs to the total POP level (A) and ranges of concentrations of the 29 OCPs (B), 9 PBDEs (C), and 209 PCBs (D) in ng/g wet weight for tuna from eight sampling sites. Whiskers represent minimum and maximum values for each site. Diamonds represent the mean values. The horizontal line represents the 50th percentile, and the box represents the 25th and 75th percentiles.
Specific Pollutant Congeners Account for a Large Fraction of the OCPs and PBDEs at Certain Sites
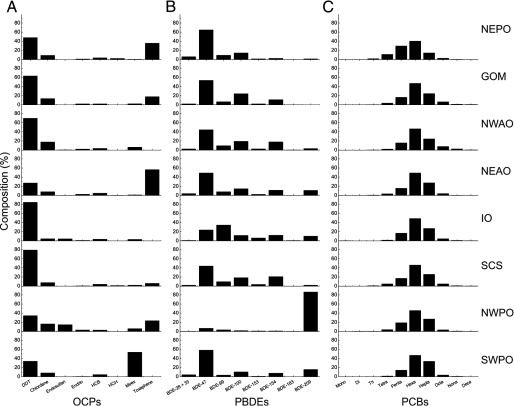
For both OCPs and PBDEs, the congeners comprising each group varied by location (Figure 4A,B; see also Figure S3C). For example, among OCPs, the major contributor to the total levels were typically the DDTs, representing 48–88% of the total OCP levels across nine sites (Figure 4A; see also Figure S3C). However, in the NEAO and the SWPO, toxaphene (56%) and mirex (53%) had the highest relative fractions of total OCPs (Figure 4A). Similarly, among PBDEs, the single congeners BDE-47 (44–65%) and BDE-100 (14–26%) were the major contributors among the most contaminated sites in the NEPO, GOM, NWAO, and NEAO (Figure 4B). However, in the least contaminated sites, the NWPO fish had 86% of the total PBDEs contributed by BDE-209, whereas in the IO the major contributor was BDE-99 (34%).
Figure 4.
Relative contributions of individual congeners to the total of each pollutant class. The percentage of each congener group relative to the total levels of OCPs (A), PBDEs (B), or PCBs (C). The POP compounds were grouped according to their occurrence in technical mixtures or common chemical structures. Due to co-elution of BDE-28 and BDE-33, the sum of both congeners is shown. The PCB congeners were grouped according to their amount of backbone chlorination. Note: DDT, dichloro-diphenyl-trichloroethane; HCB, hexachlorobenzene; HCH, hexachlorocyclohexane.
The exception to this variation in congener levels by location was seen in the PCBs, which when grouped according to their backbone chlorination showed no apparent site-specific difference in relative ratios. For instance, the combined group of hexa-chlorinated biphenyls () had the highest relative contribution to the total PCB levels, ranging from 40% to 51% across all 11 sites (Figure 4C; see also Figures S1 and S3D). These constant ratios of grouped PCB congeners were independent of the total PCB levels (Figures 3D and 4C; see also Figure S3B,D), perhaps reflecting global dispersion of PCBs and/or similar abiotic or biotic metabolism of these congeners.
Levels of TICs
Although persistent pollutants are typically present in fish at low levels, it is well known that chronic low-level POP exposure can have unanticipated effects, such as endocrine disruption or reproductive toxicity. One of these effects could be an adverse impact on the cell protection mechanisms operating in humans (and other animals). P-glycoprotein (P-gp) is a protective drug transporter that can bind a wide range of small hydrophobic molecules (Aller et al. 2009; Gottesman et al. 2002). It is typically expressed at the environmental barrier sites of animals, such as the intestine and gills, where it acts to keep harmful substances out of the body (Döring and Petzinger 2014; Sturm and Segner 2005). Interestingly, although P-gp binds some POPs (Nicklisch et al. 2016), it appears to be relatively ineffective at eliminating them, as evidenced by their ready bioaccumulation. These TICs could reduce the effectiveness of P-gp (Nicklisch et al. 2016).
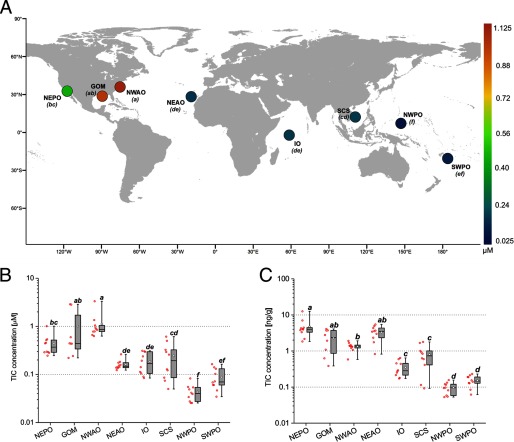
To determine environmental levels of TICs, we examined how their concentrations differed among sites (Figure 5A and Table 2). Similar to the lipid-normalized total POP concentrations, TIC concentrations were significantly different among sites [, , with ], with the lowest mean levels of detected in the NWPO and the highest mean concentrations of in the NWAO (Figure 5B). The average TIC concentrations varied as much as 28-fold across all sites (Figure 5B and Table 2). Among individual fish, the top 10 most polluted fish were from the NWAO, GOM, and NEPO, whereas the 10 least contaminated fish came from the NWPO, SWPO, and SCS (see Figure S2C). The three most contaminated fish had TIC levels ranging from 2.83 to about . On a mass basis, the geographic variation of TIC levels was also significant [, , with ] and highly similar to the total POPs (Figures 1A and 5C). Mass-based TIC levels from the most contaminated to the least contaminated fish varied almost 240-fold, ranging from 0.05 to about wet weight (see Figure S2D).
Figure 5.
Levels of transporter interfering compounds (TICs) in yellowfin tuna. (A) Sampling locations for 78 wild yellowfin tuna, color-coded for the lipid-normalized average concentration () of the 10 previously identified transporter interfering chemicals (Nicklisch et al. 2016). Letters in parenthesis represent subgroups of the sample population with means that were significantly different from each other upon and using Tukey’s post hoc analysis. Box and whisker graphs represent the lipid-normalized (B) and mass-based (C) ranges of concentrations of the P-gp interfering chemicals across the eight capture locations. Note: GOM, Gulf of Mexico; IO, Indian Ocean; NEAO, Northeast Atlantic Ocean; NEPO, Northeast Pacific Ocean; NWAO, Northwest Atlantic Ocean; NWPO, Northwest Pacific Ocean; SWPO, Southwest Pacific Ocean.
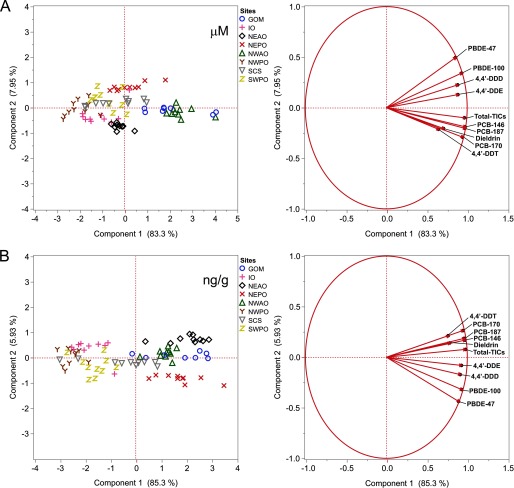
A principal components analysis of the concentrations of individual TICs and the remaining total POP concentrations (Total-TICs) revealed that 83.3% (lipid normalized) and 85.3% (mass based) of the variability was described along the first principal component (PC1) axis (Figure 6A,B). Most notable was the pattern of positive correlation across both regions and individual fish, reflecting high co-variation of the identified TICs with each other, and with the sum of the remaining POPs. Regions were largely distributed along a POP concentration gradient, with lower total POP levels (NWPO, SWPO, SCS, IO) negatively associated with PC1, whereas regions with higher POP levels (NEPO, GOM, NWAO) were positively associated with PC1.
Figure 6.
Covariation of individual TICs with the sum of the remaining POPs. Shown are the score (left panel) and loading (right panel) plots from the principal component analysis (PCA) on the TICs detected in tuna and the remaining total POPs (Total-TICs) for all 78 yellowfin tuna. Covariation of each TIC with the remaining POP levels (Total-TICs) could be observed for both lipid-normalized (A) and mass-based (B) data.
Impact of Geographic Variation on Risk-Based Consumption Levels
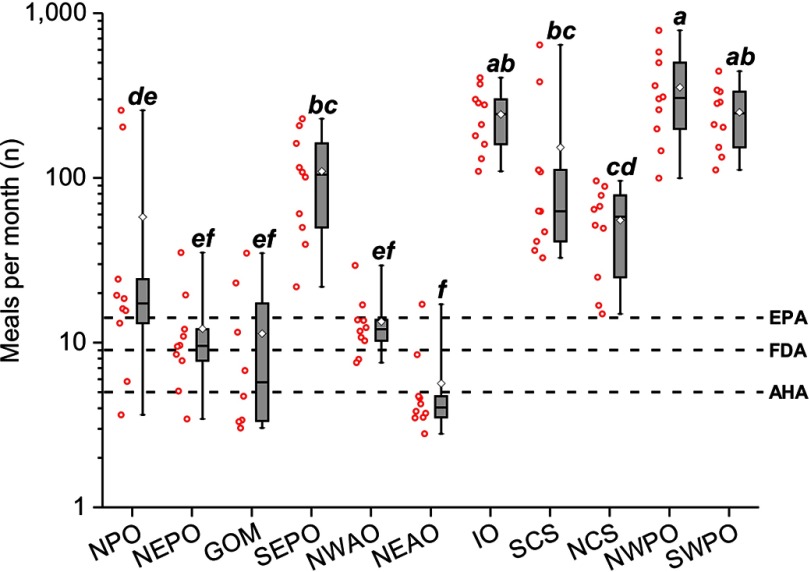
To better understand the impact of geographic variation in POP levels on potential health risks through fish consumption from different sites, we took the approach of Hites et al. (2004a), which explored the cancer risk of PCBs, toxaphene, and dieldrin in farmed versus wild salmon. The results indicated that the large variation in total POP levels would also be reflected in significant differences [, , with ] in potential meal recommendations of tuna among sites (Figure 7). Importantly, the most restrictive consumption advisories were clustered at certain sites. For instance, for 9 of 10 fish in the NEAO the calculated meal consumption advice was below the AHA or FDA recommended monthly advice on nutritional fish intake (Figure 7). Similarly, five of eight fish from the GOM had contaminant levels that might trigger advice for fish consumption limits below the minimum recommended dietary intake.
Figure 7.
Impact of geographic variation on risk-based fish consumption advisories. Ranges of risk-based consumption limits for 11 sites, calculated in meals per month and based on multiple contaminant exposure with cancerogenic health endpoints, including total PCBs (), toxaphene and dieldrin. The red hollow spheres to the left of each box plot display the individual fish values. Letters in parenthesis represent subgroups of the sample population with means that were significantly different from each other using Tukey’s post hoc analysis. The U.S. Food and Drug Administration (FDA) and American Heart Association (AHA) recommended minimum monthly fish consumption levels and the U.S. Environmental Protection Agency (EPA) threshold for unrestricted () fish meals per month are shown as dashed lines. Note: GOM, Gulf of Mexico, IO, Indian Ocean; NCS, North China Sea; NEAO, Northeast Atlantic Ocean; NEPO, Northeast Pacific Ocean; NPO, Northern Pacific Ocean; NWAO, Northwest Atlantic Ocean; NWPO, Northwest Pacific Ocean; SCS, South China Sea; SEPO, Southeast Pacific Ocean; SWPO, Southwest Pacific Ocean.
Calculated meal recommendations based on the levels measured in individual fish varied from as many as 787 meals/mo in one fish from the NWPO down to only 3 meals/mo in fish from the NEAO, GOM, NEPO, and NPO (Figure 7). Although recommended meals per month were unrestricted (i.e., ) (U.S. EPA 2000) for 67% of the tuna, 20% of the fish had recommended meal levels below FDA’s nutritional value advice for pregnant and breastfeeding women of (FDA 2014; DHHS, USDA 2015). Importantly, 13% of the fish had levels of POPs that resulted in meal levels at or below the AHA’s recommended level of
Discussion
This study demonstrates that pollutants in tuna can vary dramatically by location of capture, with implications for managing the risk of human exposure to harmful chemicals in fish. These dramatic differences likely ensue from a multitude of factors including historical deposition, atmospheric transport effects and/or local circulation patterns (Atlas and Giam 1981; Beyer et al. 2000; Jepson and Law 2016; Parnell et al. 2008; Santschi et al. 2001; Welsh and Inoue 2000). Our results indicated that simple indirect measures such as species, size, or lipid content may not necessarily be adequate to predict the differences in total levels of pollutants among fish. On a mass basis total POP levels were only modestly () correlated with lipid content and poorly correlated with size (). The correlation between lipid levels and POP content was even less robust when levels were calculated on a concentration basis. For instance, three fish with the highest POP concentrations had among lowest lipid levels (Figure 2C). However, we noticed that these fish also had high levels of PCBs, which could indicate that PCBs have a higher equilibrium concentration in tuna fat as compared to OCPs or PBDEs, and/or that a combination of exposure period, bioavailability, and pollutant distribution patterns act to enhance PCB uptake (Morgan and Lohmann 2010; van der Oost et al. 1996; Verweij et al. 2004).
Potential Sources of POPs in Yellowfin Tuna
Organochlorine pesticides.
The DDT group was largely the most prominent contributor of total OCPs across all tested sites. In the NEPO and the NEAO about 64% and 55% of the total POP levels of 12.3 and wet weight were attributed to OCPs, leading to a highly similar OCP load in these fish of 7.9 and wet weight (Table 2). Close to the site of catch in the NEPO, an estimated of DTT were discharged into the Palos Verde Shelf between the late 1950s and early 1970s (Eganhouse and Pontolillo 2008). The second highest levels of OCPs were detected in fish from the NEAO. This site of catch was close to the Canary Islands and, geographically, this archipelago is part of the African continent where DDT is still used to combat proliferation of the Anopheles mosquito that transmits malaria (UNEP 2001; Wandiga 2001). Similarly, the sub-Saharan region in the SEAO had relatively high OCP levels of wet weight, with the DDT group being the most prominent contributor (see Figure S3C). Among all 12 sites, the major contributor to total DDT levels was the metabolite 4,4′-DDE, which is in agreement with previous studies in other fish species (Covaci et al. 2006; Ueno et al. 2002, 2003).
Polybrominated diphenyl ethers.
In 1999, about 98% of the global use of Penta-PBDEs was in the United States, including the highly bioaccumulative congeners PBDE-47, -85, -99, -100, -153, and -154 (Hale et al. 2003), five of which are among the nine detected congeners in our survey. Since we measured a less migratory fish species, this could indicate that most of the penta-PBDE congeners produced and used in the United States dispersed globally. Our total PBDE levels ranged from 0.03 to wet weight and were comparable with the average values of the sum of all nine PBDEs in previous studies on U.S. fish samples (Schecter et al. 2006b, 2010c, 2010b). Among PBDEs, the major contributors in the most contaminated sites were PBDE-47 and PBDE-100. This is a similar finding to previous studies, also showing that PBDE-47 is the dominant congener in fish samples (Guo et al. 2008; Hites et al. 2004b; Schecter et al. 2010b). One exception was seen in a fish from the less contaminated site of NWPO, where nearly all the PBDE load was attributed to a single congener, PBDE-209. This is notable because PBDE-209 is not thought to accumulate in organisms due to its ability to readily metabolize into lower brominated congeners (Guo et al. 2008; Hale et al. 2003; La Guardia et al. 2007).
Polychlorinated biphenyls.
We found that the absolute levels of PCBs were highest in the NEAO ( wet weight) and the GOM (), the latter possibly being influenced by depositions from the Mississippi River (Santschi et al. 2001; Zhang et al. 2007) and/or atmospheric deposition (Dachs et al. 2002; Lohmann et al. 2007; Lohmann and Belkin 2014). Similarly, despite being banned in most of Europe since 1985 (European Communities 1985), PCB levels in fish tissue and eggs of the North and Baltic Seas are still high (Fliedner et al. 2012; Karl and Lahrssen-Wiederholt 2009), possibly explaining the high levels in the NEAO. Indeed, more recent studies in mammals and birds near the Canary Islands support our data from the NEAO, showing that PCB levels in local species are high (García-Alvarez et al. 2014; Luzardo et al. 2014).
To our knowledge this study is the first to examine all 209 PCB congeners in a large number of capture location controlled fish samples. Most previous studies on PCB levels in fish and seafood determined only a limited amount of individual PCB congeners or the sum of them (Bocio et al. 2007; Domingo and Bocio 2007; Sagratini et al. 2008; Shen et al. 2009; Storelli et al. 2003, 2008; Weijs et al. 2010). Our study revealed that among PCBs, the groups of hexa- and hepta-chlorinated PCBs had the highest relative contribution across all sites, in agreement with former studies in other fish species (Batang et al. 2016; Covaci et al. 2006; Storelli et al. 2012; Ueno et al. 2003). In addition, the pattern of polychlorinated congeners almost follows a normal distribution, despite the fact that these congeners had different ratios in the commercial Aroclor mixtures (ATSDR 2000; Frame et al. 1996). This might indicate that penta- (PCB-82 to -127), hexa- (PCB-126 to -169), and hepta-chlorinated (PCB-170 to -193) PCBs are more accumulative than lower chlorinated PCBs, including tetra-, tri-, di-, and mono-chlorinated compounds.
Health Effects of Pollutants in Fish
Many POPs examined in our study have well known acute and chronic toxic effects. We investigated three distinct chemical classes of POPs, with organochlorine pesticides (OCPs) being the most heterogeneous class in terms of their chemical structures, properties, and commercial application. Exposure to the OC pesticide DDT has been linked to a variety of adverse human health effects (Rogan and Chen 2005). Acute exposure to DDT can cause seizures and tremors (Costa et al. 2008), whereas long term exposures to DDT can induce liver and breast cancer (Cohn et al. 2007; Persson et al. 2012). In addition, reproductive and developmental defects have been reported for long term exposure to DDT and its metabolites DDE and DDD, including reduced fertility (Cocco et al. 2005), reduced duration of lactation (Karmaus et al. 2005), and reduced birth weight (Longnecker et al. 2001).
The other two classes of POPs investigated in this study, PBDEs and PCBs, belong to the group of poly halogenated compounds. Their structural similarity to the thyroid hormones triiodothyronine (T3) and thyroxine (T4) is thought to be responsible for their pronounced endocrine-disrupting effects, causing developmental, neurological, and behavioral alternations in humans (Boas et al. 2006, 2012; Diamanti-Kandarakis et al. 2009). In addition, the 12 PCB congeners with dioxin-like (co-planar) structure (ATSDR 2000) are known to cause a broad range of adverse health effects ranging from simple chloracne to serious reproductive abnormalities and immune deficiencies (Schecter et al. 2006a).
TICs in Yellowfin Tuna
Among the major contributors to the total pollutant levels were the compounds that we previously identified as inhibitors of an important cell defense protein (Nicklisch et al. 2016). Transporters have already been demonstrated to be important proteins in determining the uptake of hydrophobic xenobiotics such as pharmaceuticals into the human body (Ambudkar et al. 1999; International Transporter Consortium et al. 2010; Leslie et al. 2005), and they may be equally important for understanding the behavior of environmental chemicals in humans and other animals (Epel et al. 2008; Kurelec 1992, 1997; Kurth et al. 2015; Smital et al. 2004). The observation that some persistent organic pollutant congeners could act as inhibitors of these proteins suggests that real world pollutant mixtures, containing high levels of TICs, might act to enhance the accumulation of chemicals that would otherwise be eliminated.
In our previous in vitro studies, TIC mixtures inhibited human and mouse P-gp at levels of 7 and (Nicklisch et al. 2016). To extrapolate these results to the levels observed in fish, we calculated the lipid-normalized levels of the sum of total POPs and the transporter-inhibiting compounds. Notably, among the most contaminated fish, 3 had TIC concentrations in excess of , and 18 fish were above , representing 23% of all the fish caught. It is important to note that our results could underestimate total TIC load because the tuna also had many other POPs for which transporter interactions are presently unknown, with total concentrations of and because there are other potential TICs that we did not measure in this study (Bain et al. 1997; Bircsak et al. 2013; Dankers et al. 2013; Luckenbach and Epel 2005; Sreeramulu et al. 2007). Thus, these results suggest that there might be a relatively narrow margin of safety for exposure to TICs from highly contaminated fish, particularly in vulnerable populations.
The principal component analysis of the inhibitory POP congeners showed that fish contaminated with any of these single compounds was also likely to have a high overall load in total POPs. The mechanisms for this association are not completely understood. One possibility is that this could simply reflect their co-variation with the other pollutants in the environment. An alternative explanation, related to their effect on P-gp, could be that exposure to these TICs somehow promotes the accumulation or slows the elimination of other POPs (Epel et al. 2008; Kurelec 1997; Smital and Kurelec 1997; Sreeramulu et al. 2007). However, given that most studies to date identify POPs as inhibitors rather than substrates of transporters, it is as yet unclear how these mixture effects would occur. Future studies, to identify environmental substrates, and examine the effect of TIC exposure on substrate uptake, are needed to better answer this question.
Implications of Geographic Variation for Minimizing Human Exposure to Harmful Chemicals through Fish
Regional differences in POP levels could have important implications for efforts to reduce human exposure. Although variation among different species has prompted advisories to limit consumption of certain types of fish, geographic effects within a species are usually not accounted for in these advisories. Consistent with the differences in total POP levels, we found dramatic differences in the potential consumption limits that might be recommended among sites. Indeed, these differences were greater than those previously reported for farmed versus wild salmon (Hites et al. 2004a). Given that fish consumption has well appreciated health benefits (Cohen et al. 2005), it seems that identifying and designating where wild fish are captured could be an important tool to minimize fish pollutant risks. At present, the United States imports more than half of its total fish supply from other countries (FAO 2014, 2016; NOAA 2014) and fish of different origins can become mixed in the food supply (Jacquet and Pauly 2008; Warner et al. 2013). Our study underscores how traceability through the supply chain, and spatially stratified monitoring of POP levels, are necessary for management of fish pollutant risk.
Supplemental Material
Acknowledgments
We thank E. Parnell for his discussion of the statistical analyses.
This work was supported by the National Institutes of Health (grant no. ES021985) and by the National Science Foundation (grant no. 1314480) to A.H., and by a Waitt Foundation grant to A.H. and S.S.
References
- AHA (American Heart Association). 2015. Fish and Omega-3 Fatty Acids. http://www.heart.org/HEARTORG/HealthyLiving/HealthyEating/HealthyDietGoals/Fish-and-Omega-3-Fatty-Acids_UCM_303248_Article.jsp [accessed 4 September 2016].
- Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, et al. . 2009. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 323:1718–1722, PMID: 19325113, 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. 1999. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol 39:361–398, PMID: 10331089, 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- Atlas E, Giam CS. 1981. Global transport of organic pollutants: ambient concentrations in the remote marine atmosphere. Science 211:163–165, 10.1126/science.211.4478.163. [DOI] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry). 2000. “Toxicological Profile for Polychlorinated Biphenyls (PCBs).” Atlanta, GA:ATSDR. [PubMed] [Google Scholar]
- Bain LJ, McLachlan JB, LeBlanc GA. 1997. Structure-activity relationships for xenobiotic transport substrates and inhibitory ligands of P-glycoprotein. Environ Health Perspect 105:812–818, PMID: 9347896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batang ZB, Alikunhi N, Gochfeld M, Burger J, Al-Jahdali R, Al-Jahdali H, et al. . 2016. Congener-specific levels and patterns of polychlorinated biphenyls in edible fish tissue from the central Red Sea coast of Saudi Arabia. Sci Total Environ 572:915–925, 10.1016/j.scitotenv.2016.07.207. [DOI] [PubMed] [Google Scholar]
- Beyer A, Mackay D, Matthies M, Wania F, Webster E. 2000. Assessing long-range transport potential of persistent organic pollutants. Environ Sci Technol 34:699–703, 10.1021/es990207w. [DOI] [Google Scholar]
- Bircsak KM, Richardson JR, Aleksunes LM. 2013. Inhibition of human MDR1 and BCRP transporter ATPase activity by organochlorine and pyrethroid insecticides. J Biochem Mol Toxicol 27:157–164, PMID: 23169446, 10.1002/jbt.21458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block BA, Jonsen ID, Jorgensen SJ, Winship AJ, Shaffer SA, Bograd SJ, et al. . 2011. Tracking apex marine predator movements in a dynamic ocean. Nature 475:86–90, PMID: 21697831, 10.1038/nature10082. [DOI] [PubMed] [Google Scholar]
- Boas M, Feldt-Rasmussen U, Main KM. 2012. Thyroid effects of endocrine disrupting chemicals. Mol Cell Endocrinol 355:240–248, PMID: 21939731, 10.1016/j.mce.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Boas M, Feldt-Rasmussen U, Skakkebæk NE, Main KM. 2006. Environmental chemicals and thyroid function. Eur J Endocrinol 154:599–611, PMID: 16645005, 10.1530/eje.1.02128. [DOI] [PubMed] [Google Scholar]
- Bocio A, Domingo JL, Falcó G, Llobet JM. 2007. Concentrations of PCDD/PCDFs and PCBs in fish and seafood from the Catalan (Spain) market: estimated human intake. Environ Int 33:170–175, PMID: 17049987, 10.1016/j.envint.2006.09.005. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2009. Fourth National Report on Human Exposure to Environmental Chemicals. Atlanta, GA:CDC. [Google Scholar]
- CDC. 2015. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables. Atlanta, GA:CDC. [Google Scholar]
- Cocco P, Fadda D, Ibba A, Melis M, Tocco MG, Atzeri S, et al. . 2005. Reproductive outcomes in DDT applicators. Environ Res 98:120–126, PMID: 15721892, 10.1016/j.envres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Cohen J, Bellinger DC, Connor WE, Kris-Etherton PM, Lawrence RS, Savitz DA, et al. . 2005. A quantitative risk–benefit analysis of changes in population fish consumption. Am J Prev Med 29:325–325, PMID: 16242599, 10.1016/j.amepre.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Cohn BA, Wolff MS, Cirillo PM, Sholtz RI. 2007. DDT and breast cancer in young women: new data on the significance of age at exposure. Environ Health Perspect 115:1406–1414, PMID: 17938728, 10.1289/ehp.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly JP, Pedersen CJ. 1988. A thermodynamic-based evaluation of organic chemical accumulation in aquatic organisms. Environ Sci Technol 22:99–103, PMID: 22195516, 10.1021/es00166a011. [DOI] [PubMed] [Google Scholar]
- Costa LG, Giordano G, Guizzetti M, Vitalone A. 2008. Neurotoxicity of pesticides: a brief review. Front Biosci 13:1240–1249, PMID: 17981626, 10.2741/2758. [DOI] [PubMed] [Google Scholar]
- Covaci A, Gheorghe A, Hulea O, Schepens P. 2006. Levels and distribution of organochlorine pesticides, polychlorinated biphenyls and polybrominated diphenyl ethers in sediments and biota from the Danube Delta, Romania. Environ Pollut 140:136–149, PMID: 16112310, 10.1016/j.envpol.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Dachs J, Lohmann R, Ockenden WA, Méjanelle L, Eisenreich SJ, Jones KC. 2002. Oceanic biogeochemical controls on global dynamics of persistent organic pollutants. Environ Sci Technol 36:4229–4237, PMID: 12387392, 10.1021/es025724k. [DOI] [PubMed] [Google Scholar]
- Dankers ACA, Roelofs MJ, Piersma AH, Sweep FC, Russel FG, van den Berg M, et al. . 2013. Endocrine disruptors differentially target ATP-binding cassette transporters in the blood-testis barrier and affect Leydig cell testosterone secretion in vitro. Toxicol Sci 136:382–391, 10.1093/toxsci/kft198. [DOI] [PubMed] [Google Scholar]
- DHHS, USDA (U.S. Department of Health and Human Services, U.S. Department of Agriculture). 2015. 2015–2020 Dietary Guidelines for Americans. 8th Edition Washington, DC:DHHS, USDA. [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. . 2009. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev 30:293–342, PMID: 19502515, 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo JL, Bocio A. 2007. Levels of PCDD/PCDFs and PCBs in edible marine species and human intake: a literature review. Environ Int 33:397–405, PMID: 17270272, 10.1016/j.envint.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Döring B, Petzinger E. 2014. Phase 0 and phase III transport in various organs: combined concept of phases in xenobiotic transport and metabolism. Drug Metab Rev 46:261–282, PMID: 24483608, 10.3109/03602532.2014.882353. [DOI] [PubMed] [Google Scholar]
- Eganhouse RP, Pontolillo J. 2008. DDE in sediments of the Palos Verdes shelf, California: in situ transformation rates and geochemical fate. Environ Sci Technol 42:6392–6398, PMID: 18800506, 10.1021/es7029619. [DOI] [PubMed] [Google Scholar]
- Epel D, Luckenbach T, Stevenson CN, MacManus-Spencer LA, Hamdoun A, Smital T. 2008. Efflux transporters: newly appreciated roles in protection against pollutants. Environ Sci Technol 42:3914–3920, PMID: 18589945, 10.1021/es087187v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Communities. 1985. Council Directive 76/769/EEC on the approximation of the laws, regulations and administrative provisions of the Member States relating to restrictions on the marketing and use of certain dangerous substances and preparations. Off J Eur Communities, Chapter 13.
- FAO (Food and Agriculture Organization of the United Nations). 2014. The State of World Fisheries and Aquaculture. Rome, Italy:FAO. [Google Scholar]
- FAO. 2016. The State of World Fisheries and Aquaculture 2016. Contributing to Food Security and Nutrition for All. Rome, Italy:FAO. [Google Scholar]
- FDA (US Food and Drug Administration). 2014. What Pregnant Women and Parents Should Know, https://www.fda.gov/downloads/Food/FoodborneillnessContaminants/Metals/UCM400358.pdf [accessed 12 June 2017].
- Fliedner A, Rüdel H, Jürling H, Müller J, Neugebauer F, Schröter-Kermani C. 2012. Levels and trends of industrial chemicals (PCBs, PFCs, PBDEs) in archived herring gull eggs from German coastal regions. Environ Sci Eur 24:7, 10.1186/2190-4715-24-7. [DOI] [Google Scholar]
- Frame GM, Cochran JW, Bøwadt SS. 1996. Complete PCB congener distributions for 17 aroclor mixtures determined by 3 HRGC systems optimized for comprehensive, quantitative, congener-specific analysis. J High Resolut Chromatogr 19:657–668, 10.1002/jhrc.1240191202. [DOI] [Google Scholar]
- Froese R, Pauly D, 2000. FishBase2000: Concepts, Design and Data Sources. Los Banos, Laguna, Phillippines:International Center for Living Aquative Resources Management. [Google Scholar]
- García-Alvarez N, Martín V, Fernández A, Almunia J, Xuriach A, Arbelo M, et al. . 2014. Levels and profiles of POPs (organochlorine pesticides, PCBs, and PAHs) in free-ranging common bottlenose dolphins of the Canary Islands, Spain. Sci Total Environ 493:22–31, PMID: 24937489, 10.1016/j.scitotenv.2014.05.125. [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Fojo T, Bates SE. 2002. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2:48–58, 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- Guo Y, Meng XZ, Tang HL, Mai BX, Zeng EY. 2008. Distribution of polybrominated diphenyl ethers in fish tissues from the Pearl River Delta, China: levels, compositions and potential sources. Environ Toxicol Chem 27:576–582, 10.1897/07-366. [DOI] [PubMed] [Google Scholar]
- Hale RC, Alaee M, Manchester-Neesvig JB, Stapleton HM, Ikonomou MG. 2003. Polybrominated diphenyl ether flame retardants in the North American environment. Environ Int 29:771–779, PMID: 12850095, 10.1016/S0160-4120(03)00113-2. [DOI] [PubMed] [Google Scholar]
- Hites RA, Foran JA, Carpenter DO, Hamilton MC, Knuth BA, Schwager SJ. 2004a. Global assessment of organic contaminants in farmed salmon. Science 303:226–229, 10.1126/science.1091447. [DOI] [PubMed] [Google Scholar]
- Hites RA, Foran JA, Schwager SJ, Knuth BA, Hamilton MC, Carpenter DO. 2004b. Global assessment of polybrominated diphenyl ethers in farmed and wild salmon. Environ Sci Technol 38:4945–4949, PMID: 15506184, 10.1021/es049548m. [DOI] [PubMed] [Google Scholar]
- Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, et al. (International Transporter Consortium) . 2010. Membrane transporters in drug development. Nat Rev Drug Discov 9:215–236, PMID: 20190787, 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISSF (International Seafood Sustainability Foundation). 2017. Status of the World Fisheries for Tuna. ISSF Technical Report 2016-05B Washington, DC:ISSF. [Google Scholar]
- Jacquet JL, Pauly D. 2008. Trade secrets: renaming and mislabeling of seafood. Mar Policy 32:309–318, 10.1016/j.marpol.2007.06.007. [DOI] [Google Scholar]
- Jepson PD, Law RJ. 2016. Persistent pollutants, persistent threats. Science 352:1388–1389, 10.1126/science.aaf9075. [DOI] [PubMed] [Google Scholar]
- Karl H, Lahrssen-Wiederholt M. 2009. Dioxin and dioxin-like PCB levels in cod-liver and -muscle from different fishing grounds of the North- and Baltic Sea and the North Atlantic. J Verbr Lebensm 4:247–255, 10.1007/s00003-009-0308-5. [DOI] [Google Scholar]
- Karmaus W, Davis S, Fussman C, Brooks K. 2005. Maternal concentration of dichlorodiphenyl dichloroethylene (DDE) and initiation and duration of breast feeding. Paediatr Perinat Epidemiol 19:388–398, 10.1111/j.1365-3016.2005.00658.x. [DOI] [PubMed] [Google Scholar]
- Kelly BC, Ikonomou MG, Blair JD, Morin AE, Gobas FAPC. 2007. Food web-specific biomagnification of persistent organic pollutants. Science 317:236–239, 10.1126/science.1138275. [DOI] [PubMed] [Google Scholar]
- Kikkawa BS, Cushing JW. 2002. Growth of yellowfin tuna (Thunnus albacares) in the equatorial western Pacific Ocean. In: 15th Meeting of the Standing Committee on Tuna and Billfish, 22–27 July 2002, Honolulu, Hawai‘i Mᾱnoa, Hawai‘i:School of Ocean and Earth Science at the University of Hawai‘i. [Google Scholar]
- Knutsen HK, Kvalem HE, Thomsen C, Frøshaug M, Haugen M, Becher G, et al. . 2008. Dietary exposure to brominated flame retardants correlates with male blood levels in a selected group of Norwegians with a wide range of seafood consumption. Mol Nutr Food Res 52:217–227, 10.1002/mnfr.200700096. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM, Harris WS, Appel LJ, (American Heart Association Nutrition Committee). 2002. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 106:2747–2757, PMID: 12438303, 10.1161/01.CIR.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- Kurelec B. 1992. The multixenobiotic resistance mechanism in aquatic organisms. Crit Rev Toxicol 22:23–43, PMID: 1352103, 10.3109/10408449209145320. [DOI] [PubMed] [Google Scholar]
- Kurelec B. 1997. A new type of hazardous chemical: the chemosensitizers of multixenobiotic resistance. Environ Health Perspect 105:855–860, 10.1289/ehp.97105s4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth D, Brack W, Luckenbach T. 2015. Is chemosensitisation by environmental pollutants ecotoxicologically relevant? Aquat Toxicol 167:134–142, PMID: 26281775, 10.1016/j.aquatox.2015.07.017. [DOI] [PubMed] [Google Scholar]
- La Guardia MJ, Hale RC, Harvey E. 2007. Evidence of debromination of decabromodiphenyl ether (BDE-209) in biota from a wastewater receiving stream. Environ Sci Technol 41:6663–6670, PMID: 17969678, 10.1021/es070728g. [DOI] [PubMed] [Google Scholar]
- Leslie EM, Deeley RG, Cole SPC. 2005. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol 204:216–237, PMID: 15845415, 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Lohmann R, Belkin IM. 2014. Organic pollutants and ocean fronts across the Atlantic Ocean: a review. Prog Oceanogr 128:172–184, 10.1016/j.pocean.2014.08.013. [DOI] [Google Scholar]
- Lohmann R, Breivik K, Dachs J, Muir D. 2007. Global fate of POPs: current and future research directions. Environ Pollut 150:150–165, PMID: 17698265, 10.1016/j.envpol.2007.06.051. [DOI] [PubMed] [Google Scholar]
- Lohmann R, Jurado E, Pilson MEQ, Dachs J. 2006. Oceanic deep water formation as a sink of persistent organic pollutants. Geophys Res Lett 33:L12607, 10.1029/2006GL025953. [DOI] [Google Scholar]
- Loke M, Geslani C, Takenaka B, Leung P. 2012. An overview of seafood consumption and supply sources: Hawai‘i versus U.S. Coll Trop Agric Hum Resour Univ Hawai‘i at Mᾱnoa . Econ Issues EI-22:1–9. [Google Scholar]
- Longnecker MP, Klebanoff MA, Zhou H, Brock JW. 2001. Association between maternal serum concentration of the DDT metabolite DDE and preterm and small-for-gestational-age babies at birth. Lancet 358:110–114, PMID: 11463412, 10.1016/S0140-6736(01)05329-6. [DOI] [PubMed] [Google Scholar]
- Luckenbach T, Epel D. 2005. Nitromusk and polycyclic musk compounds as long-term inhibitors of cellular xenobiotic defense systems mediated by multidrug transporters. Environ Health Perspect 113:17–24, PMID: 15626642, 10.1289/ehp.7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzardo OP, Ruiz-Suárez N, Henríquez-Hernández LA, Valerón PF, Camacho M, Zumbado M, et al. . 2014. Assessment of the exposure to organochlorine pesticides, PCBs and PAHs in six species of predatory birds of the Canary Islands, Spain. Sci Total Environ 472:146–153, PMID: 24291140, 10.1016/j.scitotenv.2013.11.021. [DOI] [PubMed] [Google Scholar]
- Minnesota Department of Health. 2016. How Much is a Serving of Fish? http://www.health.state.mn.us/divs/eh/fish/eating/serving.html [accessed 9 July 2016].
- Morgan EJ, Lohmann R. 2010. Dietary uptake from historically contaminated sediments as a source of PCBs to migratory fish and invertebrates in an urban estuary. Environ Sci Technol 44:5444–5449, 10.1021/es100450f. [DOI] [PubMed] [Google Scholar]
- Nicklisch SCT, Rees SD, McGrath AP, Gökirmak T, Bonito LT, Vermeer LM, et al. . 2016. Global marine pollutants inhibit P-glycoprotein: environmental levels, inhibitory effects, and cocrystal structure. Sci Adv 15:e1600001, 10.1126/sciadv.1600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOAA (National Oceanic and Atmospheric Administration). 2014. Imports and Exports of Fishery Products, Annual Summary. Washington, DC:NOAA. [Google Scholar]
- Parnell PE, Groce AK, Stebbins TD, Dayton PK. 2008. Discriminating sources of PCB contamination in fish on the coastal shelf off San Diego, California (USA). Mar Pollut Bull 56:1992–2002, PMID: 18848709, 10.1016/j.marpolbul.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Persson EC, Graubard BI, Evans AA, London WT, Weber JP, LeBlanc A, et al. . 2012. Dichlorodiphenyltrichloroethane and risk of hepatocellular carcinoma. Int J Cancer 131:2078–2084, PMID: 22290210, 10.1002/ijc.27459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan WJ, Chen A. 2005. Health risks and benefits of bis(4-chlorophenyl)-1,1,1-trichloroethane (DDT). Lancet 366:763–773, PMID: 16125595, 10.1016/S0140-6736(05)67182-6. [DOI] [PubMed] [Google Scholar]
- Sagratini G, Buccioni M, Ciccarelli C, Conti P, Cristalli G, Giardinà D, et al. . 2008. Levels of polychlorinated biphenyls in fish and shellfish from the Adriatic Sea. Food Addit Contam Part B Surveill 1:69–77, PMID: 24784539, 10.1080/19393210802236919. [DOI] [PubMed] [Google Scholar]
- Santschi PH, Presley BJ, Wade TL, Garcia-Romero B, Baskaran M. 2001. Historical contamination of PAHs, PCBs, DDTs, and heavy metals in Mississippi River Delta, Galveston Bay and Tampa Bay sediment cores. Mar Environ Res 52:51–79, PMID: 11488356, 10.1016/S0141-1136(00)00260-9. [DOI] [PubMed] [Google Scholar]
- Schecter A, Birnbaum L, Ryan JJ, Constable JD. 2006a. Dioxins: an overview. Environ Res 101:419–428, PMID: 16445906, 10.1016/j.envres.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Schecter A, Colacino J, Haffner D, Patel K, Opel M, Päpke O, et al. . 2010a. Perfluorinated compounds, polychlorinated biphenyls, and organochlorine pesticide contamination in composite food samples from Dallas, Texas, USA. Environ Health Perspect 118:796–802, 10.1289/ehp.0901347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Colacino J, Patel K, Kannan K, Yun SH, Haffner D, et al. . 2010b. Polybrominated diphenyl ether levels in foodstuffs collected from three locations from the United States. Toxicol Appl Pharmacol 243:217–224, 10.1016/j.taap.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Schecter A, Haffner D, Colacino J, Patel K, Päpke O, Opel M, et al. . 2010c. Polybrominated diphenyl ethers (PBDEs) and hexabromocyclodecane (HBCD) in composite U.S. food samples. Environ Health Perspect 118:357–362, 10.1289/ehp.0901345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Päpke O, Harris TR, Tung KC, Musumba A, Olson J, et al. . 2006b. Polybrominated diphenyl ether (PBDE) levels in an expanded market basket survey of U.S. food and estimated PBDE dietary intake by age and sex. Environ Health Perspect 114:1515–1520, 10.1289/ehp.9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Yu C, Ying Y, Zhao Y, Wu Y, Han J, et al. . 2009. Levels and congener profiles of PCDD/Fs, PCBs and PBDEs in seafood from China. Chemosphere 77:1206–1211, PMID: 19800652, 10.1016/j.chemosphere.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Smital T, Kurelec B. 1997. Inhibitors of the multixenobiotic resistance mechanism in natural waters: in vivo demonstration of their effects. Environ Toxicol Chem 16:2164–2170, . [DOI] [Google Scholar]
- Smital T, Luckenbach T, Sauerborn R, Hamdoun AM, Vega RL, Epel D. 2004. Emerging contaminants—pesticides, PPCPs, microbial degradation products and natural substances as inhibitors of multixenobiotic defense in aquatic organisms. Mutat Res 552:101–117, 10.1016/j.mrfmmm.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Smith AG, Gangolli SD. 2002. Organochlorine chemicals in seafood: occurrence and health concerns. Food Chem Toxicol 40:767–779, PMID: 11983271, 10.1016/S0278-6915(02)00046-7. [DOI] [PubMed] [Google Scholar]
- Sreeramulu K, Liu R, Sharom FJ. 2007. Interaction of insecticides with mammalian P-glycoprotein and their effect on its transport function. Biochim Biophys Acta 1768:1750–1757, PMID: 17490606, 10.1016/j.bbamem.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Storelli MM, Barone G, Giacominelli-Stuffler R, Marcotrigiano GO. 2012. Contamination by polychlorinated biphenyls (PCBs) in striped dolphins (Stenella coeruleoalba) from the Southeastern Mediterranean Sea. Environ Monit Assess 184:5797–5805, PMID: 21960363, 10.1007/s10661-011-2382-2. [DOI] [PubMed] [Google Scholar]
- Storelli MM, Casalino E, Barone G, Marcotrigiano GO. 2008. Persistent organic pollutants (PCBs and DDTs) in small size specimens of bluefin tuna (Thunnus thynnus) from the Mediterranean Sea (Ionian Sea). Environ Int 34:509–513, PMID: 18164060, 10.1016/j.envint.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Storelli MM, Giacominelli-Stuffler R, Storelli A, Marcotrigiano GO. 2003. Polychlorinated biphenyls in seafood: contamination levels and human dietary exposure. Food Chem 82:491–496, 10.1016/S0308-8146(03)00119-5. [DOI] [Google Scholar]
- Sturm A, Segner H. 2005. P-glycoproteins and xenobiotic efflux transport in fish. In: Biochemistry and Molecular Biology of Fishes, Vol. 3 Mommsen TP, Moon TW, eds. Amsterdam, the Netherlands:Elsevier B.V, 495–533. [Google Scholar]
- Ueno D, Iwata H, Tanabe S, Ikeda K, Koyama J, Yamada H. 2002. Specific accumulation of persistent organochlorines in bluefin tuna collected from Japanese coastal waters. Mar Pollut Bull 45:254–261, PMID: 12398393, 10.1016/S0025-326X(02)00109-1. [DOI] [PubMed] [Google Scholar]
- Ueno D, Kajiwara N, Tanaka H, Subramanian A, Fillmann G, Lam PK, et al. . 2004. Global pollution monitoring of polybrominated diphenyl ethers using skipjack tuna as a bioindicator. Environ Sci Technol 38:2312–2316, 10.1021/es035323k. [DOI] [PubMed] [Google Scholar]
- Ueno D, Takahashi S, Tanaka H, Subramanian AN, Fillmann G, Nakata H, et al. . 2003. Global pollution monitoring of PCBs and organochlorine pesticides using skipjack tuna as a bioindicator. Arch Environ Contam Toxicol 45:378–389, 10.1007/s00244-002-0131-9. [DOI] [PubMed] [Google Scholar]
- Ueno D, Watanabe M, Subramanian A, Tanaka H, Fillmann G, Lam PK, et al. . 2005. Global pollution monitoring of polychlorinated dibenzo-p-dioxins (PCDDs), furans (PCDFs) and coplanar polychlorinated biphenyls (coplanar PCBs) using skipjack tuna as bioindicator. Environ Pollut 136:303–313, 10.1016/j.envpol.2004.12.036. [DOI] [PubMed] [Google Scholar]
- UNEP (United Nations Environment Programme). 2001. Final Act of the Conference of Plenipotentiaries on the Stockholm Convention on Persistent Organic Pollutants. Stockholm, Sweden:UNEP. [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 2000. Guidance for Assessing Chemical Contaminant Data for Use in fish Advisories, Vol. 2: Risk Assessment and Fish Consumption Limits. 3rd Edition Washington, DC:U.S. EPA. [Google Scholar]
- van der Oost R, Opperhuizen A, Satumalay K, Heida H, Vermeulen NPE. 1996. Biomonitoring aquatic pollution with feral eel (Anguilla anguilla) I. Bioaccumulation: biota-sediment ratios of PCBs, OCPs, PCDDs and PCDFs. Aquat Toxicol 35:21–46, 10.1016/0166-445X(96)00002-1. [DOI] [Google Scholar]
- Verweij F, Booij K, Satumalay K, van der Molen N, van der Oost R. 2004. Assessment of bioavailable PAH, PCB and OCP concentrations in water, using semipermeable membrane devices (SPMDs), sediments and caged carp. Chemosphere 54:1675–1689, PMID: 14675846, 10.1016/j.chemosphere.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Wandiga SO. 2001. Use and distribution of organochlorine pesticides. The future in Africa. Pure Appl Chem 73:1147–1155, 10.1351/pac200173071147. [DOI] [Google Scholar]
- Warner K, Timme W, Lowell B, Hirshfield M. 2013. Oceana Study Reveals Seafood Fraud Nationwide. Washington, DC:Oceana. [Google Scholar]
- Weijs L, Das K, Neels H, Blust R, Covaci A. 2010. Occurrence of anthropogenic and naturally-produced organohalogenated compounds in tissues of Black Sea harbour porpoises. Mar Pollut Bull 60:725–731, PMID: 20031175, 10.1016/j.marpolbul.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Welsh SE, Inoue M. 2000. Loop Current rings and the deep circulation in the Gulf of Mexico. J Geophys Res Oceans 105:16951–16959, 10.1029/2000JC900054. [DOI] [Google Scholar]
- Young FVK. 1986. The Chemical & Physical Properties of Crude Fish Oils for Refiners & Hydrogenators. Fish Oil Bull 18(1):19. [Google Scholar]
- Zhang S, Zhang Q, Darisaw S, Ehie O, Wang G. 2007. Simultaneous quantification of polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), and pharmaceuticals and personal care products (PPCPs) in Mississippi river water, in New Orleans, Louisiana, USA. Chemosphere 66:1057–1069, PMID: 16884762, 10.1016/j.chemosphere.2006.06.067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.