Abstract
Background
Early-phase trials in relapsed neuroblastoma patients historically used objective “response” of measureable disease (RECIST, without bone/bone marrow assessment) to select agents for further study. Historical cohorts may be small and potentially biased; relapse studies from international registries are outdated. Using our large recent cohort of relapsed/refractory neuroblastoma patients from COG modern-era early-phase trials, we determined outcome and quantified parameters for designing future studies.
Methods
The first early-phase COG trial enrollment (sequential) of 383 distinct relapsed/refractory neuroblastoma patients on 23 Phase 1, 3 Phase 1/2, and 9 Phase 2 trials (8/2002–1/2014) was analyzed for progression-free survival (PFS), overall survival (OS), and time-to-progression (TTP). High-risk neuroblastoma planned frontline therapy included hematopoietic stem cell transplant (~two-thirds received ≥1 HSCT); 13.2% received dinutuximab.
Results
From time of patient’s first early-phase trial enrollment (n=383): 1-year/4-year PFS were 21±2%/6±1%; 1-year/4-year OS were 57±3%/20±2%, respectively; median TTP was 58 days (interquartile range: 31–183 days, n=350); median follow-up was 25.3 months (n=33 without relapse/progression). Median time from diagnosis to first relapse/progression (TTFR) was 18.7 months (range: 1.4–64.8 months) (n=176). MYCN amplification (p=0.003, p<0.0001) and 11q LOH (p=0.02, p=0.03) were prognostic of worse PFS and OS, respectively, after early-phase trial enrollment.
Conclusions
This recent COG relapsed/refractory neuroblastoma cohort is inclusive and representative. This is the first meta-analysis of PFS/TTP/OS in the context of modern therapy. These results will inform design of future phase 2 studies by providing: historical context during the search for more effective agents, and factors prognostic of PFS/OS after relapse to stratify randomization.
Keywords: phase 2 design, endpoints, prognostic, RECIST, INRC, historical standard
Introduction
Neuroblastoma is the most common extracranial solid tumor of childhood. Current best available treatment (induction chemotherapy, surgery, radiation, hematopoietic stem cell transplant (HSCT), and immunotherapy) cures only half of patients with high-risk neuroblastoma1,2; this therapy is very toxic, and better treatments are needed. Novel treatments for patients with relapsed/refractory neuroblastoma are being studied; however, the methods of evaluating efficacy vary. In early-phase clinical trials, RECIST is the most common approach to assessment of response; however, RECIST does not address the most frequent sites of recurrent neuroblastoma, bone and bone marrow3. The International Neuroblastoma Response Criteria (INRC) include bone marrow and bone disease4,5, albeit not well quantified by INRC criteria, leading to variation in interpretation of disease burden and potential poor correlation with the true disease state.
During 8/2002–1/2014, the Children’s Oncology Group (COG) conducted nine Phase 2 trials of novel single agent or combination therapies on which patients with neuroblastoma were treated. RECIST criteria were applied to determine the tumor response; however, only two (ANBL0322, ANBL0421) of the nine Phase 2 trials met the objective response rate bar for success as prospectively defined per protocol6,7. This highlights the need for additional agents to be tested in the Phase 2 setting, and suggests that assessment of progression-free survival (PFS), time-to-progression (TTP), and overall survival (OS) would provide important insights into the true effects of new agents/combinations that may not be apparent if RECIST-based evaluations are used exclusively. Longer follow-up may be necessary to observe benefit, as reflected by endpoints PFS, OS, and TTP.
The goals of this study were: a) to estimate PFS, OS, and TTP in a large cohort of relapsed/refractory neuroblastoma patients treated with modern-era early-phase therapy to provide historical context; and, b) to identify factors prognostic of PFS and OS, from the time of early-phase trial enrollment. PFS, OS, and TTP have been estimated from a large International Neuroblastoma Risk Groups (INRG) study of neuroblastoma patients in first relapse (n=2,266)8 who were diagnosed from 1990–2002, i.e., they were not treated with modern-era therapy. Herein we provide more relevant, updated estimates of PFS, OS, and TTP. It was not a study objective, nor was it possible, to summarize response rates across trials; response criteria changed over time, but the definition of progressive disease did not. We suggest that TTP and PFS endpoints could be used to measure potential therapeutic benefit in neuroblastoma studies.
Patients and Methods
To be eligible for analysis, patients had to be diagnosed with neuroblastoma and eligible/enrolled on a COG Phase 1 or 2 trial from 11/2002–1/2014 (when all COG frontline neuroblastoma trials included HSCT). All consecutive eligible patients were included. Only a patient’s first early-phase COG trial enrollment was analyzed.
Phase 1 and Phase 2 trial eligibility criteria
Inclusion criteria for the trials were: refractory (non-responsive) or relapsed/progressing neuroblastoma (histologically verified or present in bone marrow with elevated urinary catecholamines at diagnosis), no known standard therapy, performance status ≥50%, ≥30 days since last dose of investigational drug/immunotherapy, ≥21 days since last myelosuppressive therapy, ≥7 days since last biologic therapy, full recovery from the toxicity of prior therapy, and adequate organ function. In all but one study (ANBL0421), there were no limitations on the number of prior relapses or prior therapeutic regimens. Patients who were pregnant or breastfeeding were excluded. Furthermore, in six Phase 2 trials after 2005, patients were stratified by disease: a) measureable by CT or MRI scans; or, b) evaluable, as assessed by 123I-MIBG scintigraphy. Patients or parents/guardians provided informed consent for trial enrollment, and trials were approved by either the local institution’s Institutional Review Board (IRB) or the NCI’s Pediatric Central IRB.
Phase 1 and Phase 2 trial therapy
Thirty-five COG trials were included: 23 Phase 1, three Phase 1/2, and nine Phase 2 (Table 1, Supplementary Table 1). The number of agents per trial were: single agent (24), two agents (7), three agents (3), and four agents (1). The number of trials by type of treatment were: single agent, cytotoxic (5); two agents, including a cytotoxic (9); single agent, targeted (14); two agents, targeted (2); retinoids (2); and, immunotherapy (3). The planned time to first assessment of response was a median of 28 days from enrollment (range: 21–63 days) (Table 1).
Table 1.
List of COG early-phase trials analyzed
| COG Study |
Phase | Treatment | Timing of Response Assessment* After: |
Days from study enrollment to first response assessment |
Number of patients analyzed* |
|---|---|---|---|---|---|
| ADVL0211 | 1 | G3139, Cytotoxic Chemotherapy | cycle 1,3,5,7,… | 21 | 5 |
| ADVL0212 | 1 | Depsipeptide | course 1 and 2 | 28 | 1 |
| ADVL0214 | 1 | Tarceva, Tarceva + Temozolomide | cycle 2,4,6,8,,… | 56 | 2 |
| ADVL0215 | 1 | Decitabine, Doxorubicin, Cyclophosphamide | each course | 28 | 12 |
| ADVL0314 | 1 | Bevacizumab | cycle 1,3,5,7,… | 28 | 2 |
| ADVL0316 | 1 | 17-AAG | course 1 | 21 | 1 |
| ADVL0319 | 1 | Lenalidomide | cycle 1,3,5,7,… | 28 | 1 |
| ADVL0414 | 1 | Temozolomide, Irinotecan, Vincristine | course 2,4,8,12,16,… | 42 | 2 |
| ADVL0415 | 1 | Oxaliplatin, Irinotecan | cycle 1,3,5,7,… | 21 | 1 |
| ADVL0416 | 1 | SAHA, 13 Cis-Retinoic Acid | cycle 1,3,5,7,… | 28 | 5 |
| ADVL0516 | 1 | Dasatinib | cycle 1,3,5,7,… | 28 | 1 |
| ADVL0517 | 1 | Ispinesib | cycle 1,3,5,7,… | 28 | 1 |
| ADVL0714 | 1 | VEGF Trap | cycle 2,6,10,14,,… | 28 | 1 |
| ADVL0813 | 1 | IMC-A12, (Temsirolimus | cycle 1,3,5,7,… | 28 | 2 |
| ADVL0815 | 1 | Pazopanib | cycle 1,2,4,6,8,… | 28 | 1 |
| ADVL0816 | 1 | Obatoclax/Vincristine/Doxorubicin/Dexrazoxane | cycle 1,3,5,7,… | 28 | 3 |
| ADVL0911 | 1 | Seneca Valley Virus | 4 weeks after 2nd infusion, then Weeks 8,16,28,40,52,… | 56 | 2 |
| ADVL0916 | 1 | Vorinostat, Bortezomib | each cycle | 21 | 3 |
| ADVL0918 | 1 | Temsirolimus, Irinotecan, Temozolomide | cycle 2,4,6,9,12,… | 42 | 13 |
| ADVL1013 | 1 | MK-2206 | cycle 1,3,5,8,11,… | 28 | 3 |
| ADVL1014 | 1 | REOLYSIN | cycle 1,3,5,8,11,… | 28 | 1 |
| ADVL1111 | 1 | c-Met Inhibitor, Tivantinib | cycle 1,3,5,8,11,… | 28 | 2 |
| ADVL1112 | 1 | Imetelstat | cycle 1,3,5,8,11,… | 21 | 4 |
| ADVL0413 | 1/2 | Sorafenib | cycle 2,4,6,8,,… | 56 | 1 |
| ADVL0812 | 1/2 | MLN8237 | cycle 1,3,5,7,9,… | 21 | 16 |
| ADVL0912 | 1/2 | PF-02341066 | cycle 1,3,5,7,10,13,… | 28 | 24 |
| ANBL0321 | 2 | Fenretinide | courses 2,4,7,11,15,19,23 | 42 | 59 |
| ANBL0322 | 2 | hu14.18-IL2 | 2 monthly courses | 56 | 35 |
| ANBL0421 | 2 | Irinotecan, Temozolomide | courses 3 and 6 | 63 | 55 |
| ANBL0621 | 2 | ABT-751 | cycle 2,4 6,8,10,14,… | 42 | 71 |
| ADVL0421 | 2 | Oxaliplatin | course 2,4,6,8,… | 42 | 10 |
| ADVL0524 | 2 | Ixabepilone | cycles 1,3,5,7,… | 21 | 9 |
| ADVL0525 | 2 | Pemetrexed | cycle 1,3,5,7,… | 21 | 4 |
| ADVL0821 | 2 | IMC-A12 | cycle 1,2,3,5,7,… | 28 | 20 |
| ADVL0122 | 2 | Gleevec | course 2,4,6,9,12,15,… | 56 | 10 |
This is the subset of patients enrolled on the trial who met the eligibility criteria for this analysis.
Prior therapy and risk factors at diagnosis
Prior therapy was known only if the patient was previously treated on a COG trial, i.e., unknown if prior therapy was administered “as per” COG protocols, or on trials from other groups/consortia. Risk factor data and TTFR (from diagnosis) were unknown if the patient did not enroll on a COG frontline trial or biology study.
Statistical Considerations
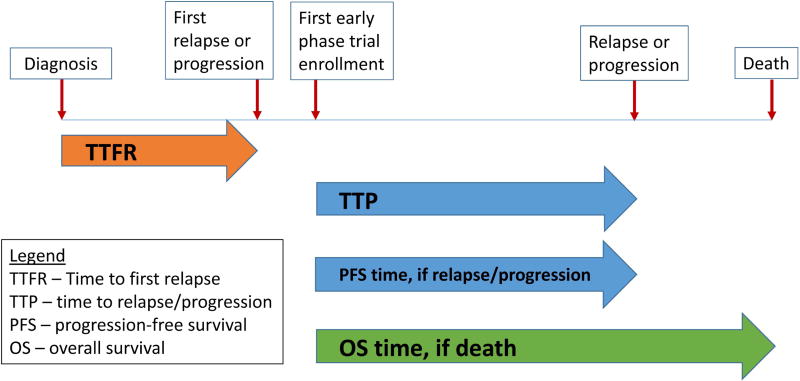
Analyses were conducted as intent-to-treat. Time to progression for PFS and TTP was calculated from the time of early-phase trial enrollment until the first occurrence of neuroblastoma relapse, progression, or death from neuroblastoma (Figure 1). For PFS, observations were censored if none of these events occurred. Overall survival (OS) time was calculated from early-phase trial enrollment until death from any cause, or censored at last contact.
Figure 1.
Schematic portrayal of TTFR (time to first relapse/progression after diagnosis), TTP (time to progression after first enrollment on an early-phase trial), PFS time (if neuroblastoma relapse, progression, or death from neuroblastoma), and OS time (if death).
Kaplan-Meier curves, with standard errors according to Peto, were generated9,10, and risk factor subgroups compared with a log rank test. The proportional hazards (PH) assumption was investigated, and a Cox PH regression model (backwards selection) was used to identify prognostic factors11, using dummy variables for the ‘missing’ category to avoid loss of sample size due to missingness. Using factors previously validated as prognostic of outcome in newly diagnosed neuroblastoma patients, we tested them for prognostic ability in relapsed/refractory patients from the time of first early-phase trial enrollment: COG risk group (low/intermediate vs high), International Neuroblastoma Staging System (INSS) stage (1,2,3,4S vs 4)4,12, age at diagnosis (<547 vs ≥547 days)13,14,15, MYCN status (not amplified vs amplified)16,17, ploidy (hyperdiploid vs diploid)17,18,19, International Neuroblastoma Pathology Classification (INPC) (favorable vs unfavorable)20,21, mitosis-karyorrhexis index (MKI) (low/intermediate vs high)22, grade (differentiating vs undifferentiated)23, 11q (no loss of heterozygosity [LOH], LOH)24,25, 1p (no LOH, LOH)24,25, prior transplant (yes vs no), and time from diagnosis to first relapse/progression (TTFR)8,26. To facilitate clinical utility of TTFR, an ‘optimal’ TTFR cut-off was sought. Patients were randomly allocated to separate Test and Validation sets. Recursive partitioning was performed, using a Cox model for OS to test cut-offs at 12,15,18,21,24,27,30,33, and 36 months. The cut-off with the largest hazard ratio (HR) (reference level: TTFR above the cut-off) among those with a significant p-value was selected from the Test set, to be confirmed in the Validation set.
Results
Prior Therapy
Before enrolling on a COG early-phase trial, 98 (26%) of 383 patients received therapy on COG frontline trials (Table 2). Outcome for patients who did versus did not enroll on a COG frontline trial or biology study was similar (PFS: p=0.8; OS: p=0.3). One hundred eighty (64%) of 281 patients received at least one transplant. Fifty-one (13.2%) patients received an anti-GD2 antibody on a COG trial: dinutuximab as post-consolidation therapy (44)27, dinutuximab for relapsed/refractory disease (2), and hu14.18-IL2 fusion molecule (10).
Table 2.
Therapy prior to inclusion in early-phase trial cohort (n=136 of 383)
| Protocol | Treatment | Number of patients |
|---|---|---|
| Frontline therapy | ||
| 321P3 | Autologous purged BMT after induction and conditioning with etoposide, cisplatin, and TBI | 1 |
| P9641 | Surgery, observation; salvage chemotherapy | 5 |
| A3961 | 4–8 cycles of carboplatinum, etoposide, cyclophosphamide, doxorubicin | 2 |
| A3973 | Purged v. Unpurged PBSC Transplant after Dose Intensive Induction Therapy | 57 |
| ANBL00P1 | Tandem High Dose Chemotherapy with Stem Cell Rescue Following Induction | 2 |
| ANBL02P1 | Induction Incorporating Dose-Intensive Topotecan and Cyclophosphamide | 6 |
| ANBL0531 | 2–8 cycles of chemotherapy | 1 |
| ANBL0532 | Randomized Trial of Single vs. Tandem Myeloablative Consolidation Therapy | 24 |
| Maintenance therapy | ||
| ANBL0032 | chimeric antibody 14.18 (Ch14.18), GM-CSF, IL-2, and Isotretinoin | 42 |
| ANBL0931 | chimeric antibody 14.18 (Ch14.18), GM-CSF, IL-2, and Isotretinoin | 2 |
| Phase 1 or 2 therapy | ||
| P9761 | irinotecan | 2 |
| P9462 | Topotecan, cyclophosphamide | 5 |
| A0935A | ch14.18 with GM-CSF, IL-2 in GD2 Positive Malignancies post ABMT or PBSC Rescue | 2 |
| ADVL0018 | hu14.18-IL2 Fusion Protein in Patients with GD2 Expressing Tumors | 6 |
| ADVL0016 | ZD1839 IressaTM, an Oral Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor | 1 |
| ADVL0921 | MLN8237 (IND# 102984), a Selective Aurora A Kinase Inhibitor | 8 |
| ADVL1011 | JAK Inhibition with Ruxolitinib | 4 |
| ANBL1021 | hu14.18-IL2 immunocytokine + GM-CSF and Isotretinoin | 8 |
Patient Characteristics
Within the subsets of patients with known risk factor data at initial diagnosis: 214/233 patients (92%) were high-risk, 205/234 patients (88%) had INSS stage 4 disease, 218/235 (93%) were ≥18 months of age at diagnosis, 32/195 (16%) had tumors with MYCN amplification, 91/182 (50%) had tumors that were diploid, 165/177 (93%) were unfavorable INPC, 29/124 (23%) had high MKI, 14/140 (10%) had differentiating grade, 14/31 (45%) had 11q LOH, and 8/32 (25%) had 1p LOH (Table 3).
Table 3.
Survival of modern-era relapsed/refractory neuroblastoma patients, overall and according to standard risk factors determined at the time of diagnosis (n=383 patients)
| Number of patients n (%) |
PFS from start of early-phase trial enrollment |
OS from start of early-phase trial enrollment |
|||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1-yr ±SE (%) |
4-yr ±SE (%) |
p-value | 1-yr ±SE (%) |
4-yr ±SE (%) |
p-value | ||
|
| |||||||
| Overall | 383 | 21±2 | 6±1 | NA | 57±3 | 20±2 | NA |
|
| |||||||
| Risk Group | |||||||
| Low | 12 (5) | 28±14 | 9±9 | 55±15 | 28±14 | ||
| Intermediate | 7 (3) | 29±17 | 0 | 0.9 | 57±19 | 29±22 | 0.8 |
| High | 214 (92) | 20±3 | 6±2 | 58±3 | 15±3 | ||
| unknown | 150 | ||||||
|
| |||||||
| Era* of first early-phase enrollment | |||||||
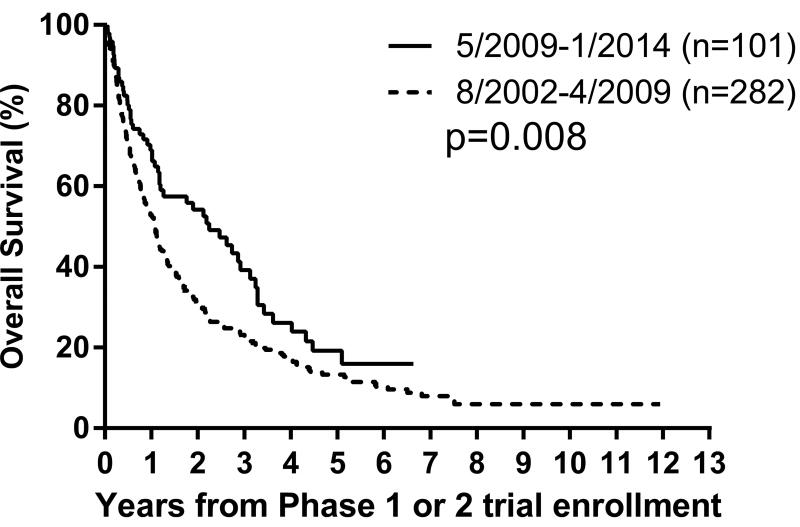
| 8/02 – 4/09 (pre-dinutuximab) | 282 (74) | 19±2 | 5±1 | 0.1 | 53±3 | 17±2 | 0.008 |
| 5/09 – 1/14 (post-dinutuximab) | 101 (26) | 28±5 | 10±4 | 69±5 | 26±6 | ||
|
| |||||||
| INSS stage# | |||||||
| 1,2,3,4S | 29 (12) | 18±7 | 5±4 | 0.6 | 46±10 | 15±8 | 0.5 |
| 4 | 205 (88) | 22±3 | 6±2 | 59±4 | 17±3 | ||
| unknown | 149 | ||||||
|
| |||||||
| Age at diagnosis# | |||||||
| <18 months | 17 (7) | 35±12 | 28±11 | 0.1 | 46±12 | 32±12 | 0.4 |
| ≥18 months | 218 (93) | 20±3 | 5±2 | 59±3 | 16±2 | ||
| unknown | 148 | ||||||
|
| |||||||
| MYCN status# | |||||||
| Not amplified | 163 (84) | 23±3 | 7±2 | 0.003 | 62±4 | 21±3 | <0.0001 |
| Amplified | 32 (16) | 13±6 | 0 | 30±8 | 0 | ||
| unknown | 188 | ||||||
|
| |||||||
| Ploidy# | |||||||
| Hyperdiploid | 91 (50) | 20±4 | 3±2 | 0.5 | 49±5 | 21±5 | 0.8 |
| Diploid | 91 (50) | 24±5 | 10±3 | 59±5 | 15±4 | ||
| unknown | 201 | ||||||
|
| |||||||
| Histology# | |||||||
| Favorable | 12 (7) | 25±13 | 17±11 | 0.5 | 57±15 | 29±14 | 0.4 |
| Unfavorable | 165 (93) | 19±3 | 5±2 | 55±4 | 16±3 | ||
| unknown | 206 | ||||||
|
| |||||||
| MKI# | |||||||
| Low/Intermediate | 95 (77) | 16±4 | 6±3 | 0.2 | 54±5 | 15±4 | 0.3 |
| High | 29 (23) | 30±9 | 5±5 | 40±9 | 9±6 | ||
| unknown | 259 | ||||||
|
| |||||||
| Grade of differentiation# | |||||||
| Differentiating | 14 (10) | 14±10 | 0 | 1.0 | 57±13 | 7±7 | 0.5 |
| Undifferentiated | 126 (90) | 20±4 | 7±2 | 54±5 | 15±3 | ||
| unknown | 243 | ||||||
|
| |||||||
| 11q# | |||||||
| No LOH | 17 (55) | 30±13 | 0 | 0.02 | 67±12 | 10±9 | 0.03 |
| LOH | 14 (45) | 0 | 0 | 31±13 | 0 | ||
| unknown | 352 | ||||||
|
| |||||||
| 1p# | |||||||
| No LOH | 24 (75) | 19±9 | 0 | 0.4 | 55±11 | 6±5 | 0.3 |
| LOH | 8 (25) | 0 | 0 | 50±18 | 0 | ||
| unknown | 351 | ||||||
|
| |||||||
| Prior transplant | |||||||
| No | 101 (36) | 26±5 | 8±3 | 0.2 | 58±5 | 27±5 | 0.5 |
| Yes | 180 (64) | 18±3 | 4±2 | 61±4 | 16±3 | ||
| unknown | 102 | ||||||
|
| |||||||
| TTFR# | |||||||
| <30 months | 146 (83) | 15±3 | 4±2 | 0.3 | 48±4 | 11±3 | 0.055 |
| ≥30 months | 30 (17) | 21±8 | 0 | 72±8 | 17±8 | ||
| unknown | 207 | ||||||
At the time of diagnosis
NA – Not applicable
Efficacy of dinutuximab identified 4/2009
Outcome
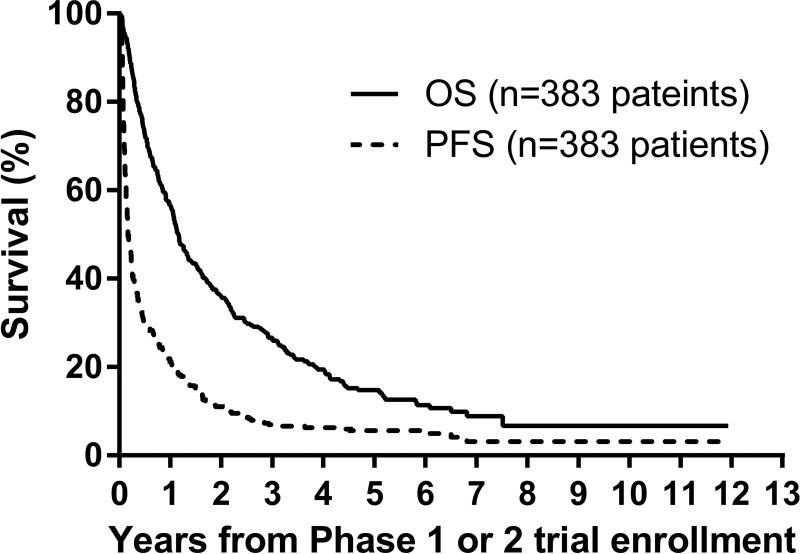
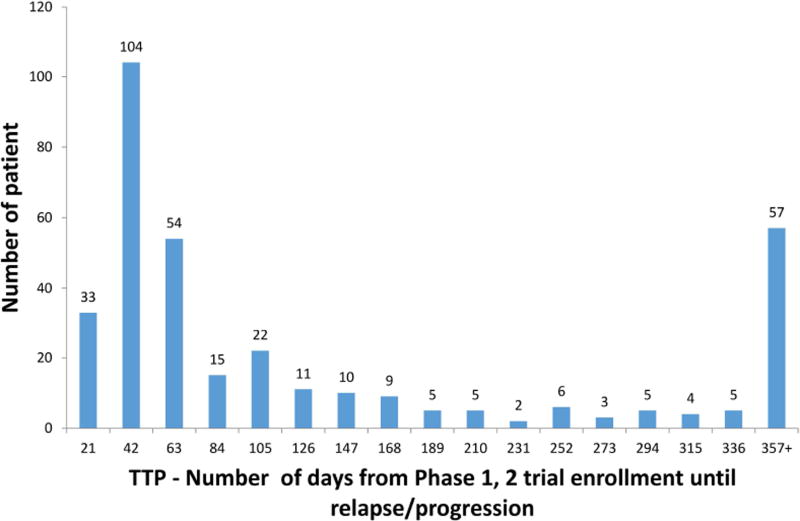
From the time of diagnosis, median TTFR was 18.7 months (range: 44 days, 5.4 years) n=176 patients with known TTFR). The majority (117/176; 66%) enrolled on their first COG early-phase trial >30 days after first relapse. From the time of early-phase trial enrollment, 1-year PFS/OS were 21±2%/57±3%, respectively, and at 4-years were 6±1%/20±2% (n=383) (Figure 2A, Table 3). From the time of early-phase trial enrollment, median TTP was 58 days (interquartile range: 31–183 days, n=350 relapses) (Figure 2B). Median follow-up time was 25.3 months (range: 0.1–145 months) in 33 patients without an event.
Figure 2.
A. Progression-free and overall survival curves from modern-era COG early-phase trials for treatment of relapsed or refractory neuroblastoma: n=383 patients (first early-phase trial enrollment). Survival time is calculated starting from the time of first enrollment onto the early-phase trial.
B. Distribution of time from early-phase trial enrollment until relapse/progression (TTP) (the n=350 subset who relapsed/progressed out of the overall 383 patients)
C–E. Overall survival curves for 383 patients on modern-era COG early phase trials for treatment of relapsed or refractory neuroblastoma.
C. By Era: 8/2002–4/2009 versus 5/2009–2/2014, p=0.008;
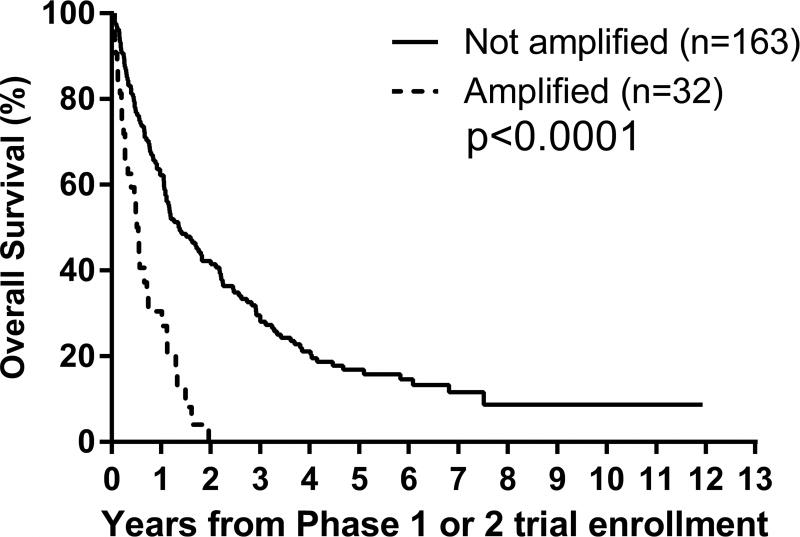
D. By MYCN status: amplified versus not amplified, p<0.0001;
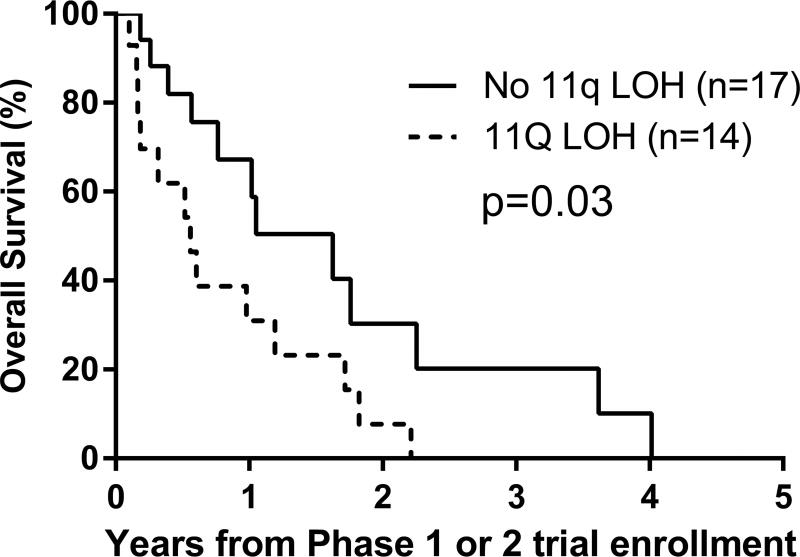
E. By 11q status: LOH versus no LOH, p=0.03.
Overall survival time is calculated starting from the time of first enrollment onto the COG early-phase trial.
F–G. Progression-free survival curves for 383 patients on modern-era COG early-phase trials for treatment of relapsed or refractory neuroblastoma.
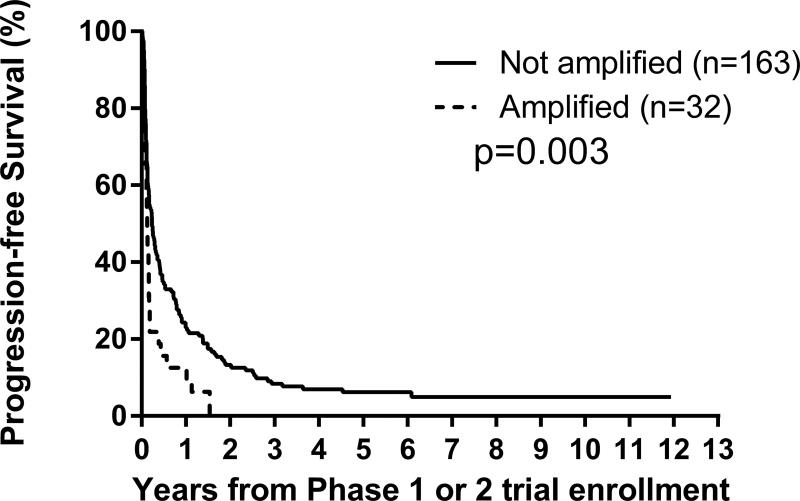
F. By MYCN status: amplified versus not amplified, p=0.003;
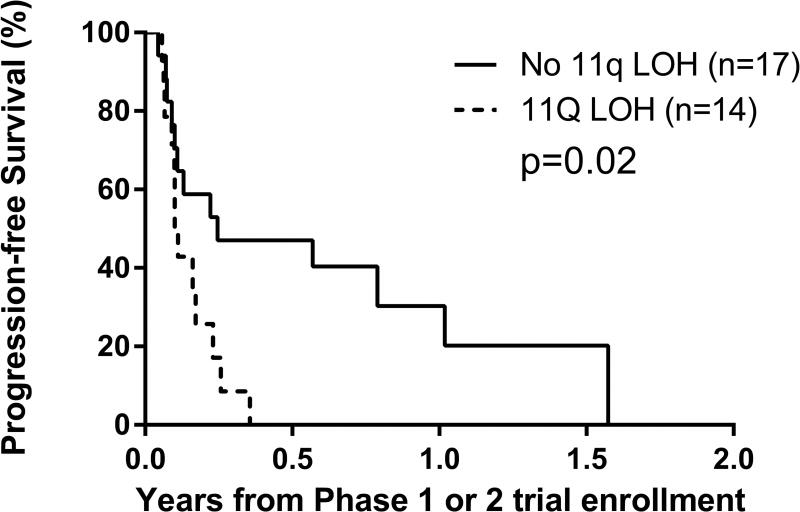
G. By 11q status: LOH versus no LOH, p=0.02.
Progression-free survival time is calculated starting from the time of first enrollment onto the COG early-phase trial.
Prognostic factors
Univariate analyses: Factors prognostic of worse PFS were MYCN amplification (p=0.003) and 11q LOH (p=0.02), and of worse OS were Era (p=0.008), MYCN amplification (p<0.0001) and 11q LOH (p=0.03) (Figures 2C–2G; Table 3). An optimal TTFR cut-off prognostic of OS could not be identified in either the Test set (n=88) or the overall cohort (n=176) with known TTFR (Supplementary Table 2). Using a TTFR cut-off of 30 months from diagnosis to first relapse, TTFR was not prognostic of PFS (p=0.3) or OS (p=0.055). The PH assumption was not violated for any factors.
In multivariable analysis, MYCN (p<0.0001, p=0.001) and 11q (p=0.02, p=0.01) were independently prognostic for PFS and OS, respectively (n=195) (Table 4).
Table 4.
Multivariable Cox models of PFS and OS
| PFS (n=195**) |
OS* (n=195**) |
||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Factor |
p-value | Hazard Ratio |
95% CI on Hazard Ratio |
Factor | p-value | Hazard Ratio |
95% CI on Hazard Ratio |
|
| |||||||
| MYCN amplification | <0.0001 | 3.4 | (2.2, 5.3) | MYCN amplification | 0.001 | 2.0 | (1.3, 2.9) |
| 11q LOH | 0.02 | 2.6 | (1.2, 5.9) | 11q LOH | 0.01 | 2.8 | (1.3, 6.3) |
| 11q unknown | 0.7 | 0.9 | (0.5, 1.6) | 11q unknown | 0.6 | 1.2 | (0.6, 2.1) |
CI = confidence interval
“Era” was tested in the OS model but was not statistically significant.
Patients for whom both factors were unknown were excluded.
Discussion
We report the outcome of a historical cohort of patients with relapsed/refractory neuroblastoma that is representative of patients currently enrolled onto early-phase clinical trials in North America. A patient’s first enrollment on an early-phase COG trial from 11/2002–1/2014 was included. The eligibility criteria of the trials were fairly inclusive, and similar to currently enrolling COG and non-COG early-phase trials. The proportions of patients by prognostic factors at diagnosis were as anticipated. It is therefore possible to use the PFS and TTP results of this study as historical context when designing future trials of anti-tumor agents. OS is an objective (“hard”) endpoint, but its ability to measure the treatment of interest is limited (“diluted”) by additional therapy received after the treatment of interest. PFS and TTP are able to measure the treatment effect of interest without the diluting effect of intervening treatments.
In the absence of clinical symptoms, the more frequently assessments of disease burden are performed, the sooner a relapse/progression can be identified. Kushner et al demonstrated the superiority of 123I-MIBG for detecting relapse in routine (every 2–4 months) monitoring of high-risk patients28. In early-phase trials, the times to relapse/progression may artificially cluster around the times that disease assessments are typically performed or dictated by the protocol. In this study, many patients relapsed/progressed within a short period of time (median TTP=58 days; Figure 2B). A limitation of our study, and a reality of clinical care, is that a relapse/progression is likely observed at the times of designated disease assessments between courses (e.g., 21–28 days or 42–56 days) rather than the true (earlier) time of relapse/progression, resulting in variability of PFS and TTP measurements. Future meta-analyses would be aided by having more uniform timing of disease assessments across trials. Another limitation of our study is that, due to lack of clear protocol definitions of “refractory”, we were unable to discriminate the refractory versus relapsed/progressing patients. To address these issues, a National Cancer Institute funded international collaboration is developing standard definitions for refractory disease and recommended time points for disease assessment.29
A limitation of this historical cohort is a potential selection bias in terms of a disease burden slightly lower than the population of relapsed/refractory neuroblastoma patients. Patients must have been well enough to meet eligibility criteria or travel to a referral institution. This historical cohort appears to be representative of relapsed patients who enrolled on early-phase trials between 2002 and 2014.
A limitation of this study is that prior therapy and baseline risk factor data were not available unless the patient had enrolled on a COG frontline protocol. However, this did not hinder estimation of PFS, TTP, and OS. The outcome for patients with missing data was similar to those who had enrolled on a frontline COG study, suggesting there was no bias. Also, despite the reduced sample size, there was sufficient power to identify factors prognostic of PFS and OS. For the 44% of patients with known risk factor (Table 3) and prior treatment (Table 2) data, most were COG-classified as high-risk at diagnosis. It is reasonable to assume the remaining patients were high-risk too, except for a few patients who developed metastatic progression following initial diagnosis of non-high-risk neuroblastoma. Patients with non-high-risk neuroblastoma who developed non-metastatic recurrence were not eligible for the trials in this study because they have available curative options. Among patients with known prior therapy, ~two-thirds received at least one transplant, slightly lower than anticipated. Fewer (13.2%) patients in this study received dinutuximab than expected in future relapsed/refractory cohorts, because most of our historical cohort received frontline therapy before the efficacy of dinutuximab was identified in 2009.27 Since 2009, the majority of high-risk patients treated in North America have received dinutuximab. The outcome for dinutuximab-treated patients who relapsed/progressed should be tested in a prospective fashion in a larger anti-GD2 antibody-treated cohort.
In a large INRG study of neuroblastoma patients in first relapse (n=2,266), London et al demonstrated that many risk factors prognostic of OS from the time of diagnosis30 remain prognostic of OS after relapse: age, INSS stage, MYCN status, and TTFR (optimal cut-off: 30 months)8,26. However, in the current study, we were unable to identify an optimal TTFR cut-off. Using a 30-month cut-off, the ~24% difference in the 1-year OS was considered clinically significant, but the sample size was too small for sufficient power to detect this difference. The ~6% difference in the 1-year PFS for TTFR (<30 months versus ≥30 months) was not considered clinically significant. The INRG study included the subset of newly diagnosed patients who relapsed/progressed, in contrast to the present COG cohort, comprised of patients in first, second, or subsequent relapse who survived long enough, and were considered well enough, to enroll on a relapse trial. The INRG and COG cohorts differ in other ways, perhaps influencing results: a) therapeutic differences over time (COG [2002–2014] had more modern treatment options for recurrent disease than INRG [1990–2002]); b) therapeutic differences between countries (INRG: 11 countries; COG: primarily North America); c) sample size (INRG: n=2,266; COG: n=383); and, d) a higher proportion of high-risk patients in the COG cohort. Importantly, both the INRG and COG analyses identify MYCN status as independently prognostic of OS after relapse. Garaventa et al identified age ≥18 months, higher stage, elevated LDH, amplified MYCN, and an abdominal primary as prognostic of worse OS after first relapse/progression.31 In a large study of patients who survived more than 5-years after diagnosis, Cotterill et al identified age >1 year with stage 4 disease and prior relapse as prognostic of subsequent relapse/progression.32
In this COG study, 66% of patients took >30 days after first relapse to enroll on their first COG early-phase study, suggesting some received non-COG-study therapy after first relapse. In a retrospective single institution study, Lau et al showed that shorter TTFR was prognostic of worse OS. Despite salvage regimens that were effective in extending overall survival time, the ultimate outcome remained dismal.33 Similarly, the outcomes for our refractory/relapsed neuroblastoma cohort remain dismal (4-year PFS/OS of 6±1%/20±2%OS, respectively) (Figure 2A), highlighting the importance of continued clinical trials aimed at identifying improved therapies for these patients.
The randomized Phase 2 trial remains the ideal approach for evaluating the efficacy of a new agent; our results support stratification by MYCN status, and possibly also 11q status. (The prognostic value of 11q status in relapsed neuroblastoma patients should be confirmed in a larger cohort.) Barring a randomized trial, a single-arm trial could test superiority of a new agent compared to an efficacy ‘bar’ (e.g., 2-year PFS >11%) selected based on the curves from this study. However, historical controls are limited by differences in frontline therapy, supportive care and approaches to disease assessments, and may not be representative of the outcome of patients given modern-era treatment. This limitation is evidenced herein by the superior outcome of patients enrolled after 5/2009 as compared to those before 5/2009. Despite the limitations of historical controls, for a rare disease like neuroblastoma, there may be situations where this is a reasonable study design to identify agents that warrant further investigation.
In summary, a representative historical cohort of relapsed/refractory neuroblastoma patients who enrolled on recent COG early-phase trials has been used to identify post-relapse prognostic factors and establish a historical context for PFS and TTP. Our study is the first meta-analysis of these outcomes in the context of modern therapy. In some cases, response alone may not reflect the efficacy of a given agent for patients with neuroblastoma. Randomized phase 2 trials can be stratified by post-relapse prognostic factors like MYCN amplification, and use INRC response together with PFS or TTP as endpoints, where the choice of endpoint(s) depends on a given trial’s objectives and therapies. These data also show that outcome for children with relapsed/progressive neuroblastoma remains dismal, emphasizing the importance of developing more effective agents for this population.
Supplementary Material
Acknowledgments
Funding sources
NIH/NCI Grants U10 CA180899 and U10 CA98413 (Children’s Oncology Group Statistics and Data Center), U10 CA98543 (Children’s Oncology Group Chair’s Grant), U10 CA01015, U10 CA07502, and U10 CA12502 (Children’s Oncology Group Phase 1/Pilot Consortium)
We thank the patients and families for their participation on COG studies. We thank the Study Chairs of the Phase 1 and 2 trials included in this analysis (Supplementary Table 3), as well as Mark Krailo and Charlotte Ahern for performing the design and statistical analysis of many of the Phase 1 trials.
Footnotes
Author Contributions Statement
Contributions were made by the authors in the following areas: Conceptualization (WL, RB, JP), Methodology (WL, DG), Software (WL, DG, CVR), Validation (WL, DG), Formal analysis (WL, DG, CVR), Investigation (WL, RB, BW, EF, DG, JP), Resources (WL, BW, AN), Data curation (WL, DG, CVR, AN), Writing original draft (WL), Writing – review and editing (WL, RB, BW, EF, JP), Visualization (WL, RB, DG, JP), Supervision (WL, RB, AN, JP), Project administration (WL, RB, AN, JP), and Funding acquisition (JP). W London is responsible for the overall content as guarantor.
Conflicts of Interest Statement
All authors (WL, RB, BW, EF, DG, CVR, AN, and JP) declare that they have no conflicts of interest.
References
- 1.Maris JM, Hogarty MD, Bagatell R, et al. Neuroblastoma. Lancet. 2007;369:2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 2.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 3.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J National Cancer Inst. 2000 Feb 2;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 4.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993 Aug;11(8):1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 5.Castel V, Garcia-Miguel P, Canete A, et al. Prospective evaluation of the International Neuroblastoma Staging System (INSS) and the International Neuroblastoma Response Criteria (INRC) in a multicentre setting. Eur J Cancer. 1999 Apr;35(4):606–611. doi: 10.1016/s0959-8049(98)00395-5. [DOI] [PubMed] [Google Scholar]
- 6.Shusterman S, London WB, Gillies SD, et al. Antitumor Activity of Hu14.18-IL2 in Patients With Relapsed/Refractory Neuroblastoma: A Children's Oncology Group (COG) Phase II Study. J Clin Oncol. 2010 Nov;28(33):4969–4975. doi: 10.1200/JCO.2009.27.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagatell R, London WB, Wagner LM, et al. Phase II Study of Irinotecan and Temozolomide in Children With Relapsed or Refractory Neuroblastoma: A Children's Oncology Group Study. J Clin Oncol. 2011 Jan;29(2):208–213. doi: 10.1200/JCO.2010.31.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.London WB*, Castel V*, Monclair T, et al. Clinical and biologic features predictive of survival after relapse of neuroblastoma: A report from the International Neuroblastoma Risk Group (INRG) Project. J Clin Oncol. 2011 Aug 20;29(24):3286–92. doi: 10.1200/JCO.2010.34.3392. [* = share first authorship] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan ELMP. In: Nonparametric estimation from incomplete observations. Association JotAS, editor. 1958. pp. 457–481. [Google Scholar]
- 10.Peto R, Pike M, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976;34:585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox DR. Regression models and life-tables. JRSSB. 1972;34:187–220. [Google Scholar]
- 12.Brodeur GM, Seeger RC, Barrett A, et al. International criteria for diagnosis, staging, and response to treatment in patients with neuroblastoma. J Clin Oncol. 1988;6:1874–1881. doi: 10.1200/JCO.1988.6.12.1874. [DOI] [PubMed] [Google Scholar]
- 13.London WB, Castleberry RP, Matthay KK, et al. Evidence for an age cut-off greater than 365 days for neuroblastoma risk group stratification in the Children’s Oncology Group. J Clin Oncol. 2005 Sep 20;23(27):6459–65. doi: 10.1200/JCO.2005.05.571. [DOI] [PubMed] [Google Scholar]
- 14.London WB, Boni L, Simon T, et al. The role of age in neuroblastoma risk stratification: The German, Italian, and Children’s Oncology Group perspectives. Cancer Letters. 2005 Oct 18;228(1–2):257–66. doi: 10.1016/j.canlet.2004.12.054. [DOI] [PubMed] [Google Scholar]
- 15.Moroz V, Machin D, Faldum A, et al. Changes over three decades in outcome and the prognostic influence of age-at-diagnosis in young patients with neuroblastoma: A report from the International Neuroblastoma Risk Group Project. Eur J Cancer. 2011 Mar;47(4):561–71. doi: 10.1016/j.ejca.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 16.Seeger RC, Brodeur GM, Sather H, et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985;313:1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- 17.Schneiderman J, London WB, Brodeur GM, et al. Clinical significance of MYCN amplification and ploidy in “Favorable” stage neuroblastoma. J Clin Oncol. 2008 Feb 20;26(6):913–8. doi: 10.1200/JCO.2007.13.9493. [DOI] [PubMed] [Google Scholar]
- 18.George RE, London WB, Cohn SL, et al. Hyperdiploidy plus nonamplified MYCN confers a favorable prognosis in children 12 to 18 months old with disseminated neuroblastoma: A Pediatric Oncology Group study. J Clin Oncol. 2005 Sep 20;23(27):6466–73. doi: 10.1200/JCO.2005.05.582. [DOI] [PubMed] [Google Scholar]
- 19.Bagatell R, Rumcheva P, London WB, et al. Outcomes of children with intermediate-risk neuroblastoma after treatment stratified by MYCN status and tumor cell ploidy. J Clin Oncol. 2005 Dec 1;23(34):8819–27. doi: 10.1200/JCO.2004.00.2931. [DOI] [PubMed] [Google Scholar]
- 20.Shimada H, Chatten J, Newton WA, Jr, et al. Histopathologic prognostic factors in neuroblastic tumors: definition of subtypes of ganglioneuroblastoma and an age-linked classification of neuroblastomas. J Natl Cancer Inst. 1984;73:405–416. doi: 10.1093/jnci/73.2.405. [DOI] [PubMed] [Google Scholar]
- 21.Shimada H, Ambros IM, Dehner LP, et al. The International Neuroblastoma Pathology Classification (the Shimada System) Cancer. 1999;86:364–372. [PubMed] [Google Scholar]
- 22.Wang LL, Teshiba R, Ikegaki N, et al. Age-Dependent Prognostic Effect by Mitosis-Karyorrhexis Index (MKI) in Neuroblastoma: A Report from the Children’s Oncology Group. Ped Develop Path. 2014 Nov-Dec;17(6):441–9. doi: 10.2350/14-06-1505-OA.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sano H, Bonadio J, Gerbing RB, et al. International Neuroblastoma Pathology Classification adds independent prognostic information beyond the prognostic contribution of age. Eur J Cancer. 2006 May;42(8):1113–1119. doi: 10.1016/j.ejca.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 24.Attiyeh EF, London WB, Mosse YP, et al. Chromosome 1p and 11q deletions and outcome in neuroblastoma: a Children’s Oncology Group study. N Engl J Med. 2005 Nov 24;353(21):2243–53. doi: 10.1056/NEJMoa052399. [DOI] [PubMed] [Google Scholar]
- 25.Schleiermacher G, Mosseri V, London WB, et al. Segmental chromosomal alterations have prognostic impact in neuroblastoma: A report from the INRG project. Br J Cancer. 2012 Oct 9;107(8):1418–22. doi: 10.1038/bjc.2012.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lau L, London WB. Recurrent Neuroblastoma. In: Shimada H, editor. Neuroblastoma – Present and Future. 2012. pp. 29–52. InTech, Web. [Google Scholar]
- 27.Yu AL, Gilman AL, Ozkaynak MF, et al. for the Children’s Oncology Group (COG) Anti-GD2 Antibody with GM-CSF, IL2 and Isotretinoin for Neuroblastoma. N Engl J Med. 2010 Sep 30;363(14):1324–34. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park JR, Bagatell R, Cohn SL, et al. Advances in Neuroblastoma Research meeting, Abstract #61. Cairns, Australia: Jun, 2016. Revisions to the International Neuroblastoma Response Criteria: A consensus statement from the NCI-Clinical Trials Planning Meeting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kushner BH, Kramer K, Modak S, et al. Sensitivity of surveillance studies for detecting asymptomatic and unsuspected relapse of high-risk neuroblastoma. J Clin Oncol. 2009 Mar;27(7):1041–6. doi: 10.1200/JCO.2008.17.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohn SL*, Pearson ADJ*, London WB, et al. for the INRG Task Force. The International Neuroblastoma Risk Group (INRG) Classification System. J Clin Oncol. 2009 Jan 10;27(2):289–97. doi: 10.1200/JCO.2008.16.6785. [* = share first authorship] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garaventa A, Parodi S, De Bernardi B, et al. Outcome of children with neuroblastoma after progression or relapse. A retrospective study of the Italian neuroblastoma registry. Eur J Cancer. 2009;45:2835–42. doi: 10.1016/j.ejca.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Cotterill SJ, Pearson AD, Pritchard J, et al. Late relapse and prognosis for neuroblastoma patients surviving 5 years or more: a report from the European Neuroblastoma Study Group "Survey". Med Pediatr Oncol. 2001;36(1):235–8. doi: 10.1002/1096-911X(20010101)36:1<235::AID-MPO1057>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 33.Lau L, Tai D, Weitzman S, et al. Factors influencing survival in children with recurrent neuroblastoma. J Pediatr Hematol Oncol. 2004;26:227–32. doi: 10.1097/00043426-200404000-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.