Abstract
Autophagy is a cell digestion process that determines cell fate by promoting cell survival or inducing cell death in a cell context-dependent manner. Several classical signaling pathways, such as phosphoinositide-3-kinase and mammalian target of rapamycin, tightly regulate autophagy. 14-3-3 proteins regulate various signaling pathways by phosphorylation-dependent binding with partner proteins. 14-3-3 proteins also regulate autophagy by binding with autophagy-related proteins such as Beclin-1 and hVPS34. This review summarizes the role of 14-3-3 proteins in the control of autophagy in cancer, neurodegenerative diseases and other pathological conditions.
Keywords: 14-3-3 proteins, autophagy, cancer, neurodegenerative diseases
Introduction
Autophagy is a conserved process that plays a crucial role in many biological processes and pathological conditions, including adaptation to stress, metabolism, inflammation, neurodegenerative disordersand cancer [1]. Autophagy is classified into three subtypes: macroautophagy, microautophagy and chaperone-mediated autophagy [2]. Microautophagy is a process in which cytoplasmic content is directly engulfed into the lysosome [3]. Chaperone-mediated autophagy has been reported only in mammalian cells. In chaperone-mediated autophagy, the chaperone complex HSC70 recognizes the cytoplasmic content that contains the Lys-Phe-Glu-Arg-Gln (KFERQ) pentapeptide recognition sequence and subsequently binds to lysosome-associated membrane protein type 2A (LAMP-2A) for degrading the recognized cytoplasmic content [4]. Macroautophagy is referred to as autophagy that is a highly conserved process from yeast to mammals, and it has critical effect on various pathological conditions such as kidney diseases [5], cancers [6], immune responses [7], and infections [8] though the regulation of cell survival and cell death.
Overview of autophagy
Autophagy, also known as “self-eating” is a process in which damaged cell organelles and misfolded proteins are sequestered into autophagosomes, which then fuse with lysosomes to form autolysosomes. In autolysosomes, cytoplasmic constituents are degraded by lysosomal hydrolases for cyclic utilization. The formation of double-membrane autophagosomes involves the following steps: induction, nucleation, elongation, and formation a complete isolation membrane [9]. The ULK1/2-Atg13-FIP200 complex is required for the formation of phagophore membranes. For nucleation, the phagophore membrane phosphoinositide-3-kinase (PI3K)/Vps34 complex containing Vps34, Vps15, Beclin-1 is recruited. The Atg12-Atg5-Atg16 complex and Atg8/light chain 3-II (LC3-II) are two ubiquitin-like conjugation systems involved in the elongation and expansion steps in autophagosome formation. Mitogen-activated protein kinase (MAPK) and PI3K inhibit autophagy by activating the mammalian target of rapamycin (mTOR) complex. However, P53 has the opposite effect. The protein 5’ adenosine monophosphate-activated protein kinase (AMPK) activates autophagy by upregulating the ULK1 complex. The process of autolysosome formation is shown in Figure 1.
Figure 1.
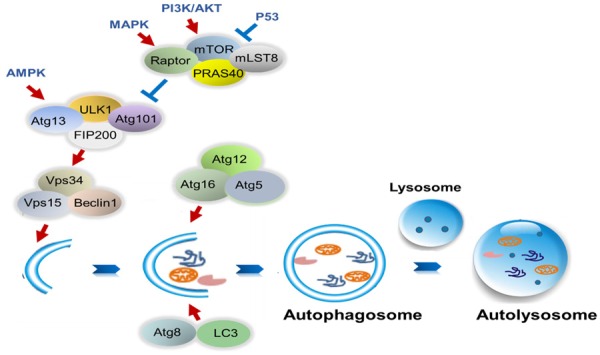
Model of regulation of autophagy process. The ULK1-Atg13-FIP200 complex promotes the recruitment of PI3K/Vps34 complex to phagophore membranes. The Atg12-Atg5-Atg16 complex and Atg8/LC3 are involved inthe elongation and expansion steps of autophagosome formation. MAPK and PI3K inhibit autophagy by activating the mTOR complex; however, P53 has the opposite effect. AMPK activates autophagy by upregulating the ULK1 complex.
Role of autophagy in neurodegenerative diseases
A common features of neurodegenerative diseases is the accumulation of a large number of misfolded proteins in the cytoplasm. Autophagy is essential for the maintenance of local homeostasis in axon terminals, and impaired autophagy induces axon atrophy [10]. Mutated PTEN induced putative kinase 1 (PINK1) is one of the main genes that causes Parkinson’s disease. The full-length PINK1 protein interacts with Beclin-1 to promote autophagy; however, the activity of Beclin-1 is decreased and autophagy is inhibited when the PINK1 protein is mutated [11]. Ravikumar et al found that in fly and mouse models of Huntington’s disease, the inhibition of mTOR induces autophagy and eliminates the accumulation of polyglutamine [12]. Under normal conditions, the β-amyloid proteins are degraded by autophagy. By contrast, in Alzheimer’s disease, β-amyloid proteins are aggravated because the autophagic flux is impaired [13]. Thus, autophagy plays a key role in neurodegenerative diseases.
Role of autophagy in cancers
The cancer is one of the diseases of serious threat to human health and life nowadays.Many studies have reported that autophagy plays a dual role in cancer. Tang et al demonstrated that in a model of liver cancer induced by HBV infection, the expression of Beclin-1 mRNA and protein ishigher in liver cancer tissue than in the healthy liver tissue [14]. Another study also showed that in many patients with colorectal or gastric cancers, the expression of Beclin-1 is detected in more than 80% of cancer tissue though immunohistochemistry. By contrast, the expression of Beclin-1 islow in normal mucosal cells of colon and stomach [15]. These results indicate that Beclin-1 may promote the development of cancer by inducing autophagy. Ren et al showed that autophagy is associated with tumor resistance. The expression of LC3-II is found to be higher in A549 cells resistant to cisplatin. When combined with 3-methyladenine (3MA; an inhibitor of autophagy), cisplatin induces more apoptosis in A549 cells [16]. Similar results were observed in another study, in which cisplatin treatment resulted in higher Beclin-1 expression and autophagosome formation in rat C6 glioma, human U251 glioma, and mouse L929 fibrosarcoma cell lines. Treatment with bafilomycin A1 (BafA1), wortmannin, or chloroquine enhanced DNA fragmentation and apoptotic cell death induced by cisplatin. Further research revealed that inhibition of AMPK enhanced cancer cell death induced by cisplatin, whereas mTOR siRNA had the opposite effect [17]. The findings of the aforementioned studies suggest that autophagy reduces the sensitivity of cells to chemotherapy drugs in cancer. By contrast, Li et al found that GFP-LC3 punctate are increased in gastric cancer cells during treatment with harmine. LY294002, an inhibitor of PI3K, significantly increased the harmine cytotoxicity to the cells by enhancing the expression of Beclin-1 and LC3-II. In addition, 3-MA and BafA1 improved the viability of gastric cancer cells treated with harmine [18]. Lysine (K)-Specific Demethylase 2B (KDM2B) is a member of the JmjC-domain-containing histone demethylase (JHDM) family, which functions as a histone lysine demethylase. KDM2B knockdown inhibited gastric cancer cell proliferation by activating autophagy. In a mouse xenograft tumor model, tumor growth was also inhibited by the downregulation of KDM2B [19]. Hu et al demonstrated that endoplasmic reticulum stress enhanced cell apoptosis by inhibiting the PI3K/AKT/mTOR signaling pathway, thus triggering autophagy in chemical-resistant ovarian cancer cells treated with cisplatin [20]. In summary, the findings demonstrate that autophagy plays a pro-apoptotic role in cancer.
Under different experimental conditions, autophagy is regulated by mTOR, AMPK, MAPK, and PI3K signaling pathways to promote cell death or facilitate cell survival. 14-3-3 proteins are commonly expressed in eukaryotes and are involved in numerous cell biology processes such as autophagy.
Overview of 14-3-3 proteins
The 14-3-3 protein family contains highly conserved acid proteins that were initially separated from bovine brain tissue [21]. 14-3-3 proteins are mainly detected in the cytoplasm, nucleus, and cell membrane of eukaryotic cells, and are distributed in the Golgi apparatus, mitochondria and chloroplast [22]. These proteins have seven isoforms (β, ε, η, γ, τ, σ and ζ) encoded by different genes in mammalian cells. The expression of 14-3-3 isoforms varies in different tissue types [23,24].
14-3-3 proteins function as homodimers and heterodimers. They bind to target proteins and alter the modification, intercellular localization, and activity of target proteins, mostly by interacting with specific phosphothreonine and phosphoserine motifs [25]. 14-3-3 proteins are crucial regulators of many biological processessuch as cell cycle progression, intracellular protein trafficking, apoptosis, DNA damage response, DNA replication, and transcriptional regulation. The regulatory roles of 14-3-3 proteins in biological processes are shown in Figure 2. 14-3-3ε, γ, and ζ activate Raf, PI3K, and MSK1/2 to regulate the ERK signaling pathway in human fibroblasts, breast cells, prostate cells, and hematopoietic stem cells [26-28]. Overexpression of 14-3-3ζ promotes cancer cell proliferation whereas the downregulation of 14-3-3ζ inhibits cell proliferation by inducing the mitochondria-dependent apoptosis pathway [29-31]. Our previous study demonstrated that human umbilical cord mesenchymal stem cell-exosomal 14-3-3ζ activates YAP by phosphorylation at the Ser127 site and promoted the formation of YAP and p-LATS complex, which restrictes excessive cell expansion and collagen deposition during cutaneous regeneration [32]. In a mous model, difopein, a general 14-3-3 antagonist, and 14-3-3 siRNA induced human glioma cell apoptosis and suppressed tumor growth [33]. These findings suggest that 14-3-3 proteins play a critical role in the regulation of diverse cellular responses.
Figure 2.
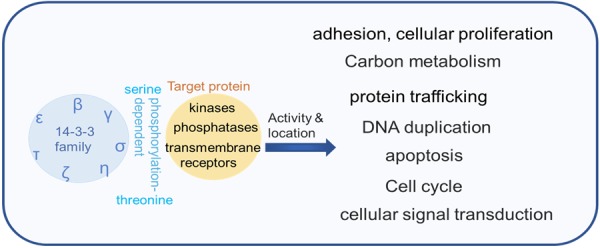
Model of the biological process of 14-3-3 proteins regulation. The 14-3-3 family contains seven isoforms (β, ε, η, γ, τ, σ, and ζ), all of which bind to target proteins and alter the modification, intercellular localization, and activity of target proteins by interacting with specific phosphothreonine and phosphoserine motifs, and then regulating the biological processes.
Regulation of autophagy by 14-3-3 proteins in cancers
Although the role of 14-3-3 proteins in human osteosarcoma is somewhat unclear [34], 14-3-3 proteins are known to promote tumorigenesis by inhibiting autophagy in lung cancer [35], glioma [36], renal cell carcinoma [37], and cervical cancer [38]. However, these proteins also promote prosurvival autophagy activationin human glioblastoma [40] and pancreatic cancer under certain conditions [41].
Overexpression 14-3-3τ in human osteosarcoma U2OS cells enhances the transcript levels of Beclin-1 and its protein expression. By contrast, 14-3-3τ depletion inhibites the expression of Beclin-1, and 14-3-3τ depletion significantly decreases GFP-LC3 punctate in U2OS cells induced by serum starvation or rapamycin. Further research showed that 14-3-3τ induces autophagy by controlling of the E2F1-Beclin-1 signal axis [34]. However, the effects of autophagy are largely unclear.
In human lung cancer, AKT phosphorylates both Beclin-1 and vimentin, and 14-3-3 forms a complex with Beclin-1 and vimentin to promote tumorigenesis by inhibiting autophagy [35]. In human glioma U251 cells, CLIC4 siRNA enhances autophagy and triggers endoplasmic reticulum (ER) stress-induced apoptosis under starvation conditions by interacting with 14-3-3ε to indirectly activate Beclin-1 [36]. In renal cell carcinoma, the overexpression of pyruvate kinase M2 (PKM2) activates mTORC1 signaling by phosphorylating the mTORC1 inhibitor AKT1 substrate 1 (AKT1S1) on S202/203, and promotes the release of AKT1S1 from Raptor, thereby binding itself to to 14-3-3 and promoting cell growth by inhibiting autophagy [37]. Wang et al also demonstrated that AKT inhibites autophagy by phosphorylating Beclin-1 at the S295 site and enhancing the formation of Beclin1/14-3-3/vimentin complex to promote tumorigenesis in human cervical HeLa cells [38]. This finding indicates that 14-3-3 proteins promote tumorigenesis by inhibiting autophagy.
Glioblastomas are the most life-threatening human brain tumors. Surgical resection combined with chemotherapy and radiotherapy has a certain therapeutic effect, but these treatment are associated with a high degree of tumor recurrence [39].Fettweis et al indicated that MAPKAPK2 (MK2) induces the phosphorylation of TSC2 at serine 1254 and an interaction with 14-3-3ζ at 15 min after irradiation; both these events promote pro-survival autophagy activation in human glioblastoma LN18, U87-MG and U373 cell lines. By contrast, the MK2 inhibitor reverses this interaction. About 4h post-treatment, the recruitment of 14-3-3ζ by Receptor Interacting Protein 3 (RIP3) is in competition with that of TSC2; thus, TSC2 is inactivated though dephosphorylation, thereby inhibiting cell survival [40]. In human pancreatic cancer cells, GSK3 inhibitors CHIR99021 and SB216763 activate autophagy, and BafA1 and ATG5 siRNA increase cell apoptosis though GSK3 knockdown, indicating that autophagy has a protective effect against pancreatic cancer. Further investigation shows that GSK3 inhibitors promote the expression of dephosphorylation transcription factor EB (TFEB), which is a key regulator of autophagy and lysosomal biogenesis, leading to its dissociation from 14-3-3 and its transfer to the nucleus [41]. This finding demonstrates that 14-3-3 proteins promote cell survival by activating autophagy in some cancers under certain conditions.
14-3-3 proteins reduce the level of autophagy to induce cancer cell apoptosis. In cervical cancer, the PI3K pathway is frequently aberrantly activated. However, the antitumor effect of BKM120, a PI3K inhibitor is largely ineffective. Kim et al demonstrated that BKM120 causes the dissociation of fork head box O3 (FOXO3a) from 14-3-3ζ to induce autophagy in PIK3CA mutant cervical cancer cells [42]. Xu et al demonstrated that ULK mediates the binding of guanine nucleotide exchange factor DENND3 to 14-3-3 through phosphorylation at serine 554 and 572 and its GEF activityis subsequently upregulated to induce autophagy by promoting the interaction between Rab12 and LC3 in HeLa cells under starvation conditions [43]. The overexpression of 14-3-3ζ blocks C2-ceramide-dependent autophagy by interacting with hVps34 and decreasing its activity in HeLa cells, whereas rapamycin promotes the dissociation of hVps34 from 14-3-3 and induces autophagy. Knockdown of 14-3-3ζ by RNA interference has the same result [44]. Thus, 14-3-3 proteins play a key role in cancers by regulating autophagy through interaction with certain proteins shown in Table 1 and Figure 3.
Table 1.
Role of autophagy in cancers regulated by 14-3-3 proteins
| Experimental model | 14-3-3 isoform | Target protein | Directly binding | Level of autophagy | Role of autophagy | Reference |
|---|---|---|---|---|---|---|
| U2OS cells | 14-3-3τ | Beclin 1 | Unclear | Increased | [34] | |
| Lung cancers | 14-3-3 | Beclin 1/vimentin | Yes | Decreased | Tumorigenesis | [35] |
| U251 cells | 14-3-3τ | Beclin 1 | No | Increased | Apoptosis | [36] |
| CLIC4 | Yes | |||||
| RCCs cancer | 14-3-3 | AKT1S1 | Yes | Decreased | Tumorigenesis | [37] |
| HeLa cells | 14-3-3 | Beclin1 S295/vimentin | Yes | Decreased | Tumorigenesis | [38] |
| LN18, U87-MG, U373 cells | 14-3-3ζ | TSC2 S1254 | Yes | Increased | Pro-survival | [40] |
| PANC1, MIA PaCa-2 | 14-3-3 | TFEB | Yes | Increased | Pro-survival | [41] |
| Cervical cancer cells | 14-3-3ζ | FOXO3a | Yes | Decreased | Apoptosis | [42] |
| HeLa cells | 14-3-3 | DENND3 serine 554 and 572 Rab12/LC3 | Yes | Unclear | [43] | |
| No | Increased | |||||
| HeLa cells | 14-3-3ζ | hVPS34 | Yes | Decreased | Apoptosis | [44] |
Abbreviations: CLIC4, chloride intracellular channel 4; AKT1S1, AKT1 substrate 1; TSC2, Tuberous Sclerosis 2; TFEB, transcription factor EB; FOXO3a, fork head box O3; DENND3, DENN domain-containing protein 3.
Figure 3.
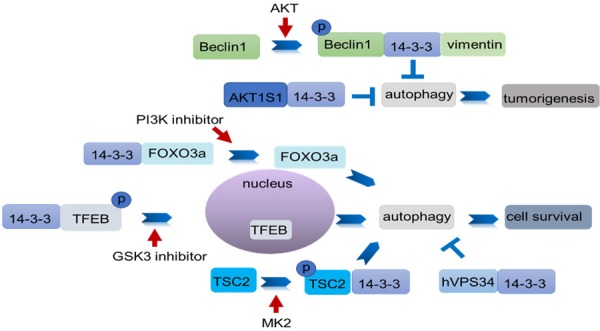
Model of 14-3-3 regulation of autophagy in cancers. 14-3-3 proteins inhibit or promote autophagy by binding with autophagy-related proteins to promote cancer cell survival or apoptosis. AKT inhibits autophagy by phosphorylating Beclin-1 at the S295 site and enhancing the formation of the Beclin-1/14-3-3/vimentin complex to promote tumorigenesis. AKT1S1 binds with 14-3-3 to promote cancer cell growth by inhibiting autophagy. PI3K inhibitors promote the dissociation of FOXO3a from 14-3-3ζ to induce autophagy. The GS3K inhibitor induces TFEB dephosphorylation and its dissociation from 14-3-3 and subsequently induces TFEB activation and nuclear translocation to activate autophagy. MK2 induces TSC2 phosphorylation and formation of TSC2/14-3-3 complex to promote autophagy. By contrast, 14-3-3 inhibits autophagy by binding with hVPS34 to induce cell apoptosis.
Regulation of autophagy by 14-3-3 proteins in neurodegenerative diseases
In neurodegenerative diseases, 14-3-3 proteins alleviate cell injury by indirectly inhibiting autophagy. Under some conditions, 14-3-3 proteins have the opposite effect.
14-3-3ε knockdown by siRNA increases autophagy and aggravates the injury of PC12 cell during rotenone treatment [45]. Another study suggested that 14-3-3ε has a neuroprotective effect on cerebral I/R injury. Delivery of 14-3-3ε into the brain by TAT reduces the formation of autophagosomes and attenuates I/R-induced neuronal apoptosis. Rapamycin inhibits the neuroprotective effect of TAT-14-3-3ε and aggravates I/R-induced brain infarct in SD rats, whereas 3-MA further increases this effect [46].
Gypenoside XVII (GP-17) promotes the elimination of Aβ40, AβPP, and Aβ42 in PC12 cells expressing the Swedish mutant of APP695 (APP695swe) and APP/PS1 mice by upregulating the expression of ATG5, LC3-II and LAMP-1. Furthermore, GP-17 induces autophagy and lysosome biogenesis by promoting the dissociation of transcription factor EB (TFEB) from TFEB/14-3-3 complexes, which leads to TFEB activation and its nuclear translocation [47]. Transthyretin (TTR) is a neuroprotective molecule that serves as a carrier protein of thyroxine (T4) and retinol through the binding protein (RBP) in plasma and cerebrospinal fluid (CSF) [48]. Vieira et al demonstrated that in TTR-/- mice, the expression of 14-3-3ζ is decreased in the hippocampus in an age-dependent manner.The reduced expression of 14-3-3ζ is attributable to increase autophagy [49].
TFE3 is phosphorylated at Ser211 by mTORC1 and is retained in the cytosol through its interaction with active Rag GTPases and 14-3-3 in ARPE-19 cells under nutrient-rich conditions. Under starvation conditions, mTORC1 and Rags are inactivated which induces the dissociation of TFE3 from 14-3-3, and TFE3 is translocated to the nucleus, where it binds to the CLEAR elements to increase lysosomal degradation and autophagy induction in ARPE-19 cells [50].Thus, in neurodegenerative diseases, 14-3-3 proteins regulate autophagy (Table 2), which promotes the degradation of accumulated protein.
Table 2.
Role of autophagy in neurodegenerative diseases regulated by 14-3-3 proteins
| Experimental model | 14-3-3 isoform | Target protein | Directly binding | Level of autophagy | Neurotoxicity | References |
|---|---|---|---|---|---|---|
| PC12 cells | 14-3-3ε | —— | Unclear | Decreased | Attenuated | [45] |
| Cerebral I/R injury | 14-3-3ε | —— | Unclear | Decreased | Attenuated | [46] |
| PC12 cells | 14-3-3 | TFEB | Unclear | Decreased | Aggravated | [47] |
| AF5 cells | 14-3-3ζ | —— | Unclear | Decreased | Aggravated | [49] |
| ARPE-19 cells | 14-3-3 | TFE3 | Yes | Decreased | Aggravated | [51] |
Regulation of autophagy by 14-3-3 proteins in other diseases
Inflammation is a common pathological process. Gómez-Suárez et al suggested that the binding of 14-3-3ζ with Raptor triggers autophagy to promote the degradation of 14-3-3η, the inhibitor of phosphoinositide-dependent kinase 1 (PDK1) resulting in AKT phosphorylation at Thr308 in the mucosa of colitic mice and SW480 cells treated with IFNγ/TNFα. However, inhibition of 14-3-3 function promotes cell death during inflammation [51]. Another study showed similar results, the levels of p14-3-3ζ (Ser58) is increased in the colonic mucosa of C57BL/6J mice treated with IFNγ/TNFα, and p14-3-3ζ inhibits mTOR through its interaction with Raptor, which triggers autophagy and enhances AKT activation, which leads to chromogranin A production [52]. These findings suggestes that autophagy plays a cytoprotective role in intestinal inflammation.
Under normal nutrient conditions, TFEB is phosphorylated at Ser211 by mTORC1 to promote the association of TFEB with 14-3-3 and the retention of TFEB in the cytosol. The inhibition of mTORC1 by LY294002, rapamycin, wortmannin, and PP242 leads to the dissociation of TFEB from 14-3-3 and its translocation to the nucleus, where TFEB interacts with the LAMTOR-RRAG-MTORC1 complex to activate autophagy in ARPE-19 cells [53].
Conclusions and prospects
Autophagy generally occurs during physiological and pathological processes; thus, it is critical for cellular responses to nutrient deprivation, stress, infection, and the development of diseases such as neurodegenerative diseases and cancer [9]. Depending on the conditions, autophagy may exert a beneficial or harmful effect. The inhibition of autophagy improves the chemosensitivity of glioma cells to cisplatin [54]. Autophagy is elevated in hypoxic regions of tumors and protects tumor cells against various stressors [55]. There are many key regulatory proteins involved in autophagy; 14-3-3 proteins play an additional role in regulating autophagy by binding with other proteins. In cancer cells, 14-3-3 inhibits autophagy by interacting with Beclin-1 to promote tumorigenesis. In Alzheimer’s disease, gypenoside XVII (GP-17) releases TFEB from TFEB/14-3-3 complexes, which leads to TFEB nuclear translocation and the induction of autophagy to reduce aggregated amyloid-β. Different isoforms of 14-3-3 proteins may have different effects on diseases. The specific isoform that mediates the autophagy-regulating role of 14-3-3 proteins in diseases warrants further investigation. Moreover, 14-3-3 proteins play an anti-apoptotic role by regulating Bcl-2, thus inhibiting autophagy [56]. Therefore, revealing the molecular processes of the 14-3-3 proteins involved in autophagy may help to increase the therapeutic effects on diseases such as cancer and neurodegenerative diseases.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant no. 81272481), the Major Research Plan of Jiangsu Higher Education (Grant no. 15KJA320001), Jiangsu Province for Outstanding Sci-tech Innovation Team in Colleges and Universities (Grant no. SJK2013-10), and Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Disclosure of conflict of interest
None.
References
- 1.Choi HS, Jeong EH, Lee TG, Kim SY, Kim HR, Kim CH. Autophagy inhibitionwith monensin enhances cell cycle arrest and apoptosis induced by mTOR orepidermal growth factor receptor inhibitors in lung cancer cells. Tuberc Respir Dis (Seoul) 2013;75:9–17. doi: 10.4046/trd.2013.75.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine B, Klionsky DJ. Development by selfdigestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 3.Mortimore GE, Lardeux BR, Adams CE. Regulation of microautophagy and basal protein turnover in rat liver. Effects of short-term starvation. J Biol Chem. 1988;263:2506–2512. [PubMed] [Google Scholar]
- 4.Kaushik S, Cuervo AM. Chaperone-mediated autophagy: a unique way to enter thelysosome world. Trends Cell Biol. 2012;22:407–417. doi: 10.1016/j.tcb.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmitt R, Melk A. Molecular mechanisms of renal aging. Kidney Int. 2017;92:569–579. doi: 10.1016/j.kint.2017.02.036. [DOI] [PubMed] [Google Scholar]
- 6.Tian M, Chen Y, Tian D, Qiao X, Ma Z, Li J. Beclin1 antagonizes LAPTM4B-mediated EGFR overactivation in gastric cancer cells. Gene. 2017;626:48–53. doi: 10.1016/j.gene.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Wei Y, Liu W, Hu W, Liu G, Wu C, Liu W, Zeng H, He C, Shi H. Genome-wide analysis of autophagy-related genes in banana highlights MaATG8s in cell death and autophagy in immune response to Fusarium wilt. Plant Cell Rep. 2017;36:1237–1250. doi: 10.1007/s00299-017-2149-5. [DOI] [PubMed] [Google Scholar]
- 8.Enkhtaivan G, Muthuraman P, Kim DH. Inhibitory effect of 2,4-dichlorophenoxyacetic acid on ROS, autophagy formation, and mRNA replication for influenza virus infection. J Mol Recognit. 2017;30 doi: 10.1002/jmr.2616. [DOI] [PubMed] [Google Scholar]
- 9.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Komatsu M, Wang QJ, Holstein GR, Friedrich VL Jr, Iwata J, Kominami E, Chait BT, Tanaka K, Yue Z. Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proc Natl Acad Sci U S A. 2007;104:14489–14494. doi: 10.1073/pnas.0701311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michiorri S, Gelmetti V, Giarda E, Lombardi F, Romano F, Marongiu R, Nerini-Molteni S, Sale P, Vago R, Arena G, Torosantucci L, Cassina L, Russo MA, Dallapiccola B, Valente EM, Casari G. The Parkinson-associated protein PINK1 interacts with Beclin1 and promotes autophagy. Cell Death Differ. 2010;17:962–974. doi: 10.1038/cdd.2009.200. [DOI] [PubMed] [Google Scholar]
- 12.Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O’Kane CJ, Rubinsztein DC. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36:585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- 13.Nixon RA. Autophagy, amyloidogenesis and Alzheimer disease. Cell Sci. 2007;120:4081–4091. doi: 10.1242/jcs.019265. [DOI] [PubMed] [Google Scholar]
- 14.Tang H, Da L, Mao Y, Li Y, Li D, Xu Z, Li F, Wang Y, Tiollais P, Li T, Zhao M. Hepatitis B virus X protein sensitizes cells to starvation-induced autophagy via up-regulation of Beclin 1 expression. Hepatology. 2009;49:60–71. doi: 10.1002/hep.22581. [DOI] [PubMed] [Google Scholar]
- 15.Ahn CH, Jeong EG, Lee JW, Kim MS, Kim SH, Kim SS, Yoo NJ, Lee SH. Expression of beclin-1, an autophagy-related protein, in gastric and colorectal cancers. APMIS. 2007;115:1344–1349. doi: 10.1111/j.1600-0463.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 16.Ren JH, He WS, Nong L, Zhu QY, Hu K, Zhang RG, Huang LL, Zhu F, Wu G. Acquired cisplatin resistance in human lung adenocarcinoma cells is associated with enhanced autophagy. Cancer Biother Radiopharm. 2010;25:75–80. doi: 10.1089/cbr.2009.0701. [DOI] [PubMed] [Google Scholar]
- 17.Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, Cattoretti G, Levine B. Promotion of tumorigenesis by heterozygous disruption of the beclin1 autophagy gene. J Clin Invest. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li C, Wang Y, Wang C, Yi X, Li M, He X. Anticancer activities of harmine by inducing a prodeath autophagy and apoptosis in human gastric cancer cells. Phytomedicine. 2017;28:10–18. doi: 10.1016/j.phymed.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Zhao E, Tang C, Jiang X, Weng X, Zhong X, Zhang D, Hou J, Wang F, Huang M, Cui H. Inhibition of cell proliferation and induction of autophagy by KDM2B/FBXL10 knockdown in gastric cancer cells. Cell Signal. 2017;36:222–229. doi: 10.1016/j.cellsig.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Hu JL, Hu XL, Guo AY, Wang CJ, Wen YY, Cang SD. Endoplasmic reticulum stress promotes autophagy and apoptosis and reverses chemoresistance in human ovarian cancer cells. Oncotarget. 2017;8:49380–49394. doi: 10.18632/oncotarget.17673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radwan O, Wu X, Govindarajulu M, Libault M, Neece DJ, Oh MH, Berg RH, Stacey G, Taylor CG, Huber SC, Clough SJ. 14-3-3 proteins SGF14c and SGF14l play critical roles during soybean nodulation. Plant Physiol. 2012;160:2125–2136. doi: 10.1104/pp.112.207027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu H, Subramanian RR, Masters SC. 14-3-3 proteins: structure, function, and regulation. Annu Rev Pharmacol Toxicol. 2000;40:617–647. doi: 10.1146/annurev.pharmtox.40.1.617. [DOI] [PubMed] [Google Scholar]
- 23.Kilani RT, Medina A, Aitken A, Jalili RB, Carr M, Ghahary A. Identification of different isoforms of 14-3-3 protein family in human dermal and epidermal layers. Mol Cell Biochem. 2008;314:161–169. doi: 10.1007/s11010-008-9777-6. [DOI] [PubMed] [Google Scholar]
- 24.Yaffe MB, Rittinger K, Volinia S, Caron PR, Aitken A, Leffers H, Gamblin SJ, Smerdon SJ, Cantley LC. The structural basis for 14-3-3: phosphopeptide binding specificity. Cell. 1997;91:961–971. doi: 10.1016/s0092-8674(00)80487-0. [DOI] [PubMed] [Google Scholar]
- 25.Muslin AJ, Tanner JW, Allen PM, Shaw AS. Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell. 1996;84:889–897. doi: 10.1016/s0092-8674(00)81067-3. [DOI] [PubMed] [Google Scholar]
- 26.Tzivion G, Gupta VS, Kaplun L, Balan V. 14-3-3 proteins as potential oncogenes. Semin Cancer Biol. 2006;16:203–213. doi: 10.1016/j.semcancer.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Neal CL, Yu D. 14-3-3zeta as a prognostic marker and therapeutic target for cancer. Expert Opin Ther Targets. 2010;14:1343–1354. doi: 10.1517/14728222.2010.531011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin M, Morrison CD, Jones S, Mohamed N, Bacher J, Plass C. Copy number gain and oncogenic activity of YWHAZ/14-3-3zeta in head and neck squamous cell carcinoma. Int J Cancer. 2009;125:603–611. doi: 10.1002/ijc.24346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maxwell SA, Cherry EM, Bayless KJ. Akt, 14-3-3zeta, and vimentin mediate a drugresistant invasive phenotype in diffuse large B-cell lymphoma. Leuk Lymphoma. 2011;52:849–864. doi: 10.3109/10428194.2010.551793. [DOI] [PubMed] [Google Scholar]
- 30.Li Z, Zhao J, Du Y, Park HR, Sun SY, Bernal-Mizrachi L, Aitken A, Khuri FR, Fu H. Downregulation of 14-3-3zeta suppresses anchorageindependent growth of lung cancer cells through anoikis activation. Proc Natl Acad Sci U S A. 2008;105:162–167. doi: 10.1073/pnas.0710905105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chatterjee D, Goldman M, Braastad CD, Darnowski J, Wyche JH, Pantazis P, Goodglick L. Reduction of 9-nitrocamptothecin-triggered apoptosis in DU-145 human prostate cancer cells by ectopic expression of 14-3-3zeta. Int J Oncol. 2004;25:503–509. [PubMed] [Google Scholar]
- 32.Zhang B, Shi Y, Gong A, Pan Z, Shi H, Yang H, Fu H, Yan Y, Zhang X, Wang M, Zhu W, Qian H, Xu W. HucMSC exosome-delivered 14-3-3ζ orchestrates self-control of the Wnt response via modulation of YAP during cutaneous regeneration. Stem Cells. 2016;34:2485–2500. doi: 10.1002/stem.2432. [DOI] [PubMed] [Google Scholar]
- 33.Cao W, Yang X, Zhou J, Teng Z, Cao L, Zhang X, Fei Z. Targeting 14-3-3 protein, difopein induces apoptosis of human glioma cells and suppresses tumor growth in mice. Apoptosis. 2010;15:230–241. doi: 10.1007/s10495-009-0437-4. [DOI] [PubMed] [Google Scholar]
- 34.Wang B, Ling S, Lin WC. 14-3-3T auregulates Beclin 1 and is required for autophagy. PLoS One. 2010;5:e10409. doi: 10.1371/journal.pone.0010409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kidd ME, Shumaker DK, Ridge KM. The Role of vimentin intermediate filaments in the progressionof lung cancer. Am J Respir Cell Mol Biol. 2014;50:1–6. doi: 10.1165/rcmb.2013-0314TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong J, Kong X, Zhang H, Yu C, Xu Y, Kang J, Yu H, Yi H, Yang X, Sun L. Inhibition of CLIC4 enhances autophagy and triggers mitochondrial and ER stress-induced apoptosis in human glioma U251 cells under starvation. PLoS One. 2012;7:e39378. doi: 10.1371/journal.pone.0039378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He CL, Bian YY, Xue Y, Liu ZX, Zhou KQ, Yao CF, Lin Y, Zou HF, Luo FX, Qu YY, Zhao JY, Ye ML, Zhao SM, Xu W. Pyruvate kinase M2 activates mTORC1 by phosphorylating AKT1S1. Sci Rep. 2016;6:21524. doi: 10.1038/srep21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang RC, Wei Y, An Z, Zou Z, Xiao G, Bhagat G, White M, Reichelt J, Levine B. Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation. Science. 2012;338:956–959. doi: 10.1126/science.1225967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310:1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 40.Fettweis G, Di Valentin E, L’homme L, Lassence C, Dequiedt F, Fillet M, Coupienne I, Piette J. RIP3 antagonizes a TSC2-mediated pro-survival pathway in glioblastoma cell death. Biochim Biophys Acta. 2017;1864:113–124. doi: 10.1016/j.bbamcr.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 41.Marchand B, Arsenault D, Raymond-Fleury A, Boisvert FM, Boucher MJ. Glycogen synthase kinase-3 (GSK3) inhibition induces prosurvival autophagic signals in human pancreatic cancer cells. J Biol Chem. 2015;290:5592–5605. doi: 10.1074/jbc.M114.616714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim HJ, Lee SY, Kim CY, Kim YH, Ju W, Kim SC. Subcellular localization of FOXO3a as a potential biomarker of response to combined treatment with inhibitors of PI3K and autophagy in PIK3CA-mutant cancer cells. Oncotarget. 2017;8:6608–6622. doi: 10.18632/oncotarget.14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu J, Fotouhi M, McPherson PS. Phosphorylation of the exchange factor DENND3 by ULK in response to starvation activates Rab12 and induces autophagy. EMBO Rep. 2015;16:709–718. doi: 10.15252/embr.201440006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pozuelo-Rubio M. Regulation of autophagic activity by 14-3-3ζ proteins associated with class III phosphatidylinositol-3-kinase. Cell Death Differ. 2011;18:479–492. doi: 10.1038/cdd.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sai Y, Peng K, Ye F, Zhao X, Zhao Y, Zou Z, Cao J, Dong Z. 14-3-3 proteins in the regulation of rotenone-induced neurotoxicity might be via its isoform 14-3-3 Epsilon’s involvement in autophagy. Cell Mol Neurobiol. 2013;33:1109–1121. doi: 10.1007/s10571-013-9977-9. [DOI] [PubMed] [Google Scholar]
- 46.Zhu Y, Bu Q, Liu X, Hu W, Wang Y. Neuroprotective effect of TAT-14-3-3ε fusion protein against cerebral ischemia/reperfusion injury in rats. PLoS One. 2014;9:e93334. doi: 10.1371/journal.pone.0093334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meng X, Luo Y, Liang T, Wang M, Zhao J, Sun G, Sun X. Gypenoside XVII enhances lysosome biogenesis and autophagy flux and accelerates autophagic clearance of amyloid-β through TFEB activation. J Alzheimers Dis. 2016;52:1135–1150. doi: 10.3233/JAD-160096. [DOI] [PubMed] [Google Scholar]
- 48.Kanai M, Raz A, Goodman DS. Retinolbinding protein: the transport protein for vitamin A in human plasma. J Clin Invest. 1968;47:2025–2044. doi: 10.1172/JCI105889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vieira M, Saraiva MJ. Transthyretin regulates hippocampal 14-3-3ζ protein levels. FEBS Lett. 2013;587:1482–1488. doi: 10.1016/j.febslet.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 50.Martina JA, Diab H, Lishu L, Jeong-A L, Patange S, Raben N, Puertollano R. The nutrientresponsive transcription factor TFE3, promotes autophagy, lysosomal biogenesis, and clearance of cellular debris. Sci Signal. 2014;7:ra9. doi: 10.1126/scisignal.2004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gómez-Suárez M, Gutiérrez-Martínez IZ, Hernández-Trejo JA, Hernández-Ruiz M, Suárez-Pérez D, Candelario A, Kamekura R, Medina-Contreras O, Schnoor M, Ortiz-Navarrete V, Villegas-Sepúlveda N, Parkos C, Nusrat A, Nava P. 14-3-3 proteins regulate Akt Thr308 phosphorylation in intestinal epithelial cells. Cell Death Differ. 2016;23:1060–1072. doi: 10.1038/cdd.2015.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hernández-Trejo JA, Suárez-Pérez D, Gutiérrez-Martínez IZ, Fernandez-Vargas OE, Serrano C, Candelario-Martínez AA, Meraz-Ríos MA, Citalán-Madrid AF, Hernández-Ruíz M, Reyes-Maldonado E, Valle-Rios R, Feintuch-Unger JH, Schnoor M, Villegas-Sepúlveda N, Medina-Contreras O, Nava P. The pro-inflammatory cytokines IFNγ/TNFα increase chromogranin A-positive neuroendocrinecells in the colonic epithelium. Biochem J. 2016;473:3805–3818. doi: 10.1042/BCJ20160390. [DOI] [PubMed] [Google Scholar]
- 53.Martina JA, Chen Y, Gucek M, Puertollano R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy. 2012;8:903–914. doi: 10.4161/auto.19653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma B, Yuan Z, Zhang L, Lv P, Yang T, Gao J, Pan N, Wu Q, Lou J, Han C, Zhang B. Long noncoding RNA AC023115.3 suppresses chemoresistance of glioblastoma by reducing autophagy. Biochim Biophys Acta. 2017;1864:1393–1404. doi: 10.1016/j.bbamcr.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 55.Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, Mukherjee C, Shi Y, Gélinas C, Fan Y, Nelson DA, Jin S, White E. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]


