Abstract
Background
Given the current epidemic of human papillomavirus (HPV)-related oropharyngeal cancer (OPC), a screening strategy is urgently needed. The presence of serum antibodies to HPV16 early (E) antigens is associated with an increased risk for OPC. The purpose of this study was to evaluate the diagnostic accuracy of antibodies to a panel of HPV16 E antigens in screening for OPC.
Methods
This case-control study included 378 patients with OPC; 153 patients with non-oropharyngeal head and neck cancer (non-OPC); and 782 healthy control subjects. Tumor HPV status was determined by p16 immunohistochemistry and HPV in situ hybridization. HPV16 E antibody levels in serum were identified by ELISA. A trained binary logistic regression model based on the combination of all E antigens was pre-defined and applied to the dataset. We calculated sensitivity/specificity of the assay to distinguish HPV-related OPC from controls. Logistic regression analysis was used to calculate odds ratios (OR) with 95% confidence intervals (CI) for the association of head and neck cancer with antibody status.
Results
Of the 378 patients with OPC, 348 had p16-positive OPC. HPV16 E antibody levels were significantly higher among patients with p16-positive OPC but not among patients with non-OPC or among controls. Serology showed high sensitivity and specificity for HPV-related OPC (binary classifier: sensitivity, 83% and specificity, 99% for p16-positive OPC).
Conclusions
A trained binary classification algorithm that incorporates information about multiple E antibodies showed high sensitivity and specificity and may be advantageous for risk stratification in future screening trials.
Keywords: Human papillomavirus, oropharyngeal cancer, head and neck neoplasms, serum antibodies, papillomavirus oncogene proteins
Introduction
Human papillomavirus (HPV)-related oropharyngeal cancer (OPC) has now reached epidemic proportions in many areas of the world, and the incidence is increasing substantially every year. As a result, in the United States (U.S.), the incidence of OPC in men is now greater than the incidence of cervical cancer in women and is expected to continue to increase over the next several decades in the U.S. and parts of Europe and Asia.1-6
Primary prevention of OPC through vaccination against HPV is possible; however, population-level effects will take decades to realize. If current incidence trends continue and no changes are made in screening or early detection, rates of HPV-related OPC are not expected to decrease until 2060.2 It is currently not possible to detect precursor lesions for HPV-related OPC or even early-stage HPV-related OPC, and no effective screening paradigm exists. If early-stage disease could be detected, modern transoral surgical techniques would allow for localized treatment of the tonsils or base of tongue to reduce cancer morbidity and mortality, a hallmark of a successful screening program. Additionally, if a group at high risk for HPV-related cancers were confirmed, strategies for HPV-related cancer prevention, such as immune modulation, could be explored.
Serum antibodies to HPV16 early (E) antigens have emerged as promising biomarkers that could aid in identifying individuals at high risk for OPC who could then go on to further screening. Detection of these antibodies has been shown to be both a marker of increased risk for OPC and other HPV-related cancers and a prognostic marker for patients with OPC and is rare among individuals without known cancer.7-13 In a previous study, we showed that compared to seronegative patients, patients seropositive for HPV16 E antibodies had approximately 250 times the risk of HPV-positive OPC.9 In the study reported here, we improved upon our previous work by exploring the diagnostic accuracy of a panel of HPV16 E antibodies for HPV-related OPC in a completely independent and much larger cohort of patients with more robust tumor HPV testing.
Methods
Study design and participants
This case-control study included participants in a prospective molecular epidemiological study of head and neck cancer conducted at The University of Texas MD Anderson Cancer Center who were recruited from February 2003 through January 2013. All patients with newly diagnosed, histologically confirmed, previously untreated squamous cell carcinoma of the head and neck (sites included were oral cavity, oropharynx, hypopharynx, and larynx) who had tumor p16 expression data available were included in the present analysis. Control subjects were healthy visitors to the institution with no previous history of cancer other than non-melanoma skin cancer. Control subjects were frequency matched on age (±5 years) and sex to case subjects. None of the patients or control subjects included here were included in our previous studies of HPV serology.9,10 Written informed consent was obtained from all participants. At the time of recruitment, participants provided demographic and exposure information as well as blood samples for biological testing. For cases, this occurred prior to the initiation of treatment. Blood samples were collected using a standard protocol and stored at -80°C until use. The study was approved by the MD Anderson Institutional Review Board.
Determination of HPV status by p16 immunohistochemistry (IHC) and HPV in situ hybridization (ISH)
Tumor p16 expression was evaluated in paraffin-embedded tumor tissue using IHC performed with the CINtec histology kit (Ventana Medical Systems, Tucson, AZ), per our routine clinical practice. Tumor p16 expression is considered a valid surrogate marker for OPC tumor HPV status and is the marker used to determine HPV status for OPC in the new American Joint Committee on Cancer (AJCC) staging system (8th edition)14,15; therefore, patients with OPC whose tumors exhibited positive p16 expression were considered to have HPV-positive tumors. HPV ISH was performed using the ISH-catalyzed signal-amplification method for biotinylated probes for HPV types 16, 18, 31, 33, and 51 (Enzo, Farmingdale, NY).
HPV DNA cloning and expression
Plasmids containing HPV16 genes16 were expressed as C-terminal glutathione S-transferase (GST)-fusion proteins using human HeLa cell lysate17 (Thermo Scientific, Waltham, MA) per the manufacturer's instructions. The HPV16 E2 gene was expressed as N- and C-terminal fragments for optimal protein expression.7 GST was expressed to serve as a negative control protein. All recombinant DNA research was performed according to the guidelines of the National Institutes of Health under institutional biologic safety review and approval.
Enzyme-linked immunosorbent assay (ELISA)
ELISAs were performed at Arizona State University essentially as described,18 with modifications.19 Protein was expressed from template cDNA and captured onto 96-well plates coated with anti-GST antibodies (GE Healthcare, Piscataway, NJ). Sera were diluted 1:100 and blocked with Escherichia coli lysate and 0.2% milk-PBST. Horseradish peroxidase-labeled anti-human IgG antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) were added at 1:10,000 and detected using Supersignal ELISA Femto chemiluminescent substrate (Thermo Scientific). Luminescence was detected as relative light units (RLU) on a Glomax 96 microplate luminometer (Promega, Madison, WI) at 425 nm. To control for nonspecific and GST-specific antibodies, the ratio of RLU for individual HPV-specific antibodies to RLU for the control GST-antigen was measured. Samples were analyzed simultaneously in duplicate and investigators were blinded with respect to case-control and HPV status of the samples. Quality control measures were previously published.9
Statistical analysis
Since few patients had tumors of the hypopharynx or larynx, patients with such tumors were grouped with patients with oral cavity tumors, and this group is hereafter referred to as patients with non-oropharyngeal cancer (“non-OPC”). Patients with p16-positive and p16-negative OPC were analyzed separately because of the clinical significance of p16 expression in OPC. The analysis was repeated for patients concordant for p16-positive/HPV-ISH-positive OPC. Patients with non-OPC were analyzed together regardless of p16/ISH status as they are considered HPV-unrelated. Cutoff values for positivity were determined using 247 control subjects from a previous study who were not included in the present analysis.9 Cutoff values were defined as three standard deviations from the mean RLU ratio for each individual antigen and were as follows: E1, 2.2; NE2, 2.4; CE2, 2.3; E4, 2.3; E5, 1.7; E6, 2.4; E7, 2.2; L1, 1.9; and L2, 1.9. A sample with an RLU ratio equal to or above the cutoff value was considered positive for that antigen.
A trained binary logistic regression classifier based on the combined RLU ratio of all E antigens9,20 was applied to the dataset. The binary classifier was implemented in MATLAB R2014b. Our classifier used to calculate the probability of being Positive or Negative for HPV-related OPC based on the combination of the different levels of antibodies against these antigens. We applied the maximum likelihood estimation (MLE) to fit the binary logistic regression to a training data set, which results in the optimization of each of the weights in the logistic regression. The decision boundary for a binary logistic regression lies where the prediction probability is 0.5.21 Therefore, we have defined a probability of being Positive for HPV-related OPC at ≥ 0.5. In order to evaluate the predictive power of our classifier, our trained model was applied to an independent data set.20 Subsequently, our trained and validated binary logistic regression model was applied to the dataset presented in this case-control study. A more detailed description of the classifier is included as an appendix (Appendix A).
Test sensitivity, specificity, area under the curve (AUC), Cohen's kappa coefficient, and diagnostic accuracy were calculated to evaluate the ability of the assay to correctly classify p16-positive and p16-positive/HPV ISH-positive OPC. Diagnostic accuracy was calculated as the proportion of subjects correctly classified by the test in the total study population.
Significant differences between groups were determined using chi-square or Fisher's exact tests for categorical variables and Student's t-test or Mann-Whitney test for continuous variables. McNemar's chi-square test was used to determine significant differences in sensitivity and specificity between the classifier and individual antibodies. Odds ratios (OR) with 95% confidence intervals (CI) were calculated using logistic regression. The hierarchical backward elimination approach was used to select variables for inclusion in the multivariable models. Variables that were not significant at p<0.05 by the Wald statistic were removed one by one and a decision to include each variable was based on the likelihood ratio test. None of the interaction terms tested were significant and therefore not included in the analysis. None of the variables included in the models violated the assumption of linearity. The Hosmer-Lemeshow goodness-of-fit test was used to determine overall model fit. Variables included were sex, smoking, and alcohol use for the model of non-OPC vs. controls and smoking and alcohol use for the models of p16-positive OPC and p16-positive/ISH-positive OPC vs. controls. Observations with missing values were dropped from the multivariable analysis.
Statistical significance was set at p<0.05 and all tests were 2-sided. Stata 12.0 (StataCorp LP, College Station, TX), was used for all statistical analyses. MATLAB and Stata script are available upon request.
Results
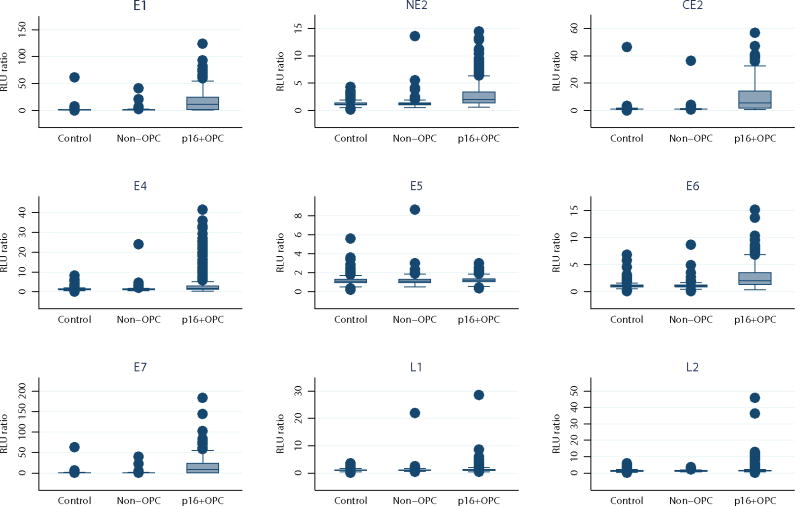
Figure 1 and Supplemental Table 1 show the distribution of antibody levels for 782 control subjects, 153 patients with non-OPC, 348 patients with p16-positive OPC, and 30 patients with p16-negative OPC included in this study. Compared with controls, patients with p16-positive OPC had significantly higher levels of all antibodies measured; there was no significant difference in antibody levels between control subjects and patients with non-OPC. The top two outliers for antibody levels among patients with non-OPC were one patient with an oral cavity tumor and one patient with a larynx tumor, both of whom had HPV-related (p16-positive and HPV ISH-positive) non-OPC, a rare entity. Although median levels of E1, E2, and L2 were significantly higher among patients with p16-negative OPC than among controls, these results should be interpreted with caution given the small sample size (Supplemental Table 1). Only 2% of controls and 5% of patients with non-OPC were E6 and/or E7 antibody-positive, while 74% of patients with p16-positive OPC and 76% of patients with p16-positive/ISH-positive OPC were (Table 1). Using the binary classifier, 1% of controls, 3% of patients with non-OPC, 83% of patients with p16-positive OPC, and 85% of patients with p16-positive/ISH-positive OPC had positive results (Table 1). We did not see a difference in seropositivity for the binary classifier when p16-positive OPC was stratified by stage (AJCC 7th ed. TNM I/II, 85% vs III/IV, 83%; p=1.0) although seropositive patients were more likely to present with T1-T3 tumors and N2c-N3 disease (Chi-square p=0.06 and p=0.05, respectively). Due to the low number of p16-positive OPC cases with stage I/II disease we did not do any further analyses to determine a possible association between seropositivity and stage at diagnosis.
Figure 1.
RLU ratio for each antibody among controls, patients with non-oropharyngeal head and neck cancer (non-OPC), and patients with p16-positive (p16+) oropharyngeal cancer (OPC). None of the non-OPC vs. control RLU ratios were significant at p<0.05 and all of the p16+ OPC vs. control RLU ratios were significant at P<0.001. P values were calculated using the Mann-Whitney test. E: early protein; L: late protein.
Table 1. Demographic and exposure characteristics of cases and controls.
| Characteristic | Controls (N=782) | Non-OPC (N=153) | Pa | p16+ OPC (N=348) | Pa | P16+/HPV ISH+ OPC (N=271)b | Pa |
|---|---|---|---|---|---|---|---|
| Age, mean (SD), years | 55.6 (11.8) | 54.1 (15.2) | 0.253c | 56.7 (8.8) | 0.088c | 56.6 (9.1) | 0.143c |
| Age, median (IQR), years | 55 (49-63) | 54 (44-64) | 0.219 | 56 (51-62) | 0.853 | 56 (50-63) | 0.814 |
| Sex, no. (%) | 0.014 | 0.011 | 0.006 | ||||
| Male | 622 (79.5) | 108 (70.6) | 299 (85.9) | 236 (87.1) | |||
| Female | 160 (20.5) | 45 (29.4) | 49 (14.1) | 35 (12.9) | |||
| Race, no. (%) | 0.118 | 0.202 | 0.252 | ||||
| White | 690 (88.2) | 128 (83.7) | 316 (90.8) | 246 (90.8) | |||
| Other | 92 (11.8) | 25 (16.3) | 32 (9.2) | 25 (9.2) | |||
| Smoking, no. (%) | <0.001 | 0.021 | 0.045 | ||||
| Never | 303 (51.4) | 59 (42.5) | 128 (43.7) | 100 (44.1) | |||
| Former | 217 (36.8) | 36 (25.9) | 113 (38.6) | 87 (38.3) | |||
| Current | 69 (11.7) | 44 (31.7) | 52 (17.8) | 40 (17.6) | |||
| Missing | 193 | 14 | 55 | 44 | |||
| Pack-years of smoking, no. (%) | 0.004 | 0.053 | 0.157 | ||||
| ≤10 | 410 (69.6) | 79 (56.8) | 183 (63.1) | 145 (64.4) | |||
| >10 | 179 (30.4) | 60 (43.2) | 107 (36.9) | 80 (35.6) | |||
| Missing | 193 | 14 | 58 | 46 | |||
| Alcohol use, no. (%) | 0.229 | <0.001 | <0.001 | ||||
| Never | 267 (45.3) | 52 (37.4) | 78 (26.6) | 62 (27.3) | |||
| Former | 105 (17.8) | 27 (19.4) | 79 (27.0) | 57 (25.1) | |||
| Current | 217 (36.8) | 60 (43.2) | 136 (46.4) | 108 (47.6) | |||
| Missing | 193 | 14 | 55 | 44 | |||
| p16/HPV ISH status, no. (%) | |||||||
| p16+/ISH+ | -- | 11 (8.9) | 271 (80.4) | 271 (100.0) | |||
| p16+/ISH- | -- | 22 (17.9) | 66 (19.6) | -- | |||
| p16-/ISH+ | -- | 0 | -- | -- | |||
| p16-/ISH- | -- | 90 (73.2) | -- | -- | |||
| Missing ISH | -- | 30 | 11 | -- | |||
| HPV antibody status, no. (%) | |||||||
| E1+ | 52 (6.7) | 12 (7.8) | 0.593 | 256 (73.6) | <0.001 | 204 (75.3) | <0.001 |
| NE2+ | 14 (1.8) | 5 (3.3) | 0.236 | 128 (36.8) | <0.001 | 106 (39.1) | <0.001 |
| CE2+ | 7 (0.9) | 5 (3.3) | 0.017 | 235 (67.5) | <0.001 | 193 (71.2) | <0.001 |
| E4+ | 14 (1.8) | 8 (5.2) | 0.010 | 110 (31.6) | <0.001 | 95 (35.1) | <0.001 |
| E5+ | 48 (6.1) | 11 (7.2) | 0.625 | 37 (10.6) | 0.008 | 29 (10.7) | 0.013 |
| E6+ | 9 (1.2) | 4 (2.6) | 0.157 | 146 (42.0) | <0.001 | 114 (42.1) | <0.001 |
| E7+ | 11 (1.4) | 5 (3.3) | 0.104 | 224 (64.4) | <0.001 | 177 (65.3) | <0.001 |
| E6+ and/or E7+ | 18 (2.3) | 7 (4.6) | 0.111 | 259 (74.4) | <0.001 | 205 (75.7) | <0.001 |
| L1+ | 11 (1.4) | 4 (2.6) | 0.287d | 27 (7.8) | <0.001 | 24 (8.9) | <0.001 |
| L2+ | 57 (7.3) | 10 (6.5) | 0.741 | 39 (11.2) | 0.029 | 36 (13.3) | <0.001 |
| Binary classifier positivee | 8 (1.0) | 4 (2.6) | 0.110 | 288 (82.8) | <0.001 | 231 (85.2) | <0.001 |
p values are for comparison to controls
A subset of p16+ OPC cases that are also HPV ISH+; note that HPV ISH was unavailable for 11 p16+ OPC
Adjusted for unequal variances
Fisher's exact test
Binary classifier is based on a combination of antibodies based on receiver operating characteristics analysis; ≥0.5 is considered positive
E: early protein; HPV: human papillomavirus; IQR: interquartile range; ISH: in situ hybridization; L: late protein; OPC: oropharyngeal cancer; SD: standard deviation
Demographic and exposure characteristics of the study population are shown in Table 1. Controls and patients with non-OPC had similar low rates of positivity for the tested antibodies; patients with p16-positive OPC had much higher rates of positivity (Table 1). Among the 66 patients with p16-positive, HPV ISH-negative OPC, 16 of the 21 tested (76%) had HPV16 DNA identified in tumor tissue by polymerase chain reaction, while among the 22 patients with p16-positive, HPV ISH-negative non-OPC, only 2 of the 10 tested (20%) had HPV16 DNA identified in tumor tissue (data not shown).
Evaluation of HPV16 E antibodies as a potential diagnostic biomarker for HPV-related OPC
The performance of HPV16 E antibodies as a marker for HPV-related OPC is shown in Table 2. Overall, the antibody assay performed well. The sensitivity of the binary classifier was 83% and the specificity was 99%, resulting in correct classification of 94% of individuals with p16-positive OPC. Compared with individual antibodies, the binary classifier had superior sensitivity (p<0.001 for all) and performed better with respect to specificity for E1, E5, and E6 and/or E7 (p<0.001 for E1 and E5; p=0.012 for E6 and/or E7; data not shown). The classifier performed equally well for p16-positive/ISH-positive OPC (sensitivity, 85%, diagnostic accuracy, 95%).
Table 2.
Sensitivity, specificity, AUC, diagnostic accuracy, and Cohen's kappa coefficient of HPV16 serology to identify HPV-related OPC.
| Sensitivity (95% CI), % | Specificity (95% CI), % | AUC (95% CI),% | Diagnostic accuracy, % | Kappa | |
|---|---|---|---|---|---|
|
|
|||||
| E antibodies by HPV status | |||||
| P16+ OPC vs. controls | |||||
| E1 | 73.6 (68.6-78.1) | 93.4 (91.4-95.0) | 83.5 (81.0-85.9) | 87.3 | 0.69 |
| NE2 | 36.8 (31.7-42.1) | 98.2 (97.0-99.0) | 67.5 (64.9-70.1) | 79.3 | 0.42 |
| CE2 | 67.5 (62.3-72.4) | 99.1 (98.2-99.6) | 83.3 (80.8-85.8) | 89.4 | 0.73 |
| E4 | 31.6 (26.8-36.8) | 98.2 (97.0-99.0) | 64.9 (62.4-67.4) | 77.7 | 0.36 |
| E5 | 10.6 (07.6-14.4) | 93.9 (91.9-95.4) | 52.2 (50.4-54.1) | 68.2 | 0.06 |
| E6 | 42.0 (36.7-47.3) | 98.8 (97.8-99.5) | 70.4 (67.8-73.0) | 81.3 | 0.48 |
| E7 | 64.4 (59.1-69.4) | 98.6 (97.5-99.3) | 81.5 (78.9-84.0) | 88.1 | 0.69 |
| E6 and/orE7 | 74.4 (69.5-78.9) | 97.7 (96.4-98.6) | 86.1 (83.7-88.4) | 91.9 | 0.77 |
| Binary classifierya | 82.8 (78.4-86.6) | 99.0 (98.0-99.6) | 90.9 (88.8-92.9) | 94.0 | 0.85 |
| P16+/HPV ISH+ OPC vs. controls | |||||
| E1 | 75.3 (69.7-80.3) | 93.4 (91.4-95.0) | 84.3 (81.6-87.0) | 88.7 | 0.70 |
| NE2 | 39.1 (33.3-45.2) | 98.2 (97.0-99.0) | 68.7 (65.7-71.6) | 83.0 | 0.46 |
| CE2 | 71.2 (65.4-76.5) | 99.1 (98.2-99.6) | 85.2 (82.4-87.9) | 91.9 | 0.77 |
| E4 | 35.1 (29.4-41.1) | 98.2 (97.0-99.0) | 66.6 (63.7-69.5) | 82.0 | 0.41 |
| E5 | 10.7 (72.8-15.0) | 93.9 (91.9-95.4) | 52.3 (50.3-54.3) | 72.5 | 0.06 |
| E6 | 42.0 (36.1-48.2) | 98.8 (97.8-99.5) | 70.5 (67.5-73.4) | 84.2 | 0.50 |
| E7 | 65.3 (59.3-71.0) | 98.6 (97.5-99.3) | 82.0 (79.1-84.8) | 90.0 | 0.71 |
| E6 and/or E7 | 75.6 (70.1-80.6) | 97.7 (96.4-98.6) | 86.7 (84.1-89.3) | 92.0 | 0.78 |
| Binary classifiera | 85.2 (80.4-89.2) | 99.0 (98.0-99.6) | 92.1 (90.0-94.3) | 95.4 | 0.88 |
Binary classifier is based on a combination of antibodies based on ROC analysis; ≥·5 is considered positive
AUC: area under the curve; CI: confidence interval
Association between HPV16 E antibodies and non-OPC and p16-positive OPC
Presence of antibodies significantly increased the risk for p16-positive OPC but not non-OPC (Table 3). Specifically, the classifier showed the strongest association with HPV-related OPC (OR, 453; 95% CI, 199-1030 for p16-positive OPC and OR, 565; 95% CI, 239-1335 for p16-positive/ISH-positive OPC). In contrast, none of the associations between antibody status and non-OPC were significant.
Table 3.
Association of HPV antibodies with non-OPC, p16-positive OPC, and p16-positive/HPV ISH-positive OPC.
| Non-OPC vs. controls | p16-positive OPC vs. controls | P16+/HPV ISH+ OPC vs. controlsa | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Pos. vs. neg. | Crude OR (95% CI) | Adjusted OR (95% CI)b | Crude OR (95% CI) | Adjusted OR (95% CI) c | Crude OR (95% CI) | Adjusted OR (95% CI) c |
| E1 | 1.2 (0.6-2.3) | 0.9 (0.4-2.0) | 39.1 (27.1-56.5) | 43.2 (27.9-66.7) | 42.7 (28.8-63.4) | 48.6 (30.5-77.4) |
| NE2 | 1.9 (0.7-5.2) | 1.0 (0.2-4.2) | 31.9 (18.0-56.5) | 43.0 (20.4-90.4) | 35.2 (19.7-63.1) | 46.7 (22.0-99.3) |
| CE2 | 3.7 (1.2-11.9) | 2.3 (0.5-10.5) | 230.2 (105.8-500.9) | 289.9 (113.5-740.2) | 273.9 (124.4-603.2) | 354.4 (135.5-926.8) |
| E4 | 3.0 (1.2-7.3) | 2.8 (0.98-8.1) | 25.4 (14.3-45.1) | 26.8 (13.2-54.5) | 29.6 (16.5-53.1) | 31.5 (15.3-64.7) |
| E5 | 1.2 (0.6-2.3) | 0.9 (0.4-2.0) | 1.8 (1.2-2.8) | 2.2 (1.3-3.5) | 1.8 (1.1-3.0) | 2.1 (1.3-3.6) |
| E6 | 2.3 (0.7-7.6) | 1.1 (0.2-6.0) | 62.1 (31.1-123.9) | 79.7 (34.0-186.7) | 62.4 (31.0-125.6) | 81.6 (34.5-193.2) |
| E7 | 2.4 (0.8-6.9) | 1.4 (0.4-5.3) | 126.6 (67.1-238.8) | 106.0 (53.8-208.7) | 132.0 (69.2-251.7) | 111.8 (56.0-223.3) |
| E6 and/or E7 | 2.0 (0.8-5.0) | 1.1 (0.3-3.5) | 123.5 (73.0-209.0) | 113.8 (63.0-205.4) | 131.8 (76.6-227.0) | 124.4 (67.6-228.9) |
| L1 | 1.9 (0.6-6.0) | 1.7 (0.4-7.6) | 5.9 (2.9-12.0) | 9.6 (3.9-23.9) | 6.8 (3.3-14.1) | 10.9 (4.3-27.5) |
| L2 | 0.9 (0.4-1.8) | 0.9 (0.4-2.0) | 1.6 (1.0-2.5) | 2.2 (1.3-3.6) | 1.9 (1.3-3.0) | 2.8 (1.7-4.6) |
| Binary classifierd | 2.6 (0.8-8.7) | 1.2 (0.2-5.9) | 464.4 (219.4-983.1) | 452.7 (198.9-1030.1) | 558.7 (257.9-1210.6) | 564.8 (238.8-1335.4) |
The subset of p16+ OPC cases that are also HPV ISH+
Adjusted for sex, smoking, and alcohol status (never vs. former vs. current)
Adjusted for smoking and alcohol status (never vs. former vs. current)
Binary classifier is based on a combination of antibodies based on receiver operating characteristics analysis; ≥0.5 is considered positive
CI: confidence interval; E: early protein; HPV: human papillomavirus; L: late protein OPC: oropharyngeal cancer; OR: odds ratio
Discussion
In this large case-control study, we found that patients with HPV-related OPC were significantly more likely than cancer-free controls to have HPV16 E antibodies and a trained binary classification algorithm had high sensitivity and specificity for HPV-related OPC. Antibody positivity among patients with non-OPC occurred at rates similar to those observed in controls.
Association between seropositivity for HPV16 E antibodies and HPV-related cancers, including OPC has been observed in previous studies by our group and others.8,9,12,13,22-24 In data from the European Prospective Investigation Into Cancer and Nutrition cohort, which included 638 patients with head and neck cancer and 1,599 control subjects, antibodies to E6 were present in fewer than 1% of controls and 35% of OPC cases; furthermore, antibodies could be detected more than 10 years before diagnosis.8 Using the same multiplex assay, these results were validated in Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO) where antibody levels were found to remain elevated and stable for up to 13 years prior to diagnosis.12 Moreover, the 10-year cumulative incidence of OPC among seropositive individuals 6.2% among men and 1.3% among women.12 In a previous study of 256 OPC cases and 250 controls, we found that seropositivity for E2, E6, and/or E7 was associated with OPC risk (OR, 249.1; 95% CI, 99.3-624.9).9 Our current results are consistent with these previous findings. In the present study of a separate and larger cohort with comprehensive tumor HPV classification, we applied a trained algorithm for classification of HPV status based on our previous cohort and found that in our current cohort the classifier had a sensitivity and specificity of 83% and 99%, respectively, with an associated greater than 450-fold increased risk for p16-positive OPC. The improved performance of the classifier over E6 and/or E7, which had a sensitivity of 74% and a specificity of 98%, supports the use of a multipanel assay for screening. While our assay shows promise as a biomarker for detection of HPV-related OPC in this case-control study, further studies are needed. Specifically, the ability of the marker to identify cases prior to clinical diagnosis in cohort studies, implementation of screening tools in those testing positive to determine detection and false referral rate in prospective screening studies, and finally, studies to determine cost-effectiveness and reduction in the burden of cancer in the screened population.25,26 Consequently, we are using these data to support the design of a trial evaluating HPV16 E antibody-based screening for OPC risk stratification in men in their 50s (a high HPV-related OPC incidence group) and evaluating novel imaging techniques for early detection of OPC (ClinicalTrials.gov Identifier: NCT02897427).
P16 by IHC is an accepted surrogate marker for tumor HPV status for OPC but is a poor marker for tumor HPV status in non-OPC.27-30 Our results add to the evidence that while non-OPC tumors may overexpress p16, they are not driven by HPV. We found that while 31% of patients with non-OPC had p16-positive tumors, the HPV16 E antibody positivity rate of these patients more closely resembled that of controls and patients with p16-negative OPC than that of patients with p16-positive OPC. In fact, there was no significant difference in antibody status between patients with p16-positive and p16-negative non-OPC, while there was a significant difference in antibody status between patients with p16-positive non-OPC and p16-positive OPC (positivity for any E antigen: p=0.601 and p<0.001, respectively). Using the trained binary classifier, four patients with non-OPC were classified as antibody positive, and of those, three had p16-positive tumors (two of whom also had HPV ISH-positive tumors) and may have had true HPV-related non-OPC. Our findings indicate that non-OPC tumors, including the majority of those that are p16-positive, are unlikely to be attributable to HPV; however, as reported in the literature, we cannot discount the possibility that a small fraction of non-OPC tumors may be related to HPV.28-30
This work expands on a previous study by our group but is substantially different from that study. In the work presented herein, we analyzed a completely different and larger cohort, we included patients with non-OPC to serve as an HPV-negative comparison group, and we used p16 IHC and HPV ISH in place of polymerase chain reaction to determine HPV status. As in our previous work, investigators performing the serology assay were blinded with respect to case-control and HPV status. Finally, based on our previous work, we applied a trained algorithm to predict HPV positivity based on combined antibody levels, and use of this algorithm improved the specificity of the assay to 99%, a level of specificity necessary for targeted population-based screening of a (currently) low-incidence cancer.
Limitations of this study include the inability to isolate the effects of individual antibodies as most seropositive subjects were positive for multiple antibodies and the potential misclassification of tumor HPV status. Although we determined tumor HPV status of OPC by using p16 by IHC, a marker currently in use as a surrogate for the HPV status of OPC in clinical trials as well as the 8th edition of the American Joint Committee on Cancer (AJCC) Staging Manual,14,15,31-34 misclassification of HPV status may have occurred. Our data suggest that this is unlikely to have occurred in more than a few cases. Of the 367 patients with OPC who had p16 and ISH data available, only 1% (n=4) had p16-negative, HPV ISH-positive tumors. Although 18% (n=66) had p16-positive, HPV ISH-negative tumors, the limited sensitivity of ISH makes it likely that most of these cases were truly HPV positive. The large point estimates with wide confidence intervals in the logistic regression analysis suggest that our study is biased due to sparse data as a result of the low number of seropositive controls. Nevertheless, our results indicate a strongly positive and clinically relevant association between seropositivity and diagnosis of HPV-related OPC.
Antibodies to HPV16 E antigens are a promising marker for HPV-related OPC, and we generated a sensitive and specific algorithm for classifying patients with respect to HPV16 E antigen positivity. Such a classifier could be used to identify at-risk individuals who would benefit from further screening. Screening methods such as transoral optical imaging and neck ultrasonography are currently being investigated as noninvasive methods for detecting OPC at earlier stages, which would allow earlier treatment to prevent significant morbidity and mortality. Whether HPV16 E antibodies ultimately prove to be adequate for selecting at-risk populations for careful follow-up or allow detection of disease at earlier stages merits further investigation in a prospective clinical trial.
Supplementary Material
Acknowledgments
Stephanie Deming of the Department of Scientific Publications at MD Anderson provided editorial assistance.
Funding sources: We acknowledge funding contributions from The University of Texas MD Anderson Christopher and Susan Damico Chair in Viral Associated Malignancies. This work was supported by generous philanthropic contributions, including the contribution from the Lyda Hill Foundation, to The University of Texas MD Anderson HPV-Related Cancers Moon Shot Program. This research was accomplished within the Oropharynx Program at The University of Texas MD Anderson Cancer Center and funded in part through the Stiefel Oropharyngeal Research Fund. This work was also supported by the National Institutes of Health (R01ES011740 and R01CA131274) to Qingyi Wei. Statistical analyses were performed, in part, by the MD Anderson Cancer Center Biostatistics Resource Group, which is supported by the National Institutes of Health through MD Anderson's Cancer Center Support Grant (P30CA016672).
Footnotes
Conflict of interest disclosures: Dr. Anderson has served as a consultant and has stock options with Provista Dx and FlexBioTech. No other authors have any conflicts to report.
Author contributions: KRD: formal analysis, data curation, writing-original draft, and visualization; KSA: conceptualization, methodology, validation, resources, writing-original draft, supervision; MSF: investigation, writing-review & editing; DC: software, formal analysis, writing-review & editing; JN: formal analysis, writing-review & editing; NL: formal analysis, writing-review & editing; QW: resources, writing-review & editing, and funding acquisition; GL: data curation, writing-review & editing; EMS: conceptualization, resources, supervision, project administration, writing-original draft, and funding acquisition.
Precis: The presence of serum antibodies to HPV16 early antigens may be used to identify at-risk individuals who would benefit from further screening. A trained algorithm that incorporates information about multiple early antibodies showed high sensitivity and specificity and may be advantageous for risk stratification in future screening trials.
References
- 1.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33:3235–3242. doi: 10.1200/JCO.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal A, Simard EP, Dorell C, et al. Annual report to the nation on the status of cancer, 1975-2009, featuring the burden and trends in human papillomavirus (HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105:175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurdgelashvili G, Dores GM, Srour SA, Chaturvedi AK, Huycke MM, Devesa SS. Incidence of potentially human papillomavirus-related neoplasms in the United States, 1978 to 2007. Cancer. 2013;119:2291–2299. doi: 10.1002/cncr.27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31:4550–4559. doi: 10.1200/JCO.2013.50.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers - United States, 2008-2012. MMWR Morb Mortal Wkly Rep. 2016;65:661–666. doi: 10.15585/mmwr.mm6526a1. [DOI] [PubMed] [Google Scholar]
- 7.Anderson KS, Wong J, D'Souza G, et al. Serum antibodies to the HPV16 proteome as biomarkers for head and neck cancer. Br J Cancer. 2011;104:1896–1905. doi: 10.1038/bjc.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreimer AR, Johansson M, Waterboer T, et al. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol. 2013;31:2708–2715. doi: 10.1200/JCO.2012.47.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson KS, Dahlstrom KR, Cheng JN, et al. HPV16 antibodies as risk factors for oropharyngeal cancer and their association with tumor HPV and smoking status. Oral Oncol. 2015;51:662–667. doi: 10.1016/j.oraloncology.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahlstrom KR, Anderson KS, Cheng JN, et al. HPV serum antibodies as predictors of survival and disease progression in patients with HPV-positive squamous cell carcinoma of the oropharynx. Clin Cancer Res. 2015;21:2861–2869. doi: 10.1158/1078-0432.CCR-14-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang Kuhs KA, Anantharaman D, Waterboer T, et al. Human papillomavirus 16 E6 antibodies in individuals without diagnosed cancer: A pooled analysis. Cancer Epidemiol Biomarkers Prev. 2015;24:683–689. doi: 10.1158/1055-9965.EPI-14-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreimer AR, Johansson M, Yanik EL, et al. Kinetics of the human papillomavirus type 16 E6 antibody response prior to oropharyngeal cancer. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djx005. 10.1093/jnci/djx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holzinger D, Wichmann G, Baboci L, et al. Sensitivity and specificity of antibodies against HPV16 E6 and other early proteins for the detection of HPV16-driven oropharyngeal squamous cell carcinoma. Int J Cancer. 2017;140:2748–2757. doi: 10.1002/ijc.30697. [DOI] [PubMed] [Google Scholar]
- 14.Edge S, Greene F, Byrd D, Brookland R, Washington M, Gershenwald J, editors. American Joint Committee on Cancer staging manual. 8th. New York: Springer; 2017. [Google Scholar]
- 15.Lydiatt WM, Patel SG, O'Sullivan B, et al. Head and neck cancers-major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:122–137. doi: 10.3322/caac.21389. [DOI] [PubMed] [Google Scholar]
- 16.Anderson KS, Sibani S, Wallstrom G, et al. Protein microarray signature of autoantibody biomarkers for the early detection of breast cancer. J Proteome Res. 2011;10:85–96. doi: 10.1021/pr100686b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Festa F, Rollins SM, Vattem K, et al. Robust microarray production of freshly expressed proteins in a human milieu. Proteomics Clin Appl. 2013;7:372–377. doi: 10.1002/prca.201200063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson KS, Wong J, Vitonis A, et al. P53 autoantibodies as potential detection and prognostic biomarkers in serous ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:859–868. doi: 10.1158/1055-9965.EPI-09-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Souza G, Gross ND, Pai SI, et al. Oral human papillomavirus (HPV) infection in HPV-positive patients with oropharyngeal cancer and their partners. J Clin Oncol. 2014;32:2408–2415. doi: 10.1200/JCO.2014.55.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson KS, Gerber JE, D'Souza G, et al. Biologic predictors of serologic responses to HPV in oropharyngeal cancer: The HOTSPOT study. Oral Oncol. 2015;51:751–758. doi: 10.1016/j.oraloncology.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Witten I, Frank E, Hall M, Pal C. Data mining: Practical machine learning tools and techniques. 4th. Cambridge, MA: Morgan Kaufmann; 2016. [Google Scholar]
- 22.Herrero R, Castellsague X, Pawlita M, et al. Human papillomavirus and oral cancer: The International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95:1772–1783. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 23.Anantharaman D, Gheit T, Waterboer T, et al. Human papillomavirus infections and upper aero-digestive tract cancers: The ARCAGE study. J Natl Cancer Inst. 2013;105:536–545. doi: 10.1093/jnci/djt053. [DOI] [PubMed] [Google Scholar]
- 24.Lang Kuhs KA, Pawlita M, Gibson SP, et al. Characterization of human papillomavirus antibodies in individuals with head and neck cancer. Cancer Epidemiol. 2016;42:46–52. doi: 10.1016/j.canep.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pepe MS, Etzioni R, Feng Z, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054–1061. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]
- 26.Baker SG, Kramer BS, McIntosh M, Patterson BH, Shyr Y, Skates S. Evaluating markers for the early detection of cancer: overview of study designs and methods. Clin Trials. 2006;3:43–56. doi: 10.1191/1740774506cn130oa. [DOI] [PubMed] [Google Scholar]
- 27.Jordan RC, Lingen MW, Perez-Ordonez B, et al. Validation of methods for oropharyngeal cancer HPV status determination in US cooperative group trials. Am J Surg Pathol. 2012;36:945–954. doi: 10.1097/PAS.0b013e318253a2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lingen MW, Xiao W, Schmitt A, et al. Low etiologic fraction for high-risk human papillomavirus in oral cavity squamous cell carcinomas. Oral Oncol. 2013;49:1–8. doi: 10.1016/j.oraloncology.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Chung CH, Zhang Q, Kong CS, et al. P16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol. 2014;32:3930–3938. doi: 10.1200/JCO.2013.54.5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zafereo ME, Xu L, Dahlstrom KR, et al. Squamous cell carcinoma of the oral cavity often overexpresses p16 but is rarely driven by human papillomavirus. Oral Oncol. 2016;56:47–53. doi: 10.1016/j.oraloncology.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rischin D, Young RJ, Fisher R, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol. 2010;28:4142–4148. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harari PM, Harris J, Kies MS, et al. Postoperative chemoradiotherapy and cetuximab for high-risk squamous cell carcinoma of the head and neck: Radiation therapy oncology group RTOG-0234. J Clin Oncol. 2014;32:2486–2495. doi: 10.1200/JCO.2013.53.9163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fakhry C, Zhang Q, Nguyen-Tan PF, et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J Clin Oncol. 2014;32:3365–3373.32. doi: 10.1200/JCO.2014.55.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edge S, Greene F, Byrd D, Brookland R, Washington M, Gershenwald J, editors. American joint committee on cancer staging manual. 8th. New York: Springer; 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.