Abstract
Best known as chaperones, heat shock proteins (HSPs) also have roles in cell signalling and regulation of metabolism. Rodent studies demonstrate that heat treatment, transgenic overexpression and pharmacological induction of HSP72 prevent high-fat diet-induced glucose intolerance and skeletal muscle insulin resistance. Overexpression of skeletal muscle HSP72 in mice has been shown to increase endurance running capacity nearly twofold and increase mitochondrial content by 50%. A positive correlation between HSP72 mRNA expression and mitochondrial enzyme activity has been observed in human skeletal muscle, and HSP72 expression is markedly decreased in skeletal muscle of insulin resistant and type 2 diabetic patients. In addition, decreased levels of HSP72 correlate with insulin resistance and non-alcoholic fatty liver disease progression in livers from obese patients. These data suggest the targeted induction of HSPs could be a therapeutic approach for preventing metabolic disease by maintaining the body's natural stress response. Exercise elicits a number of metabolic adaptations and is a powerful tool in the prevention and treatment of insulin resistance. Exercise training is also a stimulus for increased HSP expression. Although the underlying mechanism(s) for exercise-induced HSP expression are currently unknown, the HSP response may be critical for the beneficial metabolic effects of exercise. Exercise-induced extracellular HSP release may also contribute to metabolic homeostasis by actively restoring HSP72 content in insulin resistant tissues containing low endogenous levels of HSPs.
This article is part of the theme issue ‘Heat shock proteins as modulators and therapeutic targets of chronic disease: an integrated perspective’.
Keywords: aerobic capacity, skeletal muscle, heat treatment, inflammation, mitochondria
1. Introduction
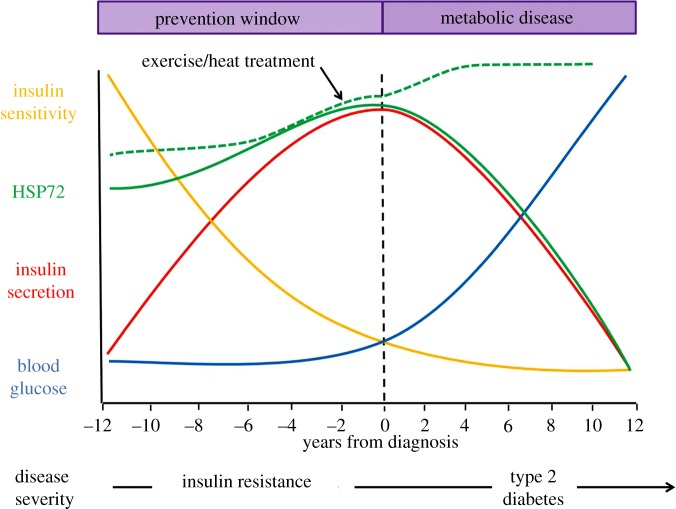
Insulin resistance is a condition that impacts at least 86 million U.S. adults aged 20 or older [1]. Insulin resistance occurs when the islet cells in the pancreas secrete insulin but the hormone no longer effectively triggers glucose uptake in metabolic tissues. The inability of metabolic tissues to take up glucose results in hyperglycaemia and hyperinsulinaemia, both hallmark symptoms of insulin resistance. Most individuals with insulin resistance go undiagnosed and the condition can persist for 10–12 years. This decade plus of time can be especially damaging as insulin resistance is an independent risk factor for obesity, cardiovascular disease, hypertension and type 2 diabetes. This time frame represents a critical intervention window where progression towards metabolic dysfunction and type 2 diabetes can be prevented and reversed (figure 1).
Figure 1.
Targeting heat shock proteins in the prevention of insulin resistance. Schematic depicting the timeline of metabolic disease from insulin resistance to type 2 diabetes. Insulin resistance can persist for 10–12 years prior to clinical diagnosis of type 2 diabetes, a time period that represents an increased risk for cardiovascular disease, obesity and type 2 diabetes. During insulin resistance, insulin secretion (red line) from pancreatic beta cells increases in an effort to maintain blood glucose (blue line). Insulin sensitivity declines (yellow line) resulting in a gradual increase in blood glucose and the development of type 2 diabetes. Insulin resistance represents a window of time when progression towards more severe metabolic disease can be prevented by lifestyle interventions like diet and exercise. HSPs are robustly induced by exercise, and HSP72 mRNA and protein expression are significantly reduced in the skeletal muscle of type 2 diabetic patients. However, very little is known about HSP expression patterns and regulation during insulin resistance. We hypothesize that HSP72 expression, in particular, may demonstrate an inverted parabolic relationship wherein initial increases in HSP72 combat metabolic dysfunction, but expression levels eventually peak and decline with disease severity and time spent under metabolic strain (solid green line). Exercise and heat treatment represent potential targeted therapies that could maintain and even increase HSP expression to prevent metabolic disease (dashed green line).
Growing evidence suggests the heat shock response and/or heat shock proteins (HSPs) could play an important role in preventing insulin resistance and the development of type 2 diabetes. HSPs are a highly conserved family of proteins best identified for their role as molecular chaperones [2]. They play a critical role in maintaining cellular function via regulation of protein folding and degradation. Not surprisingly, changes in their expression profile and cellular localization are linked to numerous disease states. Several studies suggest that induction, transcription and translation of these cytoprotective HSPs decline with chronic disease like non-alcoholic fatty liver disease (NAFLD) [3], Huntington's disease [4] and type 2 diabetes [5]. Conversely, induction and/or transgenic overexpression of HSPs results in ample metabolic benefit in animal models of obesity/metabolic disease [5–11]. Less clear, however, are the factors that regulate HSP expression in the pathological development of metabolic disease. In particular, very little is known regarding skeletal muscle HSP expression levels throughout the progression of obesity, insulin resistance and type 2 diabetes.
As skeletal muscle is the primary tissue responsible for insulin-stimulated glucose uptake [12], many researchers have investigated changes in skeletal muscle HSP expression during obesity, insulin resistance and type 2 diabetes (summarized in table 1). Skeletal muscle HSP72 (the corollary of HSP70 in animals) expression is inversely related to body fat percentage and blood glucose in healthy subjects [9,15]. Additionally, both HSP72 mRNA and protein expression are significantly reduced in the skeletal muscle of type 2 diabetic patients and subjects with insulin resistance [5,13,14,17,18]. Therefore, many have asserted that HSP72 expression levels are tightly correlated to adiposity and decrease through the progression from obesity to metabolic disease (i.e. insulin resistance and type 2 diabetes). It is also possible that glucose levels may partially regulate HSP expression levels [21–23].
Table 1.
Changes in skeletal muscle HSP72 levels during metabolic disease. HFD, high-fat diet; n.a., not available.
| investigators | model | muscle | status | duration HFD | HSP72 levels |
|---|---|---|---|---|---|
| Kurucz et al. [13] | human | vastus lateralis | type 2 diabetic | n.a. | HSP72 mRNA reduced |
| Bruce et al. [14] | human | vastus lateralis | type 2 diabetic | n.a. | HSP72 mRNA reduced |
| Chung et al. [5] | human and rodent | not reported | obese and insulin resistant | unknown for humans, 16 weeks for rodents | HSP72 protein reduced in humans, not reported in rodents |
| Gupte et al. [6] | rodent | soleus | impaired glucose tolerance | 6 weeks | HSP72 protein unaffected by diet |
| Gupte et al. [7] | rodent | soleus and extensor digitorum longus | impaired glucose tolerance | 12 weeks | HSP72 protein unaffected by diet |
| Henstridge et al. [15] | human | vastus lateralis | healthy | n.a. | HSP72 protein content inversely related to body fat percentage |
| Kavanagh et al. [16] | primate | biceps femoris | healthy and insulin resistant | 16 weeks and 6 years | HSP72 protein increased at 16 weeks and reduced at 6 years |
| Rodrigues-Krause et al. [17] | human | vastus lateralis | type 2 diabetic | n.a. | HSP72 protein reduced |
| Henstridge et al. [8] | rodent | quadriceps | obese/insulin resistant | 10 weeks | no change in HSP72 protein reported |
| Matos et al. [18] | human | vastus lateralis | healthy, obese–insulin sensitive and obese–insulin resistant | n.a. | basal and exercise-induced HSP72 protein reduced in both obese–sensitive and obese–insulin resistant subjects |
| Silverstein et al. [11] | rodent | gastrocnemius | aged mice; heat or geranylgeranylacetone treated | 14 weeks | changes in basal HSP72 protein not reported; HSP72 induction positively impacted metabolic measures |
| Marineli et al. [19] | rodent | soleus | impaired glucose tolerance | 12 weeks | HSP72 protein increased |
| Bock et al. [20] | rodent | soleus | insulin resistant | 28 weeks | HSP72 protein unaffected by diet |
| Kavanagh et al. [9] | primate | vastus lateralis | aged or young, both healthy and pre-diabetic/diabetic | n.a. | HSP72 protein inversely related to fasting blood glucose |
| Rogers et al. [10] | rodent | extensor digitorum longus (EDL) and soleus | high-capacity or low-capacity runners (HCR and LCR respectively) | 3 days | basal HSP72 protein unaffected by diet; HSP72 induction via heat blunted in LCRs in EDL |
Interestingly, multiple studies using animals fed a high-fat diet (HFD) highlight that this relationship is much more complex (table 1). For instance, investigations in primates and rodents show that short-term high-fat feeding (16 and 6–12 weeks, respectively) results in hallmark symptomology of insulin resistance but does not significantly reduce skeletal muscle HSP72 expression [6,7,10,16,19,20]. In fact, HSP72 expression may increase after short-term high-fat feeding, suggesting a possible compensatory response to combat metabolic dysfunction [16,19]. However, long-term high-fat feeding (6 years) appears to cause significant reductions in skeletal muscle HSP72 expression similar to the phenomenon described in type 2 diabetics [16]. Therefore, it is possible that skeletal muscle HSP72 expression can be characterized as an inverted parabolic relationship wherein initial increases in skeletal muscle HSP72 combat metabolic dysfunction, but these levels will eventually peak and decline depending on the severity and time spent under metabolic strain (solid green line, figure 1).
Discrepancies in the data regarding skeletal muscle HSP72 reductions during obesity, insulin resistance and type 2 diabetes may also be due to the model being used and the muscle type analysed. For example, investigations reporting significant reductions in HSP72 expression during obesity, insulin resistance and type 2 diabetes primarily analysed the vastus lateralis muscle from human subjects [13,14,17,18]. Alternatively, primate and rodent investigations observing no significant reductions in HSP72 expression in response to short-term high-fat feeding analysed the biceps femoris, soleus, and extensor digitorum longus muscles [6,7,10,16,19,20]. Thus, it is also possible that organismal differences and/or muscle fibre type differences, variations in muscle oxidative capacity, and muscle size could contribute to inter-study data variations (table 1). It is critical that future investigators address these inconsistencies when designing studies to address the role of HSP72 expression in metabolic disease. A greater understanding of the regulation of skeletal muscle HSPs during insulin resistance will allow future development of targeted therapies to maintain and even increase HSP expression to prevent metabolic disease (figure 1, dashed green line).
2. Heat shock protein mechanisms of action in insulin resistance
The complex, integrative and multi-organ nature of the HSP response makes the identification of specific mechanisms of action difficult. For instance, the most widely known HSP, HSP72, has varying roles and mechanisms of action in heart muscle, skeletal muscle, adipose tissue and the liver. Recent studies suggest decreasing inflammation, improving mitochondrial function/oxidative capacity, and maintaining proteostasis could be viable mechanisms of action for HSPs in metabolic tissues.
(a). Anti-inflammatory properties of HSP72
The ability of HSPs to decrease inflammation has centred on the proinflammatory protein c-Jun terminal kinase (JNK). Importantly, JNK activation is increased with the progression of insulin resistance and diabetes [24–29], while HSP72 expression is correspondingly decreased [5,13,14,17]. This inverse relationship between JNK activation and HSP expression also occurs during the progression from NAFLD to non-alcoholic steatohepatitis [3]. This relationship is of no coincidence. JNK activation indirectly inhibits HSP expression by maintaining heat shock factor 1 (HSF1), the primary HSP transcription factor, in its inactive monomeric state [30,31]. Beyond inactivation of HSF1 and HSP expression, there are other downstream targets of JNK that potentiate insulin resistance.
JNK is thought to drive insulin resistance through inhibitory phosphorylation of insulin receptor substrate 1 (IRS-1), a key protein in the insulin signalling cascade [25]. In addition, JNK can downregulate peroxisome proliferator-activated receptor α/fibroblast growth factor 21 (PPARα/FGF21) signalling in hepatocytes, leading to reduced fatty acid oxidation and the development of insulin resistance [32]. JNK activation also inhibits mitochondrial respiration, increases reactive oxygen species (ROS) production and causes apoptosis [33–37]. Previous studies suggest that HSP72 induction directly inhibits JNK activation, thereby improving insulin sensitivity and glucose tolerance at both skeletal muscle-specific and systemic levels [5,6,8,38,39]. For example, work by our laboratory has demonstrated that in vivo heat treatments decrease JNK activation in skeletal muscle of aged and HFD-fed rats [7,40]. Pharmacological activation of HSP72 also causes reduced JNK activation in skeletal muscle and liver [6,38]. Finally, overexpression of HSP72 in skeletal muscle decreased JNK activation in mice fed a HFD and was associated with beneficial metabolic outcomes [5]. In each instance, lowering of JNK activation resulted in improvements in insulin sensitivity and glucose tolerance, highlighting the importance of this HSP-mediated mechanism for insulin action.
HSP72 is proposed to regulate JNK activation through multiple mechanisms, including direct inhibition via protein–protein interaction with JNK [41], and/or inhibition of upstream JNK signalling pathways [42,43]. Evidence also exists suggesting that activation of HSP72 in the liver may decrease inflammation independently of JNK inhibition. Specifically, pharmacological activation of HSP72 decreases steatosis without decreasing JNK activation in HFD-fed rodents [44]. Although no change in JNK activation was observed, increased HSP72 expression resulted in inhibition of tumour necrosis factor α (TNFα) in the liver of rodents fed a HFD.
HSP72 may also play additional anti-inflammatory roles extracellularly or via localization in macrophages. For instance, HSP72 decreases during NAFLD progression in human Kupffer cells, liver-specific macrophages [3]. Interestingly, heat-induced upregulation of HSP72 in Kupffer cells coincides with suppression of TNFα [45,46]. Additionally, in myeloid cells, JNK activity is considered essential for activation of macrophages and a release of pro-inflammatory cytokines [47,48]. The ability of extracellular HSP72 to inhibit pro-inflammatory cytokine release from macrophages, lymphocytes and other immune cells [49–54] could be critical in decreasing local inflammation and attenuating the development of insulin resistance.
(b). HSP72 regulation of mitochondrial integrity and function
Mitochondrial dysfunction is a primary contributor to the development of metabolic disease and is therefore a possible target for therapy [55–57]. Our laboratory and others have shown that heat treatment improves skeletal muscle mitochondrial function by improving fatty acid oxidation [7], increasing mitochondrial enzyme activity [7,58,59], and increasing mitochondrial biogenesis [60]. Transgenic overexpression of HSP72 in skeletal muscle also increases mitochondrial enzyme activity, mitochondrial content and endurance running capacity [5,8]. Thus, it is possible that the beneficial mitochondrial adaptations stemming from heat treatment are a result of HSP72 induction.
HSP72 induction may mediate mitochondrial improvements by regulating mitophagy, the targeted degradation of mitochondria through autophagy. For instance, mice lacking skeletal muscle HSP72 demonstrate a reduced ability to degrade mitochondria through mitophagy [61]. Additionally, these mice exhibit enlarged, dysmorphic mitochondria with reduced muscle respiratory capacity and increased lipid accumulation. Thus, activation of HSP72 may improve mitochondrial quality by enhancing the degradation of dysfunctional mitochondria.
(c). HSP72 regulation of the unfolded protein response and proteostasis
Cellular stress causes unfolded proteins to accumulate in the endoplasmic reticulum (ER), which activates the unfolded protein response (UPR) [62–64]. This response is important for cellular adaptation to ER stress and prevention of ER-stress-induced apoptosis [65,66]. ER stress and chronic activation of the UPR causes inflammation and contributes to the development of insulin resistance [67–76].
While HSP family proteins have been shown to be a part of the UPR [77–79], new evidence has also identified the cytoplasmic HSP72 as a part of the UPR. Specifically, HSP72 interacts with and upregulates inositol-requiring enzyme 1α (IRE1α) signalling. Activation of IRE1α by HSP72 enhances cell survival through prevention of ER-stress-induced apoptosis [80]. This mechanism may be important in HSP72-mediated metabolic improvements, since activation of IRE1α also has been shown to suppress lipogenesis [81].
HSP72 may also impact metabolic health through the protein's additional responsibilities as a cellular chaperone. During stress, HSP72 is essential to refold misfolded proteins and to maintain proteostasis. HSP72 may maintain proteostasis by regulating proteosomal degradation and autophagy [82,83]. Degradation pathways via proteasomes and autophagy are well established, but it was recently demonstrated that mitochondria also function as sites for protein degradation [84]. Specifically, the chaperone HSP104 detangles protein aggregates allowing mitochondrial transporters to import proteins in the outer and inner mitochondrial membrane. Proteases in the mitochondrial matrix are then able to degrade the newly imported unfolded proteins. Importantly, defects in HSP70 activity resulted in increased transport of misfolded proteins into the mitochondria, causing increased mitochondrial damage and ROS production. This phenomenon was confirmed both in yeast and in human retinal pigment epithelium cells [84]. It is tempting to speculate that defects in HSP72 activity could contribute to mitochondrial dysfunction by triggering this alternative mitochondrial-dependent degradation pathway. This alternative pathway may contribute to the swollen, rounded appearance of the mitochondria during metabolic disease, as well as decreased ability for the mitochondria to function as a respiratory organelle. Future research investigating this mechanism in metabolic organs will be necessary.
(d). Heat shock transcription factor regulation of oxidative capacity
One of the most important heat shock response functions in metabolic tissue may actually lie upstream of HSP72. HSP72 overexpression leads to an increase in mitochondrial content, oxidative capacity and insulin sensitivity [5,55–57,85]. Similarly, the absence of HSP72 expression results in mitochondrial dysfunction and insulin resistance [61]. In addition to increasing HSP72 content, and thereby the ability to enhance mitochondrial quality control, exercise also increases peroxisome proliferator-activated receptor γ coactivator 1-α (PGC1α) expression [86–88]. PGC1α is the primary transcriptional coactivator for mitochondrial biosynthesis [89,90]. Interestingly, recent investigations reveal that the upstream regulatory elements of the PPARGC1A gene contain a heat shock element (HSE) binding sequence. This HSE sequence provides a docking site for the primary HSP transcription factor, heat shock factor 1 (HSF1). Indeed, chromatin immunoprecipitation analyses show that HSF1 and PGC1α co-occupy the HSE sequence on the promoter of the PPARGC1A gene [91]. Through a myriad of HSF1 activation and knockdown experiments, the Mueller lab has provided compelling evidence that HSF1 is a primary regulator of mitochondrial biogenesis, enzymatic function and whole-body metabolism [91,92]. These data exemplify the elegant coordination of HSF1 downstream targets (i.e. HSPs and PGC1α) in regulating mitochondrial biogenesis, quality control, and enzymatic function under conditions of metabolic demand and/or chronic disease. Importantly, future research is needed to delineate the specific contributions of varying downstream HSF1 targets, as well as potential direct effects of HSF1 itself, with regard to metabolic outcomes. This information, combined with a greater understanding of HSP mechanisms of action in metabolic tissue, may provide novel therapeutic targets to ameliorate metabolic dysfunction.
3. Exercise-induced heat shock protein response
Exercise is a primary treatment modality for patients exhibiting symptoms of metabolic dysfunction. Specifically, regular exercise training is known to decrease metabolic and cardiovascular disease risk factors in patients suffering from obesity and metabolic dysfunction [93,94]. Exercise is also a potent inducer of HSP expression [78], with HSP72 showing the most robust and consistent upregulation with exercise. HSP72 induction via heat treatment, pharmacologic intervention, and transgenic overexpression results in metabolic effects similar to exercise in models of obesity and insulin resistance [5–7,40,78]. Thus, exercise-induced HSP72 expression may contribute to the beneficial metabolic effects observed with exercise training. There is already a significant amount of information available about exercise and HSPs; however, little is known regarding the role of exercise-induced HSP72 expression in treating metabolic disease.
(a). Complexity of the exercise heat shock protein response
The direct cause of exercise-induced HSP upregulation, primarily of HSP72, remains unknown. It is hypothesized that a variety of biochemical, metabolic and/or physical stressors may stimulate HSP72 expression post-exercise. For instance, common challenges to tissues during exercise such as mechanical stress, acidosis, hypoxia, ischaemia, ROS formation, and calcium signalling changes are shown to independently cause HSP induction [95–103]. Additionally, increased metabolic stress via depletion of bioenergetic substrates (i.e. glycogen) is shown to potentiate exercise-induced HSP72 expression [104]. A similar potentiation effect is observed when exercise bouts are completed in a hot environment, but this effect is blunted in a cold environment [105]. Thus, it appears that elevations in HSP72 expression post-exercise are a result of not one, but many physiological stressors associated with exercise.
Adding complexity is the understanding that exercise-induced HSP expression is training modality, intensity and duration dependent. In skeletal muscle, elevations in HSP72 expression occur with both aerobic and resistance training [106,107]. Importantly, HSP72 expression is dependent on exercise intensity. For instance, HSP72 expression displays a positive relationship with exercise intensity during both aerobic and resistance training [104,106,108,109]. This relationship also exists when comparing exercise intensity and metabolic outcomes [110], supporting the potential contribution of HSP72 induction to the metabolic benefits associated with exercise.
HSP72 expression also varies based on the duration of the training regimen (i.e. acute versus chronic training). Acute exercise bouts cause dramatic elevations in HSP72 within 24 h [106], while chronic training regimens typically result in minimal elevations in HSP72 post-exercise [102]. Similarly, untrained subjects exhibit lower basal HSP72 expression and a higher degree of change in HSP expression post-exercise compared with fit subjects [102,111]. The minimal degree of change in HSP72 expression observed during long-duration training protocols and in fit subjects is likely a result of adaptation to exercise. This phenomenon, referred to as the repeated bout effect [112,113], is exemplified by the lack of potentiated HSP induction in recurring exercise bouts (specifically HSP72 and HSP27) [114]. However, cessation of exercise in trained subjects will cause basal HSP expression to return to levels comparable to those observed pre-exercise [115].
(b). Aerobic capacity and exercise training impact heat shock protein expression and induction
Recently, our laboratory has published data suggesting that intrinsic aerobic capacity, or the ability of the body to take up and use oxygen, is coupled to HSP induction and metabolic flexibility [10]. Low aerobic capacity increases susceptibility to developing metabolic dysfunction. Importantly, it is estimated that 50–70% of one's aerobic capacity is attributable to inheritable traits [116]. This genetic/phenotypic phenomenon is exemplified by rodent models selectively bred for high-capacity or low-capacity running (HCR and LCR respectively) [117]. Specifically, these models have drastic differences in susceptibility to metabolic complications [118–121]. For instance, the HSP72 response is blunted in LCR rodents after heat treatment and they require the heat intervention to maintain metabolic flexibility/protection when acutely challenged with a HFD [10]. Conversely, HCR rodents maintain the ability to upregulate HSP72 expression in skeletal muscle via heat treatment and display metabolic flexibility/protection independent of intervention when metabolically challenged. These data suggest that intrinsic aerobic capacity is coupled to the HSP72 response in skeletal muscle and that these two factors are primary contributors to whole-body metabolic health. As mentioned, unfit subjects with metabolic dysfunction, and most likely low aerobic capacity, have markedly low levels of HSP72 expression compared with healthy controls [5,13,14]. Thus, chronic exercise may restore basal HSP72 expression levels to that of healthy subjects. The restoration of basal HSP72 expression via exercise may directly impact organ-specific insulin sensitivity.
(c). Tissue-specific heat shock protein expression and induction
As mentioned, exercise increases skeletal muscle HSP72 expression. However, the levels of both basal HSP72 expression and exercise-induced HSP72 expression are dependent on muscle fibre type. For instance, muscles predominantly composed of type I fibres have higher basal HSP72 expression compared with muscles composed of type II fibres [122,123]. Furthermore, the magnitude of HSP72 upregulation is much greater in type II muscle fibres post-exercise compared with type I fibres [106,124]. This may explain the intensity dependent increases in HSP72 expression post-exercise, as higher intensity activities cause the recruitment of fast-twitch muscle fibres, resulting in a greater overall change in HSP72 expression. As type II muscle fibres are inherently glycolytic and have a high dynamic range of HSP72 expression, this invites the possibility that the positive metabolic effects seen with HSP72 overexpression may be primarily mediated by changes in type II fast-twitch muscles.
Exercise is also known to increase HSP72 expression in the liver, kidney, lungs, heart and brain [125–127]. During states of metabolic dysfunction, HSP72 expression in the liver is of primary concern owing to the organ's role in maintaining whole-body metabolic homeostasis. Pharmacologic HSP72 induction in the liver is shown to improve insulin sensitivity and glucose tolerance in models fed an HFD [38]. This protective effect may stem from the enhancement of HSP72-mediated mitochondrial quality control and the restoration of the insulin signalling pathway in hepatocytes—both of which occur with exercise and HSP72 upregulation in skeletal muscle. Thus, exercise-induced HSP72 expression in the liver may act to restore liver insulin sensitivity by mechanisms similar to those observed in skeletal muscle. However, future studies are needed to confirm this notion.
Interestingly, exercise also results in the release of extracellular HSPs (eHSPs) from the hepatosplanchnic viscera and brain into the circulation [104,128], and other potential sites of origin include epithelial cells [129] and immune cells [130,131]. eHSP72 function in general is associated with activation of the immune system [132], and in contrast to the anti-inflammatory actions of intracellular/cytosolic HSP72 (iHSP72), can induce activation of proinflammatory pathways. Based on this antagonistic action of HSP72 on the inflammatory response, the Chaperone Balance Hypothesis contends that the balance between eHSP72 and iHSP72 (eHSP72/iHSP72) could determine the extent of tissue inflammation, and thereby also influence the pathogenesis of insulin resistance and type 2 diabetes [133]. According to this hypothesis, an intervention that lowers the eHSP72/iHSP72 ratio could in effect improve insulin sensitivity. Long-term exercise training in effect results in decreased eHSP72 and increased iHSP72 expression (as in skeletal muscle), supporting this hypothesis. Importantly, the eHSP72/iHSP72 ratio could be a valuable biomarker for assessment of the inflammatory response in insulin resistance and diabetes.
However, the specific tissue contributions, mechanism(s) of action, and physiological consequences of eHSPs during exercise remain unknown. It is hypothesized that exercise-induced eHSPs may provide metabolic crosstalk between organs, contribute to exercise adaptation, and/or act as a stress-sensor or stress-messenger [78,104,128]. Exercise-induced eHSP release may also contribute to metabolic homeostasis by actively restoring HSP72 content in insulin resistant tissues containing low endogenous levels of HSPs. For example, existing evidence suggests that HSPs can be produced in tissues like muscle and adipose and released in the circulation via exosomes, small membrane vesicles that are secreted by numerous cell types [134]. In this manner, intracellular HSP72 transmission mediated by exosomes represents a novel mechanism for maintenance of HSP72 expression among different tissues. Future studies are needed to characterize the physiological outcomes of eHSPs both in healthy subjects and those with metabolic dysfunction.
4. Summary
Current lifestyle interventions for obesity and metabolic disease include dietary modification and exercise training. While effective at reducing body mass and enhancing insulin sensitivity, compliance is often low in patient populations and therefore alternative approaches are needed. Acute or short-term passive heating (≤3 weeks) has been investigated with promising improvements in metabolic parameters in humans [135]. In 1999, Philip Hooper performed the first study to suggest heat therapy (HT) could be beneficial for metabolic disease. In diabetic patients, fasting plasma glucose and haemoglobin A1c (HbA1c) levels were significantly decreased after only three weeks of HT by water immersion (30 min, 6 days/week) in which core body temperature was increased by an average of 0.8°C each session [135]. Despite this exciting phenomenon, only a handful of studies have examined the effects of HT in obese and/or type 2 diabetic patients [136–139].
Importantly, the first comprehensive investigation of long-term heat treatment in young, sedentary humans was recently performed [140]. Brunt et al. [140] found that eight weeks of repeated hot water immersion resulted in increased endothelial function (measured via flow-mediated dilation), reduced arterial stiffness, reduced mean arterial and diastolic blood pressure, and reduced carotid intima media thickness. Incredibly, these cardiovascular adaptations were on par with what is typically observed with exercise training in previously sedentary subjects. Despite ample evidence in animal studies demonstrating the beneficial effects of heat treatment on whole body metabolism and the anti-inflammatory and neuroprotective functions of HSP72 in vivo, heat treatment studies in insulin resistant or diabetic patients are lacking. Mild heat therapy treatment in patients with heart failure is remarkably effective [141], and the most promising application of mild, chronic heat treatment in humans could be in combination with exercise training. Novel, integrative research studies to examine both cellular mechanisms and systemic metabolic adaptations of heat therapy in humans could lead to new interventions for insulin resistance, obesity and cardiometabolic disease.
Data accessibility
This article has no additional data.
Authors' contributions
A.E.A., A.T.V.S. and P.C.G. wrote and edited all sections of the manuscript. P.C.G. had final editorial approval of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by National Institutes of Health Grants AG-031575 (P.C.G.), and core support was provided by National Institute of Child Health and Human Development Grant HD-002528 and the National Institute of General Medical Sciences P20 GM103418. A.E.A. and A.T.V.S. are supported by Madison and Lila Self Graduate Fellowships through the University of Kansas.
Reference
- 1.CDC. 2014. National Diabetes Statistics Report, 2014. Atlanta, GA: Division of Diabetes Translation, National Center for Chronic Disease Prevention and Health Promotion, US Department of Health & Human Services. https://stacks.cdc.gov/view/cdc/23442/cdc_23442_DS1.pdf.
- 2.Morimoto RI. 1988. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 12, 3788–3796. ( 10.1101/gad.12.24.3788) [DOI] [PubMed] [Google Scholar]
- 3.Di Naso FC, et al. 2015. Obesity depresses the anti-inflammatory HSP70 pathway, contributing to NAFLD progression. Obesity 23, 120–129. ( 10.1002/oby.20919) [DOI] [PubMed] [Google Scholar]
- 4.Labbadia J, et al. 2011. Altered chromatin architecture underlies progressive impairment of the heat shock response in mouse models of Huntington disease. J. Clin. Invest. 121, 3306–3319. ( 10.1172/JCI57413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung J, et al. 2008. HSP72 protects against obesity-induced insulin resistance. Proc. Natl Acad. Sci. USA 105, 1739–1744. ( 10.1073/pnas.0705799105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupte AA, Bomhoff GL, Morris JK, Gorres BK, Geiger PC. 2009. Lipoic acid increases heat shock protein expression and inhibits stress kinase activation to improve insulin signaling in skeletal muscle from high-fat-fed rats. J. Appl. Physiol. 106, 1425–1434. ( 10.1152/japplphysiol.91210.2008) [DOI] [PubMed] [Google Scholar]
- 7.Gupte AA, Bomhoff GL, Swerdlow RH, Geiger PC. 2009. Heat treatment improves glucose tolerance and prevents skeletal muscle insulin resistance in rats fed a high-fat diet. Diabetes 58, 567–578. ( 10.2337/db08-1070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henstridge DC, et al. 2014. Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes 63, 1881–1894. ( 10.2337/db13-0967) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kavanagh K, Davis AT, Jenkins KA, Flynn DM. 2016. Effects of heated hydrotherapy on muscle HSP70 and glucose metabolism in old and young vervet monkeys. Cell Stress Chaperones 21, 717–725. ( 10.1007/s12192-016-0699-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogers RS, et al. 2016. Deficiency in the heat stress response could underlie susceptibility to metabolic disease. Diabetes 65, 3341–3351. ( 10.2337/db16-0292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silverstein MG, Ordanes D, Wylie AT, Files DC, Milligan C, Presley TD, Kavanagh K. 2015. Inducing muscle heat shock protein 70 improves insulin sensitivity and muscular performance in aged mice. J. Gerontol. A 70, 800–808. ( 10.1093/gerona/glu119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz LD, Glickman MG, Rapoport S, Ferrannini E, DeFronzo RA. 1983. Splanchnic and peripheral disposal of oral glucose in man. Diabetes 32, 675–679. ( 10.2337/diab.32.7.675) [DOI] [PubMed] [Google Scholar]
- 13.Kurucz I, Morva A, Vaag A, Eriksson KF, Huang X, Groop L, Koranyi L. 2002. Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes 51, 1102–1109. ( 10.2337/diabetes.51.4.1102) [DOI] [PubMed] [Google Scholar]
- 14.Bruce CR, Carey AL, Hawley JA, Febbraio MA. 2003. Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes 52, 2338–2345. ( 10.2337/diabetes.52.9.2338) [DOI] [PubMed] [Google Scholar]
- 15.Henstridge DC, et al. 2010. The relationship between heat shock protein 72 expression in skeletal muscle and insulin sensitivity is dependent on adiposity. Metabolism 59, 1556–1561. ( 10.1016/j.metabol.2010.01.027) [DOI] [PubMed] [Google Scholar]
- 16.Kavanagh K, Wylie AT, Chavanne TJ, Jorgensen MJ, Voruganti VS, Comuzzie AG, Kaplan JR, McCall CE, Kritchevsky SB. 2012. Aging does not reduce heat shock protein 70 in the absence of chronic insulin resistance. J. Gerontol. A 67, 1014–1021. ( 10.1093/gerona/gls008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodrigues-Krause J, Krause M, O'Hagan C, De Vito G, Boreham C, Murphy C, Newsholme P, Colleran G. 2012. Divergence of intracellular and extracellular HSP72 in type 2 diabetes: does fat matter? Cell Stress Chaperones 17, 293–302. ( 10.1007/s12192-011-0319-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Matos MA, et al. 2014. Exercise reduces cellular stress related to skeletal muscle insulin resistance. Cell Stress Chaperones 19, 263–270. ( 10.1007/s12192-013-0453-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.da Silva Marineli R, Moura CS, Moraes EA, Lenquiste SA, Lollo PC, Morato PN, Amaya-Farfan J, Marostica MR Jr. 2015. Chia (Salvia hispanica L.) enhances HSP, PGC-1α expressions and improves glucose tolerance in diet-induced obese rats. Nutrition 31, 740–748. ( 10.1016/j.nut.2014.11.009) [DOI] [PubMed] [Google Scholar]
- 20.Bock PM, Jr, et al. 2016. Oral supplementations with L-glutamine or L-alanyl-L-glutamine do not change metabolic alterations induced by long-term high-fat diet in the B6.129F2/J mouse model of insulin resistance. Mol. Cell. Biochem. 411, 351–362. ( 10.1007/s11010-015-2597-6) [DOI] [PubMed] [Google Scholar]
- 21.Febbraio MA, et al. 2004. Glucose ingestion attenuates the exercise-induced increase in circulating heat shock protein 72 and heat shock protein 60 in humans. Cell Stress Chaperones 9, 390–396. ( 10.1379/CSC-24R1.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma W, et al. 2015. Glucose regulates heat shock factor 1 transcription activity via mTOR pathway in HCC cell lines. Cell Biol. Int. 39, 1217–1224. ( 10.1002/cbin.10493) [DOI] [PubMed] [Google Scholar]
- 23.Zhu G, Yin F, Wang L, Wei W, Jiang L, Qin J. 2016. Modeling type 2 diabetes-like hyperglycemia in C. elegans on a microdevice. Integr. Biol. 8, 30–38. ( 10.1039/c5ib00243e) [DOI] [PubMed] [Google Scholar]
- 24.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. 2002. A central role for JNK in obesity and insulin resistance. Nature 420, 333–336. ( 10.1038/nature01137) [DOI] [PubMed] [Google Scholar]
- 25.Lee YH, Giraud J, Davis RJ, White MF. 2003. c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. J. Biol. Chem. 278, 2896–2902. ( 10.1074/jbc.M208359200) [DOI] [PubMed] [Google Scholar]
- 26.Prada PO, et al. 2005. Western diet modulates insulin signaling, c-Jun N-terminal kinase activity, and insulin receptor substrate-1ser307 phosphorylation in a tissue-specific fashion. Endocrinology 146, 1576–1587. ( 10.1210/en.2004-0767) [DOI] [PubMed] [Google Scholar]
- 27.Tuncman G, Hirosumi J, Solinas G, Chang L, Karin M, Hotamisligil GS. 2006. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc. Natl Acad. Sci. USA 103, 10 741–10 746. ( 10.1073/pnas.0603509103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu C, et al. 2002. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3kinase activity in muscle. J. Biol. Chem. 277, 50 230–50 236. ( 10.1074/jbc.M200958200) [DOI] [PubMed] [Google Scholar]
- 29.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li Z-W, Karin M, Shoelson SE. 2001. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkβ. Science 293, 1673–1677. ( 10.1126/science.1061620) [DOI] [PubMed] [Google Scholar]
- 30.Kline MP, Morimoto RI. 1997. Repression of the heat shock factor 1 transcriptional activation domain is modulated by constitutive phosphorylation. Mol. Cell. Biol. 17, 2107–2115. ( 10.1128/MCB.17.4.2107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kyriakis JM, Avruch J. 1996. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J. Biol. Chem. 271, 24 313–24 316. ( 10.1074/jbc.271.40.24313) [DOI] [PubMed] [Google Scholar]
- 32.Vernia S, et al. 2014. The PPARα-FGF21 hormone axis contributes to metabolic regulation by the hepatic JNK signaling pathway. Cell Metab. 20, 512–525. ( 10.1016/j.cmet.2014.06.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. 2008. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J. Biol. Chem. 283, 13 565–13 577. ( 10.1074/jbc.M708916200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim B-J, Ryu S-W, Song B-J. 2006. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J. Biol. Chem. 281, 21 256–21 265. ( 10.1074/jbc.M510644200) [DOI] [PubMed] [Google Scholar]
- 35.Win S, Than TA, Fernandez-Checa JC, Kaplowitz N. 2014. JNK interaction with Sab mediates ER stress induced inhibition of mitochondrial respiration and cell death. Cell Death Dis. 5, e989 ( 10.1038/cddis.2013.522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaanine AH, Jeong D, Liang L, Chemaly ER, Fish K, Gordon RE, Hajjar RJ. 2012. JNK modulates FOXO3a for the expression of the mitochondrial death and mitophagy marker BNIP3 in pathological hypertrophy and in heart failure. Cell Death Dis. 3, 265 ( 10.1038/cddis.2012.5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Win S, Than TA, Le BHA, García-Ruiz C, Fernandez-Checa JC, Kaplowitz N. 2015. Sab (Sh3bp5) dependence of JNK mediated inhibition of mitochondrial respiration in palmitic acid induced hepatocyte lipotoxicity. J. Hepatol. 62, 1367–1374. ( 10.1016/j.jhep.2015.01.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adachi H, et al. 2010. An acylic polyisoprenoid derivative, geranylgeranylacetone protects against visceral adiposity and insulin resistance in high-fat-fed mice. Am. J. Physiol. 299, E764–E771. ( 10.1152/ajpendo.00075.2010) [DOI] [PubMed] [Google Scholar]
- 39.Morino S, et al. 2009. Mild electrical stimulation with heat shock ameliorates insulin resistance via enhanced insulin signaling. PLoS ONE 3, e4068 ( 10.1371/journal.pone.0004068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupte AA, Bomhoff GL, Touchberry CD, Geiger PC. 2011. Acute heat treatment improves insulin-stimulated glucose uptake in aged skeletal muscle. J. Appl. Physiol. 110, 451–457. ( 10.1152/japplphysiol.00849.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park HS, Lee JS, Huh SH, Seo JS, Choi EJ. 2001. Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. EMBO J. 20, 446–456. ( 10.1093/emboj/20.3.446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daviau A, Proulx R, Robitaille K, Di Fruscio M, Tanguay RM, Landry J, Patterson C, Durocher Y, Blouin R. 2006. Down-regulation of the mixed-lineage dual leucine zipper-bearing kinase by heat shock protein 70 and its co-chaperone CHIP. J. Biol. Chem. 281, 31 467–31 477. ( 10.1074/jbc.M607612200) [DOI] [PubMed] [Google Scholar]
- 43.Lee K-H, Lee C-T, Kim YW, Han SK, Shim Y-S, Yoo C-G. 2005. Preheating accelerates mitogen-activated protein (MAP) kinase inactivation post-heat shock via a heat shock protein 70-mediated increase in phosphorylated MAP kinase phosphatase-1. J. Biol. Chem. 280, 13 179–13 186. ( 10.1074/jbc.M410059200) [DOI] [PubMed] [Google Scholar]
- 44.Zeng XY, et al. 2015. Identification of matrine as a promising novel drug for hepatic steatosis and glucose intolerance with HSP72 as an upstream target. Br. J. Pharmacol. 172, 4303–4318. ( 10.1111/bph.13209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang M, Wang X, Yuan Y, Zhou Q, Tong C, Jiang W. 2009. Different effect of glutamine on macrophage tumor necrosis factor-alpha release and heat shock protein 72 expression in vitro and in vivo. Acta Biochim. Biophys. Sin. 41, 171–177. ( 10.1093/abbs/gmn020) [DOI] [PubMed] [Google Scholar]
- 46.Yonezawa K, Yamamoto Y, Yamamoto H, Ishikawa Y, Uchinami H, Taura K, Nakajima A, Yamaoka Y. 2001. Suppression of tumor necrosis factor-α production and neutrophil infiltration during ischemia–reperfusion injury of the liver after heat shock preconditioning. J. Hepatol. 35, 619–627. ( 10.1016/S0168-8278(01)00191-X) [DOI] [PubMed] [Google Scholar]
- 47.Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA, Davis RJ. 2013. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science 339, 218 ( 10.1126/science.1227568) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perry RJ, et al. 2015. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160, 745–758. ( 10.1016/j.cell.2015.01.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martín-Cordero L, García J, Hinchado M, Bote E, Ortega E. 2013. Influence of exercise on NA- and Hsp72-induced release of IFNγ by the peritoneal suspension of macrophages and lymphocytes from genetically obese Zucker rats. J. Physiol. Biochem. 69, 125–131. ( 10.1007/s13105-012-0196-5) [DOI] [PubMed] [Google Scholar]
- 50.Braian C, Hogea V, Stendahl O. 2013. Mycobacterium tuberculosis-induced neutrophil extracellular traps activate human macrophages. J. Innate Immun. 5, 591–602. ( 10.1159/000348676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campisi J, Fleshner M. 2003. Role of extracellular HSP72 in acute stress-induced potentiation of innate immunity in active rats. J. Appl. Physiol. 94, 43 ( 10.1152/japplphysiol.00681.2002) [DOI] [PubMed] [Google Scholar]
- 52.Asea A, Kraeft S-K, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. 2000. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat. Med. 6, 435–442. ( 10.1038/74697) [DOI] [PubMed] [Google Scholar]
- 53.Basu S, Binder RJ, Ramalingam T, Srivastava PK. 2001. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 14, 303–313. ( 10.1016/S1074-7613(01)00111-X) [DOI] [PubMed] [Google Scholar]
- 54.Sondermann H, Becker T, Mayhew M, Wieland F, Hartl F-U. 2000. Characterization of a receptor for heat shock protein 70 on macrophages and monocytes. Biol. Chem. 381, 1165–1174. ( 10.1515/BC.2000.144) [DOI] [PubMed] [Google Scholar]
- 55.Morino K, Petersen KF, Shulman GI. 2006. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 55(Suppl. 2), S9–S15. ( 10.2337/db06-S002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patti ME, et al. 2003. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc. Natl Acad. Sci. USA 100, 8466–8471. ( 10.1073/pnas.1032913100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. 2004. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N. Engl. J. Med. 350, 664–671. ( 10.1056/NEJMoa031314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen HW, Chen SC, Tsai JL, Yang RC. 1999. Previous hyperthermic treatment increases mitochondria oxidative enzyme activity and exercise capacity in rats. Kaohsiung J. Med. Sci. 15, 572–580. [PubMed] [Google Scholar]
- 59.Tamura Y, Kitaoka Y, Matsunaga Y, Hoshino D, Hatta H. 2015. Daily heat stress treatment rescues denervation-activated mitochondrial clearance and atrophy in skeletal muscle. J. Physiol. 593, 2707–2720. ( 10.1113/JP270093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu C-T, Brooks GA. 2012. Mild heat stress induces mitochondrial biogenesis in C2C12 myotubes. J. Appl. Physiol. 112, 354–361. ( 10.1152/japplphysiol.00989.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Drew BG, et al. 2014. HSP72 is a mitochondrial stress sensor critical for Parkin action, oxidative metabolism, and insulin sensitivity in skeletal muscle. Diabetes 63, 1488–1505. ( 10.2337/db13-0665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hotamisligil GS. 2006. Inflammation and metabolic disorders. Nature 444, 860–867. ( 10.1038/nature05485) [DOI] [PubMed] [Google Scholar]
- 63.Peng G, Li L, Liu Y, Pu J, Zhang S, Yu J, Zhao J, Liu P. 2011. Oleate blocks palmitate-induced abnormal lipid distribution, endoplasmic reticulum expansion and stress, and insulin resistance in skeletal muscle. Endocrinology 152, 2206–2218. ( 10.1210/en.2010-1369) [DOI] [PubMed] [Google Scholar]
- 64.Deldicque L, Cani PD, Philp A, Raymackers J-M, Meakin PJ, Ashford ML, Delzenne NM, Francaux M, Baar K. 2010. The unfolded protein response is activated in skeletal muscle by high-fat feeding: potential role in the downregulation of protein synthesis. Am. J. Physiol. Endocrinol. Metab. 299, E695–E705. ( 10.1152/ajpendo.00038.2010) [DOI] [PubMed] [Google Scholar]
- 65.Özcan U, Yilmaz E, Özcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS. 2006. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313, 1137–1140. ( 10.1126/science.1128294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hetz C. 2012. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 13, 89–102. ( 10.1038/nrm3270) [DOI] [PubMed] [Google Scholar]
- 67.Özcan U, et al. 2004. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461. ( 10.1126/science.1103160) [DOI] [PubMed] [Google Scholar]
- 68.Wang D, Wei Y, Pagliassotti MJ. 2006. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology 147, 943–951. ( 10.1210/en.2005-0570) [DOI] [PubMed] [Google Scholar]
- 69.Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, Sanyal AJ. 2008. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology 134, 568–576. ( 10.1053/j.gastro.2007.10.039) [DOI] [PubMed] [Google Scholar]
- 70.Gregor MF, Yang L, Fabbrini E, Mohammed BS, Eagon JC, Hotamisligil GS, Klein S. 2009. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes 58, 693–700. ( 10.2337/db08-1220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rutkowski DT, et al. 2008. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev. Cell 15, 829–840. ( 10.1016/j.devcel.2008.10.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rahman SM, Schroeder-Gloeckler JM, Janssen RC, Jiang H, Qadri I, Maclean KN, Friedman JE. 2007. CCAAT/enhancing binding protein β deletion in mice attenuates inflammation, endoplasmic reticulum stress, and lipid accumulation in diet-induced nonalcoholic steatohepatitis. Hepatology 45, 1108–1117. ( 10.1002/hep.21614) [DOI] [PubMed] [Google Scholar]
- 73.Schroeder-Gloeckler JM, et al. 2007. CCAAT/enhancer-binding protein β deletion reduces adiposity, hepatic steatosis, and diabetes in Leprdb/db mice. J. Biol. Chem. 282, 15 717–15 729. ( 10.1074/jbc.M701329200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee A-H, Scapa EF, Cohen DE, Glimcher LH. 2008. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 320, 1492–1496. ( 10.1126/science.1158042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ning J, Hong T, Ward A, Pi J, Liu Z, Liu H-Y, Cao W. 2011. Constitutive role for IRE1α-XBP1 signaling pathway in the insulin-mediated hepatic lipogenic program. Endocrinology 152, 2247–2255. ( 10.1210/en.2010-1036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pagliassotti MJ, Kim PY, Estrada AL, Stewart CM, Gentile CL. 2016. Endoplasmic reticulum stress in obesity and obesity-related disorders: an expanded view. Metabolism 65, 1238–1246. ( 10.1016/j.metabol.2016.05.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu G, Lee AS. 2015. Role of the unfolded protein response, GRP78 and GRP94 in organ homeostasis. J. Cell. Physiol. 230, 1413–1420. ( 10.1002/jcp.24923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Henstridge DC, Febbraio MA, Hargreaves M. 2016. Heat shock proteins and exercise adaptations. Our knowledge thus far and the road still ahead. J. Appl. Physiol. 120, 683–691. ( 10.1152/japplphysiol.00811.2015) [DOI] [PubMed] [Google Scholar]
- 79.Wu J, et al. 2011. The unfolded protein response mediates adaptation to exercise in skeletal muscle through a PGC-1α /ATF6α complex. Cell Metab. 13, 160–169. ( 10.1016/j.cmet.2011.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gupta S, Deepti A, Deegan S, Lisbona F, Hetz C, Samali A. 2010. HSP72 protects cells from ER stress-induced apoptosis via enhancement of IRE1α-XBP1 signaling through a physical interaction. PLoS Biol. 8, e1000410 ( 10.1371/journal.pbio.1000410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.So J-S, et al. 2012. Silencing of lipid metabolism genes through IRE1α-mediated mRNA decay lowers plasma lipids in mice. Cell Metab. 16, 487–499. ( 10.1016/j.cmet.2012.09.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grune T, Catalgol B, Licht A, Ermak G, Pickering AM, Ngo JK, Davies KJ. 2011. HSP70 mediates dissociation and reassociation of the 26S proteasome during adaptation to oxidative stress. Free Radical Biol. Med. 51, 1355–1364. ( 10.1016/j.freeradbiomed.2011.06.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marques C, Guo W, Pereira P, Taylor A, Patterson C, Evans PC, Shang F. 2006. The triage of damaged proteins: degradation by the ubiquitin-proteasome pathway or repair by molecular chaperones. FASEB J. 20, 741–743. ( 10.1096/fj.05-5080fje) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ruan L, Zhou C, Jin E, Kucharavy A, Zhang Y, Wen Z, Florens L, Li R. 2017. Cytosolic proteostasis through importing of misfolded proteins into mitochondria. Nature 543, 443–446. ( 10.1038/nature21695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Henstridge DC, Whitham M, Febbraio MA. 2014. Chaperoning to the metabolic party: the emerging therapeutic role of heat-shock proteins in obesity and type 2 diabetes. Mol. Metab. 3, 781–793. ( 10.1016/j.molmet.2014.08.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. 2002. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 16, 1879–1886. ( 10.1096/fj.02-0367com) [DOI] [PubMed] [Google Scholar]
- 87.Goto M, Terada S, Kato M, Katoh M, Yokozeki T, Tabata I, Shimokawa T. 2000. cDNA cloning and mRNA analysis of PGC-1 in epitrochlearis muscle in swimming-exercised rats. Biochem. Biophys. Res. Commun. 274, 350–354. ( 10.1006/bbrc.2000.3134) [DOI] [PubMed] [Google Scholar]
- 88.Norrbom J, Sundberg CJ, Ameln H, Kraus WE, Jansson E, Gustafsson T. 2004. PGC-1α mRNA expression is influenced by metabolic perturbation in exercising human skeletal muscle. J. Appl. Physiol. 96, 189–194. ( 10.1152/japplphysiol.00765.2003) [DOI] [PubMed] [Google Scholar]
- 89.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. 1998. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839. ( 10.1016/S0092-8674(00)81410-5) [DOI] [PubMed] [Google Scholar]
- 90.Wu Z, et al. 1999. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98, 115–124. ( 10.1016/S0092-8674(00)80611-X) [DOI] [PubMed] [Google Scholar]
- 91.Ma X, Xu L, Alberobello AT, Gavrilova O, Bagattin A, Skarulis M, Liu J, Finkel T, Mueller E. 2015. Celastrol protects against obesity and metabolic dysfunction through activation of a HSF1-PGC1α transcriptional axis. Cell Metab. 22, 695–708. ( 10.1016/j.cmet.2015.08.005) [DOI] [PubMed] [Google Scholar]
- 92.Xu L, Ma X, Bagattin A, Mueller E. 2016. The transcriptional coactivator PGC1α protects against hyperthermic stress via cooperation with the heat shock factor HSF1. Cell Death Dis. 7, e2102 ( 10.1038/cddis.2016.22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goodpaster BH, Katsiaras A, Kelley DE. 2003. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes 52, 2191–2197. ( 10.2337/diabetes.52.9.2191) [DOI] [PubMed] [Google Scholar]
- 94.Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, Janssen I. 2000. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann. Intern. Med. 133, 92–103. ( 10.7326/0003-4819-133-2-200007180-00008) [DOI] [PubMed] [Google Scholar]
- 95.Benjamin IJ, Horie S, Greenberg ML, Alpern RJ, Williams RS. 1992. Induction of stress proteins in cultured myogenic cells. Molecular signals for the activation of heat shock transcription factor during ischemia. J. Clin. Invest. 89, 1685–1689. ( 10.1172/JCI115768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Benjamin IJ, Kroger B, Williams RS. 1990. Activation of the heat shock transcription factor by hypoxia in mammalian cells. Proc. Natl Acad. Sci. USA 87, 6263–6267. ( 10.1073/pnas.87.16.6263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fischer CP, Hiscock NJ, Basu S, Vessby B, Kallner A, Sjoberg LB, Febbraio MA, Pedersen BK. 2006. Vitamin E isoform-specific inhibition of the exercise-induced heat shock protein 72 expression in humans. J. Appl. Physiol. 100, 1679–1687. ( 10.1152/japplphysiol.00421.2005) [DOI] [PubMed] [Google Scholar]
- 98.Goto K, et al. 2003. Effects of heat stress and mechanical stretch on protein expression in cultured skeletal muscle cells. Pflugers Arch. 447, 247–253. ( 10.1007/s00424-003-1177-x) [DOI] [PubMed] [Google Scholar]
- 99.Koh TJ, Escobedo J. 2004. Cytoskeletal disruption and small heat shock protein translocation immediately after lengthening contractions. Am. J. Physiol. Cell Physiol. 286, C713–C722. ( 10.1152/ajpcell.00341.2003) [DOI] [PubMed] [Google Scholar]
- 100.Marber MS, Latchman DS, Walker JM, Yellon DM. 1993. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation 88, 1264–1272. ( 10.1161/01.CIR.88.3.1264) [DOI] [PubMed] [Google Scholar]
- 101.Mosser DD, Kotzbauer PT, Sarge KD, Morimoto RI. 1990. In vitro activation of heat shock transcription factor DNA-binding by calcium and biochemical conditions that affect protein conformation. Proc. Natl Acad. Sci. USA 87, 3748–3752. ( 10.1073/pnas.87.10.3748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smolka MB, Zoppi CC, Alves AA, Silveira LR, Marangoni S, Pereira-Da-Silva L, Novello JC, Macedo DV. 2000. HSP72 as a complementary protection against oxidative stress induced by exercise in the soleus muscle of rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279, R1539–R1545. [DOI] [PubMed] [Google Scholar]
- 103.Weitzel G, Pilatus U, Rensing L. 1985. Similar dose response of heat shock protein synthesis and intracellular pH change in yeast. Exp. Cell Res. 159, 252–256. ( 10.1016/S0014-4827(85)80054-9) [DOI] [PubMed] [Google Scholar]
- 104.Febbraio MA, Ott P, Nielsen HB, Steensberg A, Keller C, Krustrup P, Secher NH, Pedersen BK. 2002. Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J. Physiol. 544, 957–962. ( 10.1113/jphysiol.2002.025148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Skidmore R, Gutierrez JA, Guerriero V Jr, Kregel KC. 1995. HSP70 induction during exercise and heat stress in rats: role of internal temperature. Am. J. Physiol. 268, R92–R97. [DOI] [PubMed] [Google Scholar]
- 106.Milne KJ, Noble EG. 2002. Exercise-induced elevation of HSP70 is intensity dependent. J. Appl. Physiol. 93, 561–568. ( 10.1152/japplphysiol.00528.2001) [DOI] [PubMed] [Google Scholar]
- 107.Murlasits Z, Cutlip RG, Geronilla KB, Rao KM, Wonderlin WF, Alway SE. 2006. Resistance training increases heat shock protein levels in skeletal muscle of young and old rats. Exp. Gerontol. 41, 398–406. ( 10.1016/j.exger.2006.01.005) [DOI] [PubMed] [Google Scholar]
- 108.Liu Y, Lormes W, Baur C, Opitz-Gress A, Altenburg D, Lehmann M, Steinacker JM. 2000. Human skeletal muscle HSP70 response to physical training depends on exercise intensity. Int. J. Sports Med. 21, 351–355. ( 10.1055/s-2000-3784) [DOI] [PubMed] [Google Scholar]
- 109.Liu Y, Lormes W, Wang L, Reissnecker S, Steinacker JM. 2004. Different skeletal muscle HSP70 responses to high-intensity strength training and low-intensity endurance training. Eur. J. Appl. Physiol. 91, 330–335. ( 10.1007/s00421-003-0976-2) [DOI] [PubMed] [Google Scholar]
- 110.Houmard JA, Tanner CJ, Slentz CA, Duscha BD, McCartney JS, Kraus WE. 2004. Effect of the volume and intensity of exercise training on insulin sensitivity. J. Appl. Physiol. 96, 101–106. ( 10.1152/japplphysiol.00707.2003) [DOI] [PubMed] [Google Scholar]
- 111.Morton JP, Maclaren DP, Cable NT, Campbell IT, Evans L, Kayani AC, McArdle A, Drust B. 2008. Trained men display increased basal heat shock protein content of skeletal muscle. Med. Sci. Sports Exerc. 40, 1255–1262. ( 10.1249/MSS.0b013e31816a7171) [DOI] [PubMed] [Google Scholar]
- 112.Clarkson PM, Byrnes WC, Gillisson E, Harper E. 1987. Adaptation to exercise-induced muscle damage. Clin. Sci. 73, 383–386. ( 10.1042/cs0730383) [DOI] [PubMed] [Google Scholar]
- 113.Newham DJ, Jones DA, Clarkson PM. 1987. Repeated high-force eccentric exercise: effects on muscle pain and damage. J. Appl. Physiol. 63, 1381–1386. [DOI] [PubMed] [Google Scholar]
- 114.Thompson HS, Clarkson PM, Scordilis SP. 2002. The repeated bout effect and heat shock proteins: intramuscular HSP27 and HSP70 expression following two bouts of eccentric exercise in humans. Acta Physiol. Scand. 174, 47–56. ( 10.1046/j.1365-201x.2002.00922.x) [DOI] [PubMed] [Google Scholar]
- 115.Gjovaag TF, Dahl HA. 2006. Effect of training and detraining on the expression of heat shock proteins in m. triceps brachii of untrained males and females. Eur. J. Appl. Physiol. 98, 310–322. ( 10.1007/s00421-006-0281-y) [DOI] [PubMed] [Google Scholar]
- 116.Bouchard C, Dionne FT, Simoneau JA, Boulay MR. 1992. Genetics of aerobic and anaerobic performances. Exerc. Sport Sci. Rev. 20, 27–58. ( 10.1249/00003677-199200200-00002) [DOI] [PubMed] [Google Scholar]
- 117.Koch LG, Britton SL. 2005. Divergent selection for aerobic capacity in rats as a model for complex disease. Integr. Comp. Biol. 45, 405–415. ( 10.1093/icb/45.3.405) [DOI] [PubMed] [Google Scholar]
- 118.Morris EM, et al. 2017. Aerobic capacity mediates susceptibility for the transition from steatosis to steatohepatitis. J. Physiol. 595, 4909–4926. ( 10.1113/JP274281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Noland RC, et al. 2007. Artificial selection for high-capacity endurance running is protective against high-fat diet-induced insulin resistance. Am. J. Physiol. 293, E31–E41. ( 10.1152/ajpendo.00500.2006) [DOI] [PubMed] [Google Scholar]
- 120.Spargo FJ, McGee SL, Dzamko N, Watt MJ, Kemp BE, Britton SL, Koch LG, Hargreaves M, Hawley JA. 2007. Dysregulation of muscle lipid metabolism in rats selectively bred for low aerobic running capacity. Am. J. Physiol. 292, E1631–E1636. ( 10.1152/ajpendo.00702.2006) [DOI] [PubMed] [Google Scholar]
- 121.Thyfault JP, et al. 2009. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J. Physiol. 587, 1805–1816. ( 10.1113/jphysiol.2009.169060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Larkins NT, Murphy RM, Lamb GD. 2012. Absolute amounts and diffusibility of HSP72, HSP25, and αB-crystallin in fast- and slow-twitch skeletal muscle fibers of rat. Am. J. Physiol. Cell Physiol. 302, C228–C239. ( 10.1152/ajpcell.00266.2011) [DOI] [PubMed] [Google Scholar]
- 123.Locke M, Noble EG, Atkinson BG. 1991. Inducible isoform of HSP70 is constitutively expressed in a muscle fiber type specific pattern. Am. J. Physiol. 261, C774–C779. [DOI] [PubMed] [Google Scholar]
- 124.Hernando R, Manso R. 1997. Muscle fibre stress in response to exercise: synthesis, accumulation and isoform transitions of 70-kDa heat-shock proteins. Eur. J. Biochem. 243, 460–467. ( 10.1111/j.1432-1033.1997.0460a.x) [DOI] [PubMed] [Google Scholar]
- 125.Atalay M, Oksala NK, Laaksonen DE, Khanna S, Nakao C, Lappalainen J, Roy S, Hanninen O, Sen CK. 2004. Exercise training modulates heat shock protein response in diabetic rats. J. Appl. Physiol. 97, 605–611. ( 10.1152/japplphysiol.01183.2003) [DOI] [PubMed] [Google Scholar]
- 126.Lappalainen Z, Lappalainen J, Oksala NK, Laaksonen DE, Khanna S, Sen CK, Atalay M. 2010. Exercise training and experimental diabetes modulate heat shock protein response in brain. Scand. J. Med. Sci. Sports 20, 83–89. ( 10.1111/j.1600-0838.2008.00872.x) [DOI] [PubMed] [Google Scholar]
- 127.Lollo PC, Moura CS, Morato PN, Amaya-Farfan J. 2013. Differential response of heat shock proteins to uphill and downhill exercise in heart, skeletal muscle, lung and kidney tissues. J. Sports Sci. Med. 12, 461–466. [PMC free article] [PubMed] [Google Scholar]
- 128.Lancaster GI, Moller K, Nielsen B, Secher NH, Febbraio MA, Nybo L. 2004. Exercise induces the release of heat shock protein 72 from the human brain in vivo. Cell Stress Chaperones 9, 276–280. ( 10.1379/CSC-18R.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Broquet AH, Thomas G, Masliah J, Trugnan G, Bachelet M. 2003. Expression of the molecular chaperone Hsp70 in detergent-resistant microdomains correlates with its membrane delivery and release. J. Biol. Chem. 278, 21 601–21 606. ( 10.1074/jbc.M302326200) [DOI] [PubMed] [Google Scholar]
- 130.Hunter-Lavin C, Davies EL, Bacelar MM, Marshall MJ, Andrew SM, Williams JH. 2004. Hsp70 release from peripheral blood mononuclear cells. Biochem. Biophys. Res. Commun. 324, 511–517. ( 10.1016/j.bbrc.2004.09.075) [DOI] [PubMed] [Google Scholar]
- 131.Lancaster GI, Febbraio MA. 2005. Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J. Biol. Chem. 280, 23 349–23 355. ( 10.1074/jbc.M502017200) [DOI] [PubMed] [Google Scholar]
- 132.Whitham M, Fortes MB. 2008. Heat shock protein 72: release and biological significance during exercise. Front. Biosci. 13, 1328–1339. ( 10.2741/2765) [DOI] [PubMed] [Google Scholar]
- 133.Krause M, Heck TG, Bittencourt A, Scomazzon SP, Newsholme P, Curi R, Homem de Bittencourt PI Jr. 2015. The chaperone balance hypothesis: the importance of the extracellular to intracellular HSP70 ratio to inflammation-driven type 2 diabetes, the effect of exercise, and the implications for clinical management. Mediators Inflamm. 2015, 249205 ( 10.1155/2015/249205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Takeuchi T, Suzuki M, Fujikake N, Popiel HA, Kikuchi H, Futaki S, Wada K, Nagai Y. 2015. Intercellular chaperone transmission via exosomes contributes to maintenance of protein homeostasis at the organismal level. Proc. Natl Acad. Sci. USA 112, E2497–E2506. ( 10.1073/pnas.1412651112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hooper PL. 1999. Hot-tub therapy for type 2 diabetes mellitus. N. Engl. J. Med. 341, 924–925. ( 10.1056/NEJM199909163411216) [DOI] [PubMed] [Google Scholar]
- 136.Beever R. 2010. The effects of repeated thermal therapy on quality of life in patients with type II diabetes mellitus. J. Altern. Complement. Med. 16, 677–681. ( 10.1089/acm.2009.0358) [DOI] [PubMed] [Google Scholar]
- 137.Biro S, Masuda A, Kihara T, Tei C. 2003. Clinical implications of thermal therapy in lifestyle-related diseases. Exp. Biol. Med. 228, 1245–1249. ( 10.1177/153537020322801023) [DOI] [PubMed] [Google Scholar]
- 138.Rivas E, Newmire DE, Crandall CG, Hooper PL, Ben-Ezra V. 2016. An acute bout of whole body passive hyperthermia increases plasma leptin, but does not alter glucose or insulin responses in obese type 2 diabetics and healthy adults. J. Therm. Biol. 59, 26–33. ( 10.1016/j.jtherbio.2016.04.010) [DOI] [PubMed] [Google Scholar]
- 139.Rivas E, Newmire DE, Ben-Ezra V. 2016. Obese type 2 diabetics have a blunted hypotensive response to acute hyperthermia therapy that does not affect the perception of thermal stress or physiological strain compared to healthy adults. Physiol. Behav. 165, 374–382. ( 10.1016/j.physbeh.2016.08.026) [DOI] [PubMed] [Google Scholar]
- 140.Brunt VE, Howard MJ, Francisco MA, Ely BR, Minson CT. 2016. Passive heat therapy improves endothelial function, arterial stiffness and blood pressure in sedentary humans. J. Physiol. 594, 5329–5342. ( 10.1113/JP272453) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tei C, et al. 2016. Waon therapy for managing chronic heart failure—results from a multicenter prospective randomized WAON-CHF study. Circ. J. 80, 827–834. ( 10.1253/circj.CJ-16-0051) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.