Abstract
Carbon nanodots (CNDs) have attracted great attention due to their superior solubility, biocompatibility, tunable photoluminescence, and opto-electronic properties. This work describes a new fluorescence-based spectroelectrochemistry approach to simultaneously study the photoluminescence and wavelength dependent photocurrent of microwave synthesized CNDs. The fluorescence of CNDs has a selective quench upon a reversible redox couple, ferricyanide/ferrocyanide,− reaction during the cyclic voltammetry. The CNDs modified gold slide electrode demonstrates wavelength dependent photocurrent generation during the fluorescence-electrochemical study, suggesting potential application in photoelectronics. UV-Vis absorption and electrochemistry are used to quantify the energy gap of the CNDs, and then to calibrate a Hückel model for the CNDs electronic energy levels. The Hückel (or tight binding) model treatment of an individual CND as a molecule combines the conjugated π states (C=C) with the functional groups (C=O, C-O, and COOH) associated with the surface electronic states. This experimental and theoretical investigation of the CNDs provides a new perspective on the optoelectronic properties of CNDs and should aid in their development for practical use in biomedicine, chemical sensing, and photoelectric devices.
Graphical abstract
A new perspective on optoelectronic properties of CNDs is obtained from a novel fluorescence spectroelectrochemitry and comprehensive energy gap investigation.
Introduction
Carbon-based nanodots (CNDs) are reported to be composed of polyatomic carbon domains surrounded by amorphous carbon frames and have been synthesized by chemical ablation, electrochemical carbonization, laser ablation, hydrothermal/solvothermal treatment, and microwave irradiation techniques.1, 2 There is continued interest in CNDs because of their physicochemical properties of good solubility, low toxicity, and biocompatibility, along with their favorable optoelectronic properties of strong fluorescence, phosphorescence, chemiluminescence, and photoinduced electron transfer.2–6 As such, CNDs have been found to have potential applications in biomedicine (bioimaging, biosensor, and biomedicine delivery system), chemical sensing, and photoelectric devices (solar cells, supercapacitor, photocatalysis and light-emitting devices).2–4 For the mechanism for light emission in CNDs,7, 8 some workers have proposed that the bandgap transitions responsible for fluorescence arise from conjugated π-domains consisting of sp2 hybridized islands rich in π-electrons, bond disorder induced energy gaps,9, 10 or giant red-edge effects that give rise to strong excitation wavelength dependent fluorescence.11, 12 These mechanisms are similar to those used to understand the emissive properties of single-layer graphene and graphene oxides.13, 14 Other workers ascribe the light emission characteristics to quantum confinement effects,15 size-dependent optical properties,16 surface-related defect sites,17 and radiative recombination of excited surface states.18 The lack of consensus on the relevant photophysical properties of CNDs is likely caused by variations in CND size and surface state properties, resulting from the many different synthetic routes used in their preparation. A poor understanding about the structure of CNDs in terms of their functional groups, defects, adsorbates, and electronic structure continues to impede an agreed upon mechanism.
This work uses a new combined fluorescence-electrochemical approach to investigate the optoelectronic properties of CNDs. Although numerous spectroelectrochemical techniques have been developed, such as electrochemical fluorescence spectroscopy,19, 20 electrochemical surface/tip-enhanced Raman spectroscopy,21, 22 and ultraviolet-visible (UV-Vis) absorption spectroelectrochemistry,23, 24 the simultaneous study of fluorescence and electrochemical measurements which focus on the effort of chemically reversible reactions on CNDs is rare. Here, water-soluble luminescent CNDs were synthesized by a simple one-step microwave route and were characterized by transmission electron microscopy (TEM), atomic force microscopy (AFM), X-ray photoelectron spectroscopy (XPS), Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy, X-ray powder diffraction (XRD), UV-Vis spectroscopy, fluorescence spectroscopy, pH dependent zeta potential, and quantum yield measurements. Their potential application in bioimaging was assessed from their excitation-dependent fluorescence, and their potential use as chemiluminescent sensors was evaluated by examining the effect of the ferricyanide/ferrocyanide redox couple on their fluorescence spectrum. We also examined the excitation wavelength dependence of the photocurrent (action spectrum) generated by CNDs that were immobilized on gold slide electrodes to assess their potential application in photoelectric devices. Optical and electrochemical measurements were used to measure the energy gap of the CNDs, and Hückel level calculations of the HOMO (highest occupied molecular orbital) and LUMO (lowest unoccupied molecular orbital) were fitted to the energy gap measurements by treating a CND as a molecule.
Materials and Methods
Synthesis of CNDs
A microwave assisted synthesis of CNDs was performed using citric acid and urea as precursors.25 Briefly, 1.0 g of urea (Aldrich) and 1.0 g of citric acid (ACROS Organics) were simultaneously added to 1.0 mL of deionized water to form a homogeneous solution and then heated in a microwave synthesizer (CEM Corp 908005 Microwave Reactor Discovery System) at a power of 150 W for 12 minutes. After cooling, the aqueous reactant mixture was purified using a centrifuge (Solvall Legend XFR Floor Model Centrifuge) at 3500 r/min for 20 min to remove large and aggregated particles. The dark-brown solution was further purified using a dialysis membrane (Scientific Fisher) with a molecular weight cut off of 1000 Da for 24 hours. To obtain the solid sample, the resulting solution was finally dried with a freeze dryer (Labconco Free Zone 6 Freeze Dryer) for 24 hours.
CNDs Characterization
Atomic force microscope (AFM, Agilent Technologies 5600 LS Series) and transmission electron microscopy (TEM, Carl Zeiss Libra 120 Plus) were used to study the size of the CNDs. Fourier transform infrared spectroscopy (FTIR, Varian 670), Raman spectroscopy (Horiba XploRA One Raman Confocal Microscope System), X-ray photoelectron spectroscopy (XPS, Thermo Fisher ESCALAB 250 Xi), and X-ray powder diffraction (XRD, Agilent Technologies Oxford Gemini) were used to determine the elemental composition and chemical structure of the CNDs. A Zetasizer nano-ZX (Malvern Instruments ZEN3600) was used to study the stability of the CNDs as a function of pH (Fisher Scientific pH 2100). Ultraviolet-visible spectroscopy (UV-Vis spectroscopy, Varian Cary 6000i) and fluorescence spectroscopy (Varian Cary Eclipse) were used to investigate the absorbance and fluorescence properties of the CNDs, respectively.
Cell Culture and Bioimaging
HepG2 cells were obtained from American Type Culture Collection (ATCC) and cultured in an Eagle’s Minimum Essential Medium [2 mM L-glutamine, 1 mM sodium pyruvate, and 1500 mg/L sodium bicarbonate] (ATCC) containing 10% Fatal Bovine Serum (Fisher Scientific, USA), 1% antibiotic with 100 UI/mL Penicillin and 100 µg/mL Streptomycin (Fisher Scientific, USA). The cells were incubated in a humidified incubator with 5% CO2 at 37 °C. Trypsin/EDTA (Fisher Scientific, USA) was used to passage the cells serially. The cells were seeded (150,000 cells/dish) in petri dishes with cover slips in the dish. After culturing for 24 hours, the cells were treated with CNDs at a concentration of 0.3 mg/mL. Untreated cells without CNDs were used as a control. The cells were imaged using a confocal microscope (Carl Zeiss Libra 120 Plus Z1) by mounting the cover slips onto the glass slides. All the images were taken at 10× magnification and at a scale bar of 20 µm.
Fluorescence Spectroelectrochemistry
The electrochemical-fluorescence technique comprises a Biologic VMP3 electrochemical workstation with a two-electrode testing system (a platinum wire as the counter electrode and a gold electrode for the fluorescence spectroelectrochemistry experiment (Fisher Scientific) as the working electrode (replaced by immobilized CNDs gold slide electrode for the photocurrent generation experiment) and the fluorescence spectrophotometer (the excitation wavelength could be varied from 200–700 nm by using a 450 W xenon lamp with an excitation monochromator (the area of illumination is about 5 mm in diameter) and emission could be collected from 300–1000 nm by an emission monochromator, Agilent).
Preparation of Immobilized CNDs at Gold Slide Electrode Surfaces
The gold coated slides (20 nm Au deposited on 8 mm×8 mm glass slide by Physical Vapor Deposition (Kurt Lesker PVD75 E-Beam Evaporator System) were first cleaned with O2 plasma (South Bay Technologies PC2000 Plasma Cleaner) for 15 minutes. The slides were then incubated in a mixture of 1 mM 11-mercaptodecanoic acid (HSC10COOH, Aldrich) and 8-mercapto-octanol (HSC8OH, Aldrich) in an absolute ethanol solution (ACROS Organics) with 1:5 mole ratio overnight to form a self-assembled monolayer (SAM) by alkyl thiols. After SAM formation, the gold slides were incubated in a 0.5 mM 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC, TCI)/ N-hydroxysuccinimide (NHS, Aldrich) for 2 hours to activate the carboxylic acid groups.26, 27 Next, the gold slides were rinsed with deionized water and immediately moved to a freshly prepared 3 mL solution containing 0.3 mg/mL of CNDs for 2 hours. The gold slides were rinsed with deionized water and dried before experiments.
Energy Gap and Molecular Orbital Energy Level Calculation
The optical band gap was determined using an indirect band gap calculation.28 The UV-Vis absorption spectrum calculation was processed by plotting versus hν, where A is the measured absorbance, h is the Plank constant, ν is the frequency, and hν is equal to 1240/wavelength in unit of eV. has a linear relationship with hν with a slope of D and the optical band gap is the x-intercept. Cyclic voltammetry (CV) was used to obtain the HOMO and LUMO energy levels of the CNDs.29 The electrochemical measurement was performed using a three-electrode electrochemical cell with a gold working electrode, a Ag/AgCl reference electrode, and a platinum counter electrode (Fisher Scientific) in a 4.0 mL acetonitrile (Fisher Scientific) solution containing 0.1 M tetrabutylammonium hexafluorophosphate (Fisher Scientific) as the supporting electrolyte and 1 mL of deionized water containing 0.3 mg CNDs. Cyclic voltammetry of the sample was run at a scan rate of 100 mV/s under room temperature. The electrochemical data were used to determine the HOMO and LUMO energy levels; namely, EHOMO= –(Eonset,ox+4.66)eV, and ELUMO=–(Eonset,red+4.66)eV, where Eonset,ox and Eonset,red are the onset of the oxidation and reduction potentials,29 respectively (Note that the formal potential of the Fc+/Fc redox couple was estimated as −5.06 eV in the Fermi scale when the formal potential of the Fc+/Fc redox is 0.40 V versus Ag/AgCl. See details in supplementary information). The Hückel method was used to reproduce the energy gap by assuming a molecular structure of CNDs and adjusting the coulomb and resonance integral values to obtain a HOMO-LUMO gap that is consistent with the energy gap values obtained from the spectral and electrochemical measurements.30 These parameters were used to calculate the different molecular orbital energy levels (See details in supplementary information).
Results and Discussion
CND Synthesis and Characterization
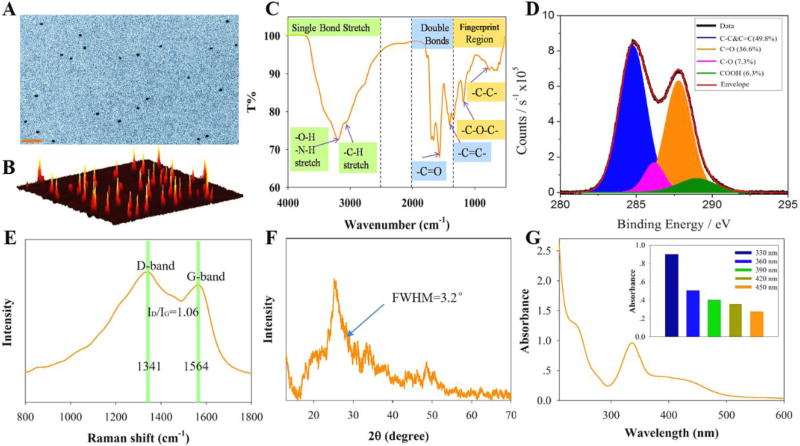
A one-step microwave route was used to synthesize CNDs from citric acid and urea.25 Transmission electron microscopy (Figure 1A) and atomic force microscopy (Figure 1B), with associated height profile analyses (Fig. S1), indicate that the CNDs are spherical and well dispersed, and have an average size of about 3 nm. FTIR spectra of the CNDs (Figure 1C) display broad bands at 3100–3400 cm−1 which are assigned to ν(O-H) and ν(N-H); functionalities that help explain the hydrophilicity and stability of the CNDs in aqueous media. The IR transitions at 768, 1184, 1402 and 1566 cm−1 are assigned to ν(C-C), ν(C-O), ν(C=C) and ν(C=O), respectively.31 The presence of these functionalities at surfaces is corroborated by XPS data (Figure 1D) of the CNDs, which could be fitted by four components: C=C & C-C (49.8%, 284.8 eV), C=O (36.6%, 287.8 eV), C-O (7.3%, 286.5 eV), and COOH (6.3%, 289.0 eV). Associated with the high resolution O (Fig. S2 and Table S1) and N (Fig. S3 and Table S2) XPS spectrum and their simulated peak fits, the survey XPS spectrum analysis shows the atomic ratio of C:O:N is estimated to be 54:27:17 (Table S3). Raman spectra of the CNDs (Figure 1E) show both the D bands at 1341 cm−1 (sp3-hybridized) and G bands at 1564 cm−1 (sp2-hybridized) with an intensity ratio ID/IG of about 1.06, suggesting the presence of a disordered graphite structure.32, 33 The main diffraction peak in the XRD spectrum (Figure 1F) appears at 25.2° with a full width at half maximum (FWHM) of about 3.2°,which corresponds to an interlayer distance of 0.35 nm between the planar carbon based sheets for graphite structure region.34 The zeta potential of the CNDs was −22.3 mV at a pH of 5.86 (Table S4), owing to the presence of carboxylate groups on the surface; further corroborating that the CNDs are highly dispersed and stable in the aqueous system.35, 36 In summation, the structure of the CNDs in this study are spheroidal with an average size of 3 nm and composed of a disordered graphite structure with surrounding amorphous carbon frames and functional groups on the surfaces.
Figure 1.
The CNDs are characterized using different techniques: A) transmission electron micrograph (scale bar is 20 nm), B) atomic force microscopy profile of CNDs distributed on a mica surface, C) Fourier transform infrared spectrum, D) X-ray photoelectron spectrum (C1 signal), E) Raman spectrum, F) X-ray diffraction data, and G) UV-Vis absorption spectrum of CNDs.
Figure 1G shows the absorption spectrum of the CNDs over the spectral range of 200 to 600 nm. There is no obvious absorption feature found above 600 nm. A shoulder/peak in the spectrum at about 236 nm is consistent with π-π* transitions of C-C and C=C bonds in sp2 hybrid regions and the main peak at 331 nm is consistent with n-π* transitions of the C=O moieties.37, 38 The emission of the CNDs occurs over a spectral region from 400 nm to wavelengths longer than 600 nm (see Figure 2A). Relative quantum yield measurements, using quinine sulfate as the reference, give a quantum yield of 8.5% (Table S5) for the as-prepared CNDs.39 Other than the lack of well-defined vibronic structure, these spectra are consistent with that expected for large π-conjugated catacondensed hydrocarbons.
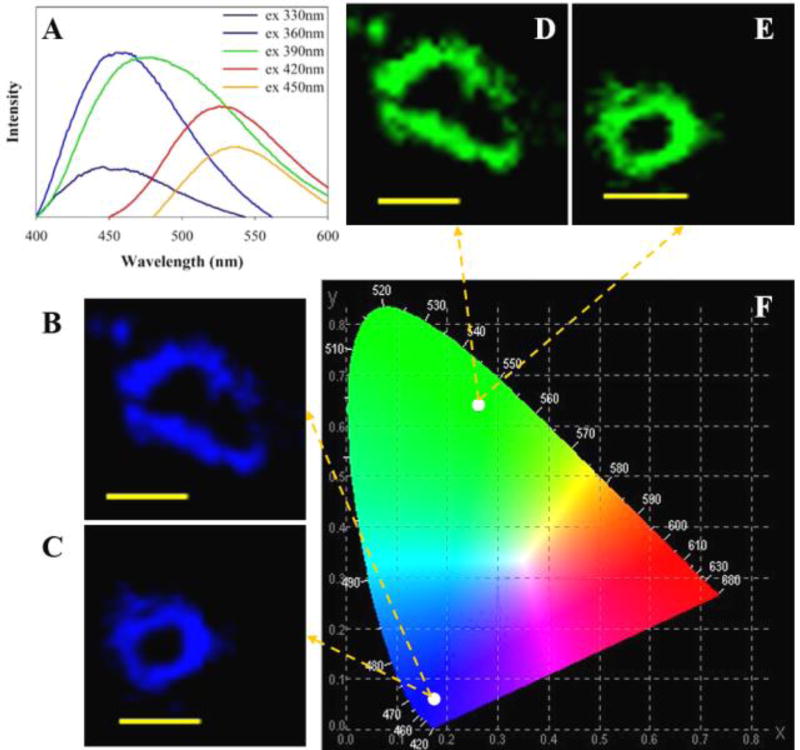
Figure 2.
(A) Fluorescence emission spectra of CNDs in deionized water. (B, C) Confocal images of HepG2 cells that are cultured with CNDs (0.3 mg/mL) for 24 hours; taken at 330 nm excitation. All of the images have a scale bar of 20 µm. (D, E) Confocal images as in panels B and C, but with 450 nm excitation. (F) Calculation of chromaticity coordinates with the emission results under excitation wavelengths of 330 and 450 nm.
CND Fluorescence and Cell Imaging
CNDs can act as blue/green fluorophores in imaging applications.40 Figure 2A shows the fluorescence emission spectra of the synthesized CNDs in deionized water at five different excitation wavelengths (λex=330, 360, 390, 420, and 450 nm). The maximum emission intensity occurs with 360 nm excitation and has a peak emission at 454 nm. The apparent red-shift in the photoluminescence spectra with changing excitation wavelength is in agreement with other reports.41, 42 The potential application of CNDs as a bioimaging agent is confirmed by the uptake of fluorescent CNDs by HepG2 cells.43 Spinning disk confocal microscopy was used to monitor the cellular uptake phenomenon by HepG2 cells after treatment with CNDs for 24 hours (Figure 2). The strong blue and green fluorescence indicates that the CNDs are internalized by the HepG2 cells (see more Fig. S4). Furthermore, the cells were grown in the presence of CNDs (0.3 mg/mL) for 72 hours without any significant cytotoxicity (Fig. S4). The strong blue and green fluorescence was consistent with a chromaticity coordinates calculation (CIE Chromaticity Diagram) that was performed after inputting the emission data obtained from excitation at 330 nm and 450 nm. These results suggest that the, as prepared, CNDs are good candidates for cell imaging agents.
Fluorescence Spectroelectrochemistry
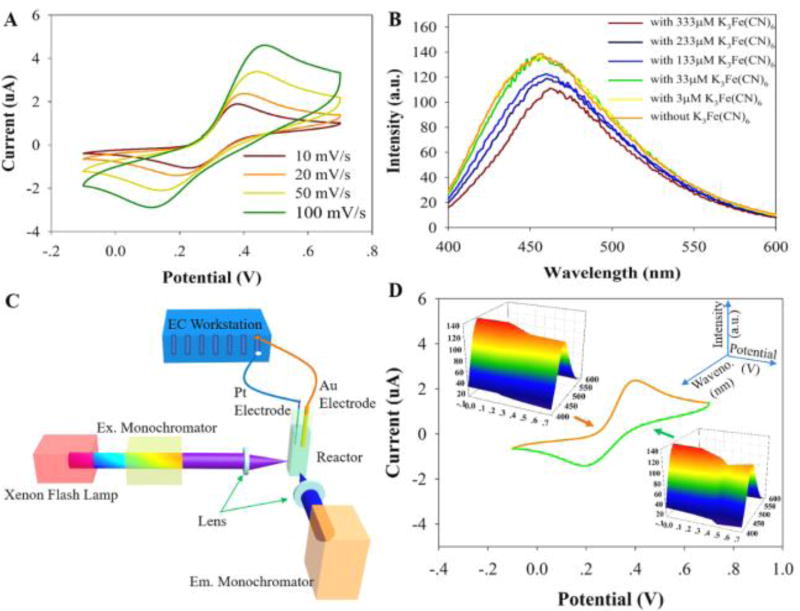
A ferricyanide/ferrocyanide redox couple was chosen as the redox probe for spectroelectrochemical measurements because it is a reversible system with spectroscopically distinguishable redox forms.44, 45 Figure 3A shows cyclic voltammograms (CV) of a ferri-/ferrocyanide solution at scan rates of 10, 20, 50, and 100 mV/s. Figure 3B shows the fluorescence intensity of the CNDs solution (using 360 nm excitation) with increasing potassium ferricyanide (K3Fe(CN)6) concentration. At low concentrations (<33 µM) of K3Fe(CN)6 the fluorescence of the CNDs is not affected strongly; however, from 33 µM to 333 µM of K3Fe(CN)6, the fluorescence intensity of the CNDs decreases and the emission peak red-shifts. This fluorescence quenching canarise from two phenomena. The main one is arising from the reaction of CNDs with Fe(CN)63−(CNDs+Fe(CN)63−→ CNDs*+Fe(CN)64−) which may influence the surface electronic state transitions caused by incomplete passivation. Since the energy gap of surface states is larger than that of the core states (details are shown in the energy gap calculation part) and smaller energy gap induces larger emission wavelength, hence, the red-shift of the fluorescence peaks present with increasing concentrations of K3Fe(CN)6.46, 47 The other factor is probably an optical filtering effect caused by the ferricyanide that absorbs light at a peak wavelength of 420 nm, while ferrocyanide does not (Fig. S5). Note that the fluorescence spectrum of CNDs before and after addition of various concentrations of ferrocyanide does not change (Fig. S6), suggesting that the redox process between K4Fe(CN)6 and K3Fe(CN)6 (the oxidation of ferrocyanide to ferricyanide and the reduction of ferricyanide to ferrocyanide) is responsible for the change in fluorescence intensity of the CNDs.
Figure 3.
(A) Cyclic voltammogram (CV) of a mixture of 333 µM K4Fe(CN)6 and 0.1 M KCl solution between −0.1 V and 0.7 V at 10, 20, 50 and 100 mV/s scan rates. (B) Fluorescence spectrum of solution including 50 µg/mL CNDs and 0.1 M KCl after addition of K3Fe(CN)6 with different concentrations (3, 33, 133, 233, and 333 µM). (C) Schematic view of the setup used for coupling electrochemistry with a fluorescence spectrophotometer. (D) CV of 333 µM K4Fe(CN)6 in 0.1 M KCl between −0.1 V and 0.7 V at scan rate of 20 mV/s with the inserted three-dimensional spectra of fluorescence signal of CNDs during the CV experiment (solution includes 50 µg/mL CNDs, 333 µM K4Fe(CN)6, and 0.1 M KCl).
To record voltammograms forK4Fe(CN)6and the concurrent, real-time influence of K3Fe(CN)6 on the fluorescence properties, a scan rate of 20 mV/s and 333 µM K4Fe(CN)6 were selected for the fluoro-electrochemical studies; see Figure 3C and 3D. Figure 3D shows the fluoroelectrochemical data for the redox cycling of ferrocyanide and ferricyanide. As the ferrocyanide is oxidized to ferricyanide near 0.45 V (vs. Ag/AgCl), the fluorescence peaks of the CNDs decrease (Figure 3D). In addition, the fluorescence peaks of the CNDs increase in the potential range from 0.45 to 0.2 V (vs. Ag/AgCl) on the reverse scan, suggesting the consumption of the electrogenerated ferricyanide. Note that, from 0.2 V of the reverse scan up to 0.24 V of the forward scan, the fluorescence peaks of CNDs are about 98.5 % of that shown in Fig. S6. In summary, these results demonstrate that the CNDs can be used as a chemiluminescence sensor for obtaining electrochemical properties using fluorescence spectrometry.
Photocurrent Generation from CNDs Immobilized Gold Slide
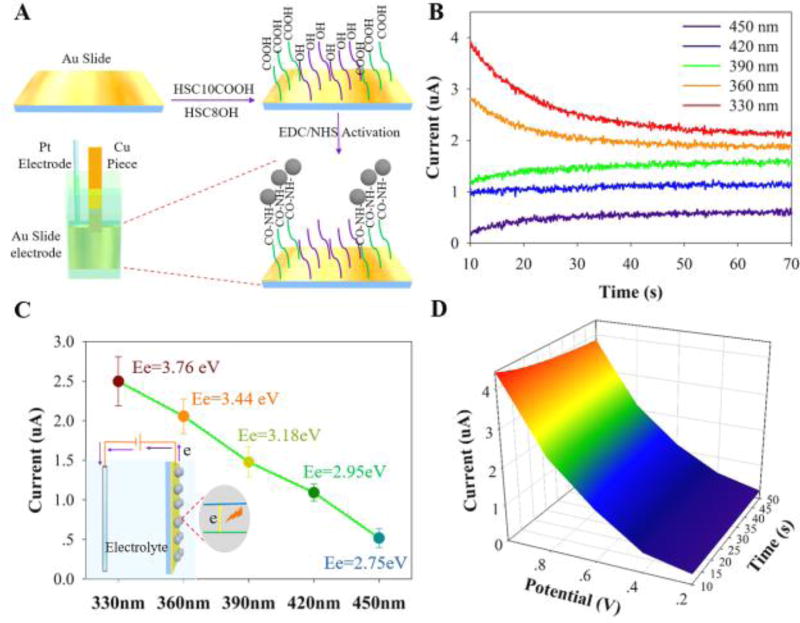
The immobilization of CNDs at the gold slide surface was verified by AFM (Fig. S1).The CNDs are clearly visualized as the white spots on the AFM image. Using a similar way for analysing immobilized proteins on analkyl thiol SAM,48 a detailed analysis of the spots of the AFM image indicates that CNDs are uniformly distributed at the slide surface with a surface coverage about 26.3±0.6% of the slide surface area. By subtracting the average height of the white spots (~ 6 nm)with the thickness of -SC10CO-SAM (1–1.1 nm) and roughness of the gold slide (1–2 nm), the thickness of CNDs is estimated to be about3 nm (Fig. S1), which is consistent with results obtained by TEM and AFM images of CNDs on mica surface (Figure 1), suggesting a monolayer of CNDs immobilized to the gold surface covalently linked to the -SC10CO-SAM.
The action spectrum of the CNDs immobilized on a gold slide electrode (Figure 4A) was obtained by measuring the photocurrent in a 3 mL 0.1 M Na2SO4 electrolyte solution under monochromatic light irradiation.The Na2SO4 based electrolyte solution was N2-degassed before the photocurrent experiment. Figure 4B shows photocurrent measurements (at an applied voltage of 0.8 V) of the CNDs treated electrodes at five different irradiation wavelengths (Fig. S7, S8 show the chronoamperometry (CA) measurement and a control experiment for the untreated gold slide electrode). Note that the resulting current from the photo-excitation processes occurring at the electrode was monitored as a function of time by the CA measurement in which the potential of the working electrode from chronoamperometry is stepped, and after 50 seconds of the decay time from initial to steady-state current, we calculated the net photocurrent attributed by CNDs as Figure 4D shown by subtracting CA measurements with an applied voltage of 0.8 V of the gold slide electrode without CNDs immobilization (Fig. S8) from CA measurements of the gold slide electrode with CNDs immobilization (Fig. S7) before and after light irradiation with different incident wavelength (330–450 nm). The highest photocurrent (~2.5 µA) was obtained for an incident wavelength of 330 nm (corresponding to photon energy, Ee=3.76 eV) while the lowest photocurrent (~0.5 µA) was obtained for an incident wavelength of 450 nm (Ee=2.75 eV) (Figure 4C). Since no photocurrent was observed from the bare gold electrode under thephoto-excitation process, the photocurrent action spectrum demonstrates that the CNDs are the photoactive species responsible for the photocurrent generation. Sincethe intensity of incident light is independent of its wavelength, the incident wavelength dependent photocurrent generation by CNDs may be arising from two likely factors. First is an increase in the incident photon to charge carrier efficiency that arises from higher light absorbance of CNDs at shorter wavelengths, as concluded from their absorption spectrum (Figure 1G insert),49 and the second is the somewhat higher energy of the photoinjected electrons at shorter wavelengths.
Figure 4.
(A) Illustration of the protocol for the self-assembled monolayer (SAM) formation and CNDs immobilization on the gold slide electrode. Note that the fluoro-electrochemical setup is the same as that in Figure 3C except the gold electrode was changed into the immobilized CNDs gold slide electrode which is electrically connected through a piece of copper tape. (B) Chronoamperometry (CA) photocurrent measurements of CNDs immobilized on the electrode at an applied voltage of 0.8 V. The different irradiation wavelengths are shown in the legend, ranging from 330–450 nm. (C) The long time photocurrent of the CND films is plotted for the different incident wavelengths, with photon energy, Ee=hc/λ, indicated. The inset shows a schematic of photocurrent generation of CNDs upon excitation. (D) The chronoamperometry measurement with different applied bias potentials of the gold slide electrode with CNDs immobilization under an incident wavelength of 330 nm.
In analogy to a semiconductor-electrolyte interface, CNDs generate electron-hole pairs (e−-h+) that can then undergo various relaxation pathways, including electron-hole pair radiative recombination, charge transfer to the substrate, and charge transfer to an acceptor in the electrolyte.50–52 The photocurrent measured in this system, without a redox couple in solution, corresponds to a net electron transfer from the CNDs to the gold slide electrode; thus the photocurrent is anodic. Figure 4D and Fig. S9 show that the voltage dependence of the photocurrent generated by the CNDs under excitation at 330 nm increases monotonically with increasing positive bias.
It was observed that photocurrent decays with time especially under the high energy incident light (330 nm) at high bias voltage (0.8 V). To exam if there was a degradation of CND layer at the gold slide electrode, multiple photocurrent measurements were carried out at different times using the same electrode; no signifcant magnitude changes of the photocurrents were observed, suggesting the stability of the CND layer at the gold slide electrode. The decay of photocurrent with time (~50 s to get stable) may be attributed to the need for establishing an equilibrium status of the semiconductor-electrolyte interface, because the higher energy light exciation,the more electron-hole pairs (e−-h+) separation at the interface (coupling with a double layer capacitance behavior with an external electrical field) would take longer time (see Fig. S7) to reach the equilibrium status for stable photocurrent generation. More insightful understanding (e.g. electron-hole pair generation and recombination, charge transfer, and analysis of mobility in CNDs) of the photocurrent decay would be of great interest for a real photovoltaic device development, which, however, is beyond the scope of this study.
The increase in photocurrent at higher bias potentials can be explained using a Fowler-Nordheim model53 for the photocurrent. In this model, the photocurrent is proportional to the following dimensionless tunnelling probability:53
| (1) |
where Ip is the photocurrent, q is the electron charge, h is Planck’s constant, m* is the effective mass, φB is the tunnelling barrier height, V0 is the open circuit voltage, αVa is the fraction of the applied voltage across the barrier (thus affecting the electric field), and L is the length over which the electric field applies. Hence, higher applied bias voltages result in a higher photocurrent from the CNDs. The photoinduced excitation and subsequent charge transport to the gold slide electrode demonstrate the potential for CNDs to be used in photovoltaic and other optoelectronic device applications.
Energy Gap Investigation and Analysis
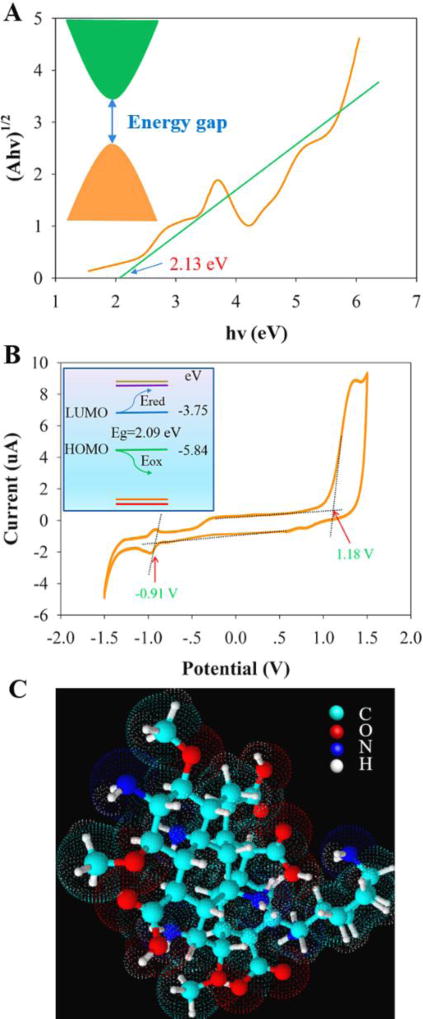
The electronic energy levels of the carbon atoms combine to form delocalized bands of energy states,54 including a valence band maximum, (in analogy to HOMO), and conduction band minimum, (in analogy to LUMO). From the UV-Vis absorption spectrum (Figure 1G), the optical band gap Eo of the CNDs is estimated to be 2.13±0.06 eV (Figure 5A).28 After adjusting for the exciton binding energy Eb (about 6.1–13.6 meV),55, 56 the energy gap from the optical measurement is estimated to be about 2.12±0.06 eV. In addition, electrochemical measurements can be used to determine the HOMO and LUMO positions by treating an individual CND as a molecule. The onset of the reduction (Ered) and oxidation (Eox) potentials for the CNDs give energies of −3.75±0.04 eV of LUMO energy level and −5.84±0.04 eV of HOMO energy level, respectively (Figure 5B, Fig S10). These values give an HOMO-LUMO energy gap for the CNDs of 2.09±0.08 eV,29, 57 which is close to the energy gap value obtained from the spectral measurements.
Figure 5.
(A) UV-Vis absorption spectrum of the CNDs used to estimate the optical band gap (E0). (B) A cyclic voltammogram of the CNDs is shown, and the inset illustrates its relation to the energy levels. (C) A proposed molecule-like structure (with a formula C36H58N6O11) of individual CNDs based on the characterization results.
The Hückel method30 was used to calculate the molecular orbital levels, for common values of the coulomb and resonance integral values (see Table S6). The molecular structure of the CNDs (Figure 5C) was generated from a graphite structure surrounded by amorphous carbon atoms and surface functional groups, which were chosen to correspond to the atomic ratio of C:O:N obtained by XPS. The core size was then increased until a HOMO-LUMO energy gap of 2.07 eV was reached. At the same given parameters, different orbital energy levels (LUMO+2, LUMO+1, LUMO, and HOMO, HOMO-1, HOMO-2) could be obtained; see Fig. S11).30 The calculated LUMO (−3.71 eV) and HOMO (−5.78 eV) electronic state energies of the molecular CND are consistent with that obtained by the electrochemical experiments (about −3.75±0.04 and −5.84±0.04 eV).
The molecular orbital model has been applied to understand the fluorescence properties of graphene oxide nanodots previously,58, 59 and the dominant fluorescence mechanism was found to originate from the electronic transitions among/between the non-oxidized carbon regions and the boundary of oxidized carbon atom regions involving the functionalized groups C-O, C=O and COOH. Compared to the graphene oxide quantum dots, previous studies indicate the CNDs have a less defined π-electron core and a larger proportion of functional groups, thus little quantum confinement effect on photoluminescence.60 In this work, the π-core region of the CNDs is small, as determined from the XRD data, yet the CND size distribution is mostly uniform. The molecular orbital energy levels that are derived from Hückel theory show good agreement with the observed fluorescence of the CNDs between 400 nm (photo energy of 3.10 eV) and 600 nm (photo energy of 2.06 eV) (Figure 2A) which may correspond to the potential electronic transitions from LUMO to HOMO (gap of 2.07 eV), to HOMO-1 (energy difference of 3.92 eV), and to HOMO-2 (3.94 eV), and/or from LUMO+1 to HOMO (2.65 eV), and LUMO+2 to HOMO (2.66 eV). Here, the responsible atom groups include non-oxidized carbon (C=C), the boundary oxidized carbon (C=O and C-O), and functionalized carbon (COOH), with electronic transition moments for C=C, C=O, C-O and O-H being 3.24 D, 2.93 D, 3.43 D and 2.30 D, respectively (details in SI and Table S7). Since the photoluminescence could be proposed to be proportional to the time constant and the number of electrons from high to low energy level, here, the Hückel model treatment combines the conjugated π states (C=C) with the functional groups (C=O, C-O, and O=C-OH), which can be dominant factors in the excitation-dependent emission of CNDs. Note that the electric vector is aligned parallel with (or perpendicular to) the transition moment of the bonds resulting in higher (or lower) light absorbance. This indirectly affects the electronic transition probability and photoluminescence properties.58, 61 For the photoluminescence origin of amorphous CNDs, the light emission characteristics has been generally ascribed to surface-related defect sites, and radiative recombination of excited surface states.60 In this study, based on the energy gap calculation and the observation of the fluorescence-based spectro-electrochemistry analysis, we propose that the photoluminescence arises from a combination of the C=C core regions, the surface functional groups (C=O, C-O, and COOH), and the surface electronic state transitions. However, the question regarding the effect of the C=C core-region, e.g. the size dependent quantum confinement, and its contribution to the origin of fluorescence from such microwave synthesized CNDs is open; and it may be explored by controlling the core sizes while maintaining the surface states.
Conclusions
A new fluorescence spectroelectrochemistry approach was developed to investigate the real-time electrochemical-fluorescence properties of microwave synthesized CNDs, and a chemically reversible redox couple was used to reveal the influence of redox chemistry on the optoelectronic properties of the CNDs. The effect of different excitation wavelengths from a fluorescence spectrophotometer on the photocurrent generated by the CNDs modified gold slide electrodes upon different applied bias voltages was investigated systematically, suggesting wavelength dependence on photocurrent generation from the CNDs as electron donorsto the gold electrode. The optical band gap of the CNDs obtained by the UV-Vis absorption spectrum is in reasonable agreement with the HOMO-LUMO energy gap found from electrochemical measurements. A theoretical Hückel model was adopted to obtain the molecular-like CND structure and was fitted to match the experimental energy gap between HOMO and LUMO energy levels. From the both experimental and theoretical energy gap analysis and the observation of the fluorescence-based spectroelectrochemistry, the excitation dependent fluorescence of the CNDs may be attributed to a combination of the core C=C, surface functionalities (C=O, C-O, and COOH), and/or surface electronic state transitions. This study provides a new perspective on the optoelectronic properties of the CNDs, which should motivate and facilitate their broad applications in biomedicine (e.g. bioimaging, antioxidation) and photoelectric devices.
Supplementary Material
Acknowledgments
JW etal at Joint School of Nanoscience and Nanoengineering (JSNN) acknowledge the partial support by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R15HL129212. This work was performed at the JSNN, a member of Southeastern Nanotechnology Infrastructure Corridor (SENIC) and National Nanotechnology Coordinated Infrastructure (NNCI), which is supported by the National Science Foundation (ECCS-1542174). DHW and BB acknowledge support through the US Department of Energy (Grant# ER46430).
Footnotes
Electronic Supplementary Information (ESI) available: [Additional AFM, XPS data, Zeta potential, quantum yield, cell imaging, control results of CNDs and details of three kinds of energy gap calculations]. See DOI: 10.1039/x0xx00000x
Contributions
JW conceived and developed the concept; ZZ and WZ designed the research plan and conducted most experiments; DMA did the bioimaging studies; BB conducted the XPS experiments; AS performed the AFM experiments; TM built the gold slide; YL, ZJ and HC performed XRD and UV-Vis experiments; DHW supervised the XPS and energy gap analysis. JW supervised the whole research. ZZ, WZ and JW co-wrote the manuscript; All authors discussed the results and commented on the manuscript.
References
- 1.Zhu S, Meng Q, Wang L, Zhang J, Song Y, Jin H, Zhang K, Sun H, Wang H, Yang B. Angewandte Chemie International Edition. 2013;52:3953–3957. doi: 10.1002/anie.201300519. [DOI] [PubMed] [Google Scholar]
- 2.Lim SY, Shen W, Gao Z. Chemical Society Reviews. 2015;44:362–381. doi: 10.1039/c4cs00269e. [DOI] [PubMed] [Google Scholar]
- 3.Zheng XT, Ananthanarayanan A, Luo KQ, Chen P. Small. 2015;11:1620–1636. doi: 10.1002/smll.201402648. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Hu A. Journal of Materials Chemistry C. 2014;2:6921–6939. [Google Scholar]
- 5.Li X, Rui M, Song J, Shen Z, Zeng H. Advanced Functional Materials. 2015;25:4929–4947. [Google Scholar]
- 6.Xu Q, Wei J, Wang J, Liu Y, Li N, Chen Y, Gao C, Zhang W, Sreeprased TS. RSC Advances. 2016;6:28745–28750. [Google Scholar]
- 7.Wang L, Zhu S-J, Wang H-Y, Qu S-N, Zhang Y-L, Zhang J-H, Chen Q-D, Xu H-L, Han W, Yang B, Sun H-B. ACS Nano. 2014;8:2541–2547. doi: 10.1021/nn500368m. [DOI] [PubMed] [Google Scholar]
- 8.Hola K, Bourlinos AB, Kozak O, Berka K, Siskova KM, Havrdova M, Tucek J, Safarova K, Otyepka M, Giannelis EP, Zboril R. Carbon. 2014;70:279–286. [Google Scholar]
- 9.Luo Z, Vora PM, Mele EJ, Johnson ATC, Kikkawa JM. Applied Physics Letters. 2009;94:111909. [Google Scholar]
- 10.Baker SN, Baker GA. Angewandte Chemie International Edition. 2010;49:6726–6744. doi: 10.1002/anie.200906623. [DOI] [PubMed] [Google Scholar]
- 11.Cushing SK, Li M, Huang F, Wu N. ACS Nano. 2014;8:1002–1013. doi: 10.1021/nn405843d. [DOI] [PubMed] [Google Scholar]
- 12.Zhu S, Shao J, Song Y, Zhao X, Du J, Wang L, Wang H, Zhang K, Zhang J, Yang B. Nanoscale. 2015;7:7927–7933. doi: 10.1039/c5nr01178g. [DOI] [PubMed] [Google Scholar]
- 13.Eda G, Lin Y-Y, Mattevi C, Yamaguchi H, Chen H-A, Chen IS, Chen C-W, Chhowalla M. Advanced Materials. 2010;22:505–509. doi: 10.1002/adma.200901996. [DOI] [PubMed] [Google Scholar]
- 14.Krishnamoorthy K, Veerapandian M, Mohan R, Kim S-J. Applied Physics A. 2012;106:501–506. [Google Scholar]
- 15.Li H, He X, Kang Z, Huang H, Liu Y, Liu J, Lian S, Tsang CHA, Yang X, Lee S-T. Angewandte Chemie International Edition. 2010;49:4430–4434. doi: 10.1002/anie.200906154. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y-P, Zhou B, Lin Y, Wang W, Fernando KAS, Pathak P, Meziani MJ, Harruff BA, Wang X, Wang H, Luo PG, Yang H, Kose ME, Chen B, Veca LM, Xie S-Y. Journal of the American Chemical Society. 2006;128:7756–7757. doi: 10.1021/ja062677d. [DOI] [PubMed] [Google Scholar]
- 17.Cao L, Meziani MJ, Sahu S, Sun Y-P. Accounts of Chemical Research. 2013;46:171–180. doi: 10.1021/ar300128j. [DOI] [PubMed] [Google Scholar]
- 18.Liu R, Wu D, Liu S, Koynov K, Knoll W, Li Q. Angewandte Chemie International Edition. 2009;48:4598–4601. doi: 10.1002/anie.200900652. [DOI] [PubMed] [Google Scholar]
- 19.Lei C, Hu D, Ackerman EJ. Chemical Communications. 2008:5490–5492. doi: 10.1039/b812161c. [DOI] [PubMed] [Google Scholar]
- 20.Chang Y-L, Palacios RE, Fan F-RF, Bard AJ, Barbara PF. Journal of the American Chemical Society. 2008;130:8906–8907. doi: 10.1021/ja803454x. [DOI] [PubMed] [Google Scholar]
- 21.Zaleski S, Cardinal MF, Klingsporn JM, Van Duyne RP. The Journal of Physical Chemistry C. 2015;119:28226–28234. [Google Scholar]
- 22.Zeng Z, Liu Y, Wei J. TrAC Trends in Analytical Chemistry. 2016;75:162–173. [Google Scholar]
- 23.Gründer Y, Mosselmans JFW, Schroeder SLM, Dryfe RAW. The Journal of Physical Chemistry C. 2013;117:5765–5773. [Google Scholar]
- 24.Gadgil B, Damlin P, Dmitrieva E, Aaritalo T, Kvarnstrom C. RSC Advances. 2015;5:42242–42249. [Google Scholar]
- 25.Qu S, Wang X, Lu Q, Liu X, Wang L. Angewandte Chemie International Edition. 2012;51:12215–12218. doi: 10.1002/anie.201206791. [DOI] [PubMed] [Google Scholar]
- 26.Sanders M, Lin Y, Wei J, Bono T, Lindquist RG. Biosensors and Bioelectronics. 2014;61:95–101. doi: 10.1016/j.bios.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Zeng Z, Shi X, Mabe T, Christie S, Gilmore G, Smith AW, Wei J. Analytical Chemistry. 2017;89:5221–5229. doi: 10.1021/acs.analchem.6b04493. [DOI] [PubMed] [Google Scholar]
- 28.Yu D, Yang Y, Durstock M, Baek J-B, Dai L. ACS Nano. 2010;4:5633–5640. doi: 10.1021/nn101671t. [DOI] [PubMed] [Google Scholar]
- 29.Cardona CM, Li W, Kaifer AE, Stockdale D, Bazan GC. Advanced Materials. 2011;23:2367–2371. doi: 10.1002/adma.201004554. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Jiang S, Glusac K, Powell DH, Anderson DF, Schanze KS. Journal of the American Chemical Society. 2002;124:12412–12413. doi: 10.1021/ja027639i. [DOI] [PubMed] [Google Scholar]
- 31.Hou H, Banks CE, Jing M, Zhang Y, Ji X. Advanced Materials. 2015;27:7861–7866. doi: 10.1002/adma.201503816. [DOI] [PubMed] [Google Scholar]
- 32.Lim CS, Hola K, Ambrosi A, Zboril R, Pumera M. Electrochemistry Communications. 2015;52:75–79. [Google Scholar]
- 33.Muthulingam S, Bae KB, Khan R, Lee I-H, Uthirakumar P. RSC Advances. 2015;5:46247–46251. [Google Scholar]
- 34.Cui Q, Xu J, Wang X, Li L, Antonietti M, Shalom M. Angewandte Chemie International Edition. 2016;55:3672–3676. doi: 10.1002/anie.201511217. [DOI] [PubMed] [Google Scholar]
- 35.Zhang M, Bai L, Shang W, Xie W, Ma H, Fu Y, Fang D, Sun H, Fan L, Han M, Liu C, Yang S. Journal of Materials Chemistry. 2012;22:7461–7467. [Google Scholar]
- 36.Zhang J, Abbasi F, Claverie J. Chemistry – A European Journal. 2015;21:15142–15147. doi: 10.1002/chem.201502158. [DOI] [PubMed] [Google Scholar]
- 37.Cuong TV, Pham VH, Tran QT, Hahn SH, Chung JS, Shin EW, Kim EJ. Materials Letters. 2010;64:399–401. [Google Scholar]
- 38.Luo Z, Lu Y, Somers LA, Johnson ATC. Journal of the American Chemical Society. 2009;131:898–899. doi: 10.1021/ja807934n. [DOI] [PubMed] [Google Scholar]
- 39.Zhu H, Wang X, Li Y, Wang Z, Yang F, Yang X. Chemical Communications. 2009:5118–5120. doi: 10.1039/b907612c. [DOI] [PubMed] [Google Scholar]
- 40.Bhunia SK, Saha A, Maity AR, Ray SC, Jana NR. Scientific Reports. 2013;3:1473. doi: 10.1038/srep01473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X, Zhang S, Kulinich SA, Liu Y, Zeng H. Scientific Reports. 2014;4:4976. [Google Scholar]
- 42.Fu M, Ehrat F, Wang Y, Milowska KZ, Reckmeier C, Rogach AL, Stolarczyk JK, Urban AS, Feldmann J. Nano Letters. 2015;15:6030–6035. doi: 10.1021/acs.nanolett.5b02215. [DOI] [PubMed] [Google Scholar]
- 43.Yang Z-C, Wang M, Yong AM, Wong SY, Zhang X-H, Tan H, Chang AY, Li X, Wang J. Chemical Communications. 2011;47:11615–11617. doi: 10.1039/c1cc14860e. [DOI] [PubMed] [Google Scholar]
- 44.Schroll CA, Chatterjee S, Heineman WR, Bryan SA. Analytical Chemistry. 2011;83:4214–4219. doi: 10.1021/ac200551n. [DOI] [PubMed] [Google Scholar]
- 45.Ibañez D, Garoz-Ruiz J, Heras A, Colina A. Analytical Chemistry. 2016;88:8210–8217. doi: 10.1021/acs.analchem.6b02008. [DOI] [PubMed] [Google Scholar]
- 46.Amjadi M, Manzoori JL, Hallaj T, Sorouraddin MH. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2014;122:715–720. doi: 10.1016/j.saa.2013.11.097. [DOI] [PubMed] [Google Scholar]
- 47.Strauss V, Margraf JT, Dolle C, Butz B, Nacken TJ, Walter J, Bauer W, Peukert W, Spiecker E, Clark T, Guldi DM. Journal of the American Chemical Society. 2014;136:17308–17316. doi: 10.1021/ja510183c. [DOI] [PubMed] [Google Scholar]
- 48.Wei J, Liu H, Dick AR, Yamamoto H, He Y, Waldeck DH. Journal of the American Chemical Society. 2002;124:9591–9599. doi: 10.1021/ja025518c. [DOI] [PubMed] [Google Scholar]
- 49.Kongkanand A, Tvrdy K, Takechi K, Kuno M, Kamat PV. Journal of the American Chemical Society. 2008;130:4007–4015. doi: 10.1021/ja0782706. [DOI] [PubMed] [Google Scholar]
- 50.Miao R, Luo Z, Zhong W, Chen S-Y, Jiang T, Dutta B, Nasr Y, Zhang Y, Suib SL. Applied Catalysis B: Environmental. 2016;189:26–38. [Google Scholar]
- 51.Wu W, Zhan L, Fan W, Song J, Li X, Li Z, Wang R, Zhang J, Zheng J, Wu M, Zeng H. Angewandte Chemie. 2015;127:6640–6644. [Google Scholar]
- 52.Hao Y, Gan Z, Zhu X, Li T, Wu X, Chu PK. The Journal of Physical Chemistry C. 2015;119:2956–2962. [Google Scholar]
- 53.Mohite A, Lin J-T, Sumanasekera G, Alphenaar BW. Nano Letters. 2006;6:1369–1373. doi: 10.1021/nl060333f. [DOI] [PubMed] [Google Scholar]
- 54.Likhtenshtein G. Electron Spin Interactions in Chemistry and Biology. 1. Springer International Publishing; 2016. [Google Scholar]
- 55.Harb M. Physical Chemistry Chemical Physics. 2015;17:25244–25249. doi: 10.1039/c5cp03924j. [DOI] [PubMed] [Google Scholar]
- 56.Moses D, Wang J, Heeger AJ, Kirova N, Brazovski S. Proceedings of the National Academy of Sciences. 2001;98:13496–13500. doi: 10.1073/pnas.241497098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zou Y, Najari A, Berrouard P, Beaupré S, Réda Aïch B, Tao Y, Leclerc M. Journal of the American Chemical Society. 2010;132:5330–5331. doi: 10.1021/ja101888b. [DOI] [PubMed] [Google Scholar]
- 58.Shang J, Ma L, Li J, Ai W, Yu T, Gurzadyan GG. Scientific Reports. 2012;2:792. doi: 10.1038/srep00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Galande C, Mohite AD, Naumov AV, Gao W, Ci L, Ajayan A, Gao H, Srivastava A, Weisman RB, Ajayan PM. Scientific Reports. 2011;1:85. doi: 10.1038/srep00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cayuela A, Soriano ML, Carrillo-Carrion C, Valcarcel M. Chemical Communications. 2016;52:1311–1326. doi: 10.1039/c5cc07754k. [DOI] [PubMed] [Google Scholar]
- 61.Itoh T. Chemical Reviews. 2012;112:4541–4568. doi: 10.1021/cr200166m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.