Abstract
Background
Fragile X-associated tremor/ataxia Syndrome (FXTAS) is an adult-onset disorder associated with premutation alleles of the FMR1 gene. The disorder is characterized by progressive action tremor, gait ataxia, and cognitive decline. FXTAS pathology includes dystrophic white matter and intranuclear inclusions in neurons and astrocytes. We previously demonstrated that the transport of iron into the brain is altered in FXTAS; therefore, we also expect an alteration of iron metabolism in brain areas related to motor control. Iron is essential for cell metabolism, but uncomplexed iron leads to oxidative stress and contributes to the development of neurodegenerative diseases.
Objectives
We investigated a potential iron modification in the striatum, the structure that participates in motor learning and performance, in FXTAS.
Methods
We used samples of putamen obtained from 9 FXTAS and 9 control cases to study iron localization using Perl’s method, and iron-binding proteins using immunostaining.
Results
We found increased iron deposition in neuronal and glial cells, which accumulate iron, in the putamen in FXTAS. We also found a generalized decreased of the amount of the iron-binding proteins, transferrin and ceruloplasmin, in the putamen, and decreased numbers of neurons and glial cells that contained ceruloplasmin. However, we found increased levels of iron, transferrin, and ceruloplasmin in microglial cells, indicating the attempt by the immune system to remove the excess iron.
Conclusions
Overall, there is a deficit in proteins that eliminate extra iron from the cells with the concomitant increased in the deposit of cellular iron.
Keywords: Fragile X, repeat-expansion disorder, CGG, dementia, FXTAS, neurodegeneration, motor disorder
Background
Fragile X-associated tremor/ataxia Syndrome (FXTAS) is a late-onset neurodegenerative disorder associated with premutation alleles (55-200 CGG repeats) of the FMR1 gene; larger expansions (> 200 CGG repeats; full mutation) give rise to fragile X syndrome (FXS), the most common inherited form of cognitive impairment. Carriers of premutation alleles are common in the general population, with an estimated frequency as high as 1 in 130 females and 1 in 250 males [1,2]. However, only about 40% of male carriers and 14% of female carriers will eventually develop FXTAS [3,4]. FXTAS is characterized by progressive action tremor, gait ataxia, cognitive decline, Parkinsonism, neuropathy, and autonomic dysfunction [5]. Central nervous system (CNS) pathology includes dystrophic white matter and intranuclear inclusions in neurons and astrocytes [6-8]. Although neurological symptoms of FXTAS have only been observed in adults, it is now clear that children carrying premutation alleles may also have forms of clinical involvement that include anxiety, attention deficit hyperactivity disorder, and autism spectrum disorders related to the premutation [9,10]. Additionally, premutation CGG-repeat expansions in the mouse Fmr1 gene have been shown to alter embryonic neocortical development [11].
Premutation carriers display a form of gene dysregulation that is quite distinct from the gene silencing observed with FXS, manifested by substantially increased levels of FMR1 mRNA, and normal or moderately decreased levels of FMRP. The extent of this altered expression is a function of the size of the CGG-repeat expansion within the premutation range, with larger CGG-repeat expansions associated with higher levels of mRNA and lower levels of protein [12]. Evidence from both human and animal studies implicates a direct toxic gain-of-function of the premutation CGG (pre-CGG) repeat-containing FMR1 mRNA [12-15]. Consistent with this hypothesis, the characteristic intranuclear inclusions found in neuronal and glial cells of FXTAS cases have been demonstrated to contain FMR1 mRNA but not FMRP [16,17]. Interestingly, FMRpolyG peptides produced by out-of-phase translation are observed in the nucleus [Todd et al., 2014 - PMID 23602499]; however, their functional significance is not known.
Iron is essential for many facets of cell metabolism, including DNA synthesis, mitochondrial respiration, synthesis of neurotransmitters, and signal transduction in the CNS. In particular, iron is required for many reactions that modulate CNS function including the synthesis of tyrosine hydroxylase, myelin, and neurotransmitters such as dopamine, norepinephrine, and serotonin [18,19]. On the other hand, uncomplexed iron reacts with molecular oxygen to generate the reactive oxygen species that lead to oxidative stress [20,21], and contributes to the development of neurodegenerative diseases. Post-transcriptional regulation of genes involved in iron metabolism is mediated by the interaction of iron regulatory proteins (IRPs) with RNA stem-loop sequence elements known as iron-responsive elements (IREs). Indeed, IREs regulate the expression of iron-binding proteins (IBPs). Processes that alter the transcription and/or translation of IRP/IBPs will result in altered iron metabolism, and therefore in the level of oxidative stress, itself a risk factor for neurodegeneration [22]. What is generally unknown about such disorders is whether iron dysregulation is a cause or consequence of the disease.
We have previously demonstrated that the transportation of iron from the circulation into the brain through the spinocerebral fluid and the blood brain barrier is altered in FXTAS [23]. We demonstrated both greatly increased iron deposition and alterations of the levels of specific IBPs/IRPs within the choroid plexus and brain capillaries [23]. Based on the observed altered iron transport into the CNS we hypothesized that iron levels may be altered within specific brain areas of the FXTAS brain. Since the cerebellum is involved in movement control and the dentate nucleus is the major outflow tract of the cerebellum, and lesions in these structures lead to gait ataxia and action tremor, the two core features of FXTAS, we first analyzed iron deposition in cerebellum. However, only 20-25% cases demonstrated a mild increase of iron in the cerebellar cortex and the dentate nuclei of the cerebellum [24]. One of the cerebral structures involved in motor control is the striatum, which participates in the control of motor learning, motor performance and sequences of movement. The striatum is also involved in cognition, emotions, and learning. We here investigate a potential iron dysregulation in the striatum, specifically in the putamen of subjects with FXTAS.
Materials
Sample collection
Samples from 9 FXTAS subjects and 9 control subjects were obtained from the FXTAS brain repository at the University of California, Davis, School of Medicine. Additional control tissue was obtained from the Pathology Department at the University of California, Davis, Health System. Control tissue was obtained from subjects who did not have any significant neurological history. Tissue specimens were obtained through consented autopsies with institutional review board approval. FXTAS cases were clinically diagnosed based on the presence of intention tremor, cerebellar ataxia, Parkinsonism, memory and executive function deficits, and autonomic dysfunction, and confirmed with the posterior presence of intranuclear ubiquitin inclusions in brain cells.
Perl’s staining
A block of cerebellar tissue (2 × 2 × 0.25 μm2) containing putamen for each case was immersed in 20% sucrose (Fisher, USA) and embedded in OCT (Fisher). Blocks were cut into 12 μm sections using a cryostat. Sections were left to dry for 10-30 min at 37° C before being washed with deionized water. Slides were submerged in a solution containing a 1:1 ratio of 20% hydrochloric acid (Fisher) and 10% potassium ferrocyanide (Fisher) for 20 minutes at room temperature. The presence of blue deposits (iron bound to hemosiderin) was confirmed using a microscope (Nikon Eclipse E200). Slices were counterstained with nuclear fast red (Ricca Chemical Company, USA) for 5 min. Slides were washed with water, dehydrated with ethanol, and cleared with xylene before being coverslipped with Permount (Fisher).
Analysis
A 0.5 cm2 area of the putamen was imaged using a Keyence BZ9000 microscope. Each 0.5 cm2 image was a merge of 36 single 20x images. Analysis was conducted using the NIH program ImageJ. The desired blue color (iron) was measured and the percentage of area occupied by iron was compared between FXTAS and control subjects using T-test analysis. A p value of 0.05 was used for statistical significance.
Results
Iron accumulates in the putamen in FXTAS
We used random samples of putamen obtained from 9 FXTAS and 9 control cases to study iron localization. We first attempted to detect ferrous iron (Fe2+) using Turnbull’s staining, however we did not obtained visible staining. This may indicate that Fe2+ is not accumulated in FXTAS, however the lack of staining it is most likely a consequence of the oxidation of Fe2+ to Fe3+ during tissue preparation and histochemical analysis [25]. We next used Perl’s method which detects ferric iron (Fe3+) bound to hemosiderin [26]. We found intracellular iron deposits in the putamen in most control and FXTAS subjects (Fig. 1A-H). The fractional area of putamen occupied by iron deposits in FXTAS cases was approximately 29-fold greater than in controls (1.16 % ± 0.4sem in FXTAS; 0.04 % ± 0.1sem in control; p = 0.03; Fig.1I).
Figure 1.
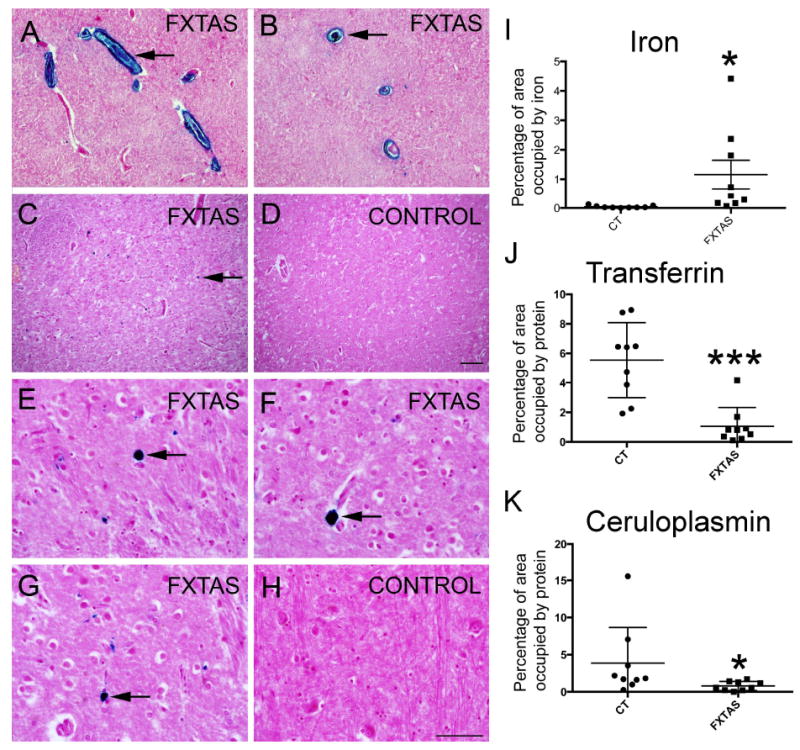
Iron bound to hemosiderin (blue) accumulated in the putamen in FXTAS. Iron (blue) in sagittal (A) and coronal (B) sections of capillaries showed a great amount of iron in the walls and within the capillaries. Iron was present in the putamen parenchyma in FXTAS (C, E-G) but not in control tissue (D, H). Arrows point to iron deposits. Tissue is co-stained with nuclear fast red. I-K: The amount of iron was increased while the amount of Tf and Cp was decreased in the putamen in FXTAS. Iron: blue, Perl’s method; Tf and Cp: brown, immunostaining. Tf: Transferrin; Cp: ceruloplasmin. Scale bar: A-D: 200 μm; E-H: 100 μm.
Transferrin and ceruloplasmin levels are decreased in the putamen in FXTAS
To further investigate potential iron dysregulation in the brains of subjects with FXTAS, we performed immunostaining with antibodies against iron-binding proteins (Fig. 1). We first analyzed the distribution and amount of (Tf), which was observed to be intracellular. We observed a 5-fold decrease in the amount of Tf in the putamen for FXTAS subjects compared to controls (1.06 % ± 0.3 in FXTAS; 5.57 % ± 0.7 in control; p=0.0002, Fig. 1J). The immunostaining with an antibody against ceruloplasmin (Cp) revealed that the ceruloplasmin levels in the putamen were quite heterogeneous across all cases. However, we found that there was a downward trend in the amount of Cp in FXTAS cases compared to control subjects that approached but did not meet significance (0.78 % ± 1.9 in FXTAS; 3.85 % ± 1.5 in control; p=0.07, Fig. 1C).
The number of cells containing detectable levels of iron bound to hemosiderin is substantially increased in the putamen in FXTAS cases
We also determined whether there was a differential accumulation of iron in the different types of cells populating the putamen, by co-staining tissue with the Perl’s method and antibodies against specific cellular markers (NeuN for neurons, S100 for astrocytes, and Sox10 for oligodendrocytes). We found that there were marginally more neurons and substantially more oligodendrocytes containing iron deposition in FXTAS than in control cases (Neurons: 12.21 % ± 4.0 in FXTAS, 4.5 % ± 0.9 in control, p = 0.08, Fig. 2A,D; oligodendrocytes: 20.41 % ± 3.7 in FXTAS, 3.66 % ± 0.6 in control, P = 0.0008, Fig. 2C,D). The average fraction of astrocytes containing iron in FXTAS was also increased in FXTAS but this increase did not meet significance (5.1 ± 1.5 in FXTAS, 2.9 ±0.2 in control, P = 0.23, Fig. 2B,D).
Figure 2.
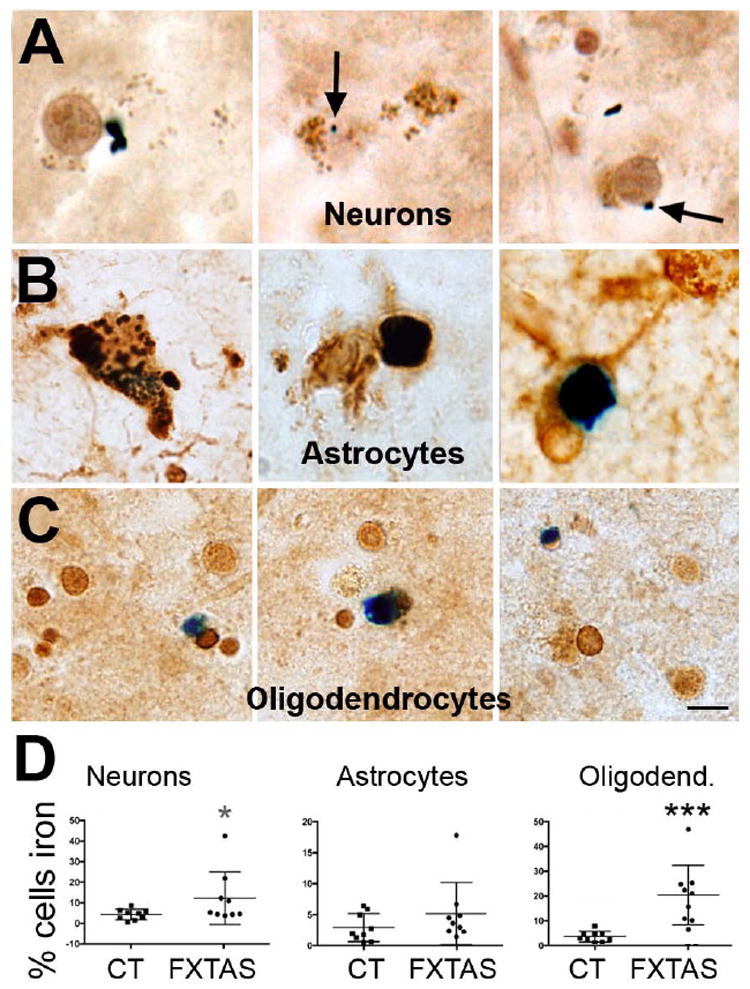
The number of oligodendrocytes containing iron drastically increased, while the number of neurons containing iron marginally increased in FXTAS. However, the number of astrocytes containing iron was similar in FXTAS and control cases. A, B, C: Representative cell-types (A: neurons; B: astrocytes; C: oligodendrocytes) containing iron in FXTAS. D: Number of cells containing iron for each control and FXTAS case included in this study. Iron: blue, Perl’s method. CT: control. Calibration bar: 5 μm.
The number of neurons and glial cells that contain ceruloplasmin, but not transferrin, is decreased in FXTAS
To assess whether there was a differential cellular accumulation of iron-related proteins, we co-stained tissue with antibodies against specific cellular markers (NeuN for neurons, S100 for astrocytes, and Sox10 for oligodendrocytes) and an antibody against Tf. We quantified the number of each cell type expressing Tf and compared this number between the FXTAS and the control cases. We found that all cell types analyzed presented with equal numbers of Tf-expressing cells in FXTAS and control cases, with Tf-expressing astrocytes and neurons accounting for approximately 50% of the total of each cell type (Fig. 3) and Tf-expressing oligodendrocytes for 70% of total oligodendrocytes. By contrast, there was a generalized decreased in the number of cells that contained Cp. Nearly all cells contained Cp in the control cases (neurons: 97.36 % ± 0.4; astrocytes: 97.73 % ± 0.6; oligodendrocytes: 98.50 % ± 0.3), however the number of cells containing Cp was decreased in FXTAS (neurons: 39.81 % ± 6.1, Fig 4A,D; astrocytes: 50.66 % ± 4.5, Fig. 4B,D; oligodendrocytes: 83.43 %± 1.2; all p > 0.0001, Fig 4).
Figure 3.
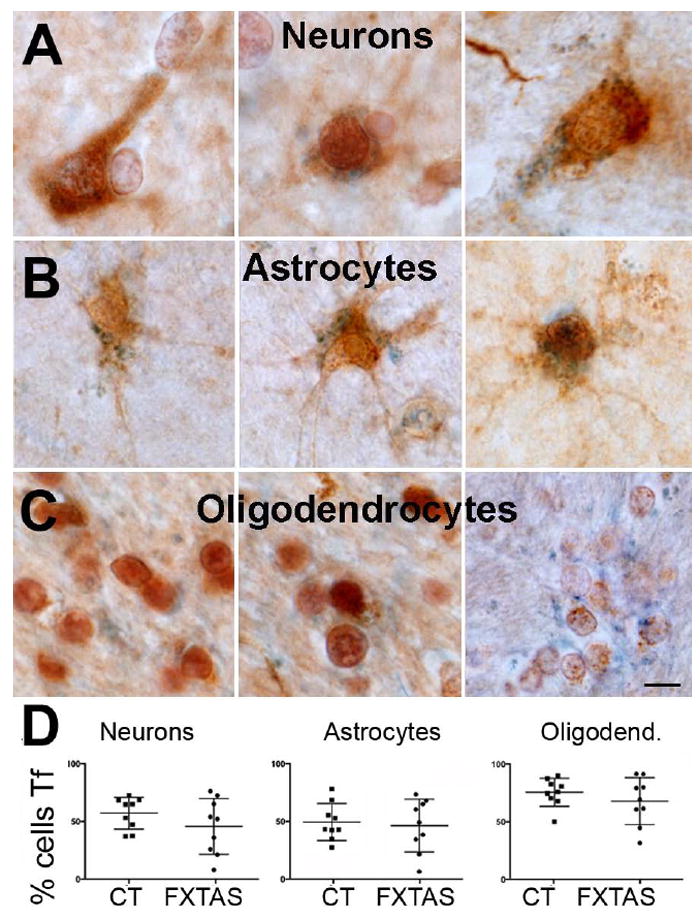
The number of neurons, astrocytes and oligodendrocytes containing Tf was similar in FXTAS and control cases. A, B, C: Representative cell-types (A: neurons; B: astrocytes; C: oligodendrocytes) containing Tf in FXTAS. D: Number of cells containing Tf for each control and FXTAS case included in this study. Tf: blue. Tf: Transferrin. CT: control. Calibration bar: 5 μm.
Figure 4.
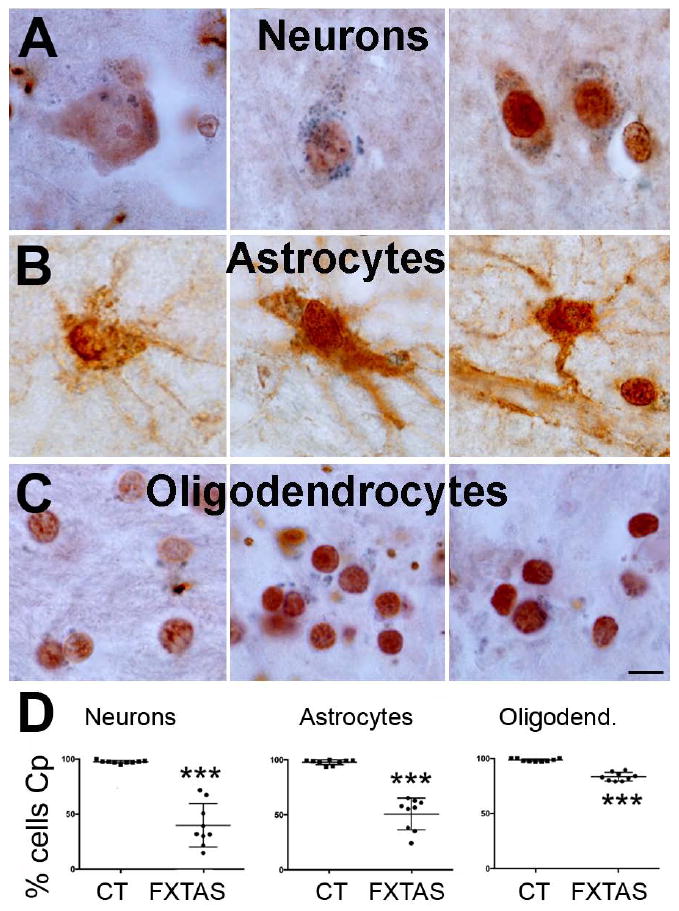
The number of neurons, astrocytes and oligodendrocytes containing Cp drastically decreased in FXTAS when compared to control cases. A, B, C: Representative cell-types (A: neurons; B: astrocytes; C: oligodendrocytes) containing Cp in FXTAS. D: Number of cells containing Cp for each control and FXTAS case included in this study. Cp: blue. Cp: ceruloplasmin. CT: control. Calibration bar: 5 μm.
The number of microglial cells containing iron, transferrin and ceruloplasmin is increased in FXTAS
We analyzed the number of microglial cells (immune cells of the CNS), containing iron, Tf and Cp, and found that there was an increase in the number of microglial cells that contained iron and both proteins.
The number of microglial cells that contained iron was more than double in FXTAS when compared to control cases (11.9 ± 2.4 in FXTAS, 4.5 ± 0.9 in control, P = 0.01, Fig 5A,D). In addition, while the increase of microglial cells containing Cp greatly increased (7 fold; 66.15 % ± 2.8 in FXTAS; 9.41 % ± 2.2 in control; p < 0.0001, Fig. 5C,D), the increase in the number of cells that contained Tf was significant, albeit more modest (1.7 fold; 28.74 % ± 0.04 in FXTAS; 16.57 % ± 0.01 in control; p = 0.02, Fig. 5B,D).
Figure 5.
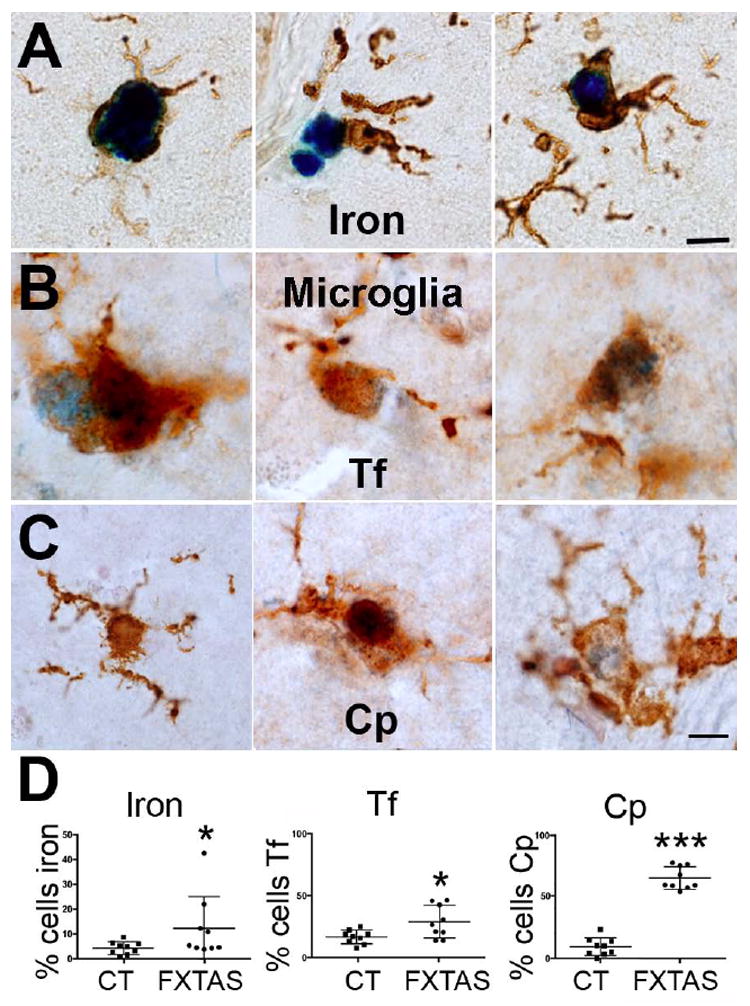
The number of microglial cells containing iron, Tf, and and Cp was increased in the putamen in FXTAS. A-C: Representative microglial cells containing iron, Tf and Cp. D-F: Number of microglial cells containing iron, Tf and Cp for each control and FXTAS case included in this study. Iron, Tf and Cp: blue. Tf: Transferrin. Cp: ceruloplasmin; CT: control. Calibration bar: 5 μm.
Summarizing, we found increased iron deposition and the number of neuronal and glial cells that accumulate iron in the putamen FXTAS. We also found a generalized decreased of the amount of Tf and Cp present in the putamen together a decreased in the number of neurons and glial cells that contained Cp. However, we found an increased in the accumulation of iron, Tf, and Cp in microglial cells in the FXTAS putamen. Overall, there is a deficit in proteins that eliminate extra iron from the cells with the concomitant increased in the deposit of cellular iron.
Discussion
We here demonstrate an excessive deposition of iron bound to hemosiderin in the putamen of patients with FXTAS. Hemosiderin is a degradation product of ferritin, and is prominent during conditions of iron overload [27]. Alteration of iron metabolism in the central nervous system is known to cause motor impairment and cognitive deficits [28], similar to those present in FXTAS. Iron enters the brain through the blood brain barrier and the choroid plexus cerebrospinal fluid barrier. We initially asked if iron levels were altered in peripheral blood in FXTAS and analyzed peripheral blood in three cases of FXTAS but did not find any alteration in iron levels or iron related proteins (data not published). On we the other hand, previously demonstrated an alteration of iron transport into the brain in the choroid plexus, which suggests that levels of iron may be altered in the brain parenchyma [23]. Brain regions affected in FXTAS include the cerebellum, where the number of Purkinje cells is reduced and present with simple and twin inclusions [29]; and the putamen, which is hypotrophic and presents with numerous intranuclear inclusions. We previously demonstrated that increased iron deposition in the cerebellar cortex and cerebellar dentate nucleus cannot be used as a FXTAS distinguishing feature, because whereas some FXTAS cases presented with an increase in iron deposition when compared to control, most did not [24].
In order to understand iron metabolism in the putamen of FXTAS patients, we first analyzed levels of iron in capillaries of the putamen and found deposition of iron in the blood vessels in FXTAS but not in control cases. This observation indicates that a similar alteration of iron metabolism to that in the choroid plexus cerebrospinal fluid barrier in FXTAS also takes place in the blood brain barrier. These data indicate that an alteration in iron transport into the brain is a characteristic feature of FXTAS. We found substantial iron bound to hemosiderin in the parenchyma of the putamen in FXTAS cases but not in control tissue. Studies of MRI patients with FXTAS rendered evidence of subcortical gray matter degeneration involving the striatum, including symmetric hypo-intensity in the putamen and caudate in T2-weighted SE images was present in all four patients examined, indicative of iron accumulation [30,31]. Our data are in agreement with previous MRI data.
We do not know if this accumulation of iron in the putamen is a consequence or a cause of the RNA toxicity that occurs in FXTAS, but we hypothesize that most likely this finding is a consequence of RNA toxicity. Processes that alter the transcription and/or translation of IRPs or IBPs will result in altered iron metabolism and increased oxidative stress. Concomitantly, we discovered a decrease in the expression of Cp, but not of Tf. Tf binds to iron and transports it into the cell while Cp regulates the exit of excess iron from the cell [32]. The decrease in the level of Cp proteins in the cells of the putamen explains the iron accumulation within these cells.
Since iron is one of the major sources of intracellular reactive oxygen species (ROS), accumulation of non-heme iron is believed to play a major role in degeneration of the basal ganglia both in normal aging and neurodegenerative diseases. We found that all the major cell types accumulate iron in the putamen, but neurons and oligodendrocytes are most affected by this alteration, and therefore these cells are going to be subjected to high ROS and the consequent degeneration, decreasing the number of cells within the putamen and the level of fiber myelination. High ROS and increased cell death are in agreement with previous reports demonstrating pronounced atrophy in FXTAS [30]. White matter degeneration has been described as the most prominent neuropathologic characteristic of FXTAS [7,33]. The very pronounced accumulation of iron in oligodendrocytes may be the link between FMR1 mRNA gain of function and the white matter pathology characteristic of FXTAS. Microglial cells greatly accumulate iron and iron-binding proteins, indicating the attempt by the immune system to clean the parenchyma from excess iron and unutilized iron-binding proteins and therefore to normalize iron metabolism in FXTAS.
Iron deposition has been linked to other degenerative diseases. Neurodegeneration with brain iron accumulation (NBIA) is a group of syndromes characterized by excess iron accumulation in the globus pallidus and to a lesser degree in the substantia nigra. It clinically presents as a neurodegenerative disease with progressive hypo- and/or hyperkinetic movement disorders and a variable degree of pyramidal, cerebellar, peripheral nerve, autonomic, cognitive and psychiatric involvement, and visual dysfunction [34,35]. Other diseases that present with an increased iron deposition include Parkinson’s and Alzheimer diseases, amyotrophic lateral sclerosis, restless legs syndrome, and prion diseases [36]. Interestingly, Parkinsonism and dementia are common in those with FXTAS, and restless legs syndrome is more common in those with the premutation compared to controls [2,28]. Due the accumulation of iron in FXTAS, the use of iron-chelating agents such as deferiprone may be adequate for treatment of iron-related motor symptoms symptoms.
Overall, we demonstrated that iron metabolism is altered in the putamen in FXTAS. This alteration has implications for understanding the pathophysiology of FXTAS and for the development of new clinical treatments.
Acknowledgments
We acknowledgment Randi Hagerman for her critical review of the manuscript.
Funding sources: This work was supported by the National Institute of Health grants MH094681 (Dr. Martínez-Cerdeño), HD040661 (Dr. P Hagerman), HD036071 (covered brain collection), and by the Department of Pathology and Laboratory Medicine at UC Davis and the Shriners Hospitals.
Financial Disclosures
VMC is funded by NIH, Shriners Hospitals and UC Davis grants.
PJH is funded by NIH, DoD, UC Davis grants, and private donors.
JA, HR, BS, AH, MS, BS, MD, DJ, and VMC: None
Footnotes
Authors’ Roles
JA: Collaborated in project design, obtained most of the data and took images.
HR, BS, AH, MS, BS, MD, DJ: Participated in data acquisition.
PH: Collaborated in study design and writing of the manuscript
VMC: Designed study, analyzed and interpreted data, made figures, wrote the manuscript, and supervised study.
Conflict of interest: PJH holds patents for assays of CGG-repeat expansion and for FMRP ELISA; he is also in non-remunerative collaborations with Pacific Biosciences, Inc. and Roche Diagnostics. Authors otherwise declare no conflict of interest.
References
- 1.Kogan CS, Turk J, Hagerman RJ, Cornish KM. Impact of the Fragile X mental retardation 1 (FMR1) gene premutation on neuropsychiatric functioning in adult males without fragile X-associated Tremor/Ataxia syndrome: a controlled study. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:859–872. doi: 10.1002/ajmg.b.30685. [DOI] [PubMed] [Google Scholar]
- 2.Tassone F, Greco CM, Hunsaker MR, Seritan AL, Berman RF, et al. Neuropathological, clinical and molecular pathology in female fragile X premutation carriers with and without FXTAS. Genes Brain Behav. 2012;11:577–585. doi: 10.1111/j.1601-183X.2012.00779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coffey SM, Cook K, Tartaglia N, Tassone F, Nguyen DV, et al. Expanded clinical phenotype of women with the FMR1 premutation. Am J Med Genet A. 2008;146A:1009–1016. doi: 10.1002/ajmg.a.32060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Revenga L, Madrigal I, Pagonabarraga J, Xuncla M, Badenas C, et al. Penetrance of FMR1 premutation associated pathologies in fragile X syndrome families. Eur J Hum Genet. 2009;17:1359–1362. doi: 10.1038/ejhg.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagerman P. Fragile X-associated tremor/ataxia syndrome (FXTAS): pathology and mechanisms. Acta Neuropathol. 2013;126:1–19. doi: 10.1007/s00401-013-1138-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129:243–255. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- 7.Greco CM, Hagerman RJ, Tassone F, Chudley AE, Del Bigio MR, et al. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain. 2002;125:1760–1771. doi: 10.1093/brain/awf184. [DOI] [PubMed] [Google Scholar]
- 8.Greco CM, Tassone F, Garcia-Arocena D, Tartaglia N, Coffey SM, et al. Clinical and neuropathologic findings in a woman with the FMR1 premutation and multiple sclerosis. Arch Neurol. 2008;65:1114–1116. doi: 10.1001/archneur.65.8.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farzin F, Perry H, Hessl D, Loesch D, Cohen J, et al. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr. 2006;27:S137–144. doi: 10.1097/00004703-200604002-00012. [DOI] [PubMed] [Google Scholar]
- 10.Chonchaiya W, Au J, Schneider A, Hessl D, Harris SW, et al. Increased prevalence of seizures in boys who were probands with the FMR1 premutation and co-morbid autism spectrum disorder. Hum Genet. 2012;131:581–589. doi: 10.1007/s00439-011-1106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunningham CL, Martinez Cerdeno V, Navarro Porras E, Prakash AN, Angelastro JM, et al. Premutation CGG-repeat expansion of the Fmr1 gene impairs mouse neocortical development. Hum Mol Genet. 2011;20:64–79. doi: 10.1093/hmg/ddq432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, et al. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet. 2000;66:6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willemsen R, Hoogeveen-Westerveld M, Reis S, Holstege J, Severijnen LA, et al. The FMR1 CGG repeat mouse displays ubiquitin-positive intranuclear neuronal inclusions; implications for the cerebellar tremor/ataxia syndrome. Hum Mol Genet. 2003;12:949–959. doi: 10.1093/hmg/ddg114. [DOI] [PubMed] [Google Scholar]
- 14.Brouwer JR, Mientjes EJ, Bakker CE, Nieuwenhuizen IM, Severijnen LA, et al. Elevated Fmr1 mRNA levels and reduced protein expression in a mouse model with an unmethylated Fragile X full mutation. Exp Cell Res. 2007;313:244–253. doi: 10.1016/j.yexcr.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sellier C, Rau F, Liu Y, Tassone F, Hukema RK, et al. Sam68 sequestration and partial loss of function are associated with splicing alterations in FXTAS patients. EMBO J. 2010;29:1248–1261. doi: 10.1038/emboj.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tassone F, Hagerman RJ, Garcia-Arocena D, Khandjian EW, Greco CM, et al. Intranuclear inclusions in neural cells with premutation alleles in fragile X associated tremor/ataxia syndrome. J Med Genet. 2004;41:e43. doi: 10.1136/jmg.2003.012518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashem V, Galloway JN, Mori M, Willemsen R, Oostra BA, et al. Ectopic expression of CGG containing mRNA is neurotoxic in mammals. Hum Mol Genet. 2009;18:2443–2451. doi: 10.1093/hmg/ddp182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beard J. Iron deficiency alters brain development and functioning. J Nutr. 2003;133:1468S–1472S. doi: 10.1093/jn/133.5.1468S. [DOI] [PubMed] [Google Scholar]
- 19.Beard J, Erikson KM, Jones BC. Neonatal iron deficiency results in irreversible changes in dopamine function in rats. J Nutr. 2003;133:1174–1179. doi: 10.1093/jn/133.4.1174. [DOI] [PubMed] [Google Scholar]
- 20.Jomova K, Valko M. Importance of iron chelation in free radical-induced oxidative stress and human disease. Curr Pharm Des. 2011;17:3460–3473. doi: 10.2174/138161211798072463. [DOI] [PubMed] [Google Scholar]
- 21.Nunez MT, Urrutia P, Mena N, Aguirre P, Tapia V, et al. Iron toxicity in neurodegeneration. Biometals. 2012;25:761–776. doi: 10.1007/s10534-012-9523-0. [DOI] [PubMed] [Google Scholar]
- 22.Hare D, Ayton S, Bush A, Lei P. A delicate balance: Iron metabolism and diseases of the brain. Front Aging Neurosci. 2013;5:34. doi: 10.3389/fnagi.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ariza J, Steward C, Rueckert F, Widdison M, Coffman R, et al. Dysregulated iron metabolism in the choroid plexus in fragile X-associated tremor/ataxia syndrome. Brain Res. 2015;1598:88–96. doi: 10.1016/j.brainres.2014.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers H, Ariza J, Monterrubio A, Hagerman P, Martinez-Cerdeno V. Cerebellar Mild Iron Accumulation in a Subset of FMR1 Premutation Carriers with FXTAS. Cerebellum. 2016 doi: 10.1007/s12311-016-0798-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meguro R, Asano Y, Iwatsuki H, Shoumura K. Perfusion-Perls and -Turnbull methods supplemented by DAB intensification for nonheme iron histochemistry: demonstration of the superior sensitivity of the methods in the liver, spleen, and stomach of the rat. Histochem Cell Biol. 2003;120:73–82. doi: 10.1007/s00418-003-0539-y. [DOI] [PubMed] [Google Scholar]
- 26.McManus, Mowry . Staining methods: histological and histochemical. Medical Division of Harper and Brothers; New York, NY: 1960. [Google Scholar]
- 27.Brittenham GM. New advances in iron metabolism, iron deficiency, and iron overload. Curr Opin Hematol. 1994;1:101–106. [PubMed] [Google Scholar]
- 28.Hagerman R, Hagerman P. The other face of the fragile X gene: Advances in clinical and molecular understanding of the FMR1 premutation and FXTAS. Lancet Neurol. 2013 doi: 10.1016/S1474-4422(13)70125-X. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ariza J, Rogers H, Monterrubio A, Reyes-Miranda A, Hagerman PJ, et al. A Majority of FXTAS Cases Present with Intranuclear Inclusions Within Purkinje Cells. Cerebellum. 2016 doi: 10.1007/s12311-016-0776-y. [DOI] [PubMed] [Google Scholar]
- 30.Scaglione C, Ginestroni A, Vella A, Dotti MT, Nave RD, et al. MRI and SPECT of midbrain and striatal degeneration in fragile X-associated tremor/ataxia syndrome. J Neurol. 2008;255:144–146. doi: 10.1007/s00415-007-0711-8. [DOI] [PubMed] [Google Scholar]
- 31.Wang JY, Hessl D, Schneider A, Tassone F, Hagerman RJ, et al. Fragile X-Associated Tremor/Ataxia Syndrome: Influence of the FMR1 Gene on Motor Fiber Tracts in Males With Normal and Premutation Alleles. JAMA Neurol. 2013:1–8. doi: 10.1001/jamaneurol.2013.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moos T. Brain iron homeostasis. Dan Med Bull. 2002;49:279–301. [PubMed] [Google Scholar]
- 33.Filley CM, Brown MS, Onderko K, Ray M, Bennett RE, et al. White matter disease and cognitive impairment in FMR1 premutation carriers. Neurology. 2015;84:2146–2152. doi: 10.1212/WNL.0000000000001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider SA. Neurodegeneration with Brain Iron Accumulation. Curr Neurol Neurosci Rep. 2016;16:9. doi: 10.1007/s11910-015-0608-3. [DOI] [PubMed] [Google Scholar]
- 35.Kruer MC. The neuropathology of neurodegeneration with brain iron accumulation. Int Rev Neurobiol. 2013;110:165–194. doi: 10.1016/B978-0-12-410502-7.00009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Batista-Nascimento L, Pimentel C, Menezes RA, Rodrigues-Pousada C. Iron and neurodegeneration: from cellular homeostasis to disease. Oxid Med Cell Longev. 2012;2012:128647. doi: 10.1155/2012/128647. [DOI] [PMC free article] [PubMed] [Google Scholar]


