Abstract
BaxΔ2 is a functional pro-apoptotic Bax isoform having alterations in its N-terminus, but sharing the rest of its sequence with Baxα. BaxΔ2 is unable to target mitochondria due to the loss of helix α1. Instead, it forms cytosolic aggregates and activates caspase 8. However, the functional domain(s) responsible for BaxΔ2 behavior have remained elusive. Here we show that disruption of helix α1 makes Baxα mimic the behavior of BaxΔ2. However, the other alterations in the BaxΔ2 N-terminus have no significant impact on aggregation or cell death. We found that the hallmark BH3 domain is necessary but not sufficient for aggregation-mediated cell death. We also noted that the core region shared by Baxα and BaxΔ2 is required for the formation of large aggregates, which is essential for BaxΔ2 cytotoxicity. However, aggregation by itself is unable to trigger cell death without the C-terminus. Interestingly, the C-terminal helical conformation, not its primary sequence, appears to be critical for caspase 8 recruitment and activation. As BaxΔ2 shares core and C-terminal sequences with most Bax isoforms, our results not only reveal a structural basis for BaxΔ2-induced cell death, but also imply an intrinsic potential for aggregate-mediated caspase 8-dependent cell death in other Bax family members.
Keywords: Bax, BaxΔ2, Caspase 8, Apoptosis, Aggregation, Death Domain
1. INTRODUCTION
The pro-apoptotic Bcl-2 family member Bax plays a crucial role in anti-tumorigenesis (1–4). Previous studies have shown that Bax possesses a significant “plasticity”, as several functional Bax isoforms have been identified (5–14). The most prominent form of the Bax subfamily, Baxα, is ubiquitously expressed in most mammalian cells and has been well studied. Baxα has 6 exons that code for 192 amino acids. Its secondary structure is comprised of 9 alpha (α) helices with small unfolded regions in between to form a globular tertiary structure (15). Several Baxα functional domains have been identified based on their similarities with other Bcl-2 family members. These include Bcl-2 homology domains: BH3 located in exon 3, BH1 in exon 4, and BH2 in the boundary region between exons 5 and 6 (1, 3, 4, 16). The BH3 domain in all pro-death members, including the Bax subfamily, plays an essential role in inducing apoptosis. Structurally, the Baxα BH3 domain is part of a hydrophobic pocket formed mainly by helices α2, α5, and α6 encoded by exons 3, 4, and 5, respectively. The pocket is covered by helix α1, which is encoded mostly by exon 2 (15, 17–20).
The first 20 amino acids of the Bax N-terminal region, which do not share homology with any other member of the Bcl-2 family, conform a special domain known as Apoptosis-Regulating Targeting (ART). ART was initially believed to be a part of the mitochondria targeting sequence, but later it has been shown to be not essential for mitochondrial targeting but critical for preventing Baxα from becoming activated in the absence of death signals (8, 9, 21–25). The underlying mechanism is not fully understood, but it is believed that ART is responsible for maintaining the inactive monomeric form of Baxα proteins. Upon stimulation by cell death signals, Baxα monomers undergo conformational changes, form oligomers, and target mitochondria (26–33). Although the exact nature of the conformational changes is controversial, it is generally accepted that the death signal-induced exposure of the BH3 domain is critical for Baxα activation. It is also known that helices α2 to α6, encoded by exons 3 to 5, are required for Baxα oligomerization (29, 33–36), and helix α1 is essential for Baxα translocation to mitochondria (22, 37–39). Recently, spectroscopic and scattering techniques have been used to identify the structures of the active Baxα monomer, dimer, and tetramer. These findings further support the concept that the displacement of helix α1 from the BH3-domain containing hydrophobic pocket is the first step for Baxα activation, leading to oligomerization and mitochondrial translocation (34).
The exact function of the Bax C-terminal region remains controversial. The Baxα C-terminal region has a transmembrane domain (TM) contained in helix α9 (15, 34, 40, 41), which has been reported to play a critical role in targeting (2, 31, 42, 43), anchoring, and penetrating the mitochondrial membrane (40, 44–46). However, it has also been shown that alteration or deletion of the C-terminal region has little influence on the ability of Baxα to target mitochondria (22, 25, 47–50) or its pro-apoptotic activity (25, 47, 48, 50). Furthermore, although Baxβ, Baxσ, Baxε and Baxω isoforms have different C-termini, they all can release cytochrome C and trigger mitochondrial cell death in the same way as Baxα (5, 6, 12, 14).
BaxΔ2 and its subfamily members are a group of unique functional Bax isoforms initially identified in patients with a family history of mismatch repair deficiency (13, 51–53). BaxΔ2 has a guanine deletion in exon 3 (G8 → G7), which causes a frameshift and pre-mature termination of translation. However, an alternative splicing event removes most of exon 2 and restores the reading frame, resulting in a full-length functional isoform (BaxΔ2) with 10 new amino acids between the alternative splicing site and the point of the deletion. The rest of the BaxΔ2 sequence is the same as Baxα (13, 51, 53).
BaxΔ2 is pro-apoptotic and still able to form dimers with Baxα and Bcl-2 (13). However, BaxΔ2 is unable to target mitochondria due to the lack of helix α1 (13, 22, 37–39). Instead, BaxΔ2 forms cytosolic aggregates, promoting apoptosis by activating caspase 8 (13, 51, 53). However, the functional domain(s) for the aggregation and activation of caspase 8 were unknown. To understand the underlying molecular mechanism of BaxΔ2-induced apoptosis, we used computational structural modeling and cell-based approaches to analyze the functional domains that are responsible for its aggregation and cytotoxicity. Our results reveal that, apart from the impairment of mitochondrial translocation, the changes in the N-terminal of BaxΔ2, when compared to Baxα, seem to have no significant effect on its aggregation and cytotoxicity. The lack of helix α1 exposes the core of the protein and promotes BaxΔ2 to form cytosolic aggregates, which activate caspase 8 via the C-terminus in a helical structure-dependent manner to trigger cell death.
2. MATERIALS AND METHODS
Cloning
All BaxΔ2 constructs indicated in the text were generated using either Baxα or BaxΔ2 as a template for PCR-based cloning. The constructs were cloned by insertion of the corresponding PCR product in a pcDNA3.1 vector between EcoRI and KpnI sites. Each construct was in-frame tagged with a green fluorescence protein (GFP) at its C-terminus in the HindIII site. There is a 15-base pair linker between the construct and GFP gene. Deletions (Δ) were made and numbered according to the BaxΔ2 amino acid sequence. BaxΔ2[Δ13-21/G7] is a BaxΔ2 without the 10 new amino acids, while BaxΔ2[R13-21/G8] has the same 10 amino acids as Baxα (R representing replacement of the amino acids). An extra base pair was added to the latter to avoid a frameshift, which makes this construct equal to Baxα without exon 2. BaxΔ2[ARTΔ13-21/G7] is BaxΔ2 with a full-length ART sequence, but deletion of the 10 amino acids. BaxΔ2[Δ1-21] is BaxΔ2 with deletion of exon 1 and the 10 amino acids. This construct is basically Baxα without the first 2 exons. BaxΔ2[Δ13-61] is BaxΔ2 with complete deletion of exon 3, which could also be interpreted as Baxα without exons 2 and 3. An extra base pair was also added to this construct to avoid a frameshift. BaxΔ2[ΔBH3] is BaxΔ2 without the BH3 domain. BaxΔ2[Δ62-175] is BaxΔ2 without exons 4, 5, and 6. BaxΔ2[Δ110-175] is BaxΔ2 without exons 5 and 6. The beginning of the exon 5 sequence was retained to maintain the integrity of helix α5. BaxΔ2[Δ141-175] is BaxΔ2 without exon 6. C-terminal point-mutants were generated using the Transformer™ Site Directed Mutagenesis Kit (Clontech, Takara). All constructs were validated by DNA sequencing.
3D Structure Modeling and Computational Analyses
Structural models for BaxΔ2 and its mutant constructs were created using RaptorX (54–56) and I-TASSER (57–59) protein tertiary structure prediction server. Specifically, RaptorX first predicts the secondary structures, solvent accessibility, and disordered regions for the query sequence and then utilizes this information to search for templates through which to construct the 3D model of the query by Modeler (60) or Rosetta (61). I-TASSER predicts the 3D model of the query sequence through four general steps: threading template identification, iterative structure assembly simulation, model selection, and refinement. Models obtained by different methods were compared and the structural similarity was determined as TM score (range between 0 and 1). Models of Baxα were also created and compared with the known structure as control. The disorder probability of the models was determined using PrDOS (62). Analysis of conservative motifs between the C-terminal regions of BaxΔ2 and BaxΔ2ω was done with the assistance of the ClustalW server (63). Helicity probability analysis was performed using NetSurfP 1.1 (64) and GOR4 (65); helicity probability values from both software programs were averaged, then values of BaxΔ2 were subtracted from each mutant construct’s values before plotted.
Cell Culture and transfection
The Bax negative colon cancer HCT116 subline cell line was generated as described previously (53). All cells were cultured in Dulbecco’s Modification of Eagle’s Medium supplemented with 10% FBS. For transfection, cells were split in a 6-well plate, allowed to grow until 60% confluence, then transfected with the appropriate constructs, as indicated in the text, using Lipofectamine® 3000 Reagent (Invitrogen). Cells were then incubated for the time corresponding to each experiment. When required, cells were treated with 40 µM of Etoposide for 4 hours.
Immunostaining and Imaging
Bax-deficient MEFs were transfected on glass cover slides in a 6-well plate, incubated at 37 °C for 16 h, then fixed with 4% paraformaldehyde and permeabilized with 0.2% Triton-X100 in Phosphate Buffered Saline (PBS). When required, cells were incubated overnight at 4°C with a primary antibody against Tom20 (Santa Cruz Biotechnologies, 1:100 dilution) or against caspase 8 (Cell Signaling, 1:100 dilution), followed by secondary antibody Alexa Fluor® 594 (Life Technologies, 1:200 dilution) for 1 h at room temperature. Nuclei were stained with DAPI during the mounting process with ProLong® Gold antifade reagent (Invitrogen). Fluorescent imaging was performed using a Keyence BZ-X710 All-in-One Fluorescence Microscope equipped with BZ-X Viewer software. Images were analyzed using BZ-X Analyzer software and ImageJ 1.48. All cells were imaged using Z-stack, in which at least 30 sections separated by 0.2 to 0.3 µm were taken and composed by full focus. Of note, the images for mitochondrial co-localization are from individual sections, not composed. For the co-localization analysis of Caspase 8, main images correspond to individual sections and the 3D analysis was performed using the XYZ Slicing tool. Approximated size of aggregates was estimated by measuring a total of 30 random clearly individual aggregates from 3 representative cells for each construct.
Cell Death and Caspase Assays
Bax negative HCT116 subline cells were transfected with GFP-tagged BaxΔ2 derived constructs and incubated for 24 h in the absence or presence of 50 µM Caspase 8 Inhibitor z-IETD-fmk (R&D Systems) as indicated in the text. Two different cell death assays were used: floating cells assay and caspase 3 substrate assay. For the floating cell death assay, floating and attached cells were harvested separately, then analyzed under a fluorescence microscope. The number of floating cells expressing GFP was divided by the total number of GFP positive cells for the percentage of cell death. For the caspase 3 substrate cell death assay, cells were incubated with NucView™ 530 Caspase-3 Substrate at the time of transfection. Cells were harvested and analyzed under a fluorescence microscope. The number of transfected cells showing cleaved Caspase-3 Substrate (red) was divided by the total number of GFP positive cells for the percentage of apoptosis. The experiments were performed in duplicate and results from at least three independent experiments were used for statistical analysis.
Immunoblotting
Bax negative HCT116 subline cells were transfected with BaxΔ2 derived constructs and incubated for 24 h. Cells were harvested and lysed in 0.1% NP-40 buffer containing 145 mM NaCl, 5 mM MgCl2, 20 mM HEPES, pH 7.4, 1 mM EDTA, 1 mM EGTA, 10 mM Dithiothreitol (DTT) and a cocktail of protease inhibitors including Aprotinin and Leupeptin. Protein concentration for each sample was measured and equal amounts of protein were loaded into a 12% SDS-PAGE gel and then transferred onto a 0.2 µm PVDF membrane. Membranes were blotted with 5% BSA, followed by incubation with a primary antibody against GFP (Santa Cruz Biotechnology, 1:1000 dilution) or actin (Millipore, 1:3000 dilution) overnight at 4°C. The membrane was washed with washing buffer (0.2% Tween® 20 in PBS). After incubation with HRP-conjugated secondary antibody (Jackson) for 1 h followed by several washes, the bands were visualized on X-ray films by a Pierce® ECL Western Blotting Substrate developing kit (Thermo Scientific).
Statistical Analysis
All values shown in the figures represent the mean ±SD. Statistical analysis was performed using R version 3.2.4 (66). One-way ANOVA followed by Tukey’s test was performed for all cell death experiments. Student t-test was used for the Caspase 8 inhibitor experiments. P values under 0.05 were considered significant.
3. RESULTS
The loss of Helix α1 is responsible for the behavioral differences between Baxα and BaxΔ2
At the level of the coding sequence, BaxΔ2 differs from Baxα in its first three exons, while the rest of its sequence is the same as Baxα, including all BH domains (Figure 1A). To understand the structural basis that is responsible for the differences between BaxΔ2 and Baxα in terms of the mechanism of apoptosis, we predicted the BaxΔ2 structure based on the already known structure of Baxα (15) (Figure 1B). Although the results obtained from computational modeling for BaxΔ2 are completely theoretical, it provided a useful tool to help identify potential functional domains and guide the experimental design. The most striking structural difference between these two proteins appears to be in the N-terminal. BaxΔ2 seems to lack the majority of helix α1, which is encoded by exon 2 and a small part of exon 3 in Baxα (Figure 1B and 1C). In addition to the lack of helix α1, BaxΔ2 also has a shortened ART sequence (Figure 1A), which has been reported to have a negative regulatory role in Bax activation (8, 9, 21–25). Furthermore, BaxΔ2 also has 10 new amino acids in the beginning of exon 3 resulted from an alternative splicing-caused frameshift. However, the original reading frame is restored at the microsatellite and maintained throughout the sequence (Figures 1A and 1C). All these alterations seem to destabilize the N-terminal structure of BaxΔ2, as the structural disorder probability analysis using PrDOS (62) revealed that the N-terminal structure of BaxΔ2 was more disordered when compared with that of Baxα (Figure 1D). The known inactive Baxα structure is partially disordered in the first 15 amino acids but becomes ordered from amino acids 16 to 35, consistent with the presence of helix α1. By contrast, BaxΔ2 shows total disorder in the first 38 amino acids, which can be attributed to the sequence alterations in the first 3 exons (Figure 1D).
FIGURE 1. Loss of helix α1 is responsible for the behavioral differences between Baxα and BaxΔ2.
(A) Schematic representation of Baxα and BaxΔ2 sequences (not scaled). ART stands for Apoptosis-Regulating Targeting sequence. Helices α1 to α9 are labeled on the top. Helices α3, α7 and α8 are very short and not shown in the figure. G7, guanine deletion in the BaxΔ2 microsatellite region. The area marked in yellow represents the 10 new amino acids. BH1-3, are the conserved Bcl-2 homology domains. Ex1–6, exons 1 to 6 are shown on the bottom. (B) Known Baxα NMR tertiary structure (15) and a computationally predicted model of BaxΔ2 structure using RaptorX and I-Tasser. The structures are color coded by corresponding exons indicated in the key. C, C-terminus. Helix α1 is indicated by an arrow in the Baxα structure, and is missing in the BaxΔ2 model structure. (C) Comparison of the N-terminal protein sequence differences between Baxα and BaxΔ2. Only part of the protein sequence coded by exon 3 is shown in this figure. The amino acids that form helix α1 in Baxα are underlined. The 10 amino acids (aa) coded by the new reading frame caused by the alternative splicing in BaxΔ2 are bolded. L26P, mutation of leucine at position 26 to proline; L27P, mutation of leucine at position 27 to proline. (D) N-terminal disorder probability analysis for Baxα and BaxΔ2 using PrDOS. (E) Prediction of helix α1 helicity for each mutant in comparison to Baxα (“0” point). Helicity was calculated using NetSurfP and GOR4; plotted results represent the average probability values minus Baxα helicity probability values for each mutant. (F) Cell death assay of Bax-negative HCT116 cells after 24 hours of transfection with GFP-tagged constructs and treated without (NT) or with Caspase 8 Inhibitor (C8I). ***, p<0.001 for both BaxΔ2 and L26P/L27 when compared to Baxα and when NT is compared to C8I. No statistically significant difference between BaxΔ2 and L26P/L27. (G) Cellular localization of the transfected GFP-tagged proteins and mitochondria stained with anti-Tom20 antibody, 16 hours after transfection in Bax-negative HCT116 cells in the absence (−) or presence (+) of Etoposide (40 µM, 4 hours treatment prior to fixing). Blue, nuclear staining with DAPI.
Previous studies by others have shown that disruption of helix α1 prevents Baxα from targeting mitochondria (22, 37–39). Since the main difference between Baxα and BaxΔ2 is the helix α1, we hypothesized that disruption of the helical structure not only impairs Baxα’s ability to target mitochondria but also could make Baxα behave like BaxΔ2. To test this, we analyzed a previously identified Bax mutation which significantly decreased Baxα’s ability to target mitochondria (Leucine 26 to Glycine [L26G]), using the secondary structure prediction programs NetSurfP (64) and GOR4 (65) with Baxα wild type as control. We found that the mutant L26G seemed to cause disruption of helix α1 (Figure 1E). We then predicted the helicity of several other possible mutants within helix α1 (data not shown) and we found a double mutant, L26P/L27P, which showed the highest disruption of helix α1 (Figure 1E). We generated L26P/L27P construct and transfected into Bax-negative HCT116 cells. The results from cell death assay showed that Baxα[L26P/L27P] mutant has a higher cytotoxicity than wild type Baxα and similar to BaxΔ2 (Figure F).
We next examined the cellular distribution and mitochondrial co-localization of the Baxα mutant (L26P/L27P). Bax-negative HCT116 cells were transfected with Baxα wild type, L26P/L27P, and BaxΔ2, and cells were treated with or without death stimulus (Etoposide) for 4 hours. As shown in Figure 1G, Baxα remained evenly distributed in the cell in the absence of a death signal, but co-localized with mitochondria upon Etoposide stimulation. However, L26P/L27P formed cytosolic aggregates regardless of Etoposide treatment. Furthermore, the aggregates in the L26P/L27P transfected cells were similar to those in BaxΔ2, heterogenic sizes and no co-localization with mitochondria (Figure 1G). These results further confirm that helix α1 is essential to target mitochondria, and disruption of the helical structure leads Baxα to have a BaxΔ2-like behavior.
The 10 new amino acids and shortened ART are not involved in BaxΔ2 aggregation and cytotoxicity
Once we confirmed that loss of helix α1 renders BaxΔ2 to the cytosol, the next step was to test what domains or regions of the protein are responsible for the new behavioral characteristics that are not seen in Baxα: aggregation and caspase 8 activation. To determine whether the 10 new amino acids and loss of part of the ART sequence (Figure 1C) contributed to BaxΔ2 aggregation and cytotoxicity, we generated a series of BaxΔ2-derived constructs with variations in the N-terminus. The 10 new amino acids were either deleted or replaced with the original 10 amino acids from Baxα, in combination with deletion or restoration of the ART sequence (Figure 2A). All constructs were tagged with GFP at their C-termini and transfected into Bax-negative human colon cancer subline HCT116 cells. Similar levels of expression of BaxΔ2 mutant proteins were detected by immunoblotting with anti-GFP antibody (Figure 2B). All transfectants showed similar levels of cytotoxicity (Figures 2C and 2D) when compared with full-length BaxΔ2. Interestingly, all BaxΔ2 mutants were capable of forming cytosolic aggregates, regardless of the N-terminal region alterations (Figure 2E). These results indicate that BaxΔ2 aggregation and cytotoxicity are likely independent of the new 10 amino acids and the shortened ART.
FIGURE 2. The 10 new amino acids and shortened ART are not involved in BaxΔ2 aggregation and cell death.
(A) Schematic representation of BaxΔ2 and four mutant constructs (not scaled). Ex1–6 indicated on the top represent the exons. The area marked in yellow represents the 10 new amino acids region resulted from the alternative splicing-caused frameshift. BaxΔ2[Δ13-21/G7] is BaxΔ2 without the 10 new amino acids; BaxΔ2[R13-21/G8] has the original 10 amino acids from Baxα, this is equivalent to Baxα without exon 2; BaxΔ2[Δ1-21/G8] is a mutant without the first 2 exons; BaxΔ2[ARTΔ13-21/G7] contains the whole ART sequence but is missing most exon 2. (B) Immunoblot of BaxΔ2 and mutants, 24 hours after transfection in HCT116 Bax-negative subline with the GFP-tagged constructs indicated in (A), using an anti-GFP antibody; anti-actin antibody used as protein-loading control. NT, non-transfected control. (C) Cell death assay of Bax-negative HCT116 cells transfected with the constructs as indicated for 24 h. (D) Apoptotic caspase 3 activity assay of Bax-negative HCT116 cells transfected with the constructs indicated in (A) for 24 hours. The cells were incubated with cell permeable NucView™ 530 Caspase-3 Substrate. (E) Cellular localization of the transfected GFP-tagged constructs 16 hours after transfection in Bax-negative HCT116 cells. Blue, nuclear staining with DAPI.
Bax BH3 domain is essential but not sufficient for BaxΔ2 aggregation and cytotoxicity
The above observations prompted us to determine which other structural domains are required for BaxΔ2 aggregation and cytotoxicity. The BH3 domain is essential for the pro-apoptotic activity of all pro-death Bcl-2 family members (1, 3, 4, 15, 17–20). Although BaxΔ2 contains an intact BH3 domain, it was unknown whether it was responsible for BaxΔ2 aggregation and/or cytotoxicity. To test this, we generated BaxΔ2 constructs by either deleting all of exon 3 (BaxΔ2[Δ13-61]), removing only the BH3 domain (BaxΔ2[ΔBH3]), or truncating after exon 3 (BaxΔ2[Δ62-175]) (Figure 3A). The results showed that, although all BaxΔ2 mutants were expressed well (Figure 3B), none of them was able to induce apoptosis (Figures 3C and 3D) or form large cytosolic aggregates (Figure 3E). Of note, loss of the BH3 domain (ΔBH3) or exon 3 (Δ13-61) resulted in formation of evenly distributed fine granules, which appeared significantly smaller than those in BaxΔ2 when approximately measuring individual aggregates (Figure 3F). These results indicate that the BH3 domain is required for BaxΔ2 to form large aggregates and induce apoptosis. With the additional fact that the BaxΔ2[Δ62-175] mutant with an intact BH3 domain was unable to form aggregates at all or induce cell death, together, these data suggest that the BH3 domain is necessary but not sufficient for BaxΔ2 aggregation and cytotoxicity.
FIGURE 3. The BaxΔ2 BH3 domain is essential but not sufficient for the formation of large aggregates and cytotoxicity.
(A) Schematic representation of the sequences of BaxΔ2 and BaxΔ2 mutant constructs (not scaled). BaxΔ2[Δ13–16] has a deletion of both exon 2 and 3, one base pair was added in between exons 1 and 4 to make it in frame. BaxΔ2[ΔBH3] has a deletion of the BH3 domain; BaxΔ2[Δ62–175] has a deletion of exons 4 through 6. The area marked in yellow represents the 10 new amino acids. (B) Immunoblot detection of BaxΔ2 and it mutants, in Bax-negative HCT116 cells 24 hours after transfection with GFP-tagged constructs, using anti-GFP antibody; anti-actin antibody used as protein-loading control. (C) Cell death assay of Bax-negative HCT116 cells transfected with the constructs indicated in (A) for 24 hours. (D) Apoptotic caspase 3 activity assay of Bax-negative HCT116 cells transfected with the constructs indicated in (A) for 24 h. The cells were incubated with cell permeable NucView™ 530 Caspase-3 Substrate. ***, p<0.001 compared with the BaxΔ2 control. (E) Cellular localization of transfected GFP-tagged constructs 16 hours after transfection in HCT116 cells. Blue, nuclear staining with DAPI. (F) Estimation of aggregate size in imaged cells. Total 30 random clearly individual aggregates from 3 representative cells for each construct. ***, p<0.001 compared with the BaxΔ2 control.
BaxΔ2 aggregation is necessary but not sufficient for its cytotoxicity
The finding that the BH3 domain is necessary but not sufficient for BaxΔ2 aggregation or cytotoxicity indicates that other regions of BaxΔ2 should be involved. To test this scenario, we generated a series of BaxΔ2 truncated mutants, which vary in exons 4 to 6 (Figure 4A). Although protein expression levels of these mutants were high in transfected cells (Figure 4B), none of the mutants was able to induce apoptosis compared to BaxΔ2 (Figures 4C and 4D). Interestingly, expression of the BaxΔ2 mutant protein missing only exon 6 forms much larger aggregates without inducing cell death (Figure 4E, Δ141-175 and Figure 4F). This suggests that the BaxΔ2 C-terminal end is not essential for aggregation but critical for cell death. Thus, the critical domains involved in BaxΔ2 large aggregation are located in the core region of the BaxΔ2 protein encoded by exons 3 to 5, while the C-terminus encoded by exon 6 is essential for BaxΔ2 cytotoxicity.
FIGURE 4. BaxΔ2 aggregation is necessary but not sufficient for its cytotoxicity.
(A) Schematic representation of the sequences of BaxΔ2 and three BaxΔ2 mutants with modified core and C-terminal regions (not scaled). BaxΔ2[Δ110–175] is BaxΔ2 without exons 5 and 6, the beginning of exon 5 was retained to maintain the integrity of helix α5; BaxΔ2[Δ141–175] is BaxΔ2 lacking exon 6; and BaxΔ2[Δ62–140] is BaxΔ2 without exons 4 and 5. (B) Immunoblot analysis of BaxΔ2 mutants, in Baxnegative HCT116 cells transfected with GFP-tagged constructs indicated in (A), using anti-GFP antibody; anti-actin antibody used as protein-loading control. (C) Cell death assay of Bax-negative HCT116 cells transfected with the constructs as indicated for 24 hours. (D)Apoptotic caspase 3 activity assay of Baxnegative HCT116 cells transfected with the constructs indicated for 24 h. The cells were incubated with cell permeable NucView™ 530 Caspase-3 Substrate ***, p<0.001 compared with the BaxΔ2 control. (E) Immunofluorescent imaging of the GFP-tagged BaxΔ2 mutants indicated in (A), in Bax-negative HCT116 cells 16 hours after transfection. Blue, nuclear staining with DAPI. (F) Estimation of aggregate size in imaged cells. Total 30 random clearly individual aggregates from 3 representative cells for each construct. ***, p<0.001 compared with the BaxΔ2 control.
BaxΔ2 C-terminus is required for caspase 8-activation in a primary sequence-independent manner
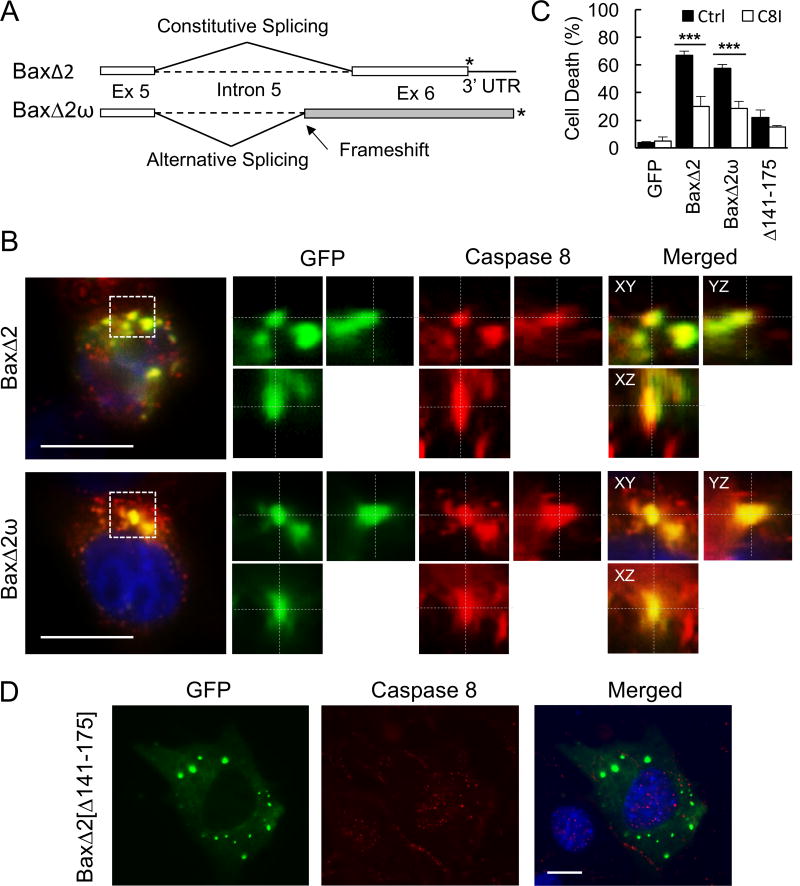
BaxΔ2 exon 6 is essential for aggregation-mediated toxicity and we previously showed that BaxΔ2-induced cell death is through activation of caspase 8 (53). Next, we wanted to know which elements of the C-terminus were critical for the activation of caspase 8. To test this idea, we used BaxΔ2ω, a BaxΔ2 family member. Like BaxΔ2, BaxΔ2ω forms cytosolic aggregates to induce cell death (51). However, BaxΔ2ω has a completely different C-terminus, due to a partial intron 5 retention caused by an alternative splicing event. The intron 5 retention in BaxΔ2ω causes a reading frame shift, resulting in a completely different sequence with an extended C-terminal (40 amino acids longer than BaxΔ2) (Figure 5A).
FIGURE 5. BaxΔ2 C-terminus is required for caspase 8 activation, but in a primary sequence independent manner.
(A) Schematic representation of the C-terminal splicing events for BaxΔ2 and BaxΔ2ω (not scaled). Alternative splicing in BaxΔ2ω causes a partial intron 5 retention (49 bp) and leads to a frameshift that generates a totally different protein sequence of C-terminus (grey color). Stop codon is indicated by an asterisk (*). (B) Cellular colocalization and 3D imaging analysis using the XYZ slicing tool, of transfected GFP-tagged BaxΔ2 and BaxΔ2ω 16 hours after transfection in Bax-negative HCT116 cells. Caspase 8 was detected with anti-caspase 8 antibody (red). Blue, nuclear staining with DAPI. (C) Quantification of cell death of Bax-negative HCT116 cells transfected with GFP, BaxΔ2, BaxΔ2ω and BaxΔ2[Δ141–175] and incubated for 24 hours in the absence (Ctrl) or presence of Caspase 8 Inhibitor (C8I). BaxΔ2[Δ141–175] is BaxΔ2 without the exon 6. ***, p<0.001 difference between Ctrl and C8I in both BaxΔ2 and BaxΔ2ω. No significant difference in BaxΔ2[Δ141–175]. (D) Cellular co-localization of BaxΔ2 C-terminal deletion mutant BaxΔ2[Δ141–175] with caspase 8 in Bax-negative HCT116 cells transfected and incubated for 16 hours and then stained with anti-caspase 8 antibody. Of note, the image of caspase 8 in (D) had to be overexposed to achieve significant detection. Blue, nuclear staining with DAPI.
To test whether the C-terminus of BaxΔ2ω is essential for activation of caspase 8, we analyzed the cellular behavior of BaxΔ2ω in comparison with BaxΔ2. Co-localization analysis showed that, like BaxΔ2, BaxΔ2ω was able to form large aggregates, which were well co-localized with clusters of caspase 8 (Figure 5B). Consistently, both BaxΔ2 and BaxΔ2ω-induced cell death was significantly reduced by caspase 8 inhibitor (Figure 5C). To further confirm that recruitment and activation of caspase 8 rely on the C-terminus, we also examined the BaxΔ2 mutant lacking the C-terminus (BaxΔ2[Δ141-175]). Loss of the C-terminus did not affect BaxΔ2 ability to form aggregates (Figure 4E), but impaired its ability to recruit caspase 8 (Figure 5D) and induce cell death (Figure 5C). These results suggest that BaxΔ2 recruitment and activation of caspase 8 rely on its C-terminus. However, such function seems to be independent of the primary amino acid sequence, as BaxΔ2ω behaves the same as BaxΔ2 even though they have distinct C-termini.
Since the primary sequence of the C-terminus appears not to be essential, we hypothesize that there may be conserved motifs and structural domains that may be responsible for the similar behavior of BaxΔ2 and BaxΔ2ω. To understand if the C-terminal region of BaxΔ2ω could form a structure similar to that of Baxα and BaxΔ2, we performed secondary and tertiary structure modeling analyses. While BaxΔ2 C-terminal structure is predicted to be the same as Baxα, presenting two helices, BaxΔ2ω is predicted to have four helices in its C-terminal region (Figure 6A). Three-dimensional structural modeling revealed the possibility of a conservative structural disposition in certain areas of the C-terminal region, despite the different number of helices (Figure 6B). Helix α8 from both proteins occupied the same space, and most of helix α9 of BaxΔ2 was well superimposed with helix α10 of BaxΔ2ω (Figure 6B). Analysis of the residue disorder probability using PrDOS (62) showed that both C-terminal regions had similar levels of disorder (Figures 6C and 6D). Taken together, these data suggest that the contribution of the C-terminus to BaxΔ2 cytotoxicity depends on conservative structural conformation, rather than the specific primary sequence.
FIGURE 6. Comparison of structure models and disorder probabilities between BaxΔ2 and BaxΔ2ω.
(A) Comparison of the sequence and C-terminal secondary structure of BaxΔ2 and BaxΔ2ω (not scaled). Exons 5 and 6 are indicated as Ex 5 and Ex 6, respectively. 3’UTR stands for 3’ untranslated region. Helices in this area are indicated as α8– α11, blue for BaxΔ2 and red for BaxΔ2ω. Stop codon is indicated by an asterisk (*). (B) Computationally modeled 3D structures of BaxΔ2 (blue) and BaxΔ2ω (red) superimposed. N and C mark the N-terminus and C-terminus, respectively. The areas circled in green are the predicted structural overlapping areas within the C-terminal regions. (C and D) Disorder probability analysis of BaxΔ2 and BaxΔ2ω C-termini using PrDOS. The last 36 amino acids for BaxΔ2 and 68 amino acids for BaxΔ2ω were analyzed.
BaxΔ2 C-terminal helix a9 is required for caspase 8-dependent cell death
To identify whether, within the predicted structurally conserved areas of the C-terminus, there are any motif(s) or residue(s) which are critical for caspase 8-dependent cell death, we performed further sequence alignment for the C-terminal regions of BaxΔ2 and BaxΔ2ω. The result showed that both proteins shared several conserved motifs, mostly clustered at the ends of helix α8 and α9 of BaxΔ2 (Figure 7A), consistent with the overlapping areas in the 3D prediction (Figure 6B). We then generated several mutants corresponding to key conservative residues (Figures 7A and 7B). The ability of these mutants to form α helices was predicted using the secondary structure prediction programs NetSurfP (64) and GOR4 (65), with BaxΔ2 as control (Figure 7D). Mutations at the end of helix α8 (L-A/S-A) seemed to have little effect on the C-terminal helicity. However, the single-point mutation L164P (L–P) in helix α9 appears to significantly disrupt the C-terminal helicity, as expected, due to proline’s ability to destabilize alpha helices. Conversely, the L164A (LT-AA mutant) had no negative effect on the helicity. Similarly, abolishment of the positive charges at the end of helix α9 (KK-AA mutant) appeared to have no negative effect on the helicity, and instead seemed to increase the helix stability. (Figure 7D).
FIGURE 7. Disruption of helix α9 significantly reduces BaxΔ2 toxicity.
(A) Peptide sequence alignment of the C-terminal regions of BaxΔ2 and BaxΔ2ω and the identified conservative motifs are highlighted in yellow. Fully conserved (*), conservative (:), and semi-conservative (.) amino acids are indicated. (B) Detailed list of mutants and the abbreviated names that are used in the following graphs. (C) Immunoblot analysis of expression of BaxΔ2 mutants in Bax-negative HCT116 cells transfected with GFP-tagged constructs for 24 hours, using anti-GFP antibody; anti-actin antibody used as protein-loading control. (D) Prediction of C-terminal helicity of each mutant in comparison to BaxΔ2 (“0” point). Helicity was calculated by subtracting BaxΔ2 helicity probability values from that of each mutant before plotting. (E) Cell death assay of the GFP-tagged BaxΔ2 and its mutants in HCT116 Bax-negative cells 24 hours after transfection and treated without (NT) or with Caspase 8 inhibitor (C8I). Statistical analysis compares the NT and C8I in each individual mutant and then NT of each mutant with the BaxΔ2 control, ***, p<0.001 and *, p<0.05.
To determine the cell death potentials of the above mutants, Bax negative HCT116 cells were transfected with the C-terminal GFP tagged mutants for 24 hours. Expression of these mutant proteins was detected by immunoblotting with anti-GFP antibody, and the results showed that all mutant proteins were detected at similar or slightly higher levels compared to BaxΔ2 (Figure 7C). However, the degrees of cell death were significantly different (Figure 7E). Mutant L-A/S-A, which was predicted to have a minimal effect on helicity, had no significant effect on BaxΔ2 cell death (pink color in Figures 7D and 7E). Double mutant LT-AA in helix α9, with no negative effect on helicity, had a moderate decrease (~15%) in cell death (orange color), compared to BaxΔ2 (black color). A similar result was observed for mutant KK-AA, with abolishment of charged amino acids at the C-terminal end (yellow color). However, mutant L-P, with the highest predicted disruption in helicity, showed a drastic decrease in cell death (red color). Consistently, double mutant L-P/KK-AA (blue color), but not LT-AA/KK-AA (green color), showed similar results as the L-P mutant (red color). Furthermore, as with BaxΔ2, the cell death caused by the toxic mutants could be significantly reduced by caspase 8 inhibitor (Figure 7E). These results indicate that disruption of helix α9 by a point-mutation significantly decreases the ability of BaxΔ2 to activate caspase 8.
In summary, our results provide a structural and molecular basis that explains the behavior differences between Baxα and BaxΔ2. Disruption of helix α1 impairs Baxα ability to target mitochondria. Lack of helix α1 also prevents BaxΔ2 from targeting mitochondria, rendering it to cytosolic accumulation and leading to aggregation and cytotoxicity. The hallmark BH3-killing domain in BaxΔ2 is necessary but not sufficient both aggregation and cell death. The core region of the protein is required for both aggregation and cell death. However, aggregation itself is not sufficient to recruit caspase 8. The C-terminal helix α9 structure seems critical for recruitment and activation of the caspase 8, independently of the specific primary sequence (Figure 8).
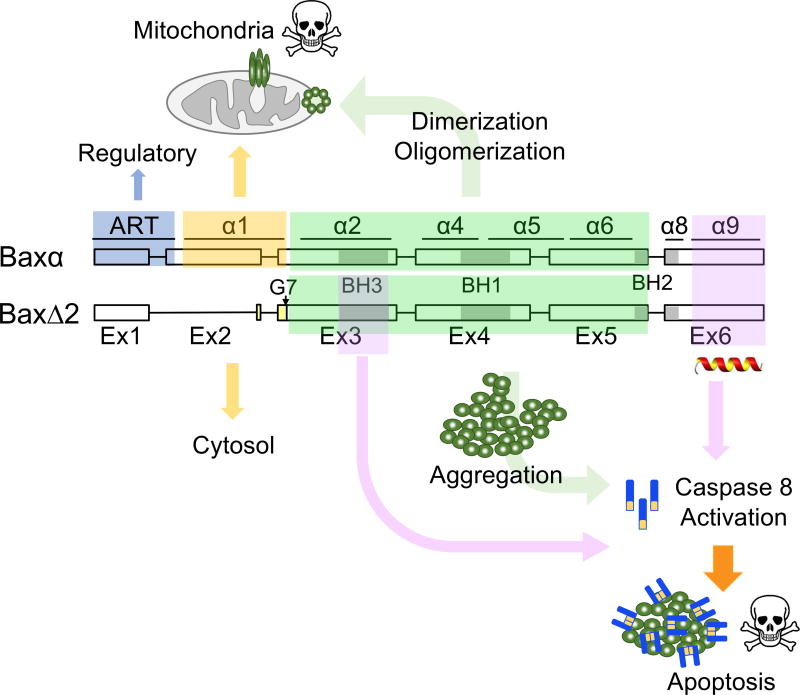
FIGURE 8. Proposed functional domains for BaxΔ2-induced cell death.
Graphical summary of the differences in cell death mechanisms between Baxα and BaxΔ2 and the critical domains for BaxΔ2 aggregation-mediated caspase 8-dependent cell death. ART plays a regulatory role in Baxα, but no apparent function in BaxΔ2. Helix α1 is responsible for Baxα to target mitochondria, but its absence renders BaxΔ2 to the cytosol. The core region of the protein, encoded by exons 3 to 5 and containing the BH3 and BH1 domains, is known to be responsible for Baxα oligomerization, and is also critical for aggregation of BaxΔ2. The role of C-terminal region, specially the helix α9, still remains controversial for Baxα, but seems essential for BaxΔ2-induced caspase 8-mediated cell death.
4. DISCUSSION
BaxΔ2 is a unique member of the Bax family (13, 51). Unlike Baxα and other Bax family members that activate the canonical mitochondrial death pathway (1–6, 8, 9, 12, 14), BaxΔ2 aggregates in cytosol to activate caspase 8, which is normally involved in the receptor death pathway (53). The underlying mechanism was previously unknown. Using the approach of computational analysis and structural modeling to help design cell based and biochemical experiments, we demonstrated that lack of helix α1 prevents BaxΔ2 from translocating to mitochondria. This leads to protein accumulation in the cytosol and the formation of large aggregates, which in turn utilize the C-terminal helical structure to activate the caspase 8-dependent death pathway (Figure 8).
Previous studies have shown that helix α1 is key for Baxα to translocate to mitochondria (22, 37–39). In addition to confirming this result, we also showed that impairment of Baxα mitochondria targeting could result in its accumulation in the cytosol and formation of large aggregates, mimicking the behavior and cytotoxicity of BaxΔ2 (Figure 1). These observations seem to differ from a previous study, in which the Baxα L26G mutant also failed to target mitochondria, but remained evenly distributed in the cytosol with low toxicity (22). The double mutant (L26P/L27P) not only impaired Baxα’s ability to target mitochondria but also promoted its aggregation and caspase activation. A possible explanation could be extrapolated from our helicity prediction data. The double mutation decreased the helicity of helix α1 more significantly than the single mutation; the disruption of helix α1 in the single mutant L26G was enough to prevent it from targeting mitochondria but might not be enough to disrupt the inactive monomeric status of the protein in the absence of a death signal. Further studies are needed to test this hypothesis.
Loss of helix α1 is responsible for the BaxΔ2 protein accumulation in the cytosol, but by itself it cannot explain the other behavioral differences in comparison with Baxα, so we analyzed the rest of the protein to determine domains responsible for these differences. We found that the shortened ART and the 10 new amino acids did not contribute to the pro-apoptotic activity of BaxΔ2. Loss of exon 2 led to a shortened ART region in BaxΔ2 (Figure 1); however, the complete restoration of ART was unable to inhibit BaxΔ2 pro-apoptotic activity (Figure 2). Absence or disruption of helix α1, seemed to result in constitutive activation regardless of the presence of ART. Little is known about the underlying mechanism of ART regulatory function, but our results suggest that it might be dependent on helix α1. Therefore, as helix α1 no longer exists in BaxΔ2, it leaves ART with no significant function in this isoform. Interestingly, the removal of the 10 new amino acids also had no significant effect on BaxΔ2 aggregation or cytotoxicity, and neither did the restoration of the original 10 amino acids (Figure 2). Since neither ART nor the 10 new amino acids affect the aggregation or pro-apoptotic activity, BaxΔ2 appears to be equivalent to Baxα without exon 2, since the rest of the sequence is the same. Indeed, both Baxα with disrupted helix α1 (Baxα[L26P/L27P]) and Baxα lacking only exon 2 (BaxΔ2[R13-21/G8]) had the same degree of aggregation and cell death as BaxΔ2 (Figures 1 and 2).
In Baxα, helix α1 covers the core region of the protein and, upon cell death signal stimulation, helix α1 is unlatched from its position and exposes the core region, which is critical for the formation of Baxα oligomers (29, 33–36). Therefore, transition from inactive to active form is needed for exerting Baxα-induced cell death. BaxΔ2, however, seems to be already in its active form regardless of death signal status. Of note, unlike Baxα, BaxΔ2 proteins are extremely unstable and susceptible to proteasomal degradation. We have also shown that BaxΔ2 is capable of binding both Baxα and Bcl-2 (13). Whether combination of fast degradation and association with other proteins can withhold BaxΔ2 activity under endogenous expression conditions remains to be explored. Another interesting notion is that, although active Baxα proteins can form oligomers in cytosol (34), they do not form large aggregates. This is most likely because Baxα proteins immediately translocate to mitochondria upon activation. Therefore, we propose that BaxΔ2 aggregation is not a gain-of-function, but an inherent consequence of its inability to target mitochondria. Sequentially, these large aggregates may serve as a platform to recruit and activate caspase 8 for cell death. The heterogeneously sized aggregates we observed were largely insoluble and could not be dissolved by detergents like NP-40 or Triton X-100, thereby remaining as insoluble precipitates that could be easily visualized under a fluorescent microscope (data not shown). This makes it practically impossible to analyze the size of these aggregates through conventional biochemical methods such as FPLC. Also, the high levels of aggregation combined with its high toxicity, even with a bacterial expression system, have made it very difficult to isolate enough soluble BaxΔ2 protein for experimental structural analyses such as NMR or CD.
Despite the difficulties to determine the size and structural nature of the BaxΔ2 aggregates, we discovered that aggregation is necessary but by itself is not sufficient to induce apoptosis. BaxΔ2 aggregation and cell death are highly interconnected, as blocking aggregation also abolished cell death (Figures 3 and 4). However, aggregation alone was not sufficient to lead to apoptosis. The BaxΔ2 mutant missing its C-terminus was able to accumulate exceptionally large aggregates in cytosol, but failed to induce significant cell death (Figure 4, Δ141-175). This suggests that the C-terminus is critical for the recruitment of caspase 8 to the aggregates. Among the C-terminus, the helical structure, and not the primary sequence, appears to be essential for this function. BaxΔ2ω, another member of the Bax family, behaves exactly the same as BaxΔ2 despite having a completely different C-terminal sequence (Figures 5 and 6). Also, disruption of helix α9 by single point mutation (L164P) significantly impaired caspase 8-dependent cell death (Figure 7). Of note, disruption of helix α9 did not totally abolish cell death, around 25% of caspase 8-independent cell death remained (Figure 7E). Similar degrees of cell death were also observed in BaxΔ2 and BaxΔ2ω when treated with Caspase 8 inhibitor (Figure 5C). These data, along with the result from the construct missing exon 6 (Figure 4, Δ141-175), indicate that the remaining cell death might be contributed by caspase 8-independent toxicity, probably resulting from high levels of protein aggregation.
We do not exclude out the possibility that truncations, especially big truncations, could cause protein misfolding or unfolding resulting in the loss of its function despite having the necessary domains. However, this seems unlikely in this case. For the C-terminal truncation (BaxΔ2[Δ145-175], the behavior of the truncated protein was very similar to that with a single-point mutation (L164P). Consistently, for the N-terminal truncation, loss of helix α1 showed a similar behavior as that of the point-mutation (L26P/L27P), both lost the ability to target mitochondria but gained the ability to aggregate and activate caspase 8-dependent cell death. It is worth to mention that, although the behavior of aggregation and cytotoxicity are similar, there is a possibility that the exact underlying mechanisms in some constructs might be different. Nevertheless, it is clear that the intrinsic function of BaxΔ2 relies on its internal structure, rather than being a result of misfolding or unfolding of the protein.
Based on all these results, we propose that upon aggregate formation, BaxΔ2 C-terminus serves as an adaptor to recruit caspase 8 and bring them to close proximity for activation. However, where and how helix α9 interacts with caspase 8 remains to be solved. The sequence or structural homology of C-terminal is shared by many Bax family members such as Baxα, Baxσ, Baxψ and Baxω, in addition to the BaxΔ2 subfamily. Although most, if not all, Bax family members do not usually require caspase 8 for cell death, our results imply the possibility that the Bax family members may have an intrinsic ability to activate the caspase 8 pathway if they fail to target mitochondria. Future studies are needed to explore this possibility. Undoubtedly, the “plasticity” of the Bax family is crucial to ensure its pro-death role in both canonical and noncanonical cell death.
Highlights.
Disruption of helix α1 makes Baxα mimic BaxΔ2 behavior
BaxΔ2 aggregation is essential but not sufficient for cytotoxicity
The C-terminus is critical for caspase 8-dependent cell death
The C-terminal helix α9 structure is the key for caspase 8 recruitment
The Bax family may have an intrinsic capability for caspase 8 activation
Acknowledgments
We would like to thank Dr. Oscar Juarez for his insightful input for this project, and Lily Huang for her valuable experimental assistance.
FUNDING
This work is supported by NIH grant (R15CA195526). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest with the contents of this article.
AUTHOR CONTRIBUTIONS
AM, SW and JX contributed to experimental design and data interpretation. AM, AN, JL, YZ, HZ, and AD performed and analyzed the experiments. SW performed structural modeling. AM, AN, BX and NM contributed to computational analysis and interpretation of data. AM and JX wrote the manuscript. All authors reviewed the results and approved the final version of the manuscript.
References
- 1.Antonsson B, Martinou J-C. The Bcl-2 Protein Family. Exp. Cell Res. 2000;256:50–57. doi: 10.1006/excr.2000.4839. [DOI] [PubMed] [Google Scholar]
- 2.Wolter KG, Hsu Y-T, Smith CL, Nechushtan A, Xi X-G, Youle RJ. Movement of Bax from the Cytosol to Mitochondria during Apoptosis. J. Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dewson G. Interplay of Bcl-2 Proteins Decides the Life or Death Fate. Open Cell Signal. J 2011 [Google Scholar]
- 4.Gross a, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 5.Fu NY, Sukumaran SK, Kerk SY, Yu VC. Baxβ: A Constitutively Active Human Bax Isoform that Is under Tight Regulatory Control by the Proteasomal Degradation Mechanism. Mol. Cell. 2009;33:15–29. doi: 10.1016/j.molcel.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 6.Zhou M, Demo SD, McClure TN, Crea R, Bitler CM. A Novel Splice Variant of the Cell Death-promoting Protein BAX. J. Biol. Chem. 1998;273:11930–11936. doi: 10.1074/jbc.273.19.11930. [DOI] [PubMed] [Google Scholar]
- 7.Maia S, Haining WN, Ansén S, Xia Z, Armstrong SA, Seth NP, Ghia P, Boer ML, den, Pieters R, Sallan SE, Nadler LM, Cardoso AA. Gene Expression Profiling Identifies BAX-delta as a Novel Tumor Antigen in Acute Lymphoblastic Leukemia. Cancer Res. 2005;65:10050–10058. doi: 10.1158/0008-5472.CAN-05-1574. [DOI] [PubMed] [Google Scholar]
- 8.Cartron P-F, Oliver L, Martin S, Moreau C, LeCabellec M-T, Jezequel P, Meflah K, Vallette FM. The expression of a new variant of the pro-apoptotic molecule Bax, Baxpsi, is correlated with an increased survival of glioblastoma multiforme patients. Hum. Mol. Genet. 2002;11:675–687. doi: 10.1093/hmg/11.6.675. [DOI] [PubMed] [Google Scholar]
- 9.Jin KL, Graham SH, Mao XO, He X, Nagayama T, Simon RP, Greenberg DA. Bax κ, a novel Bax splice variant from ischemic rat brain lacking an ART domain, promotes neuronal cell death. J. Neurochem. 2001;77:1508–1519. doi: 10.1046/j.1471-4159.2001.00361.x. [DOI] [PubMed] [Google Scholar]
- 10.Thomas AL, Price C, Martin SG, Carmichael J, Murray JC. Identification of two novel mRNA splice variants of bax. Cell Death Differ. 1999 doi: 10.1038/sj.cdd.4400474. [DOI] [PubMed] [Google Scholar]
- 11.Apte SS, Mattei M-G, Olsen BR. Mapping of the human BAX gene to chromosome 19q13.3–q13.4 and isolation of a novel alternatively spliced transcript, BAXδ. 1995 doi: 10.1016/0888-7543(95)80180-T. [DOI] [PubMed] [Google Scholar]
- 12.Shi B, Triebe D, Kajiji S, Iwata KK, Bruskin A, Mahajna J. Identification and Characterization of Baxε, a Novel Bax Variant Missing the BH2 and the Transmembrane Domains. Biochem. Biophys. Res. Commun. 1999;254:779–785. doi: 10.1006/bbrc.1998.0130. [DOI] [PubMed] [Google Scholar]
- 13.Haferkamp B, Zhang H, Lin Y, Yeap X, Bunce A, Sharpe J, Xiang J. BaxΔ2 Is a Novel Bax Isoform Unique to Microsatellite Unstable Tumors. J. Biol. Chem. 2012;287:34722–34729. doi: 10.1074/jbc.M112.374785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitt E, Paquet C, Beauchemin M, Dever-Bertrand J, Bertrand R. Characterization of Bax-ς, a Cell Death-Inducing Isoform of Bax. Biochem. Biophys. Res. Commun. 2000;270:868–879. doi: 10.1006/bbrc.2000.2537. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki M, Youle RJ, Tjandra N. Structure of Bax: Coregulation of Dimer Formation and Intracellular Localization. Cell. 2000;103:645–654. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 16.Yin X-M, Oltvai ZN, Korsmeyer SJ. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature. 1994;369:321–323. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]
- 17.Shangary S, Oliver CL, Tillman TS, Cascio M, Johnson DE. Sequence and helicity requirements for the proapoptotic activity of Bax BH3 peptides. Mol. Cancer Ther. 2004;3:1343–54. [PubMed] [Google Scholar]
- 18.Czabotar PE, Westphal D, Dewson G, Ma S, Hockings C, Fairlie WD, Lee EF, Yao S, Robin AY, Smith BJ, Huang DCS, Kluck RM, Adams JM, Colman PM. Bax Crystal Structures Reveal How BH3 Domains Activate Bax and Nucleate Its Oligomerization to Induce Apoptosis. Cell. 2013;152:519–531. doi: 10.1016/j.cell.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 19.Wang K, Gross A, Waksman G, Korsmeyer SJ. Mutagenesis of the BH3 Domain of BAX Identifies Residues Critical for Dimerization and Killing. Mol. Cell. Biol. 1998;18:6083–6089. doi: 10.1128/mcb.18.10.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.George NM, Evans JJD, Luo X. A three-helix homo-oligomerization domain containing BH3 and BH1 is responsible for the apoptotic activity of Bax. Genes & Dev. 2007;21:1937–1948. doi: 10.1101/gad.1553607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goping IS, Gross A, Lavoie JN, Nguyen M, Jemmerson R, Roth K, Korsmeyer SJ, Shore GC. Regulated Targeting of BAX to Mitochondria. J. Cell Biol. 1998;143:207–215. doi: 10.1083/jcb.143.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cartron PF, Priault M, Oliver L, Meflah K, Manon S, Vallette FM. The N-terminal end of Bax contains a mitochondrial-targeting signal. J. Biol. Chem. 2003 doi: 10.1074/jbc.M208955200. [DOI] [PubMed] [Google Scholar]
- 23.Wood DE, Newcomb EW. Cleavage of Bax Enhances Its Cell Death Function. Exp. Cell Res. 2000;256:375–382. doi: 10.1006/excr.2000.4859. [DOI] [PubMed] [Google Scholar]
- 24.Toyota H, Yanase N, Yoshimoto T, Moriyama M, Sudo T, Mizuguchi J. Calpain-induced Bax-cleavage product is a more potent inducer of apoptotic cell death than wildtype Bax. 2003 doi: 10.1016/S0304-3835(02)00552-9. [DOI] [PubMed] [Google Scholar]
- 25.Arokium H, Camougrand N, Vallette FM, Manon S. Studies of the Interaction of Substituted Mutants of BAX with Yeast Mitochondria Reveal That the C-terminal Hydrophobic α-Helix Is a Second ART Sequence and Plays a Role in the Interaction with Anti-apoptotic BCL-x L. J. Biol. Chem. 2004;279:52566–52573. doi: 10.1074/jbc.M408373200. [DOI] [PubMed] [Google Scholar]
- 26.Cartron P-F, Gallenne T, Bougras G, Gautier F, Manero F, Vusio P, Meflah K, Vallette FM, Juin P. The First α Helix of Bax Plays a Necessary Role in Its Ligand-Induced Activation by the BH3-Only Proteins Bid and PUMA. Mol. Cell. 2004;16:807–818. doi: 10.1016/j.molcel.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 27.Gross A, Jockel J, Wei MC, Korsmeyer SJ, Antonsson B, Cheng E, Levine B, Boise L, Thompson C, Hardwick J, Jong D, de, Prins F, Mason D, Reed J, Ommen G, van, Kluin P, Evan G, Lewis G, Ramsay G, Bishop J, Evan G, Wyllie A, Gilbert C, Littlewood T, Land H, Brooks M, Waters C, Penn L, Hancock D, Farrow S, Brown R, Graef I, Holsinger L, Diver S, Schreiber S, Crabtree G, Hockenbery D, Nunez G, Milliman C, Schreiber R, Korsmeyer S, Hsu Y, Youle R, Hsu Y, Wolter K, Youle R, Knudson C, Korsmeyer S, Krajewski S, Tanaka S, Takayama S, Schibler M, Fenton W, Reed J, Kroemer G, Lee J, Hapel A, Ihle J, Li P, Nijhawan D, Budihardjo I, Srinivasula S, Ahmad M, Alnemri E, Wang X, Liu J, Farmer J, Lane W, Friedman J, Weissman I, Schreiber S, Martin G, Bollag G, McCormick F, Abo A, McCarthy N, Whyte M, Gilbert C, Evan G, Muchmore S, Nguyen M, Millar D, Yong V, Korsmeyer S, Shore G, Oltvai Z, Milliman C, Korsmeyer S, Partis M, Griffiths D, Roberts G, Beechey R, Pastorino J, Chen S, Tafani M, Snyder J, Farber J, Reed J, Sattler M, Schlesinger P, Gross A, Yin X, Yamamoto K, Saito M, Waksman G, Korsmeyer S, Sedlak T, Oltvai Z, Yang E, Wang K, Boise L, Thompson C, Korsmeyer S, Simonian P, Grillot D, Merino R, Nuñez G, Simonian P, Grillot D, Merino R, Nuñez G, Spencer D, Wandless T, Schreiber S, Crabtree G, Thompson C, Thornberry N, Heiden M Vander, Chandel N, Williamson E, Schumacker P, Thompson C, Wang K, Yin X, Chao D, Milliman C, Korsmeyer S, Wolter K, Hsu Y, Smith C, Nechushtan A, Xi X, Youle R, Xiang J, Chao D, Korsmeyer S, Zha H, Aime-Sempe C, Sato T, Reed J. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsai C-J, Liu S, Hung C-L, Jhong S-R, Sung T-C, Chiang Y-W. BAX-Induced Apoptosis Can Be Initiated through a Conformational Selection Mechanism. Structure. 2015;23:139–148. doi: 10.1016/j.str.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 29.Westphal D, Kluck RM, Dewson G. Building blocks of the apoptotic pore: how Bax and Bak are activated and oligomerize during apoptosis. Cell Death Differ. 2014;21:196–205. doi: 10.1038/cdd.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, Tu H-C, Kim H, Cheng EH-Y, Tjandra N, Walensky LD. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nechushtan A, Smith CL, Hsu Y-T, Youle RJ. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grosse L, Wurm CA, Bru ser C, Neumann D, Jans DC, Jakobs S, Alkhaja A, Jans D, Nikolov M, Vukotic M, Lytovchenko O, Ludewig F, Schliebs W, Riedel D, Urlaub H, Jakobs S, Deckers M, Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod J, Mazzei G, Maundrell K, Gambale F, Sadoul R, Martinou J, Baines C, Kaiser R, Sheiko T, Craigen W, Molkentin J, Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K, Metzger K, Frezza C, Annaert W, D’Adamio L, Derks C, Dejaegere T, Pellegrini L, D’Hooge R, Scorrano L, Strooper B De, Edlich F, Banerjee S, Suzuki M, Cleland M, Arnoult D, Wang C, Neutzner A, Tjandra N, Youle R, Eskes R, Desagher S, Antonsson B, Martinou J, Estaquier J, Arnoult D, Frezza C, Cipolat S, Brito OM, de, Micaroni M, Beznoussenko G, Rudka T, Bartoli D, Polishuck R, Danial N, Strooper B De, Scorrano L, Gillies L, Du H, Peters B, Knudson C, Newmeyer D, Kuwana T, Green D, Kroemer G, Gross A, Jockel J, Wei M, Korsmeyer S, Harner M, Korner C, Walther D, Mokranjac D, Kaesmacher J, Welsch U, Griffith J, Mann M, Reggiori F, Neupert W, Hell S, Hoppins S, Collins S, Cassidy-Stone A, Hummel E, Devay R, Lackner L, Westermann B, Schuldiner M, Weissman J, Nunnari J, Hotchkiss R, Strasser A, McDunn J, Swanson P, Hsu Y, Wolter K, Youle R, Jakobs S, Wurm C, Jans D, Wurm C, Riedel D, Wenzel D, Stagge F, Deckers M, Rehling P, Jakobs S, Kluck R, Bossy-Wetzel E, Green D, Newmeyer D, Kolmakov K, Belov V, Bierwagen J, Ringemann C, Muller V, Eggeling C, Hell S, Kroemer G, Galluzzi L, Brenner C, Kuwana T, Mackey M, Perkins G, Ellisman M, Latterich M, Schneiter R, Green D, Newmeyer D, Lee Y, Jeong S, Karbowski M, Smith C, Youle R, Liu X, Kim C, Yang J, Jemmerson R, Wang X, Malsburg K von der, Muller J, Bohnert M, Oeljeklaus S, Kwiatkowska P, Becker T, Loniewska-Lwowska A, Wiese S, Rao S, Milenkovic D, Hutu D, Zerbes R, Schulze-Specking A, Meyer H, Martinou J, Rospert S, Rehling P, Meisinger C, Veenhuis M, Warscheid B, Martinez-Caballero S, Dejean L, Kinnally M, Oh K, Mannella C, Kinnally K, Martinou J, Youle R, Mattson M, Meier P, Finch A, Evan G, Moldoveanu T, Follis A, Kriwacki R, Green D, Montessuit S, Somasekharan S, Terrones O, Lucken-Ardjomande S, Herzig S, Schwarzenbacher R, Manstein D, Bossy-Wetzel E, Basanez G, Meda P, Martinou J, Nechushtan A, Smith C, Hsu Y, Youle R, Nechushtan A, Smith C, Lamensdorf I, Yoon S, Youle R, Parone P, James D, Cruz S, Da, Mattenberger Y, Donze O, Barja F, Martinou J, Peng R, Tong J, Li H, Yue B, Zou F, Yu J, Zhang L, Renault T, Manon S, Salvador-Gallego R, Mund M, Cosentino K, Schneider J, Unsay J, Schraermeyer U, Engelhardt J, Ries J, García-Sáez A, Schafer B, Quispe J, Choudhary V, Chipuk J, Ajero T, Du H, Schneiter R, Kuwana T, Schellenberg B, Wang P, Keeble J, Rodriguez-Enriquez R, Walker S, Owens T, Foster F, Tanianis-Hughes J, Brennan K, Streuli C, Gilmore A, Schinzel A, Kaufmann T, Schuler M, Martinalbo J, Grubb D, Borner C, Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes S, Mannella C, Korsmeyer S, Tait S, Green D, Todt F, Cakir Z, Reichenbach F, Youle R, Edlich F, Todt F, Cakir Z, Reichenbach F, Emschermann F, Lauterwasser J, Kaiser A, Ichim G, Tait S, Frank S, Langer H, Edlich F, Vaux D, Korsmeyer S, Wolter K, Hsu Y, Smith C, Nechushtan A, Xi X, Youle R, Wurm C, Neumann D, Lauterbach M, Harke B, Egner A, Hell S, Jakobs S, Yamaguchi R, Lartigue L, Perkins G, Scott R, Dixit A, Kushnareva Y, Kuwana T, Ellisman M, Newmeyer D, Yang R, Zhao G, Liang S, Zhang Y, Sun L, Chen H, Liu D, Youle R, Karbowski M, Yuan J, Yankner B, Zhou L, Chang D. Bax assembles into large ring-like structures remodeling the mitochondrial outer membrane in apoptosis. EMBO J. 2016;35:402–413. doi: 10.15252/embj.201592789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subburaj Y, Cosentino K, Axmann M, Pedrueza-Villalmanzo E, Hermann E, Bleicken S, Spatz J, García-Sáez AJ. Bax monomers form dimer units in the membrane that further self-assemble into multiple oligomeric species. Nat. Commun. 2015;6:8042. doi: 10.1038/ncomms9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sung T-C, Li C-Y, Lai Y-C, Hung C-L, Shih O, Yeh Y-Q, Jeng U-S, Chiang Y-W. Solution Structure of Apoptotic BAX Oligomer: Oligomerization Likely Precedes Membrane Insertion. Structure. 2015;23:1878–1888. doi: 10.1016/j.str.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Lamb HM, Hardwick JM. Unlatched BAX Pairs for Death. Cell. 2013;152:383–384. doi: 10.1016/j.cell.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Z, Zhu W, Lapolla SM, Miao Y, Shao Y, Falcone M, Boreham D, McFarlane N, Ding J, Johnson AE, Zhang XC, Andrews DW, Lin J. Bax Forms an Oligomer via Separate, Yet Interdependent, Surfaces. J. Biol. Chem. 2010;285:17614–17627. doi: 10.1074/jbc.M110.113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cartron P-F, Moreau C, Oliver L, Mayat E, Meflah K, Vallette FM. Involvement of the N-terminus of Bax in its intracellular localization and function. FEBS Lett. 2002;512:95–100. doi: 10.1016/s0014-5793(02)02227-5. [DOI] [PubMed] [Google Scholar]
- 38.Cartron PF, Arokium H, Oliver L, Meflah K, Manon S, Vallette FM. Distinct domains control the addressing and the insertion of bax into mitochondria. J. Biol. Chem. 2005;280:10587–10598. doi: 10.1074/jbc.M409714200. [DOI] [PubMed] [Google Scholar]
- 39.Sani MA, Dufourc EJ, Gröbner G. How does the Bax-α1 targeting sequence interact with mitochondrial membranes? The role of cardiolipin. Biochim. Biophys. Acta - Biomembr. 2009;1788:623–631. doi: 10.1016/j.bbamem.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 40.del Mar Martínez-Senac M, Corbalán-García S, Gómez-Fernández JC. Conformation of the C-Terminal Domain of the Pro-Apoptotic Protein Bax and Mutants and Its Interaction with Membranes. Biochemistry. 2001;40:9983–9992. doi: 10.1021/bi010667d. [DOI] [PubMed] [Google Scholar]
- 41.Gómez-Fernández JC. Functions of the C-terminal domains of apoptosis-related proteins of the Bcl-2 family. Chem. Phys. Lipids. 2014;183:77–90. doi: 10.1016/j.chemphyslip.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Valentijn AJ, Upton J-P, Bates N, Gilmore AP. Bax targeting to mitochondria occurs via both tail anchor-dependent and -independent mechanisms. Cell Death Differ. 2008;15:1243–1254. doi: 10.1038/cdd.2008.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schinzel A, Kaufmann T, Schuler M, Martinalbo J, Grubb D, Borner C. Conformational control of Bax localization and apoptotic activity by Pro168. J. Cell Biol. 2004;164:1021–1032. doi: 10.1083/jcb.200309013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Annis MG, Soucie EL, Dlugosz PJ, Cruz-Aguado JA, Penn LZ, Leber B, Andrews DW, Annis M, Zamzami N, Zhu W, Penn L, Kroemer G, Leber B, Andrews D, Antonsson B, Montessuit S, Lauper S, Eskes R, Martinou J, Arnoult D, Gaume B, Karbowski M, Sharpe J, Cecconi F, Youle R, Belzacq A, Vieira H, Verrier F, Vandecasteele G, Cohen I, Prevost M, Larquet E, Pariselli F, Petit P, Kahn A, Rizzuto R, Brenner C, Kroemer G, Cartron P, Priault M, Oliver L, Meflah K, Manon S, Vallette F, Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou J, Duelli D, Lazebnik Y, Epand R, Martinou J, Montessuit S, Epand R, Yip C, Eskes R, Desagher S, Antonsson B, Martinou J, Garcia-Saez A, Mingarro I, Perez-Paya E, Salgado J, Goping I, Gross A, Lavoie J, Nguyen M, Jemmerson R, Roth K, Korsmeyer S, Shore G, Heuck A, Hotze E, Tweten R, Johnson A, Kim P, Annis M, Dlugosz P, Leber B, Andrews D, Kuwana T, Mackey M, Perkins G, Ellisman M, Latterich M, Schneiter R, Green D, Newmeyer D, Lindeberg M, Zakharov S, Cramer W, Marzo I, Brenner C, Zamzami N, Jurgensmeier J, Susin S, Vieira H, Prevost M, Xie Z, Matsuyama S, Reed J, Kroemer G, Nechushtan A, Smith C, Hsu Y, Youle R, Nouraini S, Six E, Matsuyama S, Krajewski S, Reed J, Ruffolo S, Shore G, Sharpe J, London E, Soucie E, Annis M, Sedivy J, Filmus J, Leber B, Andrews D, Penn L, Steere B, Eisenberg D, Suzuki M, Youle R, Tjandra N, Terrones O, Antonsson B, Yamaguchi H, Wang H, Liu J, Lee R, Herrmann A, Basanez G, Wolter K, Hsu Y, Smith C, Nechushtan A, Xi X, Youle R, Yethon J, Epand R, Leber B, Epand R, Andrews D, Zha J, Weiler S, Oh K, Wei M, Korsmeyer S. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J. 2005;24:2096–2103. doi: 10.1038/sj.emboj.7600675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boohaker RJ, Zhang G, Lee MW, Nemec KN, Santra S, Perez JM, Khaled AR. Rational Development of a Cytotoxic Peptide To Trigger Cell Death. Mol. Pharm. 2012;9:2080–2093. doi: 10.1021/mp300167e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee MW, Bassiouni R, Sparrow NA, Iketani A, Boohaker RJ, Moskowitz C, Vishnubhotla P, Khaled AS, Oyer J, Copik A, Fernandez-Valle C, Perez JM, Khaled AR. The CT20 peptide causes detachment and death of metastatic breast cancer cells by promoting mitochondrial aggregation and cytoskeletal disruption. Cell Death Dis. 2014;5:e1249. doi: 10.1038/cddis.2014.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tremblais K, Oliver L, Juin P, Thérese Le Cabellec M, Meflah K, Vallette FM. The C-Terminus of bax Is Not a Membrane Addressing/Anchoring Signal. Biochem. Biophys. Res. Commun. 1999;260:582–591. doi: 10.1006/bbrc.1999.0904. [DOI] [PubMed] [Google Scholar]
- 48.Priault M, Cartron P-F, Camougrand N, Antonsson B, Vallette FM, Manon S. Investigation of the role of the C-terminus of Bax and of tc-Bid on Bax interaction with yeast mitochondria. Cell Death Differ. 2003;10:1068–1077. doi: 10.1038/sj.cdd.4401270. [DOI] [PubMed] [Google Scholar]
- 49.Oliver L, Priault M, Tremblais K, LeCabellec M-T, Meflah K, Manon S, Vallette FM. The substitution of the C-terminus of bax by that of bcl-xL does not affect its subcellular localization but abrogates its pro-apoptotic properties. FEBS Lett. 2000;487:161–165. doi: 10.1016/s0014-5793(00)02330-9. [DOI] [PubMed] [Google Scholar]
- 50.Basañez G, Nechushtan A, Drozhinin O, Chanturiya A, Choe E, Tutt S, Wood KA, Hsu Y, Zimmerberg J, Youle RJ. Bax, but not Bcl-xL, decreases the lifetime of planar phospholipid bilayer membranes at subnanomolar concentrations. Proc. Natl. Acad. Sci. U. S. A. 1999;96:5492–7. doi: 10.1073/pnas.96.10.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haferkamp B, Zhang H, Kissinger S, Wang X, Lin Y, Schultz M, Xiang J. BaxΔ2 Family Alternative Splicing Salvages Bax Microsatellite-Frameshift Mutations. Genes Cancer. 2013;4:501–12. doi: 10.1177/1947601913515906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang H, Tassone C, Lin N, Mañas A, Zhao Y, Xiang J. Detection of Bax Microsatellite Mutations and BaxDelta2 Isoform in Human Buccal Cells. J. Cell Sci. Ther. 2015 doi: 10.4172/2157-7013.S8-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang H, Lin Y, Manas A, Zhao Y, Denning MF, Ma L, Xiang J. BaxDelta2 Promotes Apoptosis through Caspase-8 Activation in Microsatellite-Unstable Colon Cancer. Mol. Cancer Res. 2014;12:1225–1232. doi: 10.1158/1541-7786.MCR-14-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Källberg M, Margaryan G, Wang S, Ma J, Xu J. RaptorX server: A Resource for Template-Based Protein Structure Modeling. 2014:17–27. doi: 10.1007/978-1-4939-0366-5_2. [DOI] [PubMed] [Google Scholar]
- 55.Källberg M, Wang H, Wang S, Peng J, Wang Z, Lu H, Xu J. Template-based protein structure modeling using the RaptorX web server. Nat. Protoc. 2012;7:1511–1522. doi: 10.1038/nprot.2012.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang S, Li W, Liu S, Xu J. RaptorX-Property: a web server for protein structure property prediction. Nucleic Acids Res. 2016 doi: 10.1093/nar/gkw306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y, Murzin A, Bateman A, Ginalski K, Rychlewski L, Baker D, Sali A, Skolnick J, Fetrow J, Kolinski A, Zemla A, Venclovas C, Moult J, Fidelis K, Zhang Y, Skolnick J, Battey J, Kopp J, Bordoli L, Read R, Clarke N, Schwede T, Tosatto S, Pettitt C, McGuffin L, Jones D, Wallner B, Elofsson A, Fischer D, Cozzetto D, Kryshtafovych A, Ceriani M, Tramontano A, Wu S, Zhang Y, Zhang Y, Skolnick J, Wu S, Skolnick J, Zhang Y, Zhang Y, Karplus K, Barrett C, Hughey R, Altschul S, Madden T, Schaffer A, Zhang J, Zhang Z, Miller W, Lipman D, Needleman S, Wunsch C, Smith T, Waterman M, Zhang Y, Kolinski A, Skolnick J, Zhang Y, Kihara D, Skolnick J, Zhang Y, Skolnick J, Zhang Y, Skolnick J, Feig M, Rotkiewicz P, Kolinski A, Skolnick J, Brooks C, Canutescu A, Shelenkov A, Dunbrack R, Berman H, Westbrook J, Feng Z, Gilliland G, Bhat T, Weissig H, Shindyalov I, Bourne P, Kabsch W, Betancourt M, Skolnick J, Reichl L. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang J, Yan R, Roy A, Xu D, Poisson J, Zhang Y. The I-TASSER Suite: protein structure and function prediction. Nat. Methods. 2014;12:7–8. doi: 10.1038/nmeth.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat. Protoc. 2010;5:725–38. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Webb B, Sali A, Webb B, Sali A. Current Protocols in Bioinformatics. John Wiley & Sons, Inc; Hoboken, NJ, USA: 2014. Comparative Protein Structure Modeling Using MODELLER; pp. 5.6.1–5.6.32. [DOI] [PubMed] [Google Scholar]
- 61.Rohl CA, Strauss CEM, Misura KMS, Baker D. Protein Structure Prediction Using Rosetta. Methods Enzymol. 2004;383:66–93. doi: 10.1016/S0076-6879(04)83004-0. [DOI] [PubMed] [Google Scholar]
- 62.Ishida T, Kinoshita K. PrDOS: prediction of disordered protein regions from amino acid sequence. Nucleic Acids Res. 2007;35:W460–4. doi: 10.1093/nar/gkm363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Larkin MA, Blackshields G, Brown NP, Chenna R, Mcgettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 64.Petersen B, Petersen T, Andersen P, Nielsen M, Lundegaard C. A generic method for assignment of reliability scores applied to solvent accessibility predictions. BMC Struct. Biol. 2009;9:51. doi: 10.1186/1472-6807-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garnier J, Gibrat JF, Robson B. GOR secondary structure prediction method version IV. Methods Enzym. 1996;266:540–553. doi: 10.1016/s0076-6879(96)66034-0. [DOI] [PubMed] [Google Scholar]
- 66.R Core Team. R: a language and environment for statistical computing. R Found. Stat. Comput. Vienna, Austria. 2016 URL https//www.R-project.org/