Abstract
α-Ketoamides undergo redox-annulations with cyclic secondary amines, such as 1,2,3,4-tetrahydroisoquinoline, pyrrolidine, piperidine, and morpholine. Catalytic amounts of benzoic acid significantly accelerate these transformations. This approach provides polycyclic imidazolidinone derivatives in typically good yields.
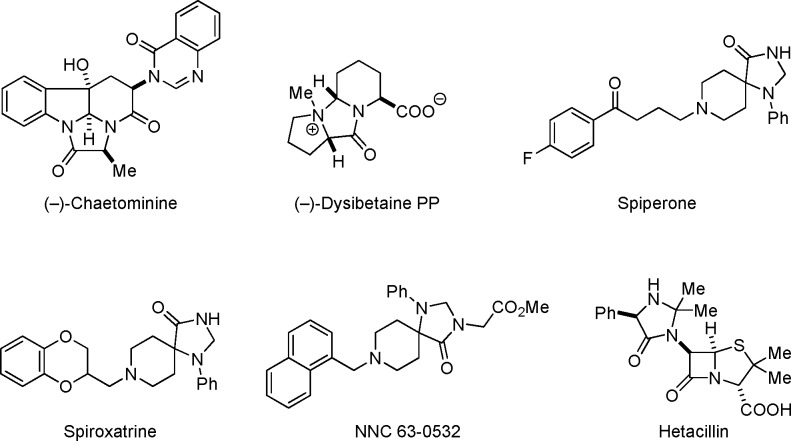
Imidazolidinones are frequently encountered as substructures of natural products and synthetic, biologically active compounds (Figure 1).1−3 Among the most common methods used to build the imidazolidinone motif are condensations of α-aminoacetamide derivatives with aldehydes or ketones, various cycloadditions, ring expansions, and others.1 Methods have also emerged that are particularly suitable for the preparation of ring-fused imidazolidinones (Scheme 1). One such approach involves an oxidative intramolecular coupling of α-aminoacetamide derivatives (eq 1).4 A decarboxylative strategy involving the condensation of proline with α-ketoamides to build bicyclic imidazolidinones containing a pyrrolidine ring has also been established (eq 2).5,6 Here we report a redox-neutral annulation approach to polycyclic imidazolidinones (eq 3).
Figure 1.
Selected 4-imidazolidinones.
Scheme 1. Selected Routes to Polycyclic 4-Imidazolidinones.
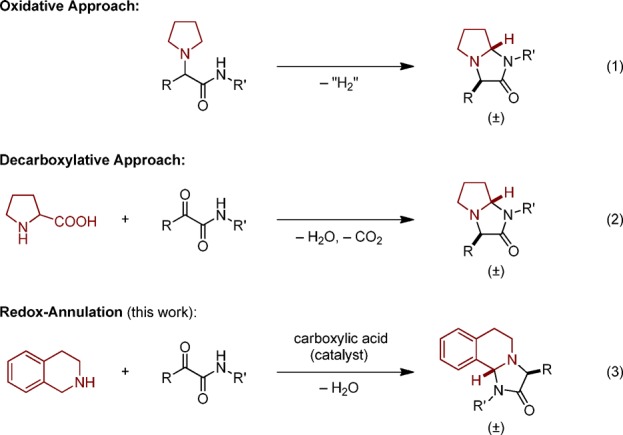
We7 and others8 have developed a range of redox-neutral annulation reactions that proceed via the condensation of a secondary amine with an aldehyde/ketone possessing a pendent (pro)nucleophile. These annulations feature concurrent N-alkylation and the functionalization of an amine α-C–H bond.9,10 The majority of these transformations proceed through azomethine ylide intermediates, utilize carboxylic acids as catalysts or promoters, and result in the formation of a new six-membered ring.11 Although there are examples of redox-neutral amine α-C–H bond functionalizations of secondary amines that give rise to the formation of new five-membered rings, typically via (3 + 2) cycloaddition of azomethine ylide intermediates12 or 1,5-electrocyclic ring-closure of conjugated azomethine ylides,13,14 this chemistry remains underdeveloped and has rarely been applied to C–N bond formation.13c,13i We reasoned that such an annulation could be applied to the synthesis of bi- or polycyclic imidazolidinones via the condensation of cyclic amines with α-ketoamides (Scheme 1, eq 3).15
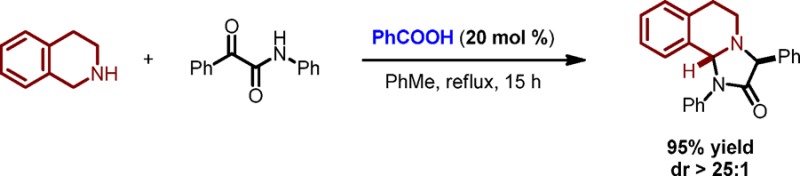
1,2,3,4-Tetrahydroisoquinoline (THIQ) and 2-oxo-N,2-diphenylacetamide (1a) were selected as model substrates in order to evaluate the proposed annulation process (Table 1). A 2:1 mixture of THIQ and 1a, upon heating under reflux in toluene for 2 days, resulted in an incomplete reaction and the isolation of desired product 2a as a single diastereomer in 50% yield (entry 1). Utilization of catalytic amounts of benzoic acid (20 mol %) resulted in a significant improvement (entry 2). Complete consumption of 1a was observed within 7 h, and 2a was obtained in 95% yield. Replacement of benzoic acid with either acetic acid or 2-ethylhexanoic acid (2-EHA) facilitated the formation of 2a in similar yields but required prolonged reaction times (entries 3 and 4). A reaction that was performed at 50 °C remained incomplete after 44 h and led to product in moderate yield (entry 5). A reduction of the amount of THIQ to 1.5 equiv was well tolerated (entry 6), whereas further reduction to 1.2 equiv led to a slight drop in yield (entry 7). Notably, the reaction performed equally well in the absence of molecular sieves (entry 8).
Table 1. Evaluation of Reaction Conditionsa.
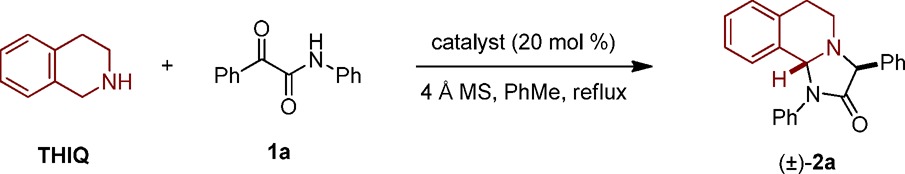
| entry | THIQ (equiv) | catalyst | time (h) | yield (%) |
|---|---|---|---|---|
| 1 | 2 | 48 | 50 | |
| 2 | 2 | PhCOOH | 7 | 95 |
| 3 | 2 | AcOH | 23 | 92 |
| 4 | 2 | 2-EHA | 21 | 91 |
| 5b | 2 | PhCOOH | 44 | 56 |
| 6 | 1.5 | PhCOOH | 12 | 93 |
| 7 | 1.2 | PhCOOH | 12 | 88 |
| 8c | 1.5 | PhCOOH | 15 | 95 |
Reactions were performed on a 0.2 mmol scale. All yields correspond to isolated yields. dr >25:1 in all cases.
Reaction was run at 50 °C and remained incomplete.
Without 4 Å MS.
The scope of the redox-annulation was explored under the optimized conditions of Table 1 (entry 8). A range of α-ketoamides with different substitution patterns were investigated (Scheme 2). The corresponding 4-imidazolidinone products 2 were isolated in good to excellent yields. Both aromatic and aliphatic substituents on the amide nitrogen were tolerated. Likewise, nonenolizable and enolizable α-ketoamides participated in the annulation reaction. In the case of the primary amide-derived product 2n, which was obtained in 53% yield, a competing pathway was identified. Specifically, the corresponding transamidation product was obtained in 38% yield.16 An enantiomerically pure α-ketoamide, derived from (S)-1-phenylethan-1-amine, provided product 2o in 86% yield as a 1.3:1 mixture of diastereomers.
Scheme 2. Ketoamide Scope.
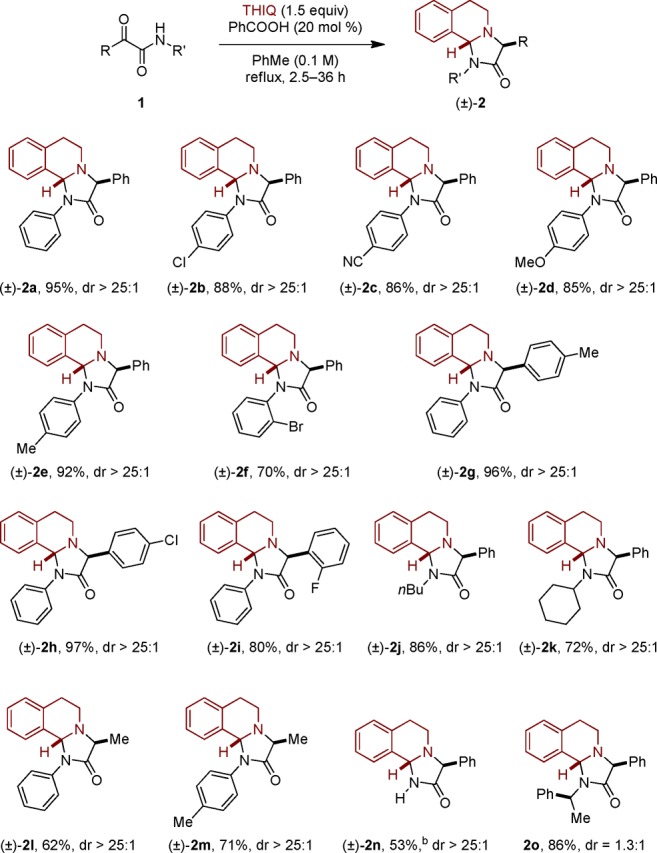
Reactions were performed on a 0.5 mmol scale. All yields correspond to isolated yields.
Transamidation product (1-(3,4-dihydroisoquinolin-2(1H)-yl)-2-phenylethane-1,2-dione) was obtained in 38% yield.
The scope of the amine component is summarized in Scheme 3. Benzylic amines other than THIQ, including the sterically hindered 1-phenyl-THIQ, readily formed annulation products upon reaction with α-ketoamide 1a. Amines with attenuated reactivities, such as pyrrolidine and azepane, provided 4-imidazolidinone products in good yields. Particularly challenging substrates such as piperidine, morpholine, and thiomorpholine underwent the title reaction at elevated temperatures.
Scheme 3. Amine Scope.
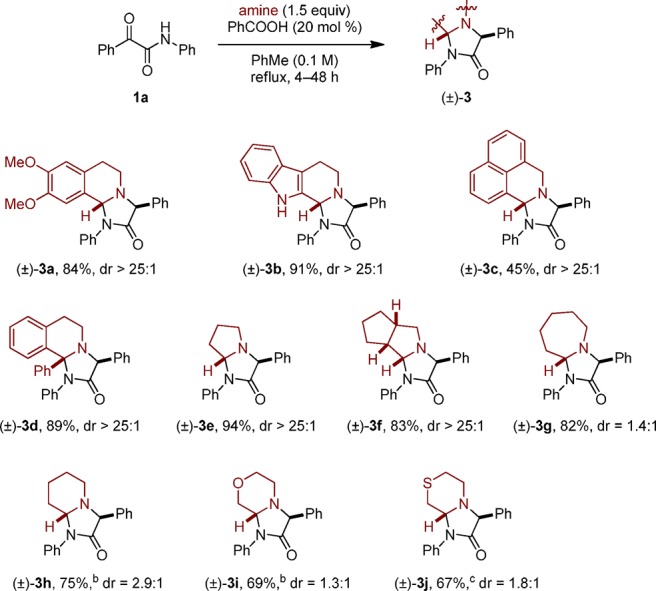
Reactions were performed on a 0.5 mmol scale. All yields correspond to isolated yields.
Reaction was performed in PhMe (0.25 M) under microwave irradiation for 30 min at 220 °C.
Reaction was performed in PhMe (0.25 M) under microwave irradiation for 1 h at 220 °C.
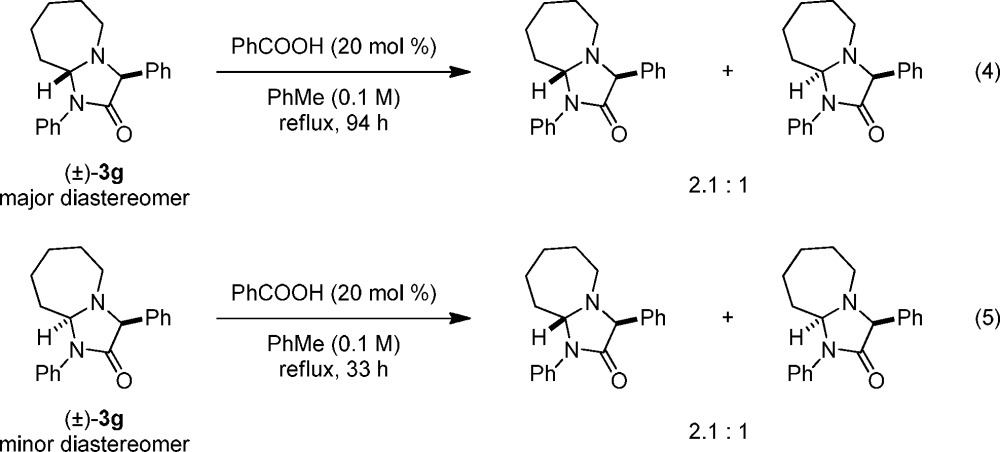
As shown in Schemes 2 and 3, reactions involving THIQ, related benzylic amines, and pyrrolidine underwent redox-annulations with α-ketoamides in highly diastereoselective fashion. In contrast, reactions with azepane, piperidine, morpholine, and thiomorpholine were poorly diastereoselective. We suspected that the aminal stereogenic center might be configurationally unstable under the reaction conditions. Thus, product ratios may reflect the different thermodynamic stabilities of the two diastereomers. To test this hypothesis, the readily available pure diastereomers of product 3g were exposed to the reaction conditions (eqs 4 and 5). Upon extended heating, both mixtures converged to a final 2.1:1 ratio of diastereomers. These experiments establish that product isomerization can indeed occur under the reaction conditions.
 |
4 |
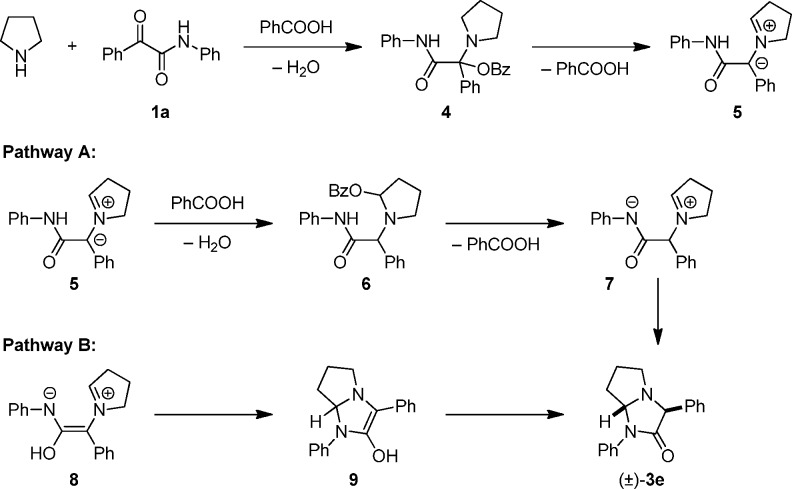
Two plausible mechanistic scenarios are shown in Scheme 4, depicting pyrrolidine and α-ketoamide 1a as prototypical examples. Based on previous investigations, the initial formation of N,O-acetal 4 appears highly likely. Again based on precedent, 4 could lose benzoic acid to form azomethine ylide 5. Following the general mechanism of other redox-annulations,115 could reengage benzoic acid to form N,O-acetal 6. The latter ultimately undergoes ring closure to final product 3e with loss of benzoic acid, possibly via the zwitterionic intermediate 7 (pathway A). In an alternate scenario, conjugated azomethine ylide 8, which represents a tautomer of azomethine ylide 5, undergoes ring closure in what is formally a 1,5-electrocyclization.14g The resulting intermediate 9 then undergoes tautomerization to product 3e (pathway B).17
Scheme 4. Mechanistic Considerations.
In conclusion, we have achieved high-yielding syntheses of polycyclic imidazolidinones via redox-annulations of cyclic amines with a range of α-ketoamides. These reactions are efficiently catalyzed by benzoic acid and are rare examples of redox-neutral transformations in which an amine α-C–H bond is replaced by a C–N bond in the context of five-membered ring formation.
Acknowledgments
Financial support from the NIH–NIGMS (R01GM101389) is gratefully acknowledged. We thank Dr. Tom Emge (Rutgers University) for crystallographic analysis and Dr. Wazo Myint (Rutgers University) for assistance with NMR assignments.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.7b03309.
Author Present Address
§ (D.S.) Department of Chemistry, University of Florida, Gainesville, FL 32611.
Author Contributions
∥ Z.Z. and X.L. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Review on the synthetic and medicinal chemistry of 4-imidazolidinones:Blackmore T.; Thompson P. Heterocycles 2011, 83, 1953. 10.3987/REV-11-707. [DOI] [Google Scholar]
- For a general review on the synthesis of aminal-type structures, see:; Hiersemann M. In Comprehensive Organic Functional Group Transformations II; Katritzky A. R. T., Richard J. K., Ed.; Elsevier Ltd.: Oxford, UK, 2005; Vol. 4, p 411. [Google Scholar]
- Selected reports on natural and synthetic, biologically active 4-imidazolidinones:; a Smissman E.; Inloes R.; El-Antably S.; Shaffer P. J. Med. Chem. 1976, 19, 161. 10.1021/jm00223a028. [DOI] [PubMed] [Google Scholar]; b Leysen J.; Gommeren W.; Laduron P. Biochem. Pharmacol. 1978, 27, 307. 10.1016/0006-2952(78)90233-2. [DOI] [PubMed] [Google Scholar]; c Nelson D.; Taylor E. Eur. J. Pharmacol. 1986, 124, 207. 10.1016/0014-2999(86)90147-0. [DOI] [PubMed] [Google Scholar]; d Nikam S.; Martin A.; Nelson D. J. Med. Chem. 1988, 31, 1965. 10.1021/jm00118a017. [DOI] [PubMed] [Google Scholar]; e Rasmussen G.; Bundgaard H. Int. J. Pharm. 1991, 71, 45. 10.1016/0378-5173(91)90066-W. [DOI] [Google Scholar]; f Pinza M.; Farina C.; Cerri A.; Pfeiffer U.; Riccaboni M. T.; Banfi S.; Biagetti R.; Pozzi O.; Magnani M.; Dorigotti L. J. Med. Chem. 1993, 36, 4214. 10.1021/jm00078a011. [DOI] [PubMed] [Google Scholar]; g Thomsen C.; Hohlweg R. Br. J. Pharmacol. 2000, 131, 903. 10.1038/sj.bjp.0703661. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Ijzendoorn D. R.; Botman P. N. M.; Blaauw R. H. Org. Lett. 2006, 8, 239. 10.1021/ol052598r. [DOI] [PubMed] [Google Scholar]; i Toumi M.; Couty F.; Marrot J.; Evano G. Org. Lett. 2008, 10, 5027. 10.1021/ol802155n. [DOI] [PubMed] [Google Scholar]; j Vale N.; Prudencio M.; Marques C.; Collins M.; Gut J.; Nogueira F.; Matos J.; Rosenthal P.; Cushion M.; Rosario V.; Mota M.; Moreira R.; Gomes P. J. Med. Chem. 2009, 52, 7800. 10.1021/jm900738c. [DOI] [PMC free article] [PubMed] [Google Scholar]; k Vale N.; Nogueira F.; Rosario V.; Gomes P.; Moreira R. Eur. J. Med. Chem. 2009, 44, 2506. 10.1016/j.ejmech.2009.01.018. [DOI] [PubMed] [Google Scholar]
- a Vasvari-Debreczy L.; Beckett A.; Vutthikongsirigool W. Tetrahedron 1981, 37, 4337. 10.1016/0040-4020(81)85031-4. [DOI] [Google Scholar]; b Papadopoulos A.; Lewall B.; Steckhan E.; Ginzel K.; Knoch F.; Nieger M. Tetrahedron 1991, 47, 563. 10.1016/S0040-4020(01)87046-0. [DOI] [Google Scholar]; c Yu H.; Shen J. RSC Adv. 2015, 5, 9815. 10.1039/C4RA15019H. [DOI] [Google Scholar]; d Ren X.; O’Hanlon J.; Morris M.; Robertson J.; Wong L. ACS Catal. 2016, 6, 6833. 10.1021/acscatal.6b02189. [DOI] [Google Scholar]
- Wu J.-s.; Jiang H.-j.; Yang J.-g.; Jin Z.-n.; Chen D.-b. Tetrahedron Lett. 2017, 58, 546. 10.1016/j.tetlet.2016.12.079. [DOI] [Google Scholar]
- Examples of condensation-based approaches to polycyclic 4-imidazolidinones:; a Katritzky A. R.; He H.-Y.; Wang J. J. Org. Chem. 2002, 67, 4951. 10.1021/jo010842w. [DOI] [PubMed] [Google Scholar]; b Ferraz R.; Gomes J. R. B.; de Oliveira E.; Moreira R.; Gomes P. J. Org. Chem. 2007, 72, 4189. 10.1021/jo0703202. [DOI] [PubMed] [Google Scholar]
- a Zhang C.; De C. K.; Mal R.; Seidel D. J. Am. Chem. Soc. 2008, 130, 416. 10.1021/ja077473r. [DOI] [PubMed] [Google Scholar]; b Zhang C.; Das D.; Seidel D. Chem. Sci. 2011, 2, 233. 10.1039/C0SC00432D. [DOI] [Google Scholar]; c Dieckmann A.; Richers M. T.; Platonova A. Y.; Zhang C.; Seidel D.; Houk K. N. J. Org. Chem. 2013, 78, 4132. 10.1021/jo400483h. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Richers M. T.; Deb I.; Platonova A. Y.; Zhang C.; Seidel D. Synthesis 2013, 45, 1730. 10.1055/s-0033-1338852. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Richers M. T.; Breugst M.; Platonova A. Y.; Ullrich A.; Dieckmann A.; Houk K. N.; Seidel D. J. Am. Chem. Soc. 2014, 136, 6123. 10.1021/ja501988b. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Jarvis C. L.; Richers M. T.; Breugst M.; Houk K. N.; Seidel D. Org. Lett. 2014, 16, 3556. 10.1021/ol501509b. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Kang Y.; Chen W.; Breugst M.; Seidel D. J. Org. Chem. 2015, 80, 9628. 10.1021/acs.joc.5b01384. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Ma L.; Seidel D. Chem. - Eur. J. 2015, 21, 12908. 10.1002/chem.201501667. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Chen W.; Seidel D. Org. Lett. 2016, 18, 1024. 10.1021/acs.orglett.6b00151. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Zhu Z.; Seidel D. Org. Lett. 2017, 19, 2841. 10.1021/acs.orglett.7b01047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Zheng L.; Yang F.; Dang Q.; Bai X. Org. Lett. 2008, 10, 889. 10.1021/ol703049j. [DOI] [PubMed] [Google Scholar]; b Mahato S.; Haque M. A.; Dwari S.; Jana C. K. RSC Adv. 2014, 4, 46214. 10.1039/C4RA05045B. [DOI] [Google Scholar]; c Li J.; Qin C.; Yu Y.; Fan H.; Fu Y.; Li H.; Wang W. Adv. Synth. Catal. 2017, 359, 2191. 10.1002/adsc.201601423. [DOI] [Google Scholar]; d Li J.; Fu Y.; Qin C.; Yu Y.; Li H.; Wang W. Org. Biomol. Chem. 2017, 15, 6474. 10.1039/C7OB01527E. [DOI] [PubMed] [Google Scholar]
- Selected reviews on amine C–H functionalization, including redox-neutral approaches:; a Murahashi S.-I. Angew. Chem., Int. Ed. Engl. 1995, 34, 2443. 10.1002/anie.199524431. [DOI] [Google Scholar]; b Matyus P.; Elias O.; Tapolcsanyi P.; Polonka-Balint A.; Halasz-Dajka B. Synthesis 2006, 2006, 2625. 10.1055/s-2006-942490. [DOI] [Google Scholar]; c Campos K. R. Chem. Soc. Rev. 2007, 36, 1069. 10.1039/B607547A. [DOI] [PubMed] [Google Scholar]; d Murahashi S.-I.; Zhang D. Chem. Soc. Rev. 2008, 37, 1490. 10.1039/b706709g. [DOI] [PubMed] [Google Scholar]; e Li C.-J. Acc. Chem. Res. 2009, 42, 335. 10.1021/ar800164n. [DOI] [PubMed] [Google Scholar]; f Jazzar R.; Hitce J.; Renaudat A.; Sofack-Kreutzer J.; Baudoin O. Chem. - Eur. J. 2010, 16, 2654. 10.1002/chem.200902374. [DOI] [PubMed] [Google Scholar]; g Yeung C. S.; Dong V. M. Chem. Rev. 2011, 111, 1215. 10.1021/cr100280d. [DOI] [PubMed] [Google Scholar]; h Pan S. C. Beilstein J. Org. Chem. 2012, 8, 1374. 10.3762/bjoc.8.159. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Mitchell E. A.; Peschiulli A.; Lefevre N.; Meerpoel L.; Maes B. U. W. Chem. - Eur. J. 2012, 18, 10092. 10.1002/chem.201201539. [DOI] [PubMed] [Google Scholar]; j Zhang C.; Tang C.; Jiao N. Chem. Soc. Rev. 2012, 41, 3464. 10.1039/c2cs15323h. [DOI] [PubMed] [Google Scholar]; k Jones K. M.; Klussmann M. Synlett 2012, 2012, 159. 10.1055/s-0031-1290117. [DOI] [Google Scholar]; l Peng B.; Maulide N. Chem. - Eur. J. 2013, 19, 13274. 10.1002/chem.201301522. [DOI] [PubMed] [Google Scholar]; m Platonova A. Y.; Glukhareva T. V.; Zimovets O. A.; Morzherin Y. Y. Chem. Heterocycl. Compd. 2013, 49, 357. 10.1007/s10593-013-1257-6. [DOI] [Google Scholar]; n Prier C. K.; Rankic D. A.; MacMillan D. W. C. Chem. Rev. 2013, 113, 5322. 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]; o Girard S. A.; Knauber T.; Li C.-J. Angew. Chem., Int. Ed. 2014, 53, 74. 10.1002/anie.201304268. [DOI] [PubMed] [Google Scholar]; p Haibach M. C.; Seidel D. Angew. Chem., Int. Ed. 2014, 53, 5010. 10.1002/anie.201306489. [DOI] [PubMed] [Google Scholar]; q Wang L.; Xiao J. Adv. Synth. Catal. 2014, 356, 1137. 10.1002/adsc.201301153. [DOI] [Google Scholar]; r Vo C.-V. T.; Bode J. W. J. Org. Chem. 2014, 79, 2809. 10.1021/jo5001252. [DOI] [PubMed] [Google Scholar]; s Seidel D. Org. Chem. Front. 2014, 1, 426. 10.1039/C4QO00022F. [DOI] [PMC free article] [PubMed] [Google Scholar]; t Qin Y.; Lv J.; Luo S. Tetrahedron Lett. 2014, 55, 551. 10.1016/j.tetlet.2013.11.051. [DOI] [Google Scholar]; u Seidel D. Acc. Chem. Res. 2015, 48, 317. 10.1021/ar5003768. [DOI] [PMC free article] [PubMed] [Google Scholar]; v Beatty J. W.; Stephenson C. R. J. Acc. Chem. Res. 2015, 48, 1474. 10.1021/acs.accounts.5b00068. [DOI] [PMC free article] [PubMed] [Google Scholar]; w Mahato S.; Jana C. K. Chem. Rec. 2016, 16, 1477. 10.1002/tcr.201600001. [DOI] [PubMed] [Google Scholar]; x Qin Y.; Zhu L.; Luo S. Chem. Rev. 2017, 117, 9433. 10.1021/acs.chemrev.6b00657. [DOI] [PubMed] [Google Scholar]; y Cheng M.-X.; Yang S.-D. Synlett 2017, 28, 159. 10.1055/s-0036-1588342. [DOI] [Google Scholar]
- Selected reviews on various types of redox-neutral transformations:; a Burns N. Z.; Baran P. S.; Hoffmann R. W. Angew. Chem., Int. Ed. 2009, 48, 2854. 10.1002/anie.200806086. [DOI] [PubMed] [Google Scholar]; b Mahatthananchai J.; Bode J. W. Acc. Chem. Res. 2014, 47, 696. 10.1021/ar400239v. [DOI] [PubMed] [Google Scholar]; c Ketcham J. M.; Shin I.; Montgomery T. P.; Krische M. J. Angew. Chem., Int. Ed. 2014, 53, 9142. 10.1002/anie.201403873. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Huang H.; Ji X.; Wu W.; Jiang H. Chem. Soc. Rev. 2015, 44, 1155. 10.1039/C4CS00288A. [DOI] [PubMed] [Google Scholar]
- For detailed discussions on the mechanisms of these transformations, see refs (7c,7e−7g) and (9u) and the following reports:; a Xue X.; Yu A.; Cai Y.; Cheng J.-P. Org. Lett. 2011, 13, 6054. 10.1021/ol2025247. [DOI] [PubMed] [Google Scholar]; b Ma L.; Paul A.; Breugst M.; Seidel D. Chem. - Eur. J. 2016, 22, 18179. 10.1002/chem.201603839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Examples of redox-neutral α-C–H functionalizations of secondary amines in the context of (3 + 2) cycloadditions:; a Ardill H.; Grigg R.; Sridharan V.; Surendrakumar S.; Thianpatanagul S.; Kanajun S. J. Chem. Soc., Chem. Commun. 1986, 602. 10.1039/c39860000602. [DOI] [Google Scholar]; b Ardill H.; Dorrity M. J. R.; Grigg R.; Leon-Ling M. S.; Malone J. F.; Sridharan V.; Thianpatanagul S. Tetrahedron 1990, 46, 6433. 10.1016/S0040-4020(01)96013-2. [DOI] [Google Scholar]; c Ardill H.; Fontaine X. L. R.; Grigg R.; Henderson D.; Montgomery J.; Sridharan V.; Surendrakumar S. Tetrahedron 1990, 46, 6449. 10.1016/S0040-4020(01)96014-4. [DOI] [Google Scholar]; d Wang B.; Mertes M. P.; Mertes K. B.; Takusagawa F. Tetrahedron Lett. 1990, 31, 5543. 10.1016/S0040-4039(00)97892-4. [DOI] [Google Scholar]; e Wittland C.; Arend M.; Risch N. Synthesis 1996, 1996, 367. 10.1055/s-1996-4208. [DOI] [Google Scholar]; f Marx M. A.; Grillot A.-L.; Louer C. T.; Beaver K. A.; Bartlett P. A. J. Am. Chem. Soc. 1997, 119, 6153. 10.1021/ja9621051. [DOI] [Google Scholar]; g Grigg R.; Sridharan V.; Thornton-Pett M.; Wang J.; Xu J.; Zhang J. Tetrahedron 2002, 58, 2627. 10.1016/S0040-4020(02)00129-1. [DOI] [Google Scholar]; h Parmar N. J.; Pansuriya B. R.; Labana B. M.; Kant R.; Gupta V. K. RSC Adv. 2013, 3, 17527. 10.1039/c3ra42220h. [DOI] [Google Scholar]; i Rahman M.; Bagdi A. K.; Mishra S.; Hajra A. Chem. Commun. 2014, 50, 2951. 10.1039/c4cc00454j. [DOI] [PubMed] [Google Scholar]; j Mantelingu K.; Lin Y.; Seidel D. Org. Lett. 2014, 16, 5910. 10.1021/ol502918g. [DOI] [PMC free article] [PubMed] [Google Scholar]; k Pavan Kumar C. S.; Harsha K. B.; Mantelingu K.; Rangappa K. S. RSC Adv. 2015, 5, 61664. 10.1039/C5RA10030E. [DOI] [Google Scholar]; l Safaei-Ghomi J.; Masoomi R. RSC Adv. 2015, 5, 15591. 10.1039/C4RA16020G. [DOI] [Google Scholar]; m Yang H.-T.; Tan Y.-C.; Ge J.; Wu H.; Li J.-X.; Yang Y.; Sun X.-Q.; Miao C.-B. J. Org. Chem. 2016, 81, 11201. 10.1021/acs.joc.6b02193. [DOI] [PubMed] [Google Scholar]; n Zheng K.-L.; Shu W.-M.; Ma J.-R.; Wu Y.-D.; Wu A.-X. Org. Lett. 2016, 18, 3526. 10.1021/acs.orglett.6b01369. [DOI] [PubMed] [Google Scholar]; o Du Y.; Yu A.; Jia J.; Zhang Y.; Meng X. Chem. Commun. 2017, 53, 1684. 10.1039/C6CC08996H. [DOI] [PubMed] [Google Scholar]; p Zheng K.-L.; You M.-Q.; Shu W.-M.; Wu Y.-D.; Wu A.-X. Org. Lett. 2017, 19, 2262. 10.1021/acs.orglett.7b00769. [DOI] [PubMed] [Google Scholar]
- Examples of redox-neutral α-C–H bond annulations of secondary amines that result in the formation of 5-membered rings:; a Grigg R.; Nimal Gunaratne H. Q.; Henderson D.; Sridharan V. Tetrahedron 1990, 46, 1599. 10.1016/S0040-4020(01)81969-4. [DOI] [Google Scholar]; b Soeder R. W.; Bowers K.; Pegram L. D.; Cartaya-Marin C. P. Synth. Commun. 1992, 22, 2737. 10.1080/00397919208021537. [DOI] [Google Scholar]; c Grigg R.; Kennewell P.; Savic V.; Sridharan V. Tetrahedron 1992, 48, 10423. 10.1016/S0040-4020(01)88345-9. [DOI] [Google Scholar]; d Deb I.; Seidel D. Tetrahedron Lett. 2010, 51, 2945. 10.1016/j.tetlet.2010.03.086. [DOI] [Google Scholar]; e Kang Y.; Richers M. T.; Sawicki C. H.; Seidel D. Chem. Commun. 2015, 51, 10648. 10.1039/C5CC03390J. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Cheng Y.-F.; Rong H.-J.; Yi C.-B.; Yao J.-J.; Qu J. Org. Lett. 2015, 17, 4758. 10.1021/acs.orglett.5b02298. [DOI] [PubMed] [Google Scholar]; g Yang Z.; Lu N.; Wei Z.; Cao J.; Liang D.; Duan H.; Lin Y. J. Org. Chem. 2016, 81, 11950. 10.1021/acs.joc.6b01781. [DOI] [PubMed] [Google Scholar]; h Rong H.-J.; Cheng Y.-F.; Liu F.-F.; Ren S.-J.; Qu J. J. Org. Chem. 2017, 82, 532. 10.1021/acs.joc.6b02562. [DOI] [PubMed] [Google Scholar]; i Purkait A.; Roy S. K.; Srivastava H. K.; Jana C. K. Org. Lett. 2017, 19, 2540. 10.1021/acs.orglett.7b00832. [DOI] [PubMed] [Google Scholar]
- Selected reviews on azomethine ylide chemistry:; a Padwa A.1,3-Dipolar Cycloaddition Chemistry; Wiley: New York, N. Y., 1984; Vol. 1. [Google Scholar]; b Padwa A., Ed. 1,3-Dipolar Cycloaddition Chemistry; Wiley: New York, 1984; Vol. 2. [Google Scholar]; c Padwa A.; Pearson W. H.. Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products; Wiley: Chichester, 2002; Vol. 59. [Google Scholar]; d Najera C.; Sansano J. M. Curr. Org. Chem. 2003, 7, 1105. 10.2174/1385272033486594. [DOI] [Google Scholar]; e Coldham I.; Hufton R. Chem. Rev. 2005, 105, 2765. 10.1021/cr040004c. [DOI] [PubMed] [Google Scholar]; f Pandey G.; Banerjee P.; Gadre S. R. Chem. Rev. 2006, 106, 4484. 10.1021/cr050011g. [DOI] [PubMed] [Google Scholar]; g Pinho e Melo T. M. V. D. Eur. J. Org. Chem. 2006, 2006, 2873. 10.1002/ejoc.200500892. [DOI] [Google Scholar]; h Bonin M.; Chauveau A.; Micouin L. Synlett 2006, 2006, 2349. 10.1055/s-2006-949626. [DOI] [Google Scholar]; i Nair V.; Suja T. D. Tetrahedron 2007, 63, 12247. 10.1016/j.tet.2007.09.065. [DOI] [Google Scholar]; j Najera C.; Sansano J. M. Top. Heterocycl. Chem. 2008, 12, 117. 10.1007/7081_2007_099. [DOI] [Google Scholar]; k Stanley L. M.; Sibi M. P. Chem. Rev. 2008, 108, 2887. 10.1021/cr078371m. [DOI] [PubMed] [Google Scholar]; l Nyerges M.; Toth J.; Groundwater P. W. Synlett 2008, 2008, 1269. 10.1055/s-2008-1072743. [DOI] [Google Scholar]; m Pineiro M.; Pinho e Melo T. M. V. D. Eur. J. Org. Chem. 2009, 2009, 5287. 10.1002/ejoc.200900644. [DOI] [Google Scholar]; n Burrell A. J. M.; Coldham I. Curr. Org. Synth. 2010, 7, 312. 10.2174/157017910791414472. [DOI] [Google Scholar]; o Anac O.; Gungor F. S. Tetrahedron 2010, 66, 5931. 10.1016/j.tet.2010.05.058. [DOI] [Google Scholar]; p Adrio J.; Carretero J. C. Chem. Commun. 2011, 47, 6784. 10.1039/c1cc10779h. [DOI] [PubMed] [Google Scholar]; q Adrio J.; Carretero J. C. Chem. Commun. 2014, 50, 12434. 10.1039/C4CC04381B. [DOI] [PubMed] [Google Scholar]; r Hashimoto T.; Maruoka K. Chem. Rev. 2015, 115, 5366. 10.1021/cr5007182. [DOI] [PubMed] [Google Scholar]; s Meyer A.; Ryan J. Molecules 2016, 21, 935. 10.3390/molecules21080935. [DOI] [Google Scholar]; See also ref (9u).
- For a review on the chemistry of ketoamides, see:De Risi C.; Pollini G. P.; Zanirato V. Chem. Rev. 2016, 116, 3241. 10.1021/acs.chemrev.5b00443. [DOI] [PubMed] [Google Scholar]
- Lanigan R. M.; Sheppard T. D. Eur. J. Org. Chem. 2013, 2013, 7453. 10.1002/ejoc.201300573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An alternative mechanism has also been proposed:Wang J.-Y.; Hu Y.; Wang D.-X.; Pan J.; Huang Z.-T.; Wang M.-X. Chem. Commun. 2009, 422. 10.1039/B816007D. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.