Abstract
Variation in parental age can have important consequences for offspring fitness and the structure of populations and disease transmission. However, our understanding of the effects of parental age on offspring in natural populations is limited. Here, we investigate consequences of parental age for offspring fitness and test for age-assortative mating in a short-lived bird, the house wren (Troglodytes aedon). Offspring immunoresponsiveness increased with maternal age and decreased with paternal age, but the strength of these effects varied with the age of one’s mate. Offspring immunoresponsiveness was augmented most with older mothers and younger fathers. Thus, we expected this combination of ages to yield the highest offspring fitness. However, offspring recruitment, longevity, and lifetime reproductive success were greatest when both parents were of above-average age. Consistent with the interactive effects of parental age on offspring fitness, we detected positive age-assortative mating among breeding pairs. Our results suggest that selection favors age-assortative mating, but in different ways depending on how parental ages affect offspring. We suggest that, in this short-lived species, selection for combinations of parental ages that maximize offspring immune responses is likely weaker than selection to produce breeding adults.
Keywords: house wren, immune response, life history, nonrandom mating, recruitment, Troglodytes aedon
Graphical abstract
Introduction
Older individuals often have greater breeding productivity than younger individuals across vertebrate taxa. Indeed, long-term ecological studies of wild populations have revealed changes in reproductive output with age in red deer (Cervus elaphus) with a maximum age of ca. 23 years (Clutton-Brock ‘84; Wilson et al. 2007) and Soay sheep (Ovis aries) with a maximum age of ca. 16 years (Wilson et al. 2007). Such a pattern has also been reported in long-lived birds such as the blue-footed booby (Sula nebouxii; maximum age ca. 14 years; Torres et al. 2011; see also Bradley and Safran 2014 for review), and moderately-aged birds such as the great tit (Parus major; maximum age ca. 9 years; Bouwhuis et al. 2009). However, increased reproductive output with age does not always result in higher annual fitness (sensu Coulson et al. 2006) of older individuals (e.g., Bouwhuis et al. 2010; Marshall et al. 2010). For example, offspring of older parents were recently found to be less fit than those of younger parents in some avian species, including the common tern (Sterna hirundo; Bouwhuis et al. 2015a) and the house sparrow (Passer domesticus; Schroeder et al. 2015). Moreover, results of previous studies suggest that parental ages can interact to affect offspring in humans (Fisch et al. 2003) and birds (Drummond and Rodríguez 2015), whereby the effect of the age of one parent depends on the age of the other (but see Richard et al. 2005 for an exception in common lizards, Lacerta vivipara). It is unclear whether parental age affects breeding performance over just a few years, as may be the case for shorter-lived species that might not experience significant senescence, and whether parental ages interact to influence offspring fitness in such species.
We recently found that increasing paternal age negatively affects offspring immune responsiveness to phytohaemagglutinin (PHA) and survival in a short-lived bird, the house wren (Troglodytes aedon), in which 86% of adults breed at only one or two years of age, and 96% at three years of age or younger (Supplementary Fig. 1). PHA is a plant-derived mitogen that, upon subcutaneous injection, stimulates a cytokine-induced proinflammatory response and the local infiltration of leukocytes (Martin et al. 2006; Vinkler et al. 2014; Bílková et al. 2015). This results in measurable tissue swelling, the magnitude of which is generally accepted as a reflection of cutaneous immune activity, derived from the innate and adaptive axes of the vertebrate immune system (Martin et al. 2006; Forsman et al. 2010; Vinkler et al. 2014). The magnitude of the swelling induced by PHA among nestlings has been found to predict their recruitment as breeding adults into local populations in a number of species, including our study species (Cichoń and Dubiec 2005; Moreno et al. 2005; López-Rull et al. 2011; Bowers et al. 2014). We recently found that increases in a male’s age were associated with a reduction in the PHA responsiveness of his genetic offspring, but not the offspring within his nest sired by males outside the pair bond and whose only contribution was sperm (Bowers et al. 2015c; see also Saino et al. 2002 for a similar result in another species). Although males that survive to above-average ages may have higher-quality genes (i.e., those that promote survival to old age) than younger males, on average, they also experience an ever-increasing load of deleterious, germ-line mutations with age (Hansen and Price ‘99; Radwan 2003; Velando et al. 2011; Preston et al. 2015). This should reduce the fitness of their progeny and, thus, the extent to which they are favored as mates (Hansen and Price ‘95; Beck and Promislow 2007; Schroeder et al. 2015). Female preferences for male age may, therefore, be subject to opposing selective forces, favoring younger males with respect to their lower mutational load, but older males with respect to their demonstrated fitness (Brooks and Kemp 2001).
Variation in parental age has widespread implications for sexual selection and the evolution of mating decisions (Kokko ‘97; Beck et al. 2002; Hunt et al. 2006; Evans et al. 2011; Ramos et al. 2014; Hsu et al. 2015). Given the negative effect of paternal age on offspring immunity that we observed, and the positive effect that maternal age and experiences can have on offspring immune activity (Grindstaff et al. 2003, 2006; Sadd and Schmid-Hempel 2007; Martyka et al. 2011; Bowers et al. 2012; 2015b), combinations of maternal and paternal ages may elicit effects on offspring survival after they have left the nest. Thus, we posit that maternal and paternal age interact to affect offspring fitness, potentially favoring age-assortative mating. Despite the occurrence of assortative mating in a variety of contexts and species (e.g., Fawcett and Johnstone 2003; Jiang et al. 2013), the strength of assortment varies widely among taxa, and little is known about the origin and maintenance of this diversity. A recent meta-analysis (Jiang et al. 2013) revealed evidence of positive assortment in a diverse set of taxa, but suggested that classical assumptions about the forces favoring assortative mating should be revisited, particularly with empirical data (see also Kirkpatrick and Nuismer 2004).
Here, we test for interactive effects of maternal and paternal age on breeding productivity and offspring fitness in the house wren. We predicted an interaction between parental ages in their effects on offspring cutaneous immune activity, longevity, and lifetime reproduction. We also test for assortative mating with respect to age among breeding pairs. We predicted that, if combinations of parental ages influence offspring fitness, the direction of these effects should predict the degree of age-assortative mating. Specifically, if offspring viability is maximized when parents differ widely in age, then parents should mate disassortatively with respect to age; alternatively, if parents of similar age produce offspring with increased reproductive value, then breeding pairs should contain members that are more similar in age than expected under random mating.
Materials and methods
House wrens are small, secondary-cavity-nesting songbirds that are distributed widely across the Americas (see Johnson 2014 for in-depth review of their biology). We studied a population breeding in secondary deciduous forest in McLean County, Illinois, USA (40.665°N, 88.89°W) from 2004–2013 as part of a long-term project that began in 1980. Since 2004, there have been 820 nestboxes available for house wrens. This species readily accepts nestboxes for nesting, and ca. 95% of the nests in this population are produced in the nestboxes provided (Drilling and Thompson ‘88). Nestboxes in this study were spaced 30 m apart along north-south transects separated by 60 m and mounted atop 48.3-cm diameter aluminum predator baffles on 1.5-m poles (Lambrechts et al. 2010 provide further details on nestboxes). Only the female incubates eggs and broods nestlings, but both parents deliver food to offspring during nestling development, which lasts ca. 15 days (Bowers et al. 2016). Breeding seasons extend from late April to August. The initiation of new nests is characterized by two peaks of activity, with the first, main peak of activity in early May and the second in late June (Bowers et al. 2016). Mate fidelity across reproductive events within seasons is low, as breeders generally switch mates from earlier- to later-season nests (Drilling and Thompson ‘91; Poirier et al. 2003), and interannual mate retention is very rare (there were 598 nests from 2004–2013 for which we knew the identity of both parents, and there were four pairs that bred together in two different years).
Nestboxes were checked at least twice weekly during the breeding season to document the initiation and status of nests. We captured breeding pairs, either inside nestboxes or using mistnets next to the box, once females completed egg laying and commenced incubation or shortly thereafter while provisioning nestlings. We measured the body mass (± 0.1 g) and tarsus length (± 0.1 mm) of adults, and banded them with a unique U. S. Geological Survey leg band.
As hatching approached, we visited nests daily to document when this occurred. We then visited nests periodically during the nestling stage to monitor their progress, and nestlings were weighed and banded 11–13 days posthatching, by which time body mass is at an asymptote (Bowers et al. 2015a). At this time, we administered a PHA skin test in nestlings by injecting PHA into the left wing web. Injection of PHA causes a proinflammatory response that results in tissue swelling. To do this, we measured wing-web thickness (± 0.01 mm; Mitutoyo no. 547–500, Mitutoyo, Aurora, IL, USA) as the mean of three measures prior to injection of the web with a 50-µL solution of PHA (L8754; Sigma-Aldrich, St. Louis, MO, USA) dissolved in phosphate-buffered saline (PBS; concentration = 5 mg PHA/mL PBS). We measured the same site 24 h later (three measures), and used the difference between the pre- and post-injection means as the response to PHA. Although this single measure does not comprehensively reflect an individual’s ability to combat infection and disease, it is a functionally relevant measure that positively predicts recruitment into the population as breeding adults (Bowers et al. 2014; see also Cichón and Dubiec 2005; Moreno et al. 2005; López-Rull et al. 2011 for similar results in other species). Following the administration of the PHA test, we visited nests daily to determine fledging dates (ca. 14–17 days posthatching), and attempted to capture all breeding adults in subsequent years through the 2016 season to document the recruitment and reproductive success of these offspring as breeding adults.
We assessed the distribution of parental ages (Supplementary Fig. 1) among the nests for which we measured offspring PHA responsiveness (N = 3,908 nestlings from 720 broods). Our data for parental ages reflect minimum ages, as most adults are not produced as offspring in our nestboxes (75 of 556 total females and 131 of 445 total males were produced in the nestboxes); thus, we assume that adults captured for the first time breeding on the study area are one year of age. This is a safe assumption, because breeders generally do not go undetected for more than one year; of all offspring produced on the study that recruited as a breeding adult from 1980–2014 (N = 1,248), 82% were captured at one year of age, and 97% by two years of age. Thus, although some newly banded adults were likely to be greater than one year of age, this should represent a small percentage of cases, and would add only noise to the patterns we observe. We used SAS (version 9.4) for all analyses, all tests are two-tailed (α = 0.05), and we converted data to z-scores prior to analysis to obtain standardized parameter estimates (Schielzeth 2010).
We first tested whether clutch sizes varied with maternal and paternal ages using a linear mixed model in PROC MIXED with the age of both parents as crossed fixed effects, and we included year and maternal and paternal identities as random effects. We also included breeding date (i.e., clutch-initiation date) and a measure of territory quality as covariates to control for environmental variation (Wilkin and Sheldon 2009; Pigeon et al. 2013). Because the nestboxes used by these birds are fixed in space, we used a proxy of territory quality quantified as the number of clutches produced in a given nestbox over the 10 years preceding its use in the current study (see also Bowers et al. 2017). Approximately 20–30% of females and 25–45% of males breeding on the study site in a given year return to breed the next year (Johnson 2014); however, birds rarely use the same nestbox in successive years, such that turnover in the use of these territories is high and that these estimates of territory quality are independent of the reproductive success of focal birds in future years (Bowers et al. 2017). Thus, this is a reliable index of territory quality in our study species that reflects the attractiveness of nest sites and the productivity of birds that use them (see also Janiszewski et al. 2013), and it is not confounded by variation in parental ages or breeding date. For example, this measure of territory quality positively predicted the body mass of nestlings produced in the current study (linear mixed model with nest as a random effect: estimate ± SE = 0.075 ± 0.033, F1, 523 = 5.05, P = 0.025), but this measure of territory quality was not correlated with either breeding date (estimate ± SE = –0.002 ± 0.041, F1, 538 = 0.00, P = 0.953) or with female or male age (correlation with female age: estimate ± SE = 0.058 ± 0.041, F1, 538 = 2.01, P = 0.156; correlation with male age: estimate ± SE = 0.030 ± 0.042, F1, 538 = 0.50, P = 0.479). We used a similar model to analyze fledging success, but with clutch size as an additional covariate. We controlled for clutch size in this analysis to reflect the number of fledglings produced, while holding constant the number of eggs laid (sensu Hodges et al. 2015). We also tested whether parental ages predicted breeding date using a linear mixed model with parental ages as fixed effects and year and maternal and paternal identities as random effects.
We tested whether offspring responsiveness to PHA varies with parental age using a similar linear mixed model as that above, with the same fixed and random effects, with the addition of nest identity as an added random effect to account for the non-independence of nestlings within broods. Nestling PHA responsiveness is positively correlated with measures of body condition (Sakaluk et al. 2014); thus, we also included nestling body mass as a covariate in this analysis to control for individual differences in the condition of nestlings (we used raw body mass as a covariate because we lacked data on tarsus length, a commonly used proxy for body size, for a number of nestlings). We used a similar model to test whether combinations of parental ages similarly affect nestling body mass, a trait that is also predictive of offspring recruitment, longevity, and future reproductive success in our study species (Bowers et al. 2014, 2015a) and in others (Bouwhuis et al. 2015b; Rollinson and Rowe 2015). We then analyzed the recruitment of individual young as breeding adults in the local population in subsequent years using a generalized linear mixed model with a binary response (recruited = 1, did not recruit = 0) and logit link function (PROC GLIMMIX). Because we predicted that the effects of parental age on offspring PHA responsiveness would also predict offspring recruitment, we retained the same fixed and random effects for this analysis as for PHA responsiveness. We also used a similar model to analyze effects of parental age on offspring longevity (i.e., number of years as a breeder) and lifetime reproductive success (i.e., total number of fledglings produced) in the population. For graphical purposes, we present surface plots of fitted values from the interaction between maternal and paternal ages. Plotting these ages on a surface required that we obtained sufficient replication for each combination of parental ages; to achieve this, we pooled parents aged three years and older (see Supplementary Fig. 1), and this approach produces similar results with respect to offspring PHA responsiveness and recruitment as those presented below.
We then tested for assortative mating among breeding pairs with respect to age. To do this, we analyzed maternal age as a dependent variable with male age as an independent variable, with breeding date as a covariate, in a linear mixed model (PROC MIXED) that included year and maternal and paternal identities and random effects. Because we did not know the exact age of many of the adults in our sample, we also tested this in a sample of nests produced from 1980–2014 by pairs of birds in which both parents were produced on the study area (N = 96 nests) and in which the age of all parents was known.
Results
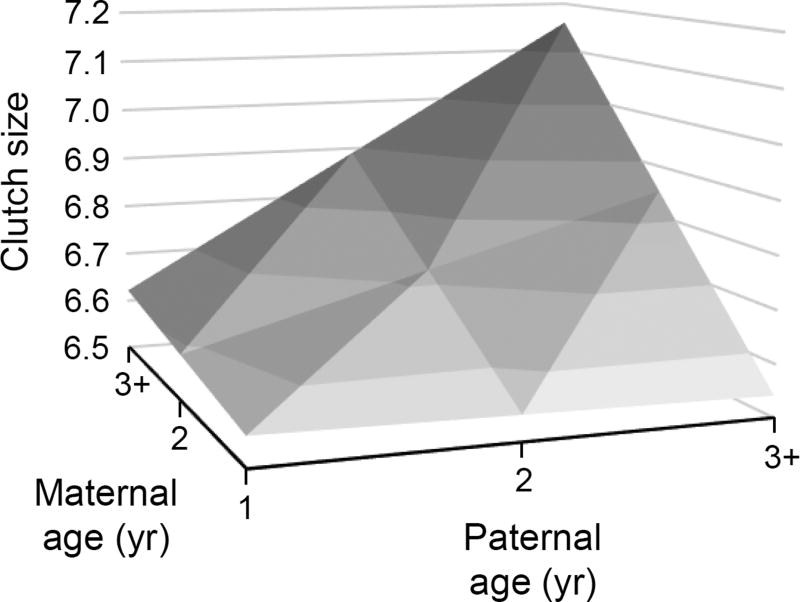
There was an interaction between maternal and paternal age in their effect on clutch size (Table 1a, Fig. 1). Clutch size increased with maternal age when females were paired with older males (estimate ± SE = 0.200 ± 0.076, F1, 65.2 = 6.93, P = 0.011; Fig. 1), but not when paired with younger males. Thus, the largest clutch sizes were produced by older females, but only when they were paired with similar-aged males (Fig. 1). Although clutch size predicted the number of young fledged from a given nest, parental ages did not interact to influence the number of fledglings produced per egg laid, nor did the age of either parent affect this (Table 1b). Breeding date was not associated with the age of either parent (maternal age: estimate ± SE = –0.051 ± 0.036, F1, 590 = 2.02, P = 0.156; paternal age: estimate ± SE = –0.033 ± 0.037, F1, 590 = 0.77, P = 0.380; maternal × paternal age: estimate ± SE = –0.010 ± 0.037, F1, 589 = 0.08, P = 0.783).
Table 1.
Effects on clutch size and fledging success.
| Estimate ± SE | F | df | P | |
|---|---|---|---|---|
|
|
||||
| (a) Clutch size | ||||
| Maternal age | 0.044 ± 0.033 | 1.81 | 1, 462.4 | 0.180 |
| Paternal age | −0.029 ± 0.031 | 0.85 | 1, 534.2 | 0.356 |
| Territory quality | −0.063 ± 0.032 | 3.96 | 1, 517.5 | 0.047 |
| Breeding date | −0.598 ± 0.030 | 403.50 | 1, 242.9 | < 0.001 |
| Maternal × Paternal age | 0.061 ± 0.030 | 4.07 | 1, 511.7 | 0.044 |
| Intercept | −0.128 ± 0.059 | |||
| (b) Fledging success | ||||
| Maternal age | 0.014 ± 0.036 | 0.14 | 1, 392.5 | 0.706 |
| Paternal age | −0.052 ± 0.036 | 2.17 | 1, 523.0 | 0.141 |
| Territory quality | −0.034 ± 0.037 | 0.85 | 1, 501.9 | 0.358 |
| Breeding date | −0.086 ± 0.046 | 3.45 | 1, 386.5 | 0.064 |
| Clutch size | 0.608 ± 0.050 | 150.40 | 1, 526.8 | < 0.001 |
| Maternal × Paternal age | 0.041 ± 0.035 | 1.38 | 1, 527.2 | 0.241 |
| Intercept | −0.091 ± 0.059 | |||
Fig. 1.
Effects of maternal and paternal age on clutch size. Plotted are predicted values fitted by the interaction between maternal and paternal ages, with ages 3+ representing individuals three years of age and older.
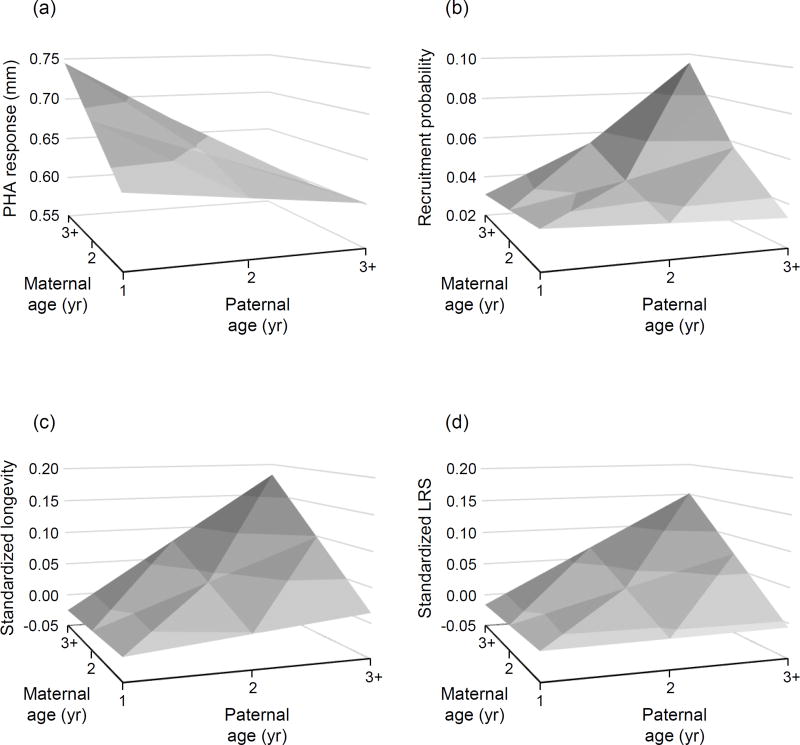
Cutaneous immune activity of offspring was influenced by particular combinations of parental ages (Table 2a, Fig. 2a). Overall, offspring immune responsiveness increased with maternal age while decreasing with paternal age, but the strength of these effects varied with partner age. The positive effect of maternal age was most pronounced when females were paired with yearling males (estimate ± SE = 0.149 ± 0.043, F1, 281 = 12.13, P < 0.001; Fig. 2a), but disappeared with increases in paternal age (Fig. 2a). On the other hand, paternal age had no effect on offspring immune responsiveness when females were one year of age, but a negative effect on offspring immune responsiveness when females were three years of age and older (estimate ± SE = –0.215 ± 0.087, F1, 60.7 = 6.19, P = 0.016; Fig. 2a). These effects do not appear to be a product of parental experience, as we assessed within-parent changes in age and their effect on offspring immune responsiveness, but there was no evidence for an effect of increasing maternal experience (F2, 473 = 0.80, P = 0.448), paternal experience (F2, 472 = 0.39, P = 0.674), or an interaction between the two (F3, 460 = 0.66, P = 0.575). Although there was a tendency for older females to rear heavier nestlings, there was no interaction between maternal and paternal ages in their effect on nestling body mass (Table 2b).
Table 2.
Effects on offspring cutaneous immune activity and body mass.
| Estimate ± SE | F | df | P | |
|---|---|---|---|---|
|
|
||||
| (a) Immune activity | ||||
| Maternal age | 0.085 ± 0.030 | 7.92 | 1, 329 | 0.005 |
| Paternal age | −0.057 ± 0.031 | 3.31 | 1, 387 | 0.070 |
| Territory quality | 0.026 ± 0.032 | 0.64 | 1, 541 | 0.423 |
| Nestling body mass | 0.079 ± 0.018 | 18.90 | 1, 2822 | < 0.001 |
| Breeding date | 0.041 ± 0.033 | 1.50 | 1, 439 | 0.221 |
| Maternal × Paternal age | −0.089 ± 0.030 | 8.68 | 1, 506 | 0.003 |
| Intercept | 0.002 ± 0.161 | |||
| (b) Body mass | ||||
| Maternal age | 0.052 ± 0.028 | 3.34 | 1, 501 | 0.068 |
| Paternal age | −0.024 ± 0.028 | 0.75 | 1, 407 | 0.386 |
| Territory quality | 0.027 ± 0.028 | 0.92 | 1, 702 | 0.338 |
| Breeding date | −0.210 ± 0.027 | 60.50 | 1, 509 | < 0.001 |
| Maternal × Paternal age | 0.040 ± 0.028 | 2.06 | 1, 687 | 0.151 |
| Intercept | 0.009 ± 0.095 | |||
Fig. 2.
Effects of maternal and paternal age on offspring. Plotted are effects of parental age on (a) cutaneous immune responsiveness to PHA, (b) the probability of recruiting as a breeding adult, (c) longevity, and (d) lifetime reproductive success of offspring in the local population. Plotted are predicted values fitted by the interaction between maternal and paternal ages, with ages 3+ representing individuals three years of age and older.
As expected, there was also an interaction between parental ages in their effect on the recruitment of offspring as breeding adults (Table 3a, Fig. 2b). However, this interaction term was of the opposite sign of that for immune activity, as the manner in which parental ages affected recruitment was different from their effect on immune responses. Offspring recruitment was higher when their parents were of similar age than when their parents’ ages differed more widely (Fig. 2b), and the age of each parent had a positive effect on offspring recruitment when paired with an older mate (effect of maternal age when paired with an older male: estimate ± SE = 0.363 ± 0.124, F1, 73.17 = 8.51, P = 0.005; effect of paternal age when paired with an older female: estimate ± SE = 0.498 ± 0.160, F1, 629 = 9.74, P = 0.002; Fig. 2b). In fact, the recruitment of offspring was greatest for the oldest of breeding pairs (Fig. 2b). Consistent with effects of parental age on offspring recruitment, offspring longevity and lifetime reproductive success were predicted similarly by an interaction between parental ages (Table 3b,c; Fig. 2c,d)
Table 3.
Effects on recruitment, longevity, and lifetime reproductive success.
| Estimate ± SE | F | df | P | |
|---|---|---|---|---|
|
|
||||
| (a) Recruitment | ||||
| Maternal age | 0.021 ± 0.089 | 0.06 | 1, 3840 | 0.810 |
| Paternal age | 0.010 ± 0.092 | 0.01 | 1, 3840 | 0.914 |
| Territory quality | −0.155 ± 0.092 | 2.85 | 1, 2859 | 0.092 |
| Nestling body mass | 0.074 ± 0.095 | 0.61 | 1, 871.1 | 0.436 |
| Breeding date | −0.570 ± 0.110 | 26.90 | 1, 532.1 | < 0.001 |
| Maternal × Paternal age | 0.209 ± 0.076 | 7.56 | 1, 3840 | 0.006 |
| Intercept | −3.455 ± 0.113 | |||
| (b) Longevity | ||||
| Maternal age | 0.010 ± 0.016 | 0.43 | 1, 3813 | 0.512 |
| Paternal age | 0.015 ± 0.016 | 0.82 | 1, 3781 | 0.366 |
| Territory quality | −0.024 ± 0.016 | 2.23 | 1, 2629 | 0.135 |
| Nestling body mass | 0.009 ± 0.016 | 0.31 | 1, 1527 | 0.579 |
| Breeding date | −0.076 ± 0.016 | 22.90 | 1, 330.4 | < 0.001 |
| Maternal × Paternal age | 0.041 ± 0.016 | 6.43 | 1, 3836 | 0.011 |
| Intercept | −0.009 ± 0.020 | |||
| (c) Reproductive success | ||||
| Maternal age | 0.033 ± 0.034 | 0.98 | 1, 755 | 0.323 |
| Paternal age | −0.005 ± 0.035 | 0.02 | 1, 847 | 0.896 |
| Territory quality | −0.014 ± 0.035 | 0.15 | 1, 792 | 0.697 |
| Nestling body mass | 0.045 ± 0.035 | 1.73 | 1, 1399 | 0.189 |
| Breeding date | −0.139 ± 0.034 | 16.20 | 1, 302 | < 0.001 |
| Maternal × Paternal age | 0.083 ± 0.035 | 5.73 | 1, 750 | 0.017 |
| Intercept | 0.325 ± 0.044 | |||
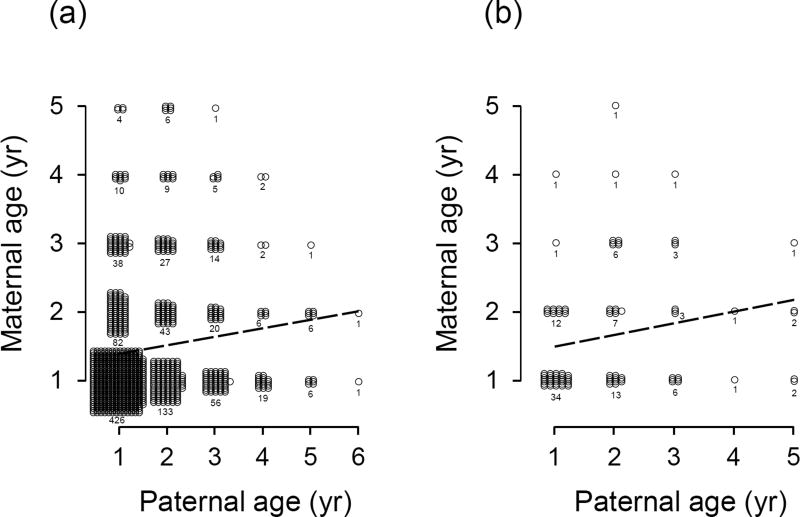
Among the breeding pairs that produced these offspring, there was a significant, positive correlation between maternal and paternal age (estimate ± SE = 0.132 ± 0.040, F1, 583 = 10.96, P = 0.001; Fig. 3a), while controlling for breeding date (association between breeding date and maternal age: estimate ± SE = –0.005 ± 0.020, F1, 245 = 0.06, P = 0.811). There was also no interaction between paternal age and breeding date (estimate ± SE = 0.009 ± 0.017, F1, 225 = 0.26, P = 0.610), indicating that the strength of the age-assortative mating was consistent across the range of the breeding season. This relationship was not manifested by an overabundance of yearling pairs, as re-analyzing the age-assortative mating without yearling pairs produces a qualitatively similar result (estimate ± SE = 0.147 ± 0.054, F1, 334 = 7.34, P = 0.007). Analyzing the correlation between maternal and paternal ages using a non-parametric correlation (not accounting for non-independence of multiple broods produced by a single individual) also produces a qualitatively similar result (Spearman’s rank correlation ρ596 = 0.129, P = 0.002). Many pairs in this analysis consisted of 1-year-old females paired with 1-year-old males, most of which were not produced on the study area and, consequently, contained some uncertainty about their age. We also analyzed this using a set of nests in which both members of the breeding pair were produced on the study area to obtain a sample of pairs in which the exact age of both adults was known without error (N = 96 breeding pairs from 1980–2014). Consistent with the association we detected among the larger set of nests, the positive correlation between maternal and paternal age persisted among all pairs of known-age birds (estimate ± SE = 0.195 ± 0.075, F1, 26.63 = 6.77, P = 0.015; Fig. 3b), and this was also the case while controlling for breeding date (association between breeding date and maternal age: estimate ± SE = –0.229 ± 0.071, F1, 24.68 = 10.32, P = 0.004; interaction between age and breeding date: estimate ± SE = 0.003 ± 0.062, F1, 16.57 = 0.00, P = 0.965).
Fig. 3.
Correlation between maternal and paternal ages. Plotted are the ages of parents at (a) nests that produced offspring in the current study (e.g., for which we measured immune responsiveness), and (b) nesting pairs in which both birds were produced on the study area from 1980–2014 and whose exact age was known. Points are jittered, and sample sizes are given below each parental age combination.
Discussion
Although previous studies have found that older individuals often have greater breeding productivity than younger individuals, at least to a point, little attention has been paid to the possibility that male and female ages may interact to affect offspring fitness. We detected significant interactions between parental ages in their effect on a number of important life-history traits, including the number of eggs a female produces and the cutaneous immune activity and fitness of her offspring. Overall, the longevity and lifetime reproductive success of offspring was higher when their parents were of above-average age; these components of offspring fitness were also greater when their parents were similar in age than when their parents differed more widely in age. Parental experience is commonly invoked in studies assessing effects of parental age on offspring; however, a correlation between parental age and breeding productivity does not necessarily reflect improvements with parental experience, as the selective disappearance of poor-quality individuals at young ages can create a positive correlation between parental age and productivity even in the absence of an effect of prior experience. We did not detect within-individual changes in nestling immune responsiveness in this study, and analysis of a larger set of nestlings indicates no within-individual improvement in breeding productivity as parents age (unpublished data). This suggests that age obtained in our study population reflects an individual’s intrinsic quality, whereby higher-quality individuals who live longer than average also produce offspring who live longer than average and have increased lifetime reproductive success (Fig. 2).
We also detected positive assortative mating with respect to age; this was the case among adults producing the offspring we measured here, and was also the case among a smaller set of known-age recruits who were produced on the study area. Assortative mating should be favored when similarities in particular traits within breeding pairs is associated with increased fitness. Correlated trait values between mates are commonly observed across animal taxa (r = 0.28, on average, in Jiang et al. 2013), and the strength of these correlations varies widely, but little is known about the origin and maintenance of this diversity. True assortative mating necessitates that some degree of choice be expressed by males, yet strong choosiness by males should generally be selected against (Servedio and Lande 2006; Servedio 2007; Jennions and Kokko 2010). Nonetheless, theory predicts that male mate choice can evolve when males can perceive their own intrasexual competitive ability and pursue females for which they are competitive, particularly if the traits upon which choice is based confer increased fertility or offspring viability (Fawcett and Johnstone 2003; Servedio and Lande 2006; Kokko and Jenions 2008). For both females and males, age is likely to be an honest signal of individual “quality,” defined here as the ability to achieve old age, and may be a trait that both females and males can readily assess, and pair accordingly.
We found that offspring with an older mother and a younger father had a heightened form of immune activity that is comprised of both innate and adaptive axes of the vertebrate immune system (Martin et al. 2006; Forsman et al. 2010). Positive assortative mating was previously detected in the barn owl (Tyto alba) with respect to a secondary sexual character (black plumage spots; Roulin ‘99) that also signals resistance to ectoparasites (Roulin et al. 2001). Given that immune activity of offspring in the current study was greatest when they had an older mother and a younger father, and that this trait is predictive of offspring recruitment into the breeding population, we might expect selection to favor disassortative mating with respect to age (e.g., to enhance allelic diversity among offspring, sensu Bonneaud et al. 2006; Ortego et al. 2009), consistent with previous findings that offspring fitness may be greatest with diversity in parental age (Drummond and Rodríguez 2015). This was not the case, however, as breeding pairs tended to contain members that were more similar to each other in age than expected under random sampling.
It is important to note that a number of processes can lead individuals to mate assortatively, such that assortative mating occurs incidentally (Jiang et al. 2013). In birds such as our study species, there are at least two main mechanisms that could generate “apparent” assortment (sensu Arnqvist et al. ‘96) with respect to age. First, interannual mate fidelity could lead to a correlation in age between breeding pairs, even if between-pair differences in age combinations have no effect on parents’ inclusive fitness. However, this could not have generated the patterns we observed in the current study because mate fidelity across seasons is very low (see Methods). Second, assortment with respect to age may arise incidentally if older females and males tend to breed earlier than younger females and males, even if mating is random with respect to age. This may be common in territorial and migratory species, but it could not explain the patterns observed in the current study. The age-assortative mating we document here occurs across the entire range of time in the breeding season during which clutches of eggs are produced (typically ca. 3 months; Bowers et al. 2016), a span over which young and old birds breed in sympatry. Thus, our data indicate that pairs of similar age form irrespective of when mating occurs.
Expectations about the selective pressures favoring assortative mating have traditionally been based on assumptions about stabilizing and disruptive selection (Jiang et al. 2013), whereby stabilizing selection favors negative assortative mating and disruptive selection favors positive assortative mating. If, however, classical notions that disruptive and stabilizing selection drive assortative mating are unlikely to be generally applicable (Jiang et al. 2013), then the processes favoring assortative mating deserve further empirical attention, particularly with respect to the consequences of assortment for parents’ inclusive fitness in a natural setting.
In conclusion, we detected interactive effects of parental age on offspring phenotype and fitness. Our results suggest that selection favors age-assortative mating, albeit perhaps in different ways depending on how parental ages affect offspring. In our short-lived study species, we posit that selection for combinations of parental ages that produce reproductively active adults likely trumps any selection for combinations of parental age that maximizes offspring immune activity. Future work will shed light on the processes generating and maintaining variation within and among taxa in the strength of assortative mating and its consequences for sexual selection and mating systems.
Supplementary Material
Supplementary Figure 1. Distribution of parental ages.
Acknowledgments
Grant information: funding for this study was provided by the School of Biological Sciences, College of Arts and Sciences, Honors Program, and Graduate School of Illinois State University; the Phi Sigma Biological Honor Society; the Sigma Xi Society; the American Ornithologists’ Union; the Animal Behavior Society; the American Museum of Natural History’s Frank M. Chapman Fund; the Champaign County Audubon Society; the Whitehall Foundation; Research Internships in Science and Engineering (RISE) from the Deutscher Akademischer Austauschdienst; the National Science Foundation (DEB 7707246, DEB 8104687, INT 8410955, BSR 8615296, INT 9123572, IBN 0316580, IOS 0718140, IOS 1118160); and the National Institutes of Health (R15HD076308-01).
We thank 1980–2016 Wren Crew members for data collection and the Davis, Sears, and Butler families; the ParkLands Foundation; and the Illinois Great Rivers Conference of the United Methodist Church for use of their properties. Funding was provided by the School of Biological Sciences, College of Arts and Sciences, Honors Program, and Graduate School of Illinois State University; the Phi Sigma Biological Honor Society; the Sigma Xi Society; the American Ornithologists’ Union; the Animal Behavior Society; the American Museum of Natural History’s Frank M. Chapman Fund; the Champaign County Audubon Society; the Whitehall Foundation; Research Internships in Science and Engineering (RISE) from the Deutscher Akademischer Austauschdienst; the National Science Foundation (DEB 7707246, DEB 8104687, INT 8410955, BSR 8615296, INT 9123572, IBN 0316580, IOS 0718140, IOS 1118160); and the National Institutes of Health (R15HD076308-01). All activities complied with current laws of the United States, the Illinois State University Institutional Animal Care and Use Committee, and U.S. Geological Survey permit 09211.
Literature Cited
- Arnqvist G, Rowe L, Krupa JJ, Sih A. Assortative mating by size: a meta-analysis of mating patterns in water striders. Evol Ecol. 1996;10:265–284. [Google Scholar]
- Beck CW, Promislow DEL. Evolution of female preference for younger males. PLoS ONE. 2007;2:e939. doi: 10.1371/journal.pone.0000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck CW, Shapiro B, Choksi S, Promislow DEL. A genetic algorithm approach to study the evolution of female preferences based on male age. Evol Ecol Res. 2002;4:275–292. [Google Scholar]
- Bílková B, Vinklerová J, Vinkler M. The relationship between health and cell-mediated immunity measured in ecology: phytohaemagglutinin skin-swelling test mirrors blood cellular composition. J Exp Zool Part A. 2015;323:767–777. doi: 10.1002/jez.1990. [DOI] [PubMed] [Google Scholar]
- Bonneaud C, Chastel O, Federici P, Westerdahl H, Sorci G. Complex Mhc-based mate choice in a wild passerine. Proc R Soc B. 2006;273:1111–1116. doi: 10.1098/rspb.2005.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwhuis S, Sheldon BC, Verhulst S, Charmantier A. Great tits growing old: selective disappearance and the partitioning of senescence to stages within the breeding cycle. Proc R Soc B. 2009;276:2769–2777. doi: 10.1098/rspb.2009.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwhuis S, Charmantier A, Verhulst S, Sheldon BC. Trans-generational effects on ageing in a wild bird population. J Evol Biol. 2010;23:636–642. doi: 10.1111/j.1420-9101.2009.01929.x. [DOI] [PubMed] [Google Scholar]
- Bouwhuis S, Vedder O, Becker PH. Sex-specific pathways of parental age effects on offspring lifetime reproductive success in a long-lived seabird. Evolution. 2015a;69:1760–1771. doi: 10.1111/evo.12692. [DOI] [PubMed] [Google Scholar]
- Bouwhuis S, Vedder O, Garroway CJ, Sheldon BC. Ecological causes of multilevel covariance between size and first-year survival in a wild bird population. J Anim Ecol. 2015b;84:208–218. doi: 10.1111/1365-2656.12264. [DOI] [PubMed] [Google Scholar]
- Bowers EK, Smith RA, Hodges CJ, Zimmerman LM, Thompson CF, Sakaluk SK. Sex-biased terminal investment in offspring induced by maternal immune challenge in the house wren (Troglodytes aedon) Proc R Soc B. 2012;279:2891–2898. doi: 10.1098/rspb.2012.0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Hodges CJ, Forsman AM, Vogel LA, Masters BS, Johnson BGP, Johnson LS, Thompson CF, Sakaluk SK. Neonatal body condition, immune responsiveness, and hematocrit predict longevity in a wild bird population. Ecology. 2014;95:3027–3034. doi: 10.1890/14-0418.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Thompson CF, Sakaluk SK. Persistent sex-by-environment effects on offspring fitness and sex-ratio adjustment in a wild bird population. J Anim Ecol. 2015a;84:473–486. doi: 10.1111/1365-2656.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Bowden RM, Sakaluk SK, Thompson CF. Immune activation generates corticosterone-mediated terminal reproductive investment in a wild bird. Am Nat. 2015b;185:769–783. doi: 10.1086/681017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Forsman AM, Masters BS, Johnson BGP, Johnson LS, Sakaluk SK, Thompson CF. Increased extra-pair paternity in broods of aging males and enhanced recruitment of extra-pair young in a migratory bird. Evolution. 2015c;69:2533–2541. doi: 10.1111/evo.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Grindstaff JL, Soukup SS, Drilling NE, Eckerle KP, Sakaluk SK, Thompson CF. Spring temperatures influence selection on breeding date and the potential for phenological mismatch in a migratory bird. Ecology. 2016;97:2880–2891. doi: 10.1002/ecy.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Thompson CF, Sakaluk SK. Maternal natal environment and breeding territory predict the condition and sex ratio of offspring. Evol Biol. 2017;44:11–20. doi: 10.1007/s11692-016-9380-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RJ, Safran RJ. Conceptual revision and synthesis of proximate factors associated with age-related improvement in reproduction. Ethology. 2014;120:1–16. [Google Scholar]
- Brooks R, Kemp DJ. Can older males deliver the good genes? Trends Ecol Evol. 2001;16:308–313. doi: 10.1016/s0169-5347(01)02147-4. [DOI] [PubMed] [Google Scholar]
- Cichón M, Dubiec A. Cell-mediated immunity predicts the probability of local recruitment in nestling blue tits. J Evol Biol. 2005;18:962–966. doi: 10.1111/j.1420-9101.2005.00910.x. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH. Reproductive effort and terminal investment in iteroparous animals. Am Nat. 1984;123:212–229. [Google Scholar]
- Coulson T, Benton TG, Lundberg P, Dall SRX, Kendall BE, Gaillard J-M. Estimating individual contributions to population growth: evolutionary fitness in ecological time. Proc R Soc B. 2006;273:547–555. doi: 10.1098/rspb.2005.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drilling NE, Thompson CF. Natal and breeding dispersal in house wrens (Troglodytes aedon) Auk. 1988;105:480–491. [Google Scholar]
- Drilling NE, Thompson CF. Mate switching in multibrooded house wrens. Auk. 1991;108:60–70. [Google Scholar]
- Drummond H, Rodríguez C. Viability of booby offspring is maximized by having one young parent and one old parent. PLoS ONE. 2015;10:e0133213. doi: 10.1371/journal.pone.0133213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SR, Gustafsson L, Sheldon BC. Divergent patterns of age-dependence in ornamental and reproductive traits in the collared flycatcher. Evolution. 2011;65:1623–1636. doi: 10.1111/j.1558-5646.2011.01253.x. [DOI] [PubMed] [Google Scholar]
- Fawcett TW, Johnstone RA. Mate choice in the face of costly competition. Behav Ecol. 2003;14:771–779. [Google Scholar]
- Fisch H, Hyun G, Golden R, Hensle TW, Olsson CA, Liberson GL. The influence of paternal age on Down syndrome. J Urology. 2003;169:2275–2278. doi: 10.1097/01.ju.0000067958.36077.d8. [DOI] [PubMed] [Google Scholar]
- Forsman AM, Sakaluk SK, Thompson CF, Vogel LA. Cutaneous immune activity, but not innate immune responsiveness, covaries with mass and environment in nestling house wrens (Troglodytes aedon) Physiol Biochem Zool. 2010;83:512–518. doi: 10.1086/649894. [DOI] [PubMed] [Google Scholar]
- Grindstaff JL, Brodie ED, III, Ketterson ED. Immune function across generations: integrating mechanism and evolutionary process in maternal antibody transmission. Proc R Soc Lond B. 2003;270:2309–2319. doi: 10.1098/rspb.2003.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindstaff JL, Hasselquist D, Nilsson J-Å, Sandell M, Smith HG, Stjernman M. Transgenerational priming of immunity: maternal exposure to a bacterial antigen enhances offspring humoral immunity. Proc R Soc B. 2006;273:2551–2557. doi: 10.1098/rspb.2006.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TF, Price DK. Good genes and old age: do old mates provide superior genes? J Evol Biol. 1995;8:759–778. [Google Scholar]
- Hansen TF, Price DK. Age- and sex-distribution of the mutation load. Genetica. 1999;106:251–262. doi: 10.1023/a:1003988101586. [DOI] [PubMed] [Google Scholar]
- Hodges CJ, Bowers EK, Thompson CF, Sakaluk SK. Cascading costs of reproduction in female house wrens induced to lay larger clutches. J Evol Biol. 2015;28:1383–1393. doi: 10.1111/jeb.12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y-H, Schroeder J, Winney I, Burke T, Nakagawa S. Are extra-pair males different from cuckolded males? A case study and a meta-analytic examination. Mol Ecol. 2015;24:1558–1571. doi: 10.1111/mec.13124. [DOI] [PubMed] [Google Scholar]
- Hunt J, Jennions MD, Spyrou N, Brooks R. Artificial selection on male longevity influences age-dependent reproductive effort in the black field cricket Teleogryllus commodus. Am Nat. 2006;168:E72–E86. doi: 10.1086/506918. [DOI] [PubMed] [Google Scholar]
- Janiszewski T, Minias P, Wojciechowski Z. Occupancy reliably reflects territory quality in a longlived migratory bird, the white stork. J Zoology. 2013;291:178–184. [Google Scholar]
- Jennions MD, Kokko H. Sexual selection. In: Westneat DF, Fox CW, editors. Evolutionary Behavioral Ecology. Oxford: Oxford University Press; 2010. pp. 343–364. [Google Scholar]
- Jiang Y, Bolnick DI, Kirkpatrick M. Assortative mating in animals. Am Nat. 2013;181:E125–E138. doi: 10.1086/670160. [DOI] [PubMed] [Google Scholar]
- Johnson LS. House wren (Troglodytes aedon) In: Poole A, editor. The Birds of North America Online. 2. Ithaca, NY: Cornell Lab of Ornithology and American Ornithologists’ Union; 2014. [DOI] [Google Scholar]
- Kirkpatrick M, Nuismer SL. Sexual selection can constrain sympatric species. Proc R Soc Lond B. 2004;271:687–693. doi: 10.1098/rspb.2003.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokko H. Evolutionarily stable strategies of age-dependent sexual advertisement. Behav Ecol Sociobiol. 1997;41:99–107. [Google Scholar]
- Kokko H, Jennions MD. Parental investment, sexual selection and sex ratios. J Evol Biol. 2008;21:919–948. doi: 10.1111/j.1420-9101.2008.01540.x. [DOI] [PubMed] [Google Scholar]
- Lambrechts MM, Adriaensen F, Ardia DR, Artemyev AV, Atiénzar F, Bańbura J, et al. The design of artificial nestboxes for the study of secondary hole-nesting birds: a review of methodological inconsistencies and potential biases. Acta Ornithol. 2010;45:1–26. [Google Scholar]
- López-Rull I, Celis P, Salaberria C, Puerta M, Gil D. Post-fledging recruitment in relation to nestling plasma testosterone and immunocompetence in the spotless starling. Funct Ecol. 2011;25:500–508. [Google Scholar]
- Marshall DJ, Heppell SS, Munch SB, Warner RR. The relationship between maternal phenotype and offspring quality: do older mothers really produce the best offspring? Ecology. 2010;91:2862–2873. doi: 10.1890/09-0156.1. [DOI] [PubMed] [Google Scholar]
- Martin LB, II, Han P, Lewittes J, Kuhlman JR, Klasing KC, Wikelski M. Phytohemagglutinin-induced skin swelling in birds: histological support for a classic immunoecological technique. Funct Ecol. 2006;20:290–299. [Google Scholar]
- Martyka R, Rutkowska J, Cichoń M. Sex-specific effects of maternal immunization on yolk antibody transfer and offspring performance in zebra finches. Biol Lett. 2011;7:50–53. doi: 10.1098/rsbl.2010.0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno J, Merino S, Sanz JJ, Arriero E, Morales J, Tomás G. Nestling cell-mediated immune response, body mass and hatching date as predictors of local recruitment in the pied flycatcher Ficedula hypoleuca. J Avian Biol. 2005;36:251–260. [Google Scholar]
- Ortego J, Calabuig G, Bonal R, Muñoz A, Aparicio JM, Cordero PJ. Temporal variation of heterozygosity-based assortative mating and related benefits in a lesser kestrel population. J Evol Biol. 2009;22:2488–2495. doi: 10.1111/j.1420-9101.2009.01865.x. [DOI] [PubMed] [Google Scholar]
- Pigeon G, Bélisle M, Garant D, Cohen AA, Pelletier F. Ecological immunology in a fluctuating environment: an integrative analysis of tree swallow nestling immune defense. Ecol Evol. 2013;3:1091–1103. doi: 10.1002/ece3.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier NE, Whittingham LA, Dunn PO. Effects of paternity and mate availability on mate switching in house wrens. Condor. 2003;105:816–821. [Google Scholar]
- Preston BT, Jalme MS, Hingrat Y, Lacroix F, Sorci G. The sperm of aging male bustards retards their offspring’s development. Nat Comm. 2015;6:6146. doi: 10.1038/ncomms7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwan J. Male age, germline mutations and the benefits of polyandry. Ecol Lett. 2003;6:581–586. [Google Scholar]
- Ramos AG, Nunziata SO, Lance SL, Rodríguez C, Faircloth BC, Gowaty PA, Drummond H. Interactive effects of male and female age on extra-pair paternity in a socially monogamous seabird. Behav Ecol Sociobiol. 2014;68:1603–1609. [Google Scholar]
- Richard M, Lecomte J, De Fraipont M, Clobert J. Age-specific mating strategies and reproductive senescence. Mol Ecol. 2005;14:3147–3155. doi: 10.1111/j.1365-294X.2005.02662.x. [DOI] [PubMed] [Google Scholar]
- Rollinson N, Rowe L. Persistent directional selection on body size and a resolution to the paradox of stasis. Evolution. 2015;69:2441–2451. doi: 10.1111/evo.12753. [DOI] [PubMed] [Google Scholar]
- Roulin A. Nonrandom pairing by male barn owls (Tyto alba) with respect to a female plumage trait. Behav Ecol. 1999;10:688–695. [Google Scholar]
- Roulin A, Riols C, Dijkstra C, Ducrest A-L. Female plumage spottiness signals parasite resistance in the barn owl (Tyto alba) Behav Ecol. 2001;12:103–110. [Google Scholar]
- Sadd BM, Schmid-Hempel P. Facultative but persistent trans-generational immunity via the mother’s eggs in bumblebees. Current Biol. 2007;17:R1046–R1047. doi: 10.1016/j.cub.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Saino N, Ambrosini R, Martinelli R, Møller AP. Mate fidelity, senescence in breeding performance and reproductive trade-offs in the barn swallow. J Anim Ecol. 2002;71:309–319. [Google Scholar]
- Sakaluk SK, Wilson AJ, Bowers EK, Johnson LS, Masters BS, Johnson BGP, Vogel LA, Forsman AM, Thompson CF. Genetic and environmental variation in condition, cutaneous immunity, and haematocrit in house wrens. BMC Evol Biol. 2014;14:424. doi: 10.1186/s12862-014-0242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schielzeth H. Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol. 2010;1:103–113. [Google Scholar]
- Schroeder J, Nakagawa S, Rees M, Mannarelli M-E, Burke T. Reduced fitness in progeny from old parents in a natural population. Proc Natl Acad Sci USA. 2015;112:4021–4025. doi: 10.1073/pnas.1422715112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servedio MR. Male versus female mate choice: sexual selection and the evolution of species recognition via reinforcement. Evolution. 2007;61:2772–2789. doi: 10.1111/j.1558-5646.2007.00247.x. [DOI] [PubMed] [Google Scholar]
- Servedio MR, Lande R. Population genetic models of male and mutual mate choice. Evolution. 2006;60:674–685. [PubMed] [Google Scholar]
- Torres R, Drummond H, Velando A. Parental age and lifespan influence offspring recruitment: a long-term study in a seabird. PLoS One. 2011;6:e27245. doi: 10.1371/journal.pone.0027245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velando A, Noguera JC, Drummond H, Torres R. Senescent males carry premutagenic lesions in sperm. J Evol Biol. 2011;24:693–697. doi: 10.1111/j.1420-9101.2010.02201.x. [DOI] [PubMed] [Google Scholar]
- Vinkler M, Bainová H, Albrecht T. Functional analysis of the skin-swelling response to phytohaemagglutinin. Funct Ecol. 2010;24:1081–1086. [Google Scholar]
- Vinkler M, Svobodová J, Gabrielová B, Bainová H, Bryjová A. Cytokine expression in phytohaemagglutinin-induced skin inflammation in a galliform bird. J Avian Biol. 2014;45:43–50. [Google Scholar]
- Wilkin TA, Sheldon BC. Sex differences in the persistence of natal environmental effects on life histories. Current Biol. 2009;19:1998–2002. doi: 10.1016/j.cub.2009.09.065. [DOI] [PubMed] [Google Scholar]
- Wilson AJ, Nussey DH, Pemberton JM, Pilkington JG, Morris A, Pelletier F, Clutton-Brock TH, Kruuk LEB. Evidence for a genetic basis of aging in two wild vertebrate populations. Current Biol. 2007;17:2136–2142. doi: 10.1016/j.cub.2007.11.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Distribution of parental ages.