Abstract
CD34 selection significantly improves GVHD-free survival in allogeneic hematopoietic cell transplantation (allo-HSCT). Specific information regarding long-term prognosis and risk factors for late mortality after CD34-selected allo-HSCT is lacking, however. We conducted a single-center landmark analysis in 276 patients alive without relapse 1 year after CD34-selected allo-HSCT for AML (n=164), ALL (n=33), or MDS (n=79). At 5 years' follow-up after the 1-year landmark (range 0.03-13 years), estimated RFS was 73% and OS 76%. The 5-year cumulative incidence of relapse and NRM were 11% and 16%, respectively. In multivariate analysis, HCT-CI score ≥ 3 correlated with marginally worse RFS (HR 1.78, 95% CI 0.97-3.28, p=0.06) and significantly worse OS (HR 2.53, 95% CI 1.26-5.08, p=0.004). Despite only 24% of patients with acute GVHD within 1 year, this also significantly correlated with worse RFS and OS, with increasing grades of acute GVHD associating with increasingly poorer survival on multivariate analysis (p<0.0001). Of 63 deaths after the landmark, GVHD accounted for 27% of deaths and was the most common cause of late mortality, followed by relapse and infection. While prognosis is excellent for patients alive without relapse 1 year after CD34-selected allo-HSCT, risks of late relapse and NRM persist, particularly due to GVHD.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is an established therapy for hematologic malignancies, including relapsed/refractory or high-risk acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL) and myelodysplastic syndrome (MDS). Yet transplant recipients remain at risk for relapse, and for late complications and non-relapse mortality (NRM). In particular, graft-versus-host disease (GVHD) is a potentially devastating complication. The use of T-cell depletion (TCD) of allografts by CD34+ cell selection has improved GVHD-free survival in acute leukemia and MDS without need for post-transplant immunosuppression and without increased risk of relapse.1-10
Previous analyses of multicenter data reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) to evaluate prognosis for recipients of myeloablative allo-HSCT who survive without relapse past the early post-transplant period have found that long-term survival for these patients is excellent.11, 12 Possible risk factors for late mortality include chronic GVHD, relapsed/refractory disease, and poor performance status. These registry studies included patients who received both T-cell replete and TCD allografts, which in many cases employed older methods of TCD. Prognostic information is lacking for patients who undergo TCD transplantation using contemporary techniques and survive without relapse past the early post-HSCT period. This study therefore focused on long-term prognosis in these patients to identify barriers to long-term survival after CD34-selected allo-HSCT.
Methods
Patients
The study included adult recipients of first allo-HSCT with a CD34+ cell–selected peripheral blood stem cell transplant (PBSCT) at Memorial Sloan Kettering Cancer Center for AML, MDS, or ALL between February 2000 and June 2013. All patients and donors provided written informed consent for treatment. The MSKCC Institutional Review and Privacy Board approved this retrospective study. High-resolution DNA-specific oligonucleotide typing characterized HLA-A, -B, -C, -DRB1, and -DQB1 loci. Clinical outcomes, including acute and chronic GVHD, relapse, and causes of death, were captured in real time per standard clinical practice.
Transplant procedures
All patients received myeloablative conditioning. TBI-based regimens included the following: TBI 1,375 cGy in 11 fractions over 4 days, followed by thiotepa 5 mg/kg/day for 2 days with either (1) fludarabine 25 mg/m2/day for 5 days beginning on the first day of thiotepa or (2) cyclophosphamide 60 mg/kg/day for 2 days starting after thiotepa.2, 3 Chemotherapy-based preparative regimens consisted of intravenous busulfan 0.8 mg/kg/dose every 6 hours for 10 or 12 doses, melphalan 70 mg/m2/day for 2 doses, and fludarabine 25 mg/m2/day for 5 doses; or clofarabine 20 mg/m2/day for 5 days, melphalan 70 mg/m2/day for 2 days, and thiotepa 5 mg/kg/day for 2 days (or 10 mg/kg for 1 day), each regimen specified by institutional protocol 10-050 (NCT01119066).6
CD34+ cell selection of PBSCs was accomplished by positive selection of CD34+ stem cells using the ISOLEX 300i Magnetic Cell Separator (Baxter, Deerfield, IL) followed by sheep RBC (sRBC) rosette depletion or, beginning in 2006 and exclusively after May 2010, using the CliniMACS CD34 Reagent System (Miltenyi Biotech, Gladbach, Germany) without need for additional sRBC rosette depletion of residual T-cells. Allografts were infused within 24–48 hours of completion of cytoreduction. Patients received no pharmacologic GVHD prophylaxis. Graft rejection prophylaxis consisted of antithymocyte globulin (Table 1). Patients received supportive care and opportunistic infection prophylaxis according to institutional guidelines.
Table 1. Patient and transplant characteristics.
| Characteristic | Value | Value |
|---|---|---|
| All patients transplanted | Patients in landmark cohort | |
|
| ||
| n=416 | n=276 | |
|
| ||
| Median age (range), years | 54 (18–73) | 54 (18–72) |
|
| ||
| Male, n (%) | 230 (55) | 150 (54) |
|
| ||
| Disease, n (%) | ||
| AML | 252 (61) | 164 (59) |
| ALL | 57 (14) | 33 (12) |
| MDS | 107 (26) | 79 (29) |
|
| ||
| KPS, n (%) | ||
| 90–100 | 222 (53) | 160 (58) |
| 80 | 147 (35) | 94 (34) |
| <80 | 27 (6) | 14 (5) |
| Data not available | 20 (5) | 8 (3) |
|
| ||
| HCT-CI, n (%) | ||
| 0 | 88 (21) | 72 (26) |
| 1–2 | 130 (31) | 90 (33) |
| ≥ 3 (range, 3–10) | 198 (48) | 114 (41) |
|
| ||
| Conditioning regimen, n (%) | ||
| TBI/thiotepa/fludarabine | 85 (20) | 53 (19) |
| TBI/thiotepa/cyclophosphamide | 79 (19) | 53(19) |
| Busulfan/melphalan/fludarabine | 241 (58) | 163 (59) |
| Clofarabine/melphalan/thiotepa | 11 (3) | 8 (3) |
|
| ||
| Donor type, n (%) | ||
| 8/8-matched related | 155 (37) | 108 (39) |
| 8/8-matched unrelated | 159 (38) | 101 (37) |
| Mismatched related | 11 (3) | 8 (3) |
| 7/8-matched, n | 9 | 6 |
| 6/8-matched, n | 2 | 2 |
| Mismatched unrelated | 91 (22) | 59 (21) |
| 7/8-matched, n | 85 | 55 |
| 6/8-matched, n | 6 | 4 |
|
| ||
| CD34 selection method, n (%) | ||
| ISOLEX 300i and sRBC | 241 (58) | 158 (57) |
| CliniMACS | 175 (42) | 118 (43) |
|
| ||
| Antithymocyte globulin (ATG), n (%) | ||
| Any ATG | 376 (90) | 248 (90) |
| Rabbit ATG (2.5-10 mg/kg) | 301 (72) | 202 (73) |
| Equine ATG (30–45 mg/kg) | 73 (18) | 46 (17) |
| Rabbit and equine ATG | 2 (<1) | 0 (0) |
|
| ||
| AML | n=251 | n=164 |
|
| ||
| Disease status at HSCT, n (%) | ||
| CR1 | 171 (68) | 116 (71) |
| CR2 or greater | 68 (27) | 40 (24) |
| PR | 9 (4) | 6 (4) |
| Refractory | 3 (1) | 2 (1) |
|
| ||
| Risk group, n (%) | ||
| Favorable | 20 (8) | 18 (11) |
| Intermediate | 139 (55) | 95 (58) |
| Poor | 91 (36) | 51 (31) |
| Unknown | 1 (<1) | 0 |
|
| ||
| Therapy-related AML, n (%) | 34 (14) | 22 (13) |
|
| ||
| FLT3-ITD or -TKD, n (%) | ||
| Positive | 26 (10) | 19 (12) |
| Negative | 67 (27) | 43 (26) |
| Data not available | 157 (63) | 102 (62) |
|
| ||
| Isolated NPM1 mutation, n (%) | ||
| Yes | 5 (2) | 4 (2) |
| No | 63 (25) | 42 (26) |
| Data not available | 183 (73) | 118 (72) |
|
| ||
| ALL | n=57 | n=33 |
|
| ||
| Disease status at HSCT, n (%) | ||
| CR1 | 43 (75) | 27 (82) |
| CR2 or greater | 13 (23) | 5 (15) |
| Refractory | 1 (2) | 1 (3) |
|
| ||
| Cytogenetic risk, n (%) | ||
| Standard/good | 23 (40) | 13 (39) |
| Poor | 34 (60) | 20 (61) |
|
| ||
| Phenotype, n (%) | ||
| B-cell | 46 (81) | 27 (82) |
| BCR-ABL1+ | 28 (49) | 18 (55) |
| T-cell | 9 (16) | 5 (15) |
| B/T-cell or NK | 2 (4) | 1 (3) |
|
| ||
| MDS | n=108 | n=79 |
|
| ||
| Disease status at HSCT, n (%) | ||
| CR | 22 (20) | 14 (18) |
| Non-CR | 86 (80) | 65 (82) |
| <5% blasts | 69 (64) | 53 (67) |
| 5–10% blasts | 14 (13) | 9 (11) |
| Unknown | 3 (3) | 3 (4) |
|
| ||
| Therapy received pre-HSCT, n (%) | 91 (84) | 67 (85) |
|
| ||
| IPSS-R at diagnosis, n (%) | ||
| Very low | 5 (5) | 3 (4) |
| Low | 21 (19) | 15 (19) |
| Intermediate | 31 (29) | 28 (35) |
| High | 19 (18) | 15 (19) |
| Very high | 21 (19) | 12 (15) |
| Data not available | 11 (10) | 6 (8) |
|
| ||
| IPSS-R at HSCT, n (%) | ||
|
| ||
| Very low | 5 (5) | 4 (5) |
| Low | 36 (33) | 30 (38) |
| Intermediate | 38 (35) | 25 (32) |
| High | 17 (16) | 11 (14) |
| Very high | 9 (8) | 6 (8) |
| Data not available | 3 (3) | 3 (4) |
|
| ||
| Therapy-related MDS, n (%) | 24 (22) | 13 (16) |
Study definitions and statistical analysis
GVHD was diagnosed clinically and confirmed histologically where appropriate. Acute and chronic GVHD were evaluated using International Bone Marrow Transplant Registry13 and National Institutes of Health consensus criteria,14 respectively. Cause of death was determined using the algorithm devised by Copelan et al., which employs a hierarchy of events to identify the underlying cause of death: For instance, if a patient dies of GvHD after donor lymphocyte infusion for relapse, relapse is the primary cause of death; if a patient dies of infection while on treatment for GvHD, GvHD is the cause of death.15 Disease risk and relapse were categorized using standard guidelines.16-18 In AML, as evaluation of molecular features including FLT3, NPM1, CEBPA, and c-kit mutational analysis became standard practice only during the course of the period studied, patients were stratified by cytogenetic features with incorporation of molecular data where available.
Kaplan-Meier methods estimated overall survival (OS) and relapse-free survival (RFS). Relapse and non-relapse mortality (NRM) were estimated with cumulative incidence functions. Given small numbers of patients with GVHD onset after the landmark, incidence of GVHD was characterized descriptively. All patients including those who underwent second allo-HSCT were included in accounts of GVHD. Association of characteristics with RFS and OS was evaluated using Cox regression. Variables significant to p≤0.05 were evaluated for association in a multivariable Cox regression model. Association between donor type and GVHD by 1 year was assessed using the Chi-square test. Competing risks analysis was performed using R version 3.2.4 (www.R-project.org). All other statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
Patient characteristics
A total of 416 patients underwent CD34-selected allogeneic PBSCT for AML, MDS, or ALL. Of these, 276 patients were alive without relapse at the designated 1-year landmark and were included in the analysis. Of those excluded, 114 had died before 1 year; the remaining 26 were alive with relapse at 1 year. Table 1 summarizes patient and transplant characteristics. Median CD34+ cell dose was 7.6 × 106 cells/kg (range 0.6–31.2 × 106), and median CD3+ T-cell dose was 1.9 × 103 cells/kg (range 0–63.0 × 103). Twenty-one patients (8%) received donor lymphocyte infusion (DLI) before the 1-year landmark, in 17 cases (81%) for mixed chimerism without relapse, in 2 cases for minimal residual disease, and in 2 cases to promote immune reconstitution in the setting of severe viral infection (JC virus and HHV-6). Nine patients in the landmark cohort (3%) had received a CD34-selected cell boost for poor graft function or secondary graft failure.
Table 1 summarizes disease-specific characteristics. In AML, 71% of patients were transplanted in CR1. The majority of patients had poor- (n=51, 31%) or intermediate-risk (n=95, 58%) disease. Mutations were present in 19/62 patients (31%) analyzed for FLT3 (internal tandem duplication or tyrosine kinase domain variant), 4/46 (9%) for isolated NPM1 mutations, and 0/22 for CEBPA mutations. Thirteen (8%) had c-kit mutation testing; one of these (8%) was positive, in conjunction with inversion of chromosome 16. Eighty-two percent of patients with MDS underwent allo-HSCT with morphologic evidence of disease, although the majority of patients (67%) had fewer than 5% blasts. In ALL, 82% of patients (n=27) were in CR1 at time of allo-HSCT. Sixty-one percent (n=20) had poor-risk cytogenetics, primarily B-ALL associated with t(9;22) in 18 patients. Twenty-one patients (8%) received post-transplant therapy for prevention of relapse, 17 with 5-azacitidine for high-risk myeloid disease and 4 with a tyrosine kinase inhibitor for Philadelphia chromosome–positive ALL.
Survival
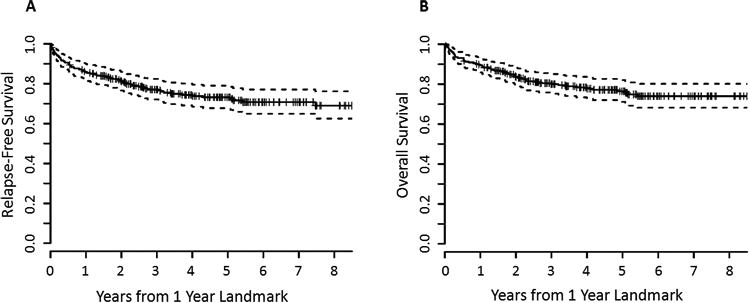
At median 5 years' follow-up after the 1-year landmark (range, 0.03–13 years), estimated 5-year RFS for the entire cohort was 73% (95% confidence interval [CI] 67–78%), and estimated 5-year OS was 76% (95% CI 70–81%) (Figure 1). In comparison, in patients surviving to 100 days without relapse, 5-year RFS was 56% (95% CI 61–61%) and OS 59% (95% CI 54–64%). Those surviving to 180 days without relapse had 5-year RFS of 61% (95% CI 56–67%) and OS 64% (95% CI 59–70%). Among all 416 patients transplanted, 5-year RFS from time of HSCT was 49% (95% CI 44–54%) and OS 52% (95% CI 48–58%) (Supplementary Figure).
Figure 1. Relapse-free and overall survival for all patients.
Kaplan-Meier estimates of (A) relapse-free survival and (B) overall survival for the entire cohort, measured in years after the 1-year landmark. Estimated 5-year RFS was 73% (95% CI 67–78%), and estimated 5-year OS was 76% (95% CI 70–81%).
In AML, estimated 5-year RFS was 77% (95% CI 69–83%), and OS was 80% (95% CI 72–85%) after the 1-year landmark. For MDS, estimated 5-year RFS and OS were 70% (95% CI 57–80%) and 71% (95% CI 57–81%), respectively. Estimated 5-year RFS and OS in ALL were 62% (95% CI 42–76%) and 72% (95% CI 53–84%), respectively.
Graft-versus-host disease
Before the 1-year landmark, 25 patients (9%) had developed grade I, 24 (9%) had developed grade II, and 16 (6%) grade III–IV acute GVHD. The remaining 211 patients (76%) had no acute GVHD at 1 year. Of the patients with grade III–IV acute GVHD, 3 had received DLI, though all had some GVHD preceding DLI. One patient had developed acute GVHD 32 days after a CD34-selected cell boost. Eighteen patients (7%) had developed chronic GVHD before 1 year, with 8 mild (3%), 6 moderate (2%), and 4 (1%) severe.
After the landmark, there were 4 cases (1%) of new acute GVHD, 3 of which followed a second allo-HSCT for relapse. None of these patients died of GVHD. Four patients (1%) developed new chronic GVHD after 1 year, which was mild in 2 patients and severe in 2 patients, of whom both died of GVHD. Recipients of mismatched donor grafts overall were at higher risk of grade ≥ II acute GVHD or moderate to severe chronic GVHD, which developed in 14% of patients receiving grafts from HLA-matched donors and 23% of patients with mismatched donors (p=0.05).
Relapse and non-relapse mortality
There were 28 relapses after the landmark with an overall 5-year cumulative incidence of relapse of 11% (95% CI,7–15%): 15 in AML (9%, 95% CI 5–14%), 4 in ALL (13%, 95% CI 1–26%), and 9 in MDS (13%, 95% CI 4–21%). Median time to relapse in patients with disease recurrence after the landmark was 25 months post-transplant (range, 12–119 months). Among patients with relapse events, 11 were alive at last follow-up, including 6 who underwent second allo-HSCT. There were 46 deaths without relapse, with a 5-year cumulative incidence of NRM of 16% (95% CI 12–21%).
Table 2 details causes of late mortality. GVHD, both acute and chronic, was the most common cause of death, accounting for 17 of 63 total deaths (27%), followed closely by relapse (n=16, 25%) and infection (n=12, 19%). Of deaths due to viral infection, 2 were attributed to EBV, 2 to JC virus, and 1 to CMV. Six patients died of other malignancies, including 3 new cancers and 3 recurrences of unrelated cancers that had previously been in remission before transplant. In contrast, among patients excluded from the landmark analysis due to death or relapse before 1 year, relapse was the most common cause of death (n=58 of 136 total deaths, 43%), followed by infection (n=39, 29%; 15 viral, 14 bacterial, 2 parasitic, 1 fungal, 7 multiple/unknown), organ failure (n=18, 13%), GVHD (n=14, 10%), primary graft failure (n=2, 1%), and other or unknown causes (n=5, 4%). In these patients, deaths from viral infection were caused by Adenovirus (n = 5) in addition to EBV (n = 5) and CMV (n = 4).
Table 2. Causes of death among patients who died after the 1-year landmark.
| Total deaths, n | 63 |
|---|---|
|
| |
| GVHD, n (%) | 17 (27) |
| Acute, n | 9 |
| Chronic, n | 6 |
| Acute/chronic, n | 2 |
|
| |
| Relapse, n (%) | 16 (25) |
| Relapse within 2 years of HSCT, n | 9 |
|
| |
| Infection, n (%) | 12 (19) |
| Viral, n | 5 |
| Multiple or unknown, n | 7 |
|
| |
| Organ failure, n (%) | 9 (14) |
| Multiple, n | 2 |
| Pulmonary, n | 3 |
| Cardiac, n | 1 |
| Liver, n | 2 |
| Renal, n | 1 |
|
| |
| Other malignancy, n (%) | 6 (10) |
| Secondary malignancy, n | 3 |
| Recurrence of other primary malignancy, n | 3 |
|
| |
| Other/unknown, n (%) | 3 (5) |
Factors associated with late prognosis
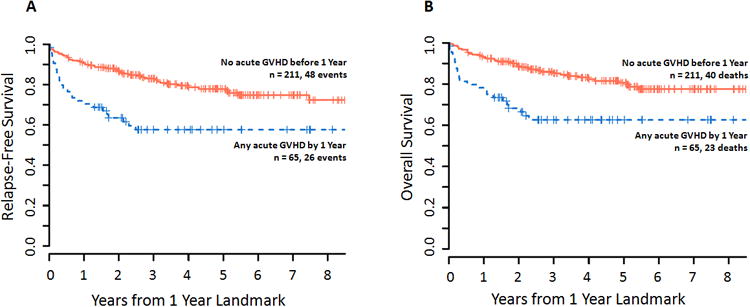
In univariate analysis (Table 3), increasing age (HR per 10 years 1.30, 95% CI 1.04–1.62, p=0.02), HCT-CI ≥ 3 (HR 2.51, 95% CI 1.28–4.90, p=0.007), and acute GVHD by 1 year (HR 2.32, 95% CI 1.39–3.88, p=0.001; figure 3), particularly grade III–IV (HR 5.61, 95% CI 2.81–11.22, p<0.0001), significantly correlated with poorer OS; similar associations were found for RFS. Chronic GVHD by 1 year did not significantly associate with RFS or OS.
Table 3. Results of univariate and multivariate Cox regression analyses for association of patient and transplant factors with relapse-free and overall survival.
| Characteristic | Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RFS | OS | RFS | OS | ||||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||
| Age, per 10 year | 1.24 (1.01, 1.51) | 0.04 | 1.30 (1.04, 1.62) | 0.02 | 1.16 (0.89, 1.50) | 0.27 | 1.17 (0.92, 1.48) | 0.19 | |
| HCT-CI | 0 | 1.0 (reference) | 0.02 | 1.0 (reference | 0.003 | 1.0 (reference) | 0.02 | 1.0 (reference) | 0.004 |
| 1–2 | 0.85 (0.43,1.67) | 1.06 (0.48,2.34) | 0.83 (0.42,1.65) | 1.05 (0.47, 2.33) | |||||
| ≥ 3 | 1.77 (0.99,3.16) | 2.51 (1.28,4.9) | 1.78 (0.97,3.28) | 2.53 (1.26, 5.08) | |||||
| KPS | 60–80 | 1.0 (reference) | 0.42 | 1.0 (reference) | 0.09 | ||||
| 90–100 | 0.82 (0.52, 1.32) | 0.65 (0.39, 1.07) | |||||||
| Donor type | MRD | 1.0 (reference) | 0.10 | 1.0 (reference) | 0.06 | ||||
| MUD | 1.03 (0.57,1.85) | 0.91 (0.47,1.74) | |||||||
| MMRD | 2.32 (0.8,6.69) | 2.6 (0.89,7.57) | |||||||
| MMUD | 1.75 (1,3.06) | 1.76 (0.97,3.19) | |||||||
| Conditioning regimen | TBI-based | 1.0 (reference) | 0.04 | 1.0 (reference) | 0.07 | 1.0 (reference) | 0.90 | ||
| Chemo-based | 1.67 (1.01, 2.76) | 1.65 (0.96, 2.83) | 1.04 (0.55, 1.97) | ||||||
| CD34 selectionmethod | ISOLEX | 1.0 (reference) | 0.88 | 1.0 (reference) | 0.61 | ||||
| CliniMACS | 1.04 (0.63, 1.71) | 0.87 (0.50, 1.51) | |||||||
| Acute GVHD by 1 year | No | 1.0 (reference) | 0.002 | 1.0 (reference) | 0.001 | ||||
| Yes | 2.17 (1.34,3.50) | 2.32 (1.39,3.88) | |||||||
| Acute GVHD grade by 1 year | 0–1 | 1.0 (reference) | <0.0001 | 1.0 (reference) | <0.0001 | 1.0 (reference) | <0.0001 | 1.0 (reference) | <0.0001 |
| 2 | 1.97 (0.97,4.02) | 2.62 (1.27, 5.39) | 2.07 (1.01, 4.27) | 2.90 (1.39, 6.04) | |||||
| 3–4 | 5.43 (2.82,10.46) | 5.61 (2.81, 11.22) | 6.14 (3.12, 12.07) | 6.14 (3.05, 12.36) | |||||
| Chronic GVHD by 1 year | No | 1.0 (reference) | 0.62 | 1.0 (reference) | 0.336 | ||||
| Yes | 1.24 (0.54,2.85) | 1.51 (0.65,3.51) | |||||||
Figure 3. Survival according to presence of acute GVHD before 1 year.
Kaplan-Meier estimates of (A) relapse-free survival and (B) overall survival according to the presence of acute GVHD of any grade, measured in years after the 1-year landmark. In univariate analysis, the presence of acute GVHD before 1 year was significantly associated with poorer RFS (HR 2.17, 95% CI 1.34–3.50, p=0.002) and poorer OS (HR 2.32, 95% CI 1.39–3.88, p=0.001).
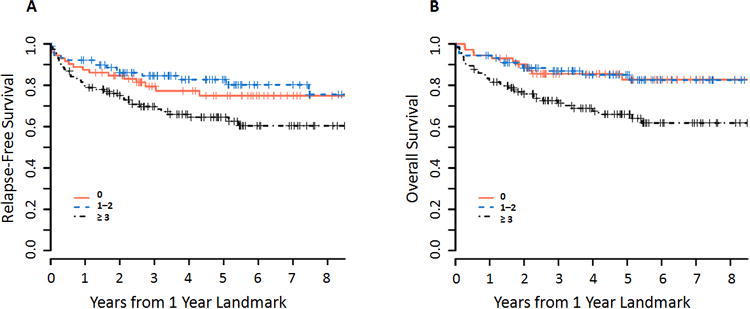
In multivariate analyses controlling for age, HCT-CI, and grade of acute GVHD by 1 year, patients with HCT-CI scores of 3 or higher had significantly worse OS (HR 2.53, 95% CI 1.26–5.08, p=0.009) and marginally worse RFS (HR 1.78, 95% CI 0.98–3.28, p=0.06), compared with those with a score of 0. Patients with an HCT-CI score of 1–2 had a similar RFS and OS (Table 3, Figure 2). Increasing grades of acute GVHD were also associated with progressively worse RFS and OS by multivariable analysis (Table 3, Figure 4), with grade III–IV portending the worst prognosis.
Figure 2. Survival according to HCT-CI.
Kaplan-Meier estimates of (A) relapse-free survival and (B) overall survival according to HCT-CI score, measured in years after the 1-year landmark. On multivariate analysis, HCT-CI score of 3 or greater was associated with marginally poorer RFS (HR 1.78, 95% CI 0.97–3.20, p=0.06) and significantly poorer OS (HR 2.53, 95% CI 1.26–5.08, p=0.009).
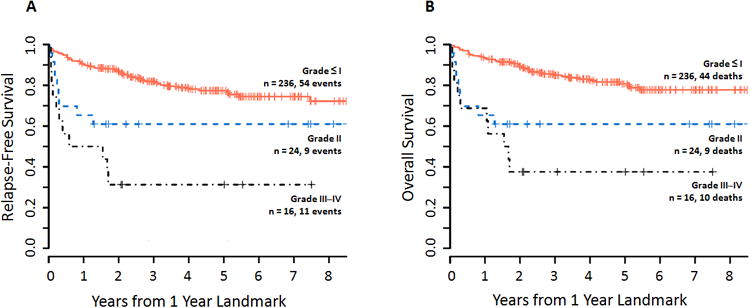
Figure 4. Survival according to grade of acute GVHD prior to 1 year.
Kaplan-Meier estimates of (A) relapse-free survival and (B) overall survival according grade of acute GVHD, measured in years after the 1-year landmark. Higher grades of acute GVHD correlated with progressively worse survival, with grade III–IV acute GVHD associated with the poorest RFS and OS. On multivariate analysis, grade II and grade III–IV aGVHD were associated with increasingly worse RFS (grade II: HR 2.07, 95% CI 1.01–4.27, p=0.05; grade III–IV: HR 6.14, 95% CI 3.12–12.07; p<0.001) and OS (grade II: HR 2.90, 95% CI 1.39–6.04, p=0.005; grade III–IV: HR 6.14, 95% CI 3.05–12.36, p<0.001).
Use of chemotherapy-based conditioning correlated with poorer RFS but not OS on univariate analysis. This association was not significant on multivariable analysis (TBI HR 1.0 [reference]; chemo HR 1.04, 95% CI 0.55–1.97, p=0.90). There was no association detected between the method of CD34 selection (ISOLEX versus CliniMACS) and RFS or OS.
Table 4 shows results of univariate analysis of the effect of disease-related factors on survival. Intermediate- or high-risk AML correlated with marginally worse RFS on univariate analysis (p=0.08), with HR of 5.16 for intermediate risk (95% CI 0.69–38.37, p=0.11) and 7.98 for poor risk (95% CI 1.05–60.39, p=0.04). The difference in OS was not significant (p=0.25). Among patients with ALL, entering transplant in CR2 or with refractory disease significantly correlated with worse RFS compared with CR1, with HR 4.56 (95% CI 1.41–14.79, p=0.01), and marginally correlated with poorer OS (HR 3.51, 95% CI 0.94–12.12, p=0.06). In MDS, none of the evaluated disease features, including pre-HSCT disease status, cytogenetic risk, or IPSS-R at diagnosis or before HSCT, corresponded with a significant difference in RFS or OS.
Table 4. Results of univariate Cox regression analysis for association of disease-specific factors with relapse-free and overall survival.
| Characteristic | RFS | OS | |||
|---|---|---|---|---|---|
|
| |||||
| HR (95% CI) | p | HR (95% CI) | p | ||
|
| |||||
| AML | |||||
|
| |||||
| Disease status at HSCT | CR1 | 1.0 (reference) | 0.76 | 1.0 (reference) | 0.67 |
| CR2 | 1.22 (0.66, 2.28) | 1.25 (0.63, 2.45) | |||
| PR/refractory | 1.35 (0.41, 4.40) | 1.57 (0.48, 5.16) | |||
|
| |||||
| Cytogenetic/ molecular risk | Favorable | 1.0 (reference) | 0.08 | 1.0 (reference) | 0.25 |
| Intermediate | 5.16 (0.69, 38.37) | 4.78 (0.64, 35.65) | |||
| Poor | 7.98 (105, 60.39) | 5.73 (0.74, 44.25) | |||
|
| |||||
| Therapy-related AML | No Yes | 1.0 (reference) 1.87 (0.86, 4.07) | 0.12 | 1.0 (reference) 1.85 (0.80, 4.25) | 0.15 |
| Yes | 1.87 (0.86, 4.07) | 1.85 (0.80, 4.25) | |||
|
| |||||
| ALL | |||||
|
| |||||
| Disease status at HSCT | CR1 | 1.0 (reference) | 0.01 | 1.0 (reference) | 0.06 |
| Non-CR1 | 4.56 (1.41, 14.79) | 3.51 (0.94, 12.12) | |||
|
| |||||
| Cytogenetic risk | Standard/good | 1.0 (reference) | 0.91 | 1.0 (reference) | 0.57 |
| Poor | 1.07 (0.34, 3.37) | 1.50 (0.37, 6.01) | |||
|
| |||||
| BCR-ABL positivity | No | 1.0 (reference) | 0.48 | 1.0 (reference) | 0.21 |
| Yes | 1.64 (0.42, 6.35) | 3.92 (0.47, 32.63) | |||
|
| |||||
| Elevated WBC count on diagnosis | No | 1.0 (reference) | 0.56 | 1.0 (reference) | 0.81 |
| Yes | 0.65 (0.16, 2.73) | 0.80 (0.13, 4.80) | |||
|
| |||||
| MDS | |||||
|
| |||||
| Disease status at HSCT | CR | 1.0 (reference) | 0.22 | 1.0 (reference) | 0.30 |
| Non-CR | 2.49 (0.58, 10.61) | 2.15 (0.50, 9.29) | |||
|
| |||||
| Cytogenetic risk | Good | 1.0 (reference) | 0.11 | 1.0 (reference) | 0.21 |
| Intermediate | 3.11 (0.97, 9.92) | 2.35 (0.71, 7.80) | |||
| Poor/very poor | 3.32 (1.02, 10.8) | 2.88 (0.87, 9.58) | |||
|
| |||||
| IPSS-R risk on diagnosis | Very low/low | 1.0 (reference) | 0.23 | 1.0 (reference) | 0.18 |
| Intermediate | 1.01 (0.28, 3.57) | 1.06 (0.25, 4.45) | |||
| High/very high | 2.09 (0.67, 6.5) | 2.46 (0.68, 8.81) | |||
|
| |||||
| IPSS-R risk at HSCT | Very low/low | 1.0 (reference) | 0.06 | 1.0 (reference) | 0.09 |
| Intermediate | 3.63 (1.26, 10.45) | 3.71 (1.14, 12.07) | |||
| High/very high | 2.39 (0.73, 7.82) | 2.89 (0.81, 10.23) | |||
Discussion
This study demonstrates that patients who receive CD34-selected allo-HSCT for AML, ALL, or MDS and survive without relapse past 1 year have an excellent prognosis. The high probability of long-term RFS and OS and low incidence of late relapse seen in our study meet or exceed those same outcomes in earlier landmark analyses of survivors of myeloablative allo-HSCT that did not distinguish between TCD and unmodified grafts.11, 12 Most recently, Lee et al. reported adult patients with acute leukemia who underwent allo-HSCT between 1990 and 2005, and were alive and relapse-free at 1 year, noting a 5-year RFS of 78% in AML and 71% in ALL,12 similar to that seen in our cohort. Of note, the median ages of patients included in that prior analysis were 37 years in AML and 29 years in ALL, with a minority of patients over 50 years, in contrast to a median age of 54 years in this study population. Prior comparisons of CD34-selected allo-HSCT with unmodified grafts have likewise shown comparable survival between the two approaches despite older patient age in the CD34-selected cohorts.8, 9 These results raise the possibility that CD34-selected allo-HSCTs might allow older patients to receive the antileukemic benefit of myeloablative conditioning without excessive risk of late mortality.
Late mortality persists due to both relapse and NRM, however; and we have identified risk factors that represent key areas of unmet need. Among patient-specific factors with prognostic significance, an HCT-CI score of 3 or greater correlated with poorer survival, a finding concordant with our analyses elsewhere of CD34-selected allo-HSCT for AML and MDS.19 Also consistent with our prior findings, patients in this cohort with an HCT-CI score of 1–2 had similar long-term survivals to those with a score of 0. This indicates that the use of CD34 selection attenuates late NRM in such patients with more modest comorbidity burdens compared with unmodified myeloablative allo-HSCT, and that such patients may be optimal candidates for consideration of CD34 selected allo-HSCT. Further research should enhance our understanding of the influence exerted by comorbidities in the CD34 selection setting, which will in turn optimize patient selection. Patients with more substantial comorbidities remain in need of additional strategies to attenuate NRM.
Although CD34 selection resulted in a low incidence of acute and chronic GVHD, those few patients who had developed acute GVHD at any time before 1 year, particularly grade III–IV, had a poor prognosis. GVHD, though uncommon after the 1-year landmark, was the most common cause of death. Even with CD34 selection, the use of mismatched donor allografts and DLI in particular expose patients to heightened risk of GVHD. We did not detect a difference in survival for patients with chronic GVHD, which has been identified as a risk factor for late mortality in the previous CIBMTR landmark analyses.11, 12 With very few patients with chronic GVHD, however, our sample was likely too small to detect a difference, and despite an extremely small number of patients with moderate to severe chronic GVHD before or after the 1-year landmark, a disproportionate number died of chronic GVHD or an acute/chronic GVHD overlap syndrome. Our data thus highlight the need for further strategies to recognize patients at heightened risk of both acute and chronic GVHD despite CD34 selection, to diagnose GVHD at an earlier stage, and to manage it effectively.
Infection, especially viral infection, was another major cause of late mortality, underscoring the role of post-transplant lymphopenia, which is more pronounced with CD34 selection.20, 21 Novel strategies to enhance immune reconstitution after CD34-selected allo-HSCT are indicated.22 Of particular interest, as the use of CD34 selection obviates the need for pharmacologic GVHD prophylaxis, it represents a suitable platform for the study and implementation of cell- and immune-based approaches to enhance post-transplant immunity without increasing risk of GVHD, including the use of cytotoxic lymphocytes directed against viral or leukemic antigens, checkpoint inhibitors, and cytokine-based therapies.
The modest contribution of many disease-related factors including cytogenetic risk and remission status on long-term prognosis indicates that the effect of such variables recedes over time in patients who are able to survive without relapse past the initial post-HSCT period. Although our analysis was limited by incomplete data regarding pre-HSCT minimal residual disease (MRD), which portends a high risk of post-HSCT relapse in both AML and ALL,23-25 this effect largely influences relapse earlier post-transplant. The specific impact on late relapse remains unknown. We also lacked complete data on molecular markers in AML for the majority of patients, including the presence of FLT3, NPM1, CEBPA, or c-kit mutations, due to the retrospective design of this analysis. Such information would have facilitated more complete risk stratification by contemporary methods and will be incorporated in future analyses.
While encouraging, these retrospective findings require prospective confirmation. To this end, TCD is currently under investigation in a national phase III randomized trial, BMT CTN 1301 (NCT02345850), comparing CD34 selection with post-transplant cyclophosphamide versus standard GVHD prophylaxis with tacrolimus and methotrexate.
In summary, for the patient who undergoes CD34-selected PBSCT and is alive without disease at the 1-year landmark, long-term survival is excellent in the absence of substantial comorbidities and GVHD. Efforts to improve identification of patients at risk for late events, and strategies to treat such patients while taking advantage of the properties of the CD34 selection platform, constitute vital areas of future research to improve even further on these outcomes.
Supplementary Material
Footnotes
Financial Disclosures: This research was supported in part by National Institutes of Health award number P01 CA23766 and NIH/NCI Cancer Center Support Grant P30 CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Pasquini MC, Devine S, Mendizabal A, Baden LR, Wingard JR, Lazarus HM, et al. Comparative outcomes of donor graft CD34+ selection and immune suppressive therapy as graft-versus-host disease prophylaxis for patients with acute myeloid leukemia in complete remission undergoing HLA-matched sibling allogeneic hematopoietic cell transplantation. J Clin Oncol. 2012;30(26):3194–201. doi: 10.1200/JCO.2012.41.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papadopoulos EB, Carabasi MH, Castro-Malaspina H, Childs BH, Mackinnon S, Boulad F, et al. T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: freedom from relapse in the absence of graft-versus-host disease. Blood. 1998;91(3):1083–90. [PubMed] [Google Scholar]
- 3.Jakubowski AA, Small TN, Young JW, Kernan NA, Castro-Malaspina H, Hsu KC, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110(13):4552–9. doi: 10.1182/blood-2007-06-093880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jakubowski AA, Small TN, Kernan NA, Castro-Malaspina H, Collins N, Koehne G, et al. T cell-depleted unrelated donor stem cell transplantation provides favorable disease-free survival for adults with hematologic malignancies. Biol Blood Marrow Transplant. 2011;17(9):1335–42. doi: 10.1016/j.bbmt.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devine SM, Carter S, Soiffer RJ, Pasquini MC, Hari PN, Stein A, et al. Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: results of the blood and marrow transplant clinical trials network protocol 0303. Biol Blood Marrow Transplant. 2011;17(9):1343–51. doi: 10.1016/j.bbmt.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro-Malaspina H, Jabubowski AA, Papadopoulos EB, Boulad F, Young JW, Kernan NA, et al. Transplantation in remission improves the disease-free survival of patients with advanced myelodysplastic syndromes treated with myeloablative T cell-depleted stem cell transplants from HLA-identical siblings. Biol Blood Marrow Transplant. 2008;14(4):458–68. doi: 10.1016/j.bbmt.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg JD, Linker A, Kuk D, Ratan R, Jurcic J, Barker JN, et al. T cell-depleted stem cell transplantation for adults with high-risk acute lymphoblastic leukemia: long-term survival for patients in first complete remission with a decreased risk of graft-versus-host disease. Biol Blood Marrow Transplant. 2013;19(2):208–13. doi: 10.1016/j.bbmt.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bayraktar UD, de Lima M, Saliba RM, Maloy M, Castro-Malaspina HR, Chen J, et al. Ex vivo T cell-depleted versus unmodified allografts in patients with acute myeloid leukemia in first complete remission. Biol Blood Marrow Transplant. 2013;19(6):898–903. doi: 10.1016/j.bbmt.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hobbs GS, Hamdi A, Hilden PD, Goldberg JD, Poon ML, Ledesma C, et al. Comparison of outcomes at two institutions of patients with ALL receiving ex vivo T-cell-depleted or unmodified allografts. Bone Marrow Transplant. 2015;50(4):493–8. doi: 10.1038/bmt.2014.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamari R, Chung SS, Papadopoulos EB, Jakubowski AA, Hilden P, Devlin SM, et al. CD34-Selected Hematopoietic Stem Cell Transplants Conditioned with Myeloablative Regimens and Antithymocyte Globulin for Advanced Myelodysplastic Syndrome: Limited Graft-versus-Host Disease without Increased Relapse. Biol Blood Marrow Transplant. 2015;21(12):2106–14. doi: 10.1016/j.bbmt.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wingard JR, Majhail NS, Brazauskas R, Wang Z, Sobocinski KA, Jacobsohn D, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29(16):2230–9. doi: 10.1200/JCO.2010.33.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SJ, Storer B, Wang H, Lazarus HM, Waller EK, Isola LM, et al. Providing personalized prognostic information for adult leukemia survivors. Biol Blood Marrow Transplant. 2013;19(11):1600–7. doi: 10.1016/j.bbmt.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowlings PA, Przepiorka D, Klein JP, Gale RP, Passweg JR, Henslee-Downey PJ, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97(4):855–64. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 14.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–56. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Copelan E, Casper JT, Carter SL, van Burik JA, Hurd D, Mendizabal AM, et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biol Blood Marrow Transplant. 2007;13(12):1469–76. doi: 10.1016/j.bbmt.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 16.O'Donnell MR, Abboud CN, Altman J, Appelbaum FR, Arber DA, Attar E, et al. Acute myeloid leukemia. J Natl Compr Canc Netw. 2012;10(8):984–1021. doi: 10.6004/jnccn.2012.0103. [DOI] [PubMed] [Google Scholar]
- 17.Alvarnas JC, Brown PA, Aoun P, Ballen KK, Barta SK, Borate U, et al. Acute Lymphoblastic Leukemia, Version 2.2015. J Natl Compr Canc Netw. 2015;13(10):1240–79. doi: 10.6004/jnccn.2015.0153. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg PL, Stone RM, Bejar R, Bennett JM, Bloomfield CD, Borate U, et al. Myelodysplastic syndromes, version 2.2015. J Natl Compr Canc Netw. 2015;13(3):261–72. doi: 10.6004/jnccn.2015.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barba P, Ratan R, Cho C, Ceberio I, Hilden P, Devlin SM, et al. The Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI) Predicts Outcomes in Patients with Acute Myeloid Leukemia and Myelodysplastic Syndromes Receiving CD34+ Selected Grafts for Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2016 doi: 10.1016/j.bbmt.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Small TN, Avigan D, Dupont B, Smith K, Black P, Heller G, et al. Immune reconstitution following T-cell depleted bone marrow transplantation: effect of age and posttransplant graft rejection prophylaxis. Biol Blood Marrow Transplant. 1997;3(2):65–75. [PubMed] [Google Scholar]
- 21.Small TN, Papadopoulos EB, Boulad F, Black P, Castro-Malaspina H, Childs BH, et al. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999;93(2):467–80. [PubMed] [Google Scholar]
- 22.Perales MA, Goldberg JD, Yuan J, Koehne G, Lechner L, Papadopoulos EB, et al. Recombinant human interleukin-7 (CYT107) promotes T-cell recovery after allogeneic stem cell transplantation. Blood. 2012;120(24):4882–91. doi: 10.1182/blood-2012-06-437236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Araki D, Wood BL, Othus M, Radich JP, Halpern AB, Zhou Y, et al. Allogeneic Hematopoietic Cell Transplantation for Acute Myeloid Leukemia: Time to Move Toward a Minimal Residual Disease-Based Definition of Complete Remission? J Clin Oncol. 2016;34(4):329–36. doi: 10.1200/JCO.2015.63.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhedin N, Huynh A, Maury S, Tabrizi R, Beldjord K, Asnafi V, et al. Role of allogeneic stem cell transplantation in adult patients with Ph-negative acute lymphoblastic leukemia. Blood. 2015;125(16):2486–96. doi: 10.1182/blood-2014-09-599894. quiz 586. [DOI] [PubMed] [Google Scholar]
- 25.Bar M, Wood BL, Radich JP, Doney KC, Woolfrey AE, Delaney C, et al. Impact of minimal residual disease, detected by flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute lymphoblastic leukemia. Leuk Res Treatment. 2014;2014:421723. doi: 10.1155/2014/421723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


