ABSTRACT
An estimated 417 million people worldwide ages 15 to 49 are infected with herpes simplex virus type 2 (HSV-2), the most common cause of genital ulcer disease. Some individuals experience frequent recurrences of genital lesions, while others only have subclinical infection, yet all risk transmitting infection to their intimate partners. A vaccine was developed that prevents shingles, which is a recurrent infection caused by varicella-zoster virus (VZV), a closely related member of the Herpesviridae family. The success of the VZV vaccine has stimulated renewed interest in a therapeutic vaccine for genital herpes. We have been evaluating a trivalent subunit antigen vaccine for prevention of genital herpes. Here, we assess the trivalent vaccine as immunotherapy in guinea pigs that were previously infected intravaginally with HSV-2. The trivalent vaccine contains HSV-2 glycoproteins C, D, and E (gC2, gD2, gE2) subunit antigens administered with CpG and alum as adjuvants. We previously demonstrated that antibodies to gD2 neutralize the virus while antibodies to gC2 and gE2 block their immune evasion activities, including evading complement attack and inhibiting activities mediated by the IgG Fc domain, respectively. Here, we demonstrate that the trivalent vaccine significantly boosts ELISA titers and neutralizing antibody titers. The trivalent vaccine reduces the frequency of recurrent genital lesions and vaginal shedding of HSV-2 DNA by approximately 50% and almost totally eliminates vaginal shedding of replication-competent virus, suggesting that the trivalent vaccine is a worthy candidate for immunotherapy of genital herpes.
KEYWORDS: antibody, complement, Genital herpes, glycoprotein D, glycoprotein C, glycoprotein E, guinea pigs, HSV-2, immune evasion, therapeutic vaccine
Introduction
The global prevalence of genital herpes is estimated at 417 million in individuals between the ages of 15 and 49, with a disproportionate burden of disease in Africa.1 HSV-1 is approximately as common as HSV-2 as the cause of first time genital herpes in resource-rich countries.2 Recurrent infections are less common after HSV-1 than HSV-2 genital infections;3 therefore, HSV-2 remains the predominant cause of recurrent genital herpes in resource-rich countries and the burden of HSV-2 infection is even greater in resource-limited countries.4 Some infected individuals have severe and frequent outbreaks of genital ulcers,5 while others have mild or subclinical infections, yet all risk transmitting genital herpes to their intimate partners.6
Complications of genital herpes include recurrent meningitis,7 which is not life threatening, and neonatal herpes, a potentially fatal infection that develops when newborns are delivered through an infected birth canal.8 Genital herpes predisposes infected individuals to HIV acquisition, while co-infection with HSV and HIV increases the risk of transmitting HIV to an uninfected partner.9 Condom use and circumcision are partially effective at preventing acquisition of genital herpes.10, 11 A vaccine that prevents genital herpes will be a welcome addition to the current prophylactic options, but no vaccine is currently available. Mathematical modeling studies estimate that an effective vaccine for genital herpes will reduce the incidence of HIV by approximately 30% to 40% over 20 y in sub-Saharan Africa.12
In infected individuals, antiviral therapy with acyclovir or valacyclovir reduces the frequency of recurrent genital lesions and the risk of transmitting infection to partners.13, 14 Vaccination to boost immunity represents an alternate approach to reduce the frequency of clinical and subclinical genital recurrences.15 Current laboratory small animal models for genital herpes vaccine studies include mice, cotton rats and guinea pigs.16-19 The murine model has advantages for immunology assays based on availability of reagents to measure CD4 and CD8 T-cell responses; however, infections do not recur spontaneously in mice, rendering this model of no value for therapeutic vaccine efficacy studies.20 The cotton rat is more difficult to handle than guinea pigs and the cotton rat model has been less extensively evaluated than the guinea pig model. Therefore, we chose the guinea pig genital infection model for therapeutic vaccine efficacy studies.
We previously reported results of a trivalent antigen vaccine consisting of HSV-2 glycoproteins C, D and E (gC2, gD2, gE2) for prevention of genital herpes in guinea pigs.21 These glycoproteins were selected to induce antibodies that block gD2 in virus entry,22 and immune evasion activities mediated by gC2 and gE2. HSV-2 gC2 inhibits the complement cascade,23 while gE2 blocks activities performed by the IgG Fc domain.19 We reported that the trivalent vaccine was 98% efficacious in preventing genital lesions compared with mock immunized animals and significantly reduced the number of days animals shed HSV-2 DNA and replication-competent virus from genital secretions.21 Here we evaluate the trivalent vaccine as immunotherapy when administered to guinea pigs that were previously infected intravaginally with HSV-2. We report that the vaccine reduces the frequency of recurrent genital lesions and vaginal shedding of HSV-2 DNA by approximately 50% compared with mock-immunized animals, and decreases the number of days animals shed replication-competent virus in genital secretions by 77%.
Results
We performed 2 separate experiments and evaluated 4 different approaches to infect guinea pigs. Our goal was to have animals develop genital lesions, yet survive so they would be available for therapeutic immunization studies. The characteristics of each group, the survival, the mean number of surviving animals that developed acute disease (days 1–14), the mean number of days with acute disease, and the mean disease score (a marker of disease severity) are shown in Table 1. Groups 1–3 had higher survival rates than group 4; however, only group 1 was significantly higher than group 4. Surviving animals had significantly more acute genital disease in group 4 than in groups 1 and 2, and the mean number of days with genital disease was significantly greater in group 4 than in the other 3 groups. The severity of disease (mean disease score) was significantly higher in group 4 than group 3, but not significantly different from groups 1 and 2. Overall, group 4 animals had more severe disease although death from acute infection was also higher. We conclude that no one group had a clear advantage over another for establishing a robust infection without high mortality before therapeutic immunization.
Table 1.
Acute disease on days 1–14 post-infection.
| Disease in surviving animals |
|||||
|---|---|---|---|---|---|
| Characteristics of each infection group | Animals included from experiment | Survival | No. animals with genital disease | Mean days with genital disease | Mean total genital disease score |
| 1) 1×104 , no acv§ | #1 | 11/12 | 5/11 | 2.3 ± 3.9# | 5.2 ± 10.3 |
| (92%) | (45%) | ||||
| 2) 1×104 PFU, acv twice daily from day 1 | #1 | 10/12 | 5/10 | 2.4 ± 3.3 | 4.6 ± 6.9 |
| (83%) | (50%) | ||||
| 3) 5×105 PFU, acv twice daily from day 1 | #1 | 10/12 | 6/10 | 2.3 ± 3.3 | 3.8 ± 6.6 |
| (83%) | (60%) | ||||
| 4) 2×104 PFU, acv once daily from day 6 | #2 | 39/72* | 32/39* | 4.6 ± 3.1* | 9.1 ± 7.5* |
| (54%) | (82%) | ||||
acv represents acyclovir;
± Standard Deviation. Survival:
group 4 compared with group 1, p < .05. Number of animals with genital disease:
group 4 compared with groups 1 and 2, p < .05. Mean number of days with genital days:
group 4 compared with groups 1, 2 and 3, p < .05. Mean total disease score (severity of disease):
group 4 compared with group 3, p < .05. The p values for survival and number of animals with genital disease were calculate using Fisher's exact test, while p values for days with disease and total disease scores were calculated using Student's t-test.
Thirty-one of 36 animals survived the acute infection in experiment #1 and 39/72 in experiment #2. Fifteen surviving animals from experiment #1 were randomly assigned to receive either the mock vaccine (CpG/alum) (n = 5) or the trivalent vaccine containing 10 μg of each subunit antigen (n = 5), or the trivalent vaccine with 10 μg of gC2, 10 μg gE2 and 15 μg gD2 (n = 5) (Table 2). Seventeen surviving animals from experiment #2 were randomly assigned to receive either the mock vaccine (n = 9) or the trivalent vaccine containing 10 μg of each subunit antigen (n = 8) (Table 2). The remaining surviving animals were assigned to receive other therapeutic vaccine formulations and are not included in this report. Immunizations were administered at 2-week intervals into the right hind limb calf muscle.23 Animals in experiment #1 were immunized on days 35, 49 and 62 while animals in experiment #2 were immunized on days 19, 33, and 47 (Table 2).
Table 2.
Animals randomized to mock or trivalent vaccine and immunization schedule.
| Number of animals randomized to trivalent group |
|||||
|---|---|---|---|---|---|
| Infection group | Experiment Number | Number of animals randomized to mock group | 10 μg each antigen | 10 μg gC2 15 μg gD2 10 μg gE2 | Days animals immunized |
| 1) 1×104 PFU, no acv§ | 1 | 2 | 2 | 2 | Days 35, 49, 62 |
| 2) 1×104 PFU, acv twice daily from day 1 | 1 | 2 | 1 | 2 | Days 35, 49, 62 |
| 3) 5×105 PFU, acv twice daily from day 1 | 1 | 1 | 2 | 1 | Days 35, 49, 62 |
| 4) 2×104 PFU, acv once daily from day 6 | 2 | 9 | 8 | 0 | Days 19, 33, 47 |
acv represents acyclovir
ELISA antibody titers were measured in experiment #2. Sera were obtained 16–18 d after intravaginal infection, which was before the first immunization, 2-weeks after the second and third immunizations, and approximately one month later at the time of the terminal bleed. ELISA titers to all 3 glycoproteins increased significantly from pre-immunization until next tested after the second immunization and remained elevated until animals were euthanized (Fig. 1).
Figure 1.
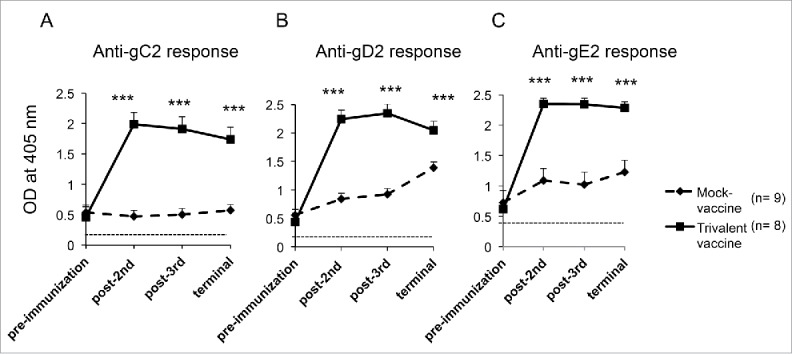
The trivalent vaccine induces robust ELISA antibody responses. Sera were evaluated at a 1:1000 dilution for (A) gC2, (B) gD2, and (C) gE2 antibodies at 16–18 d after intravaginal infection (pre immunization), after the second and third immunizations and at the end of the experiment. The dotted line represents antibody titers in uninfected, naïve guinea pigs. Statistical analysis was performed by 2-way ANOVA for repeated measures followed by Bonferroni's post-test for significance. *** p < .001 comparing mock and trivalent samples.
Neutralizing antibody titers with and without complement were evaluated in experiment #2. Sera were obtained at the terminal bleed. In the absence of complement, the 50% neutralization titer of the mock-immunized animals was 1:320, while the trivalent-immunized animals had a titer >1:1280 (Fig. 2A). In the presence of complement, the 50% end point titer for mock-immunized animals was 1:1280, while the end point titer of the trivalent group was not reached at 1:1280, but was at least 4-fold higher than the mock group based on comparing mock-immunized animals at 1:320 with trivalent-immunized animals at 1:1280 (Fig. 2B).
Figure 2.
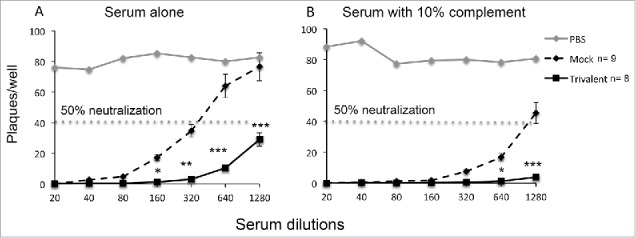
The trivalent vaccine boosts neutralizing antibody titers. (A) Sera were tested for neutralizing antibody titers in the absence of complement, or (B) in the presence of 10% human serum as a source of complement obtained from an HSV-1/HSV-2 seronegative donor. Statistical analysis was performed using nonparametric ANOVA followed by Tukey's post-test analysis. * p < .05, ** p < .01, *** p < .001 comparing mock and trivalent samples.
Animals in both experiments #1 and #2 were evaluated to determine the impact of immunization on recurrent genital lesions. Overall, 14 animals were included in the mock group and 18 in the trivalent group, which included the 5 animals in experiment #1 that received 10 μg of each antigen and the 5 that received 10 μg of gC2 and gE2 and 15 μg of gD2. The mean disease score before randomization was 15.9 ± 18.5 ( ± SD) for animals in the mock group and 10.4 ± 12.0 for animals in the trivalent group (p = 0.31, not significant). The mean number of days with genital lesions before randomization was 8.7 ± 8.3 in the mock group and 6.1 ± 5.4 in the trivalent group (p = 0.30, not significant). Therefore, the severity of disease and the mean number of days with disease did not differ significantly between the mock and trivalent groups before immunization.
Animals were scored for recurrent genital lesions beginning one day after the first immunization. The trivalent group had significantly fewer days with recurrent genital lesions (Fig. 3). The table below the figure lists the number of days that animals had recurrent lesions from the time of the first, second, or the third immunization until the end of the experiment. From the time of the first immunization, the trivalent group had 49% fewer lesion days than the mock group, after the second immunization, 47% fewer lesion days, and after the third immunization, 37% fewer lesion days. In a subset analysis, we detected no significant difference comparing the animals that were immunized with 10 μg of each antigen or 10 μg of gC2 and gE2 and 15 μg gD2. The group that receive 10 μg of each antigen had a total of 3 d with recurrent genital lesions compared with 5 d in the group that received 15 μg of gD2 (p = .72, Fisher's exact test).
Figure 3.
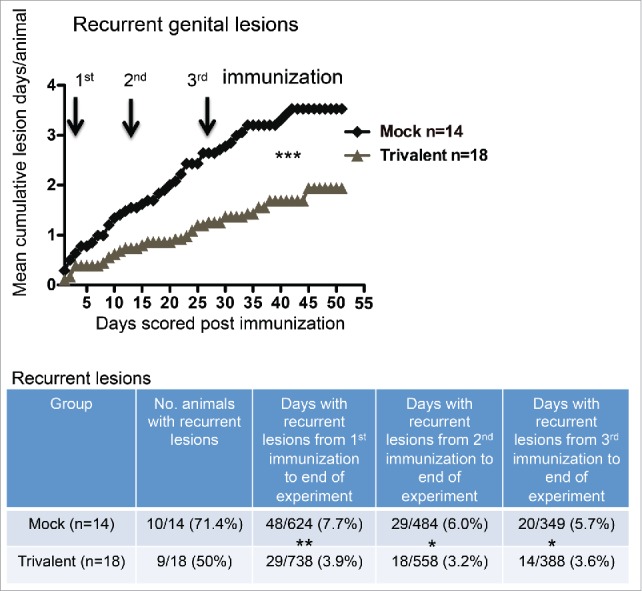
The trivalent vaccine reduces the number of days with recurrent genital lesions. Mean cumulative lesion days per guinea pig in mock and vaccinated groups. Arrows represent immunization days. The p value was calculated using Prism software by 2-way ANOVA. The table below the graph lists the number of animals that had recurrent lesions, and the total number of lesion days per group after the first, second or third immunization until the end of the experiment. The p values comparing mock and trivalent groups in the table were calculated using the Fisher's exact test: p = 0.29 comparing number of animals with recurrent disease; * p < .05, ** p < .01.
Animals in experiment #2 were evaluated for vaginal shedding of HSV-2 DNA. As a control for comparable levels of infection in the mock (n = 9) and trivalent vaccine groups (n = 8), we performed viral cultures on vaginal swabs obtained 2 d post-infection. The average titers on day 2 were not significantly different between the mock (4.3 log10 ± 1.2) and vaccine (4.3 log10 ± 0.8) groups (p = .70), indicating that the infections were comparable in the 2 groups (Fig. 4A). Vaginal swabs were obtained on 42 d during the recurrent phase of infection starting one day after the first immunization and continuing until the end of the experiment. The swabs were processed by qPCR to identify days that animals were shedding HSV-2 DNA and to quantify HSV-2 DNA copy number on days with shedding. The trivalent group had significantly fewer days with recurrent vaginal shedding of HSV-2 DNA (Fig. 4B). The table below the figure lists the number of animals that had recurrent vaginal shedding of HSV-2 DNA in each group, and the number of days animals shed HSV-2 DNA after the first, second, or third immunization until the end of the experiment. From the time of the first immunization, the trivalent group had 37% fewer days of HSV-2 DNA shedding than the mock group, while from the time of the second immunization, the trivalent group had 52% fewer days of HSV-2 DNA shedding, with a similar percent reduction after the third immunization.
Figure 4.
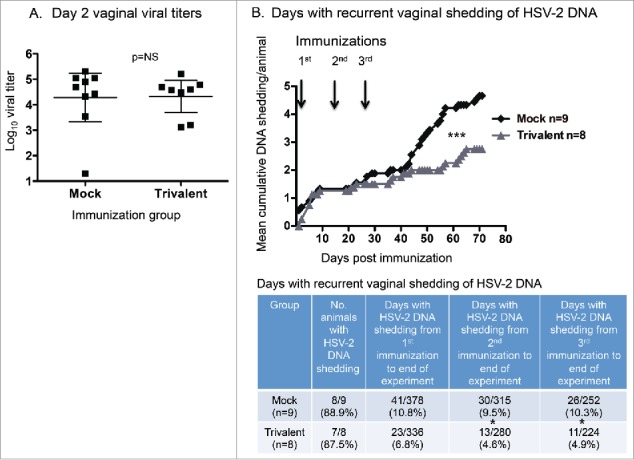
Trivalent vaccine reduces vaginal shedding of HSV-2 DNA. (A) Viral titers on vaginal swab cultures obtained 2 d post-infection. Bars represent geometric mean titers with 95% confidence intervals. The Mann-Whitney test for nonparametric data was used to calculate the p value (p = 0.51). Bars represent geometric mean titers with 95% confidence intervals. (B) Mean cumulative days of DNA shedding per guinea pig. Arrows indicate immunization days. The p value was calculated using Prism software by 2-way ANOVA. The table indicates the total days of DNA shedding per group after the first, second, or third immunization until the end of the experiment. The p values were calculated using the Fisher's exact test. The p value is 0.067 comparing the mock and trivalent groups from 1st immunization to end of experiment. NS indicates not significant; * p < .05, *** p < .001.
Cultures were performed on vaginal swabs that were positive for HSV-2 DNA (> 150 copies) after the third immunization. Only DNA positive samples were cultured based on our prior report that all 636 samples that were negative for HSV-2 DNA were also negative for replication-competent virus.21 Five of 26 swabs in the mock group grew virus compared with 1/11 in the trivalent group (Fig. 5A, B). Although this difference was not statistically significant (p = 0.22), it represents a 77% reduction in the trivalent group based on the assumption that negative swabs for HSV-2 DNA were also negative for virus. The mean log10 titer of HSV-2 DNA detected in genital swab samples in the trivalent group was 4.6-fold lower than in the mock group (Fig. 5C). The mean log10 virus titer in the 5 samples that were positive for replication-competent virus in the mock group was 2.3 log10 compared with 1 log10 in the single positive sample in the trivalent group (Fig. 5D).
Figure 5.
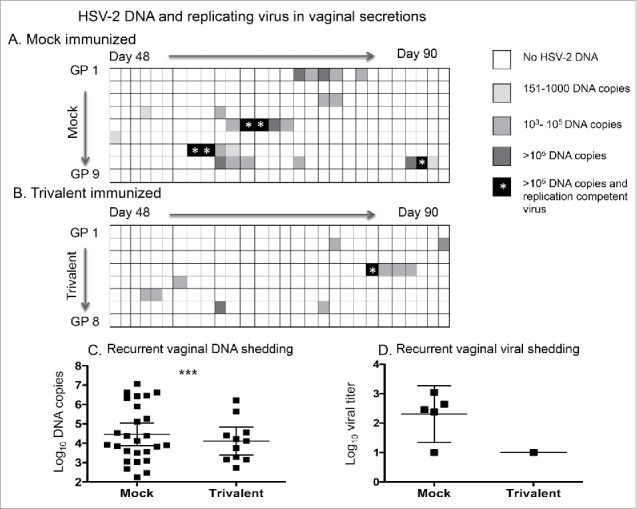
The trivalent vaccine reduces the days of HSV-2 DNA shedding and isolation of replication-competent virus in the vaginal secretions. The heat map indicates the HSV-2 DNA copy number in vaginal secretions of (A) mock-immunized, and (B) trivalent-immunized animals after the third immunization. Asterisk indicates the days that replication-competent virus was isolated from vaginal secretions. Comparing days of HSV-2 DNA shedding in mock and trivalent groups: p < .05 as calculated by Poisson regression to take into consideration that some animals shed HSV-2 DNA on multiple days while others shed on one day or not at all. (C) DNA copy number in vaginal swab samples that were positive for HSV-2 DNA. The p value comparing mock and trivalent groups was calculated using a longitudinal Poisson model to take into consideration multiple days of shedding by some animals. *** p < .001. (D) Titer of replication-competent virus isolated from vaginal swabs. Bars in (C) and (D) represent geometric mean titers with 95% confidence intervals.
Discussion
The trivalent vaccine reduced the number of days with recurrent genital lesions and vaginal shedding of HSV-2 DNA by approximately 50% from the second immunization to the end of the experiment. Therapeutic immunization with the trivalent vaccine also reduced the number of days that vaginal secretions contained replication-competent virus, thereby possibly decreasing the risk of transmission. The vaccine boosted ELISA and neutralizing antibody titers in previously infected animals. The boost in antibody immunity may explain the reduction in days with genital lesions and genital shedding of HSV-2 DNA, although the immune correlates of this protection remain unknown.
The trivalent vaccine affected lesion scores after the first immunization, yet the impact on HSV-2 DNA shedding did not appear until after the second immunization. Differences in lesion days between the mock and trivalent groups decreased, rather than increased after the third immunization. We have noted that the number of days with recurrent lesions in mock-immunized guinea pigs declines over time, which is similar to the course of natural infection in humans.24 Therefore, a larger sample size may be required to detect significant differences in lesions at these later times. Overall, the trivalent vaccine achieved the goals of a therapeutic vaccine, which were to reduce the frequency of recurrent genital lesions and the risk of transmission to intimate partners during episodes of subclinical infection.
In subjects with frequent episodes of recurrent genital herpes, valacyclovir reduces the frequency of genital shedding of HSV-2 DNA by 73%,13 while valacyclovir or acyclovir reduces the frequency of genital lesions by approximately 80%.13, 25 Therefore, daily suppressive antiviral therapy achieves some of the objectives established for a therapeutic vaccine. A possible advantage of a therapeutic vaccine over antiviral therapy is that immunizations are periodic while antiviral therapy must be taken daily to be effective.25 Other considerations that may favor vaccination over antiviral therapy for long-term treatment include that the cost of immunization will likely be lower than daily antiviral therapy, a higher likelihood of adherence to therapy, and the possibility that immunized individuals may have fewer days that they shed replication-competent virus than subjects treated with antiviral therapy. Ideally, a therapeutic vaccine will outperform antiviral therapy in all parameters evaluated; however, the considerations above suggest that even a less impressive response may be well received.
The HSV-2 MS strain used in our laboratory has a very narrow range between the Infectious Dose 50 (ID50) and Lethal Dose 50 (LD50). Our results indicate that the ID50 is approximately 1 × 104 PFU and the LD50 is approximately 2 × 104 PFU. This narrow range between ID50 and LD50 makes therapeutic vaccine trials difficult to perform using this HSV-2 strain. One option for future studies involves adjusting the timing of acyclovir treatment. Administering acyclovir on day 1 was too early to permit a robust infection, while day 3, 4 or 5 was too late to prevent death. Therefore, one consideration is to administer acyclovir on day 2 post-infection to strike a better balance between survival and disease severity. A second option is to infect with a different HSV-2 strain. The Bernstein laboratory uses an HSV-2 MS strain that causes fewer deaths while still producing acute and recurrent genital lesions, although at a reduced frequency compared with the MS strain used in the current study.21, 26 The isolate used by the Bernstein laboratory has the advantage of causing lower mortality, which is beneficial for infection of naïve animals in therapeutic vaccine trials, while the strain used in our laboratory provides a more virulent challenge, which is useful for prophylactic vaccine trials.
Human trials are in progress for prophylactic and therapeutic vaccines that use a replication-defective live virus for prevention or treatment, and subunit antigens or peptide antigens for treatment.27-29 An effective prophylactic vaccine will spare individuals the pain and suffering associated with genital herpes,5 and will have a positive impact on the HIV pandemic by eliminating genital herpes as a co-factor.12, 30-34 Obstacles faced include the disappointing performance of animal models in predicting success of vaccine candidates in human trials, and the large number of subjects required to demonstrate vaccine efficacy.35-37 In contrast, as few as 150 subjects with frequent recurrent infections may be required to prove efficacy of a therapeutic vaccine for reducing genital lesions or subclinical shedding of HSV-2 DNA.29 A challenge in developing an effective therapeutic vaccine is that vaccine-induced immunity must be more potent than natural infection, since natural infection fails to prevent recurrences. The recent success in human trials of a subunit vaccine for shingles has renewed optimism that a therapeutic vaccine for genital herpes is possible.38, 39
No consensus exists on the appropriate primary end point for a therapeutic genital herpes vaccine. Some researchers favor establishing reduction in genital lesions as the primary end point, while others prefer to focus on subclinical shedding of HSV-2 DNA, which serves as a surrogate marker for risk of transmission.40 In the current study, we noted a similar impact of the therapeutic vaccine on genital lesions and genital shedding of HSV-2 DNA. The percentage of days that animals in the trivalent group shed HSV-2 DNA was significantly lower than the mock group, but perhaps even more important was the observation that on days that the trivalent animals shed HSV-2 DNA, the chances were much reduced that the secretions contained replication-competent virus. Genital shedding of replication-competent virus may be a more relevant biomarker for risk of transmission than shedding of HSV-2 DNA because the HSV-2 DNA may be fragmented, defective, or in virions coated with antibody rendering the virus non-infectious. Subjects enrolled in clinical trials can be instructed to obtain genital swab samples that are stored in a home refrigerator or freezer until delivered on an ice pack to the clinic and are later processed for HSV DNA copy number and virus culture.41, 42
As noted in Table 2, animals in experiment #1 were infected using 3 different protocols and immunized with 2 different gD2 antigen concentrations in the trivalent vaccine, while animals in experiment #2 were immunized at different times post-infection than animals in experiment #1. All animals in experiments #1 and #2 were randomized after infection to receive either the mock or trivalent vaccine; therefore, the randomization helps offset any confounding created by the protocol design. To confirm our current findings, a follow up study is in progress using each antigen in the trivalent vaccine at a single concentration of 10 μg administered every 2 weeks beginning on day 15 post-infection. This study will use the HSV-2 MS strain from the Bernstein laboratory in an effort to reduce mortality and avoid the need to administer acyclovir, and will be performed by the independent NIH contract laboratory. Intramuscular immunization will be combined with local application of chemoattractants to increase CD8+ T cell responses in the genital tissues, which may further improve vaccine efficacy.43 A therapeutic vaccine that reduces genital lesions and days that subjects shed replication-competent virus has potential for impacting both the HSV-2 and HIV epidemics.
Materials and methods
Guinea pigs ethics statement
The Institutional Animal Care and Use Committee of the University of Pennsylvania approved the protocol (No. 805187) for use of guinea pigs. The protocol adhered to the recommendations in the Institute for Laboratory Animals Research's “Guide for the Care and Use of Laboratory Animals.” Guinea pigs appearing dehydrated (> 10% weight loss) received subcutaneous saline. Meloxicam was used for pain control once genital lesions developed. Euthasol was used to euthanize animals according to the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association.
Intravaginal infection of guinea pigs, scoring for genital lesions, and randomization procedure
Female Hartley strain guinea pigs (Charles River Laboratories) were used that weighed 350 to 400 g at the time of infection. Guinea pigs were bled from a hind limb saphenous vein at select times throughout the study. We performed 2 experiments and used HSV-2 strain MS for both.23 In experiment #1, 36 animals were divided into 3 groups of 12. The first group was infected with 1 × 104 PFU of HSV-2; the second group was infected with the same dose but was treated twice daily intraperitoneally with 50 mg/kg acyclovir starting on day 1 and continued until animals either recovered or succumbed to the acute infection; and the third group was infected with 50-fold higher titers of HSV-2 at 5 × 105 PFU and treated with acyclovir as in group 2. In experiment #2, 72 animals were infected intravaginally with 2 × 104 PFU of HSV-2 and treated with acyclovir 50 mg/kg intraperitoneally once daily starting on day 6 post-infection and continued until animals either succumbed or recovered from the acute infection (Table 1). Animals in experiment #1 were only followed for recurrent genital lesions while animals in experiment #2 were evaluated for ELISA and neutralizing antibody responses to immunization, day 2 vaginal titers after infection, recurrent genital lesions, recurrent genital shedding of HSV-2 DNA and recurrent vaginal shedding of replication competent virus.
Two investigators evaluated the guinea pigs for genital lesions. Animals were followed for genital disease throughout the experiment and scored on a scale of 0 to 4 as follows: 0 reflects no lesions, 1 represents one lesion, 2 reflects 2 or more separate lesions, 3 represents coalesced lesions, and 4 reflects ulcerated lesions. The total genital disease score is the sum of the daily scores. The daily scores assigned to each animal were based on consensus of the 2 investigators.
Randomization was performed before the first immunization. Animals were ranked based on total disease scores up to the time of randomization and placed into cohorts of 3 animals per group in experiment #1, with one animal assigned to receive mock immunization, a second to receive the trivalent vaccine containing 10 μg of each antigen, and a third to receive the trivalent vaccine with 10 μg of gC2, 10 μg gE2 and 15 μg gD2. All animals in experiment #1 had genital lesions before randomization. In experiment #2, animals were ranked based on total disease scores with 2 animals in each cohort. One animal was assigned to receive mock immunization and the other the trivalent vaccine containing 10 μg of each antigen. Within each cohort, the animal with the higher total disease score was randomly assigned to receive either the mock or trivalent vaccine. All animals in experiment #2 had genital lesions before randomization except one animal each in the mock and trivalent group.
Mock-immunized animals received CpG oligonucleotide (5′-TCGTCGTTGTCGTTTTGTCGTT-3′) (Trilink Inc.) containing 100 μg CpG/guinea pig combined with 80 μg alum (Alhydrogel, Accurate Chemical and Scientific Corp.). The trivalent group received 10 μg each of gC2, gD2 and gE2, or 10 μg gC2, 10 μg gE2 and 15 μg gD2, mixed with100 μg CpG/guinea pig and 240 μg alum in a total volume of 50 μl per immunization.23 The antigens were purified from the supernatant fluids of baculovirus infected sf9 cells and consist of the extracellular domains of gC2 (amino acids 27–426), gD2 (amino acids 26–331) and gE2 (amino acids 24–405).19, 23, 44 Individual antigens for the trivalent vaccine were mixed with CpG and alum and combined immediately before immunization. Immunizations were initiated on day 35 post-infection in experiment #1 and on day 19 in experiment #2 (Table 2). After animals were immunized, the investigators were blinded as to vaccination status for both experiments.
In experiment #2, vaginal swab samples were obtained for virus culture on day 2 and for qPCR on 714 samples collected on 7 d between days 20 and 28 after the first immunization, 7 d between days 38 and 46 after the second immunization, and 28 d between days 48 and 90 after the third immunization. Vaginal cultures were performed on samples that were positive for HSV-2 DNA by qPCR taken after the third immunization. The swabs were stored at -80°C before culture or processing for HSV-2 DNA by qPCR.
ELISA and neutralizing antibodies
ELISA plates were coated with 50 ng of gC2, gD2 or gE2 subunit antigen and incubated with guinea pig serum at a 1:1000 dilution, followed by HRP-conjugated anti-guinea pig IgG.45 Neutralizing antibody titers were determined by incubating 100 PFU of HSV-2 strain MS with serial dilutions of serum starting at 1:20 for 1 h at 37°C. For some experiments, 10% human serum from an HSV-1/HSV-2 seronegative donor was added as a source of complement to the virus-antibody mix.23 The end point neutralization titer was determined by plaque assay on Vero cells and was calculated as the serum dilution that reduced the number of plaques by 50% compared with PBS controls.23
Real-time qPCR to detect HSV-2 DNA shedding
DNeasy blood and tissue kits (Qiagen) were used to isolate DNA from 200 μl of guinea pig vaginal swab material.23 The HSV-2 DNA copy number was based on a standard curve that was generated with 50,000, 5,000, 500, 50, and 5 copies of purified HSV-2 DNA (Advanced Biotechnologies) and run in triplicate. The guinea pig samples were analyzed in duplicate. Samples were reported as negative if they contained <150 copies/ml by 40 cycles or were positive in only one of 2 wells. Primer and probe sequences for HSV-2 Us9 were: primer forward, 5′-GGCAGAAGCCTACTACTCGGAAA-3′, and reverse 5′-CCATGCGCACGAGGAAGT-3′, and probe with reporter dye 5′-FAM-CGAGGCCGCCAAC-MGBNFQ-3′ (FAM, 6-carboxyfluorescein). Assays were performed using TaqMan gene expression master mix (Applied Biosystems) and analyzed on an ABI 7500 Fast machine.
Virus cultures from vaginal swabs
Vaginal swabs were collected in one ml Dulbecco's modified Eagle's medium (DMEM) with 5% fetal bovine serum and 25 μg/ml of vancomycin.23 Cultures were performed by adding 150 μl to Vero cell monolayers in 24-well plates for 1 h at 37°C. Cells were overlaid with 0.5% methylcellulose in complete DMEM containing vancomycin, and plaques were counted 72 hours later. The limit of detection was 6.7 PFU/ml.
Disclosure of potential conflicts of interest
The authors have no conflict of interests to disclose.
Acknowledgments
Drs. Gary Cohen and Roselyn Eisenberg provided the gC2, gD2 and gE2 immunogens used in these studies. Sarah Ratcliffe provided statistical support through the Penn Center for AIDS Research Biostatistical Core that is supported by NIH NIAID grant P30 AI45008.
Funding
This work was supported by the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID) under grants R21AI105959 (Awasthi and Friedman co-PI) and RO1AI104854 (Friedman PI).
References
- [1].Looker KJ, Magaret AS, Turner KM, Vickerman P, Gottlieb SL, Newman LM. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS One 2015; 10:e114989; PMID:25608026; https://doi.org/ 10.1371/journal.pone.0140765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wald A. Genital HSV-1 infections. Sex Transm Infect 2006; 82:189-90; PMID:16731663; https://doi.org/ 10.1136/sti.2006.019935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Benedetti J, Corey L, Ashley R. Recurrence rates in genital herpes after symptomatic first-episode infection. Annals of internal medicine 1994; 121:847-54; PMID:7978697; https://doi.org/ 10.7326/0003-4819-121-11-199412010-00004 [DOI] [PubMed] [Google Scholar]
- [4].Gupta R, Warren T, Wald A. Genital herpes. Lancet 2007; 370:2127-37; PMID:18156035; https://doi.org/ 10.1016/S0140-6736(07)61908-4 [DOI] [PubMed] [Google Scholar]
- [5].Corey L, Adams HG, Brown ZA, Holmes KK. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med 1983; 98:958-72; PMID:6344712; https://doi.org/ 10.7326/0003-4819-98-6-958 [DOI] [PubMed] [Google Scholar]
- [6].Tronstein E, Johnston C, Huang ML, Selke S, Magaret A, Warren T, Corey L, Wald A. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. Jama 2011; 305:1441-9; PMID:21486977; https://doi.org/ 10.1001/jama.2011.420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tedder DG, Ashley R, Tyler KL, Levin MJ. Herpes simplex virus infection as a cause of benign recurrent lymphocytic meningitis. Ann Intern Med 1994; 121:334-8; PMID:8042822; https://doi.org/ 10.7326/0003-4819-121-5-199409010-00004 [DOI] [PubMed] [Google Scholar]
- [8].Brown ZA, Selke S, Zeh J, Kopelman J, Maslow A, Ashley RL, Watts DH, Berry S, Herd M, Corey L. The acquisition of herpes simplex virus during pregnancy. N Engl J Med 1997; 337:509-15; PMID:9262493; https://doi.org/ 10.1056/NEJM199712253372618 [DOI] [PubMed] [Google Scholar]
- [9].Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr 2004; 35:435-45; PMID:15021308; https://doi.org/ 10.1097/00126334-200404150-00001 [DOI] [PubMed] [Google Scholar]
- [10].Martin ET, Krantz E, Gottlieb SL, Magaret AS, Langenberg A, Stanberry L, Kamb M, Wald A. A pooled analysis of the effect of condoms in preventing HSV-2 acquisition. Archives of internal medicine 2009; 169:1233-40; PMID:19597073; https://doi.org/ 10.1001/archinternmed.2008.545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tobian AA, Serwadda D, Quinn TC, Kigozi G, Gravitt PE, Laeyendecker O, Charvat B, Ssempijja V, Riedesel M, Oliver AE, et al.. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med 2009; 360:1298-309; PMID:19321868; https://doi.org/ 10.1056/NEJMoa0802556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Freeman EE, White RG, Bakker R, Orroth KK, Weiss HA, Buve A, Hayes RJ, Glynn JR. Population-level effect of potential HSV2 prophylactic vaccines on HIV incidence in sub-Saharan Africa. Vaccine 2009; 27:940-6; PMID:19071187; https://doi.org/ 10.1016/j.vaccine.2008.11.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Corey L, Wald A, Patel R, Sacks SL, Tyring SK, Warren T, Douglas JM Jr, Paavonen J, Morrow RA, Beutner KR, et al.. Once-daily valacyclovir to reduce the risk of transmission of genital herpes.[see comment]. New England Journal of Medicine 2004; 350:11-20; PMID:14702423; https://doi.org/ 10.1056/ENEJMicm020035 [DOI] [PubMed] [Google Scholar]
- [14].Reitano M, Tyring S, Lang W, Thoming C, Worm AM, Borelli S, Chambers LO, Robinson JM, Corey L. Valaciclovir for the suppression of recurrent genital herpes simplex virus infection: a large-scale dose range-finding study. International Valaciclovir HSV Study Group. J Infect Dis 1998; 178:603-10. [DOI] [PubMed] [Google Scholar]
- [15].Skoberne M, Cardin R, Lee A, Kazimirova A, Zielinski V, Garvie D, Lundberg A, Larson S, Bravo FJ, Bernstein DI, et al.. An adjuvanted herpes simplex virus 2 subunit vaccine elicits a T cell response in mice and is an effective therapeutic vaccine in Guinea pigs. J Virol 2013; 87:3930-42; PMID:23365421; https://doi.org/ 10.1128/JVI.02745-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yim KC, Carroll CJ, Tuyama A, Cheshenko N, Carlucci MJ, Porter DD, Prince GA, Herold BC. The cotton rat provides a novel model to study genital herpes infection and to evaluate preventive strategies. J Virol 2005; 79:14632-9; PMID:16282463; https://doi.org/ 10.1128/JVI.79.23.14632-14639.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Boukhvalova M, McKay J, Mbaye A, Sanford-Crane H, Blanco JC, Huber A, Herold BC. Efficacy of the Herpes Simplex Virus 2 (HSV-2) Glycoprotein D/AS04 Vaccine against Genital HSV-2 and HSV-1 Infection and Disease in the Cotton Rat Sigmodon hispidus Model. J Virol 2015; 89:9825-40; PMID:26178984; https://doi.org/ 10.1128/JVI.01387-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Stanberry LR, Kern ER, Richards JT, Abbott TM, Overall JC Jr.. Genital herpes in guinea pigs: pathogenesis of the primary infection and description of recurrent disease. J Infect Dis 1982; 146:397-404; PMID:6286797; https://doi.org/ 10.1093/infdis/146.3.397 [DOI] [PubMed] [Google Scholar]
- [19].Awasthi S, Huang J, Shaw C, Friedman HM. Blocking herpes simplex virus 2 glycoprotein E immune evasion as an approach to enhance efficacy of a trivalent subunit antigen vaccine for genital herpes. J Virol 2014; 88:8421-32; PMID:24829358; https://doi.org/ 10.1128/JVI.03163-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Feldman LT, Ellison AR, Voytek CC, Yang L, Krause P, Margolis TP. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc Natl Acad Sci U S A 2002; 99:978-83; PMID:11773630; https://doi.org/ 10.1073/pnas.022301899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Awasthi S, Hook LM, Shaw CE, Pahar B, Stagray JA, Liu D, Veazey RS, Friedman HM. An HSV-2 Trivalent Vaccine Is Immunogenic in Rhesus Macaques and Highly Efficacious in Guinea Pigs. PLoS Pathog 2017; 13:e1006141; PMID:28103319; https://doi.org/ 10.1371/journal.ppat.1006141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Eisenberg RJ, Atanasiu D, Cairns TM, Gallagher JR, Krummenacher C, Cohen GH. Herpes virus fusion and entry: a story with many characters. Viruses 2012; 4:800-32; PMID:22754650; https://doi.org/ 10.3390/v4050800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Awasthi S, Lubinski JM, Shaw CE, Barrett SM, Cai M, Wang F, Betts M, Kingsley S, Distefano DJ, Balliet JW, et al.. Immunization with a Vaccine Combining Herpes Simplex Virus 2 (HSV-2) Glycoprotein C (gC) and gD Subunits Improves the Protection of Dorsal Root Ganglia in Mice and Reduces the Frequency of Recurrent Vaginal Shedding of HSV-2 DNA in Guinea Pigs Compared to Immunization with gD Alone. J Virol 2011; 85:10472-86; PMID:21813597; https://doi.org/ 10.1128/JVI.00849-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Phipps W, Saracino M, Magaret A, Selke S, Remington M, Huang ML, Warren T, Casper C, Corey L. Persistent genital herpes simplex virus-2 shedding years following the first clinical episode. J Infect Dis 2011; 203:180-7; PMID:21288817; https://doi.org/ 10.1093/infdis/jiq035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Douglas JM, Critchlow C, Benedetti J, Mertz GJ, Connor JD, Hintz MA, Fahnlander A, Remington M, Winter C, Corey L. A double-blind study of oral acyclovir for suppression of recurrences of genital herpes simplex virus infection. N Engl J Med 1984; 310:1551-6; PMID:6328298; https://doi.org/ 10.1056/NEJM198406143102402 [DOI] [PubMed] [Google Scholar]
- [26].Bernstein DI, Farley N, Bravo FJ, Earwood J, McNeal M, Fairman J, Cardin R. The adjuvant CLDC increases protection of a herpes simplex type 2 glycoprotein D vaccine in guinea pigs. Vaccine 2010; 28:3748-53; PMID:19857450; https://doi.org/ 10.1016/j.vaccine.2009.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cohen JI. Vaccination to reduce reactivation of herpes simplex virus 2. J Infect Dis 2017; 215:844-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Awasthi S, Friedman HM. Status of prophylactic and therapeutic genital herpes vaccines. Curr Opin Virol 2014; 6:6-12; PMID:24631871; https://doi.org/ 10.1016/j.coviro.2014.02.006 [DOI] [PubMed] [Google Scholar]
- [29].Bernstein DI, Wald A, Warren T, Fife K, Tyring S, Lee P, Van Wagoner N, Magaret A, Flechtner JB, Tasker S, et al.. Therapeutic vaccine for genital herpes simplex virus-2 infection: Findings from a randomized trial. J Infect Dis 2017; PMID:28329211; https://doi.org/ 10.1093/infdis/jix004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis 2002; 185:45-52; PMID:11756980; https://doi.org/ 10.1086/338231 [DOI] [PubMed] [Google Scholar]
- [31].Celum C, Wald A, Hughes J, Sanchez J, Reid S, Delany-Moretlwe S, Cowan F, Casapia M, Ortiz A, Fuchs J, et al.. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet 2008; 371:2109-19; PMID:18572080; https://doi.org/ 10.1016/S0140-6736(08)60920-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Celum C, Wald A, Lingappa JR, Magaret AS, Wang RS, Mugo N, Mujugira A, Baeten JM, Mullins JI, Hughes JP, et al.. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med 2010; 362:427-39; PMID:20089951; https://doi.org/ 10.1056/NEJMoa0904849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Freeman EE, Orroth KK, White RG, Glynn JR, Bakker R, Boily MC, Habbema D, Buvé A, Hayes R. Proportion of new HIV infections attributable to herpes simplex 2 increases over time: simulations of the changing role of sexually transmitted infections in sub-Saharan African HIV epidemics. Sex Transm Infect 2007; 83 Suppl 1:i17-24; PMID:17405782; https://doi.org/ 10.1136/sti.2006.023549 [DOI] [PubMed] [Google Scholar]
- [34].Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. Aids 2006; 20:73-83; PMID:16327322; https://doi.org/ 10.1097/01.aids.0000198081.09337.a7 [DOI] [PubMed] [Google Scholar]
- [35].Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, Tyring S, Aoki FY, Slaoui M, Denis M, et al.. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med 2002; 347:1652-61; PMID:12444179; https://doi.org/ 10.1056/NEJMoa011915 [DOI] [PubMed] [Google Scholar]
- [36].Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RL, Ewell MG, Stokes-Riner A, et al.. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med 2012; 366:34-43; PMID:22216840; https://doi.org/ 10.1056/NEJMicm1104023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Corey L, Langenberg AG, Ashley R, Sekulovich RE, Izu AE, Douglas JM Jr., Handsfield HH, Warren T, Marr L, Tyring S, et al.. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. Chiron HSV Vaccine Study Group. Jama 1999; 282:331-40. [DOI] [PubMed] [Google Scholar]
- [38].Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang SJ, Diez-Domingo J, Godeaux O, Levin MJ, McElhaney JE, Puig-Barberà J, et al.. Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N Engl J Med 2016; 375:1019-32; PMID:27626517; https://doi.org/ 10.1056/NEJMoa1603800 [DOI] [PubMed] [Google Scholar]
- [39].Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, Levin MJ, McElhaney JE, Poder A, Puig-Barberà J, et al.. Efficacy of an Adjuvanted Herpes Zoster Subunit Vaccine in Older Adults. N Engl J Med 2015; 372(22):2087-96; https://doi.org/ 10.1056/NEJMoa1501184 [DOI] [PubMed] [Google Scholar]
- [40].Schiffer JT, Mayer BT, Fong Y, Swan DA, Wald A. Herpes simplex virus-2 transmission probability estimates based on quantity of viral shedding. J R Soc Interface 2014; 11:20140160; https://doi.org/ 10.1098/rsif.2014.0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wald A, Huang ML, Carrell D, Selke S, Corey L. Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis 2003; 188:1345-51; PMID:14593592; https://doi.org/ 10.1086/379043 [DOI] [PubMed] [Google Scholar]
- [42].Wald A, Zeh J, Selke S, Ashley RL, Corey L. Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med 1995; 333:770-5; PMID:7643884; https://doi.org/ 10.1056/NEJM199509213331205 [DOI] [PubMed] [Google Scholar]
- [43].Iwasaki A. Exploiting Mucosal Immunity for Antiviral Vaccines. Annu Rev Immunol 2016; 34:575-608; PMID:27168245; https://doi.org/ 10.1146/annurev-immunol-032414-112315 [DOI] [PubMed] [Google Scholar]
- [44].Awasthi S, Mahairas GG, Shaw CE, Huang ML, Koelle DM, Posavad C, Corey L, Friedman HM. A Dual-Modality Herpes Simplex Virus 2 Vaccine for Preventing Genital Herpes by Using Glycoprotein C and D Subunit Antigens To Induce Potent Antibody Responses and Adenovirus Vectors Containing Capsid and Tegument Proteins as T Cell Immunogens. J Virol 2015; 89:8497-509; PMID:26041292; https://doi.org/ 10.1128/JVI.01089-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Awasthi S, Balliet JW, Flynn JA, Lubinski JM, Shaw CE, DiStefano DJ, Cai M, Brown M, Smith JF, Kowalski R, et al.. Protection provided by a herpes simplex virus 2 (HSV-2) glycoprotein C and D subunit antigen vaccine against genital HSV-2 infection in HSV-1-seropositive guinea pigs. J Virol 2014; 88:2000-10; PMID:24284325; https://doi.org/ 10.1128/JVI.03163-13 [DOI] [PMC free article] [PubMed] [Google Scholar]


