The unprecedented challenges of developing effective vaccines against intracellular pathogens such as HIV, malaria, and tuberculosis have resulted in more rational approaches to vaccine development. Apart from the recent advances in the design and selection of improved epitopes and adjuvants, there are also ongoing efforts to optimize delivery platforms.
KEYWORDS: T cells, antibodies, human immunodeficiency virus, vaccines
ABSTRACT
The unprecedented challenges of developing effective vaccines against intracellular pathogens such as HIV, malaria, and tuberculosis have resulted in more rational approaches to vaccine development. Apart from the recent advances in the design and selection of improved epitopes and adjuvants, there are also ongoing efforts to optimize delivery platforms. Viral vectors are the best-characterized delivery tools because of their intrinsic adjuvant capability, unique cellular tropism, and ability to trigger robust adaptive immune responses. However, a known limitation of viral vectors is preexisting immunity, and ongoing efforts are aimed at developing novel vector platforms with lower seroprevalence. It is also becoming increasingly clear that different vectors, even those derived from phylogenetically similar viruses, can elicit substantially distinct immune responses, in terms of quantity, quality, and location, which can ultimately affect immune protection. This review provides a summary of the status of viral vector development for HIV vaccines, with a particular focus on novel viral vectors and the types of adaptive immune responses that they induce.
INTRODUCTION
Since Edward Jenner first demonstrated immunity to smallpox by inoculating a 13-year-old boy with vaccinia virus over 200 years ago, various vaccines effective against numerous microorganisms have been developed. However, the challenges of developing vaccines protective against intracellular pathogens such as human immunodeficiency virus type 1 (HIV-1) has necessitated the adoption of more rational approaches to vaccine design based on the systematic design of epitopes, the use of immunogenic adjuvants, and the selection of appropriate delivery platforms. Viral vectors belong to one such platform and have inherent adjuvant capability in the form of pathogen-associated molecular patterns that can trigger innate immune responses through their engagement of specific pattern recognition receptors. More importantly, different viral vectors may exhibit distinct cellular tropism, with specific innate and adaptive immune phenotypes that render them optimally poised for inducing immunological memory against particular pathogens. Although preexistent immunity is an important factor in vector selection, a better understanding of the immune correlates of protection will ultimately guide vaccine design. In this review, we will summarize the current status of viral vector-based HIV vaccines, highlighting the effects of various vectors on vaccine immunogenicity, safety, and efficacy. This will include a description of various virus vectors that have been used in HIV vaccine development, which include nonreplicating and replicating adenovirus type 5 (Ad5), alternative-serotype adenovirus, poxvirus, lymphocytic choriomeningitis virus (LCMV), cytomegalovirus (CMV), vesicular stomatitis virus (VSV), and attenuated immunodeficiency viruses.
TARGETING SPECIFIC ARMS OF IMMUNITY AND ANTIGEN DESIGN: AN OVERVIEW
Given the complexities of HIV infection, it is increasingly clear that a successful vaccine must elicit multiple arms of adaptive immunity. One way to accomplish this goal is through the selection of distinct vaccine prime-boost platforms.
Targeting both cellular and humoral arms.
Regimens involving heterologous viral vector priming followed by recombinant protein boosting represent one of the most promising strategies to induce potent cytotoxic T lymphocytes (CTL) and antibody responses. The RV144 trial, which is the most successful HIV vaccine trial to date, resulted in 31.2% protection from HIV infection and utilized a heterologous canarypox virus vector (ALVAC strain) priming-protein boosting approach (1). Correlates of risk analysis identified elevated V1V2-specific IgG antibodies as inversely associated with infection rates (2). Similarly, a subsequent study that utilized an adenovirus priming and protein boosting regimen achieved sterilizing protection in 40 to 50% of vaccinated macaques following repeated intrarectal challenges, and this protection was correlated with Env-specific antibody titers, as well as antibody-mediated effector functions (3). Other vaccine regimens utilizing DNA, protein, or viral immunizations that induce antibody and cellular immune responses have not translated into effective HIV vaccines. These include the VAX003 and VAX004 studies, which utilized gp120 monomer, and the HVTN 505 study, which utilized a DNA priming-Ad5 boosting regimen, as well as other Ad5-based studies, such as the STEP and Phambili trials (4).
Targeting the cellular arm.
In the search for a potent HIV vaccine, vaccine regimens targeting mostly the CD8 T cell and/or the CD4 T cell components of the immune system have also been extensively explored. Viral vector-based vaccine regimens are capable of inducing robust antiviral CTL responses. The critical role of CD8 T cells in the control of HIV infection is highlighted by the strong association of robust CTL responses with control of infection in elite controllers and that experimental depletion of CD8 T cells in simian immunodeficiency virus (SIV)-infected macaques results in increased viral replication (5–7). Many of the viral vector-based vaccine modalities can induce virus-specific CTL responses that can reduce peak and/or set point viral loads following experimental SIV infection of rhesus macaques (8–11). However, such findings have not translated to the clinic, since all of these candidate HIV vaccines have failed to alter viral loads in vaccinated (and subsequently infected) individuals (1, 12, 13).
Furthermore, the important role of CD4 T cells in facilitating both the innate and adaptive immune systems has led to suggestions that vaccine modalities that preferentially induce CD4 T cell responses may be necessary for optimal vaccine-mediated protection. Indeed, it has been shown that CD4 T cells may be necessary for the effective control of certain diseases such as tuberculosis (14, 15), West Nile fever (16), and measles (17). Moreover, CD4 T cell help is required for the generation of memory CD8 T cells and high-affinity antibody responses (18–23), and poxvirus vectors, both the modified vaccinia virus Ankara (MVA) strain and the attenuated vaccinia virus strain derived from a Copenhagen vaccine strain that underwent multiple mutations in various open reading frames (NYVAC), appear to be particularly well suited for the induction of CD4 T cell responses (24–26).
However, prior studies have demonstrated that CD4 T cell-biased vaccines may be detrimental in the context of chronic viral infections (27, 28). In one of these studies, an SIV vaccine encoding a CD4 T cell epitope derived from Env resulted in increased SIV susceptibility and progression to AIDS (28). These data suggest that biased induction of CD4 T cells may be detrimental in the setting of HIV vaccination, which is not surprising, given that activated virus-specific CD4 T cells are a primary target of the virus (29). Interestingly, we showed that this phenomenon of “adding fuel to the fire” is generalizable to a distinct chronic viral infection model that does not primarily target activated CD4 T cells (27). Using the chronic LCMV mouse model, we showed that immunization with a vaccine that induces a CD4 T cell-biased response leads to fatal inflammatory disease following a challenge with chronic LCMV clone 13. This inflammatory disease induced by the CD4 T cell vaccine was prevented by transferring virus-specific CD8 T cells or antibodies, demonstrating that a balance of all arms of the adaptive immune response is critical for determining vaccine-induced protection in the context of chronic viral infection.
Lastly, it is important to mention that HIV exhibits a tremendous level of genetic heterogeneity, and therefore, a vaccine against this virus must be able to neutralize highly diverse viral strains. This challenge could be partially overcome by the in silico development of mosaic antigens that maximize immune responses to global circulating strains. A demonstration of this novel approach was reported in prior studies that demonstrate that mosaic antigens induce a greater depth and breadth of immune responses relative to consensus antigens (30, 31).
VIRAL VECTORS
Ad5 vectors.
With their ability to induce multiple arms of the immune system, viral vectors have been the most studied platforms in our search for an effective HIV vaccine. One of the earliest vectors, and thus the most studied, is Ad5. Ad5, a serotype C adenovirus, is one of the most immunogenic of the human adenoviral vectors. Several groups have shown that it induces potent humoral and cellular immunity in preclinical and clinical studies against a wide range of pathogens (32–35), as well as multiple tumor types (36, 37). Therefore, Ad5 has been used extensively in the pursuit of an HIV vaccine. Following the promising finding that Ad5 conferred protective immunity to a pathogenic SIV strain in macaques (38, 39), two clinical trials (STEP and Phambili) were set up to evaluate the ability of an Ad5 vaccine expressing HIV-1 subtype B Gag-Pol-Nef to elicit a protective cellular immune response against HIV-1 infection (12, 40). However, these trials were stopped before completion after interim analysis showed futility. Further analysis of the STEP trial also revealed a trend toward higher HIV acquisition among uncircumcized male vaccinees with preexisting Ad5 immunity (12). Another phase IIb efficacy trial (HVTN 505) that utilized priming with DNA and boosting with Ad5 expressing HIV-1 Gag-Pol-Nef antigens, as well as a modified HIV-1 Env transgene, also failed to show clinical efficacy (13).
These unexpected results of clinical trials with Ad5 have been suggested to be partly due to vaccine-induced T cell activation (41), but detailed analyses of the immunological properties of Ad5 suggest that other factors may also play a role. Studies with mice and nonhuman primates have demonstrated that the T cell responses elicited by Ad5 exhibit a partially exhausted T cell profile (42–45). Several groups have also shown that CD8 T cells induced by Ad5 are more terminally differentiated and exhibit impaired anamnestic expansion (43, 46, 47). Ad5-induced CD8 T cells also exhibit impaired central memory differentiation, evidenced by lower expression of the homeostatic survival marker CD127 and the lymphoid homing receptor CD62L than other Ad vector serotypes (42, 45). Importantly, the hallmark of exhausted CD8 T cells during chronic viral infection and cancer is the expression of inhibitory receptors such as programed cell death receptor 1 (PD-1), CTL antigen 4 (CTLA-4), T-cell immunoglobulin, mucin-3 (Tim-3), lymphocyte activation gene 3 (LAG-3), and the T-cell tyrosine-based inhibitory motif (ITIM) domain (TIGIT) (48). Intriguingly, we and others have shown that some of these inhibitory receptors, particularly PD-1, Tim-3, and CTLA-4, are permanently upregulated on Ad5-induced T cells (42, 43, 49). Those studies also demonstrated that although Ad5 induces a greater magnitude of transgene-specific CD8 T cells than other adenoviral vectors, Ad5-induced CD8 T cells are partially exhausted and show a reduced ability to secrete gamma interferon, tumor necrosis factor alpha, and interleukin-2. Recently, detailed transcriptional profiling of Ad5-induced transgene-specific CD8 T cells also showed an enrichment of transcriptomic signatures of anergy and exhaustion, further corroborating the phenotypic profile described above (49). Altogether, these features suggest that Ad5 induces a partially exhausted T cell response similar to what has been observed in chronic infection and cancer. It is important to note that despite these dysfunctional immune responses, Ad5-induced CD8 T cells still confer some protection in different challenge models, particularly in the absence of preexisting anti-Ad5 immunity (44).
The mechanisms underlying Ad5-induced immune exhaustion have not been fully elucidated. There are suggestions that multiple factors such as liver tropism (50, 51), antigen persistence or dose (42–44, 49), and impaired CD4 T cell help (45, 52) may play critical roles in the induction of T cell dysfunction.
Alternative-serotype Ad vectors.
The above results with conventional Ad5 vectors have motivated the discovery of alternative-seroptype Ad vectors. Several vaccine vectors derived from rare human adenovirus types, as well as other species such as chimpanzees and rhesus monkeys, are being developed and evaluated against multiple pathogens (53–60). Of the rarer human adenovirus types, Ad26 and Ad35 are promising. Both utilize CD46 as their primary cellular receptor, unlike Ad5, which uses the coxsackievirus and adenovirus receptor (61, 62). Seroepidemiological studies assessing both novel vectors have demonstrated that they have significantly lower seroprevalence than Ad5 in many population groups (53, 63–65).
In terms of immunogenicity, prior studies have shown that Ad26 and Ad35 tend to be slightly less immunogenic than Ad5 (42, 45). However, the quality of immune responses induced by alternative-serotype Ad vectors or Ad5 vectors is distinct. Compared to Ad5, alternative-serotype Ad vectors induce a more potent innate immune response (66) and, as mentioned earlier, a more polyfunctional (and less exhausted) T cell response. Alternative-serotype Ad vectors also induce memory CD8 T cells with a long-lived central memory T cell phenotype and are thus better poised for robust T cell expansion following antigen reexposure. When used for priming in a heterologous prime-boost regimen with Ad35 or MVA boosting, Ad26 provided partial protective efficacy against multiple intrarectal challenges with two stringent strains of SIV (SIVmac251 and SHIV-SF162P3) in rhesus monkeys (9, 67). This protection seemed to be partly mediated by both functional neutralizing and nonneutralizing antibodies. Furthermore, in multiple clinical trials, Ad26 was shown to be safe and elicit polyfunctional humoral and cellular immune responses (68–70). Ad35 has also been shown to be safe and elicit potent immune responses, albeit smaller in magnitude than those elicited by Ad5 or Ad26 (42, 44, 53, 71). Because of their high expression, many immunoinhibitory pathways have been suggested to play a role in the regulation of Ad5-induced T cell exhaustion, including PD-1, CD200, CTLA-4, CD226, and LAG-3, all of which are decreased in Ad26-induced T cells, but their precise roles following immunization remain understudied (49).
Many groups are also developing chimpanzee- and rhesus macaque-derived serotypes because of their reduced seroprevalence (60, 72–75). In addition, chimpanzee Ad3 (ChAd3), which belongs to the same subgroup as Ad5 (subgroup C), induces CD8 T cells that are more polyfunctional than those induced by Ad5 (76). Importantly, the lower prevalence of preexisting anti-ChAd3 antibody in most populations may render this vector a suitable substitute for Ad5 (55). Overall, the quest for alternative-serotype Ad vectors has the potential to elucidate novel serotypes with low preexisting immunity, high immunogenicity, and distinct tropism, which could provide vaccinologists with a well-assorted toolkit to develop vaccines against HIV and other diseases.
rLCMV vectors.
One limitation of the use of Ad vectors as vaccine platforms is their ability to elicit potent vector-specific neutralizing antibodies, which limits the efficacy of simple homologous boosting because of neutralization of the boosting homologous vaccine (77, 78). Although heterologous prime-boost vaccine regimens are often used to overcome this challenge, a single vector not hampered by preexisting humoral immunity is preferable, as it simplifies the prime-boost vaccine regimen and reduces the cost of production. To achieve this, a vector based on the arenavirus LCMV (which has been a main workhorse in basic immunology research because of its potent immunogenicity) was recently developed (79, 80). LCMV is a bisegmented negative-strand RNA virus that primarily infects rodents and has been widely used as a model to study cellular immunity (81, 82). Nonreplicating recombinant LCMV (rLCMV) vectors in which the LCMV glycoprotein (GP) gene is replaced with a vaccine transgene were shown to be highly immunogenic in mice and nonhuman primates, with the ability to target and induce dendritic cell activation and elicit persistent transgene-specific T cell responses (79, 83). The genetic absence of the GP gene in rLCMV vectors renders the virus replication defective, overriding concerns about potential LCMV-induced pathogenicity, which are especially considered in pregnant women and transplant recipients (84, 85). Interestingly, consecutive readministration of this vector as a homologous boost can lead to substantial anamnestic expansion of transgene-specific CD8 T cells and antibody responses without generating LCMV GP-specific neutralizing antibody responses (79, 80). Therefore, rLCMV provides an option for simpler, immunogenic homologous prime-boost vaccine modalities.
The unique ability of rLCMV to resist antibody neutralization is due to the absence of the gene encoding the LCMV GP in the vector. Moreover, the human seroprevalence of LCMV is reported to be <5%, compared to >20% for Ad26 and nearly 100% for Ad5 (53, 86–88). Efficacy studies have shown that an rLCMV-based vaccine protects mice against viral and tumor challenges and shows substantial protective efficacy in a model of SIV challenge of rhesus macaques when used for boosting following priming with Ad5 (83). Thus, rLCMV vectors constitute another novel tool in our armamentarium for developing protective vaccines against infectious diseases, as well as therapeutic cancer vaccines.
Poxvirus vectors.
Poxvirus vectors, in the form of the canarypox virus-based ALVAC vector used in the RV144 trial, have proven to be the most clinically successful HIV vaccine vectors to date. The RV144 trial, in which ALVAC-HIV immunity was boosted with a recombinant glycoprotein 120 subunit vaccine (AIDSVAX B/E) and is colloquially referred to as the “Thai trial,” resulted in 31% protection from HIV acquisition associated with Env V1V2-specific IgG antibodies (1, 2, 89). Prevention of HIV acquisition was more striking during the first months after vaccination, when the levels of HIV-specific antibodies were highest. This degree of protection seemed to decrease over time and was associated with reduced antibody levels. Despite this rapid waning of antibody levels, the recent RV305 trial has demonstrated that long-lived memory B cells were induced in RV144 and a robust anamnestic antibody response can be recalled with a protein boost up to 8 years later (90). To build on the success of the RV144 trial, a new HIV vaccine trial (HVTN 702) was recently started in South Africa. The HVTN 702 regimen is a modification of the RV144 trial targeting the HIV subtype C prevalent in South Africa by using the same ALVAC vector for priming but with a different adjuvant (MF59) for Env protein boosting with the aim of generating a more robust and sustained antibody response.
In total, three additional poxvirus vectors have garnered significant attention as candidate HIV vaccine vectors. These vectors can be segregated into two groups, (i) orthopoxvirus-derived NYVAC (from the Copenhagen vaccine strain) and MVA and (ii) avipoxvirus-derived ALVAC (canarypox virus) and fowlpox virus. All four vectors are replication incompetent in mammalian cells (91–93). The ALVAC vector was particularly attractive because of the lack of preexisting antivector immunity in humans. In addition to the use of ALVAC in the RV144 trial and in the recently started HVTN 702 trial, there is also an increasing focus on MVA and NYVAC. In particular, MVA has gained considerable traction as a boosting vector following Ad vector priming, where such a regimen has induced partial protection from a neutralization-resistant SIV or SHIV challenge and significant reductions in viral loads (94, 95). Ad prime-MVA boost regimens have also shown promise against malaria (73), hepatitis C virus (96), and Ebola virus (97). In all cases, robust vaccine-elicited cellular immune responses were associated with protection from infection. A head-to-head comparison of NYVAC and ALVAC expressing HIV-1 clade C antigens using an immunization regimen based on RV144 showed modest superiority of the NYVAC-based regimen with regard to multiple measures of cellular and humoral immunogenicity (98). Whether these differences reflect true superiority of NYVAC-based vectors or are too modest to confer differences in protective efficacy remains to be rigorously tested. Further efforts are ongoing to modify and rationally select poxvirus vectors to better take advantage of the utility of poxvirus vectors as both priming and boosting vectors.
A growing literature has demonstrated that the different poxvirus vectors induce qualitatively different immune responses, akin to the observations described previously examining distinct Ad serotypes. Compiled comparisons of various poxvirus vectors demonstrate that ALVAC induces particularly strong antiviral and inflammatory responses in infected human cells and immunized macaques, compared to MVA, NYVAC, and fowlpox virus (11, 99–101). These comparisons identified MVA as the second most potent activator of innate immune stimulation, with NYVAC and fowlpox virus identified as the least stimulatory. In humans, MVA was more immunogenic than fowlpox virus, and a heterologous prime-boost regimen proved to be the most effective (24, 25). Phenotyping of T cell responses in macaques following MVA or NYVAC boosting of DNA-primed responses identified a mixed CD4 and CD8 T cell response induced by MVA, while NYVAC induced a predominantly CD4 T cell response (11). However, the overall magnitudes of the T cell responses were comparable. Finally, a comparison of NYVAC priming versus ALVAC priming of macaques showed increased CD4+ T cell and antibody responses in the NYVAC-primed animals (98). The paucity of direct comparisons of the different poxvirus vectors makes it difficult to interpret how the differences in innate immune activation by these vectors might translate into differences in induced adaptive responses. However, one study has demonstrated that MVA-derived antigens are more robustly presented by mammalian cells than canarypox virus-derived antigens. The immunogenicity and modest protective efficacy of poxvirus vectors mean they are one of the most promising vector modalities available. Overall, poxviruses are perhaps the most promising HIV vaccine vectors because of their ability to reduce HIV infection rates, and current efforts are aimed toward improving the durability of the antibody responses induced by these vectors.
Replicating viral vectors.
Although any of the vectors discussed above could be developed as replicating vaccine platforms, a few new replicating vectors have garnered attention for their ability to induce immune protection against SIV in macaque studies. The impressive immune protection that is achieved by replicating virus vectors may be due to the generation of a special subset of CD8 T cells that are able to rapidly intercept the virus upon a challenge. Central memory CD8 T cells are long-lived, but they may exhibit a delay in reactivating their cytotoxic function following a viral challenge. On the other hand, effector memory CD8 T cells (which can be induced by replicating antigen) are short-lived but provide immediate cytotoxic function (102). Therefore, it has been proposed that the induction of effector memory CD8 T cells by certain replicating virus vectors may be critical for an HIV vaccine, since these responses are able to rapidly control initial infection foci before the virus becomes systemic (103). It is also important to mention that the duration of antigen stimulation can determine the levels of central memory versus effector memory CD8 T cell responses (104, 105), suggesting that viral vectors that replicate and persist for a long time may be better poised to induce effector memory CD8 T cells. Conversely, many replication-deficient adenoviral vectors, rLCMV vectors, and attenuated poxvirus vectors provide limited antigenic stimulation because of their rapid clearance by the host immune response and therefore elicit a biased central memory CD8 T cell response that preferentially localizes to lymphoid tissues (45, 47).
Of the various replication-competent vaccine vectors currently under development, recombinant human CMV (rhCMV) and replication-competent recombinant VSV (rVSV) (6, 106, 107) have been extensively characterized and are currently in clinical development.
(i) rhCMV.
As a classical member of the herpesvirus family, rhCMV persists in the host, providing a constant source of antigen necessary for the maintenance of CD8 T cells with an effector memory phenotype. Similar to novel rLCMV vectors genetically lacking LCMV GP, the immunogenicity of rhCMV vectors is not limited by preexisting antivector immunity and they can therefore be used repeatedly, even in CMV-positive monkeys, to induce effector memory CD8 T cells (108). This property is particularly relevant because CMV has a seroprevalence of up to 90% in some populations (109). The absence of significant antibody responses following rhCMV immunization means that combination with other vaccine modalities that elicit protective humoral immune responses may be required to achieve optimal vaccine-mediated protection. However, impressive findings with the rhCMV platform in animal models (as high as 50% protection seen in animal studies) may be logistically challenging to translate to the clinic, given that human CMV infection can be associated with birth defects and severe complications in immunosuppressed or organ transplant patients (110, 111). However, there are ongoing efforts to generate a modified CMV vector that could circumvent these safety challenges. It is also important to mention that CD8 T cell responses elicited by rhCMV vectors have been shown to contradict conventional major histocompatibility complex (MHC) restriction paradigms. Effector CD8 T cells induced by such vectors can recognize MHC class II-restricted epitopes, which can result in more diverse immune recognition, especially at the sites of viral entry (112). These unconventional effector CD8 T cells may be important in both prophylactic and therapeutic HIV vaccines, as they provide a new pool of effector CD8 T cells that can target viruses that have escaped most conventional CD8 T cells.
(ii) rVSV.
rVSV-based vectors have garnered renewed interest, given the recent highly successful phase III trials results of an rVSV-based vaccine for Ebola virus (113), another pathogen to which incredibly rapid immune responses are required for protection. Early testing of rVSV vectors showed promising prevention of disease progression in an intravenous SHIV (89.6P) infection model (114). However, concerns existed about the potential neurovirulence of the rVSV vector based on the original vector design (115). This is not surprising, given that VSV belongs to the rhabdovirus family, which also includes rabies virus. However, a redesigned, more attenuated, rVSV vector backbone that exhibits a lack of neurovirulence has been engineered (116, 117). Unexpectedly, the increased attenuation did not impair the immunogenicity of the transgene insert in mice and nonhuman primates, but protective efficacy has not been assessed. This revised construct has now been tested in phase I trials, where it displayed immunogenicity (6).
Efforts are ongoing to develop and test other replicating vectors that might also elicit rapid effector memory T cell responses. These efforts include recombinant replication-competent Ad4, Ad5, and Ad26 vectors (118–121), Sendai virus (122), herpes simplex virus (123), and NYVAC virus (124), many of which are in early-phase clinical and preclinical trials.
In addition, one of the most salient examples of immune protection in the SIV infection model is that induced by SIVΔnef (125). It has been shown that this attenuated SIV strain can confer substantial immune protection by a mechanism that is dependent on low-level antigen persistence that allows for the maturation of T and B cell responses (126–128). It is not completely clear what the contribution of SIVΔnef mutation is relative to immune phenotypic differentiation. Continuous SIVΔnef mutation after vaccination may provide epitope diversity to improve immune breadth and depth, but time-dependent maturation of T and B lymphocytes also seems to be critical. However, the use of attenuated immunodeficiency viruses poses reasonable concerns about the possibility of viral reversion, rendering this approach practically unfeasible in human trials.
As mentioned earlier, an advantage of replicating vectors is their enhanced antigen expression, which could potentially drive robust adaptive immune responses. High and permanent transgene expression can also be achieved by immunization with HIV-based lentiviral vectors, which integrate into the genome and provide persistent antigen expression, even in nondividing cells (129–131). One could conceptualize a model in which persistent transgene expression would be desirable to induce effector memory T cells that can quickly intercept a viral challenge at mucosal sites. Consistent with this, we have shown that priming with a replication-defective virus, followed by boosting with a highly replicating virus, is very effective at increasing the level of memory T cells. However, the reverse (priming with a highly replicating virus) results in immune exhaustion (132). Thus, the timing of prime-boost immunization is a critical aspect to consider in the next generation of prime-boost immunization regimens. Importantly, the safety consideration of using live replicating vectors is typically a factor that deters the use of these vectors in many clinical studies. Altogether, replicating viral vectors hold considerable promise as a distinct modality to elicit rapidly responding protective immunity, but the appropriate balance between immunogenicity and potential pathogenicity needs to be achieved.
AAV vectors for antibody gene delivery.
Until now, we have discussed various vaccine vectors that are used for active immunization, which refers to the induction of the host immune response after exposure to an antigen. However, a main problem with HIV vaccine design is the difficulty of generating broadly neutralizing antibodies (bNAbs). This could be circumvented by cloning bNAb genes into viral vectors to directly induce the expression of bNAbs at the injection site, bypassing all of the steps that are required for the generation of bNAbs, including isotype switching, consecutive germinal center reactions, and somatic hypermutation. Recently, an adeno-associated virus (AAV) vector that constitutively expresses HIV-specific antibodies in a humanized mouse model of HIV infection has shown promise (133, 134). This approach of “engineering immunity” has proven successful in preventing HIV infection in humanized mice, but a caveat is that humanized mice do not express a functional immune system. Further studies with macaques showed that the AAV-vectored transgene is rapidly cleared by adaptive immune responses, substantially limiting the expression of the antibody genes (135, 136). Thus, a main goal of this approach is to modulate the pathways that mediate immune tolerance to prevent the rejection of the AAV vector, allowing for durable or permanent transgene expression.
CONCLUDING REMARKS
The successful development of an HIV vaccine remains an unprecedented challenge. We have summarized here various virus vectors that are being developed in the quest to overcome this challenge (Table 1). One of the main challenges in HIV vaccine design is the high strain diversity of HIV and the difficulty of generating bNAbs by vaccination, which suggests the need for serial heterologous prime-boost immunizations. This may potentially preclude the possibility of a “single shot.” In addition, HIV can infect activated CD4 T cells that can be induced by vaccination or natural infection. Thus, a balance between protective antibody and cellular responses has been suggested to be one of the most critical aspects of HIV vaccine design (137, 138). Two main areas of difficultly remain for viral vectors. The first issue is preexisting vector immunity, and toward this end, rare-serotype vectors are constantly being characterized and developed. The second issue is how to best utilize vaccine vectors to elicit the appropriate adaptive immune responses (in terms of quantity, quality, and localization). As our knowledge of fundamental virology and immunology of vaccine vectors continues to expand, we hope to be able to translate this to successful HIV vaccine design through the rational engineering and refinement of promising vaccine modalities.
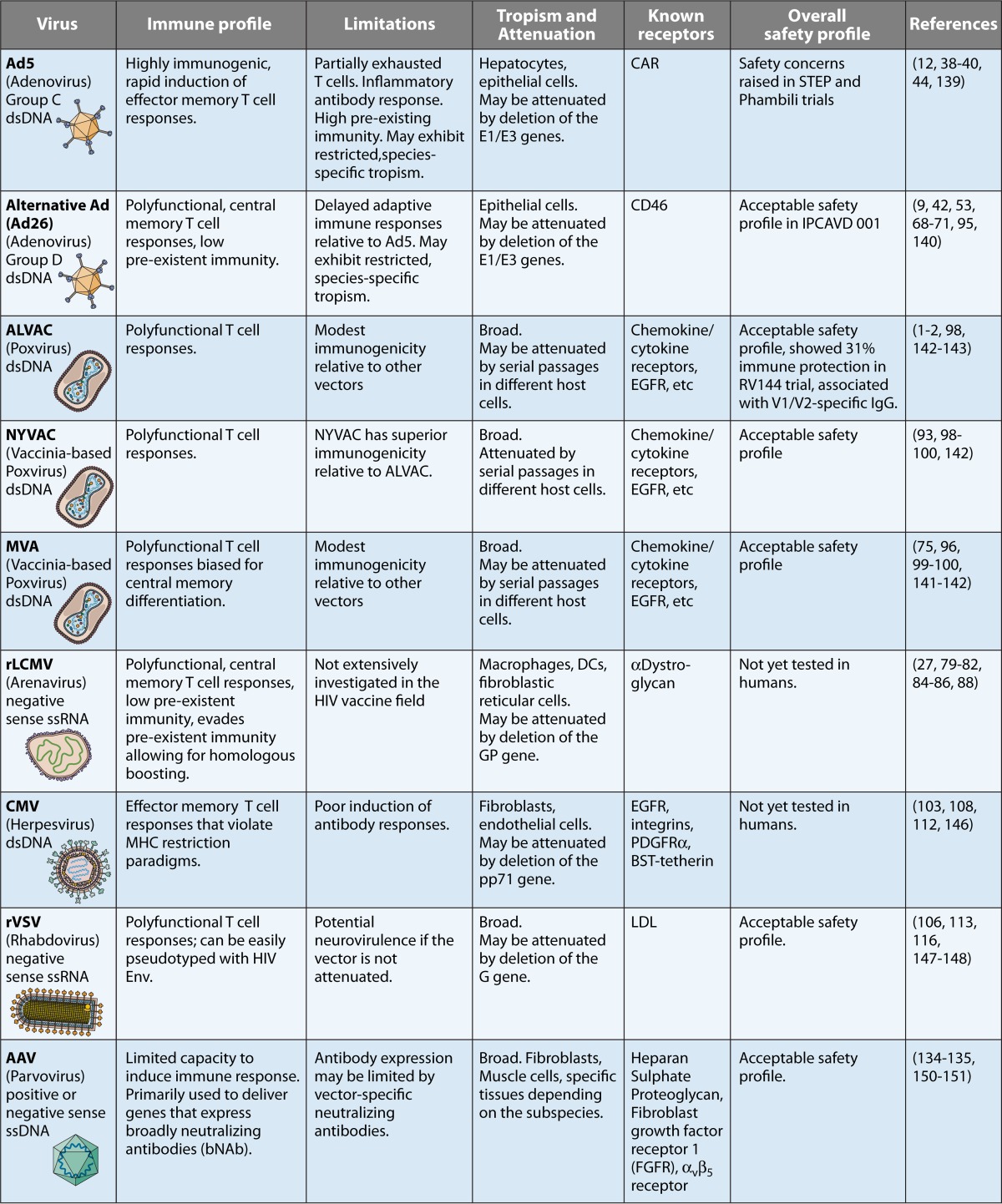
TABLE 1 .
Summary of various vaccine vectors used in AIDS vaccine development
ACKNOWLEDGMENTS
This work was supported by grants from the Chicago Centers for AIDS Research (P30 AI117943) and the NIH (1K22AI118421) to P.P.-M.
Biographies
Quazim A. Alayo obtained his M.B.B.S. degree from the University of Ibadan Medical School in Nigeria. He holds an M.Sc. degree in global health science from the University of Oxford and an M.M.Sc. degree in immunology from Harvard University. His research at the Jenner Institute at the University of Oxford focused on identifying new antigens as potential candidates for the development of malaria vaccines. During his graduate training at Harvard, Quazim worked under the supervision of Dan Barouch and Pablo Penaloza-MacMaster, and he evaluated immune responses and protection induced by various serotypes of adenovirus and poxvirus vaccine vectors and the potential of these vectors as therapeutic vaccine tools for HIV infection. Currently, Quazim is a research fellow at the Brigham and Women’s Hospital in Boston, where he is developing and assessing the impact of oncolytic herpes simplex virus vectors on the adaptive immune system in cancer virotherapy.
Pablo Penaloza-MacMaster obtained his Ph.D. with Rafi Ahmed at Emory University. His doctoral work focused on evaluating the role of inhibitory pathways during chronic viral infection. He then started a postdoctoral fellowship with Dan Barouch at Harvard University, where he focused on understanding the mechanisms of vaccine-induced immune protection and evaluating the efficacy of novel virus vaccine vectors, such as recombinant adenovirus and LCMV vectors expressing SIV antigens. He was an assistant professor at Harvard Medical School in 2015 and 2016, and then he relocated his laboratory to Northwestern University in Chicago. His laboratory currently studies the mechanisms of immune regulation during chronic viral infection and vaccination.
REFERENCES
- 1.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH, MOPH-TAVEG Investigators . 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 2.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barouch DH, Alter G, Broge T, Linde C, Ackerman ME, Brown EP, Borducchi EN, Smith KM, Nkolola JP, Liu J, Shields J, Parenteau L, Whitney JB, Abbink P, Ng’ang’a DM, Seaman MS, Lavine CL, Perry JR, Li W, Colantonio AD, Lewis MG, Chen B, Wenschuh H, Reimer U, Piatak M, Lifson JD, Handley SA, Virgin HW, Koutsoukos M, Lorin C, Voss G, Weijtens M, Pau MG, Schuitemaker H. 2015. Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science 349:320–324. doi: 10.1126/science.aab3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Day TA, Kublin JG. 2013. Lessons learned from HIV vaccine clinical efficacy trials. Curr HIV Res 11:441–449. doi: 10.2174/1570162X113116660051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, Ghrayeb J, Forman MA, Montefiori DC, Rieber EP, Letvin NL, Reimann KA. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs JD, Frank I, Elizaga ML, Allen M, Frahm N, Kochar N, Li S, Edupuganti S, Kalams SA, Tomaras GD, Sheets R, Pensiero M, Tremblay MA, Higgins TJ, Latham T, Egan MA, Clarke DK, Eldridge JH, HVTN 090 Study Group and the National Institutes of Allergy and Infectious Diseases HIV Vaccine Trials Network, Mulligan M, Rouphael N, Estep S, Rybczyk K, Dunbar D, Buchbinder S, Wagner T, Isbell R, Chinnell V, Bae J, Escamilla G, Tseng J, Fair R, Ramirez S, Broder G, Briesemeister L, Ferrara A. 2015. First-in-human evaluation of the safety and immunogenicity of a recombinant vesicular stomatitis virus human immunodeficiency virus-1 gag vaccine (HVTN 090). Open Forum Infect Dis 2:ofv082. doi: 10.1093/ofid/ofv082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ndhlovu ZM, Chibnik LB, Proudfoot J, Vine S, McMullen A, Cesa K, Porichis F, Jones RB, Alvino DM, Hart MG, Stampouloglou E, Piechocka-Trocha A, Kadie C, Pereyra F, Heckerman D, De Jager PL, Walker BD, Kaufmann DE. 2013. High-dimensional immunomonitoring models of HIV-1-specific CD8 T-cell responses accurately identify subjects achieving spontaneous viral control. Blood 121:801–811. doi: 10.1182/blood-2012-06-436295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, O’Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, Abbink P, Coffey RT, Grandpre LE, Seaman MS, Landucci G, Forthal DN, Montefiori DC, Carville A, Mansfield KG, Havenga MJ, Pau MG, Goudsmit J, Barouch DH. 2009. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, SanMiguel A, Seaman MS, Ferrari G, Forthal DN, Ourmanov I, Hirsch VM, Carville A, Mansfield KG, Stablein D, Pau MG, Schuitemaker H, Sadoff JC, Billings EA, Rao M, Robb ML, Kim JH, Marovich MA, Goudsmit J, Michael NL. 2012. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 482:89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roederer M, Keele BF, Schmidt SD, Mason RD, Welles HC, Fischer W, Labranche C, Foulds KE, Louder MK, Yang ZY, Todd JP, Buzby AP, Mach LV, Shen L, Seaton KE, Ward BM, Bailer RT, Gottardo R, Gu W, Ferrari G, Alam SM, Denny TN, Montefiori DC, Tomaras GD, Korber BT, Nason MC, Seder RA, Koup RA, Letvin NL, Rao SS, Nabel GJ, Mascola JR. 2014. Immunological and virological mechanisms of vaccine-mediated protection against SIV and HIV. Nature 505:502–508. doi: 10.1038/nature12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mooij P, Balla-Jhagjhoorsingh SS, Koopman G, Beenhakker N, van Haaften P, Baak I, Nieuwenhuis IG, Kondova I, Wagner R, Wolf H, Gómez CE, Nájera JL, Jiménez V, Esteban M, Heeney JL. 2008. Differential CD4+ versus CD8+ T-cell responses elicited by different poxvirus-based human immunodeficiency virus type 1 vaccine candidates provide comparable efficacies in primates. J Virol 82:2975–2988. doi: 10.1128/JVI.02216-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN, Step Study Protocol Team . 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammer SM, Sobieszczyk ME, Janes H, Karuna ST, Mulligan MJ, Grove D, Koblin BA, Buchbinder SP, Keefer MC, Tomaras GD, Frahm N, Hural J, Anude C, Graham BS, Enama ME, Adams E, DeJesus E, Novak RM, Frank I, Bentley C, Ramirez S, Fu R, Koup RA, Mascola JR, Nabel GJ, Montefiori DC, Kublin J, McElrath MJ, Corey L, Gilbert PB, HVTN 505 Study Team . 2013. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med 369:2083–2092. doi: 10.1056/NEJMoa1310566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol 8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 15.McShane H. 2004. Developing an improved vaccine against tuberculosis. Expert Rev Vaccines 3:299–306. doi: 10.1586/14760584.3.3.299. [DOI] [PubMed] [Google Scholar]
- 16.Sitati EM, Diamond MS. 2006. CD4+ T-cell responses are required for clearance of West Nile virus from the central nervous system. J Virol 80:12060–12069. doi: 10.1128/JVI.01650-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reich A, Erlwein O, Niewiesk S, ter Meulen V, Liebert UG. 1992. CD4+ T cells control measles virus infection of the central nervous system. Immunology 76:185–191. [PMC free article] [PubMed] [Google Scholar]
- 18.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 19.Shedlock DJ, Shen H. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 20.Sun JC, Bevan MJ. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Provine NM, Larocca RA, Penaloza-MacMaster P, Borducchi EN, McNally A, Parenteau LR, Kaufman DR, Barouch DH. 2014. Longitudinal requirement for CD4+ T cell help for adenovirus vector-elicited CD8+ T cell responses. J Immunol 192:5214–5225. doi: 10.4049/jimmunol.1302806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Provine NM, Badamchi-Zadeh A, Bricault CA, Penaloza-MacMaster P, Larocca RA, Borducchi EN, Seaman MS, Barouch DH. 2016. Transient CD4+ T cell depletion results in delayed development of functional vaccine-elicited antibody responses. J Virol 90:4278–4288. doi: 10.1128/JVI.00039-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Provine NM, Larocca RA, Aid M, Penaloza-MacMaster P, Badamchi-Zadeh A, Borducchi EN, Yates KB, Abbink P, Kirilova M, Ng’ang’a D, Bramson J, Haining WN, Barouch DH. 2016. Immediate dysfunction of vaccine-elicited CD8+ T cells primed in the absence of CD4+ T cells. J Immunol 197:1809–1822. doi: 10.4049/jimmunol.1600591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walsh SR, Seaman MS, Grandpre LE, Charbonneau C, Yanosick KE, Metch B, Keefer MC, Dolin R, Baden LR. 2012. Impact of anti-orthopoxvirus neutralizing antibodies induced by a heterologous prime-boost HIV-1 vaccine on insert-specific immune responses. Vaccine 31:114–119. doi: 10.1016/j.vaccine.2012.10.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keefer MC, Frey SE, Elizaga M, Metch B, De Rosa SC, Barroso PF, Tomaras G, Cardinali M, Goepfert P, Kalichman A, Philippon V, McElrath MJ, Jin X, Ferrari G, Defawe OD, Mazzara GP, Montefiori D, Pensiero M, Panicali DL, Corey L, NIAID HIV Vaccine Trials Network . 2011. A phase I trial of preventive HIV vaccination with heterologous poxviral-vectors containing matching HIV-1 inserts in healthy HIV-uninfected subjects. Vaccine 29:1948–1958. doi: 10.1016/j.vaccine.2010.12.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh KB, Teijaro JR, Zuniga EI, Welch MJ, Fremgen DM, Blackburn SD, von Tiehl KF, Wherry EJ, Flavell RA, Oldstone MB. 2012. Toll-like receptor 7 is required for effective adaptive immune responses that prevent persistent virus infection. Cell Host Microbe 11:643–653. doi: 10.1016/j.chom.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penaloza-MacMaster P, Barber DL, Wherry EJ, Provine NM, Teigler JE, Parenteau L, Blackmore S, Borducchi EN, Larocca RA, Yates KB, Shen H, Haining WN, Sommerstein R, Pinschewer DD, Ahmed R, Barouch DH. 2015. Vaccine-elicited CD4 T cells induce immunopathology after chronic LCMV infection. Science 347:278–282. doi: 10.1126/science.aaa2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staprans SI, Barry AP, Silvestri G, Safrit JT, Kozyr N, Sumpter B, Nguyen H, McClure H, Montefiori D, Cohen JI, Feinberg MB. 2004. Enhanced SIV replication and accelerated progression to AIDS in macaques primed to mount a CD4 T cell response to the SIV envelope protein. Proc Natl Acad Sci U S A 101:13026–13031. doi: 10.1073/pnas.0404739101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, Casazza JP, Kuruppu J, Kunstman K, Wolinsky S, Grossman Z, Dybul M, Oxenius A, Price DA, Connors M, Koup RA. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature 417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 30.Barouch DH, O’Brien KL, Simmons NL, King SL, Abbink P, Maxfield LF, Sun YH, La Porte A, Riggs AM, Lynch DM, Clark SL, Backus K, Perry JR, Seaman MS, Carville A, Mansfield KG, Szinger JJ, Fischer W, Muldoon M, Korber B. 2010. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nat Med 16:319–323. doi: 10.1038/nm.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santra S, Liao HX, Zhang R, Muldoon M, Watson S, Fischer W, Theiler J, Szinger J, Balachandran H, Buzby A, Quinn D, Parks RJ, Tsao CY, Carville A, Mansfield KG, Pavlakis GN, Felber BK, Haynes BF, Korber BT, Letvin NL. 2010. Mosaic vaccines elicit CD8+ T lymphocyte responses that confer enhanced immune coverage of diverse HIV strains in monkeys. Nat Med 16:324–328. doi: 10.1038/nm.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eloit M, Gilardi-Hebenstreit P, Toma B, Perricaudet M. 1990. Construction of a defective adenovirus vector expressing the pseudorabies virus glycoprotein gp50 and its use as a live vaccine. J Gen Virol 71:2425–2431. doi: 10.1099/0022-1317-71-10-2425. [DOI] [PubMed] [Google Scholar]
- 33.Seong YR, Choi S, Lim JS, Lee CH, Lee CK, Im DS. 2001. Immunogenicity of the E1E2 proteins of hepatitis C virus expressed by recombinant adenoviruses. Vaccine 19:2955–2964. doi: 10.1016/S0264-410X(00)00534-X. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Ewald BA, Lynch DM, Denholtz M, Abbink P, Lemckert AA, Carville A, Mansfield KG, Havenga MJ, Goudsmit J, Barouch DH. 2008. Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. J Virol 82:4844–4852. doi: 10.1128/JVI.02616-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe OD, Carter DK, Hural J, Akondy R, Buchbinder SP, Robertson MN, Mehrotra DV, Self SG, Corey L, Shiver JW, Casimiro DR, Step Study Protocol Team . 2008. HIV-1 vaccine-induced immunity in the test-of-concept step study: a case-cohort analysis. Lancet 372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang ZR, Wang HF, Zhao J, Peng YY, Wang J, Guinn BA, Huang LQ. 2007. Recent developments in the use of adenoviruses and immunotoxins in cancer gene therapy. Cancer Gene Ther 14:599–615. doi: 10.1038/sj.cgt.7701054. [DOI] [PubMed] [Google Scholar]
- 37.Gabitzsch ES, Xu Y, Balint JP Jr., Hartman ZC, Lyerly HK, Jones FR. 2010. Anti-tumor immunotherapy despite immunity to adenovirus using a novel adenoviral vector Ad5 [E1-, E2b-]-CEA. Cancer Immunol Immunother 59:1131–1135. doi: 10.1007/s00262-010-0847-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, Zhang ZQ, Simon AJ, Trigona WL, Dubey SA, Huang L, Harris VA, Long RS, Liang X, Handt L, Schleif WA, Zhu L, Freed DC, Persaud NV, Guan L, Punt KS, Tang A, Chen M, Wilson KA, Collins KB, Heidecker GJ, Fernandez VR, Perry HC, Joyce JG, Grimm KM, Cook JC, Keller PM, Kresock DS, Mach H, Troutman RD, Isopi LA, Williams DM, Xu Z, Bohannon KE, Volkin DB, Montefiori DC, Miura A, Krivulka GR, Lifton MA, Kuroda MJ, Schmitz JE, Letvin NL, Caulfield MJ, Bett AJ, Youil R. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 39.Wilson NA, Reed J, Napoe GS, Piaskowski S, Szymanski A, Furlott J, Gonzalez EJ, Yant LJ, Maness NJ, May GE, Soma T, Reynolds MR, Rakasz E, Rudersdorf R, McDermott AB, O’Connor DH, Friedrich TC, Allison DB, Patki A, Picker LJ, Burton DR, Lin J, Huang L, Patel D, Heindecker G, Fan J, Citron M, Horton M, Wang F, Liang X, Shiver JW, Casimiro DR, Watkins DI. 2006. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J Virol 80:5875–5885. doi: 10.1128/JVI.00171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gray GE, Allen M, Moodie Z, Churchyard G, Bekker LG, Nchabeleng M, Mlisana K, Metch B, de Bruyn G, Latka MH, Roux S, Mathebula M, Naicker N, Ducar C, Carter DK, Puren A, Eaton N, McElrath MJ, Robertson M, Corey L, Kublin JG, HVTN 503/Phambili Study Team . 2011. Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect Dis 11:507–515. doi: 10.1016/S1473-3099(11)70098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu H, Eller MA, Zafar S, Zhou Y, Gu M, Wei Z, Currier JR, Marovich MA, Kibuuka HN, Bailer RT, Koup RA, Robb ML, Michael NL, Kim JH, Ratto-Kim S. 2014. Preferential infection of human Ad5-specific CD4 T cells by HIV in Ad5 naturally exposed and recombinant Ad5-HIV vaccinated individuals. Proc Natl Acad Sci U S A 111:13439–13444. doi: 10.1073/pnas.1400446111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan WG, Jin HT, West EE, Penaloza-MacMaster P, Wieland A, Zilliox MJ, McElrath MJ, Barouch DH, Ahmed R. 2013. Comparative analysis of simian immunodeficiency virus gag-specific effector and memory CD8+ T cells induced by different adenovirus vectors. J Virol 87:1359–1372. doi: 10.1128/JVI.02055-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Penaloza-MacMaster P, Alayo QA, Ra J, Provine NM, Larocca R, Lee B, Barouch DH. 2016. Inhibitory receptor expression on memory CD8 T cells following ad vector immunization. Vaccine 34:4955–4963. doi: 10.1016/j.vaccine.2016.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quinn KM, Da Costa A, Yamamoto A, Berry D, Lindsay RW, Darrah PA, Wang L, Cheng C, Kong WP, Gall JG, Nicosia A, Folgori A, Colloca S, Cortese R, Gostick E, Price DA, Gomez CE, Esteban M, Wyatt LS, Moss B, Morgan C, Roederer M, Bailer RT, Nabel GJ, Koup RA, Seder RA. 2013. Comparative analysis of the magnitude, quality, phenotype, and protective capacity of simian immunodeficiency virus gag-specific CD8+ T cells following human-, simian-, and chimpanzee-derived recombinant adenoviral vector immunization. J Immunol 190:2720–2735. doi: 10.4049/jimmunol.1202861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Penaloza-MacMaster P, Provine NM, Ra J, Borducchi EN, McNally A, Simmons NL, Iampietro MJ, Barouch DH. 2013. Alternative serotype adenovirus vaccine vectors elicit memory T cells with enhanced anamnestic capacity compared to Ad5 vectors. J Virol 87:1373–1384. doi: 10.1128/JVI.02058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang TC, Millar J, Groves T, Grinshtein N, Parsons R, Takenaka S, Wan Y, Bramson JL. 2006. The CD8+ T cell population elicited by recombinant adenovirus displays a novel partially exhausted phenotype associated with prolonged antigen presentation that nonetheless provides long-term immunity. J Immunol 176:200–210. doi: 10.4049/jimmunol.176.1.200. [DOI] [PubMed] [Google Scholar]
- 47.Pillai VK, Kannanganat S, Penaloza-Macmaster P, Chennareddi L, Robinson HL, Blackwell J, Amara RR. 2011. Different patterns of expansion, contraction and memory differentiation of HIV-1 Gag-specific CD8 T cells elicited by adenovirus type 5 and modified vaccinia Ankara vaccines. Vaccine 29:5399–5406. doi: 10.1016/j.vaccine.2011.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. 2009. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Larocca RA, Provine NM, Aid M, Iampietro MJ, Borducchi EN, Badamchi-Zadeh A, Abbink P, Ng'ang'a D, Bricault CA, Blass E, Penaloza-MacMaster P, Stephenson KE, Barouch DH. 2016. Adenovirus serotype 5 vaccine vectors trigger IL-27-dependent inhibitory CD4+ T cell responses that impair CD8+ T cell function. Sci Immunol 1:eaaf7643. doi: 10.1126/sciimmunol.aaf7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teigler JE, Penaloza-MacMaster P, Obeng R, Provine NM, Larocca RA, Borducchi EN, Barouch DH. 2014. Hexon hypervariable region-modified adenovirus type 5 (Ad5) vectors display reduced hepatotoxicity but induce T lymphocyte phenotypes similar to Ad5 vectors. Clin Vaccine Immunol 21:1137–1144. doi: 10.1128/CVI.00207-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, Pink R, Buckley SM, Greig JA, Denby L, Custers J, Morita T, Francischetti IM, Monteiro RQ, Barouch DH, van Rooijen N, Napoli C, Havenga MJ, Nicklin SA, Baker AH. 2008. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 52.Lee J, Hashimoto M, Im SJ, Araki K, Jin HT, Davis CW, Konieczny BT, Spies GA, McElrath MJ, Ahmed R. 2017. Adenovirus serotype 5 vaccination results in suboptimal CD4 T helper 1 responses in mice. J Virol 91:e01132-16. doi: 10.1128/JVI.01132-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, Holterman L, Damen I, Vogels R, Thorner AR, O’Brien KL, Carville A, Mansfield KG, Goudsmit J, Havenga MJ, Barouch DH. 2007. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol 81:4654–4663. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tuboly T, Nagy E. 2001. Construction and characterization of recombinant porcine adenovirus serotype 5 expressing the transmissible gastroenteritis virus spike gene. J Gen Virol 82:183–190. doi: 10.1099/0022-1317-82-1-183. [DOI] [PubMed] [Google Scholar]
- 55.Farina SF, Gao GP, Xiang ZQ, Rux JJ, Burnett RM, Alvira MR, Marsh J, Ertl HC, Wilson JM. 2001. Replication-defective vector based on a chimpanzee adenovirus. J Virol 75:11603–11613. doi: 10.1128/JVI.75.23.11603-11613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peruzzi D, Dharmapuri S, Cirillo A, Bruni BE, Nicosia A, Cortese R, Colloca S, Ciliberto G, La Monica N, Aurisicchio L. 2009. A novel chimpanzee serotype-based adenoviral vector as delivery tool for cancer vaccines. Vaccine 27:1293–1300. doi: 10.1016/j.vaccine.2008.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Colloca S, Barnes E, Folgori A, Ammendola V, Capone S, Cirillo A, Siani L, Naddeo M, Grazioli F, Esposito ML, Ambrosio M, Sparacino A, Bartiromo M, Meola A, Smith K, Kurioka A, O’Hara GA, Ewer KJ, Anagnostou N, Bliss C, Hill AV, Traboni C, Klenerman P, Cortese R, Nicosia A. 2012. Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci Transl Med 4:115ra2. doi: 10.1126/scitranslmed.3002925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barnes E, Folgori A, Capone S, Swadling L, Aston S, Kurioka A, Meyer J, Huddart R, Smith K, Townsend R, Brown A, Antrobus R, Ammendola V, Naddeo M, O’Hara G, Willberg C, Harrison A, Grazioli F, Esposito ML, Siani L, Traboni C, Oo Y, Adams D, Hill A, Colloca S, Nicosia A, Cortese R, Klenerman P. 2012. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci Transl Med 4:115ra1. doi: 10.1126/scitranslmed.3003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ewer KJ, Lambe T, Rollier CS, Spencer AJ, Hill AVS, Dorrell L. 2016. Viral vectors as vaccine platforms: from immunogenicity to impact. Curr Opin Immunol 41:47–54. doi: 10.1016/j.coi.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 60.Abbink P, Maxfield LF, Ng’ang’a D, Borducchi EN, Iampietro MJ, Bricault CA, Teigler JE, Blackmore S, Parenteau L, Wagh K, Handley SA, Zhao G, Virgin HW, Korber B, Barouch DH. 2015. Construction and evaluation of novel rhesus monkey adenovirus vaccine vectors. J Virol 89:1512–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gaggar A, Shayakhmetov DM, Lieber A. 2003. CD46 is a cellular receptor for group B adenoviruses. Nat Med 9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- 62.Roelvink PW, Lizonova A, Lee JG, Li Y, Bergelson JM, Finberg RW, Brough DE, Kovesdi I, Wickham TJ. 1998. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J Virol 72:7909–7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barouch DH, Kik SV, Weverling GJ, Dilan R, King SL, Maxfield LF, Clark S, Ng’ang’a D, Brandariz KL, Abbink P, Sinangil F, de Bruyn G, Gray GE, Roux S, Bekker LG, Dilraj A, Kibuuka H, Robb ML, Michael NL, Anzala O, Amornkul PN, Gilmour J, Hural J, Buchbinder SP, Seaman MS, Dolin R, Baden LR, Carville A, Mansfield KG, Pau MG, Goudsmit J. 2011. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine 29:5203–5209. doi: 10.1016/j.vaccine.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kostense S, Koudstaal W, Sprangers M, Weverling GJ, Penders G, Helmus N, Vogels R, Bakker M, Berkhout B, Havenga M, Goudsmit J. 2004. Adenovirus types 5 and 35 seroprevalence in AIDS risk groups supports type 35 as a vaccine vector. AIDS 18:1213–1216. doi: 10.1097/00002030-200405210-00019. [DOI] [PubMed] [Google Scholar]
- 65.Nwanegbo E, Vardas E, Gao W, Whittle H, Sun H, Rowe D, Robbins PD, Gambotto A. 2004. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of the Gambia, South Africa, and the United States. Clin Diagn Lab Immunol 11:351–357. doi: 10.1128/CDLI.11.2.351-357.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Teigler JE, Iampietro MJ, Barouch DH. 2012. Vaccination with adenovirus serotypes 35, 26, and 48 elicits higher levels of innate cytokine responses than adenovirus serotype 5 in rhesus monkeys. J Virol 86:9590–9598. doi: 10.1128/JVI.00740-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barouch DH, Stephenson KE, Borducchi EN, Smith K, Stanley K, McNally AG, Liu J, Abbink P, Maxfield LF, Seaman MS, Dugast AS, Alter G, Ferguson M, Li W, Earl PL, Moss B, Giorgi EE, Szinger JJ, Eller LA, Billings EA, Rao M, Tovanabutra S, Sanders-Buell E, Weijtens M, Pau MG, Schuitemaker H, Robb ML, Kim JH, Korber BT, Michael NL. 2013. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell 155:531–539. doi: 10.1016/j.cell.2013.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baden LR, Walsh SR, Seaman MS, Tucker RP, Krause KH, Patel A, Johnson JA, Kleinjan J, Yanosick KE, Perry J, Zablowsky E, Abbink P, Peter L, Iampietro MJ, Cheung A, Pau MG, Weijtens M, Goudsmit J, Swann E, Wolff M, Loblein H, Dolin R, Barouch DH. 2013. First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001). J Infect Dis 207:240–247. doi: 10.1093/infdis/jis670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baden LR, Liu J, Li H, Johnson JA, Walsh SR, Kleinjan JA, Engelson BA, Peter L, Abbink P, Milner DA, Golden KL, Viani KL, Stachler MD, Chen BJ, Pau MG, Weijtens M, Carey BR, Miller CA, Swann EM, Wolff M, Loblein H, Seaman MS, Dolin R, Barouch DH. 2015. Induction of HIV-1-specific mucosal immune responses following intramuscular recombinant adenovirus serotype 26 HIV-1 vaccination of humans. J Infect Dis 211:518–528. doi: 10.1093/infdis/jiu485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baden LR, Karita E, Mutua G, Bekker LG, Gray G, Page-Shipp L, Walsh SR, Nyombayire J, Anzala O, Roux S, Laher F, Innes C, Seaman MS, Cohen YZ, Peter L, Frahm N, McElrath MJ, Hayes P, Swann E, Grunenberg N, Grazia-Pau M, Weijtens M, Sadoff J, Dally L, Lombardo A, Gilmour J, Cox J, Dolin R, Fast P, Barouch DH, Laufer DS, B003-IPCAVD004-HVTN091 Study Group. 2016. Assessment of the safety and immunogenicity of 2 novel vaccine platforms for HIV-1 prevention: a randomized trial. Ann Intern Med 164:313–322. doi: 10.7326/M15-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fuchs JD, Bart PA, Frahm N, Morgan C, Gilbert PB, Kochar N, DeRosa SC, Tomaras GD, Wagner TM, Baden LR, Koblin BA, Rouphael NG, Kalams SA, Keefer MC, Goepfert PA, Sobieszczyk ME, Mayer KH, Swann E, Liao HX, Haynes BF, Graham BS, McElrath MJ, NIAID HIV Vaccine Trials Network . 2015. Safety and immunogenicity of a recombinant adenovirus serotype 35-vectored HIV-1 vaccine in adenovirus serotype 5 seronegative and seropositive individuals. J AIDS Clin Res 6:461. doi: 10.4172/2155-6113.1000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Antrobus RD, Coughlan L, Berthoud TK, Dicks MD, Hill AV, Lambe T, Gilbert SC. 2014. Clinical assessment of a novel recombinant simian adenovirus ChAdOx1 as a vectored vaccine expressing conserved influenza A antigens. Mol Ther 22:668–674. doi: 10.1038/mt.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ewer KJ, O’Hara GA, Duncan CJ, Collins KA, Sheehy SH, Reyes-Sandoval A, Goodman AL, Edwards NJ, Elias SC, Halstead FD, Longley RJ, Rowland R, Poulton ID, Draper SJ, Blagborough AM, Berrie E, Moyle S, Williams N, Siani L, Folgori A, Colloca S, Sinden RE, Lawrie AM, Cortese R, Gilbert SC, Nicosia A, Hill AV. 2013. Protective CD8+ T-cell immunity to human malaria induced by chimpanzee adenovirus-MVA immunisation. Nat Commun 4:2836. doi: 10.1038/ncomms3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Hara GA, Duncan CJ, Ewer KJ, Collins KA, Elias SC, Halstead FD, Goodman AL, Edwards NJ, Reyes-Sandoval A, Bird P, Rowland R, Sheehy SH, Poulton ID, Hutchings C, Todryk S, Andrews L, Folgori A, Berrie E, Moyle S, Nicosia A, Colloca S, Cortese R, Siani L, Lawrie AM, Gilbert SC, Hill AV. 2012. Clinical assessment of a recombinant simian adenovirus ChAd63: a potent new vaccine vector. J Infect Dis 205:772–781. doi: 10.1093/infdis/jir850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ogwang C, Kimani D, Edwards NJ, Roberts R, Mwacharo J, Bowyer G, Bliss C, Hodgson SH, Njuguna P, Viebig NK, Nicosia A, Gitau E, Douglas S, Illingworth J, Marsh K, Lawrie A, Imoukhuede EB, Ewer K, Urban BC, Hill AVS, Bejon P, MVVC group . 2015. Prime-boost vaccination with chimpanzee adenovirus and modified vaccinia Ankara encoding TRAP provides partial protection against Plasmodium falciparum infection in Kenyan adults. Sci Transl Med 7:286re5. doi: 10.1126/scitranslmed.aaa2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Quinn KM, Zak DE, Costa A, Yamamoto A, Kastenmuller K, Hill BJ, Lynn GM, Darrah PA, Lindsay RWB, Wang L, Cheng C, Nicosia A, Folgori A, Colloca S, Cortese R, Gostick E, Price DA, Gall JGD, Roederer M, Aderem A, Seder RA. 2015. Antigen expression determines adenoviral vaccine potency independent of IFN and STING signaling. J Clin Invest 125:1129–1146. doi: 10.1172/JCI78280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barouch DH, Pau MG, Custers JHHV, Koudstaal W, Kostense S, Havenga MJE, Truitt DM, Sumida SM, Kishko MG, Arthur JC, Korioth-Schmitz B, Newberg MH, Gorgone DA, Lifton MA, Panicali DL, Nabel GJ, Letvin NL, Goudsmit J. 2004. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol 172:6290–6297. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- 78.Roberts DM, Nanda A, Havenga MJ, Abbink P, Lynch DM, Ewald BA, Liu J, Thorner AR, Swanson PE, Gorgone DA, Lifton MA, Lemckert AA, Holterman L, Chen B, Dilraj A, Carville A, Mansfield KG, Goudsmit J, Barouch DH. 2006. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 441:239–243. doi: 10.1038/nature04721. [DOI] [PubMed] [Google Scholar]
- 79.Flatz L, Hegazy AN, Bergthaler A, Verschoor A, Claus C, Fernandez M, Gattinoni L, Johnson S, Kreppel F, Kochanek S, Broek Mv, Radbruch A, Lévy F, Lambert PH, Siegrist CA, Restifo NP, Löhning M, Ochsenbein AF, Nabel GJ, Pinschewer DD. 2010. Development of replication-defective lymphocytic choriomeningitis virus vectors for the induction of potent CD8+ T cell immunity. Nat Med 16:339–345. doi: 10.1038/nm.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Penaloza MacMaster P, Shields JL, Alayo QA, Cabral C, Jimenez J, Mondesir J, Chandrashekar A, Cabral JM, Lim M, Iampietro MJ, Provine NM, Bricault CA, Seaman M, Orlinger K, Aspoeck A, Fuhrmann G, Lilja AE, Monath T, Mangeat B, Pinschewer DD, Barouch DH. 2017. Development of novel replication-defective lymphocytic choriomeningitis virus vectors expressing SIV antigens. Vaccine 35:1–9. doi: 10.1016/j.vaccine.2016.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Buchmeier MJ, Bowen MD, Peters CJ. 2001. Arenaviridae: the viruses and their replication, p 1635–1668. In Fields BN, Knipe DM, Howley PM, Griffin DE (ed), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 82.Zhou X, Ramachandran S, Mann M, Popkin DL. 2012. Role of lymphocytic choriomeningitis virus (LCMV) in understanding viral immunology: past, present and future. Viruses 4:2650–2669. doi: 10.3390/v4112650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Flatz L, Cheng C, Wang L, Foulds KE, Ko SY, Kong WP, Roychoudhuri R, Shi W, Bao S, Todd JP, Asmal M, Shen L, Donaldson M, Schmidt SD, Gall JG, Pinschewer DD, Letvin NL, Rao S, Mascola JR, Roederer M, Nabel GJ. 2012. Gene-based vaccination with a mismatched envelope protects against simian immunodeficiency virus infection in nonhuman primates. J Virol 86:7760–7770. doi: 10.1128/JVI.00599-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fischer SA, Graham MB, Kuehnert MJ, Kotton CN, Srinivasan A, Marty FM, Comer JA, Guarner J, Paddock CD, DeMeo DL, Shieh WJ, Erickson BR, Bandy U, DeMaria A Jr., Davis JP, Delmonico FL, Pavlin B, Likos A, Vincent MJ, Sealy TK, Goldsmith CS, Jernigan DB, Rollin PE, Packard MM, Patel M, Rowland C, Helfand RF, Nichol ST, Fishman JA, Ksiazek T, Zaki SR, Team LiTRI . 2006. Transmission of lymphocytic choriomeningitis virus by organ transplantation. N Engl J Med 354:2235–2249. doi: 10.1056/NEJMoa053240. [DOI] [PubMed] [Google Scholar]
- 85.Komrower GM, Williams BL, Stones PB. 1955. Lymphocytic choriomeningitis in the newborn; probable transplacental infection. Lancet 268:697–698. doi: 10.1016/S0140-6736(55)91066-7. [DOI] [PubMed] [Google Scholar]
- 86.Lledó L, Gegúndez MI, Saz JV, Bahamontes N, Beltrán M. 2003. Lymphocytic choriomeningitis virus infection in a province of Spain: analysis of sera from the general population and wild rodents. J Med Virol 70:273–275. doi: 10.1002/jmv.10389. [DOI] [PubMed] [Google Scholar]
- 87.Elbers AR, Vecht U, Osterhaus AD, Groen J, Wisselink HJ, Diepersloot RJ, Tielen MJ. 1999. Low prevalence of antibodies against the zoonotic agents Brucella abortus, Leptospira spp., Streptococcus suis serotype II, hantavirus, and lymphocytic choriomeningitis virus among veterinarians and pig farmers in the southern part of the Netherlands. Vet Q 21:50–54. doi: 10.1080/01652176.1999.9694991. [DOI] [PubMed] [Google Scholar]
- 88.Stephensen CB, Blount SR, Lanford RE, Holmes KV, Montali RJ, Fleenor ME, Shaw JF. 1992. Prevalence of serum antibodies against lymphocytic choriomeningitis virus in selected populations from two U.S. cities. J Med Virol 38:27–31. doi: 10.1002/jmv.1890380107. [DOI] [PubMed] [Google Scholar]
- 89.Rolland M, Edlefsen PT, Larsen BB, Tovanabutra S, Sanders-Buell E, Hertz T, deCamp AC, Carrico C, Menis S, Magaret CA, Ahmed H, Juraska M, Chen L, Konopa P, Nariya S, Stoddard JN, Wong K, Zhao H, Deng W, Maust BS, Bose M, Howell S, Bates A, Lazzaro M, O’Sullivan A, Lei E, Bradfield A, Ibitamuno G, Assawadarachai V, O’Connell RJ, deSouza MS, Nitayaphan S, Rerks-Ngarm S, Robb ML, McLellan JS, Georgiev I, Kwong PD, Carlson JM, Michael NL, Schief WR, Gilbert PB, Mullins JI, Kim JH. 2012. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature 490:417–420. doi: 10.1038/nature11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Easterhoff D, Moody MA, Fera D, Cheng H, Ackerman M, Wiehe K, Saunders KO, Pollara J, Vandergrift N, Parks R, Kim J, Michael NL, O’Connell RJ, Excler JL, Robb ML, Vasan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Nitayaphan S, Sinangil F, Tartaglia J, Phogat S, Kepler TB, Alam SM, Liao HX, Ferrari G, Seaman MS, Montefiori DC, Tomaras GD, Harrison SC, Haynes BF. 2017. Boosting of HIV envelope CD4 binding site antibodies with long variable heavy third complementarity determining region in the randomized double blind RV305 HIV-1 vaccine trial. PLoS Pathog 13:e1006182. doi: 10.1371/journal.ppat.1006182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Taylor J, Weinberg R, Languet B, Desmettre P, Paoletti E. 1988. Recombinant fowlpox virus inducing protective immunity in non-avian species. Vaccine 6:497–503. doi: 10.1016/0264-410X(88)90100-4. [DOI] [PubMed] [Google Scholar]
- 92.Meyer H, Sutter G, Mayr A. 1991. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J Gen Virol 72:1031–1038. doi: 10.1099/0022-1317-72-5-1031. [DOI] [PubMed] [Google Scholar]
- 93.Tartaglia J, Perkus ME, Taylor J, Norton EK, Audonnet JC, Cox WI, Davis SW, van der Hoeven J, Meignier B, Riviere M, et al. . 1992. NYVAC: a highly attenuated strain of vaccinia virus. Virology 188:217–232. doi: 10.1016/0042-6822(92)90752-B. [DOI] [PubMed] [Google Scholar]
- 94.Barouch DH, Klasse PJ, Dufour J, Veazey RS, Moore JP. 2012. Macaque studies of vaccine and microbicide combinations for preventing HIV-1 sexual transmission. Proc Natl Acad Sci U S A 109:8694–8698. 1203183109. doi: 10.1073/pnas.1203183109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barouch DH. 2013. The quest for an HIV-1 vaccine—moving forward. N Engl J Med 369:2073–2076. doi: 10.1056/NEJMp1312711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Swadling L, Capone S, Antrobus RD, Brown A, Richardson R, Newell EW, Halliday J, Kelly C, Bowen D, Fergusson J, Kurioka A, Ammendola V, Del Sorbo M, Grazioli F, Esposito ML, Siani L, Traboni C, Hill A, Colloca S, Davis M, Nicosia A, Cortese R, Folgori A, Klenerman P, Barnes E. 2014. A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory. Sci Transl Med 6:261ra153. doi: 10.1126/scitranslmed.3009185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stanley DA, Honko AN, Asiedu C, Trefry JC, Lau-Kilby AW, Johnson JC, Hensley L, Ammendola V, Abbate A, Grazioli F, Foulds KE, Cheng C, Wang L, Donaldson MM, Colloca S, Folgori A, Roederer M, Nabel GJ, Mascola J, Nicosia A, Cortese R, Koup RA, Sullivan NJ. 2014. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against Ebolavirus challenge. Nat Med 20:1126–1129. doi: 10.1038/nm.3702. [DOI] [PubMed] [Google Scholar]
- 98.García-Arriaza J, Perdiguero B, Heeney J, Seaman M, Montefiori DC, Labranche C, Yates NL, Shen X, Tomaras GD, Ferrari G, Foulds KE, McDermott A, Kao SF, Roederer M, Hawkins N, Self S, Yao J, Farrell P, Phogat S, Tartaglia J, Barnett SW, Burke B, Cristillo A, Weiss D, Lee C, Kibler K, Jacobs B, Asbach B, Wagner R, Ding S, Pantaleo G, Esteban M. 2015. Head-to-head comparison of poxvirus NYVAC and ALVAC vectors expressing identical HIV-1 clade C immunogens in prime-boost combination with Env protein in nonhuman primates. J Virol 89:8525–8539. doi: 10.1128/JVI.01265-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Teigler JE, Phogat S, Franchini G, Hirsch VM, Michael NL, Barouch DH. 2014. The canarypox virus vector ALVAC induces distinct cytokine responses compared to the vaccinia virus-based vectors MVA and NYVAC in rhesus monkeys. J Virol 88:1809–1814. doi: 10.1128/JVI.02386-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guerra S, Nájera JL, González JM, López-Fernández LA, Climent N, Gatell JM, Gallart T, Esteban M. 2007. Distinct gene expression profiling after infection of immature human monocyte-derived dendritic cells by the attenuated poxvirus vectors MVA and NYVAC. J Virol 81:8707–8721. doi: 10.1128/JVI.00444-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zanotto C, Pozzi E, Pacchioni S, Volonté L, De Giuli Morghen C, Radaelli A. 2010. Canarypox and fowlpox viruses as recombinant vaccine vectors: a biological and immunological comparison. Antiviral Res 88:53–63. doi: 10.1016/j.antiviral.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 102.Wherry EJ, Teichgräber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol 4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 103.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M Jr., Lifson JD, Picker LJ. 2011. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. 2008. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med 205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sarkar S, Teichgräber V, Kalia V, Polley A, Masopust D, Harrington LE, Ahmed R, Wherry EJ. 2007. Strength of stimulus and clonal competition impact the rate of memory CD8 T cell differentiation. J Immunol 179:6704–6714. doi: 10.4049/jimmunol.179.10.6704. [DOI] [PubMed] [Google Scholar]
- 106.Haglund K, Forman J, Kräusslich HG, Rose JK. 2000. Expression of human immunodeficiency virus type 1 Gag protein precursor and envelope proteins from a vesicular stomatitis virus recombinant: high-level production of virus-like particles containing HIV envelope. Virology 268:112–121. doi: 10.1006/viro.1999.0120. [DOI] [PubMed] [Google Scholar]
- 107.Parks CL, Picker LJ, King CR. 2013. Development of replication-competent viral vectors for HIV vaccine delivery. Curr Opin HIV AIDS 8:402–411. doi: 10.1097/COH.0b013e328363d389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak M Jr., Lifson JD, Nelson JA, Jarvis MA, Picker LJ. 2009. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med 15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. 2006. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis 43:1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 110.Pereira L, Maidji E. 2008. Cytomegalovirus infection in the human placenta: maternal immunity and developmentally regulated receptors on trophoblasts converge. Curr Top Microbiol Immunol 325:383–395. doi: 10.1007/978-3-540-77349-8_21. [DOI] [PubMed] [Google Scholar]
- 111.Britt W. 2008. Manifestations of human cytomegalovirus infection: proposed mechanisms of acute and chronic disease. Curr Top Microbiol Immunol 325:417–470. doi: 10.1007/978-3-540-77349-8_23. [DOI] [PubMed] [Google Scholar]
- 112.Hansen SG, Sacha JB, Hughes CM, Ford JC, Burwitz BJ, Scholz I, Gilbride RM, Lewis MS, Gilliam AN, Ventura AB, Malouli D, Xu G, Richards R, Whizin N, Reed JS, Hammond KB, Fischer M, Turner JM, Legasse AW, Axthelm MK, Edlefsen PT, Nelson JA, Lifson JD, Früh K, Picker LJ. 2013. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science 340:1237874. doi: 10.1126/science.1237874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Henao-Restrepo AM, Camacho A, Longini IM, Watson CH, Edmunds WJ, Egger M, Carroll MW, Dean NE, Diatta I, Doumbia M, Draguez B, Duraffour S, Enwere G, Grais R, Gunther S, Gsell PS, Hossmann S, Watle SV, Kondé MK, Kéïta S, Kone S, Kuisma E, Levine MM, Mandal S, Mauget T, Norheim G, Riveros X, Soumah A, Trelle S, Vicari AS, Røttingen JA, Kieny MP. 2017. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola ça suffit!). Lancet 389:505–518. doi: 10.1016/S0140-6736(16)32621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rose NF, Marx PA, Luckay A, Nixon DF, Moretto WJ, Donahoe SM, Montefiori D, Roberts A, Buonocore L, Rose JK. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539–549. doi: 10.1016/S0092-8674(01)00482-2. [DOI] [PubMed] [Google Scholar]
- 115.Johnson JE, Nasar F, Coleman JW, Price RE, Javadian A, Draper K, Lee M, Reilly PA, Clarke DK, Hendry RM, Udem SA. 2007. Neurovirulence properties of recombinant vesicular stomatitis virus vectors in non-human primates. Virology 360:36–49. doi: 10.1016/j.virol.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Clarke DK, Nasar F, Chong S, Johnson JE, Coleman JW, Lee M, Witko SE, Kotash CS, Abdullah R, Megati S, Luckay A, Nowak B, Lackner A, Price RE, Little P, Kalyan N, Randolf V, Javadian A, Zamb TJ, Parks CL, Egan MA, Eldridge J, Hendry M, Udem SA. 2014. Neurovirulence and immunogenicity of attenuated recombinant vesicular stomatitis viruses in nonhuman primates. J Virol 88:6690–6701. doi: 10.1128/JVI.03441-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cooper D, Wright KJ, Calderon PC, Guo M, Nasar F, Johnson JE, Coleman JW, Lee M, Kotash C, Yurgelonis I, Natuk RJ, Hendry RM, Udem SA, Clarke DK. 2008. Attenuation of recombinant vesicular stomatitis virus-human immunodeficiency virus type 1 vaccine vectors by gene translocations and G gene truncation reduces neurovirulence and enhances immunogenicity in mice. J Virol 82:207–219. doi: 10.1128/JVI.01515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Malkevitch N, Patterson LJ, Aldrich K, Richardson E, Alvord WG, Robert-Guroff M. 2003. A replication competent adenovirus 5 host range mutant-simian immunodeficiency virus (SIV) recombinant priming/subunit protein boosting vaccine regimen induces broad, persistent SIV-specific cellular immunity to dominant and subdominant epitopes in Mamu-A*01 rhesus macaques. J Immunol 170:4281–4289. doi: 10.4049/jimmunol.170.8.4281. [DOI] [PubMed] [Google Scholar]
- 119.Demberg T, Florese RH, Heath MJ, Larsen K, Kalisz I, Kalyanaraman VS, Lee EM, Pal R, Venzon D, Grant R, Patterson LJ, Korioth-Schmitz B, Buzby A, Dombagoda D, Montefiori DC, Letvin NL, Cafaro A, Ensoli B, Robert-Guroff M. 2007. A replication-competent adenovirus-human immunodeficiency virus (Ad-HIV) tat and Ad-HIV env priming/Tat and envelope protein boosting regimen elicits enhanced protective efficacy against simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J Virol 81:3414–3427. doi: 10.1128/JVI.02453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maxfield LF, Abbink P, Stephenson KE, Borducchi EN, Ng’ang’a D, Kirilova MM, Paulino N, Boyd M, Shabram P, Ruan Q, Patel M, Barouch DH. 2015. Attenuation of replication-competent adenovirus serotype 26 vaccines by vectorization. Clin Vaccine Immunol 22:1166–1175. doi: 10.1128/CVI.00510-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alexander J, Mendy J, Vang L, Avanzini JB, Garduno F, Manayani DJ, Ishioka G, Farness P, Ping LH, Swanstrom R, Parks R, Liao HX, Haynes BF, Montefiori DC, LaBranche C, Smith J, Gurwith M, Mayall T. 2013. Pre-clinical development of a recombinant, replication-competent adenovirus serotype 4 vector vaccine expressing HIV-1 envelope 1086 clade C. PLoS One 8:e82380. doi: 10.1371/journal.pone.0082380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nyombayire J, Anzala O, Gazzard B, Karita E, Bergin P, Hayes P, Kopycinski J, Omosa-Manyonyi G, Jackson A, Bizimana J, Farah B, Sayeed E, Parks CL, Inoue M, Hironaka T, Hara H, Shu T, Matano T, Dally L, Barin B, Park H, Gilmour J, Lombardo A, Excler JL, Fast P, Laufer DS, Cox JH, S001 Study Team. 2017. First-in-human evaluation of the safety and immunogenicity of an intranasally administered replication-competent Sendai virus-vectored HIV type 1 gag vaccine: induction of potent T-cell or antibody responses in prime-boost regimens. J Infect Dis 215:95–104. doi: 10.1093/infdis/jiw500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hocknell PK, Wiley RD, Wang RD, Evans TG, Bowers WJ, Hanke T, Federoff HJ, Dewhurst S. 2002. Expression of human immunodeficiency virus type 1 gp120 from herpes simplex virus type 1-derived amplicons results in potent, specific, and durable cellular and humoral immune responses. J Virol 76:5565–5580. doi: 10.1128/JVI.76.11.5565-5580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.García-Arriaza J, Perdiguero B, Heeney JL, Seaman MS, Montefiori DC, Yates NL, Tomaras GD, Ferrari G, Foulds KE, Roederer M, Self SG, Borate B, Gottardo R, Phogat S, Tartaglia J, Barnett SW, Burke B, Cristillo AD, Weiss DE, Lee C, Kibler KV, Jacobs BL, Wagner R, Ding S, Pantaleo G, Esteban M. 2017. HIV/AIDS vaccine candidates based on replication-competent recombinant poxvirus NYVAC-C-KC expressing trimeric gp140 and Gag-derived virus-like particles or lacking the viral molecule B19 that inhibits type I interferon activate relevant HIV-1-specific B and T cell immune functions in nonhuman primates. J Virol 91:e02182-16. doi: 10.1128/JVI.02182-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Daniel MD, Kirchhoff F, Czajak SC, Sehgal PK, Desrosiers RC. 1992. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science 258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 126.Alpert MD, Harvey JD, Lauer WA, Reeves RK, Piatak M Jr., Carville A, Mansfield KG, Lifson JD, Li W, Desrosiers RC, Johnson RP, Evans DT. 2012. ADCC develops over time during persistent infection with live-attenuated SIV and is associated with complete protection against SIV(mac)251 challenge. PLoS Pathog 8:e1002890. doi: 10.1371/journal.ppat.1002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Adnan S, Reeves RK, Gillis J, Wong FE, Yu Y, Camp JV, Li Q, Connole M, Li Y, Piatak M Jr., Lifson JD, Li W, Keele BF, Kozlowski PA, Desrosiers RC, Haase AT, Johnson RP. 2016. Persistent low-level replication of SIVΔnef drives maturation of antibody and CD8 T cell responses to induce protective immunity against vaginal SIV infection. PLoS Pathog 12:e1006104. doi: 10.1371/journal.ppat.1006104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Billingsley JM, Rajakumar PA, Connole MA, Salisch NC, Adnan S, Kuzmichev YV, Hong HS, Reeves RK, Kang HJ, Li W, Li Q, Haase AT, Johnson RP. 2015. Characterization of CD8+ T cell differentiation following SIVΔnef vaccination by transcription factor expression profiling. PLoS Pathog 11:e1004740. doi: 10.1371/journal.ppat.1004740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lemiale F, Asefa B, Ye D, Chen C, Korokhov N, Humeau L. 2010. An HIV-based lentiviral vector as HIV vaccine candidate: immunogenic characterization. Vaccine 28:1952–1961. doi: 10.1016/j.vaccine.2009.10.089. [DOI] [PubMed] [Google Scholar]
- 130.Asefa B, Korokhov N, Lemiale F. 2010. Heterologous HIV-based lentiviral/adenoviral vectors immunizations result in enhanced HIV-specific immunity. Vaccine 28:3617–3624. doi: 10.1016/j.vaccine.2009.12.047. [DOI] [PubMed] [Google Scholar]
- 131.Lemiale F, Korokhov N. 2009. Lentiviral vectors for HIV disease prevention and treatment. Vaccine 27:3443–3449. doi: 10.1016/j.vaccine.2009.01.055. [DOI] [PubMed] [Google Scholar]
- 132.Penaloza-MacMaster P, Teigler JE, Obeng RC, Kang ZH, Provine NM, Parenteau L, Blackmore S, Ra J, Borducchi EN, Barouch DH. 2014. Augmented replicative capacity of the boosting antigen improves the protective efficacy of heterologous prime-boost vaccine regimens. J Virol 88:6243–6254. doi: 10.1128/JVI.00406-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Deal CE, Balazs AB. 2015. Vectored antibody gene delivery for the prevention or treatment of HIV infection. Curr Opin HIV AIDS 10:190–197. doi: 10.1097/COH.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. 2011. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature 481:81–84. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Saunders KO, Wang L, Joyce MG, Yang ZY, Balazs AB, Cheng C, Ko SY, Kong WP, Rudicell RS, Georgiev IS, Duan L, Foulds KE, Donaldson M, Xu L, Schmidt SD, Todd JP, Baltimore D, Roederer M, Haase AT, Kwong PD, Rao SS, Mascola JR, Nabel GJ. 2015. Broadly neutralizing human immunodeficiency virus type 1 antibody gene transfer protects nonhuman primates from mucosal simian-human immunodeficiency virus infection. J Virol 89:8334–8345. doi: 10.1128/JVI.00908-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fuchs SP, Desrosiers RC. 2016. Promise and problems associated with the use of recombinant AAV for the delivery of anti-HIV antibodies. Mol Ther Methods Clin Dev 3:16068. doi: 10.1038/mtm.2016.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lewis GK, DeVico AL, Gallo RC. 2014. Antibody persistence and T-cell balance: two key factors confronting HIV vaccine development. Proc Natl Acad Sci U S A 111:15614–15621. doi: 10.1073/pnas.1413550111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fouts TR, Bagley K, Prado IJ, Bobb KL, Schwartz JA, Xu R, Zagursky RJ, Egan MA, Eldridge JH, LaBranche CC, Montefiori DC, Le Buanec H, Zagury D, Pal R, Pavlakis GN, Felber BK, Franchini G, Gordon S, Vaccari M, Lewis GK, DeVico AL, Gallo RC. 2015. Balance of cellular and humoral immunity determines the level of protection by HIV vaccines in rhesus macaque models of HIV infection. Proc Natl Acad Sci U S A 112:E992–E999. doi: 10.1073/pnas.1423669112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 140.Li H, Rhee EG, Masek-Hammerman K, Teigler JE, Abbink P, Barouch DH. 2012. Adenovirus serotype 26 utilizes CD46 as a primary cellular receptor and only transiently activates T lymphocytes following vaccination of rhesus monkeys. J Virol 86:10862–10865. doi: 10.1128/JVI.00928-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Grandpre LE, Duke-Cohan JS, Ewald BA, Devoy C, Barouch DH, Letvin NL, Reinherz EL, Baden LR, Dolin R, Seaman MS. 2009. Immunogenicity of recombinant modified vaccinia Ankara following a single or multi-dose vaccine regimen in rhesus monkeys. Vaccine 27:1549–1556. doi: 10.1016/j.vaccine.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Moss B. 2012. Poxvirus cell entry: how many proteins does it take? Viruses 4:688–707. doi: 10.3390/v4050688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huang Y, Duerr A, Frahm N, Zhang L, Moodie Z, De Rosa S, McElrath MJ, Gilbert PB. 2014. Immune-correlates analysis of an HIV-1 vaccine efficacy trial reveals an association of nonspecific interferon-gamma secretion with increased HIV-1 infection risk: a cohort-based modeling study. PLoS One 9:e108631. doi: 10.1371/journal.pone.0108631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cao W, Henry MD, Borrow P, Yamada H, Elder JH, Ravkov EV, Nichol ST, Compans RW, Campbell KP, Oldstone MB. 1998. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science 282:2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- 145.Mueller SN, Matloubian M, Clemens DM, Sharpe AH, Freeman GJ, Gangappa S, Larsen CP, Ahmed R. 2007. Viral targeting of fibroblastic reticular cells contributes to immunosuppression and persistence during chronic infection. Proc Natl Acad Sci U S A 104:15430–15435. doi: 10.1073/pnas.0702579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vanarsdall AL, Johnson DC. 2012. Human cytomegalovirus entry into cells. Curr Opin Virol 2:37–42. doi: 10.1016/j.coviro.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Finkelshtein D, Werman A, Novick D, Barak S, Rubinstein M. 2013. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc Natl Acad Sci U S A 110:7306–7311. doi: 10.1073/pnas.1214441110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Daddario-DiCaprio KM, Geisbert TW, Ströher U, Geisbert JB, Grolla A, Fritz EA, Fernando L, Kagan E, Jahrling PB, Hensley LE, Jones SM, Feldmann H. 2006. Postexposure protection against Marburg haemorrhagic fever with recombinant vesicular stomatitis virus vectors in non-human primates: an efficacy assessment. Lancet 367:1399–1404. doi: 10.1016/S0140-6736(06)68546-2. [DOI] [PubMed] [Google Scholar]
- 149.Adamson BJ, Fuchs JD, Sopher CJ, Flood DM, Johnson RP, Haynes BF, Kublin JG, NIAID HIV Vaccine Trials Network . 2015. A new model for catalyzing translational science: the early stage investigator mentored research scholar program in HIV vaccines. Clin Transl Sci 8:166–168. doi: 10.1111/cts.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Summerford C, Samulski RJ. 1998. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol 72:1438–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Qing K, Mah C, Hansen J, Zhou S, Dwarki V, Srivastava A. 1999. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med 5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]