Abstract
Background
Daily aspirin use has been recommended for secondary prevention of cardiovascular disease, but its use for primary prevention remains controversial.
Methods
We followed 440,277 men and women from the NIH–AARP Diet and Health Study (ages 50-71) and the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (ages 55-74) for mortality for 13 years on average. Frequency of aspirin use was ascertained through self-report, and cause of death by death certificates. We calculated multivariate hazard ratios (HRs) and 95% confidence intervals (CI) for mortality using Cox proportional hazards models for each cohort and combined by meta-analysis.
Results
We found a consistent U-shaped relationship between aspirin use and mortality in both studies, with differential risk patterns for cardiovascular mortality by disease history. Among individuals with a history of cardiovascular disease, daily aspirin use was associated with reduced cardiovascular mortality [HR=0.78 (95% CI 0.74-0.82)]. However, among those without a previous history, we observed no protection for daily aspirin users [HR=1.06 (1.02-1.11)], and elevated risk of cardiovascular mortality for those taking aspirin twice daily or more [HR=1.29 (1.19-1.39)]. Elevated risk persisted even among participants who lived beyond 5 years of follow-up and used aspirin without other nonsteroidal anti-inflammatory drugs [HR=1.31 (1.17-1.47)].
Conclusions
Results from these two large population-based U.S. cohorts confirm the utility of daily aspirin use for secondary prevention of cardiovascular mortality; however, our data suggest that caution should be exercised in more frequent use, particularly among individuals without a history of cardiovascular disease.
Keywords: Aspirin, All-cause mortality, Cardiovascular disease mortality, Cancer mortality, prospective cohort, NIH-AARP, PLCO
Introduction
Millions of individuals worldwide take aspirin with approximately 40% of the U.S. population over age 40 consuming aspirin regularly.1, 2 In randomized controlled trials, daily use of aspirin has been demonstrated to reduce cardiovascular disease incidence and mortality and all-cause mortality among individuals with established cardiovascular disease;3, 4 however, prophylactic use of aspirin for individuals without a history of cardiovascular disease remains controversial.5 Whereas the American College of Chest Physicians6 and the U.S. Preventive Services Task Force (USPSTF)7 support the use of aspirin for primary prevention among select groups, the European Society of Cardiology8 and the U.S. Food and Drug Administration (FDA)9 recommend against the use of aspirin for primary prevention of cardiovascular disease, largely due to a lack of mortality benefit and increased risk of major bleeding. Randomized trial data show that daily use of aspirin is associated with a small reduction in cardiovascular incidence but no substantial reduction in cardiovascular mortality or all-cause mortality,10-13 which may be partially due to an increased risk of fatal hemorrhagic stroke.10 The risks and benefits of aspirin for cancer prevention and cancer mortality are also unclear. Although a reduction in risk has been reported for colorectal cancer incidence,14 the evidence supporting the benefit of aspirin therapy for overall cancer incidence and mortality based on randomized trials is inconsistent.12, 13, 15-17
In making public health recommendations, experts have been divided between the potential benefit of aspirin for cardiovascular and colorectal cancer incidence and the potential harms, such as clinically important bleeding in the brain and stomach.18, 19 Risk–benefit evaluations across major causes of death in randomized trials as well as large-scale population-based cohort studies, which can provide evidence across a broader range of use, are important in guiding decisions about aspirin therapy for patient subgroups and for policy decisions.
To date, randomized trials of aspirin have largely focused on daily or every-other-day use. Much less is understood regarding the risks and benefits of aspirin use more than once daily, which is of considerable public health importance, given its extensive use and reported overutilization in many western societies.20-22 We evaluated the association between aspirin use and the risk of all-cause, cardiovascular, and cancer mortality across a range of frequencies in two large contemporary prospective cohorts in the U.S.
Methods
Study Populations
The National Institutes of Health (NIH) AARP Diet and Health Study is a large cohort study initiated between 1995 and 1996.23 A baseline questionnaire regarding demographic and lifestyle characteristics was mailed to 3.5 million members of AARP (formerly known as the American Association of Retired Persons) ages 50-71 years who resided in one of six states (California, Florida, Pennsylvania, New Jersey, North Carolina, and Louisiana) or two metropolitan areas (Atlanta, Georgia; and Detroit, Michigan). In total, 617,119 individuals returned the baseline questionnaire. A second questionnaire was mailed ∼6 months thereafter to collect additional information on risk factors, including aspirin use, and was returned by 334,908 individuals. This study was limited to the participants who completed both questionnaires themselves (n=324,522). Persons who reported poor health, a prior diagnosis of cancer, or no information on aspirin use were excluded, resulting in 297,564 participants (173,822 men, 123,742 women) for analysis. The study was approved by the Institutional Review Board of the U.S. National Cancer Institute (NCI).
The Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial is a multicenter randomized trial designed to evaluate the effectiveness of prostate, lung, colorectal, and ovarian cancer screening for disease-specific mortality.24, 25 The trial enrolled 154,952 subjects ages 55-74 years at ten screening centers in the U.S. between 1993 and 2001. Information on demographic and lifestyle characteristics, including aspirin use, was obtained through a self-administered questionnaire at baseline. This study included participants who were randomized to either arm of the trial and completed the baseline questionnaire (n=149,980). Individuals who reported a prior diagnosis of cancer or no information on aspirin use were excluded, leaving 142,713 individuals (72,017 men, 70,696 women) for analysis. The study was approval by the Institutional Review Boards at all ten study centers and the NCI.
Assessment of Aspirin Use
We ascertained frequency of aspirin use by self-report at baseline (PLCO: https://biometry.nci.nih.gov/cdas/learn/plco/early-qx/) or shortly thereafter (NIH–AARP: https://dietandhealth.cancer.gov/docs/diet_questionnaire_risk.pdf), using similar questions. Although aspirin use was also ascertained in follow-up questionnaires in both NIH–AARP and PLCO, information on the frequency of aspirin use was limited in the follow-up questionnaires and so was not incorporated into the analysis; however, overall, aspirin use was highly correlated over time in both cohorts (e.g., 80% and 77% of the NIH–AARP and PLCO participants, respectively, who were regular aspirin users at baseline reported regular aspirin use in a follow-up questionnaire administered 6-14 years later).
In both baseline questionnaires, participants were asked first about their use of aspirin or aspirin containing products in the past 12 months and then about their frequency of use: no use (NIH–AARP) or no regular use (PLCO), infrequent use (1-3 times or pills per month), weekly use (1-6 times or pills per week), once daily use (1 time or pill per day), or more than once daily use (2 or more times or pills per day). Information on dose, duration, and indication for use was not collected in either study, although based on reports by a subset of women in the Nurses' Health Study, major reasons for taking 1-6 tablets or≥7 tablets per week included headache (32% and 18%, respectively), arthritis or other musculoskeletal pain (30% and 50%, respectively), combined headache and musculoskeletal pain (16% and 15%, respectively), and cardiovascular disease prevention (9% and 8%, respectively).26 In both PLCO and NIH–AARP, use of other nonsteroidal anti-inflammatory drugs (NSAIDs) was also assessed, including use of ibuprofen in PLCO and a list of 19 non-aspirin NSAIDs in NIH–AARP. In addition, participants were specifically instructed not to report acetaminophen use in both studies.
Assessment of Disease History
Information on personal medical history was obtained through self-report. In NIH–AARP, personal history of heart disease, stroke, hypertension, diabetes, high cholesterol level, and other comorbidities such as osteoporosis, emphysema, and bone fracture after age 45, was ascertained through baseline questionnaires administered at or shortly after enrollment. History of osteoarthritis prior to 1995 (enrollment) was ascertained through a follow-up questionnaire in 2004 that was completed by 67% of participants. In PLCO, all information on personal history of coronary heart disease/heart attack, stroke, hypertension, diabetes, arthritis and other conditions such as osteoporosis, inflammatory bowel disease, diverticulitis/diverticulosis, emphysema, hepatitis, and cirrhosis, was obtained through the baseline questionnaire. The validity of self-reported data has been shown to be generally high for history of cardiovascular disease, diabetes, and other diseases (e.g., 68%-92% agreement with medical records),27-30 albeit slightly lower for hypertension (e.g., 60% agreement with medical records).31
Assessment of Death Outcomes
We ascertained death information through linkage to the National Death Index in NIH–AARP, and a mailed Annual Study Update questionnaire followed by death certificate request and periodic linkage to the National Death Index in PLCO. Studies have demonstrated relatively high sensitivity (81%-91%) and low false-positive rate (14%-28%) for cardiovascular deaths from death certificates, compared to physician review of medical records, autopsy reports (if available), and other relevant information.32, 33 Specific causes of death were classified by the International Classification of Diseases, Ninth Revision (ICD-9) in PLCO, and ICD-9 and International Classification of Diseases, 10th Revision (ICD-10) codes in NIH–AARP as follows: cancer (ICD-9 140-239; ICD-10 C00-C97, D00-D48), cardiovascular disease, including acute myocardial infarction (ICD-9 410; ICD-10 I21), stroke (ICD-9 430-438; ICD-10 I60-I69), and other cardiovascular events (ICD-9 390-404, 411-429, 440-459; ICD-10 I00-I01, I05-I13, I20, I24-I28, I31, I33-I35, I38, I40, I42, I44-I51, I70-I74, I77-I78), and all other causes.
Statistical Methods
For each cohort, we estimated hazard ratios (HRs) and 95% confidence intervals (CIs) for associations between aspirin and the mortality outcomes using Cox proportional hazards models with age as the time metric. Follow-up started at the time the questionnaire was administered and continued until death, loss to follow-up, or censoring (December 31, 2011 in NIH–AARP; December 31, 2009 or the 13th year of study follow-up in PLCO), whichever came first. We evaluated the proportional hazard assumption and no violations were observed. All analyses were performed in each cohort separately and then combined in meta-analyses using fixed and random effects models with weights based on the inverse variance. As there was little evidence for heterogeneity (P>0.01 for all models), only the fixed-effects models are presented.
Final models were adjusted for race (white, black, other), gender, smoking status (never, former, current), body mass index (BMI; <18.5, 18.5 to <25, 25 to<30, ≥30kg/m2), ibuprofen or other NSAIDs use (no use, infrequent use, weekly, 1/day, ≥2/day), history of coronary heart disease/heart attack or stroke, history of hypertension, and history of arthritis, as they were a priori confounders and/or influenced the HR estimates by more than 10%. For PLCO, study entry date was also included to account for study protocol changes (before or after April 15, 1995). For NIH–AARP, we additionally adjusted for history of high cholesterol, but it resulted in virtually no change in the risk estimates (data not shown). For both studies, we also adjusted for a summary score of co-morbidities that took into account all other reported diseases, but it also resulted in very little change in the risk estimates (data not shown). Other potential confounders, including education, physical activity, alcohol consumption, hormone replacement therapy, history of osteoporosis, and randomization arm (for PLCO) were also evaluated but did not materially influence the parameter estimates so were not included in the final models. Effect modification by sex and smoking status was also evaluated; however, no notable differences were found for any mortality outcomes, so only results from the combined analysis are presented.
To further evaluate whether pre-existing symptoms of end-stage disease influenced self-reported aspirin use at baseline and its association with mortality, we conducted sensitivity analyses. To this end, we introduced a lag time of 5 years, such that follow-up time for cohort members within the first 5 years were removed from the analysis. To limit contamination by other NSAID use, we also evaluated associations with mortality among exclusive aspirin users, who reported no use of ibuprofen or other NSAIDs.
Results
We studied a total of 440,277 participants in the NIH–AARP and the PLCO cohorts, following their mortality outcomes for an average of 13 years. During the 5,708,170 person-years of follow-up time, we identified 83,463 deaths, including 26,662 deaths from cardiovascular disease (including stroke) and 28,666 deaths from cancer, across the two cohorts. Loss to follow-up for mortality outcomes was low in both cohorts: <1% for NIH–AARP and 3.6% for PLCO. Over 26% of the NIH–AARP participants and 50% of the PLCO participants reported no use or no regular use of any aspirin products, respectively, with 22% and 21%reporting once daily useand3% and 6%reporting more than once daily use, respectively (Table 1). In both cohorts, once daily and more than once daily aspirin users were more likely to be non-Hispanic white and had a slightly higher BMI compared to non-users of aspirin (Table 1). Individuals who reported once daily aspirin use were more likely than non-users to be male and report a history of heart disease/heart attack, stroke, hypertension, or diabetes, while those who reported more than once daily use of aspirin were more likely to currently smoke and report a history of arthritis.
Table 1. Baseline Characteristics of Participants by Frequency of Aspirin Usea.
| NIH–AARP N = 297,564 | PLCO N = 142,713 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| No use | 1-3/month | 1-6/week | 1/day | 2 +/day | No use | 1-3/month | 1-6/week | 1/day | 2+/day | |
| Cohort, N (%) | 79,256 (27) | 93,609 (31) | 50,326 (17) | 66,725 (22) | 7,648 (3) | 72,630 (51) | 14,364 (10) | 18,384 (13) | 29,484 (21) | 7,851 (6) |
| Age at baseline, median (IQR) | 64 (59, 67) | 63 (58, 67) | 63 (58, 67) | 65 (60, 68) | 64 (59, 68) | 62 (58, 67) | 62 (58, 66) | 62 (58, 67) | 64 (60, 68) | 63 (59, 67) |
| Male, % | 47 | 57 | 60 | 73 | 50 | 46 | 53 | 52 | 61 | 48 |
| Non-Hispanic white, % | 90 | 92 | 94 | 95 | 94 | 87 | 86 | 92 | 91 | 90 |
| Body mass index (kg/m2) median (IQR) | 26 (24, 29) | 26 (24, 29) | 26 (24, 29) | 27 (24, 30) | 27 (24,30) | 27 (24, 30) | 27 (24, 30) | 26 (24, 29) | 27 (25, 30) | 27 (24, 31) |
| Smoking status, % | ||||||||||
| Never | 36 | 35 | 33 | 26 | 30 | 46 | 38 | 41 | 36 | 38 |
| Former | 47 | 48 | 49 | 57 | 49 | 40 | 46 | 44 | 50 | 45 |
| Current | 12 | 11 | 11 | 10 | 15 | 11 | 12 | 10 | 10 | 14 |
| History of comorbidities, % | ||||||||||
| Heart disease | 9 | 4 | 8 | 34 | 19 | 4 | 3 | 5 | 27 | 11 |
| Stroke | 2 | 1 | 1 | 4 | 4 | 2 | 1 | 1 | 6 | 4 |
| Hypertension | 37 | 32 | 37 | 51 | 48 | 30 | 28 | 32 | 46 | 42 |
| Diabetes | 8 | 6 | 7 | 12 | 11 | 7 | 6 | 6 | 12 | 9 |
| Arthritisb | 9 | 5 | 6 | 7 | 15 | 37 | 33 | 35 | 38 | 58 |
Frequency of use was defined as 1-3 times or pills per month (1-3/month), 1-6 times or pills per week (1-6/week), 1 time or pill per day (1/day), and 2 or more times or pills per day (2+/day).
Ascertained for only a subset of the NIH-AARP cohort (N = 201,387) and only includes knee or hip osteoarthritis for NIH-AARP.
NIH-AARP, the National Institutes of Health American Association of Retired Persons Diet and Health Study; PLCO, the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial
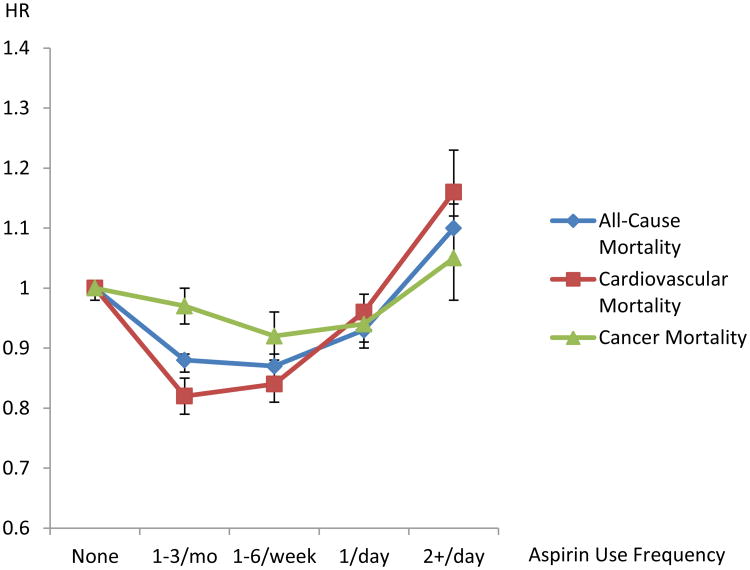
Consistent across both cohorts, a reduction in all-cause mortality was observed for individuals who reported using aspirin weekly [combined HR=0.87, 95%CI: 0.85-0.89] or once daily [combined HR=0.93 (0.91-0.94) (Table 2 and Figure 1)]. However, elevated all-cause mortality was observed among those who reported aspirin use more than once daily [combined HR=1.10 (1.07-1.14)]. To evaluate whether other factors could be responsible for this elevated risk among those reporting more than once daily use, we conducted several sensitivity analyses. The observed elevation in risk remained after excluding: 1) individuals reporting any non-aspirin NSAIDs use [combined HR=1.11 (1.06-1.16)]; 2) the first five years of follow-up [combined HR=1.09 (1.05-1.14)]; or 3) both those using other NSAIDs and the first five years of follow-up [combined HR=1.08 (1.03-1.15)]. These sensitivity analyses suggest that the observed association with more than daily use of aspirin was not likely due to concurrent use of other NSAIDs or symptoms related to pre-existing or end-stage disease.
Table 2. Riska of All-Cause, Cancer, and Cardiovascular Mortality Associated with Aspirin Useb.
| Frequency | All-Cause Mortality | Cancer Mortality | Cardiovascular Mortality | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| NIH–AARP | Person-Years | No. Cases | HR (95% CI) | No. Cases | HR (95% CI) | No. Cases | HR (95% CI) |
| None | 1,085,073 | 17,635 | 1.00 | 5,829 | 1.00 | 5,401 | 1.00 |
| 1-3/month | 1,317,360 | 15,754 | 0.86 (0.84-0.88) | 6,293 | 0.97 (0.93-1.00) | 4,141 | 0.79 (0.76-0.83) |
| 1-6/week | 701,902 | 9,440 | 0.87 (0.85-0.89) | 3,416 | 0.93 (0.89-0.97) | 2,746 | 0.84 (0.80-0.88) |
| 1/day | 891,528 | 18,495 | 0.92 (0.90-0.94) | 5,412 | 0.95 (0.91-0.98) | 7,130 | 0.98 (0.94-1.01) |
| 2+/day | 101,222 | 2,180 | 1.07 (1.02-1.12) | 615 | 1.00 (0.92-1.08) | 809 | 1.17 (1.08-1.26) |
|
| |||||||
| PLCO | |||||||
|
| |||||||
| None | 825, 224 | 9,066 | 1.00 | 3,441 | 1.0 | 2,713 | 1.0 |
| 1-3/month | 163,408 | 1,704 | 0.96 (0.91-1.02) | 681 | 0.97 (0.89-1.06) | 501 | 0.98 (0.89-1.08) |
| 1-6/week | 211,650 | 2,106 | 0.87 (0.83-0.91) | 829 | 0.90 (0.84-0.97) | 612 | 0.84 (0.77-0.92) |
| 1/day | 323,640 | 5,269 | 0.93 (0.89-0.96) | 1,573 | 0.92 (0.86-0.98) | 1,995 | 0.93 (0.87-0.99) |
| 2+/day | 87,163 | 1,406 | 1.16 (1.10-1.23) | 461 | 1.12 (1.02-1.24) | 467 | 1.15 (1.04-1.27) |
|
| |||||||
| Combined | |||||||
|
| |||||||
| None | 1,910,297 | 26,701 | 1.00 | 9,270 | 1.00 | 8,114 | 1.00 |
| 1-3/month | 1,480,768 | 17,458 | 0.88 (0.86-0.89) | 6,974 | 0.97 (0.94-1.00) | 4,642 | 0.82 (0.79-0.85) |
| 1-6/week | 913,552 | 11,546 | 0.87 (0.85-0.89) | 4,245 | 0.92 (0.89-0.96) | 3,358 | 0.84 (0.81-0.88) |
| 1/day | 1,215,168 | 23,764 | 0.93 (0.91-0.94) | 6,985 | 0.94 (0.91-0.97) | 9,125 | 0.96 (0.93-0.99) |
| 2+/day | 188,385 | 3,586 | 1.10 (1.07-1.14) | 1,076 | 1.05 (0.98-1.12) | 1,276 | 1.16 (1.10-1.23) |
Adjusting for race (white, black, other), gender, smoking status (never, former, current), body mass index (BMI; <18.5, 18.5 to <25, 25 to<30, ≥30kg/m2), use of ibuprofen or other nonsteroidal anti-inflammatory drugs, history of coronary heart disease/heart attack or stroke, history of hypertension, history of arthritis, and for the PLCO-specific analysis, study entry date due to protocol changes (before April 15,1995, on or after April 15, 1995)
Frequency of use was defined as 1-3 times or pills per month (1-3/month), 1-6 times or pills per week (1-6/week), 1 time or pill per day (1/day), and 2 or more times or pills per day (2+/day).
No. indicates number; HR, hazard ratio; CI, confidence interval; NIH-AARP, the National Institutes of Health American Association of Retired Persons Diet and Health Study; PLCO, the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial
Figure 1.
Risk of All-Cause, Cancer, and Cardiovascular Mortality Associated with Aspirin Use from the Combined Analysis of the NIH–AARP Diet and Health Study and the PLCO Cancer Screening Trial. Risks were adjusting for race (white, black, other), gender, smoking status (never, former, current), body mass index (BMI; <18.5, 18.5 to <25, 25 to<30, ≥30kg/m2), use of ibuprofen or other non-steroidal anti-inflammatory drugs, history of coronary heart disease/heart attack or stroke, history of hypertension, history of arthritis, and for the PLCO-specific analysis, study entry date due to protocol changes (before April 15, 1995, on or after April 15, 1995).
We also examined the two most common underlying causes of death, cardiovascular disease and cancer (Table 2). A small reduction in risk for cancer mortality was observed for individuals who reported weekly [combined HR=0.92 (0.89-0.96)] or daily [combined HR=0.94 (0.91-0.97)] use of aspirin, but not for those taking aspirin more than once daily [combined HR=1.05 (0.98-1.12)]. Similar to what we observed for all-cause mortality, we observed a U-shaped curve for cardiovascular disease mortality with some benefit observed for those taking aspirin weekly [combined HR=0.84 (0.81-0.88)] or daily [combined HR=0.96 (0.93-0.99)], but an increased risk for those taking aspirin more than once daily [combined 1.16 (1.10-1.23)], compared to non-users. Again, this elevated risk among those taking aspirin more than once daily remained even after excluding other NSAIDs use [combined HR=1.15 (1.06-1.25)]; the first five years of follow-up [combined HR=1.15(1.07-1.24)]; or both factors [combined HR=1.13 (1.03-1.24)], suggesting that neither concurrent NSAID use or end-stage disease were responsible for this elevated risk.
We evaluated more specific cardiovascular causes of death. Compared to non-users, individuals taking aspirin more than once daily had an increased risk of death due to stroke [combined HR=1.35 (1.17-1.57)] and acute myocardial infarction [combined HR=1.26 (1.11-1.44)], which was consistent across both cohorts (eTable 1). The elevation in mortality due to stroke was not limited to hemorrhagic stroke, although number of deaths by subtype of stroke was small (e.g., n = 68 deaths due to hemorrhagic stroke among those who took aspirin ≥2/day in both cohorts combined).Number of deaths due to other bleeding problems was also too small for a meaningful analysis (e.g., n=10 deaths due to gastrointestinal hemorrhage among those taking aspirin ≥2/day in both cohorts combined).
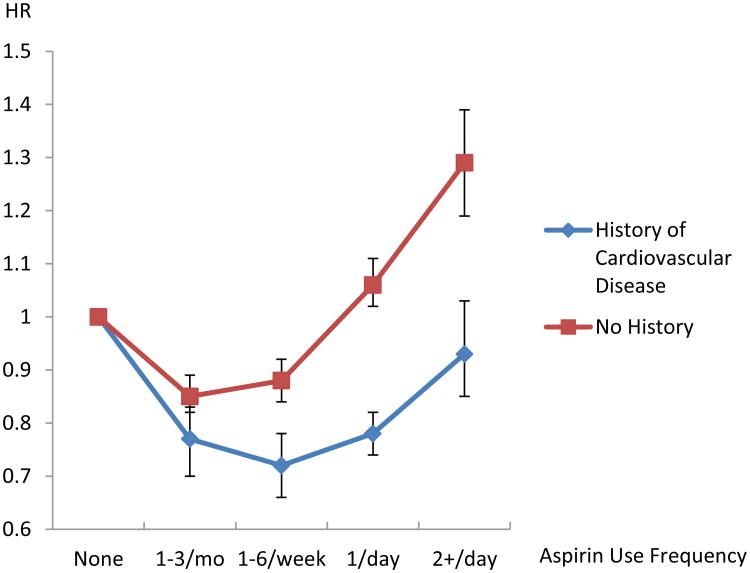
As randomized trials have shown a benefit for once daily aspirin use in the secondary prevention of heart disease, we also evaluated whether the association of aspirin use and cardiovascular mortality varied by disease history. Among individuals with a reported history of cardiovascular disease, daily aspirin use was associated with a 22% reduction in risk for cardiovascular mortality [combined HR=0.78 (0.74-0.82)], with no association observed for those taking aspirin more than once daily [combined HR=0.93 (0.85-1.03)] (Table 3 and Figure 2). However, among those without a previous history, no protective benefit was observed for daily aspirin users [combined HR=1.06 (1.02-1.11)], and an elevated risk of cardiovascular mortality was observed for those taking aspirin more than once daily [combined HR=1.29 (1.19-1.39)], particularly for deaths due to stroke [combined HR=1.54 (1.30-1.84)]. This association remained even after excluding other NSAID use [combined HR=1.31 (1.19-1.45)]; deaths occurring during the first five years of follow-up [combined HR=1.31 (1.17-1.47); or both [combined HR=1.31 (1.17-1.47)]. To evaluate whether the increased risk observed may be due to other cardiovascular risk factors, we further stratified by history of hypertension and diabetes (eTable 2). The associations persisted after further excluding persons with a history of hypertension or diabetes in addition to those with a history of cardiovascular disease [combined HR=1.28 (1.14-1.45)]. Among participants with a reported history of diabetes, a reduction in cardiovascular mortality was observed for daily aspirin use among those with a history of cardiovascular disease [combined HR=0.88 (0.79-0.97)]; however, no benefit was observed among those without a cardiovascular disease history for daily aspirin use [combined HR =1.15 (1.04-1.27)] and an elevated risk was observed for more than once daily use [combined HR=1.31 (1.08-1.57), eTable 3].
Table 3. Riska of Cardiovascular Mortality Associated with Aspirin Useb by History of Cardiovascular Disease.
| Frequency | History of Cardiovascular Diseasec | No Historyd | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| NIH–AARP | Person-Years | No. Cases | HR (95% CI) | Person-Years | No. Cases | HR (95% CI) |
| None | 96,531 | 1,660 | 1.00 | 988,542 | 3,741 | 1.00 |
| 1-3/month | 58,724 | 628 | 0.71 (0.65-0.78) | 1,258,636 | 3,513 | 0.84 (0.80-0.88) |
| 1-6/week | 55,489 | 668 | 0.72 (0.66-0.79) | 646,413 | 2,078 | 0.89 (0.84-0.93) |
| 1/day | 307,643 | 4,131 | 0.79 (0.74-0.83) | 583,885 | 2,999 | 1.10 (1.04-1.15) |
| 2 +/day | 20,111 | 332 | 0.93 (0.82-1.05) | 81,112 | 477 | 1.32 (1.20-1.45) |
|
| ||||||
| PLCO | ||||||
|
| ||||||
| None | 35,823 | 571 | 1.00 | 789,401 | 2,137 | 1.00 |
| 1-3/month | 5,465 | 97 | 1.14 (0.92-1.42) | 157943 | 398 | 0.94 (0.84-1.05) |
| 1-6/week | 12,697 | 139 | 0.68 (0.56-0.82) | 198953 | 467 | 0.86 (0.78-0.96) |
| 1/day | 95,623 | 1,216 | 0.77 (0.70-0.85) | 228017 | 773 | 0.97 (0.89-1.06) |
| 2 +/day | 10,570 | 166 | 0.95 (0.79-1.13) | 76593 | 304 | 1.23 (1.09-1.39) |
|
| ||||||
| Combined | ||||||
|
| ||||||
| None | 132,354 | 2,231 | 1.00 | 1,774,708 | 5,878 | 1.00 |
| 1-3/month | 64,189 | 725 | 0.77 (0.70-0.83) | 1,415,389 | 3,911 | 0.85 (0.82-0.89) |
| 1-6/week | 68,186 | 807 | 0.72 (0.66-0.78) | 844,322 | 2,545 | 0.88 (0.84-0.92) |
| 1/day | 403,266 | 5,347 | 0.78 (0.74-0.82) | 810,752 | 3,772 | 1.06 (1.02-1.11) |
| 2 +/day | 30,681 | 498 | 0.93 (0.85-1.03) | 157,325 | 781 | 1.29 (1.19-1.39) |
Adjusting for race (white, black, other), gender, smoking status (never, former, current), body mass index (BMI; <18.5, 18.5 to <25, 25 to<30, ≥30kg/m2), use of ibuprofen or other nonsteroidal anti-inflammatory drugs, history of hypertension, history of arthritis, and for the PLCO-specific analysis, study entry date due (before April 15,1995, on or after April 15, 1995)
Frequency of use was defined as 1-3 times or pills per month (1-3/month), 1-6 times or pills per week (1-6/week), 1 time or pill per day (1/day), and 2 or more times or pills per day (2+/day).
History of cardiovascular disease includes anyone who reported ever having coronary heart disease/heart attack or stroke.
No history includes anyone with no reported cardiovascular disease.
No. indicates number; HR, hazard ratio; CI, confidence interval; NIH-AARP, the National Institutes of Health American Association of Retired Persons Diet and Health Study; PLCO, the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial
Figure 2.
Risk of Cardiovascular Mortality Associated with Aspirin Use by History of Cardiovascular Disease from the Combined Analysis of the NIH–AARP Diet and Health Study and the PLCO Cancer Screening Trial. Risks were adjusting for race (white, black, other), gender, smoking status (never, former, current), body mass index (BMI; <18.5, 18.5 to <25, 25 to<30, ≥30kg/m2), use of ibuprofen or other non-steroidal anti-inflammatory drugs, history of coronary heart disease/heart attack or stroke, history of hypertension, history of arthritis, and for the PLCO-specific analysis, study entry date due to protocol changes (before April 15, 1995, on or after April 15, 1995).
Discussion
Across two large contemporary U.S. cohort studies, we observed a consistent U-shaped risk curve among aspirin users for mortality, with a reduced risk among daily users and an increased risk in all-cause and cardiovascular mortality among individuals who reported using aspirin more than once per day. Mortality was particularly elevated among individuals without a history of cardiovascular disease and persisted after further excluding participants with hypertension and diabetes, those who died in the first five years of follow-up, or who concurrently used other NSAIDs. Our population-based study confirms randomized trial results on the utility of daily aspirin use for secondary prevention of cardiovascular mortality in the general population, but suggests that caution should be exercised in advocating its use in individuals without a history of cardiovascular disease, where more than once daily use of aspirin may lead to more harm than benefit.
Consistent with the 2014 FDA guideline9 and findings from previous randomized trials for primary and secondary prevention,10-13 we found that daily aspirin use was associated with a reduction in cardiovascular mortality among individuals who reported a history of cardiovascular events, but not among those without a prior history. In 2016, the USPSTF updated their recommendations for aspirin for primary prevention based on an individual's estimated 10-year cardiovascular disease risk.7 However, due to limitations on information collected, we were unable to calculate individual 10-year risk estimates using the ASCVD (Atherosclerotic Cardiovascular Disease) Risk Algorithm in our study and thus, unable to evaluate mortality by risk groups as in the revised USPSTF guidelines. In our study, we also observed a reduction in cardiovascular mortality for those taking aspirin 1-6 times per week. Although randomized trials have not observed a mortality benefit for alternate day dosing,34-36 when these same trials were analyzed adjusting for non-compliance, a reduction in cardiovascular mortality was observed,37 which is consistent with our findings. The Nurse's Health Study also observed a benefit at less frequent doses.26 This is consistent with the anti-platelet effects of aspirin, which are known to last 7 to 10 days, and may be maximized at alternate or every third day dosing.38, 39 The long-term effects of aspirin use more than once daily have not been studied in randomized trials and few population-based studies have examined the effects of more than daily use; however, the Nurses' Health Study did find a nonsignificant elevation in risk of all-cause, cardiovascular, and cancer mortality among relatively young women (46-year-old on average) who reported using aspirin >14 times a week,26 which is consistent with the increased risk we observed for more than once daily use.
Aspirin, even at low doses, can have important side effects, including fatal stroke, gastrointestinal toxicity and other bleeding complications,18, 19 which have been shown to be dose-dependent.40 Low doses of aspirin have been shown to be effective in reducing platelet aggregation through the inhibition of COX-1 and the synthesis of thromboxane A2. Higher concentrations of aspirin, however, can also inhibit COX-2, which synthesizes anti-thrombotic prostacyclins, leading to paradoxical thrombosis and vasoconstriction, counterbalancing the effect of COX-1.11, 26, 41 Others have also suggested that aspirin at higher doses may conceal, rather than prevent, vascular events.42 In randomized trials,10-13 the failure of aspirin to reduce mortality among those without a history of cardiovascular disease despite a reduction in non-fatal events appears partially due to the increased risk of fatal hemorrhagic stroke.10 Indeed, we observed an increase in mortality due to stroke (including hemorrhagic stroke although the numbers were small) and to a lesser extent acute myocardial infarction among those taking aspirin more than once daily. Others have reported that the increased risk of major gastrointestinal bleeding with aspirin use should also be taken into account in considering aspirin for primary prevention.36 The lack of benefit for daily as well as more than daily use of aspirin among those without a history of cardiovascular disease in this study suggests that caution should be taken in advocating its use and that lifestyle modifications, such as changes in diet, physical activity, and smoking cessation, may offer more benefit than chemoprevention in the general population.
Our study was not a randomized trial and had only limited self-reported data on frequency of aspirin use. No data on dose was available that would have allowed us to examine differences by aspirin strength. However, we observed consistent findings across two large population-based cohorts and confirmed the results of randomized trials showing the benefit of daily aspirin for secondary prevention of cardiovascular disease mortality. Our findings for aspirin and mortality are also consistent with results from the Nurses' Health Study,26 which used a time-varying exposure variable for aspirin use and also collected information on duration, dose, and indication for use. While some underreporting of aspirin use by self-report has been noted in other studies, reporting accuracy tends to improve with more frequent and regular use.43, 44 As information on aspirin use was obtained prospectively in this study, any misclassification of aspirin use due to inaccurate reporting is likely to be non-differential, leading most likely to an attenuation of the effects of aspirin.45 Although we relied on self-reported data on comorbidities, the validity of self-reported data has been shown to be generally good for heart disease, stroke, hypertension, diabetes, and other medical conditions.27-31 It is possible that the elevated mortality risks we observed were due to other conditions not captured in the questionnaires; however, the unidentified comorbidities would need to be strongly correlated with both aspirin use and mortality to have a substantial impact on our findings. Cardiovascular mortality risk estimates for individuals taking aspirin more than once daily remained elevated even after excluding deaths in the first five years of follow-up, minimizing the likelihood that heavy aspirin users were treating symptoms related to end-stage disease.
In conclusion, our study confirms the potential utility of daily aspirin for the secondary prevention of cardiovascular mortality, but suggests caution for its use among those without a history of cardiovascular disease. Consistent findings across two large cohorts suggest that more than once daily use of aspirin does not provide a reduction in mortality and maybe associated with an increased risk of death, particularly among individuals without a history of cardiovascular disease. Although further studies are needed to confirm these findings, our study highlights the need to evaluate important long-term health risks of aspirin across a broad range of common use patterns and to fully consider the risks and benefits before making public health recommendations.
Supplementary Material
Acknowledgments
The authors thank the participants from the NIH-AARP and PLCO cohorts for their excellent cooperation in these studies. The authors also thank Mr. Adam Risch and Mr. John Commins, Information Management Services, Inc. for their assistance in the data analysis. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee.
Data availability: Investigators may apply to access the study data through the National Institutes of Health, AARP Diet and Health Study website (https://dietandhealth.cancer.gov/resource/) and the PLCO Cancer Data Access System website (https://biometry.nci.nih.gov/cdas/learn/plco/instructions/?subtype=Data-Only).
Funding Source: The National Institutes of Health AARP Diet and Health Study (NIH-AARP) and Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial are supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute (NCI), National Institutes of Health (NIH), DHHS. PLCO is also supported by contracts from the Division of Cancer Prevention, NCI, NIH, DHHS.
Footnotes
Conflict of Interest Disclosures: none declared.
References
- 1.Ajani UA, Ford ES, Greenland KJ, Giles WH, Mokdad AH. Aspirin use among U.S. adults: Behavioral Risk Factor Surveillance System. Am J Prev Med. 2006;30(1):74–77. doi: 10.1016/j.amepre.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 2.Pignone M, Anderson GK, Binns K, Tilson HH, Weisman SM. Aspirin use among adults aged 40 and older in the United States: results of a national survey. Am J Prev Med. 2007;32(5):403–407. doi: 10.1016/j.amepre.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger JS, Brown DL, Becker RC. Low-dose aspirin in patients with stable cardiovascular disease: a meta-analysis. Am J Med. 2008;121(1):43–49. doi: 10.1016/j.amjmed.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Matthys F, De BT, De BG, Stichele RV. Review of guidelines on primary prevention of cardiovascular disease with aspirin: how much evidence is needed to turn a tanker? Eur J Prev Cardiol. 2014;21(3):354–365. doi: 10.1177/2047487312472077. [DOI] [PubMed] [Google Scholar]
- 6.Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e637S–e668S. doi: 10.1378/chest.11-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bibbins-Domingo K. Aspirin Use for the Primary Prevention of Cardiovascular Disease and Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164(12):836–845. doi: 10.7326/M16-0577. [DOI] [PubMed] [Google Scholar]
- 8.Perk J, De BG, Gohlke H, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) Eur Heart J. 2012;33(13):1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 9.FDA Consumer Update. Can an Aspirin a Day Help Prevent a Heart Attack? 2014 May 5; [Google Scholar]
- 10.Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA. 2006;295(3):306–313. doi: 10.1001/jama.295.3.306. [DOI] [PubMed] [Google Scholar]
- 12.Seshasai SR, Wijesuriya S, Sivakumaran R, et al. Effect of aspirin on vascular and nonvascular outcomes: meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172(3):209–216. doi: 10.1001/archinternmed.2011.628. [DOI] [PubMed] [Google Scholar]
- 13.Sutcliffe P, Connock M, Gurung T, et al. Aspirin in primary prevention of cardiovascular disease and cancer: a systematic review of the balance of evidence from reviews of randomized trials. PLoS One. 2013;8(12):e81970. doi: 10.1371/journal.pone.0081970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376(9754):1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 15.Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294(1):47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 16.Cook NR, Lee IM, Zhang SM, Moorthy MV, Buring JE. Alternate-day, low-dose aspirin and cancer risk: long-term observational follow-up of a randomized trial. Ann Intern Med. 2013;159(2):77–85. doi: 10.7326/0003-4819-159-2-201307160-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377(9759):31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 18.DeBerardis G, Lucisano G, D'Ettorre A, et al. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA. 2012;307(21):2286–2294. doi: 10.1001/jama.2012.5034. [DOI] [PubMed] [Google Scholar]
- 19.Sostres C, Lanas A. Gastrointestinal effects of aspirin. Nat Rev Gastroenterol Hepatol. 2011;8(7):385–394. doi: 10.1038/nrgastro.2011.97. [DOI] [PubMed] [Google Scholar]
- 20.Manes C, Giacci L, Sciartilli A, D'Alleva A, De CR. Aspirin overprescription in primary cardiovascular prevention. Thromb Res. 2006;118(4):471–477. doi: 10.1016/j.thromres.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Rodondi N, Cornuz J, Marques-Vidal P, et al. Aspirin use for the primary prevention of coronary heart disease: a population-based study in Switzerland. Prev Med. 2008;46(2):137–144. doi: 10.1016/j.ypmed.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 22.VanWormer JJ, Miller AW, Rezkalla SH. Aspirin overutilization for the primary prevention of cardiovascular disease. Clin Epidemiol. 2014;6:433–440. doi: 10.2147/CLEP.S72032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions : the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154(12):1119–1125. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 24.Hayes RB, Reding D, Kopp W, et al. Etiologic and early marker studies in the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial. Control Clin Trials. 2000;21(6 Suppl):349S–355S. doi: 10.1016/s0197-2456(00)00101-x. [DOI] [PubMed] [Google Scholar]
- 25.Prorok PC, Andriole GL, Bresalier RS, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21(6 Suppl):273S–309S. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 26.Chan AT, Manson JE, Feskanich D, Stampfer MJ, Colditz GA, Fuchs CS. Long-term aspirin use and mortality in women. Arch Intern Med. 2007;167(6):562–572. doi: 10.1001/archinte.167.6.562. [DOI] [PubMed] [Google Scholar]
- 27.Barr EL, Tonkin AM, Welborn TA, Shaw JE. Validity of self-reported cardiovascular disease events in comparison to medical record adjudication and a statewide hospital morbidity database: the AusDiab study. Intern Med J. 2009;39(1):49–53. doi: 10.1111/j.1445-5994.2008.01864.x. [DOI] [PubMed] [Google Scholar]
- 28.Jackson JM, DeFor TA, Crain AL, et al. Self-reported diabetes is a valid outcome in pragmatic clinical trials and observational studies. J Clin Epidemiol. 2013;66(3):349–350. doi: 10.1016/j.jclinepi.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Jackson JM, DeFor TA, Crain AL, et al. Validity of diabetes self-reports in the Women's Health Initiative. Menopause. 2014;21(8):861–868. doi: 10.1097/GME.0000000000000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelstrup AM, Juillerat P, Korzenik J. The accuracy of self-reported medical history: a preliminary analysis of the promise of internet-based research in Inflammatory Bowel Diseases. J Crohns Colitis. 2014;8(5):349–356. doi: 10.1016/j.crohns.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Tormo MJ, Navarro C, Chirlaque MD, Barber X. Validation of self diagnosis of high blood pressure in a sample of the Spanish EPIC cohort: overall agreement and predictive values. EPIC Group of Spain. J Epidemiol Community Health. 2000;54(3):221–226. doi: 10.1136/jech.54.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goraya TY, Jacobsen SJ, Belau PG, Weston SA, Kottke TE, Roger VL. Validation of death certificate diagnosis of out-of-hospital coronary heart disease deaths in Olmsted County, Minnesota. Mayo Clin Proc. 2000;75(7):681–687. doi: 10.4065/75.7.681. [DOI] [PubMed] [Google Scholar]
- 33.Coady SA, Sorlie PD, Cooper LS, Folsom AR, Rosamond WD, Conwill DE. Validation of death certificate diagnosis for coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. J Clin Epidemiol. 2001;54(1):40–50. doi: 10.1016/s0895-4356(00)00272-9. [DOI] [PubMed] [Google Scholar]
- 34.Final report on the aspirin component of the ongoing Physicians' Health Study. Steering Committee of the Physicians' Health Study Research Group. N Engl J Med. 1989;321(3):129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 35.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352(13):1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 36.van Kruijsdijk RC, Visseren FL, Ridker PM, et al. Individualised prediction of alternate-day aspirin treatment effects on the combined risk of cancer, cardiovascular disease and gastrointestinal bleeding in healthy women. Heart. 2015;101(5):369–376. doi: 10.1136/heartjnl-2014-306342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cook NR, Cole SR, Buring JE. Aspirin in the primary prevention of cardiovascular disease in the Women's Health Study: effect of noncompliance. Eur J Epidemiol. 2012;27(6):431–438. doi: 10.1007/s10654-012-9702-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lorenz RL, Boehlig B, Uedelhoven WM, Weber PC. Superior antiplatelet action of alternate day pulsed dosing versus split dose administration of aspirin. Am J Cardiol. 1989;64(18):1185–1188. doi: 10.1016/0002-9149(89)90875-8. [DOI] [PubMed] [Google Scholar]
- 39.Feldman M, Cryer B, Rushin K, Betancourt J. A comparison of every-third-day versus daily low-dose aspirin therapy on serum thromboxane concentrations in healthy men and women. Clin Appl Thromb Hemost. 2001;7(1):53–57. doi: 10.1177/107602960100700111. [DOI] [PubMed] [Google Scholar]
- 40.United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: interim results. UK-TIA Study Group. Br Med J (Clin Res Ed) 1988;296(6618):316–320. [PMC free article] [PubMed] [Google Scholar]
- 41.Patrono C, Garcia Rodriguez LA, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 2005;353(22):2373–2383. doi: 10.1056/NEJMra052717. [DOI] [PubMed] [Google Scholar]
- 42.Cleland JG. Preventing atherosclerotic events with aspirin. BMJ. 2002;324(7329):103–105. doi: 10.1136/bmj.324.7329.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pit S, Byles J. Older Australians' medication use: self-report by phone showed good agreement and accuracy compared with home visit. J Clin Epidemiol. 2010;63(4):428–434. doi: 10.1016/j.jclinepi.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Pit SW, Byles JE, Cockburn J. Accuracy of telephone self-report of drug use in older people and agreement with pharmaceutical claims data. Drugs Aging. 2008;25(1):71–80. doi: 10.2165/00002512-200825010-00008. [DOI] [PubMed] [Google Scholar]
- 45.Burstyn I, Yang Y, Schnatter AR. Effects of non-differential exposure misclassification on false conclusions in hypothesis-generating studies. Int J Environ Res Public Health. 2014;11(10):10951–10966. doi: 10.3390/ijerph111010951. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.