Abstract
Objective
Prolonged hospitalization due to burn injury results in physical inactivity and muscle weakness. However, how these changes are distributed among body parts is unknown. The aim of this study was to evaluate the degree of body composition changes in different anatomical regions during intensive care unit hospitalization (ICUh).
Design
Retrospective chart review.
Setting
Children’s burn hospital.
Patients
Twenty-four severely burned children admitted to our institution between 2000 and 2015.
Interventions
All patients underwent a dual-energy x-ray absorptiometry (DEXA) within 2 weeks after injury and 2 weeks before discharge to determine body composition changes. No subject underwent anabolic intervention. We analyzed changes of bone mineral content, bone mineral density, total fat mass, total mass, and total lean mass of the entire body and specifically analyzed the changes between the upper and lower limbs.
Measurements and Main Results
In the 24 patients, age was 10±5 years, total body surface area burned was 59±17%, time between DEXAs was 34±21 days, and length of stay was 39±24 days. We found a significant (p<0.001) average loss of 3% of lean mass in the whole body; this loss was significantly greater (p<0.001) in the upper extremities (17%) than in the lower extremities (7%). We also observed a remodeling of the fat compartments, with a significant whole-body increase in fat mass (p<0.001) that was greater in the truncal region (p<0.0001) and in the lower limbs (p<0.05).
Conclusions
ICUh is associated with greater lean mass loss in the upper limbs of burned children. Mobilization programs should include early mobilization of upper limbs to restore upper extremity function.
Keywords: exercise, rehabilitation, patient discharge, lower extremity, upper extremity
Introduction
Sedation required for mechanical ventilation and pain control causes physical inactivity which leads to muscle breakdown [1], loss of bone mass and reduced quality of life in burn patients [2] as well as other patients in the Intensive Care Unit (ICU) [3, 4]. Muscle breakdown begins within 48 hours of immobilization and maximizes at 14 – 21 days after immobilization [5]. In pediatric burn patients, muscle breakdown in burned children remains elevated up to one year post burn with a depletion of 17% lean mass and reduction of 31% of fat stores [6]. Moreover, among pediatric ICU patients, the incidence of chronic protein-energy malnutrition has been shown to be 22% and the recorded risk for acute protein-energy malnutrition is of 8% [7]. Between 1% and 5% of muscle strength is lost per day, and as much as 40% muscle strength loss occurs during the first week in the intensive care unit (ICU) [8]. Due to profound protein catabolism, this effect is more pronounced in burn patients [9]. Despite the necessity of positive pressure ventilation for adequate pulmonary support, it is directly correlated with limb weakness [10], which results in the majority of patients with severe burns [11]. Furthermore, the severity of limb weakness correlates with the duration of the ventilation [12]. Bone mineral density (BMD) has also been shown to decrease in patients with long ICU stays, which persists even after 6 months of normal activity in non-burned patients [8].
Unfortunately, severe burn injuries are associated with long ICU stays in adult and pediatric patients [11] and ensuing losses in lean body mass. It is not previously known whether losses in body mass are greater in the upper or lower region of the body. In order to inform ICU-related exercise programs that may reduce recovery time, this study was undertaken to evaluate the degree of body composition changes in different regions of severely burned children during their ICU stays, and to correlate these findings with changes in strength.
Materials and Methods
Patients
With Institutional Review Board approval (IRB 04-157), twenty-four severely burned children admitted to our institution between 2000 and 2015 were enrolled. Parents or legal guardians provided informed consent and children provided assent, as applicable, prior to study participation. The 24 patients studied were narrowed from a starting sample population of 210 burned children with burns greater than or equal to 30% TBSA that had also received dual-energy x-ray absorptiometry (DEXA) during their acute hospitalization stay. Of the 210 children that had greater than or equal to 30% TBSA with DEXA scans, 165 children were excluded because their scans had not been performed within 2 weeks after burn injury or within 2 weeks before discharge (or with at least 2 weeks in between their admission and discharge scans). From the remaining 45 children, 21 had received supplementation with anabolic agents that could have confounded the changes in their bone, fat or muscle composition, and these children were additionally excluded (Figure 1). All children underwent standard of care treatment during hospitalization, which included early excision and grafting, feeding 1.2 to 1.6 times the measured resting energy expenditure of the patient as soon as possible to help alleviate the hypermetabolic response, fluid resuscitation, β blockers treatment for reduction in the post-burn catecholamine surge, as well as optimized ventilation [13]. Inclusion criteria for this institutional research protocol study included burns greater than or equal to 30% TBSA, burns including at least one side of the upper and lower extremity, and body composition scans (DEXAs) within 2 weeks after burn injury at admission and within 2 weeks before discharge (with a 2-week minimum period between the 2 scans). Patients who received anabolic agents, including growth hormone and oxandrolone, as well as those who underwent early exercise interventional programs, were excluded from the study due to the confounding effect of these research treatments.
Figure 1.
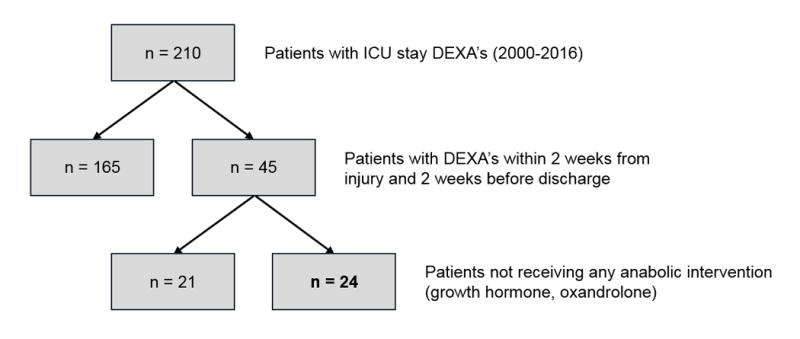
Patient consort type diagram. ICU: Intensive care unit; DEXA: Dual-energy x-ray absorptiometry.
Dual-energy x-ray absorptiometry
Body composition was determined by DEXA using a Hologic model QDR-4500-W instrument (Bedford, MA) and pediatric-specific software. The software generated total body composition data based on the different intensities of x-ray beams passing through different types of tissues [14]. The total amount of radiation a patient is exposed to per exam is 0.001 mSv (millisievert). The machine was calibrated daily using phantom loading. All scans were obtained with the patient lying motionlessly, and if movement was noted, the scans were repeated. Patients only underwent scans with synthetic sterile gowns. All objects and clothes (besides feeding tubes and arterial or venous lines) were removed for the exam. DEXA scans were performed within 2 weeks after burn injury admission and within 2 weeks before discharge. The minimum period between the 2 scans was set at 2 weeks. Bone mineral content (BMC), BMD, total mass, total fat mass, and total lean mass were analyzed for the whole body as well as individual measurements of total mass, total fat mass, and total lean mass for the upper and lower extremities and trunk. For the upper and lower extremities, the totals for each parameter were calculated by summing the values of both upper extremities or both lower extremities.
Nutritional regiments, fluid resuscitation and physical therapy
Patients received enteral nutrition starting at admission and continuing over their hospitalization stay until they could consume adequate nutrition “ad libitum”. The enteral formula used consisted of 82% carbohydrates, 15% protein, and 3% fat (Vivonex T.E.N., Nestlé Health Science, Minneapolis, MN) [15] and was delivered at a rate of 1500 kcal/m2 TBSA/day plus 1500 kcal/m2 TBSA burned/day until the first resting energy expenditure measurements were performed. After this assessment, the caloric loads were held at 1.2 – 1.6 times the measured resting energy expenditure.
Total fluid resuscitation was administered within 24 hours of admission according to the Galveston formula (5000 mL/m2 TBSA + 2000 mL/m2 total body surface area of lactated Ringer’s solution). All patients received physical therapy which included active range of motion exercises, stretching of all extremities and assisted ambulation as tolerated. Assisting devices included walkers, splints, knee immobilizers and IV poles.
Resting energy expenditure
All our patients underwent a resting energy expenditure (REE) measurement within one week from admission, and weekly REE measurements were taken until discharge. After patients had fasted from 10pm to 6am, REEs were performed within 2 hours. REE was measured using a Sensor-Medics Vmax 29 metabolic cart (Yorba Linda, CA). O2 gas, CO2 gas and air flows were calibrated using known gasses and a 3-liter syringe. The patient was tested in a supine position while under a large, ventilated hood, in a temperature-controlled room set at 30°C. Inspired and expired gases were continuously measured. The REE was calculated from the oxygen consumption and carbon dioxide production by equations described by Weir [16]. Values of carbon dioxide production (VCO2), oxygen production (VO2), and REE were included when they were at steady states for at least 5 min. The average REE was calculated from these steady-state measurements for 20 minutes. Estimated basal energy expenditure, based on age, sex, height and weight, was calculated using the Harris-Benedict equation [17] and normalized as a percentage of predicted expenditure.
Statistical Analysis
Patient demographics and REE data were summarized as means, standard deviations, counts or percentages. Mixed linear regression models were used to assess within subjects’ body composition changes during acute hospitalization, including subjects as random effects. In each model, the main predictor of each scan’s result was the time point at which the DEXA scan was performed. The DEXA scans were classified as either early (within 2 weeks of admission) or late (within 2 weeks of discharge). Time between body composition measurements differed among patients; therefore, the time between the two scans was included to account for this difference. Initially, age, sex, burn type, burn size, and percent of full thickness burn were added to the models to assess their impacts on the DEXA changes. In the final models, only “age” and “time between scans” were included because they were the only two to show statistical significance (p<0.001). A Likelihood Ratio Test (LRT) was used to assess the effects of the main predictor of lean mass loss, whether the patient was early in their hospital course or (early DEXA) or late (late DEXA). The main hypothesis of this study was that post-burn lean mass loss is greater in the upper extremities relative to the lower limbs. This hypothesis was tested by modeling upper extremity lean mass over time. To adjust for the fact that legs have greater overall lean mass, and would result in a greater gross loss of mass, leg lean mass was used as a covariate to more accurately examine the proportion of mass loss in the upper extremities. Statistical analyses were performed using R statistical software (R Core Team, 2017, version 3.3.3). Results are expressed as means ± SD. Significance was set at p< 0.05.
Results
Patient characteristics
Demographics for the patient population are summarized in Table 1. The predominant mechanism of injury was flame burn (21/24; 88%), followed by electrical injury (3/24; 12%). The time from burn to first DEXA scan was an average of 10 ± 3 days, while the average time between DEXA scans was 34 ± 20 days. Patients were ventilated for an average of 4 ± 4 days and remained in the ICU for an average of 39 ± 24 days. There was large variability of burn location in this patient study group. Patients included had burns on the head, chest, back, arms, and legs; however, all patients had at least one burn on one of their extremities. The body weights taken by the DEXA measurements and a regular scale are summarized in Table 2.
Table 1.
Patient characteristics.
| Characteristic | Value |
|---|---|
| Sex, n | |
| Female | 6 |
| Male | 18 |
| Ethnicity, n (%) | |
| Hispanic | 20 (83%) |
| Caucasian | 4 (17%) |
| Age, years | 10 ± 5 |
| TBSA burned, % | 59 ± 17 |
| TBSA burned full thickness, % | 48 ± 25 |
| LOS, days | 39 ± 24 |
| Ventilation, days | 4 ± 4 |
| Drugs, n | |
| None | 8 (34%) |
| Propranolol | 7 (29%) |
| Insulin | 3 (13%) |
| Glucagon-like-peptide | 2 (8%) |
| Fenofibrate | 2 (8%) |
| Ketoconazole | 2 (8%) |
Abbreviations: LOS, length of stay; TBSA, total body surface area
Data expressed as mean ± SD unless noted otherwise.
Table 2.
Patient weight in kilograms.
| DEXA total body weight | Gross weight | % difference | |
|---|---|---|---|
| 1st Scan | 36 ± 18 | 40 ± 22 | 7 ± 11 |
| 2nd Scan | 36 ± 17 | 36 ±17 | 0 ± 2 |
Abbreviations: DEXA, Dual-energy x-ray absorptiometry.
Data expressed as mean ± SD unless noted otherwise.
Upper extremities
The changes of upper extremity masses between the two DEXA scan time points revealed a greater reduction in total lean mass (p = 0.0007) and total mass (p = 0.0028) in the upper extremities when controlled for lower extremities mass. No differences in the change in total fat mass were noted between the upper and lower extremities (p = 0.51) (Figure 2). Even when lower extremity mass was not controlled, upper extremity masses showed considerable reductions in total lean mass and consequently total mass. Average total lean mass was reduced by 0.55 kg (16%; p < 0.0001) and total mass by 0.52 kg (12%; p < 0.0001) (Figure 3). Average total fat mass increased by 4%, though this was not statistically significant (p = 0.38).
Figure 2.
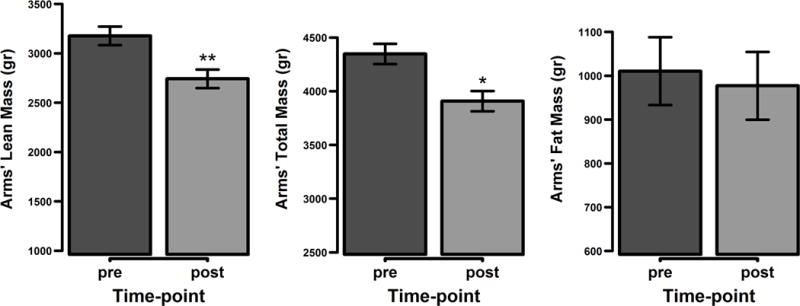
Upper extremities lean body mass changes pre vs. post adjusted for lower extremities lean body mass. Represented are the mean values with the standard error of the mean differences. * p < 0.05, ** p < 0.001
Figure 3.
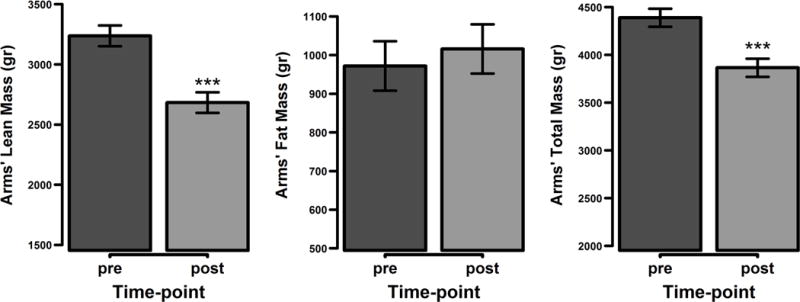
Body composition changes in the upper extremities. Represented are the mean values with the standard error of the mean differences. *** p < 0.0001
Lower extremities
The lower extremities showed an average 0.49 kg (6%) loss in total lean mass (p < 0.05). Conversely, average total fat mass in the lower extremities increased by 0.28 kg (9%; p < 0.05) (Figure 4).
Figure 4.
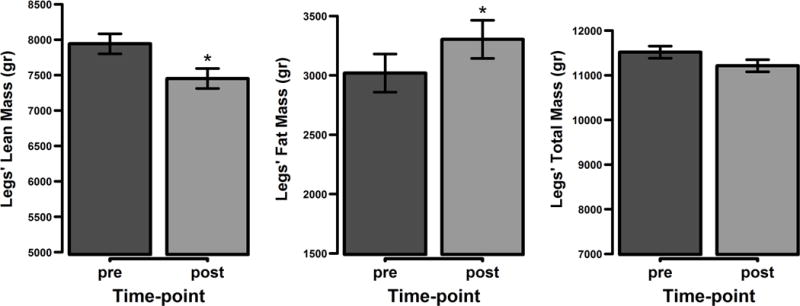
Body composition changes in the lower extremities. Represented are the mean values with the standard error of the mean differences. * p < 0.05
Truncal region
The trunk showed an average 0.65 kg (23%) increase in total fat mass and an average 0.75 kg (5%) increase in total mass (p < 0.0001 for both). Although total lean mass showed a slight increase, this finding was not significant (Figure 5).
Figure 5.
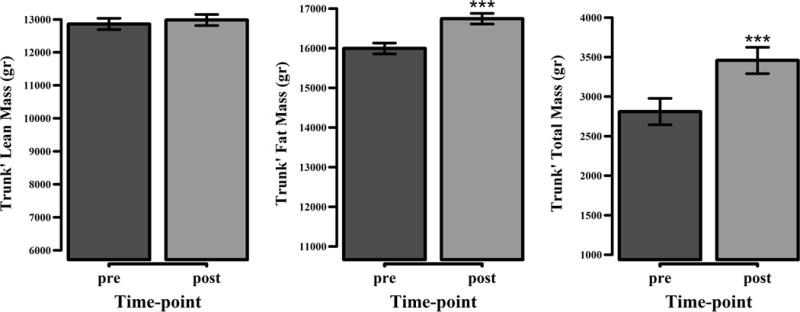
Body composition changes in the trunk. Represented are the mean values with the standard error of the mean differences. *** p < 0.0001
Whole body
Average whole body total mass was slightly reduced by 0.46 kg (1%) after the average 34 days of ICU stay, although this finding was not statistically significant (p = 0.40). Total fat mass increased by an average of 0.99 kg (13%, p < 0.001), and average total lean mass decreased by 0.86 kg (3%, p < 0.001). BMC and BMD both showed small, statistically insignificant decreases of 9% and 7%, respectively (p = 0.12 and p = 0.14). All body composition changes occurring in the study population over their ICU stays are summarized in Figure 6.
Figure 6.
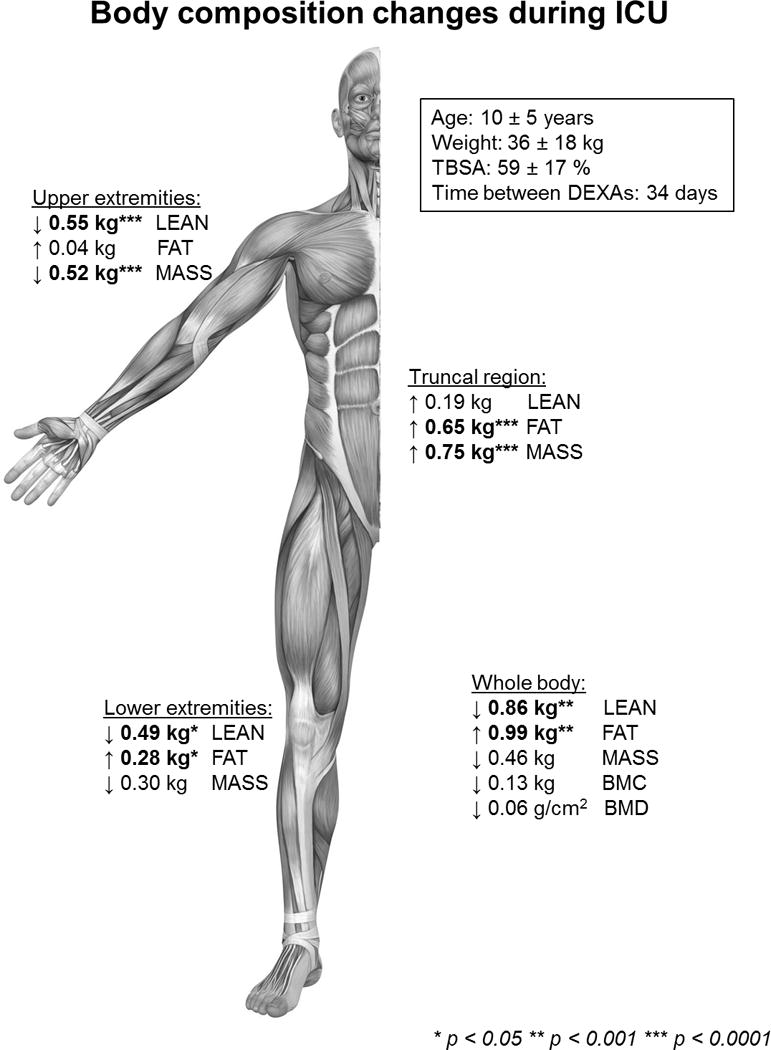
Summary of body composition changes during intensive care unit hospitalization. BMC, bone mineral content; BMD, bone mineral density; FAT, total fat mass; LEAN, lean body mass; MASS, total mass, TBSA, total body surface area burned.
Resting energy expenditure
Resting Energy Expenditures were calculated within one week of admission and again within 3 days prior to discharge. The average kcal per day given at admission was 1873 ± 606 kcal/d while the percent of predicted kcal/d was 224 ± 338%. At discharge, the average REE 1549 ± 520kcal/day and discharge percent of predicted kcal/day was 201 ± 326 %.
Discussion
This manuscript is the first study focusing on the specific morphology of body composition changes in burned children during their ICU stays. Our data show that total lean mass in the upper and lower extremities decreases significantly during ICU stay and that this reduction is significantly greater in the arms than in the legs. Additionally, they show that a considerable increase in fat mass (23% increase) occurs in the truncal region during the ICU stay, which averaged 34 days.
DEXA technology provides an ideal technique for analyzing total body composition for children because it is quick, simple to administer, minimally irradiative, and cost effective if the institution has already purchased a DEXA machine [18, 19]. Jeschke et al. reported that DEXA scans can give misleading readings of lean body composition because of the inability of the machine to differentiate lean mass from water or edema [20]. Nevertheless, in a recent large prospective study, Branski et al. showed that DEXA machines could differentiate between water and edema [19]. While burn patients suffer from edema, especially during the acute phase of injury, this effect is reduced over time [21]. Admission, early DEXA scans were performed 10 ± 3 days post burn. Although this timing could be considered too early to provide adequate resolution of edema, scans were performed at this time because muscle breakdown is known to begin within onset of immobilization.
The development of physical weakness during ICU stay is well documented in adults, but less so in children [22–24]. Chong et al. showed that 30% of pediatric ICU patients develop weakness in the ICU [25], and older children have been found to be more affected by muscle wasting than younger ones [26]. Given that the average age of the current study population was relatively young, at a mean of 10 years, the results of this study would be expected to be less affected by the effects of a wide age range.
Although no previous literature has demonstrated that total lean mass and total mass are lost a different degree than those lost in the upper and lower extremities, the results of this study showed a greater decrease of average lean mass the upper limbs compared to lower limbs, total lean mass or total mass. Due to the mobilization programs developed in the past decades, good survival results have been obtained. However, these programs concentrate on bringing the patient to ambulation as fast as possible without addressing strength in the upper limbs [27]. Several studies have documented the need for burn exercise regimens to address all body regions [28, 29]. In this patient population, a traditional mobilization programs was used to treat the patients, which consisted of 2 daily sessions of active and passive range of motion exercises and strength training for muscles of the lower limbs to restore ambulation. Perhaps, future exercise rehabilitation programs could improve patient functional outcomes by focusing on upper extremity conditioning to accommodate for the disproportionate amount of muscle lost in the upper extremities found in this study.
Moreover, these data revealed that the overall fat mass increases in the trunk, which confirms previous reports demonstrating that burn patients experience increased central fat accumulation [30, 31]. Different interventional drugs appear to reduce the muscle catabolism after severe burn injury and could prevent further loss of lean body mass [32]. Using an aggressive, early enteral nutrition protocol to obtain positive protein and energy balance in relation to the basal metabolic rate improves nitrogen balance during the acute phase of stress in two thirds of critically ill children [33]. Thus, exercise rehabilitation with nutrition intervention are important factors that may contribute to improving the morphological changes in burn children that require further study.
In this study, there were no statistically significant changes in bone mineral composition and density, consistent with previous studies showing significant changes only at several weeks post injury rather than during the limited time frame examined within the ICU stay [8].
When combined with immobilization, high serum levels of catecholamines, glucocorticoids, and pro-inflammatory cytokines contribute to the breakdown of lean muscle in severely burned children [34]. Of these factors, immobilization is most easily addressed through strength-building physical activity that can start within the first 5 days post injury [35]. Countering the loss of lean mass is extremely important since loss of 40% of lean mass loss has been shown to dramatically increase mortality rate [36]. Moreover, reducing loss of whole-body muscle has been demonstrated to provide faster and more efficient recovery [37–39].
Since early mobilization has been demonstrated to be safe in patients who are mechanically ventilated and/or have a femoral catheter [40, 41], these mobilization regimens should be performed as soon as possible, preferably in combination with exercise programs. Cycle machines that are traditionally used for building strength for ambulation in the lower limbs can also be used for upper limbs, as these are can be used by patients while still in bed. Other studies indicate that daily electrical stimulation of the muscles not only helps preserve muscle strength, but also improves strength [42]. For this reason, neuromuscular electrical stimulation can also be considered when developing ICU exercise programs for children and adults.
The major limitation of this study is the small sample size, which is attributable to the fact that many patients were not able to have a complete DEXA scan within the first 2 weeks after injury or were receiving anabolic stimulants. The small sample size exposes the study to bias, as it is less likely to reflect the larger pediatric burn population with burns greater than 30% TBSA. Additionally, individual daily calorie intake or fluid administration for resuscitation was not adjusted for possibility of confounding the degree of change in DEXA results. The scan results may also be affected by foreign objects as feeding tubes and lines which were necessary for proper patient monitoring and care. However, these objects were constituted of plastic rather than metal, making the likelihood of causing a significant alteration of the DEXA data collection less likely. Although Branski et al. [19] have demonstrate the reliability of DEXA scans in differentiating free fluid/edema from lean body mass, the degree to which patient edema may have affected the readings is unknown. This finding is especially true when taking into account the differences in weight between DEXA and gross body weight. Furthermore, DEXA data gathered at admission, shown in Table 2, in this study revealed average whole-body weight assessed by DEXA to be 7 kg less than the gross body weight, suggesting that weight due to edema was appropriately excluded.
Moreover, losses in the muscle strength of these patients, typically obtained with BIODEX testing, was not obtained because the severity of burn injury immediately after burn prevented such testing. Finally, patients receiving propranolol were excluded, but patients receiving other anti-catabolic agents were excluded. When patients receiving these newer anabolic agents were assessed, there were no differences between the patients receiving these medications and the population included in the study. As these medications become more common in the armamentarium of gold-standard burn care measures, future studies investigating patients receiving these different drug treatments are warranted.
In conclusion, this study shows that burned children experience significant reductions in whole-body and lean-body mass as well as increases in truncal fat mass during ICU stays. Loss of lean mass is proportionally greatest in the upper extremities, whereas gains in fat mass are greatest in the truncal region. Reducing bed-rest by incorporating early exercise programs that focus on all extremities, especially the upper limbs, are supported from this study to minimize loss of mass and shorten rehabilitation in burned children. Studies on the impact of these body composition changes on functional outcomes should be explored in future studies.
Acknowledgments
Source of Funding: This study was supported by the National Institutes of Health (P50GM060338, R01HD049471, R01GM056687, T32GM008256, R01GM112936, 3R01HD049471-12S1) and Shriners Hospitals for Children (SHC 84080, SHC 80100, SHC 71008) as well as a Clinical and Translational Science Award (UL1TR001439). None of the study sponsors had any role in the study design; in the collection, analysis, or interpretation of the data; in the writing of the report; or in the decision to submit the article for publication.
Copyright form disclosure: Drs. Cambiaso-Daniel, Malagaris, Hundeshagen, Voigt, Blears, Mlcak, and Herndon received support for article research from the National Institutes of Health (NIH). Dr. Mlcak’s institution received funding from the NIH (P50GM060338, RO1HD049471, RO1GM056687, T32GM008256, RO1GM112936), Shriners Hospitals for Children (84080, 80100, 71008), and a Clinical and Translational Science Award (UL1TR001439). Dr. Finnerty’s institution received funding from the NIH, Shriners Hospitals for Children, National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR), and the Department of Defense (DoD); and she received support for article research from NIH, Shriners Hospitals for Children, NIDILRR, and the DoD. Dr. Herndon’s institution received funding from NIH (P50GM060338, R01HD049471, R01GM056687, T32GM008256, R01GM112936, UL1TR001439), Shriners of North America (SHC 84080, SHC 80100, SHC 71008), NIDILR, DoD, and Gillson-Longenbaugh Foundation; and he received funding from Elsevier (royalties, ongoing). Dr. Suman’s institution received funding from the NIH, DoD, and Shriners Hospitals for Children; and he received support for article research from the NIH, DoD, NIDILRR, and Shriners Hospitals for Children.
Footnotes
Accepted as poster presentation at conference on Shock, June 3–6, 2017, Fort Lauderdale, FL, USA.
Reprints will not be reordered.
Conflicts of Interest: The authors declare no conflicts of interest.
Dr. Rivas disclosed that he does not have any potential conflicts of interest.
References
- 1.Vet NJ, et al. Optimal sedation in pediatric intensive care patients: a systematic review. Intensive Care Med. 2013;39(9):1524–34. doi: 10.1007/s00134-013-2971-3. [DOI] [PubMed] [Google Scholar]
- 2.Pereira C, et al. Post burn muscle wasting and the effects of treatments. Int J Biochem Cell Biol. 2005;37(10):1948–61. doi: 10.1016/j.biocel.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Schweickert WD, Hall J. ICU-acquired weakness. Chest. 2007;131(5):1541–9. doi: 10.1378/chest.06-2065. [DOI] [PubMed] [Google Scholar]
- 4.Hart DW, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000;232(4):455–65. doi: 10.1097/00000658-200010000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gruther W, et al. Muscle wasting in intensive care patients: ultrasound observation of the M. quadriceps femoris muscle layer. J Rehabil Med. 2008;40(3):185–9. doi: 10.2340/16501977-0139. [DOI] [PubMed] [Google Scholar]
- 6.Chao T, et al. Skeletal Muscle Protein Breakdown Remains Elevated in Pediatric Burn Survivors up to One-Year Post-Injury. Shock. 2015;44(5):397–401. doi: 10.1097/SHK.0000000000000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briassoulis G, Venkataraman S, Thompson A. Cytokines and metabolic patterns in pediatric patients with critical illness. Clin Dev Immunol. 2010;2010:354047. doi: 10.1155/2010/354047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bloomfield SA. Changes in musculoskeletal structure and function with prolonged bed rest. Med Sci Sports Exerc. 1997;29(2):197–206. doi: 10.1097/00005768-199702000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Herndon DN, et al. Muscle protein catabolism after severe burn: effects of IGF-1/IGFBP-3 treatment. Ann Surg. 1999;229(5):713–20. doi: 10.1097/00000658-199905000-00014. discussion 720–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Jonghe B, et al. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med. 2007;35(9):2007–15. doi: 10.1097/01.ccm.0000281450.01881.d8. [DOI] [PubMed] [Google Scholar]
- 11.Kraft R, et al. Burn size and survival probability in paediatric patients in modern burn care: a prospective observational cohort study. Lancet. 2012;379(9820):1013–21. doi: 10.1016/S0140-6736(11)61345-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine S, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358(13):1327–35. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
- 13.Jeschke MG, Herndon DN. Burns in children: standard and new treatments. Lancet. 2014;383(9923):1168–78. doi: 10.1016/S0140-6736(13)61093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazess RB, et al. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990;51(6):1106–12. doi: 10.1093/ajcn/51.6.1106. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez NA, et al. Nutrition in burns: Galveston contributions. JPEN J Parenter Enteral Nutr. 2011;35(6):704–14. doi: 10.1177/0148607111417446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. 1949. Nutrition. 1990;6(3):213–21. [PubMed] [Google Scholar]
- 17.Harris JB., VS . Biometric Studies of Basal Metabolism in Man. Washington, DC: Carnegie Institute of Washington; 1919. pp. 223–227. (Standard basal metabolism constants for physiologists and clinicians). [Google Scholar]
- 18.Gilsanz V. Bone density in children: a review of the available techniques and indications. Eur J Radiol. 1998;26(2):177–82. doi: 10.1016/s0720-048x(97)00093-4. [DOI] [PubMed] [Google Scholar]
- 19.Branski LK, et al. Measurement of body composition in burned children: is there a gold standard? JPEN J Parenter Enteral Nutr. 2010;34(1):55–63. doi: 10.1177/0148607109336601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeschke MG, et al. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248(3):387–400. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delman K, et al. Resuscitation with lactated Ringer’s solution after hemorrhage: lack of cardiac toxicity. Shock. 1996;5(4):298–303. [PubMed] [Google Scholar]
- 22.Topp R, et al. The effect of bed rest and potential of prehabilitation on patients in the intensive care unit. AACN Clin Issues. 2002;13(2):263–76. doi: 10.1097/00044067-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Adler J, Malone D. Early mobilization in the intensive care unit: a systematic review. Cardiopulm Phys Ther J. 2012;23(1):5–13. [PMC free article] [PubMed] [Google Scholar]
- 24.Wieczorek B, et al. Early mobilization in the pediatric intensive care unit: a systematic review. J Pediatr Intensive Care. 2015;2015:129–170. doi: 10.1055/s-0035-1563386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choong K, et al. Functional recovery following critical illness in children: the “wee-cover” pilot study. Pediatr Crit Care Med. 2015;16(4):310–8. doi: 10.1097/PCC.0000000000000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banwell BL, et al. Muscle weakness in critically ill children. Neurology. 2003;61(12):1779–82. doi: 10.1212/01.wnl.0000098886.90030.67. [DOI] [PubMed] [Google Scholar]
- 27.Clark DE, et al. Effectiveness of an early mobilization protocol in a trauma and burns intensive care unit: a retrospective cohort study. Phys Ther. 2013;93(2):186–96. doi: 10.2522/ptj.20110417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porter C, et al. Whole body and skeletal muscle protein turnover in recovery from burns. Int J Burns Trauma. 2013;3(1):9–17. [PMC free article] [PubMed] [Google Scholar]
- 29.Porter C, et al. The metabolic stress response to burn trauma: current understanding and therapies. Lancet. 2016;388(10052):1417–1426. doi: 10.1016/S0140-6736(16)31469-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herndon DN, et al. Lipolysis in burned patients is stimulated by the beta 2-receptor for catecholamines. Arch Surg. 1994;129(12):1301–4. doi: 10.1001/archsurg.1994.01420360091012. discussion 1304–5. [DOI] [PubMed] [Google Scholar]
- 31.Wolfe RR, et al. Regulation of lipolysis in severely burned children. Ann Surg. 1987;206(2):214–21. doi: 10.1097/00000658-198708000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diaz EC, et al. Effects of pharmacological interventions on muscle protein synthesis and breakdown in recovery from burns. Burns. 2015;41(4):649–57. doi: 10.1016/j.burns.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Briassoulis G, et al. Influence of an aggressive early enteral nutrition protocol on nitrogen balance in critically ill children. J Nutr Biochem. 2002;13(9):560. doi: 10.1016/s0955-2863(02)00200-0. [DOI] [PubMed] [Google Scholar]
- 34.Diaz EC, et al. Predictors of muscle protein synthesis after severe pediatric burns. J Trauma Acute Care Surg. 2015;78(4):816–22. doi: 10.1097/TA.0000000000000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodgson CL, et al. Clinical review: early patient mobilization in the ICU. Crit Care. 2013;17(1):207. doi: 10.1186/cc11820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang DW, DeSanti L, Demling RH. Anticatabolic and anabolic strategies in critical illness: a review of current treatment modalities. Shock. 1998;10(3):155–60. doi: 10.1097/00024382-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Hardee JP, et al. Early rehabilitative exercise training in the recovery from pediatric burn. Med Sci Sports Exerc. 2014;46(9):1710–6. doi: 10.1249/MSS.0000000000000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porter C, et al. The role of exercise in the rehabilitation of patients with severe burns. Exerc Sport Sci Rev. 2015;43(1):34–40. doi: 10.1249/JES.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suman OE, et al. Effects of a 12-wk resistance exercise program on skeletal muscle strength in children with burn injuries. J Appl Physiol (1985) 2001;91(3):1168–75. doi: 10.1152/jappl.2001.91.3.1168. [DOI] [PubMed] [Google Scholar]
- 40.Sricharoenchai T, et al. Safety of physical therapy interventions in critically ill patients: a single-center prospective evaluation of 1110 intensive care unit admissions. J Crit Care. 2014;29(3):395–400. doi: 10.1016/j.jcrc.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Damluji A, et al. Safety and feasibility of femoral catheters during physical rehabilitation in the intensive care unit. J Crit Care. 2013;28(4):535 e9–15. doi: 10.1016/j.jcrc.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Gerovasili V, et al. Electrical muscle stimulation preserves the muscle mass of critically ill patients: a randomized study. Crit Care. 2009;13(5):R161. doi: 10.1186/cc8123. [DOI] [PMC free article] [PubMed] [Google Scholar]


