Abstract
Background
The addition of procarbazine, lomustine, vincristine (PCV) chemotherapy to radiotherapy (RT) for patients with high-risk (≥40 y old or subtotally resected) low-grade glioma (LGG) results in an absolute median survival benefit of over 5 years. We evaluated the cost-effectiveness of this treatment strategy.
Methods
A decision tree with an integrated 3-state Markov model was created to follow patients with high-risk LGG after surgery treated with RT versus RT+PCV. Patients existed in one of 3 health states: stable, progressive, or dead. Survival and freedom from progression were modeled to reflect the results of RTOG 9802 using time-dependent transition probabilities. Health utility values and costs of care were derived from the literature and national registry databases. Analysis was conducted from the health care perspective. Deterministic and probabilistic sensitivity analysis explored uncertainty in model parameters.
Results
Modeled outcomes demonstrated agreement with clinical data in expected benefit of addition of PCV to RT. The addition of PCV to RT yielded an incremental benefit of 4.77 quality-adjusted life-years (QALYs) (9.94 for RT+PCV vs 5.17 for RT alone) at an incremental cost of $48635 ($188234 for RT+PCV vs $139598 for RT alone), resulting in an incremental cost-effectiveness ratio of $10186 per QALY gained. Probabilistic sensitivity analysis demonstrates that within modeled distributions of parameters, RT+PCV has 99.96% probability of being cost-effectiveness at a willingness-to-pay threshold of $100000 per QALY.
Conclusion
The addition of PCV to RT is a cost-effective treatment strategy for patients with high-risk LGG.
Keywords: brain tumor, chemotherapy, cost-effectiveness analysis, low-grade glioma, radiotherapy
Importance of the study
To our knowledge, there have been no reported economic evaluations of medical therapies in the setting of treatment for LGG. Given the significant clinical efficacy of the addition of PCV chemotherapy to RT in the adjuvant treatment of patients with high-risk LGG, we sought to assess the cost-effectiveness of this strategy. In this economic analysis, the addition of PCV chemotherapy to RT yielded an incremental benefit of 4.77 QALYs at an incremental cost of $48635, resulting in an incremental cost-effectiveness ratio of $10186 per QALY gained. The addition of PCV to RT is a highly clinically efficacious as well as cost-effective treatment strategy for patients with high-risk LGG, providing high value for resource utilization.
Low-grade glioma (LGG), also classified as World Health Organization (WHO) grade II glioma, comprises 5%–10% of all adult brain tumors and most commonly affects young adults. Transformation to higher-grade disease (WHO grades III–IV) commonly occurs, and progressive neurologic deterioration and ultimately premature death occur in nearly all patients. Definitive treatment consists of maximal safe surgical resection followed by adjuvant therapy.
The Radiation Therapy Oncology Group (RTOG) defines patients with high-risk LGG as those younger than 40 years old who underwent subtotal resection or biopsy, or patients who are 40 years of age and older. Historically, options for adjuvant therapy following surgery for high-risk LGG consisted of radiation therapy (RT), chemotherapy, or a combination of RT and chemotherapy, without clear consensus on optimal strategy. The phase III clinical trial RTOG 9802 investigated the effect of the addition of chemotherapy to RT, by randomizing 251 patients with high-risk LGG to RT or RT plus procarbazine/lomustine [CCNU]/vincristine (PCV) chemotherapy. Although initial results did not demonstrate a clear benefit for RT plus chemotherapy,1 final results demonstrated an overall survival (OS) and progression-free survival (PFS) benefit for combined modality therapy (median OS, 13.3 y) versus RT alone (median OS, 7.8 y).2 These results have established adjuvant radiation plus chemotherapy as the standard of care for high-risk LGG.
In the era of value-based health care, the topic of cost and, more importantly, value has increasingly entered the domain of clinical relevance. The National Comprehensive Cancer Network now incorporates an affordability measure in specific versions of its widely used treatment guidelines. Cost-effectiveness analyses represent a standard methodology of assessing value of medical therapies. Therapies that are clinically efficacious but carry high value are of benefit to both patients and the national economic well-being. In contrast, therapies that are clinically efficacious but unlikely to be cost-effective can pose difficult questions in a broader discussion of resource stewardship and patient care.3,4
To our knowledge, there are no studies in the literature reporting on the cost-effectiveness of interventions in the LGG setting. Given the significant clinical efficacy of RT+PCV as adjuvant therapy for high-risk LGG, we sought to assess its cost-effectiveness.
Methods
This study did not involve human participants or animals, and was exempt from formal review by our institutional review board.
Patients and Intervention
Our treatment schema was modeled after the RTOG 9802 phase III clinical trial.2 The base case comprised patients with high-risk LGG after maximal safe resection with indications for adjuvant therapy, who were defined as those younger than 40 years who underwent subtotal resection or biopsy, or patients 40 years of age and older. Based on the median age of patients in RTOG 9802, patients entered the model with initial starting age of 40 years. All patients were assumed to be free of progression at baseline. Patients were randomly assigned to RT alone or RT plus PCV. Radiation therapy was 54 Gy in 30 fractions of 1.8 Gy per fraction, delivered with intensity modulated technique. Procarbazine was administered at a dose of 60 mg/m2/day on days 8–21 every 8 weeks for 6 cycles. Lomustine (CCNU) was administered at a dose of 110 mg/m2 on day 1 every 8 weeks for 6 cycles. Vincristine was administered at a dose of 1.4 mg/m2 (maximum 2 mg/m2) i.v. on days 8 and 29 every 8 weeks for 6 cycles. For dose calculations in our model, patients were assumed to be 70 kg with body surface area of 1.8 mg/m2. Patients continued with their respective regimens until progression or completion of therapy.
Decision-Analytic Markov Model
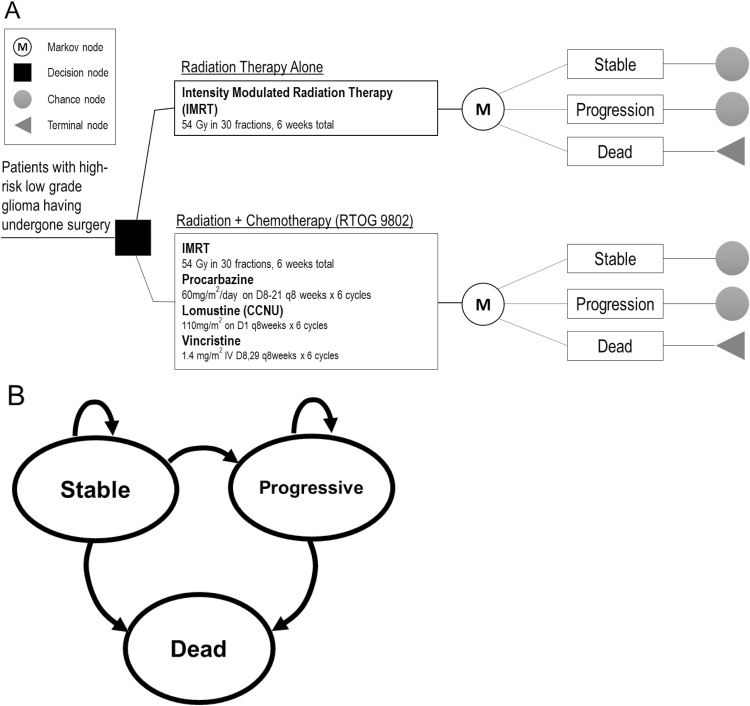
We developed a decision tree with an integrated 3-state Markov model using TreeAge Pro 2017 software to follow patients treated with RT alone versus RT plus PCV (Fig. 1A, Supplementary Figure S1) and assess cost-effectiveness. The health states were: stable, progressive, and dead (Fig. 1B). Patients were followed monthly over their remaining lifetimes. Given that the primary toxicity of patients in RTOG 9802 was hematologic, rates of grade 3 or higher hematologic toxicity were incorporated into the model. All patients entered the model with the intent to receive 6 cycles of PCV. Given the scarcity of health-economic data specific to recurrent LGGs, cost and utility values after recurrence for patients who progressed after definitive therapy were extrapolated from data in the setting of recurrent malignant gliomas, including studies of glioblastoma multiforme. Adverse events in the progressive state were not explicitly modeled but were inherently accounted for in assigned utilities and costs. After progression, patients were modeled as undergoing next-line therapy until death.
Fig. 1.
(A) Abbreviated decision tree and Markov model used to compare 2 strategies for treating high-risk LGG after surgical resection, explored in RTOG 9802. (B) Influence diagram shows a network of 3 health states linked by transitional variables.
We assessed cost-effectiveness by calculating the incremental cost-effectiveness ratio (ICER). Deterministic sensitivity analysis was conducted to explore the effects of uncertainty in our assumptions about treatment efficacy, utilities, and costs.5 Probabilistic sensitivity analysis with 10000 Monte Carlo simulations was performed to explore the influence of simultaneous changes in transition probabilities and uncertainties in utilities and cost.5,6 Economic analysis was conducted from the health care perspective.
Transition Probabilities
Overall and progression-free survivals were modeled to reflect the results of RTOG 9802 (Supplementary Figure S2). First, graphical data were extracted from the published Kaplan–Meier curves by using a validated graphical digitizer (WebPlotDigitizer v3.11; Ankit Rohatgi).7 Using R software v3.3.3, the digitized data were used to reconstruct individual patient data by finding numerical solutions to the inverted Kaplan–Meier equations using available information on the number of events and the number at risk, as per methodology described by Guyot et al.8 In order to model the patients for the duration of their lifetimes beyond the trial period, parametric models (exponential, Gompertz, log-logistic, log-normal, or Weibull distributions) were fitted to the reconstructed individual patient data, selecting the model with the best fit as determined by the model associated with the smallest Akaike information criterion value.9 Finally, a graphical validation of fit was performed between the digitized survival curve and the parametric curve.
The authors of RTOG 9802 recognized that the proportionality assumption of the Cox proportional hazards model is not reasonable for both PFS and OS, as the survival curves of the 2 treatment arms crossed, and thus applied a time-varying treatment effect to their Cox proportional hazards models to assess the effect of treatment.2 However, this time-varying effect was not elucidated in the study manuscript or supplementary materials. Accordingly, this implied that transition probabilities were not constant across all cycles of the model, therefore requiring us to model the transitions through health states by time-dependent transition probabilities.10 The baseline transition probabilities of OS and PFS between the time points (t − u) and t were estimated by one minus the ratio of the survival function at the end of the interval to the survival function at the beginning of the interval, otherwise expressed as: P(t_u) = 1 − [S(t)/S(t − u)], where u represents the cycle length of the model (month), S(t) is the survival function, and P(t) is the transition probability.11 Detailed methodology on derivation of transition probabilities is listed in the Supplementary material text. Briefly, transition probabilities from stable to dead state (pStableToDead), stable to progressive state (pStableToProgress), and progressive to dead state (pProgressToDead) under the 2 treatments were derived using age-specific mortality12,13 and an iterative, optimizing algorithm to minimize the difference between the target (actual trial data and extrapolated data beyond the trial period) and a model derived from our Markov states by using a nonlinear least-squares objective function.3 All time-dependent transition probabilities are listed in Supplementary Table S1.
Deterministic sensitivity analysis explored patient, treatment, and economic variations which could affect the cost-effectiveness of RT+PCV, including subgroups of patients limited to only grade II oligodendroglioma, oligoastrocytoma, or astrocytoma histology, presence of isocitrate dehydrogenase (IDH)1 R132H mutation,2 as well as variations in utility values and costs. Time-dependent transition probabilities for each patient subgroup were derived in a similar manner as described above for the overall trial population. For probabilistic sensitivity analysis, uncertainty (eg, 95% confidence interval) for transition probabilities could not be directly inferred from the trial data, given that details of the time-varying treatment effect used by the study authors were not reported. We chose to model uncertainty pertaining to the time-dependent transition probabilities using a β distribution centered about the time-dependent transition probability, with the lower (LL) and upper limits (UL) of the range representing the 95% CI set as 70% and 130%, respectively, of the specific time-dependent transition probability. A β distribution was fitted using mean estimated by the specific time-dependent transition probability and standard error estimated by the equation SE ≈ (UL – LL)/(2 × 1.96), per methodology described by Briggs et al.14
Costs and Utilities
The usual cost of chemotherapy in the US health care system was estimated at 121% of the cost negotiated by the Department of Veterans Affairs (VA) and reported in the Federal Supply Schedule, as suggested by the VA Health Economics Resource Center15,16 (Supplementary Table S2). Costs of radiotherapy planning and delivery, chemotherapy administration, and supportive care were derived from the national payment amount listed in the Medicare physician fee schedule for 2016 (Table 1, Supplementary Table S3).17 Cost of the progressive state reflected cost of recurrent gliomas undergoing next-line treatments.18 Uncertainty in cost was modeled by the γ distribution, which is bounded by 0 and infinity.5,14
Table 1.
Model parameters and assumptions
| Base Case and Modeled Distribution | |||
|---|---|---|---|
| Variable | RT | RT+PCV | Reference and Note |
| Transition probabilities: β distributed | |||
| Mortality in Stable State | Age-Specific Mortality | CDC-National Center for Health Statistics13 | |
| Progression from Stable State | Time-dependent Transition Probabilities (listed in full in Supplementary Table S1) | Buckner et al2 * | |
| Mortality after Progression | |||
| Serious Adverse Events | 0.007937 | 0.045170 | Buckner et al2 ,# |
| Utilities: β distributed | |||
| Stable State | 0.815 (0.643 to 0.987) | McCarter et al,19 based on HUI2 HRQL scores | |
| Progressive State | 0.731 (0.525 to 0.938) | Garside et al,20 Kovic et al21 ; decrease of 0.02 QALY per consecutive month of progression (maximum 30 cumulative decreases) | |
| Toll for Major Toxicity | −0.0898 | Nafees et al22 ; utility decrement modeled hematologic toxicity | |
| Cost per model cycle (month): γ distributed@ | |||
| Stable State& | $715 ($500 to $930) [first 24 months] $634 ($444 to $824) [months 25–60] $593 ($415 to $771) [after 60 months] | CMS National Health Expenditure Data,37 Physician Fee Schedule;17 | |
| Progressing State | $2809 ($1966 to $3652) | Wasserfallen et al18 ; modeled as cost of recurrent gliomas under treatment | |
| RT | $15509 ($10856 to $20162) | CMS Physician Fee Schedule17 ; one-time cost. See Supplementary Table S3 for micro-costing | |
| Chemotherapy | N/A | $1591 ($1113 to 2067) [first 12 months only] | Federal Supply Schedule.38 ^See Supplementary Table S4 for micro-costing |
| Toll for Major Toxicity | $13365 ($9356 to 17375) [first 12 months only] | Caggiano et al,39 Liou et al23 ; one-time cost. | |
Abbreviations: QALY, quality adjusted life years; IDH, isocitrate dehydrogenase; RT, radiotherapy; PCV, procarbazine, lomustine, vincristine; ICER, incremental cost-effectiveness ratio.
@Boundaries of γ distribution set as ±30% from base cost.
*Time-dependent transition (vs fixed or time-independent) probabilities were used, since the proportionality assumption of the Cox proportional hazards model is not reasonable for both PFS and OS due to Kaplan–Meier curves of each arm crossing with one another; uncertainty in both groups was attributed to a single arm (RT+PCV).
#Modeled grade 3 or higher hematologic toxicity; probability of treatment toxicity constrained to occur only during time of treatment (first 12 mo).
&Cost of stable state accounted for baseline medical care, plus physician visit and MRI every 3 months during first 2 years, every 6 months during years 3–5, and annually thereafter.
^Usual cost of chemotherapy in the US health care system was modeled by using 121% of the drug costs reported in the Federal Supply Schedule. Calculation of PCV dosing was based on patient with body surface area of 1.8 mg/m2 who receives procarbazine 60 mg/m2/day on D8–21 q8 weeks × 6 cycles, lomustine 110 mg/m2 on D1 q8weeks × 6 cycles, vincristine 1.4 mg/m2 i.v. D8,29 q8weeks × 6 cycles, with appropriate supportive care for chemotherapy administration.
Health state utilities of stable disease were derived from a report of health status and health-related quality of life measured at diagnosis using the Health Utilities Index through a self-assessment questionnaire.19 Health state utilities for recurrent disease were modeled using baseline values and time-dependent decrements used for economic evaluations in the malignant glioma setting.20,21 Consistent with this methodology, we assumed a decrease of 0.02 QALYs per consecutive month spent in the progressive state, with a maximum of 30 cumulative decrements. The utility toll and costs for major toxicity were modeled to reflect the impact of hematologic toxicity. Specifically, as neutropenia was the primary grade 3 or higher toxicity experienced by patients on trial, we chose to model the utility toll and costs for major toxicity with values for episodes of neutropenia derived from the literature.22,23 Minor toxicities were considered to be inherent to the disease state, and therefore were not explicitly modeled. The same utility values and costs associated with specific health states used for the overall trial population were applied to individual patient subgroups in the deterministic sensitivity analysis.
Finally, we applied an annual discounting rate of 3% to all costs and benefits incurred in the future to adjust for inflation.24
Results
Base Case
Modeled outcomes demonstrated agreement with clinical data in OS, PFS, and expected benefit of the addition of PCV to RT. In our model, 5- and 10-year OS rates for RT+PCV versus RT alone were 70% versus 65%, and 61% versus 39%, respectively; 5-year and 10-year PFS rates for RT+PCV versus RT alone were 61% versus 45%, and 49% versus 20%, respectively (Supplementary Fig. S2).
All patients were followed through the entire time horizon to the termination of the model. From time of RT initiation, patients treated with RT lived 5.17 QALYs at a cumulative cost of $139598, and those treated with RT+PCV lived 9.94 QALYs at a cumulative cost of $188232. The addition of PCV to RT resulted in an additional 11.82 life-years and 4.77 QALYs. Gains were achieved at an incremental cost of $48635. Taken together, the ICER of the addition of PCV to RT was $10186 per QALY gained (Table 2).
Table 2.
Base case analysis and deterministic sensitivity analyses exploring variations in patient subgroups, cost, and treatment effects
| Parameter | Life-Years Gained | Incremental Cost | Incremental Benefit, QALY | ICER per QALY Gained |
|---|---|---|---|---|
| Base case | 11.82 | $48 635 | 4.77 | $10 186 |
| Grade II oligodendroglioma | 16.33 | $56 542 | 6.29 | $8985 |
| Grade II oligoastrocytoma | 12.30 | $63 475 | 5.00 | $12 695 |
| Grade II astrocytoma | 4.56 | $46 003 | 1.87 | $24 617 |
| IDH1 R132H mutation (+) | 14.57 | $32 539 | 6.51 | $5000 |
| Stable and progressing utilities 0.25 | 11.82 | $48 635 | 1.41 | $34 378 |
| Stable and progressing utilities 1.0 | 11.82 | $48 635 | 5.67 | $8581 |
| RT and PCV at 50% cost | 11.82 | $40 794 | 4.77 | $8544 |
| RT and PCV at double cost | 11.82 | $64 358 | 4.77 | $13 480 |
| RT and PCV at 400% cost | 11.82 | $95 721 | 4.77 | $20 048 |
| RT and PCV at 1000% cost | 11.82 | $189 894 | 4.77 | $39 772 |
Abbreviations: QALY, quality adjusted life years; IDH, isocitrate dehydrogenase; RT, radiotherapy; PCV, procarbazine, lomustine, vincristine; ICER, incremental cost-effectiveness ratio.
Sensitivity Analysis
The ICER remained under $50000 per QALY gained in our subgroup analyses, including patients with grade II oligodendroglioma, oligoastrocytoma, astrocytoma, and presence of IDH1 R132H mutation (Table 2). In subgroups associated with more favorable prognoses compared with the base case, the ICER decreased to $8985 for grade II oligodendroglioma and $5000 for patients with IDH1 R132H mutation. In subgroups associated with poorer prognoses compared with the base case, the ICER increased to $12695 for grade II oligoastrocytoma and $24617 for grade II astrocytoma. If less optimistic utilities of 0.25 are assumed for stable and progressive states, the ICER increased to $34378. If perfect utilities of 1.0 are assumed for both stable and progressive states, the ICER expectedly decreased to $8581. If the cost of RT and PCV is reduced by 50% or increased to 200%, 400%, and 1000%, the corresponding ICERs are $8544, $13480, $20048, and $39772, respectively.
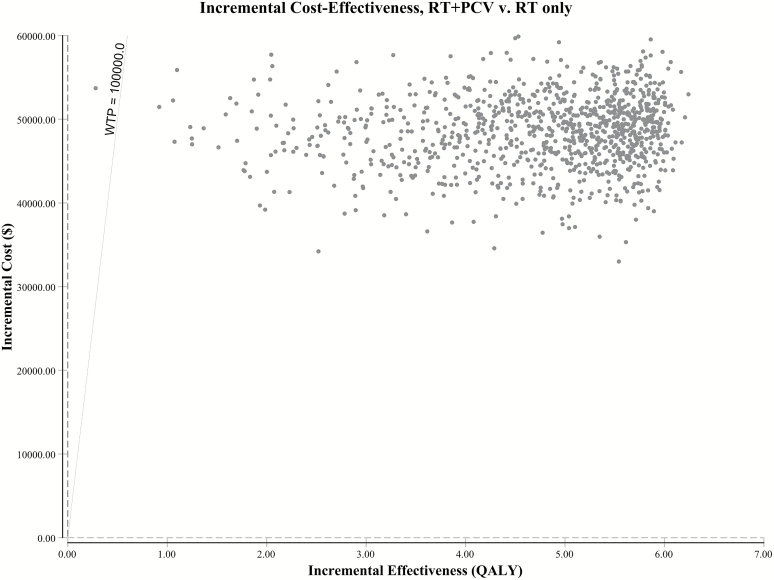
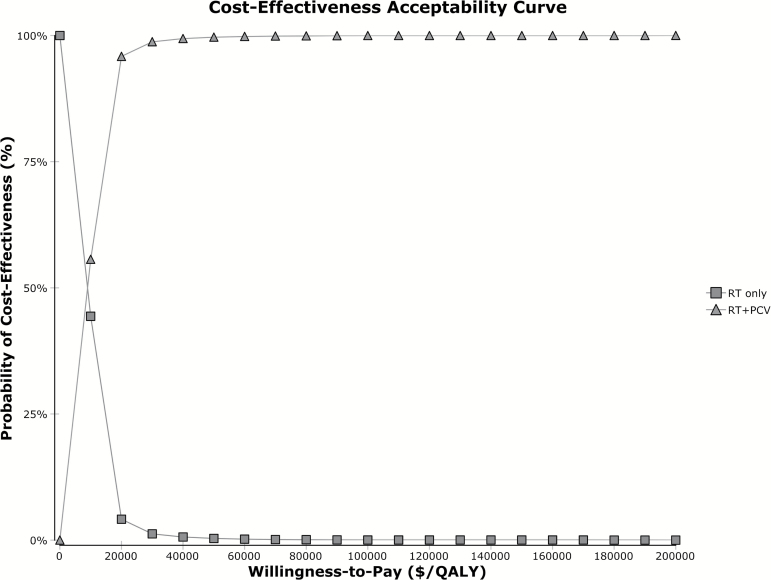
Probabilistic sensitivity analysis performed with 10000 Monte Carlo simulations showed a 99.96% chance of cost-effectiveness at a willingness-to-pay (WTP) threshold of $100000 per QALY gained (Fig. 2). The cost-effectiveness acceptability curve demonstrates that the probability of cost-effectiveness remains above 90% at WTP thresholds above $20000 per QALY (Fig. 3).
Fig. 2.
Incremental cost-effectiveness scatterplot of 10 000 Monte Carlo simulations shows 99.96% probability of cost-effectiveness at WTP threshold of $100 000 per QALY gained. Scatterplot data-points correspond to simulated incremental cost-effectiveness ratios expressed as $ per QALY gained. Abbreviations: QALY, quality adjusted life years; RT, radiotherapy; PCV, procarbazine, lomustine, vincristine.
Fig. 3.
Cost-effectiveness acceptability curve shows the effect of cost on probability of cost-effectiveness. The WTP corresponds to specific incremental cost-effectiveness ratio thresholds ($ per QALY). Abbreviations: QALY, quality adjusted life years; RT, radiotherapy; PCV, procarbazine, lomustine, vincristine.
Discussion
For interventions that provide significant clinical benefit, cost-effectiveness analyses allow for characterization of value. We performed an economic analysis on the addition of PCV to RT in the adjuvant treatment of patients with high-risk LGG, based on clinical outcomes from RTOG 9802.2 The base case analysis demonstrated that the addition of PCV to RT results in an incremental cost-effectiveness ratio of $10186 per QALY gained, well below commonly accepted standards of cost-effectiveness used in the United States. Sensitivity analysis demonstrates that this conclusion is insensitive to ranges of relevant clinical uncertainty for model input parameters, with probabilistic sensitivity analysis showing that RT plus PCV continues to have over 99% chance of being cost-effective throughout simultaneous modeled distributions of uncertainty.
A key factor contributing to this result is the magnitude, both relative and absolute, of the benefit that RT plus PCV demonstrated over RT alone in RTOG 9802 (median OS, 13.3 vs 7.8 y).2 Notably, this magnitude of benefit is similar to that observed in at least 2 other randomized clinical trials with different but comparable patients: RTOG 9402, which randomized patients with anaplastic oligodendrogliomas with presence of 1p/19q codeletions to PCV plus RT versus RT alone (median OS, 14.7 vs 7.3 y),25 as well as the European Organisation for Research and Treatment of Cancer 26951 trial, which randomized patients with anaplastic oligodendrogliomas of intrinsic glioma subtype 9 (high percentage of 1p/19q codeletions and IDH1 mutations) treated with PCV plus RT versus RT alone (median OS, 12.8 vs 5.5 y).26,27 Furthermore, based on initial results in abstract form, it is expected that the mature results of the CODEL trial will show similar magnitude of benefit in 1p/19q codeleted anaplastic glioma patients treated with RT plus temozolomide (TMZ) versus RT alone.28 Although histologically classified as having anaplastic glioma (WHO grade III), the patients carrying 1p/19q codeleted tumors from the 3 trials described above are the ones most likely to exhibit similar behavior to the study population of RTOG 9802, as genomic studies have shown that in WHO grade II or III tumors containing favorable molecular mutations such as IDH1 and 1p/19q codeletion, OS is not substantially affected by whether the tumor is histologically WHO grade II or III.29
Our model has limitations. We chose to model all patients as completing the full course of 6 cycles of PCV chemotherapy, while the median number completed on RTOG was 3–4 cycles.2 We believe this would allow for more relevant cost accounting, as most neuro-oncologists prescribing treatment do so with definitive intent in allowing patients to derive maximal benefit. Although the effect of number of chemotherapy cycles received on treatment benefit was not reported, if patients on RTOG 9802 who received the full 6 cycles of PCV experienced greater benefit than those who received fewer cycles, then our already modest ICER could potentially be an overestimate.
Some neuro-oncologists clinically utilize a regimen of RT plus TMZ, as opposed to RT plus PCV, in hopes of capturing the survival benefit observed on RTOG 9802 while dealing with the more favorable side effect profile of TMZ. We chose not to include this strategy in our analysis, as the primary evidence for this practice largely stems from the short term (5-y follow-up time) results of a single-arm phase II clinical trial, RTOG 0424,30 and extrapolation of a single-institution retrospective study in the anaplastic glioma setting.31
Clinical outcomes of patients (including after progression) were directly modeled by time-dependent transition probabilities to represent reported outcomes of patients on trial. In the setting of rare to lacking health status and economic studies specifically studying patients with recurrent LGG, we assumed that the health-economic impacts were similar to those in patients diagnosed upfront with malignant gliomas such as glioblastoma multiforme. We felt that this approach represented the best method of estimating costs and utilities associated with the progressive state. We note that patients in RTOG 9802 who were initially treated with RT alone received more therapies after progression compared with those who received RT+PCV during the study follow-up period.2 This would translate to a higher accumulated cost for patients treated with RT alone, which would decrease the incremental cost between RT+PCV and RT alone, thus decreasing the ICER. Therefore, accounting for this observation would show that the addition of PCV to RT is even more cost-effective.
Other than primary hematologic toxicity, we chose not to model other effects of treatment, including constitutional and cognitive effects. Patients on both arms of the trial actually were found to have higher Mini-Mental State Exam scores during the first 5 years after randomization compared with at baseline.2 However, we recognize that the Mini-Mental State Exam can be a relatively insensitive instrument and that conclusions regarding less severe or delayed neurocognitive effects cannot be drawn.
Our analysis was conducted from the health care perspective, which focuses on costs as experienced by both the patient as well as the private or public payer. Analyses can also be conducted from a societal perspective to account for relevant costs that may accrue outside of the formal health care sector, and thus could help inform decisions about the broad allocation of resources across an entire population.32 Such an approach would seek to account for all intervention-related downstream effects in outcomes and costs, across all parties affected, including costs related to time, transportation, impact on productivity, utilization of social services, non-health-related consumption, education, and other factors in addition to formal health care sector costs.32 This approach was not pursued, as we felt that there was insufficient information to feasibly quantify all non-health consequences in incorporating the societal perspective.
Despite these limitations, we believe our conclusions are justified. Our model was informed by high-quality data. Monthly follow-up allowed for high temporal resolution and all patients had lived out their remaining lifetimes by the end of the model time horizon. Our costing and reporting methods are transparent and in accord with the Society for Medical Decision Making task force guidelines.33 A major strength was demonstrating the feasibility of using time-dependent transition probabilities in order to model a trial where the proportional hazards assumption was invalid. The use of time-dependent transition probabilities allowed us to directly solve for optimal solutions of transitions between health states that best approximate the trial’s survival outcomes. Finally, sensitivity analyses confirmed that RT plus PCV is likely to remain cost-effective even under more unfavorable costing and treatment assumptions.
Cancer care is one of the fastest growing components of the US health care system, and so are its associated costs. Mariotto et al have reported that the total cost of cancer care is projected to increase from 125 billion to 158 billion 2010 US dollars between 2010 and 2020.34 Medical advances can provide new but increasingly expensive treatment options, which can raise difficult questions of how to effectively and efficiently allocate limited resources. Commonly accepted WTP thresholds in the United States vary from $50000 to 3 times the per capita gross domestic product, about $160000 per QALY.35 Our base case scenario yielded a cost of $10186 per QALY gained, which is well below these thresholds. For perspective, economic evaluations have shown that other clinically efficacious oncologic interventions can be associated with high ICERs beyond conventional thresholds of cost-effectiveness, such as $668368 per QALY for the addition of tumor-treating fields to standard first-line treatment with RT and TMZ for patients with newly diagnosed glioblastoma,36 or $472668 per QALY for the addition of pertuzumab to docetaxel and trastuzumab in the first-line treatment of patients with human epidermal growth factor receptor 2–overexpressing metastatic breast cancer3 (Table 3). In conclusion, the addition of PCV chemotherapy to RT is a highly efficacious as well as cost-effective treatment strategy and provides high value for resource utilization in the management of patients with high-risk LGG.
Table 3.
Cost-effectiveness of select oncologic interventions
| Intervention | Incremental Cost | Incremental Benefit, QALY | ICER per QALY Gained | Reference |
|---|---|---|---|---|
| Addition of PCV to RT in Adjuvant Therapy for High-Risk Low-Grade Glioma | $48 635 | 4.77 | $10 186 | Current study |
| Addition of Tumor-Treating Fields to RT and TMZ in First-line Treatment of GBM | $207 946* | 0.34 | $668 368* | Bernard-Arnoux et al (2016)36 |
| Addition of Bevacizumab to RT and TMZ in First-line Treatment of GBM | $59 328# | 0.13 | $450 868# | Kovic et al (2015)21 |
| Addition of Pertuzumab to Docetaxel and Trastuzumab in First-line Treatment of HER2+ Metastatic Breast Cancer | $294 747 | 0.62 | $472 668 | Durkee et al (2016)3 |
Abbreviations: GBM, glioblastoma multiforme; HER2+, human epidermal growth factor receptor 2 overexpressing.
*Adjusted from euros to US dollars.
#Adjusted from Canadian dollars to US dollars.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This work was supported by the Henry S. Kaplan Research Fund, Department of Radiation Oncology, Stanford University (to Y.Q. and B.D.); the KL2 Mentored Career Development Award of the Stanford Clinical and Translational Science Award to Spectrum (NIH KL2 TR 001083, to E.L.P.); and the Department of Veterans Affairs (to D.K.O.).
Conflict of interest statement
S.G.S. is a consultant for Nektar Therapeutics. D.K.O. is a consultant for Zoll. D.T.C. receives research support, honoraria, and salary support from Varian Medical Systems and has stock ownership in ViewRay. All other authors have no financial disclosures and no conflicts of interest.
Supplementary Material
Acknowledgments
Preliminary results of this work were selected for oral presentation at the 2017 Annual Meeting of the American Society for Radiation Oncology (ASTRO) held September 24–27, 2017 in San Diego, California.
References
- 1. Shaw EG, Wang M, Coons SW et al. Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: initial results of RTOG 9802. J Clin Oncol. 2012;30(25):3065–3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buckner JC, Shaw EG, Pugh SL et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374(14):1344–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Durkee BY, Qian Y, Pollom EL et al. Cost-effectiveness of pertuzumab in human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2016;34(9):902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bach PB. Walking the tightrope between treatment efficacy and price. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34(9):889–890. [DOI] [PubMed] [Google Scholar]
- 5. Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD; ISPOR-SMDM Modeling Good Research Practices Task Force Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med Decis Making. 2012;32(5):722–732. [DOI] [PubMed] [Google Scholar]
- 6. Doubilet P, Begg CB, Weinstein MC, Braun P, McNeil BJ. Probabilistic sensitivity analysis using Monte Carlo simulation. A practical approach. Med Decis Making. 1985;5(2):157–177. [DOI] [PubMed] [Google Scholar]
- 7. Rich JT, Neely JG, Paniello RC, Voelker CC, Nussenbaum B, Wang EW. A practical guide to understanding Kaplan–Meier curves. Otolaryngol Head Neck Surg. 2010;143(3):331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol. 2012;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diaby V, Adunlin G, Montero AJ. Survival modeling for the estimation of transition probabilities in model-based economic evaluations in the absence of individual patient data: a tutorial. Pharmacoeconomics. 2014;32(2):101–108. [DOI] [PubMed] [Google Scholar]
- 10. Gray AM, Clarke PM, Wolstenholme JL. Applied Methods of Cost-Effectiveness Analysis in Healthcare. Oxford, UK: Oxford University Press; 2011. [Google Scholar]
- 11. Briggs AH, Claxton K, Sculpher MJ. Decision Modelling for Health Economic Evaluation. Oxford, UK: Oxford University Press; 2006. [Google Scholar]
- 12. Williams C, Lewsey JD, Mackay DF, Briggs AH. Estimation of survival probabilities for use in cost-effectiveness analyses. Med Decis Making. 2017;37(4):427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. NVSS—Mortality Tables—Life Expectancy—LEWK3 https://www.cdc.gov/nchs/nvss/mortality/lewk3.htm. Accessed March 13, 2017.
- 14. Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics. 2000;17(5):479–500. [DOI] [PubMed] [Google Scholar]
- 15. Service O of A and LNACFSS. VA Federal Supply Schedule Service http://www.fss.va.gov/. Accessed March 16, 2017.
- 16. HERC: Determining the Cost of Pharmaceuticals for a Cost-Effectiveness Analysis http://www.herc.research.va.gov/include/page.asp?id=pharmaceutical-costs. Accessed March 16, 2017.
- 17. Physician Fee Schedule Search https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed December 8, 2016.
- 18. Wasserfallen JB, Ostermann S, Leyvraz S, Stupp R. Cost of temozolomide therapy and global care for recurrent malignant gliomas followed until death. Neuro Oncol. 2005;7(2):189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCarter H, Furlong W, Whitton AC et al. Health status measurements at diagnosis as predictors of survival among adults with brain tumors. J Clin Oncol. 2006;24(22):3636–3643. [DOI] [PubMed] [Google Scholar]
- 20. Garside R, Pitt M, Anderson R et al. The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation. Health Technol Assess. 2007;11(45):iii–iv, ix. [DOI] [PubMed] [Google Scholar]
- 21. Kovic B, Xie F. Economic evaluation of bevacizumab for the first-line treatment of newly diagnosed glioblastoma multiforme. J Clin Oncol. 2015;33(20):2296–2302. [DOI] [PubMed] [Google Scholar]
- 22. Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liou SY, Stephens JM, Carpiuc KT, Feng W, Botteman MF, Hay JW. Economic burden of haematological adverse effects in cancer patients: a systematic review. Clin Drug Investig. 2007;27(6):381–396. [DOI] [PubMed] [Google Scholar]
- 24. Gold MR, ed. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 25. Cairncross G, Wang M, Shaw E et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31(3):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Erdem-Eraslan L, Gravendeel LA, de Rooi J et al. Intrinsic molecular subtypes of glioma are prognostic and predict benefit from adjuvant procarbazine, lomustine, and vincristine chemotherapy in combination with other prognostic factors in anaplastic oligodendroglial brain tumors: a report from EORTC study 26951. J Clin Oncol. 2013;31(3):328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van den Bent MJ, Brandes AA, Taphoorn MJB et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31(3):344–350. [DOI] [PubMed] [Google Scholar]
- 28. Jaeckle K, Vogelbaum M, Ballman K et al. CODEL (Alliance-N0577; EORTC-26081/22086; NRG-1071; NCIC-CEC-2): phase III Randomized Study of RT vs RT+TMZ vs TMZ for newly diagnosed 1p/19q-codeleted anaplastic oligodendroglial tumors. analysis of patients treated on the original protocol design (PL02.005). Neurology. 2016;86(suppl 16):PL02.005. [Google Scholar]
- 29. Suzuki H, Aoki K, Chiba K et al. Mutational landscape and clonal architecture in grade II and III gliomas. Nat Genet. 2015;47(5):458–468. [DOI] [PubMed] [Google Scholar]
- 30. Fisher BJ, Hu C, Macdonald DR et al. Phase 2 study of temozolomide-based chemoradiation therapy for high-risk low-grade gliomas: preliminary results of Radiation Therapy Oncology Group 0424. Int J Radiat Oncol Biol Phys. 2015;91(3):497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Speirs CK, Simpson JR, Robinson CG et al. Impact of 1p/19q codeletion and histology on outcomes of anaplastic gliomas treated with radiation therapy and temozolomide. Int J Radiat Oncol Biol Phys. 2015;91(2):268–276. [DOI] [PubMed] [Google Scholar]
- 32. Sanders GD, Neumann PJ, Basu A et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103. [DOI] [PubMed] [Google Scholar]
- 33. Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB; ISPOR-SMDM Modeling Good Research Practices Task Force Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Med Decis Making. 2012;32(5):733–743. [DOI] [PubMed] [Google Scholar]
- 34. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn’t it increase at the rate of inflation? Arch Intern Med. 2003;163(14):1637–1641. [DOI] [PubMed] [Google Scholar]
- 36. Bernard-Arnoux F, Lamure M, Ducray F, Aulagner G, Honnorat J, Armoiry X. The cost-effectiveness of tumor-treating fields therapy in patients with newly diagnosed glioblastoma. Neuro Oncol. 2016;18(8):1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Medicare C for, Baltimore MS 7500 SB, Usa M. Age-and-Gender https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Age-and-Gender.html. Published August 9, 2016. Accessed March 13, 2017.
- 38. Logistics O of A and. Pharmaceutical Prices—Office of Acquisition and Logistics (OAL) https://www.va.gov/oal/business/fss/pharmPrices.asp. Accessed March 13, 2017.
- 39. Caggiano V, Weiss RV, Rickert TS, Linde-Zwirble WT. Incidence, cost, and mortality of neutropenia hospitalization associated with chemotherapy. Cancer. 2005;103(9):1916–1924. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.