Abstract
BACKGROUND
The human aorta dilates with advancing age. However, the association between progressive aortic dilation with aging and cardiac remodeling has not been established in studies of community-dwelling adults. We hypothesized that there would be a relationship between aortic size increase over the early adult lifespan with left ventricular (LV) structural remodeling and subclinical LV dysfunction in middle age, even in the absence of overt cardiovascular and valvular disease.
METHODS
Included were Coronary Artery Risk Development in Young Adults (CARDIA) study participants (n=2933) aged 23–35 years with available transthoracic echocardiography measurements during 20-years of follow-up. Multivariable linear regression models assessed sex-specific associations between 20-year change in aortic root diameter with LV structure and function.
RESULTS
Larger aortic root diameter at 20-year follow-up was associated with greater LV mass (2.77g/mm versus 2.18/mm, in men and women respectively, p<0.001). In longitudinal analyses, increase in aortic root diameter over 20-year follow up was associated with a greater 20-year increase in LV mass and LV Mass to LV end diastolic volume ratio in both sexes. In women but not in men, increased aortic root diameter over 20-years was associated with increased left atrial dimension, impaired E/E′ and impaired early diastolic longitudinal and circumferential strain rates assessed by speckle tracking echocardiography.
CONCLUSIONS
Progressive increase in aortic root diameter from early adulthood to middle age was associated with increased LV mass and LV concentric remodeling in both sexes and impaired diastolic function predominantly in women.
INTRODUCTION
Aortic remodeling with advancing age has been described in population and postmortem studies [1–5]. These aging-related changes are thought to be mediated in part, by collagen deposition in the aortic wall, aortic stiffness, progressive thinning and fragmentation of elastin fibers, ultimately resulting in aortic root enlargement [2, 6, 7]. Structural alterations in the aorta are exacerbated by chronic pressure load and exposure to cardiovascular risk factors [1, 5, 8–11]. In the Coronary Artery Risk Development in Young Adults (CARDIA) study, we have previously demonstrated that aortic root size increases from early adulthood to middle age, with a greater increase in men than women, and that key determinants that may accelerate this aging-related increase in aortic size include: obesity, cigarette smoking and blood pressure [12]. Recent studies have shown that larger aortic root diameter (AoD) independently predicts adverse cardiovascular outcomes [13–15]. However, studies evaluating the association between longitudinal aortic size increase and cardiac structural, systolic and diastolic functional indices in later life are notably lacking.
Observational studies have shown a greater incidence of heart failure with preserved ejection fraction (HFpEF) with aging, especially among women [16]. Adverse vascular-ventricular interactions, elevated LV filling pressures with predominant but not isolated diastolic dysfunction may be contributory mechanisms that are theorized to play a role in the pathophysiology of HFpEF [17, 18]. This may suggest differential arterial and ventricular interactions with aging and by gender that may be reflected in associations of change in aortic root size with markers of LV remodeling and subclinical function.
Accordingly, we posited that there would be a relationship between AoD increase over the early adult lifespan with LV structural remodeling and subclinical LV function in middle age. We tested these hypotheses in CARDIA; a community-based cohort study that provides a unique opportunity to explore these relationships over the early adult life course and also assess for race and gender specific effects.
METHODS
Study Design and Participants
The selection criteria and study design of the CARDIA study have been described in detail elsewhere [19]. Briefly, CARDIA is a multi-center bi-racial cohort study that enrolled 5,115 men and women from 4 U.S. Field Centers (Birmingham, Alabama; Oakland, California; Chicago, Illinois; and Minneapolis, Minnesota), aged 18 to 30 years at baseline (1985–1986) with 25 years of follow-up in 7 subsequent visits up until 2010–2011 (year-25 exam). For this study, we evaluated 3,239 CARDIA participants with echocardiograms done 20 years apart, 1990–1991 (year-5, baseline) and 2010–2011 (year-25, follow up). 170 participants were excluded due to suboptimal images for AoD assessment. Participants with clinical cardiovascular disease events (93) and moderate or severe valvular dysfunction (43) were excluded. The final analytic cohort had 2,933 participants (91% of total year 5 -year 25 participants). All study participants gave written informed consent and the institutional review boards of each participating institution approved study.
Echocardiographic assessments
The echocardiography protocols at CARDIA year-5 and year-25 were designed to ensure consistency across exams and followed existing American Society of Echocardiography (ASE) guidelines at each period [20–23]. Briefly, trained sonographers, following standardized protocols across all four-field centers, acquired images. Experienced readers interpreted digitized images using standard software offline image analysis system transmitted to a core-reading laboratory (video tape in year-5 to the University of California, Irvine and digitally in year-25 to the Johns Hopkins University Echocardiography Reading Center in Baltimore, Maryland). AoD was measured from 2D guided parasternal M-mode tracings at the level of the sinuses of Valsalva, using the leading edge-to-leading edge approach in end-diastole. LV and left atrial dimensions were acquired using 2D-guided M-mode obtained from parasternal views [22]. LV mass was derived according to ASE guidelines. LV volumes were estimated from apical views using Simpson’s method. LV ejection fraction (LVEF) was calculated from LV end diastolic and end systolic volumes [24]. Peak early diastolic velocity (E) and peak late diastolic velocity (A) were measured from pulsed Doppler recordings of transmitral flow. Early peak diastolic mitral annular velocity (E′) - derived from tissue Doppler as the average of septal and lateral mitral annular velocities [25].
Speckle Tracking Echocardiography
The speckle tracking echocardiography (STE) protocol and quality control procedures for CARDIA has been previously described [23, 26]. In a retrospective analysis of prospectively collected data, STE for LV myocardial strain and strain rate measures were performed offline with dedicated semi-automated 2-dimensional wall motion tracking software (Toshiba Medical systems). Three cardiac cycles were recorded for apical and parasternal views at an average frame rate of 46.2 frames per second. After manual tracing of LV endocardial border, a mid-wall region of interest was automatically defined and adapted when necessary. Lagrangian strain was derived from the change in regional length relative to the length at end-diastole: Strain = (L (t) – L0)/L0 × 100, in which L (t) is the length at time t and L0 is the length of the segment at the beginning of the QRS segment. Strain rate was defined as the rate of deformation, estimated from the strain temporal derivative. Strain rate parameters were presented as deformation per second (1/s). Global strain values were calculated as the average of segmental peak systolic strain. More negative strain values imply better systolic function whereas more negative diastolic strain rate values denote worse diastolic function.
Covariates
Assessments of risk factor variables in the CARDIA Study have been previously described [22, 27]. Briefly, the use of anti-hypertensive medication and smoking status (current smoker, former smoker and never smoker) were self-reported, assessed using validated questionnaires. A physical activity score (exercise units) was obtained from the CARDIA physical activity history—a modified version of the Minnesota Leisure Time Physical Activity Questionnaire [28]. Three seated blood pressure (BP) measurements were obtained; the mean of the second and third readings was used. Diabetes mellitus was ascertained at each examination based on one or more of a combination of history of medication use (every visit), fasting glucose ≥ 126 mg/dL, 2 hours from a glucose tolerance test (glucose≥200 mg/dL) or HbA1c ≥ 6.5 and the absence of pregnancy. Total cholesterol, high-density lipoprotein cholesterol (HDL-C), and triglycerides were determined using an enzymatic assay by Northwest Lipids Research Laboratory (Seattle, Washington). Low-density lipoprotein cholesterol (LDL-C) was derived by the Friedewald equation [29].
Statistical analysis
Descriptive characteristics of study participants were summarized for men and women at year-5 and year-25. Continuous variables were presented as means and standard deviations (SD) or median and inter quartile range (IQR), using student t-test or Wilcoxon rank-sum (Mann-Whitney) tests, as appropriate. Categorical variables were compared using chi-squared statistics for frequencies and proportions. AoD at the year-25 exam was used for cross-sectional analyses and 20-year change in AoD (ΔAoD); calculated as AoD in year-25 minus AoD in year-5) was used for longitudinal analyses. Multivariable linear regression models were used to assess the cross-sectional and longitudinal associations of AoD and ΔAoD with LV structure and function in both sexes. In cross-sectional analyses with LV structure and function as dependent variables, models were adjusted for age, sex, race, education, smoking status (current, former vs never), systolic blood pressure, diastolic blood pressure, resting heart rate, body mass index, HDL-C, LDL-C, anti-hypertensive medications (any vs. none) and physical activity (continuous). In longitudinal analyses, the relationship between LV structure and function as dependent variables and ΔAoD was assessed with models adjusted for baseline year-5 risk factors, year-5 echocardiography parameters if available, 20-year change in risk factors, chronic risk exposure (anti-hypertensive use; consistent anti-hypertensive use at year-5 and year-25, anti-hypertensive use at year-5 but not year-25 and vice versa compared to never used anti-hypertensive medication, diabetes mellitus at year-5 and new onset diabetes at year-25, smoking (current, former vs never). To assess potential collinearity of selected covariates in regression models, variance inflation factor was utilized. Finally, we examined the interaction of AoD with sex and race in its association with cardiac structural and functional parameters using interaction terms as well as stratified analyses. Statistical significance was set at a two-sided probability of p<0.05. All statistical analyses were performed using STATA (version 13.1, Stata Corp., College Station, Texas, USA).
RESULTS
Population characteristics
Baseline and follow-up population characteristics of the 2933 CARDIA participants are illustrated in Table 1. Fifty-six percent of study population was female and about fifty-five percent Caucasian. The median age at baseline and follow-up was 30 (IQR 27–33) and 51 (IQR 47–53) years, respectively. The prevalence of diabetes increased significantly from baseline to 20-year follow-up examination, together with an increase in mean body mass index and systolic and diastolic blood pressures in both men and women over 20-years of follow-up. Compared to women, men had higher blood pressures at each visit. Mean LV mass increased from baseline to 20-year follow-up in both men and women, although men had a higher mean LV mass compared to women in both examinations. Conversely, LVEF was higher in women compared to men at baseline and decreased significantly in both sexes across the 20-year follow-up period. Mean absolute AoD increased from baseline to 20-year follow-up exam, and was higher in men (29.9 ± 3.6mm versus 33.3 ± 3.8mm, p<0.001) compared to women (26.1 ± 3.1mm versus 28.7 ± 3.4mm, p<0.001) at both baseline and follow-up examination. Similarly, men had larger mean increase in aortic root diameter over 20-years compared to women (3.40 ± 3.9mm versus 2.62 ± 3.4, P<0.001).
Table 1.
Population Characteristics by Sex
| Men (n=1275) | Women (n=1658) | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Characteristics | Year -5 exam (1990–1991) | Year-25 exam (2010–2011) | P Value | Year -5 exam (1990–1991) | Year-25 exam (2010–2011) | P Value |
| Age, years | 30 (27–33) | 51 (47–53) | <0.001 | 31 (27–33) | 51 (47–53) | <0.001 |
| Caucasian, % | 764 (59.9) | 764 (59.9) | N/A | 873 (52.7) | 873 (52.7) | N/A |
| Education, yr | 14.7 ± 2.5 | 15.1 ± 2.7 | <0.001 | 14.6 ± 2.3 | 15.2 ± 2.6 | <0.001 |
| Body mass Index, Kg/m2 | 25.9 ± 4.4 | 29.3 ± 5.8 | <0.001 | 26.0 ± 6.4 | 30.6 ± 8.1 | <0.001 |
| Body Surface Area, m2 | 2.0 ± 0.2 | 2.1 ± 0.2 | <0.001 | 1.7 ± 0.2 | 1.9 ± 0.2 | <0.001 |
| Height, cm | 177.9±6.8 | 177.9±6.9 | 0.91 | 164.5±6.7 | 164.5±6.7 | 0.27 |
| Systolic Bp, mmHg | 111.1 ± 10.7 | 121.8 ± 14.0 | <0.001 | 103.8 ± 10.0 | 117.4 ± 17.0 | <0.001 |
| Diastolic Bp, mmHg | 71.2 ± 9.5 | 75.7 ± 10.5 | <0.001 | 66.6 ± 9.3 | 73.8 ± 11.6 | <0.001 |
| Pulse pressure, mmHg | 39.9 ± 8.6 | 46.1 ± 7.7 | <0.001 | 37.1 ± 7.7 | 43.6 ± 9.4 | <0.001 |
| Resting heart rate, bpm | 65.1 ± 9.2 | 65.6 ± 10.8 | 0.09 | 69.8 ± 9.4 | 67.2 ± 10.4 | <0.001 |
| HDL-C, mg/dL | 49.1 ± 13.2 | 51.1 ± 15.2 | <0.001 | 57.4 ± 13.4 | 63.7 ± 18.3 | <0.001 |
| LDL-C, mg/dL | 112.6 ± 32.8 | 113.7 ± 33.3 | <0.001 | 105.2 ± 28.9 | 111.8 ± 32.1 | <0.001 |
| Current cigarette smoker, % | 334 (26.2) | 216 (16.9) | <0.001 | 394 (23.8) | 233 (14.1) | <0.001 |
| Diabetes Mellitus, % | 5 (0.4) | 145 (11.4) | <0.001 | 39 (0.3) | 179 (7.9) | <0.001 |
| Anti-hypertensive medication use | 14 (1.1) | 297 (23.3) | <0.001 | 39 (2.4) | 434 (26.2) | <0.001 |
| LV mass, g | 172.2 ± 42.5 | 190.7 ± 50.3 | <0.001 | 128.6 ± 33.7 | 148.2 ±42.1 | <0.001 |
| LV mass index, g/m2.7 | 36.4 ± 8.7 | 40.4 ±38.7 | <0.001 | 33.6 ± 9.0 | 38.7 ± 11.5 | <0.001 |
| LV ejection fraction, % | 63.0 ± 16.8 | 60.6 ± 7.0 | <0.001 | 64.1 ± 6.2 | 62.4 ± 6.7 | <0.001 |
| LMVR, g/ml | 1.32 ± 0.31 | 1.59 ± 0.51 | <0.001 | 1.18 ± 0.25 | 1.55 ± 0.49 | <0.001 |
| 3.64 ± 0.45 | 3.83 ± 0.44 | <0.001 | 3.41 ± 0.44 | 3.58 ± 0.49 | <0.001 | |
| AoD, mm | 29.9 ± 3.6 | 33.3 ± 3.8 | <0.001 | 26.1 ± 3.1 | 28.7 ± 3.4 | <0.001 |
HDL-C indicates high density lipoprotein cholesterol; LDL, low density lipoprotein; LV, left ventricle; AoD, aortic root diameter; LV, left ventricle; LVMVR, left ventricle to end diastolic volume ratio; LAD, left atrial dimension in systole bp, blood pressure; bpm, beats per minute LV ejection fraction was derived from 1370 participants with Simpsons method Continuous variables are presented in means ± SD or median and inter-quartile range. Categorical variables are presented in frequency and proportions.
The results of sex-specific regression analyses adjusted for temporal changes in cardiovascular risk factors and therapies to control hypertension are shown in Table 2. Larger AoD at CARDIA examination year-25 was associated with higher LVM (2.77g/mm versus 2.18 g/mm, in men and women respectively), lower LVEF and higher LV mass to LV end diastolic volume ratio (LVMVR) in both sexes, p<0.05 for all). Larger AoD at CARDIA examination year-25 was also associated with impaired E′, and impaired longitudinal and circumferential early diastolic strain rates by STE. In contrast, larger AoD at examination year-25 was associated with higher LAD (p<0.001) in women only.
Table 2.
Coefficients for Multivariable Linear Regression of LV Structure and Function with AoD and 20-year Change in AoD
|
|
|
||||
|---|---|---|---|---|---|
| AoD at Year-25 | Association of 20-yr Change in AoD | ||||
| Men | Univariable | Multivariable | Univariable | Multivariable | |
| Structural indices | Coefficient (SE) | Coefficient (SE) | Coefficient (SE) | Coefficient (SE) | |
| LVM,g | 3.95 (0.4)*** | 2.77(0.36)*** | 0.88 (0.39)* | 1.68 (0.4)*** | |
| LV MVR, g/ml | 0.02 (0.004)*** | 0.01(0.004)** | 0.01 (0.004)** | 0.02 (0.01)** | |
| LAD, cm | 0.02 (0.003)*** | 0.01(0.003)* | 0.01 (0.003)** | 0.01(0.003) Ϫ | |
| Systolic Functional Indices | |||||
| LVEF, % | −0.17 (0.05)** | −0.15(0.06)** | −0.06 (0.05) | −0.17(0.1) Ϫ | |
| Ell. % | 0.02 (0.02) | −0.01(0.02) | 0.02 (0.02) | 0.0002 (0.02) | |
| Ecc, % | 0.03 (0.02) | 0.04(0.02) | 0.02 (0.02) | 0.02(0.02) | |
| Diastolic Functional Indices | |||||
| E/E′ | 0.02 (0.02) | −0.01(0.02) | 0.03 (0.02) Ϫ | −0.005(0.02) | |
| E′ | −0.1(0.02)*** | −0.06(0.02)*** | −0.02 (0.02) | −0.03(0.02) Ϫ | |
| Ell_SRE, 1/s | −0.01 (0.002)*** | −0.003(0.002) Ϫ | 0.0005 (0.002) | −0.002(0.002) | |
| ECC_SRE, 1/s | −0.01 (0.003)* | −0.006(0.003)* | −0.002 (0.002) | −0.004 (0.003) | |
| Women | |||||
| Structural indices | |||||
| LVM, g | 3.53 (0.3)*** | 2.18(0.27)*** | 0.70 (0.31)* | 1.47 (0.3)*** | |
| LV MVR, g/ml | 0.02 (0.004)*** | 0.01(0.004)*** | 0.01 (0.004)* | 0.02 (0.01)* | |
| LAD, cm | 0.03 (0.003)*** | 0.02(0.003)*** | 0.02 (0.003)*** | 0.02 (0.004)*** | |
| Systolic Functional Indices | |||||
| LVEF, % | −0.16 (0.05)** | −0.17(0.05)** | −0.06 (0.05) | −0.19(0.09) Ϫ | |
| Ell, % | 0.05 (0.02)** | −0.002(0.02) | 0.013 (0.019) | 0.002 (0.02) | |
| ECC, % | 0.04 (0.02)* | 0.01(0.02) | 0.01 (0.02) | 0.01(0.03) | |
| Diastolic functional Indices | |||||
| E/E′ | 0.07 (0.02)*** | 0.017(0.02) | 0.07 (0.02)*** | 0.04(0.02)* | |
| E′ | −0.13 (0.02)*** | −0.07(0.02)*** | −0.07 (0.02)*** | −0.07(0.02)*** | |
| Ell_SRE, 1/s | −0.01 (0.002)*** | −.008(0.002)*** | −0.004 (0.002)* | −0.089(0.002)** | |
| ECC_SRE, 1/s | −0.01 (0.002)*** | −0.009(0.003)** | −0.005 (0.002)* | −0.01 (0.003)** | |
Multivariable models showing coefficients and (standard errors) per 1mm change in AoD or AoD. For AoD Y25 model adjusted for age, sex, race, education, smoking status (current, former vs never), systolic blood pressure, diastolic blood pressure, resting heart rate, HDL-C, LDL-C, anti-hypertensive medications (any vs. none) and physical activity (continuous), and body mass index. For associations with AoD, models were adjusted for Year 5 risk factors, Year-5 echocardiography parameters, chronic risk exposure (anti-hypertensive use; consistent anti-hypertensive use at Year-5 and Year-25, anti-hypertensive use at Year-5 but not Year-25 and vice versa compared to never used anti-hypertensive medication, diabetes mellitus at Year-5 and new-onset diabetes, smoking (current, former vs never) and 20-year change in risk factors. LV indicates Left ventricular; LVM, LVEF, LV ejection fraction (%); LAD, left atrial dimension in systole (ml); E, early diastolic mitral inflow velocity; E′, mean septal and lateral mitral annular velocity; Ell, longitudinal strain; Ecc, circumferential strain; Ell_SRE, early diastolic longitudinal strain rate; ECC_SRE, early diastolic circumferential strain rate; 20-yr change in AoD represents (Year-25 minus Year-5). P<0.1Ϫ P<0.05*, P<0.01**, P<0.001***
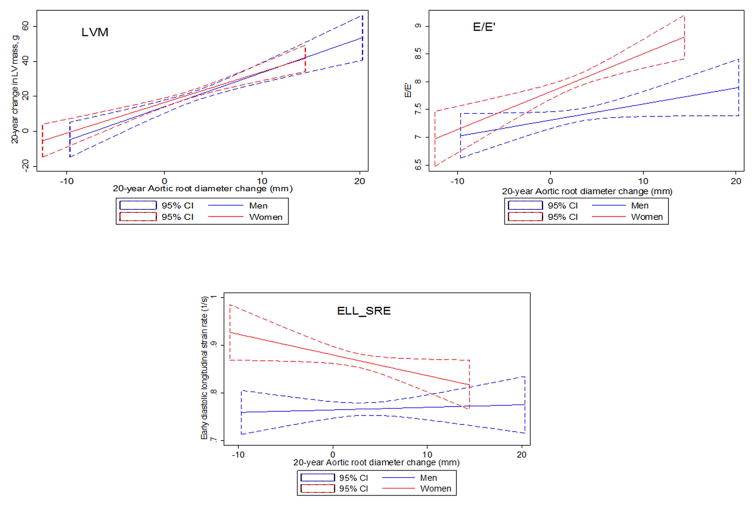
Statistical significance of gender and race interaction terms with regard to 20-year change in AoD in fully adjusted regression models are demonstrated in Table 3. As illustrated in Figure 1 and Table 3, we observed significant interaction between AoD change over 20-years and sex with respect to E/E′ (P=0.04), E′ (p=0.02) and Ell_SRE (p=0.045) but no interaction between AoD change over 20 years and sex with LVM (p=0.53) and LVMVR (p=0.15). There was no significant interaction between AoD increase over 20 years and race for any LV structure or functional parameter. As shown in Table 2, increase in AoD over 20 years was associated with greater increase in LVM (1.68 g/mm versus 1.47g/mm, in men and women respectively, p<0.001) and greater increase in LVMVR over 20-years follow-up in both sexes. We did not observe a consistent significant relationship between increased AoD over 20 years with measures of subclinical LV systolic function assessed by STE in either sex. In women only, AoD and 20-year change in AoD was associated with increased left atrial dimension, impaired E/E′ and impaired longitudinal and circumferential early diastolic strain rates by STE. Further analysis (Appendix Table 4) accounting for childbearing status did not attenuate these associations.
Table 3.
Gender and race interaction terms from fully adjusted multivariable regression of LV structure and function with 20-year change in AoD
|
|
|||||
|---|---|---|---|---|---|
| Association with 20-yr Change in AoD | |||||
|
|
|||||
| Multivariable | Gender interaction | Race interaction | |||
|
|
|
||||
| Structural indices | Coefficient | SE | P value | P value | P value |
| LVM | 1.64 | 0.24 | <0.001 | 0.59 | 0.30 |
| LV MVR | 0.02 | 0.005 | <0.001 | 0.16 | 0.51 |
| LAD | 0.01 | 0.002 | <0.001 | 0.29 | 0.88 |
| Systolic Functional Indices | |||||
| LVEF | −0. 17 | 0.07 | 0.01 | 0.81 | 0.92 |
| Ell | 0.0002 | 0.014 | 0.99 | 0.81 | 0.47 |
| ECC | 0.02 | 0.018 | 0.20 | 0.89 | 0.61 |
| Diastolic functional Indices | |||||
| E/E′ | 0.02 | 0.01 | 0.057 | 0.04 | 0.92 |
| E′ | −0.05 | 0.01 | <0.001 | 0.02 | 0.88 |
| Ell_SRE | −0.005 | 0.002 | 0.003 | 0.045 | 0.97 |
| ECC_SRE | −0.007 | 0.002 | 0.001 | 0.22 | 0.30 |
Multivariable regression models adjusted risk factors as shown in Table 2.
Table highlights p-values for gender and race interaction terms and 20-year change in AoD with respect to its association with LV structure and function
Figure 1.
Plots showing the linear regression fit and 95% confidence intervals for men (blue line) and women (red line) with left ventricular structure and functional indices on the y-axis and 20-year change in AoD on the x-axis.
DISCUSSION
Our study provides data regarding sex-specific associations between AoD change from early adulthood to middle age with cardiac structure and function. We confirm previous studies that have shown positive relationships between larger aortic root size and higher LV mass [10, 30–33]. The present study importantly extends these findings by evaluating the association of aortic size change over the early adult life course with cardiac structure and function in middle age and included assessments by Tissue Doppler and STE. We demonstrate that, in the community, increased AoD from early adulthood to middle age was associated with increased LV mass and LV concentric remodeling in both sexes and impaired diastolic function predominantly in women.
Production of elastin in the media layer of the aortic wall greatly diminishes after childhood [34]. In comparison, aortic lumen diameter increases from early adulthood in response to aging-related cyclical stress, increased body size and chronic risk factor exposure [12, 35]. Consistent with the law of Laplace, aortic luminal dilation may result in increased aortic wall stress, leading to further thinning and fracturing of elastic lamina. This logically implies a vicious cyclical pattern wherein increased aortic size with subsequent higher wall stress and concomitant vascular stiffness, could result in further aortic enlargement and acceleration of the aortic-aging processes. Data using murine animal models convincingly illustrated that aortic stiffening may precede subsequent aortic aneurysmal dilation [36]. Aortic root dilation with concomitant arterial stiffness may result in early return and increased magnitude of reflected pressure waves in late systole, leading to augmented aortic pressures, reduced coronary blood flow, myocardial fibrosis, and increased loading conditions of the ventricle with resultant compensatory LV hypertrophy. Alterations in aortic material properties that occur in mid-to-late adulthood and their relationship with echocardiography determined adverse cardiac remodeling have been primarily described in cross-sectional studies [30–33]. Several prior studies and a meta-analysis have shown associations between larger aortic size and higher LV mass [10, 30–33]. These reported associations might reflect the role of comorbidities that may also contribute to left ventricular (LV) alterations. Our study results are in concordance with these findings and importantly extend them by demonstrating increased AoD over 20-years was associated with greater 20-year increase in LV mass and LV concentric remodeling. Data from the Framingham study showed that aortic root diameter change was independently associated with incident heart failure [14], but this relationship was not statistically significant after accounting for alterations in LV mass. Taking together with our findings, a mediatory role for LV structural remodeling in the pathogenesis of heart failure, in response to increasing aortic size is supported.
Notably, we found that the relationship between increased AoD over 20-years with impaired diastolic function by Doppler and STE was found predominantly in women. HFpEF accounts for about 50% of heart failure events and is more prevalent with aging, particularly among women [17, 37–39]. The pathophysiological mechanisms underlying this sex difference are not completely understood, but may be related to differential patterns of diastolic function and vascular-ventricular coupling with aging. Studies of generally healthy populations have shown that women may have a steeper rise in arterial stiffness and wave reflections compared to men with aging [40]. Beyond sex differences in aortic material properties, there are also differences by sex with respect to myocardial fibrosis and ventricular remodeling in response to arterial loading conditions. In the Multi-Ethnic Study of Atherosclerosis, women had greater fibrosis than men and replacement myocardial fibrosis was related to LV concentric remodeling in women compared to LV dilation in men [41, 42]. Similarly, in senescent hearts, a steeper increase in LV mass was observed in women in contrast to men [43]. In young females, childbearing may represent a transient period of physiological increase in intravascular blood volume that may relate to short-term aortic dilation. In this study, accounting for childbearing status did not attenuate observed associations between aortic size and cardiac structure and function. Furthermore, sex-specific relationships with diastolic function persisted despite the mitigating effects of accounting for temporal changes in body size, blood pressure, valvular dysfunction, and overt cardiovascular disease. Since these sex-specific associations were present despite a relatively smaller increase in aortic size in women compared to men with senescence, it is plausible that these vascular-ventricular interactions are modulated by additional pathophysiological mechanisms. These sex differences may be related to neuro-hormonal, bio-molecular and genetic influences, but further studies are required to elucidate the exact pathobiological mechanisms.
STRENGTHS AND LIMITATIONS
The strengths of this study include a large biracial cohort of men and women, long-term follow up, ability to control for demographic, socioeconomic and other traditional cardiovascular risk factors and inclusion of measures of cardiac performance assessed by STE and Tissue Doppler. To our knowledge, this is the first comprehensive assessment of the relationship between longitudinal aortic size change with LV structure and function in later life in a large community-based population. We acknowledge that aortic root measurement was performed using the leading edge-to-leading edge technique which may include 1–2mm of aortic wall thickness and the use of 2D-guided M-mode may also result in underestimation of aortic root diameters. Echocardiograms were acquired 20 years apart on different equipment, interpreters, and reading centers. However, rigorous training and quality control procedures were followed and the CARDIA echocardiography exams showed good reproducibility profile [12, 22, 23].
CONCLUSIONS
In summary, this study demonstrated associations between aortic root diameter increase from early adulthood to middle age with greater 20-year increase in LV mass and LV concentric remodeling in both sexes, and impaired diastolic function predominantly in women. Our study findings support the hypothesis that aortic root dilation is associated with adverse cardiac remodeling and underscores the potential importance of sex-specific ventricular-arterial interactions in the pathogenesis of heart failure.
Acknowledgments
The Coronary Artery Risk Development in Young Adults Study (CARDIA) is supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN268200900041C from the National Heart, Lung, and Blood Institute (NHLBI), the Intramural Research Program of the National Institute on Aging (NIA), and an intra-agency agreement between NIA and NHLBI (AG0005).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lam CS, Xanthakis V, Sullivan LM, Lieb W, Aragam J, Redfield MM, et al. Aortic root remodeling over the adult life course: longitudinal data from the Framingham Heart Study. Circulation. 2010;122:884–90. doi: 10.1161/CIRCULATIONAHA.110.937839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlatmann TJ, Becker AE. Histologic changes in the normal aging aorta: implications for dissecting aortic aneurysm. Am J Cardiol. 1977;39:13–20. doi: 10.1016/s0002-9149(77)80004-0. [DOI] [PubMed] [Google Scholar]
- 3.Devereux RB, de Simone G, Arnett DK, Best LG, Boerwinkle E, Howard BV, et al. Normal limits in relation to age, body size and gender of two-dimensional echocardiographic aortic root dimensions in persons >/=15 years of age. Am J Cardiol. 2012;110:1189–94. doi: 10.1016/j.amjcard.2012.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–45. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 5.AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, et al. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension. 2013;62:934–41. doi: 10.1161/HYPERTENSIONAHA.113.01445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Virmani R, Avolio AP, Mergner WJ, Robinowitz M, Herderick EE, Cornhill JF, et al. Effect of aging on aortic morphology in populations with high and low prevalence of hypertension and atherosclerosis. Comparison between occidental and Chinese communities. Am J Pathol. 1991;139:1119–29. [PMC free article] [PubMed] [Google Scholar]
- 7.O’Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005;45:652–8. doi: 10.1161/01.HYP.0000153793.84859.b8. [DOI] [PubMed] [Google Scholar]
- 8.Vasan RS, Larson MG, Levy D. Determinants of echocardiographic aortic root size. The Framingham Heart Study. Circulation. 1995;91:734–40. doi: 10.1161/01.cir.91.3.734. [DOI] [PubMed] [Google Scholar]
- 9.Kim M, Roman MJ, Cavallini MC, Schwartz JE, Pickering TG, Devereux RB. Effect of hypertension on aortic root size and prevalence of aortic regurgitation. Hypertension. 1996;28:47–52. doi: 10.1161/01.hyp.28.1.47. [DOI] [PubMed] [Google Scholar]
- 10.Cuspidi C, Meani S, Fusi V, Valerio C, Sala C, Zanchetti A. Prevalence and correlates of aortic root dilatation in patients with essential hypertension: relationship with cardiac and extracardiac target organ damage. J Hypertens. 2006;24:573–80. doi: 10.1097/01.hjh.0000209992.48928.1f. [DOI] [PubMed] [Google Scholar]
- 11.Sawabe M, Hamamatsu A, Chida K, Mieno MN, Ozawa T. Age is a major pathobiological determinant of aortic dilatation: a large autopsy study of community deaths. J Atheroscler Thromb. 2011;18:157–65. doi: 10.5551/jat.6528. [DOI] [PubMed] [Google Scholar]
- 12.Teixido-Tura G, Almeida AL, Choi EY, Gjesdal O, Jacobs DR, Jr, Dietz HC, et al. Determinants of Aortic Root Dilatation and Reference Values Among Young Adults Over a 20-Year Period: Coronary Artery Risk Development in Young Adults Study. Hypertension. 2015;66:23–9. doi: 10.1161/HYPERTENSIONAHA.115.05156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardin JM, Arnold AM, Polak J, Jackson S, Smith V, Gottdiener J. Usefulness of aortic root dimension in persons > or = 65 years of age in predicting heart failure, stroke, cardiovascular mortality, all-cause mortality and acute myocardial infarction (from the Cardiovascular Health Study) Am J Cardiol. 2006;97:270–5. doi: 10.1016/j.amjcard.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 14.Lam CS, Gona P, Larson MG, Aragam J, Lee DS, Mitchell GF, et al. Aortic root remodeling and risk of heart failure in the Framingham Heart study. JACC Heart Fail. 2013;1:79–83. doi: 10.1016/j.jchf.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuspidi C, Facchetti R, Bombelli M, Re A, Cairoa M, Sala C, et al. Aortic root diameter and risk of cardiovascular events in a general population: data from the PAMELA study. J Hypertens. 2014;32:1879–87. doi: 10.1097/HJH.0000000000000264. [DOI] [PubMed] [Google Scholar]
- 16.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 17.Borlaug BA, Kass DA. Ventricular-vascular interaction in heart failure. Heart Fail Clin. 2008;4:23–36. doi: 10.1016/j.hfc.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular-arterial interactions. J Am Coll Cardiol. 2013;61:96–103. doi: 10.1016/j.jacc.2012.08.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–16. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 20.Gardin JM, Wagenknecht LE, Anton-Culver H, Flack J, Gidding S, Kurosaki T, et al. Relationship of cardiovascular risk factors to echocardiographic left ventricular mass in healthy young black and white adult men and women. The CARDIA study. Coronary Artery Risk Development in Young Adults. Circulation. 1995;92:380–7. doi: 10.1161/01.cir.92.3.380. [DOI] [PubMed] [Google Scholar]
- 21.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Gidding SS, Liu K, Colangelo LA, Cook NL, Goff DC, Glasser SP, et al. Longitudinal determinants of left ventricular mass and geometry: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Circ Cardiovasc Imaging. 2013;6:769–75. doi: 10.1161/CIRCIMAGING.112.000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armstrong AC, Ricketts EP, Cox C, Adler P, Arynchyn A, Liu K, et al. Quality Control and Reproducibility in M-Mode, Two-Dimensional, and Speckle Tracking Echocardiography Acquisition and Analysis: The CARDIA Study, Year 25 Examination Experience. Echocardiography. 2015;32:1233–40. doi: 10.1111/echo.12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–93. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 26.Kishi S, Teixido-Tura G, Ning H, Venkatesh BA, Wu C, Almeida A, et al. Cumulative Blood Pressure in Early Adulthood and Cardiac Dysfunction in Middle Age: The CARDIA Study. J Am Coll Cardiol. 2015;65:2679–87. doi: 10.1016/j.jacc.2015.04.042. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong AC, Gidding SS, Colangelo LA, Kishi S, Liu K, Sidney S, et al. Association of early adult modifiable cardiovascular risk factors with left atrial size over a 20-year follow-up period: the CARDIA study. BMJ Open. 2014;4:e004001. doi: 10.1136/bmjopen-2013-004001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sidney S, Jacobs DR, Jr, Haskell WL, Armstrong MA, Dimicco A, Oberman A, et al. Comparison of two methods of assessing physical activity in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 1991;133:1231–45. doi: 10.1093/oxfordjournals.aje.a115835. [DOI] [PubMed] [Google Scholar]
- 29.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 30.Cipolli JA, Souza FA, Ferreira-Sae MC, Pio-Magalhaes JA, Figueiredo ES, Vidotti VG, et al. Sex-specific hemodynamic and non-hemodynamic determinants of aortic root size in hypertensive subjects with left ventricular hypertrophy. Hypertens Res. 2009;32:956–61. doi: 10.1038/hr.2009.134. [DOI] [PubMed] [Google Scholar]
- 31.Cuspidi C, Negri F, Salvetti M, Lonati L, Sala C, Capra A, et al. Aortic root dilatation in hypertensive patients: a multicenter survey in echocardiographic practice. Blood Press. 2011;20:267–73. doi: 10.3109/08037051.2011.565556. [DOI] [PubMed] [Google Scholar]
- 32.Bella JN, Wachtell K, Boman K, Palmieri V, Papademetriou V, Gerdts E, et al. Relation of left ventricular geometry and function to aortic root dilatation in patients with systemic hypertension and left ventricular hypertrophy (the LIFE study) Am J Cardiol. 2002;89:337–41. doi: 10.1016/s0002-9149(01)02238-x. [DOI] [PubMed] [Google Scholar]
- 33.Covella M, Milan A, Totaro S, Cuspidi C, Re A, Rabbia F, et al. Echocardiographic aortic root dilatation in hypertensive patients: a systematic review and meta-analysis. J Hypertens. 2014;32:1928–35. doi: 10.1097/HJH.0000000000000286. discussion 35. [DOI] [PubMed] [Google Scholar]
- 34.Fritze O, Romero B, Schleicher M, Jacob MP, Oh DY, Starcher B, et al. Age-related changes in the elastic tissue of the human aorta. J Vasc Res. 2012;49:77–86. doi: 10.1159/000331278. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell GF. Arterial stiffness: insights from Framingham and Iceland. Curr Opin Nephrol Hypertens. 2015;24:1–7. doi: 10.1097/MNH.0000000000000092. [DOI] [PubMed] [Google Scholar]
- 36.Raaz U, Zollner AM, Schellinger IN, Toh R, Nakagami F, Brandt M, et al. Segmental aortic stiffening contributes to experimental abdominal aortic aneurysm development. Circulation. 2015;131:1783–95. doi: 10.1161/CIRCULATIONAHA.114.012377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ky B, French B, May Khan A, Plappert T, Wang A, Chirinos JA, et al. Ventricular-arterial coupling, remodeling, and prognosis in chronic heart failure. J Am Coll Cardiol. 2013;62:1165–72. doi: 10.1016/j.jacc.2013.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russo C, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MS, et al. Arterial stiffness and wave reflection: sex differences and relationship with left ventricular diastolic function. Hypertension. 2012;60:362–8. doi: 10.1161/HYPERTENSIONAHA.112.191148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mottram PM, Haluska BA, Leano R, Carlier S, Case C, Marwick TH. Relation of arterial stiffness to diastolic dysfunction in hypertensive heart disease. Heart. 2005;91:1551–6. doi: 10.1136/hrt.2004.046805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duprez DA, Jacobs DR, Jr, Lutsey PL, Herrington D, Prime D, Ouyang P, et al. Race/ethnic and sex differences in large and small artery elasticity--results of the multi-ethnic study of atherosclerosis (MESA) Ethn Dis. 2009;19:243–50. [PMC free article] [PubMed] [Google Scholar]
- 41.Ambale Venkatesh B, Volpe GJ, Donekal S, Mewton N, Liu CY, Shea S, et al. Association of longitudinal changes in left ventricular structure and function with myocardial fibrosis: the Multi-Ethnic Study of Atherosclerosis study. Hypertension. 2014;64:508–15. doi: 10.1161/HYPERTENSIONAHA.114.03697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu CY, Liu YC, Wu C, Armstrong A, Volpe GJ, van der Geest RJ, et al. Evaluation of age-related interstitial myocardial fibrosis with cardiac magnetic resonance contrast-enhanced T1 mapping: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2013;62:1280–7. doi: 10.1016/j.jacc.2013.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eng J, McClelland RL, Gomes AS, Hundley WG, Cheng S, Wu CO, et al. Adverse Left Ventricular Remodeling and Age Assessed with Cardiac MR Imaging: The Multi-Ethnic Study of Atherosclerosis. Radiology. 2016;278:714–22. doi: 10.1148/radiol.2015150982. [DOI] [PMC free article] [PubMed] [Google Scholar]