Abstract
People tend to be more prosocial after synchronizing behaviors with others, yet the underlying neural mechanisms are rarely known. In this study, participant dyads performed either a coordination task or an independence task, with their brain activations recorded via the functional near-infrared spectroscopy hyperscanning technique. Participant dyads in the coordination group showed higher synchronized behaviors and greater subsequent inclination to help each other than those in the independence group, indicating the prosocial effect of interpersonal synchrony. Importantly, the coordination group demonstrated the significant task-related brain coherence, namely the interbrain synchronization, at the left middle frontal area. The detected interbrain synchronization was sensitive to shared intentionality between participants and was correlated with the mutual prosocial inclination. Further, the task-related brain coherence played a mediation role in the prosocial effect of interpersonal synchrony. This study reveals the relevance of brain-to-brain synchronization among individuals with subsequent mutual prosocial inclination and suggests the neural mechanism associating with shared cognition for the facilitation of interpersonal synchrony on prosociality.
Keywords: interbrain synchronization, prosociality, interpersonal synchrony, shared intentionality, functional near-infrared spectroscopy
Introduction
Our everyday lives are filled with social interactions, which involve varying degrees of person-to-person synchronies, i.e. the behavior consistencies (temporal/spatial) among individuals (Richardson et al., 2007; Nessler and Gilliland, 2009). Synchronous behavior is socially important and plays a central role in establishing and promoting social cohesion (McNeill, 1995). Accumulative studies have shown that moving in synchrony with other persons fosters the prosociality to each other (Endedijk et al., 2015; Reddish et al., 2013). For example, synchronized walking, singing and tapping could lead to the increased prosocial behaviors/inclination, such as cooperation (Wiltermuth and Heath, 2009), helpfulness (Kirschner and Tomasello, 2010; Cirelli et al., 2014), trust (Launay et al., 2013), rapport (Hove and Risen, 2009), closeness (Valdesolo and DeSteno, 2011) and empathy (Koehne et al., 2016). However, relatively little is known about the underlying neural mechanisms. In this study, we ask two individuals in dyads to behave synchronously with partners, during which their brain activities were simultaneously recorded. Our aim is to explore the neural substrate of interpersonal synchrony and its effect on mutual prosociality.
While two persons are behaving in a synchronized way, their brain activities are at the same time coupled, demonstrating the interbrain synchronization (IBS) (Hasson et al., 2012). Using multiple electroencephalography (EEG) setups, researchers have captured the synchronous oscillatory activities (in alpha, delta or theta frequency bands) across individuals in time counting (Mu et al., 2016), fingertip moving (Yun et al., 2012), gesture imitating (Dumas et al., 2010) and instrument playing (Lindenberger et al., 2009; Müller et al., 2013). The synchronous brain activities were mainly detected at the frontal, central and posterior areas and were positively correlated with their behavioral synchrony (Mu et al., 2016). Besides, with the low constraints on measurements (e.g. high tolerance of head/body motion), functional near-infrared spectroscopy (fNIRS) technique has been used to examine the dynamic interbrain activities in relatively natural settings. There were enhanced synchronous brain activities among individuals in together key pressing (Funane et al., 2011), active motor imitating (Holper et al., 2012), cooperative singing/humming (Osaka et al., 2014, 2015) and coordinated group walking (Ikeda et al., 2017). Consistent with the EEG studies, the fNIRS studies found the increased IBS mainly at the frontal area (including pre-motor cortex, left inferior frontal cortex and frontal pole), and further, the detected brain synchronization was correlated with the synchronized performance.
Recently, it is shown that synchronous brain activities across two persons can occur with their prosocial behaviors. Specifically, IBS has been observed among interacting persons when they were performing prosocial tasks, i.e. behaving in cooperative ways to achieve common goals. These tasks included a building game completed by a builder and an assistant (Liu et al., 2015), a simulative plane takeoff and landing operated by a captain and a copilot (Astolfi et al., 2012), a non-computerized game, Jenga game, performed by two players (Liu et al., 2016) and a dual n-back task conducted by two teammates (Dommer et al., 2012). In other situations where individuals decide whether to show cooperation to their partners, IBS also emerged at frontal regions (King-Casas et al., 2005; Astolfi et al., 2010). The changes of connectivity pattern in the interbrain network (prefrontal areas, theta band) could predict individuals’ decision to cooperate (De Vico Fallani et al., 2010). More recently, the association between synchronous brain activities and prosociality was found in larger participant group as 4 persons (Nozawa et al., 2016) and 12 persons (Dikker et al., 2017).
There are at least two possible theories accounting for the prosocial effect of interpersonal synchrony. When individuals are performing the tasks requiring synchronized behaviors, they share intentions of achieving the common goals, which ensures that they take the joint actions of themselves and others into account at the same time (Kirschner and Tomasello, 2010). The self-other overlap following shared intentionality can predict behaviors of cooperation (Reddish et al., 2013), as well as compassion (Valdesolo and DeSteno, 2011). Interpersonal synchrony combined with shared intentionality supports prosocial behaviors (Reddish et al., 2013). It seems that the shared intentionality among individuals plays an important role in the effect of interpersonal synchrony on prosociality (Keller et al., 2014). However, other studies indicate that interpersonal synchrony increases perceived similarity among individuals (Mazzurega et al., 2011; Rabinowitch et al., 2015; Reddish et al., 2016), and the perceived similarity can serve as a mediating factor between interpersonal synchrony and prosociality (Valdesolo and DeSteno, 2011). Moreover, people are inclined to help and mate with others similar to themselves (Fessler and Holbrook, 2014; Lumsden et al., 2014). It is likely that interpersonal synchrony enhances a sense of interpersonal similarity, blurs the self-other distinction, and then raises prosociality (Hove and Risen, 2009; Valdesolo et al., 2010; Tarr et al., 2014; Rabinowitch et al., 2015).
In this study, we arranged two groups of participant dyads (i.e. the coordination and the independence groups). Participant dyads in the coordination group were asked to synchronize their behavior with partners, i.e. press keys as simultaneously as possible with partners after silent time counting (Mu et al., 2016), while dyads in the independence group perform the same task independently with respective partners (i.e. computers). Brain activities of all dyads were recorded through the fNIRS-based hyperscanning approach. This approach has been previously used to measure the IBS from two or more persons during their social interactions such as cooperation and communication (Cui et al., 2012; Cheng et al., 2015; Jiang et al., 2015; Pan et al., 2017).
Following the experimental task, we measured the mutual prosocial inclination between participants in dyads. We expected that the coordination group would show greater prosocial inclination than the independence group. Further, we expected that participants in the coordination group would demonstrate the synchronous brain activities across them. To test the intentionality and the similarity hypotheses, subjective measurements of shared intentionality and perceived similarity between participants were arranged. Noted that these two hypotheses are not mutually exclusive, given that the coordination task may at the same time induce the shared intentionality and the perceived similarity between participants. We examined which hypothesis could be more possible in elucidating the underlying mechanism for the effect of interpersonal synchrony on prosociality.
Methods
Participants
Seventy female college students took part in this study as paid volunteers. They were randomly assigned as 35 dyads, with 18 dyads in the coordination group (mean age ± s.d. = 20.67 ± 2.26 years) and 17 dyads in the independence group (mean age ± s.d. = 20.65 ± 2.09 years). Two participants in a dyad were unacquainted; they had not known each other or had never met before the experiment. All participants were right-handed, with normal or corrected-to-normal vision and no record of neurological or psychiatric disorders. Written informed consent was obtained from each participant prior to the experiment. The study procedures were approved by the University Committee on Human Research Protection of East China Normal University.
Tasks and procedures
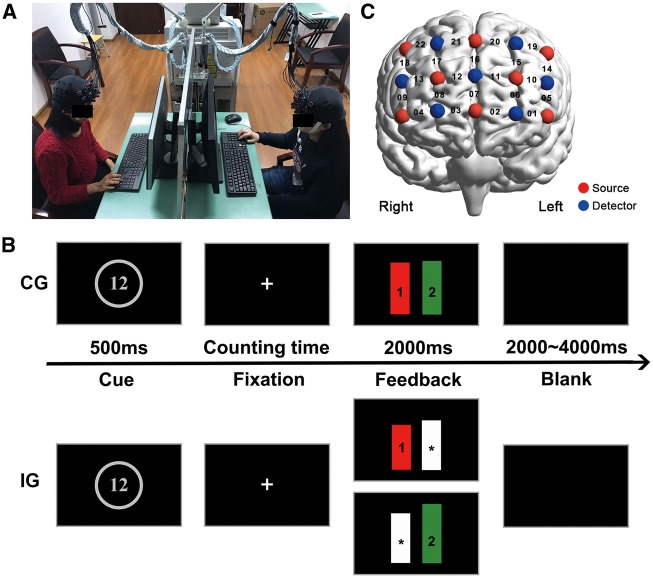
Two participants were seated on the opposite sides of a table, separated by a board (Figure 1A). They were labeled as participants #1 and #2, respectively. Monitors and keyboards were used to present stimuli and receive the responses. In this study, participants would first have a 30 s resting-state session, during which they were required to relax mind and keep motionless as much as possible (Jiang et al., 2015). Then, they would experience two phases. In phase 1, they performed either a coordination task or an independence task. The tasks were implemented using E-prime 2.0 (Psychology Software Tools Inc, Pittsburgh, PA, USA). In phase 2, they evaluated their prosociality, shared intentionality and perceived similarity to their partner. Tasks and related measurements were described as follows.
Fig. 1.
Experimental design. (A) Experimental setup. (B) Experimental tasks and procedures. Events and time flow in a trial (CG, coordination group; IG, independence group; the same denotations for the following figures). (C) Probe configuration. The integers on the cerebral cortex indicate the recording CHs.
Coordination task
In this task, participants were instructed to press keys simultaneously with their partner after counting a time in mind (Mu et al., 2016).
Each trial began with a 500 ms cue of an integer number (Figure 1B). It indicated the standard counting time. After that, there was a fixation screen reminding them of beginning to count. When finishing the time counting, they pressed keys on keyboards (‘1’ by participant #1 and ‘0’ by participant #2). Participants were wearing earplugs so that they could not hear the sounds of key pressing. Next, a feedback screen lasting 2 s was presented. It consisted of a red bar and a green bar, the heights of which indicated the duration of counting time for participants #1 and #2, respectively. The intuitionistic information would help participants recognize their mutual rhythmic synchronization and adjust their counting speed accordingly. This was followed by a blank screen (2–4 s), indicating the end of a trial. Totally, there were three blocks of 15 trials each, lasting ∼18 min.
During the whole task, two participants in dyad were not allowed to communicate with each other by verbal or gestures. This setup made participants in dyad adjust their behaviors based on the feedback, without any directly communicative interaction between them. Prior to the formal task, six practice trials (two trials for each counting time) were administered to familiarize the participants with the task procedures and to ensure that task instructions were understood.
Independence task
The procedures of the independence task were similar to that of the coordination task, except that: (i) the partner of the task was a computer instead of a human; (ii) the duration of computer’s counting time was set as the one indicated by the beginning cue, which was same for participants #1 and #2; (iii) the feedback screen consisted of a white bar with ‘*’ indicating the duration of counting time by the computer, and a red or green bar indicating the duration by participants #1 or #2, respectively.
Mutual prosocial inclination assessment
Participants read the story that a person was in trouble. The story was described as: ‘One afternoon on your way to the cinema, you are going to see an anticipated movie. At the same time, you happen to see the partner. She turns to you because she can’t find the way to the classroom. She looks very worried and anxious. As the classroom is far from your location and the route is complex, it is a little difficult for you to explain the way clearly. The partner hopes you take her to the classroom. If you help her, you will miss the long-awaited movie’. Participants were asked that how much time they would take to help the partner if they were in this context. The rating was on a time scale of ‘0 min’ to ‘50 min’ (Oswald, 2002). The mutually prosocial inclination of a dyad was calculated by averaging two participants’ rating scores.
Subjective measurements
For the shared intentionality, we extracted five questions from the rapport questionnaire (Supplementary Material) (Puccinelli and Tickle-Degnen, 2004). For example, ‘when I was interacting with my partner, there was a shared flow of thoughts and feelings’. For the perceived similarity, a self-report questionnaire was used (Supplementary Material) (Rabinowitch et al., 2015). For example, ‘how much did you feel similar to the other participants’. All questions in the subjective measurements were rated on a 9-point Likert-type scale (1 = ‘not very much’ and 9 = ‘very much’). The rating scores for these questions in the shared intentionality or in the perceived similarity were summed for each participant. The scores of the two participants in a dyad were then averaged as the score of shared intentionality or perceived similarity for that dyad.
Data acquisition
We used an ETG-7100 optical topography system (Hitachi, Japan) to simultaneously record two brains’ cortical hemodynamic activities, including oxyhemo-globin (HbO) and deoxyhemoglobin concentrations. The sample rate was 10 Hz. A 3 × 5 probe patch was placed on the participant’s prefrontal cortex (Figure 1C). The patch placement was in accordance with the international 10–20 system. The bottom row of the patch was placed on top of the participant’ eyebrows, with the middle optrode right at Fpz. The patch consisted of 22 recording channels (CHs). In this study, the virtual registration method was used to determine the correspondence between the NIRS CHs and the measured points on the cerebral cortex (Singh et al., 2005; Tsuzuki et al., 2007).
Data analysis
The task-related brain coherence and the IBS
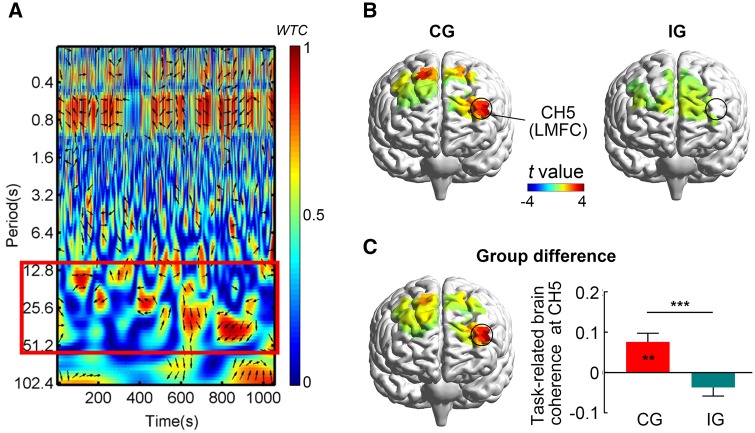
We focused on the changes in the HbO concentration because of its sensitivity in fNIRS measurements (Hoshi, 2003; Cui et al., 2012). The HbO time series on the stage of resting and time counting were collected from the NIRS CHs and the resting session was regarded as a baseline. Principle component analysis (PCA) was used to remove the global components (Zhang et al., 2016). After PCA, all data were able to be included in the subsequent analysis. In this study, the wavelet transform coherence (WTC) analysis of two time series derived from two participants in dyad was conducted to assess the IBS between them for each CH (Grinsted et al., 2004; Murphy et al., 2009). According to previous studies (Cui et al., 2012; Jiang et al., 2015), larger coherence value would be observed when two persons interact, compared with that during the resting state. Based on the same rationale, the average coherence value between 12.8 and 51.2 s (0.02 ∼ 0.08 Hz) was calculated. This frequency band was a range within the 20 s trial cycle in our coordination/independence task and also found to be more sensitive to our task based on the WTC analysis (Figure 3A, Supplementary Figure S1). Moreover, adopting the frequency band could remove high and low frequency noise as well. Therefore, we focused on this frequency band in this study.
Fig. 3.
fNIRS results. (A) IBS indicated by WTC. The coherence based on HbO signal from CH5 in a representative pair in the CG. The red border represents the frequency band of interest (12.8–51.2 s), indicating when the task was carried out. The color bar denotes the value of WTC (1 = highest coherence, 0 = lowest coherence). (B) One-sample t-test maps of task-related brain coherence for the CG and the IG. The IBS was detected only in the CG at CH5 after FDR correction (LMFC, left middle frontal cortex). (C) The independent-samples t-test map of task-related brain coherence for group difference (i.e. CG vs IG). The task-related brain coherence at CH5 was significant higher in the CG than in the IG. Error bars indicate standard errors. **P < 0.01, ***P < 0.001.
The averaged coherence values in the frequency band were calculated during the resting and time counting stages. The task-related coherence was defined as the time counting coherence minusing resting coherence. Next, task-related coherence was transformed into Fisher z-statistics. One-sample t-test for the task-related coherences in a participant group was conducted across each CH. If significant, the IBS was detected on a CH for the group. False discovery rate (FDR) correction was applied for multiple comparisons. Finally, the visualization of the task-related coherence results was performed using the xjview toolbox (http://www.alivelearn.net/xjview8/) and the BrainNet Viewer toolbox (http://www.nitrc.org/projects/bnv/) (Xia et al., 2013). Specifically, the nirs2img function in the xjview toolbox was used to convert the t values of 22 CHs (along with the corresponding Montreal Neurological Institute coordinates) into an image file (t-test map), and then the image file was visualized by BrainNet Viewer.
Movement synchronization
Movement synchronization between two participants was evaluated by the synchronization index (SI, Mardia and Jupp, 2000). For each participant, we calculated the time deviation between participant’s duration of counting time and the standard counting time for each trial. Then, the phases of participants’ time deviation series were extracted by using Hilbert transform. For a given dyad, the SI reflected the phase difference between two participants and was defined as (Tognoli et al., 2007):
where N represents the number of trials, θ1 and θ2 are the phases of participants #1 and #2, respectively. SI is a unitless number, with 0 representing the absence of synchronization and 1 representing full synchronization.
Results
Prosocial effect of interpersonal synchrony
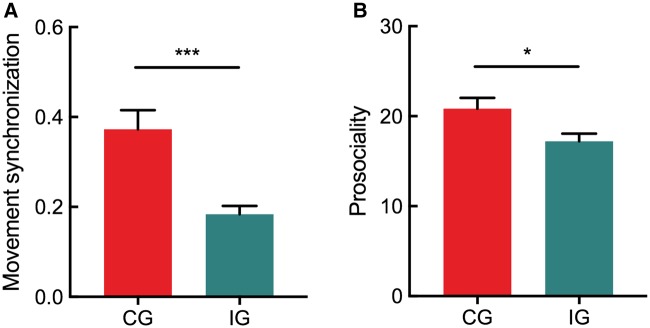
We first examined the difference of movement synchronization between the coordination and the independence groups. The result of an independent-samples t-test showed the higher SI in the coordination group (0.37 ± 0.18) than in the independence group (0.18 ± 0.08), t (33) = 3.98, p < 0.001, Cohen’s d = 1.39 (Figure 2A), indicating the higher movement synchronization in the coordination group compared with the independence group.
Fig. 2.
Behavioral performance. (A) The movement synchronization in two groups. (B) The prosociality (e.g. inclination of helping each other) in two groups. Error bars indicate standard errors. *P < 0.05, ***P < 0.001.
We then examined the difference of prosocial inclination between these two groups. The independent-samples t-test found the greater inclination of helping each other in the coordination group (20.83 ± 5.14) than in the independence group (17.21 ± 3.52), t (33) = 2.42, p < 0.05, Cohen’s d = 0.84 (Figure 2B). This result indicated that the coordination group compared with the independence group demonstrated the prosocial effect of interpersonal synchrony.
Task-related coherence in two groups
A series of one-sample t-tests was conducted to explore the task-related coherences in participant groups. For the coordination group, there was significant task-related coherence, i.e. the IBS, at CH5, t (17) = 3.60, p < 0.05, Cohen’s d = 1.19 and at CH21, t (16) = 2.73, p < 0.05, Cohen’s d = 0.91. Only the coherence at CH5 survived after FDR correction (p < 0.05) (Figure 3B). This CH was approximately located at the left middle frontal cortex (LMFC). For the independence group, no IBS was detected for any CH (before FDR correction) (Figure 3B). Further, an independent-samples t-test showed that the task-related brain coherence at CH5 was significant higher in the coordination group (0.07 ± 0.09) than that in the independence group (−0.04 ± 0.09), t (33) = 3.77, p < 0.001, Cohen’s d = 1.31 (Figure 3C). These results revealed that the coordination task elicited the IBS between two participants at the frontal area; in contrast, no IBS was detected in participants performing the independence task.
Association of the detected IBS with the prosociality
For the coordination group, the Pearson-correlation analyses were used to assess the association between the task-related coherence at CH5 and the inclination of helping the other participant in the same dyad. The correlation between the coherence and mutual inclination to helping was significantly positive, r (17) = 0.49, p < 0.05, (Figure 4). Similar analysis in the independence group did not found the significant correlation, r (16) = −0.08, p > 0.05. We further examined whether there was a significant difference between these two correlations. The statistical comparison relied on tests implemented in the R package cocor (Diedenhofen et al., 2015). Silver’s z procedure confirmed the significant difference between these two correlations (Silver et al., 2004). The result showed that the correlation in the coordination group was stronger than that in the independence group, z = 1.66, p < 0.05 (one-tailed). These results indicated that the increased mutual prosocial inclination in the coordination group was associated with the brain coherence (i.e. the IBS); such association was not found in the independence group.
Fig. 4.
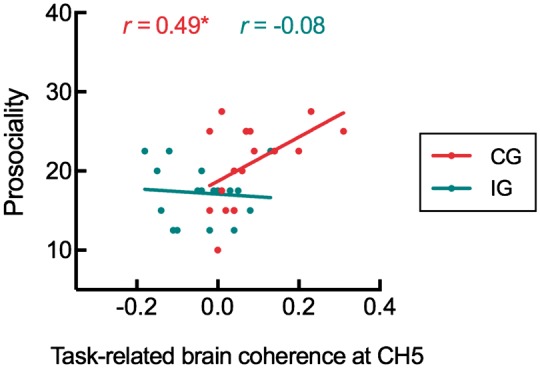
Correlation between the task-related brain coherence at CH5 and the prosociality in two groups. Positive correlative relationship was found in the CG. *P < 0.05.
The association of the detected IBS with subjective measurements
A set of independent-samples t-tests was used to examine the difference of subjective measurements between two participant groups. The results showed the higher intentionality in the coordination group (29.78 ± 4.85) than in the independence group (22.94 ± 4.19), t (33) = 4.45, p < 0.001, Cohen’s d = 1.55 (Figure 5A); such difference was not found for perceived similarity, t (33) = 0.01, p > 0.05, Cohen’s d = 0.003 (Figure 5B). Next, the correlation analyses were used to assess the association of task-related brain coherence with subjective measurements. The results of analysis in the shared intentionality showed significantly positive correlation in the coordination group, r (17) = 0.50, p < 0.05, but not in the independence group, r (16) = −0.27, p > 0.05 (Figure 5C); the correlation in the coordination group was stronger than that in the independence group, z = 2.22, p < 0.05 (one-tailed). Similar analyses for the perceived similarity revealed no correlation either in the coordination group, r (17) = 0.24, p > 0.05, or in the independence group, r (16) = −0.32, p > 0.05 (Figure 5D). In sum, these results revealed that participant dyads performing the coordination task compared with the independence task showed more shared intentionality but not perceived similarity; the increased shared intentionality in the coordination group correlated with the detected IBS.
Fig. 5.
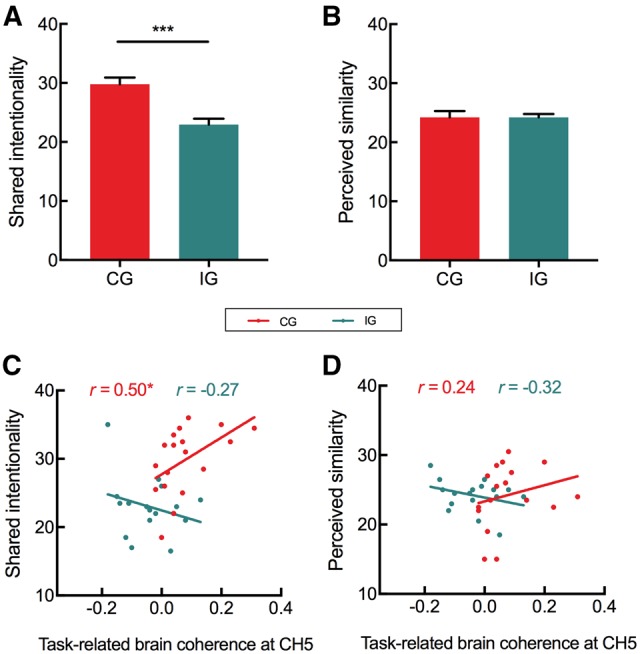
Results of subjective measures. (A) Shared intentionality in two groups. (B) Perceived similarity in two groups. (C) Correlations between task-related coherence and shared intentionality in two groups. (D) Correlations between task-related coherence and perceived similarity in two groups. *P < 0.05, ***P < 0.001.
The role of task-related coherence in the prosocial effect of interpersonal synchrony
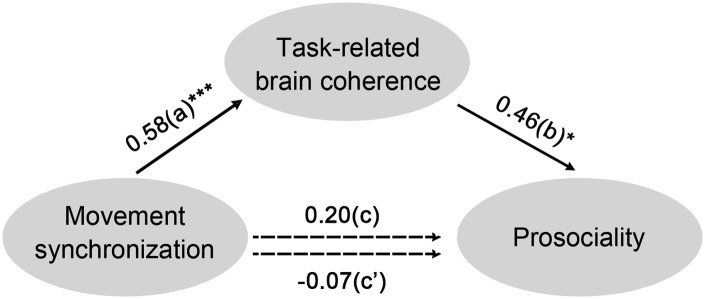
Previous analyses revealed (i) the prosocial effect of interpersonal synchrony for the coordination group relative to the independence group, (ii) the higher task-related coherence in the coordination group than in the independence group. These findings suggested that the revealed coherence might play a mediation role in the prosocial effect of interpersonal synchrony. To examine this possibility, a mediation analysis was conducted on the data from two groups, with movement synchronization, tasked-related coherence and mutual prosocial inclination as the independent variable, the mediating variable and the dependent variable, respectively. The analysis revealed a significantly mediation effect (bootstrap ab = 7.42, 95% confidence interval 0.92–17.12, Figure 6), suggesting the mediation role of the task-related brain coherence in the prosocial effect of interpersonal synchrony.
Fig. 6.
The mediation effect. The effect of movement synchronization on prosociality was significant reduced when task-related brain coherence was included in the regression model. The estimates presented here were standardized values. *P < 0.05, ***P < 0.001.
Discussion
In this study, we investigated the prosocial effect of interpersonal synchrony when two brain activations were recorded through the fNIRS-based hyperscanning technique. We found the significant task-related brain coherence, i.e. the IBS, between two participants in the coordination group but not in the independence group. The detected IBS was correlated with the mutual prosocial inclination and the shared intentionality between participants. Further, there was a mediation effect of task-related coherence in the prosocial effect of interpersonal synchrony.
First, we estimated the task-related brain synchronization among participant dyads during their task performance. The coordination group showed the significant task-related brain synchronization (i.e. the IBS), which was consistent with the previous studies (Funane et al., 2011;Mu et al., 2016). The detected IBS in this study was roughly located in the LMFC. The area has been generally considered to engage in various cognitive processes, such as response inhibition (Fu et al., 2008), working memory (Luerding et al., 2008) and self-reference (Lemogne et al., 2009). Recently, LMFC has been found to play an important role in processing relevant information about others in social interactions (Frith and Frith, 2001; Leslie et al., 2004; Amodio and Frith, 2006; Aichhorn et al., 2009; Koster-Hale et al., 2013). For example, the increased brain activity in LMFC was observed when an individual was following partner’s sight to gaze the target compared with gazing a target by himself (Schilbach et al., 2010). Similarly, there was greater LMFC activation when participants took the third-person perspective than the first-person perspective (David et al., 2006). Further, the brain activities in LMFC were found when typical children viewed other’s photos; however, the activities were absent in autistic children who might have impaired social function (Uddin et al., 2008). In a recent study, researchers found that the volume in LMFC was positively correlated with understanding of others (indexed as their ability to correctly understand others’ belief states) (Lewis et al., 2011). In our study, the detected IBS at the LMFC was positively correlated with the subjective shared intentionality in the coordination group, r (17) = 0.50, p < 0.05. Taken together, these findings indicated the possible relation of LMFC with the representation of others in social interaction.
Note that the IBS in LMFC in this study was induced by a minimal social interaction. When two participants in a dyad performing the coordination task, there was no direct communication between them, as they were visually separated by a board and wearing ear plugs. They depended only on the feedback of each trial to coordinate with the partner/computer. Therefore, compared with other social interactions (i.e. conversation, eye contact, face-to-face interaction), our task had the minimal nature of the social interaction. Previous studies have revealed that the IBS could be induced by this kind of social interaction (i.e. without direct communication between individuals), such as simultaneously key pressing (Pan et al., 2017), time counting (Funane et al., 2011;Mu et al., 2016) and social dilemma gaming (King-Casas et al., 2005; Astolfi et al., 2010). Also, it was found that there were larger brain activities in LMFC during simply watching social interactions between other people (Iacoboni et al., 2004), participating in one-way interactions (Schippers et al., 2010) and being with virtual others (Schilbach et al., 2006; Lotze et al., 2007). These findings supported the emergence of IBS, as well as the possible role of LMFC in a kind of minimal social interaction.
Second, the revealed IBS was associated with subsequent mutual prosociality (Figure 4). The task-related brain coherence played a mediating role in the prosocial effect of interpersonal synchrony (Figure 6). These results imply that when participants synchronize behaviors with other persons, their brains are immersed in interacting with others too; the interacting brains among participants affect the subsequent prosocial inclination or behaviors. It is consistent with previous studies showing that the brain activation involved in performing social interactive tasks was correlated with subsequent prosociality. In observing social exclusion, the brain activities at the medial prefrontal cortex and anterior insula were related to the following helping/comforting inclination (Masten et al., 2011). In playing public goods game, the brain activity at posterior superior temporal sulcus could predict the future trust behavior to others (Fahrenfort et al., 2012). And also in group drumming, brain activity at the caudate was associated with the helping behavior to the partner (Kokal et al., 2011). More generally, brain activation in social interactions was associated with subsequent sociocognitive processes as risky decisions (Cohen and Ranganath, 2005), social compliance (Klucharev et al., 2009) and emotional regulation (Denny et al., 2014). The alteration of neural activity in adolescents can predict the future risk-taking behavior (Qu et al., 2015), and there was a strong connection between frontal activation and subsequent relapse risk of addictive behavior (Grüsser et al., 2004). All these results revealed that functional neural activities occurring in social interaction could be associated with the future social cognition and behaviors among individuals (Konvalinka and Roepstorff, 2012).
Third, we observed the higher shared intentionality in the coordination group than in the independence group. The association between the prefrontal IBS and shared intentionality was confirmed, consistent with previous findings that the prefrontal area was involved in sharing intentionality (Van Overwalle and Baetens, 2009). Additional analysis revealed the significant correlation of intentionality scores with the prosocial inclination in the coordination group, r (17) = 0.61, P < 0.01 but not in the independence group, r (16) = 0.36, P > 0.05. The mediation analysis revealed the role of shared intentionality in the prosocial effect of interpersonal synchrony (bootstrap ab = 7.54, 95% confidence interval: 2.42–13.72). These results provide the evidence that the intentionality hypothesis can possibly account for the effect of interpersonal synchrony on prosocial inclination. As for the similarity hypothesis, we did not find a difference in perceived similarity between two groups. Previous studies also reported no more perceived similarity after synchronized movements compared with sequential movements (Kirschner, 2011). In our study, there is no correlation between perceived similarity and the revealed IBS in the coordination group. Thus, these findings suggested that the similarity hypothesis could not be an account for the prosocial effect of interpersonal synchrony in our study.
It is noted that the prosocial effect of interpersonal synchrony in this study was showed at the group level. The coordination group relative to the independence group showed higher movement synchrony and greater prosocial inclination, which was consistent with previous findings (Cirelli et al., 2014; Reddish et al., 2016). However, at the participant level, we observed the lack of correlation of movement synchronization and prosocial inclination, no matter in the coordination group (r = 0.05, P > 0.05) or in the independence group (r = 0.05, P > 0.05). We explain these results by our measurement of movement synchronization, i.e. the synchrony index, does not absolutely reflect the task-induced synchrony between two participants. One dyad may be characterized to perform in a more/less synchronized way than other dyads. Thus, the factors of experimental task and the personal characterization have confounded effects on the movement synchronization of participant dyads. Future studies need to distangle effects of the factors and accurately estimate the relation of task-induced movement synchrony to the prosocial inclination.
Several limitations should be addressed. First, we manipulated the interpersonal synchrony explicitly through instructions, requesting participants synchronize with a partner or a computer. Previous researches have investigated the dynamic progress of implicit/spontaneous synchrony through swinging handheld pendulums (Kugler and Turvey, 1987), walking side by side (Zivotofsky and Hausdorff, 2007) and rocking in rocking chairs (Richardson et al., 2007; Demos et al., 2012). Exploring the effect of interpersonal synchrony from an implicit aspect can assist us to foster a richer understanding of the prosocial effect of interpersonal synchrony. Second, the measurement of prosociality in this study was subjective report. Future research may assess the prosociality by the experimental tasks, such as the cold-pressor task (Piira et al., 2006) and the electrical stimulation task (Inui et al., 2002). Third, recent studies found that dyads’ interaction could be represented and retained by pair-specific neural synchronization as social memory, which affected the behavioral and neural performance in the next day (Koike et al., 2016). It would be worth exploring whether the effect of interpersonal synchrony on prosocial inclination/behaviors could last for a long period and if so, how long it would be. It is also noted that the optode probe set of NIRS only covered the prefrontal cortex, leaving other regions unexplored. However, previous studies revealed that movement synchrony (i.e. group drumming) elicited the brain activation at the caudate (Kokal et al., 2011), implicit movement synchrony related to neural coupling at the frontal, parietal and central regions (Yun et al., 2012), IBS in right temporo-parietal junction based on shared intentionality (Tang et al., 2016). The roles of these brain regions could be further examined by measuring from the entire brain.
In summary, our work reveals the brain-to-brain synchronization among individuals performing the coordination task. The revealed IBS predicts subsequent mutual prosociality and is sensitive to the shared intentionality rather than perceived similarity between participants. The brain synchronization plays a mediation role in the prosocial effect of interpersonal synchrony. These findings elucidate, to some extent, the neural mechanism relating to shared cognition for the facilitation of interpersonal synchrony on prosociality. Our study also exemplifies an approach of the hyperscanning technique to examine the effect of human interactions on social cognition. Future studies can investigate the underpinning neural signatures in the prosocial effect of interpersonal synchrony from the developmental perspective, as well as this effect in social deficit brains as autism spectrem disorders.
Funding
This research was supported by Peak Discipline Construction Project of Education at East China Normal University (to Y. H.); National Natural Science Foundation of China (31371052 to Y. H.).
Supplementary Material
Acknowledgements
We are grateful to Guillaume Dumas (from Institut Pasteur de Paris) and Emmanuelle Tognoli (from Florida Atlantic University) for their insightful comments and suggestions on our work. We also thank Yi Zhu and Chenbo Wang for their comments and corrections on earlier drafts.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Aichhorn M., Perner J., Weiss B., Kronbichler M., Staffen W., Ladurner G. (2009). Temporo-parietal junction activity in theory-of-mind tasks: falseness, beliefs, or attention. Journal of Cognitive Neuroscience, 21(6),1179–92. [DOI] [PubMed] [Google Scholar]
- Amodio D., Frith C. (2006). Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience, 7(4),268..http://dx.doi.org/10.1038/nrn1884 [DOI] [PubMed] [Google Scholar]
- Astolfi L., Cincotti F., Mattia D., et al. (2010). Simultaneous estimation of cortical activity during social interactions by using EEG hyperscannings. Conference Proceedings–IEEE Engineering in Medicine and Biology Society, 2814–2817. [DOI] [PubMed]
- Astolfi L., Toppi J., Borghini G., et al. (2012). Cortical activity and functional hyperconnectivity by simultaneous EEG recordings from interacting couples of professional pilots. Conference Proceedings—IEEE Engineering in Medicine and Biology Society, 2012, 4752.. [DOI] [PubMed] [Google Scholar]
- Cheng X., Li X., Hu Y. (2015). Synchronous brain activity during cooperative exchange depends on gender of partner: a fNIRS-based hyperscanning study. Human Brain Mapping, 36(6),2039–48.http://dx.doi.org/10.1002/hbm.22754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli L.K., Einarson K.M., Trainor L.J. (2014). Interpersonal synchrony increases prosocial behavior in infants. Developmental Science, 17(6),1003–11.http://dx.doi.org/10.1111/desc.12193 [DOI] [PubMed] [Google Scholar]
- Cohen M.X., Ranganath C. (2005). Behavioral and neural predictors of upcoming decisions. Cognitive, Affective, and Behavioral Neuroscience, 5(2),117–26.http://dx.doi.org/10.3758/CABN.5.2.117 [DOI] [PubMed] [Google Scholar]
- Cui X., Bryant D.M., Reiss A.L. (2012). NIRS-based hyperscanning reveals increased interpersonal coherence in superior frontal cortex during cooperation. Neuroimage, 59(3),2430–7.http://dx.doi.org/10.1016/j.neuroimage.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David N., Bewernick B.H., Cohen M.X., et al. (2006). Neural representations of self versus other: visual-spatial perspective taking and agency in a virtual ball-tossing game. Journal of Cognitive Neuroscience, 18(6),898–910.http://dx.doi.org/10.1162/jocn.2006.18.6.898 [DOI] [PubMed] [Google Scholar]
- De Vico Fallani F., Nicosia V., Sinatra R., et al. (2010). Defecting or not defecting: how to “read” human behavior during cooperative games by EEG measurements. PLoS One, 5(12),e14187.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demos A.P., Chaffin R., Begosh K.T., Daniels J.R., Marsh K.L. (2012). Rocking to the beat: effects of music and partner’s movements on spontaneous interpersonal coordination. Journal of Experimental Psychology: General, 141(1),49.. [DOI] [PubMed] [Google Scholar]
- Denny B.T., Ochsner K.N., Weber J., Wager T.D. (2014). Anticipatory brain activity predicts the success or failure of subsequent emotion regulation. Social Cognitive and Affective Neuroscience, 9(4),403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedenhofen B., Musch J., Olivier J. (2015). Cocor: a comprehensive solution for the statistical comparison of correlations. PLoS One, 10(4),e0121945.http://dx.doi.org/10.1371/journal.pone.0121945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikker S., Wan L., Davidesco I., et al. (2017). Brain-to-brain synchrony tracks real-world dynamic group interactions in the classroom. Current Biology, 27(9),1375–80.http://dx.doi.org/10.1016/j.cub.2017.04.002 [DOI] [PubMed] [Google Scholar]
- Dommer L., Jäger N., Scholkmann F., Wolf M., Holper L. (2012). Between-brain coherence during joint n-back task performance: a two-person functional near-infrared spectroscopy study. Behavioural Brain Research, 234(2),212–22. [DOI] [PubMed] [Google Scholar]
- Dumas G., Nadel J., Soussignan R., Martinerie J., Garnero L., Lauwereyns J. (2010). Inter-brain synchronization during social interaction. PLoS One, 5(8),e12166.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endedijk H.M., Ramenzoni V.C., Cox R.F., Cillessen A.H., Bekkering H., Hunnius S. (2015). Development of interpersonal synchrony between peers during a drumming task. Developmental Psychology, 51(5),714–21. [DOI] [PubMed] [Google Scholar]
- Fahrenfort J.J., Winden F.V., Pelloux B., Stallen M., Ridderinkhof K.R. (2012). Neural correlates of dynamically evolving interpersonal ties predict prosocial behavior. Frontiers in Neuroscience, 6(28),28.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler D.M., Holbrook C. (2014). Marching into battle: synchronized walking diminishes the conceptualized formidability of an antagonist in men. Biology Letters, 10(8),63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U., Frith C. (2001). The biological basis of social interaction. Current Directions in Psychological Science, 10(5),151–5.http://dx.doi.org/10.1111/1467-8721.00137 [Google Scholar]
- Fu L.P., Bi G.H., Zou Z.T., Wang Y., Ye E.M., Ma L. (2008). Impaired response inhibition function in abstinent heroin dependents: an fMRI study. Neuroscience Letters, 438(3),322–6.http://dx.doi.org/10.1016/j.neulet.2008.04.033 [DOI] [PubMed] [Google Scholar]
- Funane T., Kiguchi M., Atsumori H., Sato H., Kubota K., Koizumi H. (2011). Synchronous activity of two people’s prefrontal cortices during a cooperative task measured by simultaneous near-infrared spectroscopy. Journal of Biomedical Optics, 16(7),077011.. [DOI] [PubMed] [Google Scholar]
- Grinsted A., Moore J.C., Jevrejeva S. (2004). Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Processes in Geophysics, 11(5–6), 561–66. [Google Scholar]
- Grüsser S.M., Wrase J., Klein S., et al. (2004). Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology, 175(3),296–302. [DOI] [PubMed] [Google Scholar]
- Hasson U., Ghazanfar A.A., Galantucci B., Garrod S., Keysers C. (2012). Brain-to-brain coupling: a mechanism for creating and sharing a social world. Trends in Cognitive Sciences, 16(2),114.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holper L., Scholkmann F., Wolf M. (2012). Between-brain connectivity during imitation measured by fNIRS. Neuroimage, 63(1),212–22.http://dx.doi.org/10.1016/j.neuroimage.2012.06.028 [DOI] [PubMed] [Google Scholar]
- Hoshi Y. (2003). Functional near-infrared optical imaging: utility and limitations in human brain mapping. Psychophysiology, 40(4),511–20.http://dx.doi.org/10.1111/1469-8986.00053 [DOI] [PubMed] [Google Scholar]
- Hove M.J., Risen J.L. (2009). It’s all in the timing: interpersonal synchrony increases affiliation. Social Cognition, 27(6),949–60. [Google Scholar]
- Iacoboni M., Lieberman M.D., Knowlton B.J., et al. (2004). Watching social interactions produces dorsomedial prefrontal and medial parietal BOLD fMRI signal increases compared to a resting baseline. Neuroimage, 21(3),1167–73.http://dx.doi.org/10.1016/j.neuroimage.2003.11.013 [DOI] [PubMed] [Google Scholar]
- Ikeda S., Nozawa T., Yokoyama R., et al. (2017). Steady beat sound facilitates both coordinated group walking and inter-subject neural synchrony. Frontiers in Human Neuroscience, 11, 147.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui K., Tran T.D., Qiu Y., Wang X., Hoshiyama M., Kakigi R. (2002). Pain-related magnetic fields evoked by intra-epidermal electrical stimulation in humans. Clinical Neurophysiology, 113(2),298–304. [DOI] [PubMed] [Google Scholar]
- Jiang J., Chen C.S., Dai B.H., et al. (2015). Leader emergence through interpersonal neural synchronization. Proceedings of the National Academy of Sciences of the United States of America, 112(14),4274–9.http://dx.doi.org/10.1073/pnas.1422930112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P.E., Novembre G., Hove M.J. (2014). Rhythm in joint action: psychological and neurophysiological mechanisms for real-time interpersonal synchrony. Philosophical Transactions of the Royal B Biological Sciences, 369(1658),20130394..http://dx.doi.org/10.1098/rstb.2013.0394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Casas B., Tomlin D., Anen C., Camerer C.F., Quartz S.R., Montague P.R. (2005). Getting to know you: reputation and trust in a two-person economic exchange. Science, 308(5718),78–83. [DOI] [PubMed] [Google Scholar]
- Kirschner S., Tomasello M. (2010). Joint music making promotes prosocial behavior in 4-year-old children. Evolution and Human Behavior, 31(5),354–64.http://dx.doi.org/10.1016/j.evolhumbehav.2010.04.004 [Google Scholar]
- Klucharev V., Hytönen K., Rijpkema M., Smidts A., Fernández G. (2009). Reinforcement learning signal predicts social conformity. Neuron, 61(1),140–51. [DOI] [PubMed] [Google Scholar]
- Koehne S., Hatri A., Cacioppo J.T., Dziobek I. (2016). Perceived interpersonal synchrony increases empathy: insights from autism spectrum disorder. Cognition, 146, 8–15. [DOI] [PubMed] [Google Scholar]
- Koike T., Tanabe H.C., Okazaki S., et al. (2016). Neural substrates of shared attention as social memory: a hyperscanning functional magnetic resonance imaging study. Neuroimage, 125(1),401–12. [DOI] [PubMed] [Google Scholar]
- Kokal I., Engel A., Kirschner S., Keysers C., Avenanti A. (2011). Synchronized drumming enhances activity in the caudate and facilitates prosocial commitment–if the rhythm comes easily. PLoS One, 6(11),e27272.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konvalinka I., Roepstorff A. (2012). The two-brain approach: how can mutually interacting brains teach us something about social interaction?. Frontiers in Human Neuroscience, 6(6),215.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster-Hale J., Saxe R., Dungan J., Young L.L. (2013). Decoding moral judgments from neural representations of intentions. Proceedings of the National Academy of Sciences of the United States of America, 110(14),5648–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler P.N., Turvey M.T. (1987). Information, Natural Law, and the Self-Assembly of Rhythmic Movement. Hillsdale, NJ: Erlbaum. [Google Scholar]
- Launay J., Dean R.T., Bailes F. (2013). Synchronization can influence trust following virtual interaction. Experimental Psychology, 60(1),53–63.http://dx.doi.org/10.1027/1618-3169/a000173 [DOI] [PubMed] [Google Scholar]
- Lemogne C., le Bastard G., Mayberg H., et al. (2009). In search of the depressive self: extended medial prefrontal network during self-referential processing in major depression. Social Cognitive and Affective Neuroscience, 4(3),305–12.http://dx.doi.org/10.1093/scan/nsp008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie A.M., Friedman O., German T.P. (2004). Core mechanisms in “theory of mind”. Trends in Cognitive Sciences, 8(12),528–33. [DOI] [PubMed] [Google Scholar]
- Lewis P.A., Rezaie R., Brown R., Roberts N., Dunbar R.I. (2011). Ventromedial prefrontal volume predicts understanding of others and social network size. Neuroimage, 57(4),1624–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U., Li S.C., Gruber W., Muller V. (2009). Brains swinging in concert: cortical phase synchronization while playing guitar. BMC Neuroscience, 10(1),1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Mok C., Witt E.E., Pradhan A.H., Chen J.E., Reiss A.L. (2016). NIRS-based hyperscanning reveals inter-brain neural synchronization during cooperative Jenga game with face-to-face communication. Frontiers in Human Neuroscience, 10, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Saito H., Oi M. (2015). Role of the right inferior frontal gyrus in turn-based cooperation and competition: a near-infrared spectroscopy study. Brain and Cognition, 99, 17–23.http://dx.doi.org/10.1016/j.bandc.2015.07.001 [DOI] [PubMed] [Google Scholar]
- Lotze M., Veit R., Anders S., Birbaumer N. (2007). Evidence for a different role of the ventral and dorsal medial prefrontal cortex for social reactive aggression: an interactive fMRI study. Neuroimage, 34(1),470–8.http://dx.doi.org/10.1016/j.neuroimage.2006.09.028 [DOI] [PubMed] [Google Scholar]
- Luerding R., Weigand T., Bogdahn U., Schmidt-Wilcke T. (2008). Working memory performance is correlated with local brain morphology in the medial frontal and anterior cingulate cortex in fibromyalgia patients: structural correlates of pain–cognition interaction. Brain, 131(12),3222–31. [DOI] [PubMed] [Google Scholar]
- Lumsden J., Miles L.K., Macrae C.N. (2014). Sync or sink? Interpersonal synchrony impacts self-esteem. Frontiers in Psychology, 5(3),1064.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardia K.V., Jupp P.E. (2000). Directional Statistics. Wiley, Chichester. [Google Scholar]
- Masten C.L., Morelli S.A., Eisenberger N.I. (2011). An fMRI investigation of empathy for ‘social pain’ and subsequent prosocial behavior. Neuroimage, 55(1),381–8. [DOI] [PubMed] [Google Scholar]
- Mazzurega M., Pavani F., Paladino M.P., Schubert T.W. (2011). Self-other bodily merging in the context of synchronous but arbitrary-related multisensory inputs. Experimental Brain Research, 213(2–3),213–21. [DOI] [PubMed] [Google Scholar]
- McNeill W.H. (1995). Keeping Together in Time. Cambridge, MA: Harvard UP. [Google Scholar]
- Mu Y., Guo C., Han S. (2016). Oxytocin enhances inter-brain synchrony during social synchrony in male adults. Social Cognitive and Affective Neuroscience, 11(12),1882–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller V., Sänger J., Lindenberger U., Sporns O. (2013). Intra- and inter-brain synchronization during musical improvisation on the guitar. PLoS One, 8(9),e73852.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K., Birn R.M., Handwerker D.A., Jones T.B., Bandettini P.A. (2009). The impact of global signal regression on resting state correlations: are anti-correlated networks introduced?. Neuroimage, 44(3),893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nessler J.A., Gilliland S.J. (2009). Interpersonal synchronization during side by side treadmill walking is influenced by leg length differential and altered sensory feedback. Human Movement Science, 28(6),772–85.http://dx.doi.org/10.1016/j.humov.2009.04.007 [DOI] [PubMed] [Google Scholar]
- Nozawa T., Sasaki Y., Sakaki K., Yokoyama R., Kawashima R. (2016). Interpersonal frontopolar neural synchronization in group communication: an exploration toward fnirs hyperscanning of natural interactions. Neuroimage, 133, 484–97. [DOI] [PubMed] [Google Scholar]
- Osaka N., Minamoto T., Yaoi K., Azuma M., Osaka M. (2014). Neural synchronization during cooperated humming: a hyperscanning study using fNIRS. Procedia - Social and Behavioral Sciences, 126(1),241–3. [Google Scholar]
- Osaka N., Minamoto T., Yaoi K., Azuma M., Shimada Y.M., Osaka M. (2015). How two brains make one synchronized mind in the inferior frontal cortex: fNIRS-Based hyperscanning during cooperative singing. Frontiers in Psychology, 6(127),1811.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald P.A. (2002). The interactive effects of affective demeanor, cognitive processes, and perspective-taking focus on helping behavior. The Journal of Social Psychology, 142(1),120–32.http://dx.doi.org/10.1080/00224540209603890 [DOI] [PubMed] [Google Scholar]
- Pan Y., Cheng X., Zhang Z., Li X., Hu Y. (2017). Cooperation in lovers: an fNIRS-based hyperscanning study. Human Brain Mapping, 38(2),831–41.http://dx.doi.org/10.1002/hbm.23421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piira T., Hayes B., Goodenough B., von Baeyer C.L. (2006). Effects of attentional direction, age, and coping style on cold-pressor pain in children. Behaviour Research and Therapy, 44(6),835–48.http://dx.doi.org/10.1016/j.brat.2005.03.013 [DOI] [PubMed] [Google Scholar]
- Puccinelli N.M., Tickle-Degnen L. (2004). Knowing too much about others: moderators of the relationship between eavesdropping and rapport in social interaction. Journal of Nonverbal Behavior, 28(4),223–43.http://dx.doi.org/10.1007/s10919-004-4157-8 [Google Scholar]
- Qu Y., Galvan A., Fuligni A.J., Lieberman M.D., Telzer E.H. (2015). Longitudinal changes in prefrontal cortex activation underlie declines in adolescent risk taking. Journal of Neuroscience, 35(32),11308–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitch T.-C., Knafo-Noam A., Kotz S. (2015). Synchronous rhythmic interaction enhances children’s perceived similarity and closeness towards each other. PLoS One, 10(4),e0120878.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddish P., Fischer R., Bulbulia J., Szolnoki A. (2013). Let’s dance together: synchrony, shared intentionality and cooperation. PLoS One, 8(8),e71182.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddish P., Tong E.M., Jong J., Lanman J.A., Whitehouse H. (2016). Collective synchrony increases prosociality towards non-performers and outgroup members. British Journal of Social Psychology, 55(4),722–6. [DOI] [PubMed] [Google Scholar]
- Richardson M.J., Marsh K.L., Isenhower R.W., Goodman J.R., Schmidt R.C. (2007). Rocking together: dynamics of intentional and unintentional interpersonal coordination. Human Movement Science, 26(6),867–91. [DOI] [PubMed] [Google Scholar]
- Schilbach L., Wilms M., Eickhoff S.B., Romanzetti S., Tepest R., Bente G. (2010). Minds made for sharing: initiating joint attention recruits reward-related neurocircuitry. Journal of Cognitive Neuroscience, 22(12),2702–15. [DOI] [PubMed] [Google Scholar]
- Schilbach L., Wohlschlaeger A.M., Kraemer N.C., et al. (2006). Being with virtual others: neural correlates of social interaction. Neuropsychologia, 44(5),718–30. [DOI] [PubMed] [Google Scholar]
- Schippers M.B., Roebroeck A., Renken R., Nanetti L., Keysers C. (2010). Mapping the information flow from one brain to another during gestural communication. Proceedings of the National Academy of Sciences of the United States of America, 107(20),9388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver N.C., Hittner J.B., May K. (2004). Testing dependent correlations with nonoverlapping variables: a monte carlo simulation. The Journal of Experimental Education, 73(1),53–69.http://dx.doi.org/10.3200/JEXE.71.1.53-70 [Google Scholar]
- Singh A.K., Okamoto M., Dan H., Jurcak V., Dan I. (2005). Spatial registration of multichannel multi-subject fNIRS data to MNI space without MRI. Neuroimage, 27(4),842–51. [DOI] [PubMed] [Google Scholar]
- Tang H., Mai X., Wang S., Zhu C., Krueger F., Liu C. (2016). Interpersonal brain synchronization in the right temporo-parietal junction during face-to-face economic exchange. Social Cognitive and Affective Neuroscience, 11(1),23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr B., Launay J., Dunbar R.I. (2014). Music and social bonding: “self-other” merging and neurohormonal mechanisms. Frontiers in Psychology, 5, 1096.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognoli E., Lagarde J., DeGuzman G.C., Kelso J.A. (2007). The phi complex as a neuromarker of human social synchrony. Proceedings of the National Academy of Sciences of the United States of America, 104(19),8190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki D., Jurcak V., Singh A.K., Okamoto M., Watanabe E., Dan I. (2007). Virtual spatial registration of stand-alone MRS data to MNI space. Neuroimage, 34(4),1506–18. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Davies M.S., Scott A.A., et al. (2008). Neural basis of self and other representation in autism: an FMRI study of self-face recognition. PLoS One, 3(10),e3526..http://dx.doi.org/10.1371/journal.pone.0003526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdesolo P., DeSteno D. (2011). Synchrony and the social tuning of compassion. Emotion, 11(2),262–6.http://dx.doi.org/10.1037/a0021302 [DOI] [PubMed] [Google Scholar]
- Valdesolo P., Ouyang J., DeSteno D. (2010). The rhythm of joint action: synchrony promotes cooperative ability. Journal of Experimental Social Psychology, 46(4),693–5.http://dx.doi.org/10.1016/j.jesp.2010.03.004 [Google Scholar]
- Van Overwalle F., Baetens K. (2009). Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage, 48(3),564–84. [DOI] [PubMed] [Google Scholar]
- Wiltermuth S.S., Heath C. (2009). Synchrony and cooperation. Psychological Science, 20(1),1–5.http://dx.doi.org/10.1111/j.1467-9280.2008.02253.x [DOI] [PubMed] [Google Scholar]
- Xia M., Wang J., He Y. (2013). BrainNet viewer: a network visualization tool for human brain connectomics. PLoS One, 8(7),e68910..http://dx.doi.org/10.1371/journal.pone.0068910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun K., Watanabe K., Shimojo S. (2012). Interpersonal body and neural synchronization as a marker of implicit social interaction. Scientific Reports, 2(12),959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Noah J.A., Hirsch J. (2016). Separation of the global and local components in functional near-infrared spectroscopy signals using principal component spatial filtering. Neurophotonics, 3(1),015004..http://dx.doi.org/10.1117/1.NPh.3.1.015004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivotofsky A.Z., Hausdorff J.M. (2007). The sensory feedback mechanisms enabling couples to walk synchronously: an initial investigation. Journal of Neuroengineering and Rehabilitation, 4(1),28..http://dx.doi.org/10.1186/1743-0003-4-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.