Abstract
Type 1 Diabetes (T1D) has a strong genetic component. The Insulin dependent diabetes 22 (Idd22) locus was identified in crosses of T1D-susceptible NOD mice with the strongly T1D-resistant ALR strain. The NOD-Idd22 recombinant congenic mouse strain was generated where NOD mice carry the full Idd22 confidence interval. NOD-Idd22 mice exhibit almost complete protection from spontaneous T1D and a significant reduction in insulitis. Our goal was to unravel the mode of Idd22 based protection using in vivo and in vitro models. We determined that Idd22 did not impact immune cell diabetogenicity or β-cell resistance to cytoxicity in vitro. However, NOD-Idd22 mice were highly protected against adoptive transfer of T1D. Transferred CTL trafficked to the pancreatic lymph node (PLN) and proliferated to the same extent in NOD and NOD-Idd22 mice yet, the accumulation of pathogenic CTL in the islets was significantly reduced in NOD-Idd22 mice, correlating with disease resistance. Pancreatic endothelial cells from NOD-Idd22 animals expressed lower levels of adhesion molecules, even in response to inflammatory stimuli. Lower adhesion molecule expression resulted in weaker adherence of T cells to NOD-Idd22 endothelium as compared to NOD derived endothelium. Taken together, these results provide evidence that Idd22 regulates the ability of β-cell autoreactive T cells to traffic into the pancreatic islets and may represent a new target for pharmaceutical intervention to potentially prevent T1D.
Introduction
In both humans and mice, Type 1 diabetes (T1D) is primarily considered an autoimmune disorder characterized by the loss of insulin producing pancreatic β-cells. In the T1D-prone NOD mouse, this immune-mediated process directly results in insulin insufficiency and hyperglycemia (1). The principal pathologic lesion in T1D is a destructive immune cell infiltrate comprised largely of CD8+ and CD4+ T lymphocytes in and around the islets of Langerhans (insulitis), which is preceded by entry of macrophages and dendritic cells (2). Both CD4+ and cytotoxic CD8+ (CTL) T cell subsets are required for spontaneous T1D development in NOD mice as destruction of β-cells has been firmly established in this model as a T cell-mediated process (3–6).
Early studies of the NOD mouse identified the importance of the hematopoietic compartment in regulating T1D pathogenesis. By mating NOD mice with outcross partners that do not develop T1D, such as CBA or C57BL/10, the resulting F1 progeny were fully protected from spontaneous disease. However, if these mice were made chimeric with bone marrow from NOD mice, they developed T1D at high rates (7, 8). Further, when normally T1D-resistant mouse strains were induced to express diabetogenic T cell receptors (TCRs) through transgenesis or were administered β-cell autoreactive T cells through adoptive transfer, T1D onset was observed (9, 10). Accordingly, a large majority of the Idd loci identified to date have been acknowledged to act through hematopoietic cells (11–14).
In contrast to these results, we previously identified that ALR mice were uniquely and highly resistant to spontaneous as well as induced autoimmune diabetes (15). The ALR mouse arose from the same outbred Swiss progenitors as the T1D-prone NOD strain and based on genome wide single nucleotide polymorphism (SNP) typing, the ALR and NOD strains share more than 85% genomic identity. Islets from ALR mice are resistant to destruction by both autoreactive CTL as well as combinations of proinflammatory cytokines in vitro (15). In vivo, the lack of T1D is associated with a dearth of invasive insulitis. Importantly, in spite of their high genetic identity to NOD mice, ALR mice as well as ALR×NOD F1 progeny remain fully resistant to T1D after transfer of bone marrow from NOD. Furthermore, ALR and ALR×NOD F1 hybrids are fully protected from transfer of highly pathogenic CD8+ CTLs (G9C8 and AI4) (15) as well as the diabetogenic CD4+ BDC2.5 T cell clone (16). These data suggest that ALR mice harbor novel immune and/or non-immune mediated T1D-resistance loci that if identified could be translated into therapies for T1D.
Genetic studies designed to ascertain ALR-derived T1D resistance loci have identified genomic regions linked to dominant T1D resistance including mt-Nd2 (17) within the mitochondrial genome as well as loci on Chromosomes (Chr) 3, 8, and 17 (18). Here we investigate the region on Chr 8 termed Idd22. Through introgression of Idd22 onto the NOD background, we report that the protective mechanism of the ALR-derived allele does not act within the immune cell compartment or act directly within β-cells to increase survivability. Instead, this T1D-protective locus provides a novel means of regulating T1D by reducing the expression of adhesion molecules important for T cell adhesion to endothelial cells of the pancreatic microvasculature. This process limits the infiltration of autoreactive T cells into the pancreatic islets.
Materials and Methods
Animals
All mice used in this study were housed in specific pathogen-free facilities. These studies were approved by the Institutional Animal Care and Use Committee (IACUC) at Sanford Research, Sioux Falls, SD, the Children’s Hospital of Pittsburgh, and University of Florida. NOD/ShiLtJ (NOD), NOD.Cg-Rag1tm1Mom (NOD.Rag1−/−), NOD.Cg-Rag1tm1Mom-Tg(TcraAI4)1Dvs/DvsJ (NOD.AI4α-Rag1−/−), NOD.Cg-Rag1tm1MomTg(TcrbAI4)1Dvs/DvsJ (NOD.AI4β-Rag1−/−), NOD.NON-Thy1a/1LtJ (NOD-Thy1α), and NOD.CB17-Prkdcscid/J (NOD-scid) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). ALR/LtMx mice were bred and maintained at the Children’s Hospital of Pittsburgh and University of Florida. NOD.Cg-Rag1tm1Mom-Thy1α/α-Tg(TcraAI4)1Dvs/DvsJ (NOD.AI4α-Thy1α) mice were generated by breeding NOD.NON-Thy1a/1LtJ males to NOD.AI4α-Rag1−/− females and fixing for AI4α/α, Thy1α/α, and Rag1−/−. F1 hybrid progeny from outcrosses of NOD.AI4α-Rag1−/− or NOD.AI4α-Thy1α to NOD.AI4β-Rag1−/− (hereafter referred to as NOD.AI4α/β-Rag1−/− or NOD.AI4α/β-Thy1α/β) were created for adoptive transfer studies. Female and male NOD.AI4α/β-Rag1−/− mice develop diabetes between 3 and 5 weeks of age. Mice transgenic for H2-Kd and carrying the insulin specific CD8+ TCR transgenes (clone G9C8) on the C57BL/6 background (IS-CD8+) were maintained and used at Sanford Research, Sioux Falls, SD (19). To examine the specific contribution of the Idd22 locus on Chr 8 to T1D resistance, a recombinant inbred congenic mouse strain (RCS), NODcALR-(D8Mit293-D8Mit137)/Mx mice (NOD-Idd22) was generated as described (16). Briefly, mice with ALR genome on Chr 8 as well as the lowest percentage of contaminating ALR alleles, genome wide, were bred from the N2 to N7 generations. N8 mice were homozygous for NOD at all locations typed except for the Idd22 confidence interval on Chr 8. N10 mice in this RCS were intercrossed and the Idd22 segment was fixed for homozygosity using markers D8Mit293, D8Mit205, D8Mit74, D8Mit268, and D8Mit137 (Figure 1A).
Figure 1. Idd22 protects against spontaneous development of T1D.
A. Graphical representation of the Idd22 congenic region on Chr 8 of NOD-Idd22 mice. Idd22 is defined as the region of Chr 8 between the markers D8Mit293 to D8Mit137. White regions represent NOD type, Black regions represent ALR type, and hatched regions represent regions of undetermined origin. B. 79 NOD-Idd22 and 37 NOD females were enrolled in a spontaneous T1D incidence study and were monitored bi-weekly for development of disease. Difference in incidence (P<0.0001) was analyzed using Log-rank survival curve analysis. C. NOD-Idd22 and NOD mice were sacrificed at the indicated time points, pancreata were harvested, and severity of insulitis was scored by histology (H&E). Differences at individual time-points were analyzed by Student’s t test (*P<0.05). n≥3 per timepoint, per group. D–G. Representative micrographs of H&E stained pancreata from NOD (D and E) and NOD-Idd22 (F and G) mice at 14 weeks of age. E and G (black scale bars indicate 200 um) show enlarged images of the boxed areas of D and F (black scale bars indicate 500 um), respectively.
T1D Incidence
Development of spontaneous T1D in NOD-Idd22 mice was assessed using NOD-Idd22 (n=79) and NOD control mice (n=37). Mice were aged until onset of T1D or 40 weeks of age. Diabetes development was monitored every other week starting at 8 weeks of age using Diastix (Bayer Diagnostics, Leverkusen, Germany) as described (18). A positive test for glycosuria with Diastix was confirmed via measurement of blood glucose from the tail vein using a One Touch Ultra 2 meter (Life Scan, Inc, Milpitas, CA). A blood glucose level >250 mg/dL on 2 consecutive days was considered diagnostic of diabetes onset.
Insulitis
The degree of insulitis within the islets was examined at the following ages (in weeks): 4, 6, 8, 10, and 14 for NOD, and 4, 6, 8, 10, 14, and 40 for NOD-Idd22 mice. NOD mice were not followed until 40 weeks due to the onset of T1D. After euthanasia, the pancreata of NOD and NOD-Idd22 mice were fixed in 10% buffered formalin overnight, paraffin embedded, and stained with hematoxylin/eosin (H&E). Insulitis scores were calculated as described previously (20). Briefly, individual islets were scored on a scale of 0–4, where 0 is no visible lymphocytes, score 1 is mild peri-insulitis, score 2 indicates some intra-islet infiltration, score 3 indicates the presence of insulitis covering up to 75% of islet area, and a score of 4 indicates >75% of the islet area displaying insulitis. The average score was then calculated for the entire histological section.
Flow Cytometry of Immune Cell Subsets
Flow cytometric analysis of the peripheral blood (PBL) and spleens of NOD and NOD-Idd22 mice was performed as previously described (21). PBL samples were collected using heparin tubes and spleens were homogenized. Red blood cells were lysed using Gey’s hemolytic solution (22), and white blood cells were stained with antibodies against B220, CD3, CD4, CD8, Ly-6g, CD11b, and F4/80. The following cell types were gated according to their surface markers: B cells (CD3−B220+), helper T cells (CD3+CD4+), CTLs (CD3+CD8+), Neutrophils (Ly-6g+CD11b+), and Macrophages (CD11b+F4/80+). Flow cytometry was performed using a BD LSR Fortessa. Data were analyzed using FlowJo Software (Treestar, Ashland, Or).
Adoptive Transfer
We performed adoptive transfer studies using NOD-Scid mice as recipients according to standard methods (23, 24). Six-week old NOD or NOD-Idd22 females were used as spleen donors. This allowed for a comparison of transfer from non-diabetic T1D-resistant NOD-Idd22 with pre-diabetic NOD females. Six and nine NOD-Scid mice received NOD or NOD-Idd22 splenocytes, respectively. Blood glucose levels were measured once weekly in recipient mice until T1D onset as described above, and animals were euthanized at diagnosis or 20 weeks post transfer when T1D did not develop.
Adoptive transfer was also performed using splenocytes of NOD.AI4α/β-Rag1−/− mice or IS-CD8+ CTLs into sub-lethally irradiated (750 cGy) NOD and NOD-Idd22 recipient mice as described previously (23, 24). IS-CD8+ CTLs were pre-activated in vitro prior to transfer. Blood glucose levels were assessed 3 times weekly in recipient mice until T1D onset (defined above) or 4 weeks post adoptive transfer, at which time mice were euthanized.
Culture of Islets with Pro-inflammatory Cytokines
Complete DMEM media was prepared as follows, standard 5.5 mM glucose media DMEM (Life Technologies, Carlsbad, CA) supplemented with 10 units/ml gentamycin (Sigma-Aldrich, St. Louis, MO) and 10% (vol/vol) heat-inactivated FBS (Atlanta Biologicals, Norcross, GA). NOD, NOD-Rag1−/− or NOD-Idd22 islets were isolated as described (25) and plated in complete media alone or in complete media plus IL-1β (30 U/mL), TNFα (1000 U/mL), and IFNγ (1000 U/mL) either alone or combination for 72 hours at 37°C. At this time point, nitrite production [Griess reaction (17)], viability [Neutral red assay (17)], and glucose-stimulated insulin secretion (GSIS) (15) were assessed. NOD-Rag1−/− islets were used in order to eliminate variability due to early disease processes at this age in NOD mice. Insulin ELISA (ALPCO Diagnostics, Salem, NH) was used to determine the insulin content of the media and GSIS was calculated as [Insulin (Stimulated)- Insulin (Basal)] and expressed as ng insulin secreted/10ng DNA.
Cell Mediated Lymphocytotoxicity Assays
Cell mediated lymphocytotoxicity (CML) assays were performed as described previously (15, 26, 27). Briefly, splenocytes from NOD.AI4α/β-Rag1−/− mice were primed in complete RPMI1640 media containing IL-2 (25U/mL) and mimotope (0.1µM; sequence YFIENYLEL) for 3 days. Islets were isolated from 10–12 week old NOD-Rag1−/− or NOD-Idd22 mice, cultured overnight, dispersed into single cell suspensions and plated at a density of 40,000 cells per well in flat-bottom 96 well plates. Dispersed islet cells were exposed to IFNγ (1000U/mL) for 24 hours preceding the CML. The plated islet cells were labeled with 51Cr (1 µCi/well) for 3 h at 37°C, washed with complete DMEM media, and then activated NOD.AI4α/β-Rag1−/− splenocytes were added to the wells at a 25:1 Effector:Target ratio. T cell-islet cell co-cultures were incubated for 16 hours before the assays were stopped. Specific lysis was calculated according to the equation below (15, 26).
Immunohistochemistry
Pancreata of mice that received NOD.AI4α/β-Rag1−/−-Thy1α/β splenocytes were fresh-frozen in optimal cutting temperature (OCT) compound (Sakura Finitek, Torrance, CA) at day 3, 5, 7, and 9 post transfer, sectioned, and fixed in −20°C Acetone. Slides were quenched using Dual Endogenous Enzyme Block (DEEB, DAKO, Carpinteria, CA) and subsequently blocked using Background Sniper (Biocare Medical, Walnut Creek, CA). Slides were stained for Insulin (Clone C27C9, Cell Signaling Technologies, Danvers, MA) followed by Mach 2 Rabbit AP-polymer (Biocare Medical). Insulin staining was detected using Liquid Permanent Red (DAKO). Thy1a was then labeled with anti CD90 (Clone OX-7, Stemcell Technologies, Vancouver, Canada) using M.O.M. kit (Vector Laboratories, Inc., Burlingame, CA) according to manufacturer instructions. Detection of Thy1a was achieved using 3’3’ diaminobenzidine (Biocare Medical). Slides were counterstained with hematoxylin (Biocare Medical) and mounted with VectaMount™ Permanent Mounting Medium (Vector Laboratories).
Lymph Node Proliferation Assay
Splenocytes from NOD.AI4α/β-Thy1α/β mice were isolated as described above and stained with Cell Trace Violet (CTV) cell proliferation dye (Life Technologies) according to manufacturer’s instructions. After labeling, splenocytes were washed and resuspended in ice cold DPBS at a concentration of 1×108 cells/mL. Cells (2×107) were injected into 8–10 week old female recipients via tail-vein. Three days post transfer, mice were sacrificed and pancreatic draining lymph nodes (PLN) were collected. Lymph nodes were homogenized to single cells and stained for flow cytometric analysis. Lymph nodes were analyzed using Live/Dead Near IR (Life Technologies) to exclude dead cells. AI4 CD8+ T cells were identified with PE labeled anti-Thy1α or H-2Db tetramer loaded w)ith mimetope (YAIENYLEL) (Mimetope loaded H-2Db and irrelevant peptide (LCMV loaded control H-2Db tetramer produced by the NIH Tetramer Core Facility, Atlanta, GA) and APC-labeled anti-CD8 (Biolegend, San Diego, CA) (Supplemental Figure 1). Activation status of AI4 cells was determined by staining with antibodies against KLRG1, CD11a, CD43, CD27, IL7Rα, and CD62L. Data were analyzed using FlowJo Software.
Generation of Pancreatic Endothelial Cell (PEC) lines
Pancreata were removed from two 8–10 week old NOD or NOD-Idd22 mice and processed according to previously published methods (28) with the following modifications. After generation of the single-cell suspension, pancreatic cells were pooled and magnetically sorted via positive selection using avidin-coated magnetic beads and biotin-labeled anti-endoglin monoclonal antibody (eBioscience, San Diego, CA). Isolated endothelial cells were immortalized using sv40 large T antigen (29) and maintained in culture at 33°C. Three to five days prior to initiation of experiments, PEC cell lines were moved to 37°C to down-regulate T antigen expression. After plating, cells were allowed to grow to confluence at 37°C in DMEM supplemented with 10% heat inactivated (HI) FBS (Corning Life Sciences, Corning, NY), 2 mM L-Glutamine, and 1× Pen/Strep (Sigma-Aldrich, St. Louis, MO). Upon reaching confluency, media was aspirated and cells were treated with either media alone or media containing 10 ng/mL TNF-α for 4 hours. Cells were then trypsinized, resuspended in stain buffer (PBS + 2% HI FBS) and stained with anti-E-Selectin-PE (Clone 10E9.6, BD Biosciences), anti-P-Selectin-FITC (Clone RB40.34, BD Biosciences), anti-CD54-PE (ICAM-1) (Clone YN1/1.7.4, Affymetrix), or anti-CD106-FITC (VCAM-1) (Clone 429, Affymetrix) and fixed. Cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences).
In Vitro Hydrodynamic Flow Chamber Adhesion Assay
Flow chamber adhesion studies were performed as previously described (30). Briefly, NOD splenic CD8+ T cells were enriched via EasySep Mouse CD8+ T cell isolation kit (Stemcell Technologies, Cambridge, MA) and labelled with 200 nmol/L Cell Tracker Green (Molecular Probes). The labeled cells were resuspended at 2×105 cells/mL in HBSS with stirring at 37°C. A Glycotech flow chamber was used for visualization of the laminar plate flow chamber containing monolayers of the NOD or NOD-Idd22 PEC lines generated above on a microscope. The monolayers were washed with HBSS in triplicate prior to flow chamber assembly. The labeled cells were drawn across the endothelial monolayers at the listed shear rates using a programmable digital syringe pump and viewed with a Nikon Eclipse TE-2000 epifluorescent microscope and Hamamatsu digital camera. Real-time video was captured using SIMPLE PCI software (Compix), which allows for motion tracking analysis of individual cell rolling velocity.
Statistics
Differences in T1D incidence rates were assessed using Log-rank survival curve analysis via. Differences in pair-wise comparisons between two groups were assessed using two-tailed Student’s t test with a significance threshold of p<0.05. ANOVA was used to determine differences between multiple groups with a false discovery rate (FDR) adjusted significance threshold of p≤0.05. All statistical tests were performed using GraphPad Prism version 6 (GraphPad Sofware; San Diego, California). Where appropriate, all figures depict the Mean ± SEM.
Results
The Idd22 region provides significant protection against spontaneous T1D
To isolate the effects of Idd22 from the other ALR resistance loci, the NOD-Idd22 RCS was generated using a marker-assisted breeding strategy to rapidly fix the region of Chr 8 between the markers D8Mit293 and D8Mit137 on the NOD background (Figure 1A). As ALR and NOD mice have high genetic identity, no informative microsatellite markers were found to define the extent to which Idd22 extends proximally to the centromere.
To determine if the Idd22 locus imparted altered susceptibility to the development of T1D, diabetes progression was monitored in NOD-Idd22 and parental NOD females. As expected, approximately 90% of the NOD mice developed T1D by 20 weeks of age. In contrast, T1D incidence was significantly lower among NOD-Idd22 mice with less than 15% developing hyperglycemia by 40 weeks of age (P<0.0001, Figure 1B).
Histological analysis indicated that insulitis severity increased with age in both groups, but to a much lesser extent in NOD-Idd22 compared to NOD (Figure 1C). Insulitis was detectable in NOD control mice as early as 4 weeks of age; however, insulitis was not detectable in NOD-Idd22 mice until 6 weeks of age. At 14 weeks of age, the insulitis scores were 2.9 and 0.26 in NOD and NOD-Idd22 islets, respectively (P<0.001) (Figures 1C, D–G). Through 40 weeks of age, NOD-Idd22 mice displayed mild peri-insulitis with a minimal amount of infiltration into the islets (insulitis score of 1.4). Insulitis score was not reported for NOD mice at 40 weeks of age because these mice rarely reach this age.
Idd22 splenocytes harbor full pathogenic potential
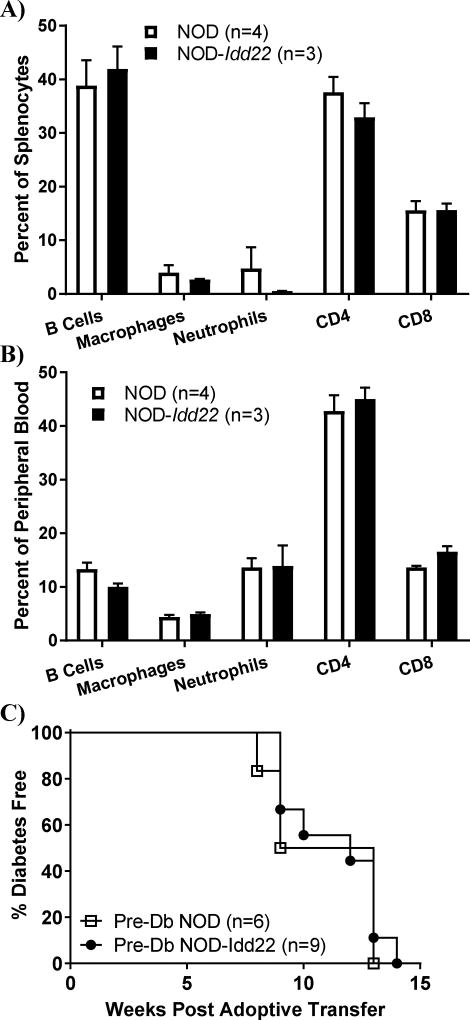
The majority of Idd loci that have been mapped using outcrosses of NOD to other inbred mouse strains were found to regulate the pathogenicity of hematopoietic cells (11–14). To determine if Idd22 resulted in a modulation of immune cell frequency, flow cytometry was performed to examine the percent of immune cell subtypes within the spleen (Figure 2A) and peripheral blood (Figure 2B). Comparison of NOD and NOD-Idd22 mice revealed no significant difference in any cell type assessed. To detect functional differences in immune cells that may be attributable to Idd22, splenocytes from NOD or NOD-Idd22 mice were adoptively transferred into NOD-Scid recipients. Transfer of splenocytes from NOD and NOD-Idd22 donors induced T1D at similar rates (100% T1D onset by 13–15 weeks post-adoptive transfer in both groups, P=0.82) (Figure 2C). These data demonstrate that splenocytes from NOD-Idd22 mice harbor pathogenic potential.
Figure 2. Idd22 does not modulate immune cell subsets.
A and B. Spleen and Peripheral Blood cell subset frequencies were analyzed via flow cytometery. No differences were found in the proportion of cell subsets in either compartment, P>0.2 for all comparison, Student’s t test. C. Splenocytes from pre-diabetic NOD and NOD-Idd22 donors were adoptively transferred into NOD-scid recipients. Recipients were monitored weekly for development of T1D. Idd22 did not modulate the ability of donor splenocytes to transfer disease, P=0.68, Log-rank survival curve test.
Idd22 does not protect islet cells against immune mediated destruction in vitro
To determine whether Idd22 imparted enhanced survivability to β-cells against inflammation induced cell death, in vitro models were utilized. We cultured NOD-Rag1−/− and NOD-Idd22 islets in the presence or absence of pro-inflammatory cytokines. Insulin secretion was similar between NOD-Rag1−/− (0.089 ng/mL) and NOD-Idd22 (0.088 ng/mL) islets in 2.7mM glucose media in the absence of cytokine treatment (Figure 3A). Insulin secretion increased in both groups in 25 mM glucose without cytokine treatment, the increase was greater in NOD-Idd22 islets compared to NOD-Rag1−/− islets (2.58 ng/mL versus 0.93 ng/mL, respectively; P<0.01). The pre-treatment of islets from either mouse strain with single cytokines (IL-1β, IFNγ, or TNFα) had no effect on insulin secretion (Supplemental Figure 2). After pre-treatment with combined proinflammatory cytokines (IL-1β, IFNγ, and TNFα (Figure 3A)) both groups had a mild, but significant (P<0.02) increase in insulin release at basal glucose levels (2.7mM). Neither NOD-Rag1−/− nor NOD-Idd22 islets pre-treated with combined proinflammatory cytokines (IL-1β, IFNγ, and TNFα (Figure 3A) or the combination of IL-1β and IFNγ (Supplemental Figure 2) responded to 25mM glucose. Insulin release after combined cytokines was comparable between the two groups after combined cytokine exposure.
Figure 3. β-cell susceptibility to immune-mediated death is not reduced by Idd22.
A. NOD-Idd22 and NOD-Rag1−/− islets were plated in media containing 5.5 mM glucose with or without combined proinflammatory cytokines for 72 hours. Islets were washed and basal levels of insulin secretion were determined after 1 hour incubation in 2.7mM Glucose containing KRBH media. Stimulated insulin secretion was then measured after incubation of islets in [25 mM] glucose KRBH media for 2 hours. Without cytokine pre-treatment, NOD-Idd22 islets produced significantly more insulin in response to high glucose (P<0.001). Pre-treatment with combined proinflammatory cytokines abolished stimulated insulin secretion in both NOD-Idd22 and NOD-Rag1−/− islets. B. Production of NO by NOD-Idd22 and NOD-Rag1−/− islets was determined after initial culture as in A. Treatment with combined proinflammatory cytokines increased nitric oxide (NO) production in both groups, however NOD-Idd22 produced less NO in response to cytokines than NOD-Rag1−/− islets (P<0.01). C. No difference was observed (P=0.89) when comparing NOD-Idd22 and NOD-Rag1−/− for islet cell viability after 72 hours of treatment with combined proinflammatory cytokines. D. Susceptibility of NOD-Idd22 and NOD-Rag1−/− islets to AI4 T cell mediated lymphocytotoxicity in vitro was measured by co-culture using a 25:1 E:T ratio. Both unprimed and primed NOD-Idd22 islet cells were found to be more susceptible to AI4 induced killing than NOD islets. P<0.05; and P<0.001, respectively, Student’s t test. Data are presented as Mean±SEM from three independent experiments performed in triplicate.
Nitric oxide (NO) production was similar between NOD-Rag1−/− and NOD-Idd22 islets in the absence of cytokine treatment (P=0.11) and increased in both groups after treatment with combined cytokines for 72 hours (P<0.0001). Compared to NOD-Rag1−/− islets, NO production by NOD-Idd22 islets was lower in response to cytokine treatment (P<0.01) (Figure 3B). However, the variance in NO levels did not result in a difference in insulin secretory function or cell viability after 72 hour cytokine treatment (Figure 3A & 3C).
We next investigated if the β-cells of NOD-Idd22 mice are resistant to direct immune-cell mediated cytotoxicity in vitro. Surprisingly, after co-culture of NOD-Rag1−/− and NOD-Idd22 islet cells with activated NOD.AI4α/β-Rag1−/− CTL (25:1 Effector:Target ratio), NOD-Idd22 islets exhibited greater susceptibility to cell mediated lymphocytotoxicity, regardless of whether the target cells were primed with IFNγ for 24 hours prior to co-culture (P<0.05) (Figure 3D). These in vitro assays suggest that NOD-Idd22 islets are equally susceptible as NOD-Rag1−/− islets to pro-inflammatory cytokine induced dysfunction and death and potentially, even more vulnerable to cell-mediated killing in vitro.
NOD-Idd22 islets are protected against activated diabetogenic CTLs in vivo
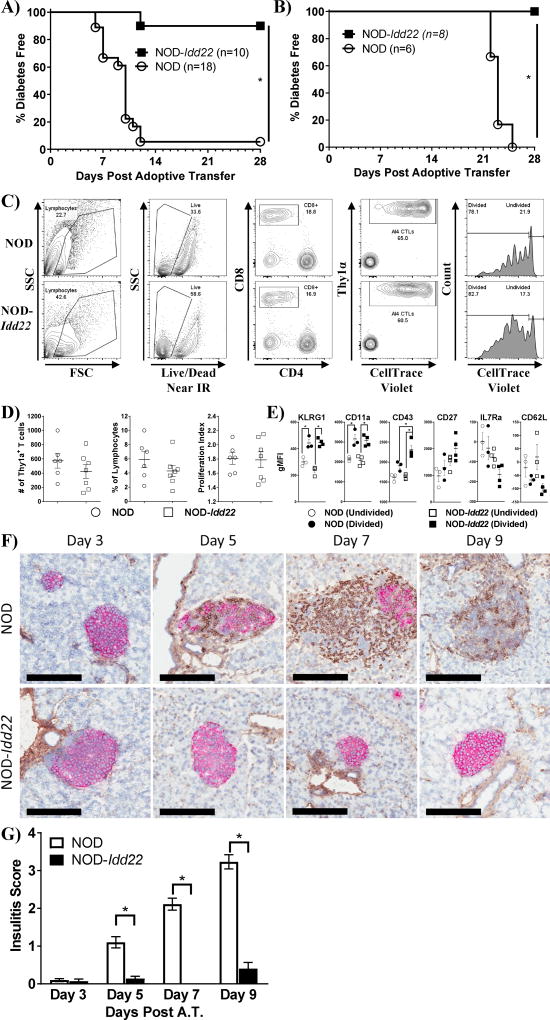
To determine whether NOD-Idd22 islets are protected from the highly diabetogenic AI4 CTL in vivo, we transferred 2×107 NOD.AI4α/β-Rag1−/− splenocytes into sub-lethally irradiated NOD and NOD-Idd22 mice. NOD-Idd22 recipients were highly resistant to T1D development after adoptive transfer of NOD.AI4α/β-Rag1−/− splenocytes, whereas greater than 90% of the NOD recipients developed T1D within 12 days post adoptive transfer (P<0.001) (Figure 4A). We also investigated whether NOD-Idd22 mice were resistant to transfer of another diabetogenic CTL clone. Confirming the results seen with transfer of AI4 CTLs, IS-CD8+ T cells failed to induce disease in NOD-Idd22 recipients by 28 days post transfer, whereas 100% of NOD recipients developed disease within 25 days (Figure 4B).
Figure 4. Activated AI4 lymphocytes fail to traffic to the islet in vivo in NOD-Idd22 recipients.
A. AI4 Splenocytes were adoptively transferred into NOD or NOD-Idd22 recipients and mice were monitored daily for development of T1D as before. AI4 splenocytes induced diabetes in >90% of NOD recipients by day 12 post transfer but failed to induce T1D in 90% of NOD-Idd22 mice through 30 days post transfer (P<0.001, logrank survival curve analysis). B. IS-CD8+ T cells (107) were adoptively transferred into recipient mice and monitored daily for development of T1D. IS-CD8 T cells failed to induce disease in NOD-Idd22 recipients by 28 days post transfer, whereas 100% of all NOD recipients developed T1D by 25 days post transfer (P<0.001, logrank survival curve analysis). C. Cell trace violet (CTV) labeled NOD.AI4α/β-Thy1α/β splenocytes were adoptively transferred into NOD-Idd22 and NOD recipients and pancreatic draining lymph nodes were biopsied three days later and analyzed via flow cytometery. A representative gating strategy for identification of transferred AI4 cytotoxic T lymphocytes (CTLs) in the pancreatic lymph node (PLN) is shown. Cells are initially selected on forward and side scatter, followed by exclusion of dead cells. AI4 cells are identified as CD8+ Thy1α+. Representative histograms show the extent of CTV dye dilution in NOD (top) and NOD-Idd22 (bottom) recipients. D. Thy1a+ frequency, number and proliferation index are identical in NOD and NOD-Idd22 recipients. Data points graphed are individual recipient mice (6 for NOD and 7 NOD-Idd22) and represent 3 individual experiments. E. The activation markers KLRG1, CD11a, CD43, and CD27 are all similar (or higher) in NOD-Idd22 recipients when compared to NOD, while, as expected, IL7Rα and CD62L expression was downregulated on proliferating cells (*P<0.05, Student’s t test). Data points graphed are individual recipient mice. Two independent experiments were performed. F. Representative micrographs of IHC stained pancreata from NOD (top row) and NOD-Idd22 (bottom row) recipients post transfer of 20×106 NOD.AI4α/β-Rag1−/−-Thy1α/β splenocytes at the indicated timepoints. NOD-Idd22 islets remain insulitis free with strong insulin staining through 9 days post transfer while NOD islets display increasing insulitis and reduction in insulin positive area and intensity through the same time period. Black scale bars indicate 200 um. G. NOD.AI4α/β-Rag1−/−-Thy1α/β splenocytes were adoptively transferred into NOD or NOD-Idd22 recipients. Pancreata were biopsied and fresh-frozen at indicated time points and stained via immunohistochemistry for insulin (Red) and Thy1α (Brown). Degree of infiltration of AI4 CTLs was determined in a similar fashion to insulitis scoring. Between 39 and 108 total islets from 5 recipient mice per group were scored and are listed in Supplemental Table.
To examine autoreactive T cell activation and proliferation in vivo, we transferred CTV labeled NOD.AI4α/β-Rag1−/−-Thy1α/β splenocytes into NOD and NOD-Idd22 recipients. Three days post transfer the PLN from recipient mice were harvested and analyzed. Transferred AI4 CTLs were identified by progressively gating on lymphocytes by forward and side scatter characteristics, live dump negative, CD8+ positive cells, and then on Thy1α+ cells (Figure 4C). No differences were observed in the frequency, number, or proliferative capacity of AI4 CTLs in the PLNs of NOD-Idd22 recipients compared to PLN of NOD mice (Figure 4D). Additionally, no differences were detected in activation markers on the AI4 CTL, including KLRG1, CD11a, CD27, IL7Rα, or CD62L in either recipient strain. Interestingly, CD43 was found to be significantly higher on proliferating AI4 CTLs transferred to NOD-Idd22 recipients as compared to NOD (Figure 4E). These data suggests that AI4 cells enter the PLN, are activated, and proliferate to similar extents in NOD and NOD-Idd22 recipients.
We then sought to determine whether activated AI4 CTLs fail to traffic to the islets or reach the islets but fail to destroy the target β-cells in vivo. We therefore quantified the extent of AI4 CTL infiltration into the islets of NOD and NOD-Idd22 animals at days 3, 5, 7, and 9 post transfer of NOD.AI4α/β-Rag1−/−-Thy1α/β splenocytes. As expected, pancreata from NOD recipient mice displayed an increase in severity of AI4 infiltration over time, with a concomitant loss of insulin intensity. In stark contrast, NOD-Idd22 pancreata displayed almost no AI4 infiltration, with no apparent reduction in insulin staining (Figure 4F–G, Supplemental Table). These data indicate Idd22 is preventing accumulation of activated, diabetogenic T cells in the islet in vivo.
Idd22 reduces the expression of adhesion molecules and firm T cell adhesion on pancreatic endothelial cells
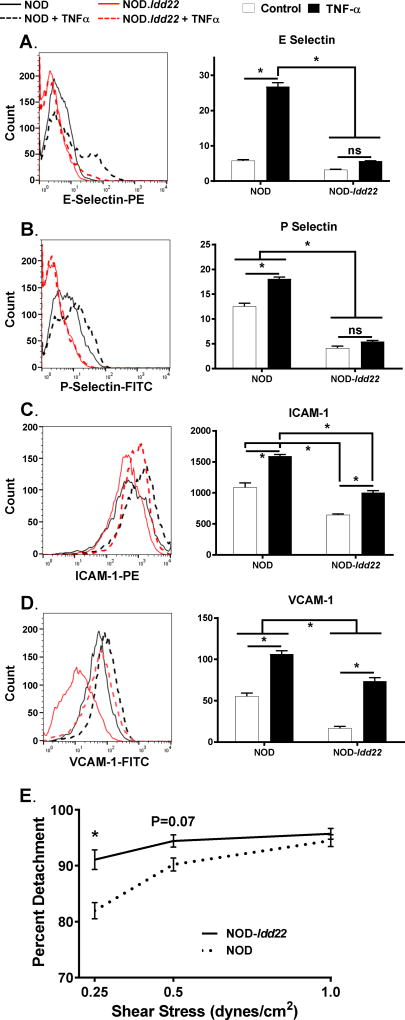
To determine whether Idd22 was modulating the ability of diabetogenic T cells to traffic to the islet by preventing adherence to the pancreatic microvasculature, we generated PEC lines from NOD and NOD-Idd22 mice. Utilizing flow cytometry, we measured the expression level of molecules critical for trafficking of lymphocytes out of the vasculature with or without TNFα stimulation. The basal expression level of E-Selectin (P=0.06) was not found to be different, however, basal levels of P-Selectin (P<0.0001), ICAM-1 (P<0.001), and VCAM-1 (P<0.001) were reduced in NOD-Idd22 when compared to NOD PECs. Furthermore, upon TNFα stimulation, NOD-Idd22 PECs failed to upregulate P selectin (P=0.21) and expressed significantly lower amounts of E-Selectin (P<0.0001), ICAM-1 (P<0.0001), and VCAM-1 (P<0.01) than NOD PECs (Figure 5A–D). To test if Idd22 acts within pancreatic endothelial cells and alters firm adhesion of diabetogenic T cells, laminal flow assays were performed to assess interactions of NOD CD8+ T cells with PEC. Enriched NOD CD8+ T cells formed strong interactions with NOD derived mPECs. In contrast, the interactions of NOD CD8+ T cells with NOD-Idd22 PECs were less stable under shearing tangential flow conditions (Figure 5E). These data suggest that Idd22 impacts the expression of adhesion molecules by PEC and reduce firm adhesion interactions leading to reduced potential for activated T cells to leave the vasculature and infiltrate the islets.
Figure 5. T cell trafficking is reduced in NOD-Idd22 mice due to reduced adhesion molecule expression and firm adhesion on NOD-Idd22 pancreatic endothelial cells.
Pancreatic Endothelial cells were isolated from NOD and NOD-Idd22 mice, pooled, and immortalized. Cells were grown to confluency and then either treated with media alone (empty bars) or media + TNF-α (black bars) for 4 hours and then stained for A. E-Selectin, B. P-Selectin, C. ICAM-1, and D. VCAM-1. Data were analyzed using ANOVA. *P<0.05 after FDR adjustment for multiple comparisons and are representative of 3 independent experiments performed in triplicate. E. Splenic NOD CTLs were isolated and stained with Cell Tracker Green, drawn across NOD or NOD-Idd22 derived PEC monolayers under indicated shear stress intensities, and monitored for the development of firm adhesion. Data are graphed as the percentage of firmly adherent cells that detached from the endothelial cell monolayers at each sheer stress. NOD endothelium (black dotted line) established stronger adhesive interactions with the T cells than did NOD-Idd22 (black solid line) endothelium. Data are compiled from 2 separate experiments performed in triplicate. (*P<0.05, ANOVA with multiple comparisons test).
Discussion
The ALR mouse was derived from the same outbred population as the NOD strain (1, 31) and shares significant genomic similarity: 85% genetic identity at SNPs typed genome-wide (32). However, unlike the NOD, ALR mice are extremely resistant against the development of both spontaneous and induced T1D. Further, ALR mice develop minimally detectable insulitis (15, 33). The genetic basis for the unusually strong resistance of ALR islets to immune-mediated destruction has been linked to four independent genetic loci: Chr 3 (Susp), 8 (Idd22), 17 (Idd16.1), and a SNP within the mitochondrial gene mt-Nd2 (18, 34). Idd22 is unique in that it is the only T1D-resistance linkage that has mapped to mouse Chr 8. In addition, since mt-Nd2 has also been linked to diabetes resistance in humans (35), other genetic variations harbored by the ALR mouse may be informative in developing new therapeutics for treatment of human disease.
To determine the mechanism of T1D protection imparted by the ALR-derived Idd22 locus, this region was introgressed onto the NOD background (Figure 1A). The resulting NOD-Idd22 mouse strain carrying an approximately 70 Mb pair congenic region of the ALR genome was found to be nearly completely protected from spontaneous T1D (Figure 1B). However, unlike the majority of T1D resistance linkages identified using outcrosses of NOD mice with other mouse strains, Idd22 does not appear to modulate the diabetogenicity of the immune system (13) (Figure 2). In addition, this protection is distinct from β-cell centric mechanisms, such as mt-Nd2 or Idd9 based protection (16, 36) as Idd22 does not appear to enhance protection of pancreatic islets against autoimmune effectors. The response of NOD-Idd22 islets to pro-inflammatory cytokine treatment and cell mediated lymphocytotoxicity assays in vitro more closely resembled NOD rather than ALR islets (15, 37, 38) (Figure 3), demonstrating that Idd22 does not contain the allele(s) responsible for the extreme resistance of ALR islets to cytotoxic mediators. Somewhat contrary to the in vitro data, histological examination of NOD-Idd22 pancreata revealed that the immune cell infiltration was significantly dampened in these congenic mice, even after transfer of highly diabetogenic T cells (Figures 1C, 1D, 4F–G, Supplemental Table). These observations indicate that the resistance to transferred disease provided by the ALR derived Idd22 allele is operating through a unique mechanism to reduce the severity of insulitis; hence, we hypothesized that the ALR derived Idd22 locus modulates a critical step in the pathogenesis of T1D, homing of autoreactive CTLs to the pancreatic islet. Although it is known that trafficking of lymphocytes to the pancreatic islet can be blocked by genetic ablation or pharmacological targeting of adhesion interactions (39–41), Idd22 is, to our knowledge, the first spontaneous Idd loci to accomplish a similar feat, even when challenged with a highly diabetogenic immune system (Figures 4 and 5).
Recruitment and homing of immune cells to the target organ is known to be a highly complex, but specific, process involving professional antigen presenting cells (APCs), vascular endothelial cells (VEC), and adhesion molecules, as well as cytokines and chemokines. The process is initiated as CTLs interact with and are activated by mature APCs presenting cognate antigen (in the case of autoimmune disorders, such as T1D, auto-antigens) in the draining lymph nodes, at which point they enter the bloodstream and travel to the specific target tissue (42). In NOD-Idd22 mice, autoreactive CTL are activated, proliferate, and then leave the PLN (Figure 4C–E). Once in the blood lumen, the CTLs loosely associate with the vascular endothelium via interactions with E-Selectins and P-Selectins and scan for their cognate antigen (43). As cognate antigen (primarily insulin in the case of T1D (44)) presented by the VECs is identified, antigen receptor engagement, along with LFA-1/ICAM-1 and CD44/hyaluronic acid interactions initiate firm adhesion of the CTL (40, 43, 45) to the vascular endothelium. Once firm adhesion to the VECs has been established, the CTL will then initiate proteolytic cleavage of CD44, weakening the intercellular interactions and undergo diapedesis through the vessel wall into the target tissue (41), at which point the CTLs will be in close proximity to the target cells. In Idd22 mice, the reduced ability of CTL to enter the islet (Figure 1C–G, 4F–H) is likely due to reduced levels of adhesion molecules expressed by vascular endothelial cells in the pancreas (Figure 5A–D). Although NOD-Idd22 PECs express intermediate levels of ICAM-1 and VCAM-1, the absence of E- and P-Selectin will reduce activation of LFA-1 on the surface of lymphocytes, preventing firm adhesion (Figure 5E), possibly through reduced ICAM-1 and VCAM-1 interactions. It is therefore plausible that Idd22 interferes with the homing of activated, autoreactive CTLs in T1D and does so by modulating VEC-CTL interactions.
A variety of experimental and a few clinical studies have provided evidence that blocking either the adhesion molecules (40, 46, 47) or chemokines (48, 49) involved in autoreactive CTL homing can result in the control of other autoimmune diseases such as rheumatoid arthritis, inflammatory bowel diseases, and psoriasis in both mice and humans. However, these therapies carry the risk of potentially dangerous adverse drug reactions (50, 51). Investigations into the specific genetic feature(s) responsible for the reduction in T cell trafficking in NOD-Idd22 mice are in progress. There are several genes of interest in the region that have previously been linked to human autoimmune disorders. Microtubule-associated serine-threonine-kinase 3 (Mast3) and Nucleotide-binding oligomerization domain containing 2 (NOD2) are intriguing candidate genes as both have previously been linked to autoimmune inflammatory bowel diseases in human patients (52–54). As Idd22 is able to control homing of CTLs without the need for exogenous intervention, the specific pathway involved may represent an attractive target for therapeutic intervention to prevent T1D development. Additional studies are therefore required to further delineate the exact mechanism by which Idd22 prevents entry of autoreactive CTLs into the pancreatic islet.
Supplementary Material
Acknowledgments
We are grateful to Wayne Orr for his generous gift of the sv40 Large T antigen construct for immortalization of the pancreas-derived endothelial cell lines, and Lynne Bauer for assistance with breeding and generating the NOD-Idd22 mouse strain.
Footnotes
Funding: Support for this work was provided by grants from the NIH AI56374 (C.E.M.) and DK074656 (C.E.M.), P01 AI042288, UC4 DK104194 American Diabetes Association (C.E.M.), and Lawson Wilkins Pediatric Endocrine Society (L.G.K.).
Duality of Interest: No potential conflicts of interest relevant to this article were reported.
Author Contributions: R.L.W., L.G.M., and C.E.M. performed the experiments, interpreted the data and wrote the manuscript. J.L., S.K., S.Z., M.A., V.M.P., A.F., I.R., C.A., A.S., A.Y., S.Y., J.G., and J.C. performed experiments and interpreted the data. C.G.K. provided insight and interpreted the data. C.E.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Leiter E, Atkinson M. NOD mice and related strains : research applications in diabetes, AIDS, cancer, and other diseases. R.G. Landes; Austin, Tex: 1998. [Google Scholar]
- 2.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kikutani H, Makino S. The murine autoimmune diabetes model: NOD and related strains. Adv Immunol. 1992;51:285–322. doi: 10.1016/s0065-2776(08)60490-3. [DOI] [PubMed] [Google Scholar]
- 4.Wicker LS, Leiter EH, Todd JA, Renjilian RJ, Peterson E, Fischer PA, Podolin PL, Zijlstra M, Jaenisch R, Peterson LB. Beta 2-microglobulin-deficient NOD mice do not develop insulitis or diabetes. Diabetes. 1994;43:500–504. doi: 10.2337/diab.43.3.500. [DOI] [PubMed] [Google Scholar]
- 5.Serreze DV, Chapman HD, Varnum DS, Gerling I, Leiter EH, Shultz LD. Initiation of autoimmune diabetes in NOD/Lt mice is MHC class I-dependent. Journal of immunology. 1997;158:3978–3986. [PubMed] [Google Scholar]
- 6.Serreze DV, Leiter EH, Christianson GJ, Greiner D, Roopenian DC. Major histocompatibility complex class I-deficient NOD-B2mnull mice are diabetes and insulitis resistant. Diabetes. 1994;43:505–509. doi: 10.2337/diab.43.3.505. [DOI] [PubMed] [Google Scholar]
- 7.Serreze DV, Leiter EH, Shultz LD. Transplantation analysis of B cell destruction in (NOD × CBA)F1 mouse bone marrow chimeras. Diabetologia. 1990;33:84–92. doi: 10.1007/BF00401045. [DOI] [PubMed] [Google Scholar]
- 8.Wicker LS, Miller BJ, Chai A, Terada M, Mullen Y. Expression of genetically determined diabetes and insulitis in the nonobese diabetic (NOD) mouse at the level of bone marrow-derived cells. Transfer of diabetes and insulitis to nondiabetic (NOD × B10) F1 mice with bone marrow cells from NOD mice. The Journal of experimental medicine. 1988;167:1801–1810. doi: 10.1084/jem.167.6.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savinov AY, Wong FS, Stonebraker AC, Chervonsky AV. Presentation of Antigen by Endothelial Cells and Chemoattraction Are Required for Homing of Insulin-specific CD8+ T Cells. Journal of Experimental Medicine. 2003;197:643–656. doi: 10.1084/jem.20021378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong FS, Visintin I, Wen L, Flavell RA, Janeway CA., Jr CD8 T cell clones from young nonobese diabetic (NOD) islets can transfer rapid onset of diabetes in NOD mice in the absence of CD4 cells. The Journal of experimental medicine. 1996;183:67–76. doi: 10.1084/jem.183.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Driver JP, Serreze DV, Chen YG. Mouse models for the study of autoimmune type 1 diabetes: a NOD to similarities and differences to human disease. Seminars in immunopathology. 2011;33:67–87. doi: 10.1007/s00281-010-0204-1. [DOI] [PubMed] [Google Scholar]
- 12.Jayasimhan A, Mansour KP, Slattery RM. Advances in our understanding of the pathophysiology of Type 1 diabetes: lessons from the NOD mouse. Clin Sci (Lond) 2014;126:1–18. doi: 10.1042/CS20120627. [DOI] [PubMed] [Google Scholar]
- 13.Thayer TC, Wilson SB, Mathews CE. Use of nonobese diabetic mice to understand human type 1 diabetes. Endocrinology and metabolism clinics of North America. 2010;39:541–561. doi: 10.1016/j.ecl.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wicker LS, Todd JA, Peterson LB. Genetic control of autoimmune diabetes in the NOD mouse. Annual review of immunology. 1995;13:179–200. doi: 10.1146/annurev.iy.13.040195.001143. [DOI] [PubMed] [Google Scholar]
- 15.Mathews CE, Graser RT, Savinov A, Serreze DV, Leiter EH. Unusual resistance of ALR/Lt mouse beta cells to autoimmune destruction: role for beta cell-expressed resistance determinants. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:235–240. doi: 10.1073/pnas.98.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Gusdon AM, Piganelli J, Leiter EH, Mathews CE. mt-Nd2(a) Modifies resistance against autoimmune type 1 diabetes in NOD mice at the level of the pancreatic beta-cell. Diabetes. 2011;60:355–359. doi: 10.2337/db10-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathews CE, Leiter EH, Spirina O, Bykhovskaya Y, Gusdon AM, Ringquist S, Fischel-Ghodsian N. mt-Nd2 Allele of the ALR/Lt mouse confers resistance against both chemically induced and autoimmune diabetes. Diabetologia. 2005;48:261–267. doi: 10.1007/s00125-004-1644-8. [DOI] [PubMed] [Google Scholar]
- 18.Mathews CE, Graser RT, Bagley RJ, Caldwell JW, Li R, Churchill GA, Serreze DV, Leiter EH. Genetic analysis of resistance to Type-1 Diabetes in ALR/Lt mice, a NOD-related strain with defenses against autoimmune-mediated diabetogenic stress. Immunogenetics. 2003;55:491–496. doi: 10.1007/s00251-003-0603-8. [DOI] [PubMed] [Google Scholar]
- 19.Varanasi V, Avanesyan L, Schumann DM, Chervonsky AV. Cytotoxic mechanisms employed by mouse T cells to destroy pancreatic beta-cells. Diabetes. 2012;61:2862–2870. doi: 10.2337/db11-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerling IC, Serreze DV, Christianson SW, Leiter EH. Intrathymic islet cell transplantation reduces beta-cell autoimmunity and prevents diabetes in NOD/Lt mice. Diabetes. 1992;41:1672–1676. doi: 10.2337/diab.41.12.1672. [DOI] [PubMed] [Google Scholar]
- 21.Thayer TC, Delano M, Liu C, Chen J, Padgett LE, Tse HM, Annamali M, Piganelli JD, Moldawer LL, Mathews CE. Superoxide production by macrophages and T cells is critical for the induction of autoreactivity and type 1 diabetes. Diabetes. 2011;60:2144–2151. doi: 10.2337/db10-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, Mathews CE. Use of chemical probes to detect mitochondrial ROS by flow cytometry and spectrofluorometry. Methods Enzymol. 2014;542:223–241. doi: 10.1016/B978-0-12-416618-9.00012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christianson SW, Shultz LD, Leiter EH. Adoptive transfer of diabetes into immunodeficient NOD-scid/scid mice. Relative contributions of CD4+ and CD8+ T-cells from diabetic versus prediabetic NOD.NON-Thy-1a donors. Diabetes. 1993;42:44–55. doi: 10.2337/diab.42.1.44. [DOI] [PubMed] [Google Scholar]
- 24.Shultz LD, Lang PA, Christianson SW, Gott B, Lyons B, Umeda S, Leiter E, Hesselton R, Wagar EJ, Leif JH, Kollet O, Lapidot T, Greiner DL. NOD/LtSz-Rag1null mice: an immunodeficient and radioresistant model for engraftment of human hematolymphoid cells, HIV infection, and adoptive transfer of NOD mouse diabetogenic T cells. Journal of immunology. 2000;164:2496–2507. doi: 10.4049/jimmunol.164.5.2496. [DOI] [PubMed] [Google Scholar]
- 25.Ablamunits V, Elias D, Cohen IR. The pathogenicity of islet-infiltrating lymphocytes in the non-obese diabetic (NOD) mouse. Clin Exp Immunol. 1999;115:260–267. doi: 10.1046/j.1365-2249.1999.00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takaki T, Marron MP, Mathews CE, Guttmann ST, Bottino R, Trucco M, DiLorenzo TP, Serreze DV. HLA-A*0201-restricted T cells from humanized NOD mice recognize autoantigens of potential clinical relevance to type 1 diabetes. Journal of immunology. 2006;176:3257–3265. doi: 10.4049/jimmunol.176.5.3257. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Grieshaber S, Mathews CE. Methods to assess beta cell death mediated by cytotoxic T lymphocytes. Journal of visualized experiments : JoVE. 2011 doi: 10.3791/2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sobczak M, Dargatz J, Chrzanowska-Wodnicka M. Isolation and culture of pulmonary endothelial cells from neonatal mice. Journal of visualized experiments : JoVE. 2010 doi: 10.3791/2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yurdagul A, Jr, Green J, Albert P, McInnis MC, Mazar AP, Orr AW. alpha5beta1 integrin signaling mediates oxidized low-density lipoprotein-induced inflammation and early atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:1362–1373. doi: 10.1161/ATVBAHA.114.303863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharp CD, Huang M, Glawe J, Patrick DR, Pardue S, Barlow SC, Kevil CG. Stromal cell-derived factor-1/CXCL12 stimulates chemorepulsion of NOD/LtJ T-cell adhesion to islet microvascular endothelium. Diabetes. 2008;57:102–112. doi: 10.2337/db07-0494. [DOI] [PubMed] [Google Scholar]
- 31.Ino T, Kawamoto Y, Sato K, Nishikawa K, Yamada A, Ishibashi K, Sekiguchi F. Selection of mouse strains showing high and low incidences of alloxan-induced diabetes. Jikken Dobutsu. 1991;40:61–67. doi: 10.1538/expanim1978.40.1_61. [DOI] [PubMed] [Google Scholar]
- 32.Yang H, Ding Y, Hutchins LN, Szatkiewicz J, Bell TA, Paigen BJ, Graber JH, de Villena FP, Churchill GA. A customized and versatile high-density genotyping array for the mouse. Nat Methods. 2009;6:663–666. doi: 10.1038/nmeth.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathews CE, Leiter EH. Resistance of ALR/Lt islets to free radical-mediated diabetogenic stress is inherited as a dominant trait. Diabetes. 1999;48:2189–2196. doi: 10.2337/diabetes.48.11.2189. [DOI] [PubMed] [Google Scholar]
- 34.Mathews CE, Dunn BD, Hannigan MO, Huang CK, Leiter EH. Genetic control of neutrophil superoxide production in diabetes-resistant ALR/Lt mice. Free radical biology & medicine. 2002;32:744–751. doi: 10.1016/s0891-5849(02)00747-5. [DOI] [PubMed] [Google Scholar]
- 35.Uchigata Y, Okada T, Gong JS, Yamada Y, Iwamoto Y, Tanaka M. A mitochondrial genotype associated with the development of autoimmune-related type 1 diabetes. Diabetes Care. 2002;25:2106. doi: 10.2337/diacare.25.11.2106. [DOI] [PubMed] [Google Scholar]
- 36.Hill NJ, Stotland A, Solomon M, Secrest P, Getzoff E, Sarvetnick N. Resistance of the target islet tissue to autoimmune destruction contributes to genetic susceptibility in Type 1 diabetes. Biology direct. 2007;2:5. doi: 10.1186/1745-6150-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DiLorenzo TP, Graser RT, Ono T, Christianson GJ, Chapman HD, Roopenian DC, Nathenson SG, Serreze DV. Major histocompatibility complex class I-restricted T cells are required for all but the end stages of diabetes development in nonobese diabetic mice and use a prevalent T cell receptor alpha chain gene rearrangement. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:12538–12543. doi: 10.1073/pnas.95.21.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graser RT, DiLorenzo TP, Wang F, Christianson GJ, Chapman HD, Roopenian DC, Nathenson SG, Serreze DV. Identification of a CD8 T cell that can independently mediate autoimmune diabetes development in the complete absence of CD4 T cell helper functions. Journal of immunology. 2000;164:3913–3918. doi: 10.4049/jimmunol.164.7.3913. [DOI] [PubMed] [Google Scholar]
- 39.Martin S, van den Engel NK, Vinke A, Heidenthal E, Schulte B, Kolb H. Dominant role of intercellular adhesion molecule-1 in the pathogenesis of autoimmune diabetes in non-obese diabetic mice. Journal of autoimmunity. 2001;17:109–117. doi: 10.1006/jaut.2001.0526. [DOI] [PubMed] [Google Scholar]
- 40.Savinov AY, Strongin AY. Targeting the T-cell membrane type-1 matrix metalloproteinase-CD44 axis in a transferred type 1 diabetes model in NOD mice. Experimental and therapeutic medicine. 2013;5:438–442. doi: 10.3892/etm.2012.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savinov AY, Burn P. Interference with islet-specific homing of autoreactive T cells: an emerging therapeutic strategy for type 1 diabetes. Drug discovery today. 2010;15:531–539. doi: 10.1016/j.drudis.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 42.Mempel TR, Henrickson SE, von Andrian UH. T-cell priming by dendriticcells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 43.Weber C. Novel mechanistic concepts for the control of leukocyte transmigration: specialization of integrins, chemokines, and junctional molecules. J Mol Med (Berl) 2003;81:4–19. doi: 10.1007/s00109-002-0391-x. [DOI] [PubMed] [Google Scholar]
- 44.Mohan JF, Unanue ER. A novel pathway of presentation by class II-MHC molecules involving peptides or denatured proteins important in autoimmunity. Mol Immunol. 2013;55:166–168. doi: 10.1016/j.molimm.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dustin ML, Bromley SK, Kan Z, Peterson DA, Unanue ER. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proceedings of the National Academy of Sciences. 1997;94:3909–3913. doi: 10.1073/pnas.94.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verdrengh M, Holmdahl R, Tarkowski A. Administration of antibodies to hyaluronanreceptor (CD44) delays the start and ameliorates the severity of collagen II arthritis. Scandinavian journal of immunology. 1995;42:353–358. doi: 10.1111/j.1365-3083.1995.tb03667.x. [DOI] [PubMed] [Google Scholar]
- 47.Davis LS, Kavanaugh AF, Nichols LA, Lipsky PE. Induction of persistent T cell hyporesponsiveness in vivo by monoclonal antibody to ICAM-1 in patients with rheumatoid arthritis. Journal of immunology. 1995;154:3525–3537. [PubMed] [Google Scholar]
- 48.Plater-Zyberk C, Hoogewerf AJ, Proudfoot AE, Power CA, Wells TN. Effect of a CC chemokine receptor antagonist on collagen induced arthritis in DBA/1 mice. Immunology letters. 1997;57:117–120. doi: 10.1016/s0165-2478(97)00075-8. [DOI] [PubMed] [Google Scholar]
- 49.Schwarz MK, Wells TN. Interfering with chemokine networks--the hope for new therapeutics. Curr Opin Chem Biol. 1999;3:407–417. doi: 10.1016/S1367-5931(99)80061-1. [DOI] [PubMed] [Google Scholar]
- 50.Ferner RE. Adverse drug reactions in dermatology. Clin Exp Dermatol. 2015;40:105–109. doi: 10.1111/ced.12572. quiz 109–110. [DOI] [PubMed] [Google Scholar]
- 51.Tavazzi E, Ferrante P, Khalili K. Progressive multifocal leukoencephalopathy: an unexpected complication of modern therapeutic monoclonal antibody therapies. Clin Microbiol Infect. 2011;17:1776–1780. doi: 10.1111/j.1469-0691.2011.03653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Labbe C, Boucher G, Foisy S, Alikashani A, Nkwimi H, David G, Beaudoin M, Goyette P, Charron G, Xavier RJ, Rioux JD. Genome-wide expression profiling implicates a MAST3-regulated gene set in colonic mucosal inflammation of ulcerative colitis patients. Inflammatory bowel diseases. 2012;18:1072–1080. doi: 10.1002/ibd.21887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Labbe C, Goyette P, Lefebvre C, Stevens C, Green T, Tello-Ruiz MK, Cao Z, Landry AL, Stempak J, Annese V, Latiano A, Brant SR, Duerr RH, Taylor KD, Cho JH, Steinhart AH, Daly MJ, Silverberg MS, Xavier RJ, Rioux JD. MAST3: a novel IBD risk factor that modulates TLR4 signaling. Genes and immunity. 2008;9:602–612. doi: 10.1038/gene.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.