Pan and Chan discuss the role of myelinating glia in axonal development and the impact of demyelination on axon degeneration.
Abstract
Axon loss and neurodegeneration constitute clinically debilitating sequelae in demyelinating diseases such as multiple sclerosis, but the underlying mechanisms of secondary degeneration are not well understood. Myelinating glia play a fundamental role in promoting the maturation of the axon cytoskeleton, regulating axon trafficking parameters, and imposing architectural rearrangements such as the nodes of Ranvier and their associated molecular domains. In the setting of demyelination, these changes may be reversed or persist as maladaptive features, leading to axon degeneration. In this review, we consider recent insights into axon–glial interactions during development and disease to propose that disruption of the cytoskeleton, nodal architecture, and other components of axon infrastructure is a potential mediator of pathophysiological damage after demyelination.
Introduction
Multiple sclerosis (MS) is canonically described as a demyelinating disease, yet the most severe clinical outcomes in MS patients are associated not with demyelination itself but with its eventual sequelae: axon loss and neurodegeneration. Although inflammatory injury has been proposed as a primary determinant of axonal damage, immunosuppressive therapies have had limited success in preventing the onset of progressive MS, which is characterized by worsening, permanent neurological disability and chronically demyelinated lesions with relatively sparse immune infiltration and profound axon loss (Bjartmar et al., 2003; Trapp and Nave, 2008; Dutta and Trapp, 2011; Hauser et al., 2017). Charcot–Marie–Tooth disease type 1, an inherited demyelinating peripheral neuropathy resulting from mutations in Schwann cell myelin genes, also involves progressive axon loss with a nonimmune etiology (Suter and Scherer, 2003; Nave et al., 2007). These clinical observations and histopathological findings, along with the intimate physical and molecular association of the axon and its myelinating glia, suggest a situation in which axonal integrity is maintained by the presence of myelin. However, the lack of adequate animal models to study demyelination in isolation has presented significant barriers toward defining these interactions. Consequently, the mechanisms of secondary degeneration in a demyelinated axon, and even the underlying assumption that the loss of myelin is detrimental to the axon, remain patently unclear.
Several mouse mutants harboring mutations in myelin-associated genes exhibit late-onset axon degeneration in the absence of inflammatory demyelination, prompting a search for basic biological mechanisms through which oligodendrocytes support axon function (Griffiths et al., 1998; Yin et al., 1998; Lappe-Siefke et al., 2003). Recent attention has been focused on the hypothesis that myelinating glia provide metabolic support to their invested axons, centered on the observation that oligodendrocyte-derived glycolytic metabolites trafficked by monocarboxylate transporter 1 can be metabolized by myelinated axons. In support of this, down-regulation of monocarboxylate transporter 1 specifically in oligodendrocytes is sufficient to impair long-term maintenance of myelinated axons, and oligodendrocytes have the capacity to sense and respond to axonal energetic demand through N-Methyl-d-aspartate receptor signaling (Fünfschilling et al., 2012; Lee et al., 2012; Saab et al., 2016). Despite these seminal observations, the significance of metabolic support has not been demonstrated in the context of demyelination, and it is conceivable that metabolic support by myelinating glia does not comprehensively account for all axonal damage that occurs in myelin disease. Properties of axonal infrastructure that are established or regulated during myelination, such as the maturation of the cytoskeleton, trafficking of cargoes, and nodal organization of voltage-gated ion channels, are perturbed or reversed during demyelination. As axon integrity is contingent on the proper functioning of these processes, their disruption may serve as a unifying model of secondary axon degeneration, onto which pathological events, such as the loss of metabolic and ion homeostasis, converge (Hirokawa et al., 2010; Kevenaar and Hoogenraad, 2015; Zhang and Rasband, 2016). Interrogating the mechanisms of injury in a demyelinated axon then becomes a question of understanding the fundamental cell biology of the axon–myelin unit. To this end, recent advances using in vivo two-photon imaging, high-pressure freezing electron microscopy, superresolution microscopy, and protein crystallography offer intricate descriptions of axon–glia interactions (Nikić et al., 2011; Xu et al., 2013; Sorbara et al., 2014; Pronker et al., 2016; D’Este et al., 2017; Snaidero et al., 2017). These new insights, and others, are examined in the context of prior studies to propose that (1) disruption of the axon cytoskeleton, axonal transport, and nodal architecture is sufficient to cause neurodegeneration; (2) myelin is involved in the regulation of these processes; and (3) demyelination could potentially lead to dysregulation of these processes and, subsequently, axon degeneration.
Molecular and cytoskeletal rearrangements during developmental myelination
Deletion of a large portion of the gene encoding myelin basic protein (MBP) in the shiverer mouse results in a hypomyelinated central nervous system (CNS) with no compact myelin formation, progressive tremors, and premature death (Chernoff, 1981; Roach et al., 1985). Unmyelinated axons in these animals exhibit no overt signs of degeneration, implying that axons tolerate a situation in which they are never myelinated better than they do a situation in which they are myelinated and subsequently demyelinated (Rosenbluth, 1980). Stated more succinctly, no myelin is better than lost myelin. It is conceivable then that myelinated axons become dependent to some capacity on their associated glia, resulting in their degeneration when perturbed by a demyelinating insult. This dependency could arise from molecular changes in the axon that are induced by myelination or as a consequence of the physical situation of an ensheathed axon and its limited access to the extracellular environment (Nave, 2010). And so, a possible approach to understanding secondary axon degeneration is to consider maturational changes in the axon during developmental myelination and how they may be maladaptive during demyelination.
Assembly and maintenance of nodal architecture
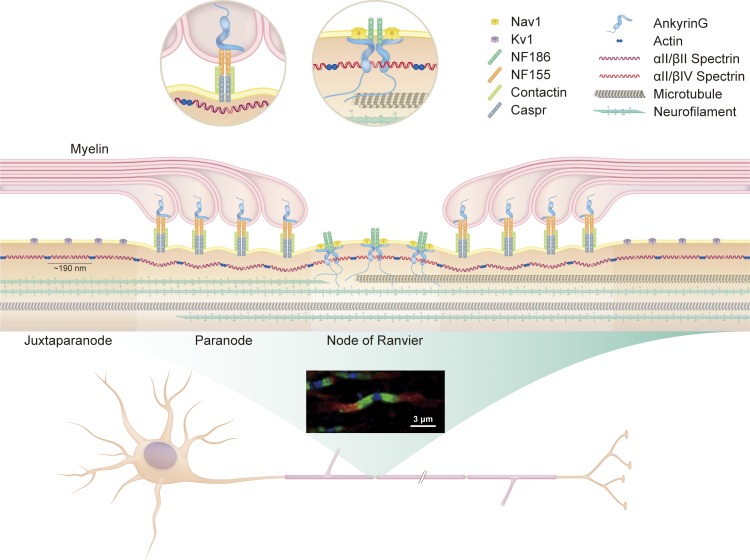
During myelination, axons undergo dramatic rearrangements of cell adhesion molecules, ion channels, and cytoskeletal proteins to produce a repeating geometric pattern of organized molecular domains: the nodes of Ranvier, the paranodal axoglial junctions, and the juxtaparanodes (Fig. 1). Collectively, these sets of domains interleave between segments of compact myelin that ensheath the internodes (Poliak and Peles, 2003; Salzer, 2003; Chang and Rasband, 2013; Normand and Rasband, 2015). Nodes of Ranvier are micron-long unmyelinated segments that serve as excitable regions of clustered voltage-gated sodium channels, enabling rapid saltatory conduction and reducing the metabolic demand of signal propagation (Trapp and Stys, 2009). During developmental myelination, Schwann cells and oligodendrocytes induce nodal clustering of the axonal cell adhesion molecule neurofascin 186 (NF186) and recruitment of ankyrin G, a cytoskeletal scaffolding protein that binds both voltage-gated sodium channels and βIV spectrin (Zhou et al., 1998; Komada and Soriano, 2002; Eshed et al., 2005; Sherman et al., 2005; Yang et al., 2007; Susuki et al., 2013; Amor et al., 2017). βIV spectrin, in turn, associates with actin and anchors the entire complex to the underlying axonal cytoskeleton (Berghs et al., 2000). Flanking either end of each node of Ranvier are the paranodes, cellular junctions between the axolemma and the terminal ends of each myelin sheath layer that are involved in preventing lateral diffusion of axonal proteins, facilitating the localization of nodal ion channels, and acting as sites for axon–glial signaling (Fig. 1). Assembly of the paranodal junction is also dependent on axon–glial interactions, with the glial cell adhesion molecule neurofascin 155 (NF155) complexing with axonal contactin and contactin-associated protein (Caspr), which, in turn, associate with the actin and αII/βII spectrin cytoskeleton through the adaptor protein 4.1B (Bhat et al., 2001; Boyle et al., 2001; Gollan et al., 2002; Ogawa et al., 2006). Finally, juxtaparanodes are the sites of voltage-gated potassium channels, whose localization depends on the interaction between axonal Caspr2 and glial TAG-1 (Poliak et al., 2003; Fig. 1).
Figure 1.
Architecture of axonal cytoskeleton and polarized molecular domains. Myelination induces architectural rearrangement of the axon into polarized molecular domains. The nodes of Ranvier are short, unmyelinated segments that contain clusters of voltage-gated sodium channels. Flanking the nodes are the paranodes, junctions between noncompacted paranodal myelin loops and the underlying axolemma. Distal to the paranodes are the juxtaparanodes, which contain voltage-gated potassium channels. The fluorescent micrograph of optic nerve axons illustrates the distinct localization of potassium channels (red, juxtaparanode), Caspr (green, paranode), and βIV spectrin (blue, node). The axon cytoskeleton facilitates structural integrity and molecular organization and acts as a conduit for axon transport. It consists primarily of neurofilaments, microtubules, actins, and spectrins. Actins and spectrins assemble into a repeating lattice with structural periodicity of 180 to 190 nm. At nodes, ankyrin adaptor proteins anchor nodal constituents such as voltage-gated ion channels to the underlying actin–spectrin cytoskeleton. At paranodes, protein 4.1B (not depicted), anchors the NF155–Caspr–Contactin complex to the underlying actin–spectrin cytoskeleton.
Disruption of nodal architecture
The assembly and maintenance of these molecular domains, and of the underlying cytoskeletal scaffold, are essential for axonal integrity and function. Mice that are deficient in Caspr have disrupted paranodal axoglial junctions and develop axonal swellings in Purkinje cells with accumulations of mitochondria, smooth endoplasmic reticulum, and disorganized microtubules and neurofilaments (Garcia-Fresco et al., 2006). These swellings begin to appear in the proximal paranodal regions during early postnatal development, and they progress to cytoskeletal fragmentation and vacuolation of degenerating axons as development continues. Shambler mice, which harbor a mutation in the Cntnap1 gene that encodes a truncated Caspr protein with no transmembrane or intracellular domains, also exhibit paranodal accumulations of membranous organelles in the sciatic nerve (Sun et al., 2009; Takagishi et al., 2016).
Genetic ablation of ankyrin G was recently reported to induce destabilization of the nodes of Ranvier and axon degeneration with cytoskeletal depletion and nodal accumulation of membranous organelles (Saifetiarova et al., 2017). Notably, these findings are contradictory to an earlier study demonstrating that ankyrin R is capable of compensating for ankyrin G loss, although variations in genetic background strains, Cre lines, or observational time windows may account for this discrepancy (Ho et al., 2014). Alternatively, degeneration in these mutants could result from perturbation of the axon initial segment, which is dependent on ankyrin G for its assembly and maintenance and is critical for regulating axodendritic polarity and axon trafficking (Ogawa et al., 2006; Hedstrom et al., 2008; Schafer et al., 2009; Sobotzik et al., 2009). Ankyrin B regulates axon transport through association with the dynactin subunit p62, and deficiency of ankyrin B leads to severe malformation of white matter tracts in vivo and impaired trafficking of synaptic vesicles and membranous organelles in vitro (Lorenzo et al., 2014). Deletion of NF186 also results in progressive nodal destabilization and axon degeneration and, notably, concurrent deletion of glial NF155, which acts in maintaining the paranodal junctions, exacerbates nodal disassembly (Taylor et al., 2017).
Spectrins are prominent constituents of the nodal cytoskeleton and considerable efforts have been made to probe their function both in the context of nodal organization and axon degeneration. Studies in Caenorhabditis elegans suggest that spectrins are important for the physical integrity of the axon, as β spectrin–deficient worms suffer mechanical axon trauma from the physical force of their body movements (Hammarlund et al., 2007; Krieg et al., 2017). Quivering mice, which lack βIV spectrin, exhibit aberrant ion channel localization, cytoskeletal abnormalities, and tortuous membranous protrusions but do not show signs of axon degeneration (Parkinson et al., 2001; Yang et al., 2004). Similarly, genetic deletion of βII spectrin in mice results in widened clusters of voltage-gated sodium channels at the nodes of Ranvier and aberrant localization of voltage-gated potassium channels to the paranodal domain, with no overt axon degeneration (Zhang et al., 2013; Amor et al., 2017). The lack of axon degeneration in these mutants may be explained by compensation of other β spectrins, four of which are expressed in the vertebrate CNS (Ho et al., 2014; Zhang et al., 2014). In support of this, ectopic expression of a spinocerebellar ataxia type 5–associated variant of human βIII spectrin in Drosophila melanogaster, which expresses only one endogenous β spectrin, is sufficient to induce progressive neurodegeneration with axonal swellings and marked defects in vesicular axon trafficking (Lorenzo et al., 2010). These impairments in axon transport could be caused by cross talk between the actin/spectrin cytoskeleton and microtubules, as treatment of cultured Drosophila neurons with the actin depolymerizer cytochalasin D is sufficient to induce microtubule fragmentation and exacerbate microtubule destabilization in response to treatment with the microtubule depolymerizer nocodazole or depletion of the microtubule-stabilizing spectraplakin protein Shot (Qu et al., 2017).
In contrast, because αII spectrin is the only α spectrin expressed in the CNS, neurons may not tolerate the loss of αII spectrin as well as β spectrins (Zhang et al., 2014). Human mutations in αII spectrin cause West syndrome, a developmental neurological disorder characterized by severe infantile epilepsy, hypomyelination, and cerebral atrophy (Saitsu et al., 2010; Tohyama et al., 2015). In mice, αII spectrin mutations are embryonically lethal from cardiac and neurological dysfunction, and the role of αII spectrin in axons remains to be characterized (Stankewich et al., 2011). Collectively, the axon pathology associated with deficiencies in nodal components imply an association between the proper organization of molecular domains and axonal integrity, consistent with the findings of aberrant localization and expression of Caspr, neurofascins, ankyrins, spectrins, and voltage-gated ion channels in postmortem human MS samples and animal models of demyelination (Wolswijk and Balesar, 2003; Arroyo et al., 2004; Craner et al., 2004; Coman et al., 2006; Howell et al., 2006; Griggs et al., 2017).
Maturation of the axon cytoskeleton
Myelination of axons induces maturation of the axon cytoskeleton, mediating radial expansion of the axon through regulation of neurofilament content and phosphorylation of the carboxy termini of medium/heavy neurofilament subunits, which is thought to promote neurofilament spacing through electrostatic repulsion (de Waegh et al., 1992; Witt and Brady, 2000; Garcia et al., 2003). Examination of retinal ganglion cell axons, which are unmyelinated within the retinal nerve fiber layer and the proximal optic nerve but myelinated for the remainder of their length, demonstrates that the distal myelinated axon is characterized by increased axon diameter, increased neurofilament content, and increased neurofilament phosphorylation (Nixon et al., 1994; Sánchez et al., 1996, 2000). Conversely, decreased neurofilament spacing, neurofilament density, and neurofilament phosphorylation are observed in the unmyelinated stem processes of dorsal root ganglion (DRG) neurons and, notably, in their nodes of Ranvier, which can be considered as short, unmyelinated axon segments (Hsieh et al., 1994). Neurofilament transport rates are locally decreased in myelinated axon segments of DRG neurons cultured with Schwann cells, suggesting that slower neurofilament transport may facilitate the accumulation of neurofilaments and subsequent increase in axon caliber (Monsma et al., 2014). Indeed, decreased rates of neurofilament transport, increased neurofilament content, and increased neurofilament phosphorylation are correlated with axon radial growth during developmental myelination (Hoffman et al., 1985; Sánchez et al., 1996, 2000). Although it was initially reported that oligodendrocyte contact without compact myelin formation is sufficient to induce axon caliber changes in the shiverer mouse, subsequent observations noted that shiverer optic nerve axons resemble premyelinated axons with decreased axon caliber, neurofilament content, and neurofilament phosphorylation and increased microtubule content, microtubule density, and rates of slow axonal transport (Sánchez et al., 1996; Brady et al., 1999). More recently, the surprising finding that parvalbumin interneurons in the neocortex are intermittently myelinated coincided with the observation that myelinated segments of these interneurons have relatively higher and lower neurofilament and microtubule content, respectively (Micheva et al., 2016). Collectively, comparisons between contiguous unmyelinated and myelinated axon segments, pre- and postmyelinated axons, and unmyelinated and myelinated axons in the shiverer mouse provide compelling evidence for the role of myelinating glia in dictating axonal cytoskeletal composition and trafficking parameters.
Cytoskeletal and axon transport deficits associated with mutations in myelin-associated genes
Proteolipid protein (PLP)
In contrast to the shiverer mouse, null mutations in several myelin-associated genes result in the absence of myelin protein constituents without significantly affecting proper myelin formation. In humans, mutations in PLP1, which encodes myelin PLP, can cause the leukodystrophy Pelizaeus–Merzbacher disease (PMD) and the axonopathy hereditary spastic paraplegia type 2 (HSP2), to varying degrees of severity. Duplications, triplications, and point mutations in the PLP1 gene lead to severe, hypomyelinating PMD, whereas deletions of the entire gene are associated with milder forms of PMD and HSP2, characterized by late onset and slow progression (Gruenenfelder et al., 2011). Plp1 mutations in mice recapitulate these observations; although spontaneous Plp1 point mutants and Plp1-overexpressing transgenic mice exhibit hypomyelination and oligodendrocyte cell death, Plp1-null mice have late-onset axonal pathology in the setting of normal compact myelin formation (Sidman et al., 1964; Nave et al., 1986; Schneider et al., 1992; Kagawa et al., 1994; Klugmann et al., 1997).
The uncoupling of axon maintenance and myelination has made the Plp1-null mutant a useful model for studying axon–glial interactions. Progressive axon degeneration in these mice begins at ∼6 to 8 wk of age, with focal axonal spheroids containing multivesicular bodies, disorganized cytoskeletal components, mitochondria, and other membranous organelles that localize to axonal compartments distal to the nodes of Ranvier (Griffiths et al., 1998). Accumulations of membranous organelles and cytoskeletal components imply a defect in axonal trafficking, which was demonstrated functionally through injection of fluorescently conjugated cholera toxin subunit B into the retina and superior colliculus. Subsequent measurements of anterograde trafficking to the superior colliculus or retrograde trafficking to the retina revealed deficits in both processes, and aggregates of cholera toxin subunit B could be directly visualized in axonal spheroids, consistent with electron micrograph observations (Edgar et al., 2004). A role for trafficking disruption is further implied by length-dependent pathology in the descending corticospinal tracts and ascending dorsal column fibers of Plp1-null animals and human HSP patients, because the distal segments of long axons are most likely to be susceptible to impaired axon transport (Garbern et al., 2002). Mice in which PLP is removed and replaced with the peripheral myelin protein P0 are phenotypically similar to Plp1-null animals but with an accelerated course of axon degeneration. These mice exhibit shorter microtubule lengths, aberrant microtubule orientations, decreased microtubule stability as assessed by the ratio of insoluble to soluble acetylated α-tubulin, and hyperphosphorylation of the microtubule-binding protein tau, suggesting that these axon-transport deficits may arise from dysregulation of axonal microtubules by myelin (Yin et al., 2016).
2′,3′-cyclic nucleotide phosphodiesterase (CNP)
Deletion of another myelin-associated gene, Cnp, results in axonal pathology virtually identical to Plp1-null mutants, albeit with more overt behavioral deficits and a shorter life span (Lappe-Siefke et al., 2003). These differences may be a consequence of the paranodal disruption and sodium channel declustering that can be observed before overt axon degeneration (Rasband et al., 2005). Although Cnp encodes a cyclic nucleotide phosphodiesterase, there are no known substrates in oligodendrocytes, and its molecular function was unknown until a recent study implicated CNP in the formation and maintenance of cytosolic channels that course through the myelin sheath (Snaidero et al., 2017). High-pressure freezing electron microscopy is a technical advance that allows for the preservation and visualization of these structures in CNP-deficient myelin in the developing optic nerve, which contains progressively fewer cytoplasmic channels as myelin compaction proceeds (Möbius et al., 2016; Snaidero et al., 2017). Remarkably, Cnp-null/shiverer heterozygotes, which have a 50% reduced expression of MBP, have a significant decrease in axonal degeneration in the spinal cord when compared with Cnp-null single mutants. This interaction is modeled as CNP maintaining the opening of cytoplasmic channels by antagonizing the force of myelin compaction by MBP, which in turn facilitates the potential trafficking of metabolites or signaling factors to the ensheathed axon.
In both Plp1 and Cnp mutants, organelle accumulation is observed at the nodes of Ranvier and adjacent compartments, which suggests that these regions are either particularly vulnerable to general disruptions in axon trafficking or that there is local regulation of axon transport that coincides with the geometry of these molecular domains (Griffiths et al., 1998; Lappe-Siefke et al., 2003; Edgar et al., 2004). Two lines of evidence argue for the second possibility. Because of random X inactivation, myelinated axons in female Plp1+/− heterozygotes are ensheathed by a mosaic of wild-type and PLP-deficient oligodendrocytes. In these chimeric mutants, axonal spheroids were associated only with PLP-deficient myelin (Griffiths et al., 1998). Similarly, neurospheres from Plp1-null mice transplanted into the dorsal white matter of shiverer mice were sufficient to form compact myelin and induce axonal swellings specifically within the graft site (Edgar et al., 2004). Alternatively, trafficked cargoes such as mitochondria may have a tendency to pause at or be actively recruited to nodal domains, manifesting as organelle accumulations in the setting of disrupted axon transport (Armstrong et al., 1987; Salzer, 2003). Furthermore, the nodes of Ranvier represent repeated constrictions in axon diameter and microtubule bundling that can obstruct axon transport, although the extent and prevalence of these properties in the CNS is unclear (Sousa and Bhat, 2007). Thus, oligodendrocytes could interact with axon trafficking either indirectly, by imposing cytoskeletal and nodal architectural changes onto the physical framework of the axon, or directly, by engaging axon transport machinery.
Myelin-associated glycoprotein (MAG)
MAG is a minor myelin constituent present on the innermost membrane of the internodal myelin sheath and Schwann cell paranodal loops, and its bidirectional signaling capability and direct apposition to the axolemma make it a suitable candidate for actuating both of these processes (Quarles, 2007, 2009). Compact myelin is formed in the absence of MAG but with subtle ultrastructural abnormalities such as the loss of the periaxonal space, redundant hypermyelination, and detachment of myelin lamellae (Li et al., 1994; Montag et al., 1994). Myelinated axons in MAG-deficient animals exhibit reduced axon caliber and neurofilament phosphorylation and progressive axon loss in both central and peripheral white matter, implying a function for MAG in cytoskeletal maturation and maintenance of axonal integrity (Fruttiger et al., 1995; Yin et al., 1998; Pan et al., 2005). This is corroborated by the observation that experimental autoimmune encephalomyelitis (EAE), a model of inflammatory demyelination, and various neurotoxic models of axon injury are exacerbated in MAG-deficient animals (Nguyen et al., 2009). Interestingly, soluble MAG-Fc in vitro confers resistance to the microtubule depolymerizer vincristine and promotes microtubule detyrosination, a posttranslational modification associated with perseverant or pharmacologically stabilized microtubules, suggesting that the neuroprotective properties of MAG are mediated through the stabilization of the axon cytoskeleton (Nguyen et al., 2009; Janke, 2014).
Although the crystal structure of MAG has recently been solved with conformational description of its five extracellular immunoglobulin-like domains, the molecular mechanism of MAG signaling has been difficult to ascertain because of the experimental inaccessibility of the periaxonal space and the multitude of potential axonal receptors, which include sialic acid–containing glycoproteins/gangliosides, the Nogo receptor, and the neurotrophin receptor p75 (Quarles, 2007, 2009; Pronker et al., 2016). Mice that are deficient in Galgt1, which encodes a biosynthetic enzyme necessary for the formation of complex gangliosides, recapitulate the axon degeneration and cytoskeletal changes of MAG-deficient mice, and MAG-induced neuroprotection against vincristine toxicity in vitro is not elicited in DRG neurons that lack complex gangliosides, suggesting that they act as the receptor for MAG in these contexts (Rasband et al., 2005; Nguyen et al., 2009; Mehta et al., 2010). However, gangliosides are not membrane-spanning molecules, and it is not known how MAG-induced signaling events are communicated from the extracellular space to the axoplasm, though cooperation with a transmembrane coreceptor is a likely possibility (Quarles, 2007, 2009). Addition of soluble MAG-Fc or heterologous expression of MAG in COS-7 cells co-cultured with DRG neurons recruits the activity of axonal kinases Cdk5 and Erk1/2, which phosphorylate neurofilaments and microtubule-associated proteins (Dashiell et al., 2002). Decreased Cdk5 and Erk1/2 activity in vivo is also observed in sciatic nerve lysates of MAG-deficient animals (Dashiell et al., 2002). Biochemical interrogation of kinesin subunit regulation in an extruded squid axoplasm preparation indicates that Cdk5, through inhibition of protein phosphatase 1–mediated activation of glycogen synthase kinase 3, indirectly promotes kinesin cargo association by preventing glycogen synthase kinase 3 phosphorylation of kinesin light chain (Morfini et al., 2002, 2004). This regulatory cascade suggests that the loss of axon contact with MAG during demyelination could potentially lead to deficits in kinesin-driven anterograde transport and other processes that are dependent on Cdk5 and Erk1/2 kinase activity. Collectively, the axon degenerative phenotypes and cytoskeletal alterations associated with mutations in myelin genes suggest a general role for myelin in maintaining axonal infrastructure, though the molecular details behind the importance of these genes are only just beginning to be unraveled. In particular, it will be intriguing to determine the extent to which the loss of PLP, CNP, and MAG is functionally similar to demyelination, a situation where interactions between myelin proteins and the axon are physically disrupted.
Cytoskeletal and axon transport deficits in animal models of demyelination
EAE
Experimental models of demyelination differ in the aspects of MS that they represent and the degree to which they can faithfully model them. EAE, typically an acute, monophasic neuroinflammatory episode produced via immunization of C57BL/6 mice with myelin oligodendroglial glycoprotein peptide, is the most widely used model because of its ease of induction, stereotyped clinical course, and autoimmune etiology (Ransohoff, 2012). In vivo two-photon excitation microscopy of the dorsal spinal cord during EAE has afforded detailed insight into the mechanisms of acute inflammatory axonal degeneration (Nikić et al., 2011; Sorbara et al., 2014). Using a stabilized spinal cord preparation that is amenable to pharmacological manipulation, sparse labeled axons were tracked over time to reveal a sequence of events beginning with axonal swellings, which are observable before demyelination, and progressing to mitochondrial dystrophy, microtubule tyrosination, and irreversible cytoskeletal fragmentation (Nikić et al., 2011; Sorbara et al., 2014). Furthermore, widespread impairments in mitochondrial transport rates were found within EAE lesions, even in myelinated axons without overt morphological disturbances. The decrease in mitochondrial transport rates was attributed to increases in pause duration, implying a deficit in motor protein association rather than transport velocity itself (Sorbara et al., 2014). All of these pathological events were spatiotemporally correlated with the apposition of microglia and macrophages, and could be attenuated and exacerbated by scavengers and donors of reactive oxygen/nitrogen species, respectively (Nikić et al., 2011; Sorbara et al., 2014). Collectively, these findings suggest that innate immune cells impair axon trafficking of membranous organelles and induce cytoskeletal insults via induction of oxidative stress, independently of the loss of myelin.
Innate and adaptive immune responses undoubtedly contribute to axon pathology in myelin disease; however, the more probable scenario is that neuroinflammation and dysregulation of the axon–myelin unit have variable degrees of contributions to axon damage, depending on the course of the disease and the nature of the lesion. In a recent study, conditional deletion of Chrm1 from oligodendrocytes, which encodes the M1 muscarinic acetylcholine receptor, was found to accelerate remyelination, attenuate axon loss, and reduce the severity of clinical symptoms during EAE (Mei et al., 2016). The increased number of surviving axons could specifically be attributed to axons with thin myelin sheaths, indicative of newly remyelinated axons. In conjunction with their observation that immune cell infiltration in EAE lesions was unchanged in Chrm1 knockouts, this suggests that remyelination is neuroprotective in inflammatory demyelination. The association between remyelination and neuroprotection in Chrm1 knockouts implicates myelin more directly as a factor for axonal integrity, in contrast to the demyelination-independent axon damage observed by Nikić et al. (2011). However, these disparate findings likely represent distinct biological phenomena that occur in parallel rather than being mutually exclusive (Nikić et al., 2011; Sorbara et al., 2014; Mei et al., 2016). EAE lesions are also variably distributed in time and space, and degenerating myelinated axons may, in fact, be distal to a demyelinated lesion not within the surgically accessed field of view. Furthermore, although EAE is a valuable tool to model a complex neuroinflammatory environment leading to myelin and axon pathology, the difficulties in uncoupling neuroprotective and immunomodulatory effects and the contribution of immune cells to axonal damage make it difficult to examine the effects of demyelination in isolation (Ransohoff, 2012). Nevertheless, it is evident that in vivo imaging of the diseased nervous system can be instrumental in establishing molecular causality and temporal sequencing of events across individual axons.
Lysolecithin and cuprizone demyelination
Stereotaxic injection of the gliotoxic detergent lysolecithin and dietary administration of the copper chelator cuprizone are two canonical nonimmune mediated models of demyelination. Although often referred to as axon-sparing manipulations, both models result in positive immunolabeling of β-amyloid precursor protein (β-APP) spheroids, a marker for pathological impairment of axon transport, because β-APP is not present at detectable levels unless accumulated into aggregates (Sachs et al., 2014; Höflich et al., 2016; Schultz et al., 2017). Whether these findings are indicative of demyelination-induced axon damage is uncertain, because lysolecithin can be toxic to neurons depending on the concentration used, duration of treatment, and targeting of the injection (Waxman et al., 1979; Foster et al., 1980; Birgbauer et al., 2004). Cuprizone induces oligodendrocyte toxicity through inhibition of mitochondrial complex IV, but the mechanism and extent of its specificity for oligodendrocytes are also unclear (Kipp et al., 2009). Furthermore, despite being described as nonimmune-mediated manipulations, these methods result in extensive recruitment of microglia to lesion sites, which can potentially act as mediators of axon injury (Sachs et al., 2014; Höflich et al., 2016; Schultz et al., 2017).
Several of these limitations can be addressed, in part, by examining myelinating co-cultures, which lack microglia, astrocytes, and immune cells and allow the experimenter to control for the direct effects of lysolecithin on unmyelinated axons. Live imaging of mitochondria transport in myelinating Schwann cell and DRG neuron co-cultures demonstrated that lysolecithin-induced demyelination of DRG neurons results in increased stationary mitochondrial volume and mitochondrial transport rate, which is partly mediated through the stress-activated transcription factor ATF3 (Kiryu-Seo et al., 2010). In contrast, decreased mitochondrial transport rates are observed in EAE, perhaps indicative of divergent responses between immune- and nonimmune-mediated demyelination models, or between in vivo and in vitro settings (Sorbara et al., 2014). Notably, although myelination also increases mitochondrial transport rate, remyelination after a lysolecithin insult reverses the demyelination-induced increase in transport rate rather than successively increasing it, implying that myelinating an unmyelinated axon and remyelinating a demyelinated axon are not equivalent processes (Kiryu-Seo et al., 2010).
Syntaphilin, a docking receptor that maintains mitochondria at microtubule-associated stationary sites along the axon, appears to be at least partly responsible for orchestrating this compensatory response (Kang et al., 2008). In vivo cuprizone-induced demyelination of the corpus callosum and in vitro lysolecithin-mediated demyelination of organotypic slice cultures of syntaphilin-deficient knockout mice demonstrate that demyelination-induced increases in stationary mitochondria volume does not occur in the absence of syntaphilin (Ohno et al., 2014). Demyelinated syntaphilin-deficient axons have an increased frequency of β-APP–positive axonal spheroids, which, remarkably, can be rescued by the sodium channel blocker flecainide in lysolecithin-treated slice cultures. The interpretation of these findings is complicated by the observation that higher doses of cuprizone cause mitochondrial deficits in liver hepatocytes and by the coadministration of rapamycin, which is intended to increase the extent of experimental demyelination in the cuprizone model but may also inhibit axonal signaling pathways that promote neuronal survival (Matsushima and Morell, 2001; Sachs et al., 2014; Garza-Lombó and Gonsebatt, 2016). As a result, it is difficult to conclude with absolute certainty whether syntaphilin deficiency is exacerbating axon damage secondary to demyelination, innate immune cell infiltration, or direct cuprizone neurotoxicity. Nevertheless, these data suggest that axon trafficking and recruitment of mitochondria to stationary sites are important compensatory responses to the increased metabolic demand imposed by maintenance of ion homeostasis in a demyelinated axon and that perturbation of this response is sufficient to cause axon degeneration (Trapp and Stys, 2009).
Diphtheria toxin (DT) ablation
To address the lack of cell-type specificity in lysolecithin and cuprizone demyelination models, transgenic strategies of specifically expressing either diphtheria toxin subunit A (DTA) or the DT receptor in oligodendrocytes have been developed (Traka et al., 2010; Pohl et al., 2011; Oluich et al., 2012). Administration of DT to a transgenic mouse line expressing the DT receptor under the MBP promoter results in inhibition of protein translation, rapid oligodendrocyte cytotoxicity, and mortality by 3 wk after injection, which, because of the biostability of the myelin sheath, occurs before overt demyelination (Oluich et al., 2012). Although the labeling of mature myelin markers appears unchanged, over half of the nodes of Ranvier in these mice have detached or everted paranodal myelin loops. Moreover, myelinated axons exhibit positive β-APP immunolabeling, and ultrastructural analysis of the dorsal spinal cord demonstrates nodal and paranodal accumulations of membranous organelles. These results suggest that a combination of paranodal axoglial junction disassembly, inhibition of protein translation in the oligodendrocyte soma, and/or oligodendrocyte cell death is sufficient to cause acute axon transport disruptions.
In contrast, genetic expression of DTA under the regulation of an inducible oligodendrocyte-specific PLP-CreERT2 driver results in a slower disease progression with marked myelin vacuolation and demyelination in the cerebellar white matter, spinal cord, and brainstem (Pohl et al., 2011). After a latency of 3 wk, DTA-expressing animals exhibit progressive motor deficits, ataxia, and weight loss over an additional 3 wk until euthanization. Axon loss, β-APP aggregates, neurofilament dephosphorylation, organelle accumulation, and microgliosis were observed in demyelinated regions and, notably, were still present in a genetic background where no functional lymphocytes are produced. A similar approach using a variant PLP/CreERT driver also results in mitochondrial accumulations in demyelinated spinal cord axons but no changes in axon numbers (Traka et al., 2010). Interestingly, these mice develop a delayed adaptive immune response 40 wk after induction, resulting in profound axon loss and severe neurological disability (Traka et al., 2016). Variations in recombination efficiency and subsequent clinical course could potentially account for these differences (Doerflinger et al., 2003; Leone et al., 2003). Cell-type–specific DT ablation represents a significant technical advance over toxic demyelination models and demonstrates that demyelination is capable of causing axon trafficking defects and subsequent degeneration in the absence of direct neurotoxicity. However, observational time windows in DT or toxic demyelination models can also be limited by early lethality or efficient remyelination, which may preclude the detection of marked secondary axon degeneration and limit the capacity of these techniques to model chronically demyelinated lesions in progressive MS. Looking forward, combining cell-type–specific demyelination approaches with mechanistic readouts used in previous studies, such as the sensing and manipulation of oxidative stress or the detailed characterization of mitochondrial morphology and trafficking, will be important future directions to better define the molecular events directly influenced by myelin loss (Kiryu-Seo et al., 2010; Nikić et al., 2011; Ohno et al., 2014; Sorbara et al., 2014).
Recent insights into the ultrastructure of the axon cytoskeleton and molecular mechanisms of axon degeneration
Superresolution imaging via stochastic optical reconstruction microscopy enabled the revelation that the subcortical axonal cytoskeleton is composed of repeating ring-like arrangements of short actin filaments (Huang et al., 2008; Xu et al., 2013). These actin filaments are capped at their barbed ends by α-adducin, an actin-binding protein involved in the regulation of actin ring diameter. α-Adducin–deficient mice exhibit progressive axon degeneration and axon diameter enlargement in CNS white matter tracts (Leite et al., 2016). Adjacent actin rings are connected longitudinally by spectrin tetramers, consisting of two α and two β subunits, which impose a structural periodicity of ∼180 to 190 nm along the axial length of the axon and appear to be both necessary and sufficient for the formation of the actin lattice (Fig. 1; Xu et al., 2013; Zhong et al., 2014). A similar periodic spatial organization is also observed for other molecular constituents within the nodes of Ranvier and adjacent domains, such as ankyrin G, NF168, Caspr, and voltage-gated sodium channels. Remarkably, dual-color stimulated emission depletion nanoscopy of teased myelinated sciatic nerve fibers demonstrates a tight periodic association between axonal proteins within the paranodal junctions and Schwann cell proteins on the paranodal myelin loops (D’Este et al., 2017). This spatial correlation, along with the adherence of these proteins to the 180- to 190-nm periodicity of the actin–spectrin cytoskeleton, suggests that actin and spectrins act as a physical scaffold for axonal transmembrane proteins and, by proxy, their glial-binding partners.
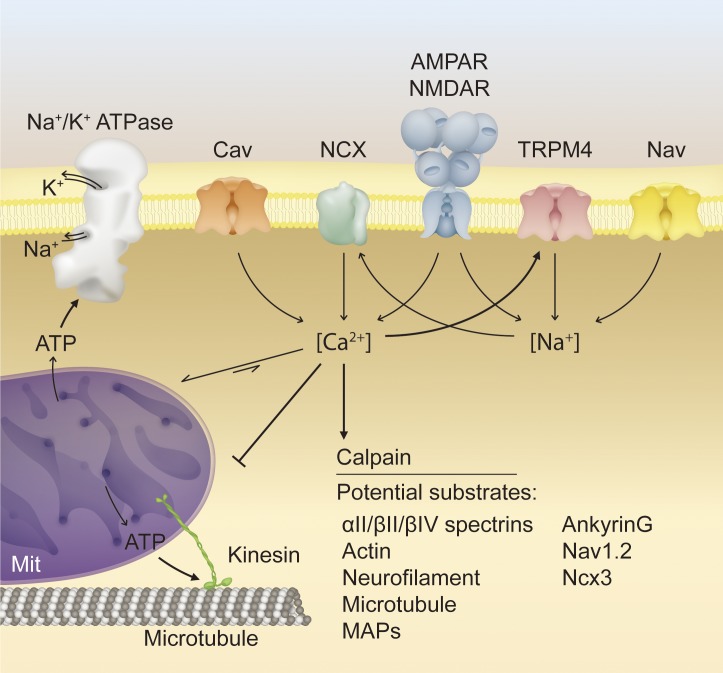
Although actin–spectrin periodicity is established in unmyelinated axons and appears to be identical between myelinated and unmyelinated axon segments, it is conceivable that a pathological insult such as demyelination could disrupt the actin–spectrin lattice (D’Este et al., 2016; He et al., 2016). αII spectrin and other spectrins are principal targets of the calcium-dependent cysteine protease calpain, which is activated in response to increases in intracellular calcium during mechanical axon trauma, ischemia/anoxia, and Wallerian degeneration (Stys et al., 1992; Saatman et al., 2003; Schafer et al., 2009; Yang et al., 2013). Impaired calcium homeostasis is a putative mechanism for neurodegeneration in MS; declustering of voltage-gated sodium channels significantly increases the metabolic burden of maintaining sodium concentration gradients through the Na+/K+ ATPase, and subsequently, high levels of intracellular sodium concentration can reverse the Na+/Ca2+ exchanger (NCX) and drive calcium influx (Fig. 2; Stys et al., 1992; Nikolaeva et al., 2005; Stirling and Stys, 2010). Alternatively, calcium-permeable glutamate receptors and voltage-gated calcium channels on the axolemma or intra-axonal calcium stores in the mitochondria and endoplasmic reticulum could contribute to increased axoplasmic calcium (Brown et al., 2001; Nikolaeva et al., 2005; Villegas et al., 2014). In either case, increased calcium concentration leads to autolytic activation of calpain and downstream cleavage of αII/βII/βIV spectrins, actin, neurofilaments, microtubules, microtubule-binding proteins, ankyrin G, NaV1.2, NCX3, and other substrates critical to neuronal function (Kamakura et al., 1983; Sato et al., 1986; Billger et al., 1988; Harris and Morrow, 1990; Hu and Bennett, 1991; Villa et al., 1998; Iwata et al., 2004; Bano et al., 2005; Vosler et al., 2008; Schafer et al., 2009; Ma, 2013). Disassembly of the axon cytoskeleton and insufficient ATP generation impairs axon trafficking, generating a pathological feedback loop with insufficiency of mitochondrial transport to demyelinated axon segments and progressive exacerbation of metabolic stress (Trapp and Stys, 2009). Intracellular calcium has also been implicated, independently of calpain activation, in the regulation of mitochondrial transport through the Rho-like GTPase Miro, inhibition of mitochondrial function, depolymerization of microtubules, and stimulation of calcium-induced calcium release (Job et al., 1981; O’Brien et al., 1997; Wang and Schwarz, 2009; Villegas et al., 2014). Collectively, these mechanisms represent convergent and synergistic pathways to impaired axon function and axon degeneration (Fig. 2).
Figure 2.
Proposed molecular mechanisms of secondary axon degeneration. Multiple pathological mechanisms converge synergistically on axon degeneration. In a demyelinated axon, disassembly of nodal architecture and its underlying cytoskeleton may lead to voltage-gated sodium channel declustering, increasing sodium ion influx and the metabolic demand of action potential conduction. Impaired axon transport, increased metabolic load, and excitotoxic stress may lead to failure of the Na+/K+ ATPase, reversal of the NCX, and entry of calcium into the axon. Increased axoplasmic calcium leads to activation of the calcium-dependent protease calpain, which cleaves many putative substrates crucial to axon function, including those associated with the axon cytoskeleton or transport machinery. Overloading of mitochondrial calcium buffering capacity and impairment of mitochondrial transport via disruption of motor proteins and microtubules inhibits ATP production and exacerbates metabolic impairment. Excitotoxic stress further contributes to increased sodium and calcium influx, transmitted through various ion channels. NMDAR, N-Methyl-d-aspartate receptor. AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; Cav, voltage-gated calcium channel; MAP, microtubule-associated protein; Mit, mitochondria; Nav, voltage-gated sodium channel.
Visualization of calcium-mediated spectrin disruption at the nanoscale level was recently achieved with stochastic optical reconstruction microscopy, demonstrating that prolonged activation of voltage-gated calcium channels in cultured hippocampal neurons is sufficient to perturb βIV spectrin periodicity at the axon initial segment (Leterrier et al., 2015). However, evidence for excitotoxic cytoskeletal degradation in the setting of demyelination is largely correlative. Altered distributions of NCX, voltage-gated sodium channels, voltage-gated calcium channels, and glutamate receptors are observed in postmortem human MS tissue and EAE mice, but functional demonstration of this sequence of events has not been pursued in demyelination to the extent it has been in models of mechanical or ischemic/anoxic axon injury (Stys et al., 1992; Shields et al., 1999; Kornek et al., 2001; Saatman et al., 2003; Craner et al., 2004; Howell et al., 2006; Black et al., 2007; Newcombe et al., 2008; Schafer et al., 2009; Yang et al., 2013). In EAE, two-photon imaging of the surgically exposed brainstem with a Förster resonance energy transfer–based ratiometric calcium indicator revealed elevated baseline calcium levels in axon segments that spatiotemporally correlated with immune cell apposition, regions of active demyelination, and subsequent cytoskeletal fragmentation (Siffrin et al., 2010). Furthermore, genetic deletion of the calcium-sensitive TRPM4 and pH-sensitive ASIC1 cation channels improves clinical outcomes and alleviates neurodegeneration in EAE, as does pharmacological blockade of ionotropic glutamate receptors, voltage-gated sodium channels, or calpain activity (Pitt et al., 2000; Lo et al., 2003; Bechtold et al., 2006; Friese et al., 2007; Hassen et al., 2008; Schattling et al., 2012). Whether demyelination is sufficient to induce ion dyshomeostasis in the absence of inflammatory injury, whether spectrins and other cytoskeletal components are degraded through these mechanisms, and whether this sequence of events can lead to functional consequences, such as impaired axon transport, remain as important, unanswered questions.
Concluding remarks
Recently, the anti–CD20 antibody ocrelizumab was approved by the Food and Drug Administration as the first treatment for primary progressive MS; despite this important milestone and its efficacy in treating relapsing/remitting MS, ocrelizumab is an immunomodulatory intervention with modest effects on clinical outcomes in primary progressive patients (Hauser et al., 2017; Montalban et al., 2017). The inability to halt progressive disease, and the existence of a small subset of primary progressive patients that present with precocious onset of permanent clinical disability, suggest that neuroinflammation is not the sole or even primary cause of axon degeneration (Trapp and Nave, 2008; Stys et al., 2012). As such, the prevention of axon and neuron loss remains a pressing, unmet need and can only be achieved through rigorous characterization of axon–glial interactions and molecular mechanisms of axon degeneration.
Maturational changes in myelinated axons during development and axon pathology associated with mutations in myelin-associated genes strongly suggest a role for myelin in regulating the axon cytoskeleton and axon transport. Furthermore, neuron-autonomous perturbation of nodal and cytoskeletal constituents or blockade of axon transport is sufficient to result in degeneration, independently of myelination (Lee et al., 1994; Zhao et al., 2001; LaMonte et al., 2002; Garcia-Fresco et al., 2006; d’Ydewalle et al., 2011; Neumann and Hilliard, 2014; Leite et al., 2016). The missing link is categorical evidence that demyelination can also result in these perturbations. Although this association has been alluded to, the interpretation of animal models of demyelination is problematic because of the inability to parse out nonspecific effects of/on axons, astrocytes, and immune cells from the direct consequences of myelin loss (Table 1). As a result, even the most fundamental question of whether demyelination is detrimental to the axon has been difficult to answer. Orthogonal approaches to inducing cell-type–specific oligodendrocyte death and demyelination with minimal disruption to the surrounding parenchyma are an active area of development, but these techniques come with experimental caveats such as the necrotic inflammation and secondary adaptive immunity of DT models and the limited efficacy in adult animals of inducible caspase models (Caprariello et al., 2012, 2015; Traka et al., 2016). Regardless, the increasingly powerful repertoire of methods to visualize neurons and glia with subdiffraction limited resolution, artifact-free ultrastructural detail, and single-cell and single-axon resolution in vivo, over time, in live animals will be indispensable tools for answering these questions (Misgeld and Kerschensteiner, 2006; Huang et al., 2010; Möbius et al., 2016; Follain et al., 2017). There are undoubtedly a considerable number of degenerative pathways, including the loss of metabolic support by myelinating glia, that converge on cytoskeletal degradation and axon transport disruption, manifesting either directly from the loss of axon–glial signaling or as indirect sequelae of maladaptive changes such as voltage-gated sodium channel declustering and ion dyshomeostasis (Table 2). It is our conviction that defining the relative contributions of each of these mechanisms will constitute the next major advances not only in understanding and treating demyelinating disease but also in detailing the intricate cell biology of the axon and its myelinating glia.
Table 1. Axon pathology secondary to mutations in myelin-associated genes and experimental demyelination models.
| Animal model | Axon phenotype | References |
|---|---|---|
| Shiverer (Mbp null) | Decreased axon caliber/neurofilament phosphorylation, minimal axon degeneration | Rosenbluth, 1980; Brady et al., 1999 |
| Plp1 null | Progressive axon loss, axonal spheroids containing multivesicular bodies, disorganized cytoskeletal components, and membranous organelles, impaired axon transport, and microtubule abnormalities | Klugmann et al., 1997; Griffiths et al., 1998; Garbern et al., 2002; Edgar et al., 2004; Yin et al., 2016 |
| Cnp null | Mislocalization of voltage-gated sodium channels and Caspr, progressive axon loss, axonal spheroids with accumulations of membranous organelles, impaired axon transport, and impaired formation and maintenance of cytoplasmic channels | Lappe-Siefke et al., 2003; Rasband et al., 2005; Snaidero et al., 2017 |
| Mag null | Decreased axon caliber/neurofilament phosphorylation, progressive axon loss, increased susceptibility to EAE/neurotoxic injury, and decreased Cdk5/Erk1/2 kinase activity | Fruttiger et al., 1995; Yin et al., 1998; Dashiell et al., 2002; Pan et al., 2005; Nguyen et al., 2009 |
| EAE | Axon loss, irreversible cytoskeletal fragmentation, impaired mitochondrial morphology and trafficking, impaired axon transport, oxidative damage, and increased axoplasmic calcium | Siffrin et al., 2010; Nikić et al., 2011; Sorbara et al., 2014; Höflich et al., 2016; Mei et al., 2016 |
| Lysolecithin | Impaired axon transport, increased mitochondrial stationary site size and transport rate, and disrupted nodal architecture | Arroyo et al., 2004; Kiryu-Seo et al., 2010; Ohno et al., 2014; Höflich et al., 2016; Schultz et al., 2017 |
| Cuprizone | Axon loss, impaired axon transport, and increased mitochondrial stationary site size | Ohno et al., 2014; Sachs et al., 2014; Höflich et al., 2016; Schultz et al., 2017 |
| DT ablation | Axon loss, impaired axon transport, and neurofilament dephosphorylation | Traka et al., 2010, 2016; Pohl et al., 2011; Oluich et al., 2012 |
Table 2. Developmental and maladaptive changes in myelination and demyelination.
| Developmental changes during myelination | Potential maladaptive changes during demyelination |
|---|---|
| Formation of nodes of Ranvier and paranodal junctions | Declustering of voltage-gated sodium channels, accumulation of intracellular sodium, failure of the Na+/K+ ATPase, accumulation of calcium through NCX reversal, and/or glutamate excitotoxicity and impairment of axon transport |
| Alterations in the phosphorylation states of cytoskeletal and axon transport-associated proteins | Dephosphorylation of microtubule-associated proteins following the loss of MAG and other myelin-derived signals, changes in microtubule stability potentially leading to cytoskeletal fragmentation or alterations in axon transport, and phosphorylation and inhibition of kinesin light chain |
| Development of the actin–spectrin cytoskeleton appears to be independent of myelination | Spectrin degradation and loss of actin–spectrin periodicity, loss of structural organization for cytoskeletal-associated proteins, declustering and mislocalization of voltage-gated sodium channels, mechanical instability, and perturbed interaction with the microtubule network leading to impaired axon transport |
| Dependence on myelinating glia for metabolic support | Progressive inability to maintain metabolic homeostasis in the setting of declustered voltage-gated sodium channels, impaired axon transport, cytoskeletal fragmentation, and aberrant ion concentration gradients |
Acknowledgments
The authors thank members of the Chan laboratory for insightful and helpful discussion and Kae-Jiun Chang and Pei-Jung Lee for their significant efforts in designing and illustrating the figures. We regret and apologize for the many important studies and reviews that were omitted from this discussion because of space limitations.
This work was supported by the National Institutes of Heath/National Institute of Neurological Disorders and Stroke (grants R01NS062796, R01NS097428, and R01NS095889), the National Multiple Sclerosis Society (grant RG5203A4), and the Rachleff family endowment.
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- APP
- amyloid precursor protein
- CNP
- 2′,3′-cyclic nucleotide phosphodiesterase
- CNS
- central nervous system
- DRG
- dorsal root ganglion
- DT
- diphtheria toxin
- DTA
- DT subunit A
- EAE
- experimental autoimmune encephalomyelitis
- HSP
- hereditary spastic paraplegia
- MAG
- myelin-associated glycoprotein
- MBP
- myelin basic protein
- MS
- multiple sclerosis
- NCX
- Na+/Ca2+ exchanger
- PLP
- proteolipid protein
- PMD
- Pelizaeus–Merzbacher disease
References
- Amor V., Zhang C., Vainshtein A., Zhang A., Zollinger D.R., Eshed-Eisenbach Y., Brophy P.J., Rasband M.N., and Peles E.. 2017. The paranodal cytoskeleton clusters Na(+) channels at nodes of Ranvier. eLife. 6:e21392 10.7554/eLife.21392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong R., Toews A.D., and Morell P.. 1987. Axonal transport through nodes of Ranvier. Brain Res. 412:196–199. 10.1016/0006-8993(87)91461-2 [DOI] [PubMed] [Google Scholar]
- Arroyo E.J., Sirkowski E.E., Chitale R., and Scherer S.S.. 2004. Acute demyelination disrupts the molecular organization of peripheral nervous system nodes. J. Comp. Neurol. 479:424–434. 10.1002/cne.20321 [DOI] [PubMed] [Google Scholar]
- Bano D., Young K.W., Guerin C.J., Lefeuvre R., Rothwell N.J., Naldini L., Rizzuto R., Carafoli E., and Nicotera P.. 2005. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell. 120:275–285. 10.1016/j.cell.2004.11.049 [DOI] [PubMed] [Google Scholar]
- Bechtold D.A., Miller S.J., Dawson A.C., Sun Y., Kapoor R., Berry D., and Smith K.J.. 2006. Axonal protection achieved in a model of multiple sclerosis using lamotrigine. J. Neurol. 253:1542–1551. 10.1007/s00415-006-0204-1 [DOI] [PubMed] [Google Scholar]
- Berghs S., Aggujaro D., Dirkx R. Jr., Maksimova E., Stabach P., Hermel J.M., Zhang J.P., Philbrick W., Slepnev V., Ort T., and Solimena M.. 2000. βIV spectrin, a new spectrin localized at axon initial segments and nodes of ranvier in the central and peripheral nervous system. J. Cell Biol. 151:985–1002. 10.1083/jcb.151.5.985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat M.A., Rios J.C., Lu Y., Garcia-Fresco G.P., Ching W., St Martin M., Li J., Einheber S., Chesler M., Rosenbluth J., et al. . 2001. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 30:369–383. 10.1016/S0896-6273(01)00294-X [DOI] [PubMed] [Google Scholar]
- Billger M., Wallin M., and Karlsson J.-O.. 1988. Proteolysis of tubulin and microtubule-associated proteins 1 and 2 by calpain I and II. Difference in sensitivity of assembled and disassembled microtubules. Cell Calcium. 9:33–44. 10.1016/0143-4160(88)90036-X [DOI] [PubMed] [Google Scholar]
- Birgbauer E., Rao T.S., and Webb M.. 2004. Lysolecithin induces demyelination in vitro in a cerebellar slice culture system. J. Neurosci. Res. 78:157–166. 10.1002/jnr.20248 [DOI] [PubMed] [Google Scholar]
- Bjartmar C., Wujek J.R., and Trapp B.D.. 2003. Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J. Neurol. Sci. 206:165–171. 10.1016/S0022-510X(02)00069-2 [DOI] [PubMed] [Google Scholar]
- Black J.A., Newcombe J., Trapp B.D., and Waxman S.G.. 2007. Sodium channel expression within chronic multiple sclerosis plaques. J. Neuropathol. Exp. Neurol. 66:828–837. 10.1097/nen.0b013e3181462841 [DOI] [PubMed] [Google Scholar]
- Boyle M.E., Berglund E.O., Murai K.K., Weber L., Peles E., and Ranscht B.. 2001. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron. 30:385–397. 10.1016/S0896-6273(01)00296-3 [DOI] [PubMed] [Google Scholar]
- Brady S.T., Witt A.S., Kirkpatrick L.L., de Waegh S.M., Readhead C., Tu P.H., and Lee V.M.. 1999. Formation of compact myelin is required for maturation of the axonal cytoskeleton. J. Neurosci. 19:7278–7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.M., Westenbroek R.E., Catterall W.A., and Ransom B.R.. 2001. Axonal L-type Ca2+ channels and anoxic injury in rat CNS white matter. J. Neurophysiol. 85:900–911. [DOI] [PubMed] [Google Scholar]
- Caprariello A.V., Mangla S., Miller R.H., and Selkirk S.M.. 2012. Apoptosis of oligodendrocytes in the central nervous system results in rapid focal demyelination. Ann. Neurol. 72:395–405. 10.1002/ana.23606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprariello A.V., Batt C.E., Zippe I., Romito-DiGiacomo R.R., Karl M., and Miller R.H.. 2015. Apoptosis of oligodendrocytes during early development delays myelination and impairs subsequent responses to demyelination. J. Neurosci. 35:14031–14041. 10.1523/JNEUROSCI.1706-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K.-J., and Rasband M.N.. 2013. Excitable domains of myelinated nerves: axon initial segments and nodes of Ranvier. Curr. Top. Membr. 72:159–192. 10.1016/B978-0-12-417027-8.00005-2 [DOI] [PubMed] [Google Scholar]
- Chernoff G.F. 1981. Shiverer: an autosomal recessive mutant mouse with myelin deficiency. J. Hered. 72:128 10.1093/oxfordjournals.jhered.a109442 [DOI] [PubMed] [Google Scholar]
- Coman I., Aigrot M.S., Seilhean D., Reynolds R., Girault J.A., Zalc B., and Lubetzki C.. 2006. Nodal, paranodal and juxtaparanodal axonal proteins during demyelination and remyelination in multiple sclerosis. Brain. 129:3186–3195. 10.1093/brain/awl144 [DOI] [PubMed] [Google Scholar]
- Craner M.J., Newcombe J., Black J.A., Hartle C., Cuzner M.L., and Waxman S.G.. 2004. Molecular changes in neurons in multiple sclerosis: altered axonal expression of Nav1.2 and Nav1.6 sodium channels and Na+/Ca2+ exchanger. Proc. Natl. Acad. Sci. USA. 101:8168–8173. 10.1073/pnas.0402765101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Este E., Kamin D., Velte C., Göttfert F., Simons M., and Hell S.W.. 2016. Subcortical cytoskeleton periodicity throughout the nervous system. Sci. Rep. 6:22741 10.1038/srep22741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Este E., Kamin D., Balzarotti F., and Hell S.W.. 2017. Ultrastructural anatomy of nodes of Ranvier in the peripheral nervous system as revealed by STED microscopy. Proc. Natl. Acad. Sci. USA. 114:E191–E199. 10.1073/pnas.1619553114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Ydewalle C., Krishnan J., Chiheb D.M., Van Damme P., Irobi J., Kozikowski A.P., Vanden Berghe P., Timmerman V., Robberecht W., and Van Den Bosch L.. 2011. HDAC6 inhibitors reverse axonal loss in a mouse model of mutant HSPB1-induced Charcot-Marie-Tooth disease. Nat. Med. 17:968–974. 10.1038/nm.2396 [DOI] [PubMed] [Google Scholar]
- Dashiell S.M., Tanner S.L., Pant H.C., and Quarles R.H.. 2002. Myelin-associated glycoprotein modulates expression and phosphorylation of neuronal cytoskeletal elements and their associated kinases. J. Neurochem. 81:1263–1272. 10.1046/j.1471-4159.2002.00927.x [DOI] [PubMed] [Google Scholar]
- de Waegh S.M., Lee V.M., and Brady S.T.. 1992. Local modulation of neurofilament phosphorylation, axonal caliber, and slow axonal transport by myelinating Schwann cells. Cell. 68:451–463. 10.1016/0092-8674(92)90183-D [DOI] [PubMed] [Google Scholar]
- Doerflinger N.H., Macklin W.B., and Popko B.. 2003. Inducible site-specific recombination in myelinating cells. Genesis. 35:63–72. 10.1002/gene.10154 [DOI] [PubMed] [Google Scholar]
- Dutta R., and Trapp B.D.. 2011. Mechanisms of neuronal dysfunction and degeneration in multiple sclerosis. Prog. Neurobiol. 93:1–12. 10.1016/j.pneurobio.2010.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar J.M., McLaughlin M., Yool D., Zhang S.-C., Fowler J.H., Montague P., Barrie J.A., McCulloch M.C., Duncan I.D., Garbern J., et al. . 2004. Oligodendroglial modulation of fast axonal transport in a mouse model of hereditary spastic paraplegia. J. Cell Biol. 166:121–131. 10.1083/jcb.200312012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshed Y., Feinberg K., Poliak S., Sabanay H., Sarig-Nadir O., Spiegel I., Bermingham J.R. Jr., and Peles E.. 2005. Gliomedin mediates Schwann cell-axon interaction and the molecular assembly of the nodes of Ranvier. Neuron. 47:215–229. 10.1016/j.neuron.2005.06.026 [DOI] [PubMed] [Google Scholar]
- Follain G., Mercier L., Osmani N., Harlepp S., and Goetz J.G.. 2017. Seeing is believing - multi-scale spatio-temporal imaging towards in vivo cell biology. J. Cell Sci. 130:23–38. 10.1242/jcs.189001 [DOI] [PubMed] [Google Scholar]
- Foster R.E., Kocsis J.D., Malenka R.C., and Waxman S.G.. 1980. Lysophosphatidyl choline-induced focal demyelination in the rabbit corpus callosum. Electron-microscopic observations. J. Neurol. Sci. 48:221–231. 10.1016/0022-510X(80)90202-6 [DOI] [PubMed] [Google Scholar]
- Friese M.A., Craner M.J., Etzensperger R., Vergo S., Wemmie J.A., Welsh M.J., Vincent A., and Fugger L.. 2007. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat. Med. 13:1483–1489. 10.1038/nm1668 [DOI] [PubMed] [Google Scholar]
- Fruttiger M., Montag D., Schachner M., and Martini R.. 1995. Crucial role for the myelin-associated glycoprotein in the maintenance of axon-myelin integrity. Eur. J. Neurosci. 7:511–515. 10.1111/j.1460-9568.1995.tb00347.x [DOI] [PubMed] [Google Scholar]
- Fünfschilling U., Supplie L.M., Mahad D., Boretius S., Saab A.S., Edgar J., Brinkmann B.G., Kassmann C.M., Tzvetanova I.D., Möbius W., et al. . 2012. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 485:517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbern J.Y., Yool D.A., Moore G.J., Wilds I.B., Faulk M.W., Klugmann M., Nave K.A., Sistermans E.A., van der Knaap M.S., Bird T.D., et al. . 2002. Patients lacking the major CNS myelin protein, proteolipid protein 1, develop length-dependent axonal degeneration in the absence of demyelination and inflammation. Brain. 125:551–561. 10.1093/brain/awf043 [DOI] [PubMed] [Google Scholar]
- Garcia M.L., Lobsiger C.S., Shah S.B., Deerinck T.J., Crum J., Young D., Ward C.M., Crawford T.O., Gotow T., Uchiyama Y., et al. . 2003. NF-M is an essential target for the myelin-directed “outside-in” signaling cascade that mediates radial axonal growth. J. Cell Biol. 163:1011–1020. 10.1083/jcb.200308159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Fresco G.P., Sousa A.D., Pillai A.M., Moy S.S., Crawley J.N., Tessarollo L., Dupree J.L., and Bhat M.A.. 2006. Disruption of axo-glial junctions causes cytoskeletal disorganization and degeneration of Purkinje neuron axons. Proc. Natl. Acad. Sci. USA. 103:5137–5142. 10.1073/pnas.0601082103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza-Lombó C., and Gonsebatt M.E.. 2016. Mammalian target of rapamycin: its role in early neural development and in adult and aged brain function. Front. Cell. Neurosci. 10:157 10.3389/fncel.2016.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan L., Sabanay H., Poliak S., Berglund E.O., Ranscht B., and Peles E.. 2002. Retention of a cell adhesion complex at the paranodal junction requires the cytoplasmic region of Caspr. J. Cell Biol. 157:1247–1256. 10.1083/jcb.200203050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths I., Klugmann M., Anderson T., Yool D., Thomson C., Schwab M.H., Schneider A., Zimmermann F., McCulloch M., Nadon N., and Nave K.A.. 1998. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 280:1610–1613. 10.1126/science.280.5369.1610 [DOI] [PubMed] [Google Scholar]
- Griggs R.B., Yermakov L.M., and Susuki K.. 2017. Formation and disruption of functional domains in myelinated CNS axons. Neurosci. Res. 116:77–87. 10.1016/j.neures.2016.09.010 [DOI] [PubMed] [Google Scholar]
- Gruenenfelder F.I., Thomson G., Penderis J., and Edgar J.M.. 2011. Axon-glial interaction in the CNS: what we have learned from mouse models of Pelizaeus-Merzbacher disease. J. Anat. 219:33–43. 10.1111/j.1469-7580.2011.01363.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund M., Jorgensen E.M., and Bastiani M.J.. 2007. Axons break in animals lacking β-spectrin. J. Cell Biol. 176:269–275. 10.1083/jcb.200611117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A.S., and Morrow J.S.. 1990. Calmodulin and calcium-dependent protease I coordinately regulate the interaction of fodrin with actin. Proc. Natl. Acad. Sci. USA. 87:3009–3013. 10.1073/pnas.87.8.3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassen G.W., Feliberti J., Kesner L., Stracher A., and Mokhtarian F.. 2008. Prevention of axonal injury using calpain inhibitor in chronic progressive experimental autoimmune encephalomyelitis. Brain Res. 1236:206–215. 10.1016/j.brainres.2008.07.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser S.L., Bar-Or A., Comi G., Giovannoni G., Hartung H.-P.P., Hemmer B., Lublin F., Montalban X., Rammohan K.W., Selmaj K., et al. OPERA I and OPERA II Clinical Investigators . 2017. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N. Engl. J. Med. 376:221–234. 10.1056/NEJMoa1601277 [DOI] [PubMed] [Google Scholar]
- He J., Zhou R., Wu Z., Carrasco M.A., Kurshan P.T., Farley J.E., Simon D.J., Wang G., Han B., Hao J., et al. . 2016. Prevalent presence of periodic actin-spectrin-based membrane skeleton in a broad range of neuronal cell types and animal species. Proc. Natl. Acad. Sci. USA. 113:6029–6034. 10.1073/pnas.1605707113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom K.L., Ogawa Y., and Rasband M.N.. 2008. AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J. Cell Biol. 183:635–640. 10.1083/jcb.200806112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N., Niwa S., and Tanaka Y.. 2010. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 68:610–638. 10.1016/j.neuron.2010.09.039 [DOI] [PubMed] [Google Scholar]
- Ho T.S.Y., Zollinger D.R., Chang K.J., Xu M., Cooper E.C., Stankewich M.C., Bennett V., and Rasband M.N.. 2014. A hierarchy of ankyrin-spectrin complexes clusters sodium channels at nodes of Ranvier. Nat. Neurosci. 17:1664–1672. 10.1038/nn.3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P.N., Griffin J.W., Gold B.G., and Price D.L.. 1985. Slowing of neurofilament transport and the radial growth of developing nerve fibers. J. Neurosci. 5:2920–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höflich K.M., Beyer C., Clarner T., Schmitz C., Nyamoya S., Kipp M., and Hochstrasser T.. 2016. Acute axonal damage in three different murine models of multiple sclerosis: A comparative approach. Brain Res. 1650:125–133. 10.1016/j.brainres.2016.08.048 [DOI] [PubMed] [Google Scholar]
- Howell O.W., Palser A., Polito A., Melrose S., Zonta B., Scheiermann C., Vora A.J., Brophy P.J., and Reynolds R.. 2006. Disruption of neurofascin localization reveals early changes preceding demyelination and remyelination in multiple sclerosis. Brain. 129:3173–3185. 10.1093/brain/awl290 [DOI] [PubMed] [Google Scholar]
- Hsieh S.T., Kidd G.J., Crawford T.O., Xu Z., Lin W.M., Trapp B.D., Cleveland D.W., and Griffin J.W.. 1994. Regional modulation of neurofilament organization by myelination in normal axons. J. Neurosci. 14:6392–6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R.-J., and Bennett V.. 1991. In vitro proteolysis of brain spectrin by calpain I inhibits association of spectrin with ankyrin-independent membrane binding site(s). J. Biol. Chem. 266:18200–18205. [PubMed] [Google Scholar]
- Huang B., Wang W., Bates M., and Zhuang X.. 2008. Three-dimensional super-resolution imaging by stochastic optical reconstruction microscopy. Science. 319:810–813. 10.1126/science.1153529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B., Babcock H., and Zhuang X.. 2010. Breaking the diffraction barrier: super-resolution imaging of cells. Cell. 143:1047–1058. 10.1016/j.cell.2010.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata A., Stys P.K., Wolf J.A., Chen X.-H., Taylor A.G., Meaney D.F., and Smith D.H.. 2004. Traumatic axonal injury induces proteolytic cleavage of the voltage-gated sodium channels modulated by tetrodotoxin and protease inhibitors. J. Neurosci. 24:4605–4613. 10.1523/JNEUROSCI.0515-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke C. 2014. The tubulin code: Molecular components, readout mechanisms, and functions. J. Cell Biol. 206:461–472. 10.1083/jcb.201406055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job D., Fischer E.H., and Margolis R.L.. 1981. Rapid disassembly of cold-stable microtubules by calmodulin. Proc. Natl. Acad. Sci. USA. 78:4679–4682. 10.1073/pnas.78.8.4679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T., Ikenaka K., Inoue Y., Kuriyama S., Tsujii T., Nakao J., Nakajima K., Aruga J., Okano H., and Mikoshiba K.. 1994. Glial cell degeneration and hypomyelination caused by overexpression of myelin proteolipid protein gene. Neuron. 13:427–442. 10.1016/0896-6273(94)90358-1 [DOI] [PubMed] [Google Scholar]
- Kamakura K., Ishiura S., Sugita H., and Toyokura Y.. 1983. Identification of Ca2+-activated neutral protease in the peripheral nerve and its effects on neurofilament degeneration. J. Neurochem. 40:908–913. 10.1111/j.1471-4159.1983.tb08072.x [DOI] [PubMed] [Google Scholar]
- Kang J.S., Tian J.H., Pan P.Y., Zald P., Li C., Deng C., and Sheng Z.H.. 2008. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell. 132:137–148. 10.1016/j.cell.2007.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevenaar J.T., and Hoogenraad C.C.. 2015. The axonal cytoskeleton: from organization to function. Front. Mol. Neurosci. 8:44 10.3389/fnmol.2015.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipp M., Clarner T., Dang J., Copray S., and Beyer C.. 2009. The cuprizone animal model: new insights into an old story. Acta Neuropathol. 118:723–736. 10.1007/s00401-009-0591-3 [DOI] [PubMed] [Google Scholar]
- Kiryu-Seo S., Ohno N., Kidd G.J., Komuro H., and Trapp B.D.. 2010. Demyelination increases axonal stationary mitochondrial size and the speed of axonal mitochondrial transport. J. Neurosci. 30:6658–6666. 10.1523/JNEUROSCI.5265-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugmann M., Schwab M.H., Pühlhofer A., Schneider A., Zimmermann F., Griffiths I.R., and Nave K.A.. 1997. Assembly of CNS myelin in the absence of proteolipid protein. Neuron. 18:59–70. 10.1016/S0896-6273(01)80046-5 [DOI] [PubMed] [Google Scholar]
- Komada M., and Soriano P.. 2002. [β]IV-spectrin regulates sodium channel clustering through ankyrin-G at axon initial segments and nodes of Ranvier. J. Cell Biol. 156:337–348. 10.1083/jcb.200110003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornek B., Storch M.K., Bauer J., Djamshidian A., Weissert R., Wallstroem E., Stefferl A., Zimprich F., Olsson T., Linington C., et al. . 2001. Distribution of a calcium channel subunit in dystrophic axons in multiple sclerosis and experimental autoimmune encephalomyelitis. Brain. 124:1114–1124. 10.1093/brain/124.6.1114 [DOI] [PubMed] [Google Scholar]
- Krieg M., Stühmer J., Cueva J.G., Fetter R., Spilker K., Cremers D., Shen K., Dunn A.R., and Goodman M.B.. 2017. Genetic defects in β-spectrin and tau sensitize C. elegans axons to movement-induced damage via torque-tension coupling. eLife. 6:e20172 10.7554/eLife.20172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMonte B.H., Wallace K.E., Holloway B.A., Shelly S.S., Ascaño J., Tokito M., Van Winkle T., Howland D.S., and Holzbaur E.L.. 2002. Disruption of dynein/dynactin inhibits axonal transport in motor neurons causing late-onset progressive degeneration. Neuron. 34:715–727. 10.1016/S0896-6273(02)00696-7 [DOI] [PubMed] [Google Scholar]
- Lappe-Siefke C., Goebbels S., Gravel M., Nicksch E., Lee J., Braun P.E., Griffiths I.R., and Nave K.A.. 2003. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat. Genet. 33:366–374. 10.1038/ng1095 [DOI] [PubMed] [Google Scholar]
- Lee M.K., Marszalek J.R., and Cleveland D.W.. 1994. A mutant neurofilament subunit causes massive, selective motor neuron death: implications for the pathogenesis of human motor neuron disease. Neuron. 13:975–988. 10.1016/0896-6273(94)90263-1 [DOI] [PubMed] [Google Scholar]
- Lee Y., Morrison B.M., Li Y., Lengacher S., Farah M.H., Hoffman P.N., Liu Y., Tsingalia A., Jin L., Zhang P.-W., et al. . 2012. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 487:443–448. 10.1038/nature11314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite S.C., Sampaio P., Sousa V.F., Nogueira-Rodrigues J., Pinto-Costa R., Peters L.L., Brites P., and Sousa M.M.. 2016. The Actin-Binding Protein α-Adducin Is Required for Maintaining Axon Diameter. Cell Reports. 15:490–498. 10.1016/j.celrep.2016.03.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone D.P., Genoud S., Atanasoski S., Grausenburger R., Berger P., Metzger D., Macklin W.B., Chambon P., and Suter U.. 2003. Tamoxifen-inducible glia-specific Cre mice for somatic mutagenesis in oligodendrocytes and Schwann cells. Mol. Cell. Neurosci. 22:430–440. 10.1016/S1044-7431(03)00029-0 [DOI] [PubMed] [Google Scholar]
- Leterrier C., Potier J., Caillol G., Debarnot C., Rueda Boroni F., and Dargent B.. 2015. Nanoscale Architecture of the Axon Initial Segment Reveals an Organized and Robust Scaffold. Cell Reports. 13:2781–2793. 10.1016/j.celrep.2015.11.051 [DOI] [PubMed] [Google Scholar]
- Li C., Tropak M.B., Gerlai R., Clapoff S., Abramow-Newerly W., Trapp B., Peterson A., and Roder J.. 1994. Myelination in the absence of myelin-associated glycoprotein. Nature. 369:747–750. 10.1038/369747a0 [DOI] [PubMed] [Google Scholar]
- Lo A.C., Saab C.Y., Black J.A., and Waxman S.G.. 2003. Phenytoin protects spinal cord axons and preserves axonal conduction and neurological function in a model of neuroinflammation in vivo. J. Neurophysiol. 90:3566–3571. 10.1152/jn.00434.2003 [DOI] [PubMed] [Google Scholar]
- Lorenzo D.N., Li M.G., Mische S.E., Armbrust K.R., Ranum L.P.W., and Hays T.S.. 2010. Spectrin mutations that cause spinocerebellar ataxia type 5 impair axonal transport and induce neurodegeneration in Drosophila. J. Cell Biol. 189:143–158. 10.1083/jcb.200905158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo D.N., Badea A., Davis J., Hostettler J., He J., Zhong G., Zhuang X., and Bennett V.. 2014. A PIK3C3-ankyrin-B-dynactin pathway promotes axonal growth and multiorganelle transport. J. Cell Biol. 207:735–752. 10.1083/jcb.201407063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M. 2013. Role of calpains in the injury-induced dysfunction and degeneration of the mammalian axon. Neurobiol. Dis. 60:61–79. 10.1016/j.nbd.2013.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima G.K., and Morell P.. 2001. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 11:107–116. 10.1111/j.1750-3639.2001.tb00385.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta N.R., Nguyen T., Bullen J.W. Jr., Griffin J.W., and Schnaar R.L.. 2010. Myelin-associated glycoprotein (MAG) protects neurons from acute toxicity using a ganglioside-dependent mechanism. ACS Chem. Neurosci. 1:215–222. 10.1021/cn900029p [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei F., Lehmann-Horn K., Shen Y.-A.A., Rankin K.A., Stebbins K.J., Lorrain D.S., Pekarek K., A Sagan S., Xiao L., Teuscher C., et al. . 2016. Accelerated remyelination during inflammatory demyelination prevents axonal loss and improves functional recovery. eLife. 5:e18246 10.7554/eLife.18246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheva K.D., Wolman D., Mensh B.D., Pax E., Buchanan J., Smith S.J., and Bock D.D.. 2016. A large fraction of neocortical myelin ensheathes axons of local inhibitory neurons. eLife. 5:e15784 10.7554/eLife.15784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misgeld T., and Kerschensteiner M.. 2006. In vivo imaging of the diseased nervous system. Nat. Rev. Neurosci. 7:449–463. 10.1038/nrn1905 [DOI] [PubMed] [Google Scholar]
- Möbius W., Nave K.A., and Werner H.B.. 2016. Electron microscopy of myelin: Structure preservation by high-pressure freezing. Brain Res. 1641(Pt A):92–100. 10.1016/j.brainres.2016.02.027 [DOI] [PubMed] [Google Scholar]
- Monsma P.C., Li Y., Fenn J.D., Jung P., and Brown A.. 2014. Local regulation of neurofilament transport by myelinating cells. J. Neurosci. 34:2979–2988. 10.1523/JNEUROSCI.4502-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag D., Giese K.P., Bartsch U., Martini R., Lang Y., Blüthmann H., Karthigasan J., Kirschner D.A., Wintergerst E.S., Nave K.A., et al. . 1994. Mice deficient for the myelin-associated glycoprotein show subtle abnormalities in myelin. Neuron. 13:229–246. 10.1016/0896-6273(94)90472-3 [DOI] [PubMed] [Google Scholar]
- Montalban X., Hauser S.L., Kappos L., Arnold D.L., Bar-Or A., Comi G., de Seze J., Giovannoni G., Hartung H.-P.P., Hemmer B., et al. ORATORIO Clinical Investigators . 2017. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N. Engl. J. Med. 376:209–220. 10.1056/NEJMoa1606468 [DOI] [PubMed] [Google Scholar]
- Morfini G., Szebenyi G., Elluru R., Ratner N., and Brady S.T.. 2002. Glycogen synthase kinase 3 phosphorylates kinesin light chains and negatively regulates kinesin-based motility. EMBO J. 21:281–293. 10.1093/emboj/21.3.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G., Szebenyi G., Brown H., Pant H.C., Pigino G., DeBoer S., Beffert U., and Brady S.T.. 2004. A novel CDK5-dependent pathway for regulating GSK3 activity and kinesin-driven motility in neurons. EMBO J. 23:2235–2245. 10.1038/sj.emboj.7600237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave K.A. 2010. Myelination and the trophic support of long axons. Nat. Rev. Neurosci. 11:275–283. 10.1038/nrn2797 [DOI] [PubMed] [Google Scholar]
- Nave K.A., Lai C., Bloom F.E., and Milner R.J.. 1986. Jimpy mutant mouse: a 74-base deletion in the mRNA for myelin proteolipid protein and evidence for a primary defect in RNA splicing. Proc. Natl. Acad. Sci. USA. 83:9264–9268. 10.1073/pnas.83.23.9264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave K.A., Sereda M.W., and Ehrenreich H.. 2007. Mechanisms of disease: inherited demyelinating neuropathies--from basic to clinical research. Nat. Clin. Pract. Neurol. 3:453–464. 10.1038/ncpneuro0583 [DOI] [PubMed] [Google Scholar]
- Neumann B., and Hilliard M.A.. 2014. Loss of MEC-17 leads to microtubule instability and axonal degeneration. Cell Reports. 6:93–103. 10.1016/j.celrep.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe J., Uddin A., Dove R., Patel B., Turski L., Nishizawa Y., and Smith T.. 2008. Glutamate receptor expression in multiple sclerosis lesions. Brain Pathol. 18:52–61. 10.1111/j.1750-3639.2007.00101.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T., Mehta N.R., Conant K., Kim K.-J.J., Jones M., Calabresi P.A., Melli G., Hoke A., Schnaar R.L., Ming G.-L.L., et al. . 2009. Axonal protective effects of the myelin-associated glycoprotein. J. Neurosci. 29:630–637. 10.1523/JNEUROSCI.5204-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikić I., Merkler D., Sorbara C., Brinkoetter M., Kreutzfeldt M., Bareyre F.M., Brück W., Bishop D., Misgeld T., and Kerschensteiner M.. 2011. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat. Med. 17:495–499. 10.1038/nm.2324 [DOI] [PubMed] [Google Scholar]
- Nikolaeva M.A., Mukherjee B., and Stys P.K.. 2005. Na+-dependent sources of intra-axonal Ca2+ release in rat optic nerve during in vitro chemical ischemia. J. Neurosci. 25:9960–9967. 10.1523/JNEUROSCI.2003-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon R.A., Paskevich P.A., Sihag R.K., and Thayer C.Y.. 1994. Phosphorylation on carboxyl terminus domains of neurofilament proteins in retinal ganglion cell neurons in vivo: influences on regional neurofilament accumulation, interneurofilament spacing, and axon caliber. J. Cell Biol. 126:1031–1046. 10.1083/jcb.126.4.1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normand E.A., and Rasband M.N.. 2015. Subcellular patterning: axonal domains with specialized structure and function. Dev. Cell. 32:459–468. 10.1016/j.devcel.2015.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien E.T., Salmon E.D., and Erickson H.P.. 1997. How calcium causes microtubule depolymerization. Cell Motil. Cytoskeleton. 36:125–135. [DOI] [PubMed] [Google Scholar]
- Ogawa Y., Schafer D.P., Horresh I., Bar V., Hales K., Yang Y., Susuki K., Peles E., Stankewich M.C., and Rasband M.N.. 2006. Spectrins and ankyrinB constitute a specialized paranodal cytoskeleton. J. Neurosci. 26:5230–5239. 10.1523/JNEUROSCI.0425-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno N., Chiang H., Mahad D.J., Kidd G.J., Liu L., Ransohoff R.M., Sheng Z.-H.H., Komuro H., and Trapp B.D.. 2014. Mitochondrial immobilization mediated by syntaphilin facilitates survival of demyelinated axons. Proc. Natl. Acad. Sci. USA. 111:9953–9958. 10.1073/pnas.1401155111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oluich L.-J., Stratton J.A., Xing Y.L., Ng S.W., Cate H.S., Sah P., Windels F., Kilpatrick T.J., and Merson T.D.. 2012. Targeted ablation of oligodendrocytes induces axonal pathology independent of overt demyelination. J. Neurosci. 32:8317–8330. 10.1523/JNEUROSCI.1053-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B., Fromholt S.E., Hess E.J., Crawford T.O., Griffin J.W., Sheikh K.A., and Schnaar R.L.. 2005. Myelin-associated glycoprotein and complementary axonal ligands, gangliosides, mediate axon stability in the CNS and PNS: neuropathology and behavioral deficits in single- and double-null mice. Exp. Neurol. 195:208–217. 10.1016/j.expneurol.2005.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson N.J., Olsson C.L., Hallows J.L., McKee-Johnson J., Keogh B.P., Noben-Trauth K., Kujawa S.G., and Tempel B.L.. 2001. Mutant β-spectrin 4 causes auditory and motor neuropathies in quivering mice. Nat. Genet. 29:61–65. 10.1038/ng710 [DOI] [PubMed] [Google Scholar]
- Pitt D., Werner P., and Raine C.S.. 2000. Glutamate excitotoxicity in a model of multiple sclerosis. Nat. Med. 6:67–70. 10.1038/71555 [DOI] [PubMed] [Google Scholar]
- Pohl H.B.F., Porcheri C., Mueggler T., Bachmann L.C., Martino G., Riethmacher D., Franklin R.J.M., Rudin M., and Suter U.. 2011. Genetically induced adult oligodendrocyte cell death is associated with poor myelin clearance, reduced remyelination, and axonal damage. J. Neurosci. 31:1069–1080. 10.1523/JNEUROSCI.5035-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliak S., and Peles E.. 2003. The local differentiation of myelinated axons at nodes of Ranvier. Nat. Rev. Neurosci. 4:968–980. 10.1038/nrn1253 [DOI] [PubMed] [Google Scholar]
- Poliak S., Salomon D., Elhanany H., Sabanay H., Kiernan B., Pevny L., Stewart C.L., Xu X., Chiu S.-Y.Y., Shrager P., et al. . 2003. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J. Cell Biol. 162:1149–1160. 10.1083/jcb.200305018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronker M.F., Lemstra S., Snijder J., Heck A.J., Thies-Weesie D.M., Pasterkamp R.J., and Janssen B.J.. 2016. Structural basis of myelin-associated glycoprotein adhesion and signalling. Nat. Commun. 7:13584 10.1038/ncomms13584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y., Hahn I., Webb S.E., Pearce S.P., and Prokop A.. 2017. Periodic actin structures in neuronal axons are required to maintain microtubules. Mol. Biol. Cell. 28:296–308. 10.1091/mbc.E16-10-0727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarles R.H. 2007. Myelin-associated glycoprotein (MAG): past, present and beyond. J. Neurochem. 100:1431–1448. [DOI] [PubMed] [Google Scholar]
- Quarles R.H. 2009. A hypothesis about the relationship of myelin-associated glycoprotein’s function in myelinated axons to its capacity to inhibit neurite outgrowth. Neurochem. Res. 34:79–86. 10.1007/s11064-008-9668-y [DOI] [PubMed] [Google Scholar]
- Ransohoff R.M. 2012. Animal models of multiple sclerosis: the good, the bad and the bottom line. Nat. Neurosci. 15:1074–1077. 10.1038/nn.3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband M.N., Tayler J., Kaga Y., Yang Y., Lappe-Siefke C., Nave K.A., and Bansal R.. 2005. CNP is required for maintenance of axon-glia interactions at nodes of Ranvier in the CNS. Glia. 50:86–90. 10.1002/glia.20165 [DOI] [PubMed] [Google Scholar]
- Roach A., Takahashi N., Pravtcheva D., Ruddle F., and Hood L.. 1985. Chromosomal mapping of mouse myelin basic protein gene and structure and transcription of the partially deleted gene in shiverer mutant mice. Cell. 42:149–155. 10.1016/S0092-8674(85)80110-0 [DOI] [PubMed] [Google Scholar]
- Rosenbluth J. 1980. Central myelin in the mouse mutant shiverer. J. Comp. Neurol. 194:639–648. 10.1002/cne.901940310 [DOI] [PubMed] [Google Scholar]
- Saab A.S., Tzvetavona I.D., Trevisiol A., Baltan S., Dibaj P., Kusch K., Möbius W., Goetze B., Jahn H.M., Huang W., et al. . 2016. Oligodendroglial NMDA Receptors Regulate Glucose Import and Axonal Energy Metabolism. Neuron. 91:119–132. 10.1016/j.neuron.2016.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatman K.E., Abai B., Grosvenor A., Vorwerk C.K., Smith D.H., and Meaney D.F.. 2003. Traumatic axonal injury results in biphasic calpain activation and retrograde transport impairment in mice. J. Cereb. Blood Flow Metab. 23:34–42. 10.1097/01.WCB.0000035040.10031.B0 [DOI] [PubMed] [Google Scholar]
- Sachs H.H., Bercury K.K., Popescu D.C., Narayanan S.P., and Macklin W.B.. 2014. A new model of cuprizone-mediated demyelination/remyelination. ASN Neuro. 6:165912923 10.1177/1759091414551955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saifetiarova J., Taylor A.M., and Bhat M.A.. 2017. Early and Late Loss of the Cytoskeletal Scaffolding Protein, Ankyrin G Reveals Its Role in Maturation and Maintenance of Nodes of Ranvier in Myelinated Axons. J. Neurosci. 37:2524–2538. 10.1523/JNEUROSCI.2661-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitsu H., Tohyama J., Kumada T., Egawa K., Hamada K., Okada I., Mizuguchi T., Osaka H., Miyata R., Furukawa T., et al. . 2010. Dominant-negative mutations in alpha-II spectrin cause West syndrome with severe cerebral hypomyelination, spastic quadriplegia, and developmental delay. Am. J. Hum. Genet. 86:881–891. 10.1016/j.ajhg.2010.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer J.L. 2003. Polarized domains of myelinated axons. Neuron. 40:297–318. 10.1016/S0896-6273(03)00628-7 [DOI] [PubMed] [Google Scholar]
- Sánchez I., Hassinger L., Paskevich P.A., Shine H.D., and Nixon R.A.. 1996. Oligodendroglia regulate the regional expansion of axon caliber and local accumulation of neurofilaments during development independently of myelin formation. J. Neurosci. 16:5095–5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez I., Hassinger L., Sihag R.K., Cleveland D.W., Mohan P., and Nixon R.A.. 2000. Local control of neurofilament accumulation during radial growth of myelinating axons in vivo. Selective role of site-specific phosphorylation. J. Cell Biol. 151:1013–1024. 10.1083/jcb.151.5.1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato C., Nishizawa K., Nakayama T., Nakamura H., Yoshimura N., Takano E., and Murachi T.. 1986. Rapid proteolysis of brain MAP-1 related cytoskeleton-associated 350kd protein by purified calpain. Cell Struct. Funct. 11:253–257. 10.1247/csf.11.253 [DOI] [PubMed] [Google Scholar]
- Schafer D.P., Jha S., Liu F., Akella T., McCullough L.D., and Rasband M.N.. 2009. Disruption of the axon initial segment cytoskeleton is a new mechanism for neuronal injury. J. Neurosci. 29:13242–13254. 10.1523/JNEUROSCI.3376-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattling B., Steinbach K., Thies E., Kruse M., Menigoz A., Ufer F., Flockerzi V., Brück W., Pongs O., Vennekens R., et al. . 2012. TRPM4 cation channel mediates axonal and neuronal degeneration in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat. Med. 18:1805–1811. 10.1038/nm.3015 [DOI] [PubMed] [Google Scholar]
- Schneider A., Montague P., Griffiths I., Fanarraga M., Kennedy P., Brophy P., and Nave K.A.. 1992. Uncoupling of hypomyelination and glial cell death by a mutation in the proteolipid protein gene. Nature. 358:758–761. 10.1038/358758a0 [DOI] [PubMed] [Google Scholar]
- Schultz V., van der Meer F., Wrzos C., Scheidt U., Bahn E., Stadelmann C., Brück W., and Junker A.. 2017. Acutely damaged axons are remyelinated in multiple sclerosis and experimental models of demyelination. Glia. 65:1350–1360. 10.1002/glia.23167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman D.L., Tait S., Melrose S., Johnson R., Zonta B., Court F.A., Macklin W.B., Meek S., Smith A.J., Cottrell D.F., and Brophy P.J.. 2005. Neurofascins are required to establish axonal domains for saltatory conduction. Neuron. 48:737–742. 10.1016/j.neuron.2005.10.019 [DOI] [PubMed] [Google Scholar]
- Shields D.C., Schaecher K.E., Saido T.C., and Banik N.L.. 1999. A putative mechanism of demyelination in multiple sclerosis by a proteolytic enzyme, calpain. Proc. Natl. Acad. Sci. USA. 96:11486–11491. 10.1073/pnas.96.20.11486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman R.L., Dickie M.M., and Appel S.H.. 1964. Mutant mice (quaking and jimpy) with deficient myelination in the central nervous system. Science. 144:309–311. 10.1126/science.144.3616.309 [DOI] [PubMed] [Google Scholar]
- Siffrin V., Radbruch H., Glumm R., Niesner R., Paterka M., Herz J., Leuenberger T., Lehmann S.M., Luenstedt S., Rinnenthal J.L., et al. . 2010. In vivo imaging of partially reversible th17 cell-induced neuronal dysfunction in the course of encephalomyelitis. Immunity. 33:424–436. 10.1016/j.immuni.2010.08.018 [DOI] [PubMed] [Google Scholar]
- Snaidero N., Velte C., Myllykoski M., Raasakka A., Ignatev A., Werner H.B., Erwig M.S., Möbius W., Kursula P., Nave K.A., and Simons M.. 2017. Antagonistic Functions of MBP and CNP Establish Cytosolic Channels in CNS Myelin. Cell Reports. 18:314–323. 10.1016/j.celrep.2016.12.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobotzik J.-M.M., Sie J.M., Politi C., Del Turco D., Bennett V., Deller T., and Schultz C.. 2009. AnkyrinG is required to maintain axo-dendritic polarity in vivo. Proc. Natl. Acad. Sci. USA. 106:17564–17569. 10.1073/pnas.0909267106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorbara C.D., Wagner N.E., Ladwig A., Nikić I., Merkler D., Kleele T., Marinković P., Naumann R., Godinho L., Bareyre F.M., et al. . 2014. Pervasive axonal transport deficits in multiple sclerosis models. Neuron. 84:1183–1190. 10.1016/j.neuron.2014.11.006 [DOI] [PubMed] [Google Scholar]
- Sousa A.D., and Bhat M.A.. 2007. Cytoskeletal transition at the paranodes: the Achilles’ heel of myelinated axons. Neuron Glia Biol. 3:169–178. 10.1017/S1740925X07000415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankewich M.C., Cianci C.D., Stabach P.R., Ji L., Nath A., and Morrow J.S.. 2011. Cell organization, growth, and neural and cardiac development require αII-spectrin. J. Cell Sci. 124:3956–3966. 10.1242/jcs.080374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling D.P., and Stys P.K.. 2010. Mechanisms of axonal injury: internodal nanocomplexes and calcium deregulation. Trends Mol. Med. 16:160–170. 10.1016/j.molmed.2010.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stys P.K., Waxman S.G., and Ransom B.R.. 1992. Ionic mechanisms of anoxic injury in mammalian CNS white matter: role of Na+ channels and Na(+)-Ca2+ exchanger. J. Neurosci. 12:430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stys P.K., Zamponi G.W., van Minnen J., and Geurts J.J.. 2012. Will the real multiple sclerosis please stand up? Nat. Rev. Neurosci. 13:507–514. 10.1038/nrn3275 [DOI] [PubMed] [Google Scholar]
- Sun X.Y., Takagishi Y., Okabe E., Chishima Y., Kanou Y., Murase S., Mizumura K., Inaba M., Komatsu Y., Hayashi Y., et al. . 2009. A novel Caspr mutation causes the shambling mouse phenotype by disrupting axoglial interactions of myelinated nerves. J. Neuropathol. Exp. Neurol. 68:1207–1218. 10.1097/NEN.0b013e3181be2e96 [DOI] [PubMed] [Google Scholar]
- Susuki K., Chang K.-J.J., Zollinger D.R., Liu Y., Ogawa Y., Eshed-Eisenbach Y., Dours-Zimmermann M.T.T., Oses-Prieto J.A., Burlingame A.L., Seidenbecher C.I., et al. . 2013. Three mechanisms assemble central nervous system nodes of Ranvier. Neuron. 78:469–482. 10.1016/j.neuron.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter U., and Scherer S.S.. 2003. Disease mechanisms in inherited neuropathies. Nat. Rev. Neurosci. 4:714–726. 10.1038/nrn1196 [DOI] [PubMed] [Google Scholar]
- Takagishi Y., Katanosaka K., Mizoguchi H., and Murata Y.. 2016. Disrupted axon-glia interactions at the paranode in myelinated nerves cause axonal degeneration and neuronal cell death in the aged Caspr mutant mouse shambling. Neurobiol. Aging. 43:34–46. 10.1016/j.neurobiolaging.2016.03.020 [DOI] [PubMed] [Google Scholar]
- Taylor A.M., Saifetiarova J., and Bhat M.A.. 2017. Postnatal loss of neuronal and glial neurofascins differentially affects node of Ranvier maintenance and myelinated axon function. Front. Cell. Neurosci. 11:11 10.3389/fncel.2017.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohyama J., Nakashima M., Nabatame S., Gaik-Siew C., Miyata R., Rener-Primec Z., Kato M., Matsumoto N., and Saitsu H.. 2015. SPTAN1 encephalopathy: distinct phenotypes and genotypes. J. Hum. Genet. 60:167–173. 10.1038/jhg.2015.5 [DOI] [PubMed] [Google Scholar]
- Traka M., Arasi K., Avila R.L., Podojil J.R., Christakos A., Miller S.D., Soliven B., and Popko B.. 2010. A genetic mouse model of adult-onset, pervasive central nervous system demyelination with robust remyelination. Brain. 133:3017–3029. 10.1093/brain/awq247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traka M., Podojil J.R., McCarthy D.P., Miller S.D., and Popko B.. 2016. Oligodendrocyte death results in immune-mediated CNS demyelination. Nat. Neurosci. 19:65–74. 10.1038/nn.4193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp B.D., and Nave K.A.. 2008. Multiple sclerosis: an immune or neurodegenerative disorder? Annu. Rev. Neurosci. 31:247–269. 10.1146/annurev.neuro.30.051606.094313 [DOI] [PubMed] [Google Scholar]
- Trapp B.D., and Stys P.K.. 2009. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol. 8:280–291. 10.1016/S1474-4422(09)70043-2 [DOI] [PubMed] [Google Scholar]
- Villa P.G., Henzel W.J., Sensenbrenner M., Henderson C.E., and Pettmann B.. 1998. Calpain inhibitors, but not caspase inhibitors, prevent actin proteolysis and DNA fragmentation during apoptosis. J. Cell Sci. 111:713–722. [DOI] [PubMed] [Google Scholar]
- Villegas R., Martinez N.W., Lillo J., Pihan P., Hernandez D., Twiss J.L., and Court F.A.. 2014. Calcium release from intra-axonal endoplasmic reticulum leads to axon degeneration through mitochondrial dysfunction. J. Neurosci. 34:7179–7189. 10.1523/JNEUROSCI.4784-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosler P.S., Brennan C.S., and Chen J.. 2008. Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol. Neurobiol. 38:78–100. 10.1007/s12035-008-8036-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., and Schwarz T.L.. 2009. The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell. 136:163–174. 10.1016/j.cell.2008.11.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman S.G., Kocsis J.D., and Nitta K.C.. 1979. Lysophosphatidyl choline-induced focal demyelination in the rabbit corpus callosum. Light-microscopic observations. J. Neurol. Sci. 44:45–53. 10.1016/0022-510X(79)90221-1 [DOI] [PubMed] [Google Scholar]
- Witt A., and Brady S.T.. 2000. Unwrapping new layers of complexity in axon/glial relationships. Glia. 29:112–117. [DOI] [PubMed] [Google Scholar]
- Wolswijk G., and Balesar R.. 2003. Changes in the expression and localization of the paranodal protein Caspr on axons in chronic multiple sclerosis. Brain. 126:1638–1649. 10.1093/brain/awg151 [DOI] [PubMed] [Google Scholar]
- Xu K., Zhong G., and Zhuang X.. 2013. Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science. 339:452–456. 10.1126/science.1232251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Weimer R.M., Kallop D., Olsen O., Wu Z., Renier N., Uryu K., and Tessier-Lavigne M.. 2013. Regulation of axon degeneration after injury and in development by the endogenous calpain inhibitor calpastatin. Neuron. 80:1175–1189. 10.1016/j.neuron.2013.08.034 [DOI] [PubMed] [Google Scholar]
- Yang Y., Lacas-Gervais S., Morest D.K., Solimena M., and Rasband M.N.. 2004. BetaIV spectrins are essential for membrane stability and the molecular organization of nodes of Ranvier. J. Neurosci. 24:7230–7240. 10.1523/JNEUROSCI.2125-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Ogawa Y., Hedstrom K.L., and Rasband M.N.. 2007. βIV spectrin is recruited to axon initial segments and nodes of Ranvier by ankyrinG. J. Cell Biol. 176:509–519. 10.1083/jcb.200610128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X., Crawford T.O., Griffin J.W., Tu Ph., Lee V.M., Li C., Roder J., and Trapp B.D.. 1998. Myelin-associated glycoprotein is a myelin signal that modulates the caliber of myelinated axons. J. Neurosci. 18:1953–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X., Kidd G.J., Ohno N., Perkins G.A., Ellisman M.H., Bastian C., Brunet S., Baltan S., and Trapp B.D.. 2016. Proteolipid protein-deficient myelin promotes axonal mitochondrial dysfunction via altered metabolic coupling. J. Cell Biol. 215:531–542. 10.1083/jcb.201607099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., and Rasband M.N.. 2016. Cytoskeletal control of axon domain assembly and function. Curr. Opin. Neurobiol. 39:116–121. 10.1016/j.conb.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Susuki K., Zollinger D.R., Dupree J.L., and Rasband M.N.. 2013. Membrane domain organization of myelinated axons requires βII spectrin. J. Cell Biol. 203:437–443. 10.1083/jcb.201308116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen K., Sloan S.A., Bennett M.L., Scholze A.R., O’Keeffe S., Phatnani H.P., Guarnieri P., Caneda C., Ruderisch N., et al. . 2014. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34:11929–11947. 10.1523/JNEUROSCI.1860-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Takita J., Tanaka Y., Setou M., Nakagawa T., Takeda S., Yang H.W., Terada S., Nakata T., Takei Y., et al. . 2001. Charcot-Marie-Tooth disease type 2A caused by mutation in a microtubule motor KIF1Bbeta. Cell. 105:587–597. 10.1016/S0092-8674(01)00363-4 [DOI] [PubMed] [Google Scholar]
- Zhong G., He J., Zhou R., Lorenzo D., Babcock H.P., Bennett V., and Zhuang X.. 2014. Developmental mechanism of the periodic membrane skeleton in axons. eLife. 3 10.7554/eLife.04581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Lambert S., Malen P.L., Carpenter S., Boland L.M., and Bennett V.. 1998. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J. Cell Biol. 143:1295–1304. 10.1083/jcb.143.5.1295 [DOI] [PMC free article] [PubMed] [Google Scholar]