Abstract
Discovery of Piezo channels and the reporting of their sensitivity to the inhibitor GsMTx4 were important milestones in the study of non-selective cationic mechanosensitive channels (MSCs) in normal physiology and pathogenesis. GsMTx4 had been used for years to investigate the functional role of cationic MSCs, especially in muscle tissue, but with little understanding of its target or inhibitory mechanism. The sensitivity of Piezo channels to bilayer stress and its robust mechanosensitivity when expressed in heterologous systems were keys to determining GsMTx4’s mechanism of action. However, questions remain regarding Piezo’s role in muscle function due to the non-selective nature of GsMTx4 inhibition toward membrane mechanoenzymes and the implication of MCS channel types by genetic knockdown. Evidence supporting Piezo like activity, at least in the developmental stages of muscle, is presented. While the MSC targets of GsMTx4 in muscle pathology are unclear, its muscle protective effects are clearly demonstrated in two recent in situ studies on normal cardiomyocytes and dystrophic skeletal muscle. The muscle protective function may be due to the combined effect of GsMTx4’s inhibitory action on cationic MSCs like Piezo and TRP, and its potentiation of repolarizing K+ selective MSCs like K2P and SAKCa. Paradoxically, the potent in vitro action of GsMTx4 on many physiological functions seems to conflict with its lack of in situ side-effects on normal animal physiology. Future investigations into cytoskeletal control of sarcolemma mechanics and the suspected inclusion of MSCs in membrane micro/nano sized domains with distinct mechanical properties will aide our understanding of this dichotomy.
The Historical Conundrum of Mechanosensitive Channels
Mechanosensitive currents were first recorded over 35 years ago in vertebrate outer hair cells, the primary auditory mechanoreceptors [1]. This was followed soon after by recordings of single mechanosensitive ion channels (MSCs) when negative pressure was applied to cell-attached patches from skeletal muscle [2]. Because the patch produces a targeted stress stimulus and high resolution channel recordings, it became the primary assay used in the early characterization of MSC conductance, ion selectivity, pressure sensitivity and identification of inhibitors. Many of these studies were performed on muscle cells due to the inherent mechanical function of these cells [2–7]. In fact, the earliest potential link between MSCs and pathology was the observation that aberrant function of these channels in patches from dystrophic muscle fibers, was a potential pathway for the elevated [Ca2+]i levels in muscular dystrophy [5].
In these early studies, emphasis was placed on single channel recordings showing higher open probability with increasing pipette pressure as evidence of cellular mechanosensitivity. However, patch analysis of the MSC gating properties suffered from poor temporal resolution and stimulus control. This assay was significantly enhanced with the advent of the pressure clamp that provided greater control of the pressure stimulus so that the channel non-stationary gating kinetics became accessible [8–10]. Application of step shaped pressure stimuli to Xenopus oocyte patches revealed a cation selective MSC displaying rapid voltage-dependent inactivation. The transient gating property was identified as inactivation rather than adaptation by monitoring the stimulus strength with patch capacitance [11] and was labile when exposed to excess mechanical stimulation. Cationic MSCs with this rapidly inactivating channel behavior appeared to be ubiquitously expressed in non-specialized mechanosensory cell types [11–13] including muscle cells [14, 15].
For years a discrepancy between the single channel properties of MSCs and their whole-cell current correlates [16] brought into question the potential artifactual nature of MSCs in patches. Also, since channel opening necessities a change in channel shape, gating is an inherently mechanical process and can be modulated by tension in its surroundings. This led to multiple reports of mechano-modulation of voltage- and ligand- gated channels [17, 18]. Thus, criteria were established to identify channels that were primarily gated by mechanical energy and whose functional roles in cells were primarily mechanosensation [19, 20]. As methods of genetic manipulation became more common, abrogation of mechanically activated whole-cell responses from both specialized sensory and non-sensory cell types by genetic knockdown/out of channels led to a growing list of MSCs ([21] and see below). However, for more than two decades the genetic identity of the ubiquitous cationic channel, displaying rapid voltage-sensitive inactivation when expressed in heterologous systems, remained a mystery.
MSC Gating Mechanisms and Molecular Identity
MSCs fall into two general physiological roles depending on their ion selectivity; inhibitory hyperpolarizing (K+ selective) and the excitatory depolarizing (non-selective cationic). They can be further categorized by their mechanosensory mechanisms. The force to open MSCs can be transmitted via ECM/cytoskeletal tethers to the channel or directly through bilayer tension and curvature changes near the channel. Channels gated through their association with the ECM/cytoskeleton are difficult to study in patches due to disruption of these supporting structural proteins. However, the patch remains an important tool for distinguishing channels that are directly mechanogated, versus mechanoactivated by secondary messengers or mechanomodulation of a channel with a different primary sensitivity.
An example of channels gated by the ECM are the tip link channels in outer hair cell cilia of the auditory system. The channel proteins TMC 1 and 2 are part of a multi-subunit structure at the tips of each cilia [22] which is tethered to the side of an adjacent cilia by cadherin like proteins [23]. Sound induced vibrations lead to motion between adjacent cilia and activation of the tethered channels. The family of DEG/ENaC/ASIC channels are MSCs present in both non-specialized and specialized mechanosensory cells [24, 25], and are gated by movement of their extracellular domains [26] that are associated with the ECM [27]. This connection to the ECM is disrupted during patch formation which is likely why these channels do not show mechanosensitivity in patches [25, 28].
Transient receptor potential (TRP) channels encompass a large family of cation selective, environmental sensors for stimuli such as temperature, intracellular pH, lipid and amphipath composition and mechanics (reviewed in [29]). Some TRP channels are activated by multistep mechano-signaling cascades while others appear to be directly mechanically gated via association with tethers or lipid tension/curvature. Recent cryo-EM structure of different TRP channels [30–32] suggests a common mechanically sensitive gating mechanism [33]. Liu and Montell, 2015 [33] suggest a model where membrane stress generated by varying stimulation sources (amphiphiles, temperature, tethered ancillary subunits, etc.) are transmitted to the core gating mechanism through the membrane. The core is composed of a dual gating mechanism where the gates are within the membrane plane at the level of the inner and outer monolayers. The sensitivity of the core to different environmental factors is dependent on surrounding channel structures that are unique to the different TRP channel types.
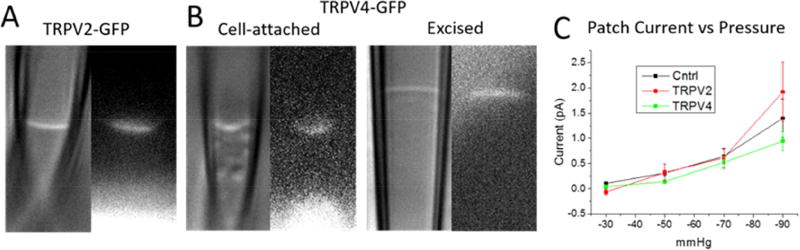
Many TRP channels appear to be directly mechanically gated at the whole-cell level. Though few reports show these channels are mechanically gated in patches or when reconstituted [34, 35], and these results have been disputed [36, 37]. We have expressed TRPV2 and 4 channels as C-terminal GFP chimeras that have been reported to be mechanosensitive in developing myotubes and tested for mechanosensitivity in the patch. All chimeras showed strong fluorescence in the patch dome, though there was no increase in patch currents over endogenous levels (Fig. 1). There was also no inhibition of patch MSCs from expression of miRNAs against these same TRP channels (data not shown), even though two of these channels have been linked to elevated cation influx in dystrophic muscle. This suggests that the TRP channels are highly sensitive to the mechanical environment constructed by cytoskeleton/ECM and membrane composition, all of which can be disrupted during patch formation [37]. This made it unlikely that the ubiquitous rapidly inactivating non-selective cation MSCs in patches belonged to the TRP channel family.
Figure 1.
TRPV 2 and 4 are not activated in the patch dome. Patches were formed from myotubes, and visualized, mechanically stimulated and recorded from as in [38]. Myotubes were transfected with either C-terminally tagged TRPV2 (A) or TRPV4 (B). Fluorescence images next to DIC images show channel proteins present in the patch domes of both cell-attached and excised inside-out patches. Patches were stretched with 500 ms negative pressure steps and the mean integrated current during the pressure steps are shown in (C). Patch currents from myotubes expressing TRPV2 and TRPV4 were not significantly different from control cell patches.
Some MSCs are easily activated in patches suggesting they are activated by lipid tension and do not require contributions from cytoskeleton. The bacterial MSCs, MscL and MscS, were the first reconstituted and cloned that are purely activated by lipid tension [39]. Lipid and amphipath sensitivity, and gating related structural changes determined from the crystal structures and simulations, showed that these channels were activated by membrane thinning [40, 41]. In eukaryotes, the K+ selective 2P domain channels (K2P) were the first MSCs isolated and cloned [42, 43] that could be directly activated by pipette pressure in excised patches [44]. While many factors modulate the mechanosensitive gating of K2P channels, such as pH and lipid composition, these channels are also directly sensitive to lipid tension [45]. Recently the crystal structure for these channels became available and it showed that gating involved the concerted movement of multiple channel elements within, and outside of, the membrane plane [46]. The model suggests that mechanical gating is dependent on extramembranous channel structures on the cytoplasmic side that associate with the local lipid headgroups of the inner leaflet. The association of these elements was sensitive to both pH and lipid composition, especially the phosphorylated state of phosphatidylinositol (PI). Another MSC activated directly by pressure applied to a patch is the stretched-activated Ca2+-activated K+ selective channels (SAKCa) [47]. A splice variant of these channels containing a cytoplasmic element called STREX imparts mechanosensitivity in patches [48]. K2P and SAKCa channels are important regulators of the membrane potential and excitability in cardiac and smooth muscle cells [49, 50].
Piezo – the Missing Link
Finally, in 2010 a ubiquitously expressed family of channels was identified called Piezo, that was cation selective and displayed rapid voltage dependent inactivation in patches and whole-cell mechanical assays [51]. Like K2P channels, Piezo has recently been reconstituted in different lipid based assays devoid of cytoskeletal intervention and shown to be directly gated by lipid tension [52, 53]. However channel gating is definitely modulated by cytoskeleton [53, 54]. These channels have become the focus of many studies into the gating mechanism of cationic MSCs and their physiological role in both specialized and non-specialized mechanosensors (for review see [55]). Two family members were identified, called Piezo 1 and 2, with the most obvious functional difference between them being the more rapid inactivation of Piezo 2 channels. Piezo channel subunits are large proteins of over 2500 amino acids and the channels were first thought to be formed from tetramers [56]. However, the recent cryo-EM structure of the Piezo1 channel clearly shows the channel is a trimer [57]. Expression of Piezo channels in heterologous systems produces channels ranging from ~20–50 pS showing rapid voltage-dependent inactivation (Piezo 1 decay time constant ≈ 50 ms at −80 mV holding potential).
An important aspect of the transient gating property is its labile nature [9]. Channels become non-inactivating with excessive mechanical stimulation likely caused by disruption of cortical cytoskeletal support and/or membrane domain structure [11]. Exogenous expression of Piezo 1 channels in heterologous cell types also show this property [58], and mutations of Piezo1 that lead to disease also disrupt the transient activation so that normal physiological mechanical stimuli lead to elevated cation current [59, 60].
GsMTx4 Mechanism of inhibition and Modulation of Piezo vs K+ selective MSCs
In 2000 a peptide inhibitor of the inactivating non-selective cation MSCs was discovered [61], called Grammostola Mechanotoxin #4 (GsMTx4), and became an important identifier and tool for investigating the physiological role of these channels [62]. The peptide was isolated by screening spider venoms against an endogenously expressed cation selective MSCs from rat astrocytes. GsMTx4 appeared to inhibit multiple types of endogenous cation selective MSCs in whole-cell current or Ca2+ influx assays ([61, 63–66] and see Fig. 3), though the identity of the channel target(s) remains uncertain.
GsMTx4 is an amphipathic peptide carrying a +5 charge primarily due to six lysine residues that surround a relatively large hydrophobic face. These physical properties, along with its highly potent inhibitory effect on Gram positive bacterial growth [67], places it in the broad spectrum of cationic antimicrobial peptides (AMP) [68]. Many AMPs gain their antimicrobial properties through their ability to disrupt bacterial membranes by inducing membrane curvature. Most AMPs do not have this same disruptive effect on eukaryotic cell membranes due to the comparatively low negative charge and lipid heterogeneity of eukaryotic membranes. However, GsMTx4 is unusual compared to other AMPs because it has weak preference between zwitterionic and anionic membranes [69]. The depth of binding in relaxed membranes appears to be critical to the mechanism of inhibition for most spider venom peptides as it enables the interaction with specific channel gating structures. However, GsMTx4 does not bind directly to channel gating elements since it is active as an all D-amino acid peptide. Rather it inhibits MSCs by changing the local membrane properties near the channel [70]. Gating-modifier spider venom peptides that selectively inhibit voltage- and ligand- gated channels have characteristic bilayer binding depths that increase the probability of interactions with the channel structures they target [71].
The discovery of Piezo channels began a new era in GsMTx4 research, because Piezo1 expression in heterologous cell types replicated the gating properties of the ubiquitous endogenous cationic channels in patches and were inhibited by GsMTx4 [72, 73]. This allowed the study of GsMTx4’s effects on the gating kinetics of a defined reproducible channel response. The first question was to explore what made GsMTx4 so much more potent at inhibiting MSCs than the many other similar peptides from animal venoms.
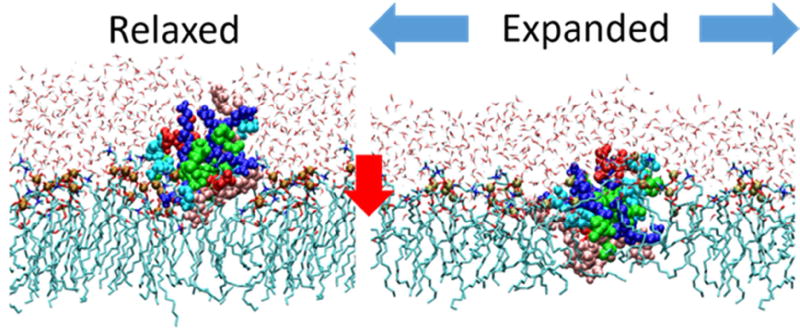
The distribution of positive charge is a critical parameter in determining the interaction properties of AMPs. To better understand GsMTx4’s effects on membranes we synthesized six lysine-glutamate peptide analogs and tested them against Piezo1 channels in patches [74]. We found several analogs with compromised inhibitory activity. Interestingly, compromised analogs did not inhibit any slower or washout any faster suggesting the kinetics of inhibition were unaffected. Further investigation into the physical interactions of GsMTx4 with membranes showed that the peptide has a superficial association with relaxed membranes. However, a distinguishing feature between the compromised and uncompromised analogs was the ease of expelling them from monolayers. Peptides that were expelled more easily from monolayers during compression in a Langmuir trough had greater inhibitory potential. The greatest expulsion differences between analogs occurred when the monolayer was at a tension, or area/lipid ratio, that resembles a bilayer. This meant that at the “monolayer-bilayer equivalence point” GsMTx4 was in rapid equilibrium between a superficial association (low area occupancy) and more deeply bound states that occupy greater area. We predicted that this creates a reservoir of mobile surface area on one monolayer which would have the effect of clamping the tension on that monolayer which was further supported by molecular dynamic simulations (Fig. 2 and [75]). A new model of GsMTx4 inhibition is proposed where the critical factors for its inhibitory potential are how deeply it binds to relaxed membranes and its availability to transition to deeper states as tension increases. Other amphipathic peptides that do not inhibit MSCs may bind deeper to relaxed membranes limiting their area reservoir and/or may specifically associate with channel structures restricting rapid depth transitions as tension changes.
Figure 2.
Simulation of GsMTx4 association with lipid monolayer. GsMTx4 peptide associates superficially to relaxed monolayer. Expansion of the monolayer causes the peptide to penetrate deeper increasing its area contribution to the monolayer surface. Dynamic changes in peptide depth acts as an area reservoir buffering tension in the exposed monolayer disrupting tension transmission to membrane embedded proteins.
MSCs can be sensitive to tension induced bilayer thickness changes as in the case of the prokaryotic MscL, or to differential tension between monolayers caused by changes in curvature. Due to viscous coupling between monolayers, GsMTx4 relief of tension in one monolayer during stretch or curvature changes should transfer stress to the opposite monolayer. Mechanosensitive channels which are sensitive to tension changes in the inner monolayer or positive curvature (bending toward cytoplasm) would experience increased tension in the presence of GsMTx4 in the outer monolayer. TREK and TRAAK channels are the only mechanosensitive channels in the K2P family. They are activated by trinitrophenol (negative curvature generator, partitions to the outer monolayer) and inhibited by chlorpromazine (positive curvature generator, partitions to inner monolayer) [44, 76]. They are also more strongly activated by negative pressure vs positive pressure in inside-out excised patches. These findings originally led to the supposition that TREK and TRAAK were activated by outer monolayer tension [44]. However, deletion mutants identified the cytoplasmically located C-term tail and its association with the inner monolayer as critical for mechanogating [77–79]. And the recent crystal structure of TRAAK [46] supports a model where inner monolayer tension gates K2P channels. Our tests with GsMTx4 also support this model, as GsMTx4 potentiates TREK1 MSC currents when applied to outside-out patches suggesting peptide relief of outer monolayer tension increases tension on the inner monolayer [74]. This may be due to a combination of GsMTx4 inducing negative curvature in the resting patches and/or acting as an outer monolayer tension clamp when pressure is applied.
The recent cryo-EM structure of Piezo channels shows large extracellular domains that move closer to the outer monolayer in the open channel state [57]. The importance of the paddle domain to gating is evidenced by disruption of channel mechanogating by insertion of GFP into different positions within this domain [53]. The “paddle” domain may associate with the outer mono layer during gating suggesting the possibility of a structural correlate in cationic MSCs to the K2P C-terminal cytoplasmic domain. With extramembranous gating elements on opposite monolayers, it is tempting to hypothesize that excitatory MSCs are gated by outer monolayer tension changes, and inhibitory MSCs are gated by tension in the inner monolayer.
Differential tension between the inner and outer monolayers caused by membrane curvature, can be produced by wedge shaped amphipaths in one monolayer through hydrolysis of either the lipid acyl chains or the headgroups [80]. Curvature can also be generated by insertion of proteins like caveolin into one monolayer or surface association of intrinsically curved proteins that belong to the Bin, Amphiphysin and Rvs families [81]. K2P channel gating is modulated by lipid composition [44, 82], and reconstituted Piezo channels in lipid vesicles were activated by LPC (positive curvature generator) addition to the cytoplasmic monolayer [52]. However, sensitivities to lipid composition can be difficult to interpret since many channels contain pockets for lipid binding that can affect gating in ways other than their effect on lipid curvature [77, 83]. A useful comparison would be to test Piezo function in the presence of non-lipid amphipaths like chlorpromazine and trinitrophenol that generate the opposite curvature in eukaryotic membranes and would be unlikely to specifically interact with the channel.
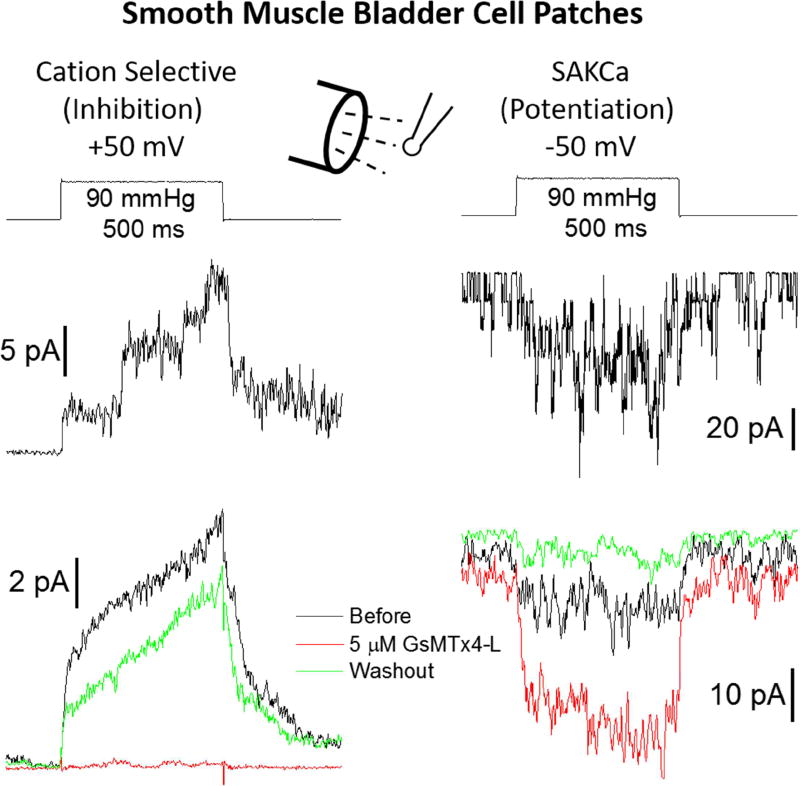
Modulation of a cell’s mechanosensitivity by changing the expression levels of excitatory and inhibitory MSCs was demonstrated by the significant reduction in mechanically activated total current elicited from N2A neuroblastoma cells (high Piezo endogenous expression) after exogenous expression of TRAAK channels [45]. Another means of modulating mechanosensitivity would exploit the different responses of cation selective and K+ selective MSCs to a single amphipath (i.e. inhibition of cation selective Piezo channels vs potentiation of K+ selective K2P channels). To determine if this may be a general mechanism for mechanosensitive modulation we tested GsMTx4 on stretch- and Ca2+- activated large conductance K+ selective channels (SAKCa) in cells known for high expression of these channels [48, 84]. We observed both cation selective and large conductance K+ selective MSCs in patches from smooth muscle bladder cells (Fig. 3) that were simultaneously inhibited (cation selective) and potentiated (K+ selective) by GsMTx4. Similar potentiation of SAKCa channels was observed in chick heart cells.
Figure 3.
Endogenous SAKCa channel recordings in human smooth muscle bladder cells and show mechanosensitivity and potentiation by GsMTx4, while endogenous cation selective MSCs are inhibited. Both SAKCa and non-selective cationic channels are commonly expressed in these cells having a conductance of 200–300 pS. The SAKCa channels are active in resting outside-out (O-O) patches. Electrophysiological recordings were performed as in [38]. The pipette was filled with 140 mM KCl while the bath saline contained 140 mM NaCl and 5 mM KCl. The magnitude and length of the pressure steps are shown above the current traces. Recordings are from a single patch with non-selective cationic channels prevalent at +50 mV (K+ reversal potential) and primarily SAKCa channel currents observed at −50 mV. Representative single channel records are shown in top panels. The mean currents from 10–15 pressure steps are shown before, during GsMTx4 application and after washout. The mean currents show the cationic MSC are inhibited by GsMTx4, but the SAKCa currents are potentiated.
Does Piezo Contribute to Endogenous Cationic MS Currents in Normal Muscle physiology and Pathology?
While Piezo 1 and 2 were shown to have low expression levels in adult mouse skeletal and cardiac muscle [51], we have observed the Piezo1 sequence is present in a cDNA library constructed from C2C12 mouse myoblast mRNA (Dr. Philip Gottlieb, person communication). In differentiated myofibers, Piezo channels may have low expression levels on the sarcolemma unless triggered to translocate from cytoplasmic pools during pathogenesis. Precedence for the translocation of channels to the surface during pathogenesis has been seen for TRPV2 [85, 86]. TRP channels undoubtedly play an important mechanosensory role in muscle [87], though their mechanosensitivity in patches is weak due to reliance on other structural or auxiliary components. This suggests that other channels on the sarcolemma produce the commonly observed MSC currents in patches from muscle cells.
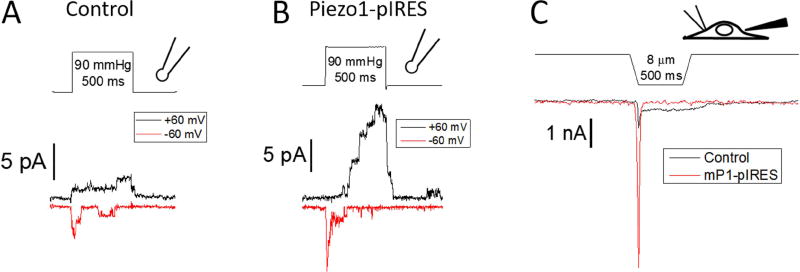
The most commonly observed cation selective MSCs in patches from mouse skeletal differentiated muscle fibers and developing myotubes show a conductance between 25–50 pS [14, 88, 89]. The 25–50 pS channels show weak inward rectification similar to the conductance reported for exogenously expressed Piezo channels [51, 72]. Voltage dependent, rapid inactivation is a hallmark of Piezo channels and is observed in ~50% of excised outside-out (O-O) patches from myotubes (Fig. 4A and see [14]), suggesting a channel with Piezo like properties is present at least during differentiation. These inactivating currents were larger in myotubes expressing Piezo1-pIRES (Fig. 4B) and showed activation latency at positive voltages as has been observed for Piezo1 mutants expressed in HEK cells [59]. In whole-cell recordings of mechanically activated currents by probe indentation, the endogenous current appears to be composed of two components – a rapid transient current and a non-inactivating current (Fig. 4C and see [38]). In Piezo1-pIRES expressing myotubes, a rapid transient current with similar gating kinetics dominates the recording.
Figure 4.
Comparison of endogenous MSCs to exogenous expression of Piezo 1 in myotubes. Pressure stimuli and patch and whole-cell recordings were performed as in [38]. (A) Endogenous currents from O-O patches show rapid, voltage dependent inactivating channels. (B) MSCs with similar gating kinetics were observed in patches from myotubes expressing Piezo1-pIRES, but the currents were typically larger. (F) The difference in current magnitude between whole-cell recordings from endogenous and mouse Piezo1-pIRES expressing myotubes stimulated by probe pressure mirrored the patch results. The control (untransfected) cells show a rapidly inactivating current on top of a non-inactivating current. Mouse Piezo1 expressing cells show a significantly larger transient current with gating kinetics similar to the rapidly inactivating component of endogenous cells.
Another distinguishing characteristic of myotube MSC currents is labile inactivation that appears to be related to disruption of the cortical cytoskeleton and/or membrane domain structure [14, 38]. In cell-attached (C-A) patches from myotubes, >90% of endogenous currents are activated relatively slowly over 500 ms pressure steps and rarely inactivate [14]. Inactivation is lost in dystrophic myotubes missing the cortical cytoskeletal element dystrophin [14], and is diminished when caveolae have been disrupted by Cav3 miRNA or cholesterol depletion with methyl-β-cyclodextran [38]. This suggests a required cytoskeletal or domain like structure possibly involving an auxiliary membrane subunits or specific lipid components. Inactivation of exogenously expressed Piezo inactivation is also shown to be labile [58]. Surprisingly, Piezo inactivation was not lost in patches from cell blebs which have disrupted cortical cytoskeleton [53]. However, blebs are known to rapidly remodel [90] and maintain cytoskeletal assemblies after isolation [91] so that the auxillary structures necessary for inactivation may have reformed in the Piezo study in blebs. Piezo channels are not the only channels with mechanically labile gating properties, however these observations are at least consistent with the presence of a Piezo like MSC.
Channel mutations that lead to disease in specific tissues can be used as indicators of the presence of the channel. Inactivation is an important function of MSCs in normal physiology and abnormal, or modified, kinetics is involved in pathology. Most dystrophies are the result of mutations to cortical cytoskeleton or genes directing the development of the cortex [92] which increase the susceptibility of MSCs to loss of inactivation leading to increased cation influx. Interestingly, the pathogenesis of Xerocytosis [59] and Arthrogryposis [93] are caused by mutations to the Piezo 1 and 2 respectively that increase channel current by changing inactivation properties. Muscular dystrophy is not the primary phenotype related to these particular mutations bringing into question the role of Piezo in muscle. However, it is possible that the specific mutations that cause Piezo channels to loose inactivation in red blood cells (Xerocytosis) and neuronal, pulmonary and connective tissue cells (Arthrogryposis) may be context sensitive. The phenotypic loss of inactivation may be related to cell-type specific cytoskeletal/membrane interactions with Piezo channels. Piezo channels with these specific mutations may function completely normally in muscle cells. Alternatively, some mutations to Piezo channels may be less detrimental to muscle cells with a normally formed cytoskeleton than to other cell types with a less robust cortical cytoskeleton. A host of other reasons such as altered targeting, redundant mechanosensory systems in muscle cells may also limit the effect of these mutations on muscle.
These findings in total are speculative, but they suggest a Piezo like current is present at least during the differentiation stage of myotubes. More definitive experiments are required to prove its expression, such as genetic silencing methods and the use of the Piezo specific activation agent YODA1 [94]. Determining the role of Piezo currents in muscle development and normal physiological stress signaling are important future lines of investigation.
Therapeutic Potential of GsMTx4
Impaired cation/Ca2+ homeostasis due to elevated sarcolemma cation flux, observed in disease states such as Duchene muscular dystrophy and cardiac ischemia [95] and hypertrophy [96], is a hallmark of muscle pathology. Significant contributors to the cation imbalance are the non-selective cationic MSCs among which TRP channel dysregulation has frequently been invoked [86, 97]. GsMTx4 has been used to implicate multiple TRP channels in muscle pathology by inhibiting whole-cell currents attributed to these channels [98]. As stated above the role of Piezo channels in muscle cell mechanosensitivity is still uncertain. However, GsMTx4 does not exclusively inhibit Piezo channels and indeed appears to affect a number of mechanosensitive channels by either inhibition or potentiation. It may also have a direct effect on membrane mechanoenzymes like Nox2 [99] and mechanosensitive GPCR signaling systems [100].
An important aspect to consider is that all disease causes structural changes ranging from organizational changes of elements that comprise subcellular structures to morphological changes in tissues. These structural changes invariably lead to changes in the underlying mechanics. Thus, while MSCs may not be the root cause of most disease states, they are often involved in the pathogenesis. For example, many changes in the cytoarchitecture of cardiomyocytes occur after ischemic injury [101–104] leading to altered sarcolemma mechanics. In Duchene Muscular Dystrophy, mutations causing loss of the cortical organizing protein dystrophin lead to tilting and twisting of the sarcomeric structures affecting the transmission of force at the cell cortex [105].
The D-form of GsMTx4 has now been tested in vivo in mouse models of cardiac ischemic reperfusion injury [106] and muscular dystrophy [107], and shown to be an effective muscle protectant. In a mouse model of ischemic reperfusion injury GsMTx4-D was injected by intravenous administration during the ischemic event and pretreatment subcutaneously for a two day prior to ischemic challenge. Both treatments showed significant reductions in infarct size, arrhythmic electrical activity and increased cardiac output up to two days post ischemia.
To determine if GsMTx4 can act directly on cardiomyocytes we tested it on isolated cardiomyocytes and observed improved contraction induced Ca2+ influx and reduce apoptotic signaling kinases after hypoxic challenge. However, in this same hypoxia test, GsMTx4 inhibited upregulation of the pro-survival Akt kinase which may be due to its cardioprotective effect. This does not negate GsMTx4-D cardioprotective effects and there are compounds currently being tested [108, 109] to specifically upregulate Akt which could be used in combination with GsMTx4 to increase its effectiveness.
While GsMTx4-D can act directly on cardiomyocytes this does not eliminate other mechanism of action. These cardioprotective effects may be due to improved blood flow to ischemic tissue during hypoxia since GsMTx4 causes vascular smooth muscle relaxation by both decreasing mechanically activated excitatory cation currents [110, 111] and down regulation of endothelin-1 stimulated increase in arterial resistance [112, 113]. It may also affect fibroblast conductive properties which are known to play a role in generation of arrhythmias following ischemia [114, 115]. In this regard GsMTx4’s potentiation of K2P and SAKCa channels may also be important aspects of its antiarrhythmic effect.
We also tested GsMTx4-D’s ability to protect skeletal muscle from eccentric contraction injury in D2-mdx mice over 6 weeks [107]. We showed that GsMTx4-D has a tissue half-life of >7 days due to the low susceptibility of D-amino acid peptides to enzymatic digestion, and its distribution to muscle increases with exercise. D2-mdx is a new mouse model of muscular dystrophy with the mdx mutation on a DBA2 mouse background which shows increased fibrosis that more closely mimics the human phenotype [116, 117]. At six weeks, the peptide injected animals had ~20% greater muscle weight than untreated animals and displayed lower susceptibility to muscle damage evidenced by >50% increase in muscle strength following eccentric contraction induced injury. However, there was no decrease in fibrosis or central nucleation. Though, similar to its use in ischemia protection, GsMTx4 could be used in combination with other compounds that are being developed for treatment of fibrosis [118].
MSCs in Normal Physiology versus Pathology
As GsMTx4 has become an important tool for elucidating the role of cationic MSCs in normal physiology and pathologenesis, an interesting dichotomy has arisen. GsMTx4 has revealed that MSCS “apparently” contribute significantly to normal short-term and developmental physiology (examples: arterial pressure regulation [110, 111, 119, 120], skeletal muscle pressor reflex [121], cardiac stretch induced slow force response [122], RBC volume regulation [123, 124], neuronal touch and pain sensation [125–127], mechanotransduction of sound by OHCs [128, 129], serotonin release by gut epithelium [130], renal epithelium luminal pressure detection [131], etc.). Interestingly, when injected over 6 weeks, the GsMTx4 exposure reached concentrations in most tissues that would inhibit MSCs (nervous system is an exception – GsMTx4 does not appear to cross the blood brain barrier [106, 107]), but with no detectable effect on normal physiology [107]. We also showed that IV injection into telemetered free-roaming ferrets had no acute effect on normal cardiac hemodynamic or electrical properties [106]. And subcutaneous injection into mice over 2 weeks that were housed with, or without, running wheels showed a 2–3 fold increased muscle concentration in the animals with running wheels, but no change in running distance [107]. However, GsMTx4 effects on pathology are apparent from both in vitro and in vivo tests.
This might suggest that most MSCs are only weakly activated during normal cell mechanics and do not play the significant role in normal physiology suggested by in vitro assays. Also other redundant mechanosensory signaling pathways may be sufficient to replace the role of MSCs. However, an alternative intriguing explanation for the lack of side-effects seen by chronic GsMTx4 exposure is restricted access of GsMTx4 to mechanical domains containing MSCs in undisturbed membranes. This could be due to various chemical or physical barriers, like caveolae, cytoskeletal corrals and lipid domain composition. GsMTx4, like many other spider venom peptides, acts by binding to the bulk membrane and then diffusing to its target where it likely needs to reach a critical concentration to buffer local tension. Membrane barriers to diffusion are likely disrupted under pathological conditions, and in nonphysiological in vitro assays like patches, and whole-cell currents elicited by swelling, shearing and pressing stimuli on cells adhering to a flat glass surface. This is suggested by the fact that GsMTx4 takes more than 10 times as long to inhibit whole-cell Piezo currents from pressing than O-O patch currents [72]. Membrane barriers might restrict GsMTx4 access to the MSCs, except under pathological conditions which could explain its lack of side-effects under normal physiological stresses.
To further investigate GsMTx4 mechanism of action we have developed an environmentally sensitive NBD (7-nitrobenz-2-oxa-1,3-diazol-4-yl) fluorescent analog of GsMTx4 that increases fluorescence with membrane depth. This analog will be useful in determining the distribution of GsMTx4 on the cell surface and its interaction with different domain structures like caveolae by measuring fluorescence energy transfer. We can also compare the kinetics of fluorescence intensity change with whole-cell current inhibition rates and determine if distribution and depth changes occur prior to channel inhibition which would support peptide access to channels changes with stress.
Conclusions
Piezo channels have filled a significant gap in our knowledge of the molecular identities of MSCs. The lability of its distinctive voltage dependent inactivation property is a critical area of study as its disruption has been tied to multiple disease states. While it has not been shown to be highly expressed in muscle cells, we presented evidence that channels with the hallmark properties of Piezo may contribute a significant component of the MSC current in differentiating myotubes. As aberrant mechanically activated cation fluxes are a significant contributor to pathology in general, and the sarcolemma is particularly sensitive to mechanical deficiencies, it will be important to clarify the role of these channels for skeletal and cardiac muscle disease treatment. Non-selective cationic MSCs like Piezo and TRP channels are likely candidates for the elevated cation flux in muscle pathology and they are both inhibited by GsMTx4-D which has demonstrated therapeutic potential in treatment of muscle disease. In addition, GsMTx4’s action as an outer monolayer area reservoir may help repolarize damaged cells through potentiation of K+ selective MSCs which appear to be sensitive to inner monolayer stress. A major question that remains is why GsMTx4 effects so many critical physiological functions in vitro with so little effect on the normal physiology in vivo, yet has potent therapeutic effects on pathology. Clarification of this dichotomy will likely come from a better understanding of cytoskeletal control of membrane stress and the dynamics of micro/nano scale membrane domains possessing distinct mechanical properties.
Acknowledgments
This work was funded by a DoD grant project No. DM102091 and a NIH grant HL054887 awarded to Dr. Frederick Sachs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests:
GsMTx4 is licensed to Tonus Therapeutics. Thomas Suchyna has part ownership in this company.
Disclaimer:
GsMTx4, in both the L and D forms, have been tested on multiple normal and disease models as discussed in section labeled MSCs in Normal Physiology versus Pathology, and has been shown to affect multiple normal physiological processes in vitro. GsMTx4 is a membrane tension modifier and therefore may target multiple sarcolemma mechanoenzymes. These efficacy tests were short-term treatments and do not address the issues of long-term toxicity. Long term toxicity tests are currently being planned for an investigatory new drug application to the FDA. Currently, non-GLP/GMP GsMTx4-D costs ~$10,000/g. We expect the costs of non-GLP/GMP and GLP/GMP peptides to decrease with larger production scales.
References
- 1.Corey DP, Hudspeth AJ. Ionic basis of the receptor potential in a vertebrate hair cell. Nature. 1979;281(5733):675–677. doi: 10.1038/281675a0. [DOI] [PubMed] [Google Scholar]
- 2.Guharay F, Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. Journal of Physiology (London) 1984;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winegar BD, Haws CM, Lansman JB. Subconductance block of single mechanosensitive ion channels in skeletal muscle fibers by aminoglycoside antibiotics. Journal of General Physiology. 1996;107:433–443. doi: 10.1085/jgp.107.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franco-Obregon A, Jr, Lansman JB. Mechanosensitive ion channels in skeletal muscle from normal and dystrophic mice. Journal of Physiology. 1994;481:299–309. doi: 10.1113/jphysiol.1994.sp020440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franco A, Jr, Lansman JB. Calcium entry through stretch-inactivated ion channels in mdx myotubes. Nature. 1990;344(6267):670–673. doi: 10.1038/344670a0. [DOI] [PubMed] [Google Scholar]
- 6.Franco A, Jr, Winegar BD, Lansman JB. Open channel block by gadolinium ion of the stretch-inactivated ion channel in mdx myotubes. Biophysical Journal. 1991;59:1164–1170. doi: 10.1016/S0006-3495(91)82332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sigurdson WJ, et al. Stretch Activation of a K + Channel in Molluscan Heart Cells. Journal of Experimental Biology. 1987;127:191–209. [Google Scholar]
- 8.McBride DW, Jr, Hamill OP. Pressure-clamp technique for measurement of the relaxation kinetics of mechanosensitive channels. Trends in Neurosciences. 1993;16:341–345. doi: 10.1016/0166-2236(93)90089-5. [DOI] [PubMed] [Google Scholar]
- 9.Hamill OP, McBride DW., Jr Rapid adaptation of single mechanosensitive channels in Xenopus oocytes. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:7462–7466. doi: 10.1073/pnas.89.16.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Besch SR, Suchyna T, Sachs F. High-speed pressure clamp. Pflugers Archiv-European Journal of Physiology. 2002;445(1):161–166. doi: 10.1007/s00424-002-0903-0. [DOI] [PubMed] [Google Scholar]
- 11.Suchyna TM, Besch SR, Sachs F. Dynamic regulation of mechanosensitive channels: capacitance used to monitor patch tension in real time. Phys. Biol. 2004;1(1–2):1–18. doi: 10.1088/1478-3967/1/1/001. [DOI] [PubMed] [Google Scholar]
- 12.Maroto R, Kurosky A, Hamill OP. Mechanosensitive Ca(2+) permeant cation channels in human prostate tumor cells. Channels (Austin) 2012;6(4):290–307. doi: 10.4161/chan.21063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soria B, et al. Single mechanosensitive and Ca2+-sensitive channel currents recorded from mouse and human embryonic stem cells. The Journal of membrane biology. 2013;246(3):215–230. doi: 10.1007/s00232-012-9523-6. [DOI] [PubMed] [Google Scholar]
- 14.Suchyna TM, Sachs F. Mechanosensitive channel properties and membrane mechanics in mouse dystrophic myotubes. J. Physiol. 2007;581(Pt 1):369–387. doi: 10.1113/jphysiol.2006.125021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suchyna T, Sachs F. The membrane/cytoskeleton interface and stretch-activated channels. In: Peter Kohl FS, Franz Michael R, editors. Cardiac Mechano-Electric Coupling and Arrhythmias. Oxford University Press; Oxford: 2011. pp. 57–65. [Google Scholar]
- 16.Morris CE, Horn R. Failure to elicit neuronal macroscopic mechanosensitive currents anticipated by single-channel studies. Science. 1991;251:1246–1249. doi: 10.1126/science.1706535. [DOI] [PubMed] [Google Scholar]
- 17.Gu CX, Juranka PF, Morris CE. Stretch-activation and stretch-inactivation of Shaker-IR, a voltage- gated K+ channel. Biophys J. 2001;80(6):2678–2693. doi: 10.1016/S0006-3495(01)76237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laitko U, Morris CE. Membrane tension accelerates rate-limiting voltage-dependent activation and slow inactivation steps in a Shaker channel. The Journal of general physiology. 2004;123(2):135–154. doi: 10.1085/jgp.200308965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ernstrom GG, Chalfie M. Genetics of sensory mechanotransduction. Annu Rev Genet. 2002;36:411–53. doi: 10.1146/annurev.genet.36.061802.101708. [DOI] [PubMed] [Google Scholar]
- 20.Christensen AP, Corey DP. TRP channels in mechanosensation: direct or indirect activation? Nature Reviews Neuroscience. 2007;8(7):510–521. doi: 10.1038/nrn2149. [DOI] [PubMed] [Google Scholar]
- 21.Árnadóttir J, Chalfie M. Eukaryotic mechanosensitive channels. Annual review of biophysics. 2010;39:111–137. doi: 10.1146/annurev.biophys.37.032807.125836. [DOI] [PubMed] [Google Scholar]
- 22.Kurima K, et al. TMC1 and TMC2 localize at the site of mechanotransduction in mammalian inner ear hair cell stereocilia. Cell reports. 2015;12(10):1606–1617. doi: 10.1016/j.celrep.2015.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maeda R, et al. Tip-link protein protocadherin 15 interacts with transmembrane channel-like proteins TMC1 and TMC2. Proceedings of the National Academy of Sciences. 2014;111(35): 12907–12912. doi: 10.1073/pnas.1402152111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanukoglu I, Hanukoglu A. Epithelial sodium channel (ENaC) family: Phylogeny, structure–function, tissue distribution, and associated inherited diseases. Gene. 2016;579(2):95–132. doi: 10.1016/j.gene.2015.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eastwood AL, Goodman MB. Insight into DEG/ENaC channel gating from genetics and structure. Physiology. 2012;27(5):282–290. doi: 10.1152/physiol.00006.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanukoglu I. ASIC and ENaC type sodium channels: Conformational states and the structures of the ion selectivity filters. The FEBS Journal. 2016 doi: 10.1111/febs.13840. [DOI] [PubMed] [Google Scholar]
- 27.Knoepp F, et al. Mechanical activation of epithelial Na+ channel relies on an interdependent activity of the extracellular matrix and extracellular N-glycans of αENaC. bioRxiv. 2017:102756. [Google Scholar]
- 28.Wemmie JA, Taugher RJ, Kreple CJ. Acid-sensing ion channels in pain and disease. Nature Reviews Neuroscience. 2013;14(7):461–471. doi: 10.1038/nrn3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue R, Jian Z, Kawarabayashi Y. Mechanosensitive TRP channels in cardiovascular pathophysiology. Pharmacol Ther. 2009;123(3):371–85. doi: 10.1016/j.pharmthera.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Liao M, et al. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504(7478):107–112. doi: 10.1038/nature12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paulsen CE, et al. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature. 2015;520(7548):511–517. doi: 10.1038/nature14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grieben M, et al. Structure of the polycystic kidney disease TRP channel Polycystin-2 (PC2) Nature Structural & Molecular Biology. 2016 doi: 10.1038/nsmb.3343. [DOI] [PubMed] [Google Scholar]
- 33.Liu C, Montell C. Forcing open TRP channels: mechanical gating as a unifying activation mechanism. Biochemical and biophysical research communications. 2015;460(1):22–25. doi: 10.1016/j.bbrc.2015.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maroto R, et al. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat. Cell Biol. 2005;7(2):179–185. doi: 10.1038/ncb1218. [DOI] [PubMed] [Google Scholar]
- 35.Yan Z, et al. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature. 2013;493(7431):221–225. doi: 10.1038/nature11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gottlieb P, et al. Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflugers Arch. 2008;455(6):1097–103. doi: 10.1007/s00424-007-0359-3. [DOI] [PubMed] [Google Scholar]
- 37.Suchyna TM, Markin VS, Sachs F. Biophysics and structure of the patch and the gigaseal. Biophys J. 2009;97(3):738–47. doi: 10.1016/j.bpj.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang H, et al. Caveolae regulation of mechanosensitive channel function in myotubes. PLoS One. 2013;8(8):e72894. doi: 10.1371/journal.pone.0072894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sukharev SI, et al. Two types of mechanosensitive channels in the E. Coli envelope: solublization and functional reconstitution. Biophysical Journal. 1993;65:177–183. doi: 10.1016/S0006-3495(93)81044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sukharev S, Anishkin A. Mechanosensitive channels: what can we learn from 'simple' model systems? Trends Neurosci. 2004;27(6):345–51. doi: 10.1016/j.tins.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Martinac B. Mechanosensitive ion channels: molecules of mechanotransduction. J Cell Sci. 2004;117(Pt 12):2449–60. doi: 10.1242/jcs.01232. [DOI] [PubMed] [Google Scholar]
- 42.Lesage F, et al. TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. The EMBO journal. 1996;15(5):1004. [PMC free article] [PubMed] [Google Scholar]
- 43.Fink M, et al. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. The EMBO Journal. 1996;15(24):6854. [PMC free article] [PubMed] [Google Scholar]
- 44.Patel AJ, et al. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO Journal. 1998;17(15):4283–4290. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brohawn SG, Campbell EB, MacKinnon R. Physical mechanism for gating and mechanosensitivity of the human TRAAK K+ channel. Nature. 2014;516(7529):126–130. doi: 10.1038/nature14013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brohawn SG, del Mármol J, MacKinnon R. Crystal structure of the human K2P TRAAK, a lipid-and mechano-sensitive K+ ion channel. Science. 2012;335(6067):436–441. doi: 10.1126/science.1213808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawakubo T, et al. Characterization of a newly found stretch-activated KCa, ATP channel in cultured chick ventricular myocytes. Am. J Physiol. 1999;276(6 Pt 2):H1827–H1838. doi: 10.1152/ajpheart.1999.276.6.H1827. [DOI] [PubMed] [Google Scholar]
- 48.Tang Q, et al. Characterization of a functionally expressed stretch-activated BKca channel cloned from chick ventricular myocytes. The Journal of membrane biology. 2003;196(3):185– 200. doi: 10.1007/s00232-003-0637-8. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi K, Naruse K. Stretch-activated BK channel and heart function. Progress in biophysics and molecular biology. 2012;110(2):239–244. doi: 10.1016/j.pbiomolbio.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Ponte CG, et al. Selective, direct activation of high-conductance, calcium-activated potassium channels causes smooth muscle relaxation. Molecular pharmacology. 2012;81(4):567–577. doi: 10.1124/mol.111.075853. [DOI] [PubMed] [Google Scholar]
- 51.Coste B, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330(6000):55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Syeda R, et al. Piezo1 Channels Are Inherently Mechanosensitive. Cell reports. 2016;17(7):1739–1746. doi: 10.1016/j.celrep.2016.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cox CD, et al. Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nature communications. 2016;7 doi: 10.1038/ncomms10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Retailleau K, et al. Piezo1 in smooth muscle cells is involved in hypertension-dependent arterial remodeling. Cell reports. 2015;13(6):1161–1171. doi: 10.1016/j.celrep.2015.09.072. [DOI] [PubMed] [Google Scholar]
- 55.Gottlieb PA. A tour de force: the discovery, properties, and function of Piezo channels. Current Topics in Membranes and Transport. 2017;79 doi: 10.1016/bs.ctm.2016.11.007. (in press) [DOI] [PubMed] [Google Scholar]
- 56.Coste B, et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483(7388):176–81. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ge J, et al. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature. 2015 doi: 10.1038/nature15247. advance online publication. [DOI] [PubMed] [Google Scholar]
- 58.Gottlieb PA, Bae C, Sachs F. Gating the mechanical channel Piezo1: a comparison between whole-cell and patch recording. Channels (Austin) 2012;6(4):282–9. doi: 10.4161/chan.21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bae C, et al. Xerocytosis is caused by mutations that alter the kinetics of the mechanosensitive channel PIEZO1. Proc Natl Acad Sci U S A. 2013;110(12):E1162–8. doi: 10.1073/pnas.1219777110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bae C, Gottlieb PA, Sachs F. Human PIEZO1: removing inactivation. Biophys J. 2013;105(4):880–6. doi: 10.1016/j.bpj.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suchyna TM, et al. Identification of a peptide toxin from Grammostola spatulata spider venom that blocks stretch activated channels. Journal of General Physiology. 2000;115:583–598. doi: 10.1085/jgp.115.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bowman CL, et al. Mechanosensitive ion channels and the peptide inhibitor GsMTx-4: History, properties, mechanisms and pharmacology. Toxicon. 2006 doi: 10.1016/j.toxicon.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yeung EW, et al. Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J. Physiol. 2005;562(Pt 2):367–380. doi: 10.1113/jphysiol.2004.075275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stiber JA, et al. Mice lacking Homer 1 exhibit a skeletal myopathy characterized by abnormal transient receptor potential channel activity. Mol Cell Biol. 2008;28(8):2637–47. doi: 10.1128/MCB.01601-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Williams IA, Allen DG. Intracellular calcium handling in ventricular myocytes from mdx mice. Am. J. Physiol Heart Circ. Physiol. 2007;292(2):H846–H855. doi: 10.1152/ajpheart.00688.2006. [DOI] [PubMed] [Google Scholar]
- 66.Dyachenko V, et al. Mechanical deformation of ventricular myocytes modulates both TRPC6 and Kir2.3 channels. Cell Calcium. 2009;45(1):38–54. doi: 10.1016/j.ceca.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 67.Jung HJ, et al. Lipid membrane interaction and antimicrobial activity of GsMTx-4, an inhibitor of mechanosensitive channel. Biochem Biophys Res Commun. 2006;340(2):633–8. doi: 10.1016/j.bbrc.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 68.Schmidt NW, Wong GC. Antimicrobial peptides and induced membrane curvature: geometry, coordination chemistry, and molecular engineering. Current Opinion in Solid State and Materials Science. 2013;17(4):151–163. doi: 10.1016/j.cossms.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Posokhov YO, et al. Is lipid bilayer binding a common property of inhibitor cysteine knot ion-channel blockers? Biophys J. 2007;93(4):L20–2. doi: 10.1529/biophysj.107.112375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suchyna TM, et al. Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature. 2004;430(6996):235–240. doi: 10.1038/nature02743. [DOI] [PubMed] [Google Scholar]
- 71.Gupta K, et al. Tarantula toxins use common surfaces for interacting with Kv and ASIC ion channels. eLife. 2015;4:e06774. doi: 10.7554/eLife.06774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bae C, Sachs F, Gottlieb PA. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry. 2011;50(29):6295–300. doi: 10.1021/bi200770q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang F, et al. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. The Journal of Physiology. 2016 doi: 10.1113/JP272718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gnanasambandam R, et al. GsMTx4: Mechanism of Inhibiting Mechanosensitive Ion Channels. Biophysical Journal. 2017;112(1):31–45. doi: 10.1016/j.bpj.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nishizawa K, et al. Effects of Lys to Glu mutations in GsMTx4 on membrane binding, peptide orientation, and self-association propensity, as analyzed by molecular dynamics simulations. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2015 doi: 10.1016/j.bbamem.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maingret F, et al. TRAAK is a mammalian neuronal mechano-gated K + channel. Journal of Biological Chemistry. 1999;274(3):1381–1387. doi: 10.1074/jbc.274.3.1381. [DOI] [PubMed] [Google Scholar]
- 77.Chemin J, et al. A phospholipid sensor controls mechanogating of the K+ channel TREK-1. The EMBO Journal. 2005;24(1):44–53. doi: 10.1038/sj.emboj.7600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim Y, et al. Localization of TREK-2 K+ channel domains that regulate channel kinetics and sensitivity to pressure, fatty acids and pH i. Pflügers Archiv European Journal of Physiology. 2001;442(6):952–960. doi: 10.1007/s004240100626. [DOI] [PubMed] [Google Scholar]
- 79.Kim Y, et al. Synergistic interaction and the role of C-terminus in the activation of TRAAK K+ channels by pressure, free fatty acids and alkali. Pflügers Archiv European Journal of Physiology. 2001;442(1):64–72. doi: 10.1007/s004240000496. [DOI] [PubMed] [Google Scholar]
- 80.Kooijman EE, et al. Modulation of membrane curvature by phosphatidic acid and lysophosphatidic acid. Traffic. 2003;4(3):162–174. doi: 10.1034/j.1600-0854.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- 81.Jarsch IK, Daste F, Gallop JL. Membrane curvature in cell biology: An integration of molecular mechanisms. J Cell Biol. 2016;214(4):375–387. doi: 10.1083/jcb.201604003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chemin J, et al. Mechanisms underlying excitatory effects of group I metabotropic glutamate receptors via inhibition of 2P domain K+ channels. The EMBO Journal. 2003;22(20):5403–5411. doi: 10.1093/emboj/cdg528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hansen SB, Tao X, MacKinnon R. Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2. 2. Nature. 2011;477(7365):495–498. doi: 10.1038/nature10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Petkov GV. Central role of the BK channel in urinary bladder smooth muscle physiology and pathophysiology. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2014;307(6):R571–R584. doi: 10.1152/ajpregu.00142.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lorin C, Vögeli I, Niggli E. Dystrophic cardiomyopathy: role of TRPV2 channels in stretch-induced cell damage. Cardiovascular research. 2015;106(1):153–162. doi: 10.1093/cvr/cvv021. [DOI] [PubMed] [Google Scholar]
- 86.Iwata Y, et al. Blockade of sarcolemmal TRPV2 accumulation inhibits progression of dilated cardiomyopathy. Cardiovascular research. 2013:cvt163. doi: 10.1093/cvr/cvt163. [DOI] [PubMed] [Google Scholar]
- 87.Zanou N, et al. Role of TRPC1 channel in skeletal muscle function. Am J Physiol Cell Physiol. 2010;298(1):C149–62. doi: 10.1152/ajpcell.00241.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Franco-Obregon A, Lansman JB. Changes in mechanosensitive channel gating following mechanical stimulation in skeletal muscle myotubes from the mdx mouse. J. Physiol. 2002;539(Pt 2):391–407. doi: 10.1113/jphysiol.2001.013043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haws CM, Lansman JB. Developmental regulation of mechanosensitive calcium channels in skeletal muscle from normal and mdx mice. Proceedings of the Royal Society of London. Series B: Biological Sciences. 1991;245:173–177. doi: 10.1098/rspb.1991.0105. [DOI] [PubMed] [Google Scholar]
- 90.Charras GT, et al. Life and times of a cellular bleb. Biophys. J. 2008;94(5):1836–1853. doi: 10.1529/biophysj.107.113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Biro M, et al. Cell cortex composition and homeostasis resolved by integrating proteomics and quantitative imaging. Cytoskeleton. 2013;70(11):741–754. doi: 10.1002/cm.21142. [DOI] [PubMed] [Google Scholar]
- 92.Kanagawa M, Toda T. The genetic and molecular basis of muscular dystrophy: roles of cell–matrix linkage in the pathogenesis. Journal of human genetics. 2006;51(11):915–926. doi: 10.1007/s10038-006-0056-7. [DOI] [PubMed] [Google Scholar]
- 93.Coste B, et al. Gain-of-function mutations in the mechanically activated ion channel PIEZO2 cause a subtype of Distal Arthrogryposis. Proceedings of the National Academy of Sciences. 2013;110(12):4667–4672. doi: 10.1073/pnas.1221400110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Syeda R, et al. Chemical activation of the mechanotransduction channel Piezo1. Elife. 2015:e07369. doi: 10.7554/eLife.07369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miyamae M, et al. Attenuation of postischemic reperfusion injury is related to prevention of [Ca2+] m overload in rat hearts. American Journal of Physiology-Heart and Circulatory Physiology. 1996;271(5):H2145–H2153. doi: 10.1152/ajpheart.1996.271.5.H2145. [DOI] [PubMed] [Google Scholar]
- 96.Clemo HF, Stambler BS, Baumgarten CM. Persistent activation of a swelling-activated cation current in ventricular myocytes from dogs with tachycardia-induced congestive heart failure. Circ. Res. 1998;83(2):147–157. doi: 10.1161/01.res.83.2.147. [DOI] [PubMed] [Google Scholar]
- 97.Gailly P. TRP channels in normal and dystrophic skeletal muscle. Current opinion in pharmacology. 2012;12(3):326–334. doi: 10.1016/j.coph.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 98.Friedrich O, et al. Mechano-regulation of the beating heart at the cellular level--mechanosensitive channels in normal and diseased heart. Prog Biophys Mol Biol. 2012;110(2–3):226–38. doi: 10.1016/j.pbiomolbio.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 99.Khairallah RJ, et al. Microtubules underlie dysfunction in duchenne muscular dystrophy. Sci Signal. 2012;5(236):ra56. doi: 10.1126/scisignal.2002829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Storch U, Schnitzler MMy, Gudermann T. G protein-mediated stretch reception. American Journal of Physiology-Heart and Circulatory Physiology. 2012;302(6):H1241–H1249. doi: 10.1152/ajpheart.00818.2011. [DOI] [PubMed] [Google Scholar]
- 101.Piper HM, García-Dorado D. Prime causes of rapid cardiomyocyte death during reperfusion. The Annals of thoracic surgery. 1999;68(5):1913–1919. doi: 10.1016/s0003-4975(99)01025-5. [DOI] [PubMed] [Google Scholar]
- 102.Otani H. Ischemic preconditioning: from molecular mechanisms to therapeutic opportunities. Antioxidants & redox signaling. 2008;10(2):207–248. doi: 10.1089/ars.2007.1679. [DOI] [PubMed] [Google Scholar]
- 103.Sun J, et al. Cardioprotective Role of Caveolae in Ischemia-Reperfusion Injury. Translational Medicine. 2013 doi: 10.4172/2161-1025.1000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Okada H, et al. Integrins protect cardiomyocytes from ischemia/reperfusion injury. The Journal of clinical investigation. 2013;123(10):4294. doi: 10.1172/JCI64216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Friedrich O, et al. Microarchitecture is severely compromised but motor protein function is preserved in dystrophic mdx skeletal muscle. Biophys J. 2010;98(4):606–16. doi: 10.1016/j.bpj.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang J, et al. GsMTx4-D is a cardioprotectant against myocardial infarction during ischemia and reperfusion. Journal of Molecular and Cellular Cardiology. 2016;98:83–94. doi: 10.1016/j.yjmcc.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ward CW, Sachs F, Suchyna TM. Short Term GsMTx4 Treatment Protects Muscle From Eccentric Contration Injury in D2-mdx Model of DMD. Submitted, 2107. [Google Scholar]
- 108.Lotz C, et al. Isoflurane Protects the Myocardium Against Ischemic Injury via the Preservation of Mitochondrial Respiration and Its Supramolecular Organization. Anesthesia & Analgesia. 2015;120(2):265–274. doi: 10.1213/ANE.0000000000000494. [DOI] [PubMed] [Google Scholar]
- 109.Qiao S, et al. MicroRNA-21 Mediates Isoflurane-induced Cardioprotection against Ischemia–Reperfusion Injury via Akt/Nitric Oxide Synthase/Mitochondrial Permeability Transition Pore Pathway. The Journal of the American Society of Anesthesiologists. 2015;123(4):786–798. doi: 10.1097/ALN.0000000000000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Spassova MA, et al. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc. Natl. Acad. Sci. U. S. A. 2006;103(44):16586–16591. doi: 10.1073/pnas.0606894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fanchaouy M, et al. Stretch-elicited calcium responses in the intact mouse thoracic aorta. Cell Calcium. 2007;41(1):41–50. doi: 10.1016/j.ceca.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 112.Johansson S, et al. Cerebrovascular endothelin-1 hyper-reactivity is associated with transient receptor potential canonical channels 1 and 6 activation and delayed cerebral hypoperfusion after forebrain ischaemia in rats. Acta Physiologica. 2015;214(3):376–389. doi: 10.1111/apha.12519. [DOI] [PubMed] [Google Scholar]
- 113.Ostrow LW, Suchyna TM, Sachs F. Stretch induced endothelin-1 secretion by adult rat astrocytes involves calcium influx via stretch-activated ion channels (SACs) Biochem Biophys Res Commun. 2011;410(1):81–6. doi: 10.1016/j.bbrc.2011.05.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Peyronnet R, Nerbonne JM, Kohl P. Cardiac mechano-gated ion channels and arrhythmias. Circulation research. 2016;118(2):311–329. doi: 10.1161/CIRCRESAHA.115.305043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rog-Zielinska EA, et al. The living scar–cardiac fibroblasts and the injured heart. Trends in molecular medicine. 2016;22(2):99–114. doi: 10.1016/j.molmed.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Coley WD, et al. Effect of genetic background on the dystrophic phenotype in mdx mice. Human molecular genetics. 2015:ddv460. doi: 10.1093/hmg/ddv460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Heydemann A, et al. Latent TGF-β–binding protein 4 modifies muscular dystrophy in mice. The Journal of clinical investigation. 2010;120(2):645. doi: 10.1172/JCI39845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Turgeman T, et al. Prevention of muscle fibrosis and improvement in muscle performance in the mdx mouse by halofuginone. Neuromuscular Disorders. 2008;18(11):857–868. doi: 10.1016/j.nmd.2008.06.386. [DOI] [PubMed] [Google Scholar]
- 119.Gilbert G, et al. Stretch-induced Ca2+ signaling in vascular smooth muscle cells depend on Ca2+ store segregation. Cardiovascular research. 2014:cvu069. doi: 10.1093/cvr/cvu069. [DOI] [PubMed] [Google Scholar]
- 120.Gonzales AL, et al. A PLC (gamma) 1-Dependent, Force-Sensitive Signaling Network in the Myogenic Constriction of Cerebral Arteries. Science signaling. 2014;7(327):ra49. doi: 10.1126/scisignal.2004732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Copp SW, et al. The mechano-gated channel inhibitor GsMTx4 reduces the exercise pressor reflex in decerebrate rats. The Journal of physiology. 2016;594(3):641–655. doi: 10.1113/JP271714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ward ML, et al. Stretch-activated channels in the heart: contributions to length-dependence and to cardiomyopathy. Prog Biophys Mol Biol. 2008;97(2–3):232–49. doi: 10.1016/j.pbiomolbio.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 123.Vandorpe DH, et al. Hypoxia activates a Ca2+-permeable cation conductance sensitive to carbon monoxide and to GsMTx-4 in human and mouse sickle erythrocytes. PLoS One. 2010;5(1):e8732. doi: 10.1371/journal.pone.0008732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stewart AK, et al. The GPA-dependent, spherostomatocytosis mutant AE1 E758K induces GPA-independent, endogenous cation transport in amphibian oocytes. Am J Physiol Cell Physiol. 2010;298(2):C283–97. doi: 10.1152/ajpcell.00444.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Woo S-H, et al. Piezo2 is required for Merkel-cell mechanotransduction. Nature. 2014;509(7502):622–626. doi: 10.1038/nature13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Park SP, et al. A tarantula spider toxin, GsMTx4, reduces mechanical and neuropathic pain. Pain. 2008;137(1):208–17. doi: 10.1016/j.pain.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 127.Alessandri-Haber N, et al. TRPC1 and TRPC6 channels cooperate with TRPV4 to mediate mechanical hyperalgesia and nociceptor sensitization. J Neurosci. 2009;29(19):6217–28. doi: 10.1523/JNEUROSCI.0893-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Beurg M, Kim KX, Fettiplace R. Conductance and block of hair-cell mechanotransducer channels in transmembrane channel–like protein mutants. The Journal of general physiology. 2014;144(1):55–69. doi: 10.1085/jgp.201411173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wu Z, et al. Mechanosensory hair cells express two molecularly distinct mechanotransduction channels. Nature Neuroscience. 2017;20(1):24–33. doi: 10.1038/nn.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang F, et al. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. The Journal of physiology. 2017;595(1):79–91. doi: 10.1113/JP272718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Peyronnet R, et al. Piezo1-dependent stretch-activated channels are inhibited by Polycystin-2 in renal tubular epithelial cells. EMBO reports. 2013;14(12):1143–1148. doi: 10.1038/embor.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]