Abstract
Background
The paradigm shift from crystalloid to plasma resuscitation of traumatic hemorrhagic shock has improved patient outcomes due in part to plasma-mediated reversal of catecholamine and inflammation-induced endothelial injury, decreasing vascular permeability and attenuating organ injury. Since sepsis induces a similar endothelial injury as seen in hemorrhage, we hypothesized that plasma resuscitation would increase 48-hour survival in a rat sepsis model.
Methods
Adult male Sprague-Dawley rats (375–425g) were subjected to 35% cecal ligation and puncture (CLP) (t=0h). Twenty-two hours post-CLP and prior to resuscitation (t=22h), animals were randomized to resuscitation with normal saline (NS, 10 cc/kg/hr) or pooled rat fresh frozen plasma (FFP, 3.33 cc/kg/hr). Resuscitation under general anesthesia proceeded for the next six hours (t=22h to t=28h); lactate was checked every 2 hours, and fluid volumes were titrated based on lactate clearance. Blood samples were obtained before (t=22 h) and after resuscitation (t=28 h), and at death or study conclusion. Lung specimens were obtained for calculation of wet-to-dry weight ratio. Fisher’s exact test was used to analyze the primary outcome of 48-hour survival. ANOVA with repeated measures was used to analyze the effect of FFP versus NS resuscitation on blood gas, electrolytes, blood urea nitrogen (BUN), creatinine, interleukin (IL)-6, IL-10, catecholamines, and syndecan-1 (marker for endothelial injury). A two-tailed alpha level of <0.05 was used for all statistical tests.
Results
Thirty-three animals were studied: 14 FFP, 14 NS, and 5 sham. Post-CLP but pre-resuscitation (t=22 h) variables between FFP and NS animals were similar and significantly deranged compared to sham animals. FFP significantly increased 48-hour survival compared to NS (n=8 [57%] vs n=2 [14%]), attenuated the post-resuscitation (t=28 h) levels of epinephrine (mean 2.2 vs 7.0 ng/ml), norepinephrine, (3.8 vs 8.9 ng/ml), IL-6 (3.8 vs 18.7 ng/ml), and syndecan-1 (21.8 vs 31.0 ng/ml) (all p<0.05), improved the post-resuscitation PO2 to FiO2 ratio (353 vs 151), and reduced the pulmonary wet-to-dry weight ratio (5.28 vs 5.94) (all p<0.05).
Conclusion
Compared to crystalloid, plasma resuscitation increased 48-hour survival in a rat sepsis model, improved pulmonary function and decreased pulmonary edema, and attenuated markers for inflammation, endothelial injury, and catecholamines.
Keywords: septic shock, fluid therapy, endothelium
Introduction
Sepsis is defined as a life-threatening syndrome of organ dysfunction secondary to infection (1). In addition to a vigilant screening program to enable early identification of at-risk individuals, successful treatment of sepsis requires three components: prompt antibiotic therapy, hemodynamic support with fluids and vasopressors, and adequate source control (2). Fluid resuscitation is an essential component, but the optimal fluid for the initial resuscitation of sepsis remains unclear (3). The most recent Surviving Sepsis Campaign guidelines recommend at least 30 ml/kg bolus of crystalloids for the hypotensive septic patient, but which crystalloid to use is not specified (2). Despite possible harm related to infusion of 0.9% sodium chloride or normal saline (NS) (4), it remains the most prescribed fluid therapy worldwide (5), especially for hypovolemic patients.
Several inflammatory conditions including trauma (6) and sepsis (7) are known to cause endothelial injury (“endotheliopathy”), resulting in increased vascular permeability to fluid and proteins resulting in organ edema and injury (8). Fluid therapy for traumatic hemorrhagic shock has undergone a paradigm shift in the last twenty years with the transition from crystalloid and artificial colloid based resuscitation to the use of plasma as the primary volume expander (9), leading to improved survival (10, 11) and decreased incidence of inflammatory (12) and edema-related complications (13). Clinical studies and animal models (14, 15, 16, 17) have demonstrated that part of this benefit is derived from plasma-mediated reversal of trauma-induced endothelial injury, restoration of endothelial integrity, and decreased vascular permeability, although the exact underlying mechanism is not fully understood. Since sepsis induces a similar endothelial injury as seen in traumatic hemorrhagic shock, plasma could confer similar benefits in the setting of sepsis. We used a rat sepsis model of cecal ligation and puncture (CLP) for this experiment, which induces a clinically-relevant polymicrobial sepsis (18,19). We hypothesized that resuscitation with rat fresh frozen plasma (FFP) would result in reduced 48-hour mortality due to reversal of catecholamine and inflammation-induced endothelial injury compared to resuscitation with normal saline (NS) in a rat CLP sepsis model.
Methods
Animals studied
After approval from the Institutional Animal Care and Use Committee of the McGovern Medical School (protocol number AWC-15-0117), this protocol was performed in accordance with the Animal Welfare Act and other Federal statutes and regulations relating to animal experiments, as well as the Guide for the Care and Use of Laboratory Animals of the National Academy of Sciences and the ARRIVE guidelines. Adult male Sprague-Dawley rats (Envigo RMS, Indianapolis, IN) weighing 375–425g were used. All animals were allowed at least 3 days of acclimation after arrival and were housed at the UTHealth Center for Laboratory Animal Medicine and Care (CLAMC) at 25°C under 12-hour day/night cycles. Animals were housed in pairs prior to CLP and individually after CLP, and had access to food and water ad libitum throughout the experiment.
Collection of donor plasma
Rat FFP was collected from donor animals as previously described (20). Briefly, rat whole blood was collected by cardiac puncture into citrated tubes and separated into components. The plasma was aliquoted and frozen within one hour of collection. Rat FFP was stored at −80°C until use. Prior to infusion, FFP from 3 different donors was thawed, warmed to room temperature, and pooled to minimize the potential impact of donor plasma variability among individual animals. Rat FFP was used instead of human FFP to eliminate potential cross-species reactions.
Experimental protocol
The experimental protocol is summarized in Figure 1. Animals undergoing CLP were randomized by computerized sequence to resuscitation with FFP or NS at 1:1 allocation and block size of 2. CLP was performed as previously described (18, 19) on a pair of animals (one NS and one FFP) per morning in the animal laboratory. Briefly, after general anesthesia was induced by isoflurane (2–3% isoflurane in 97–98% O2) via nose cone, the cecum was exposed by laparotomy. The distal 35% of the cecum was suture ligated, then punctured through-and-through with a 16-gauge needle. The cecum was gently squeezed to exude stool; this was weighed, and 150mg of stool was placed on the puncture site. The stool burden was quantified to reduce model variability. The abdomen was then closed. The animals were awakened from anesthesia and returned to their home cages in the animal housing facility to recover from surgery. A previous study reported that “milking” stool from the ascending colon to the cecum, followed by 35% cecal ligation, through-and-through puncture with a 20-gauge needle, and extrusion of all stool distal to the ligation resulted in 90% mortality by 48 hours (18).
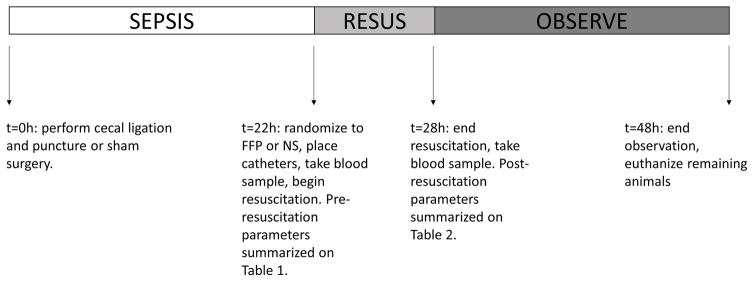
Figure 1.
Timeline of experimental protocol. CLP was performed at t=0h. Resuscitation was performed from 22 hours to 28 hours after CLP (t=22h to t=28h). Blood samples were drawn immediately before and after resuscitation. Blood samples and lung specimens were obtained at time of euthanasia. Animals were observed up to 48 hours after CLP. CLP, cecal ligation and puncture.
Fluid resuscitation was begun 22 hours after CLP (t=22h). During model development, we found that initiating fluid resuscitation at t=22h resulted in our target 90% mortality at 48 hours in the control (NS) arm. Animals were returned to the laboratory and placed under isoflurane (2% isoflurane in 98% O2) by nose cone. Catheters (3-French) were inserted into the femoral artery and vein by cut-down technique. The femoral artery catheter was used for blood sampling and was connected to a PowerLab 8-channel recorder with LabChart 8 software (AD Instruments, Colorado Springs, CO) for continuous hemodynamic monitoring. The femoral vein catheter was used for all fluid infusions. The resuscitation period lasted 6 hours. During resuscitation, isoflurane was titrated to the minimum level required to maintain zero animal movement on toe pinch. Generally, this was 2% isoflurane (in 98% O2) at the beginning of resuscitation, and ≤1% isoflurane by the end of resuscitation. All animals were breathing spontaneously. While animals were under anesthesia, death was defined as respiratory arrest.
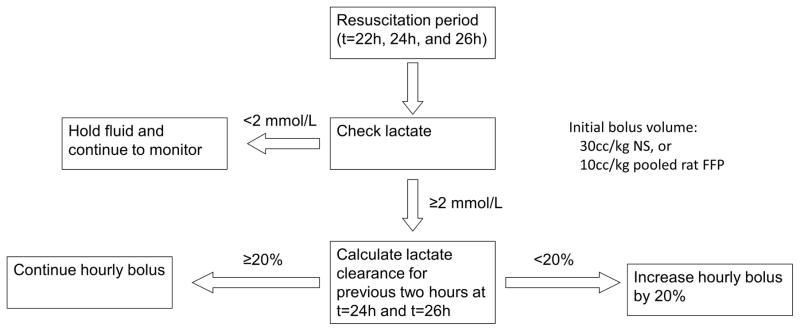
Fluids were administered as hourly boluses during the 6-hour resuscitation period. The initial bolus volume for the first two hours was 3.33 ml/kg/hr of FFP or 10 ml/kg/hr of NS, consistent with the most recent Surviving Sepsis Campaign Guidelines that 30 ml/kg of crystalloid should be administered within the first 3 hours of resuscitation (2). Afterward, further resuscitation volumes were adjusted based on the lactate clearance (Figure 2). The arterial lactate level was measured on a point-of-care device (NovaVet Statsensor Xpress, Nova Biomedical, Waltham, MA) every two hours (at t=22 h, 24h, and 26h), and fluid resuscitation was withheld for lactate ≤2 mmol/L. Otherwise, the lactate clearance was calculated as 100%*(lactateprevious – lactatecurrent) / lactateprevious. If the lactate clearance was <20% (i.e. <10% per hour), then the bolus volume for each of the next two hours was increased by 20%. If the lactate clearance was ≥20%, then the bolus volume was not changed. This resuscitation algorithm was chosen in lieu of fixed-volume resuscitation because lactate is a physiologic measure of global perfusion, and lactate clearance has been proposed as a guide for fluid resuscitation in septic patients (21). After the end of the resuscitation period (t=28 hours), animals were awakened from anesthesia and closely monitored for the next 20 hours (until t=48 hours). We monitored animals using a predefined scoring system which gave 1–4 points in each of six aspects: grooming, activity level, posture, facial expression, and presence of diarrhea or respiratory distress (Supplement) (22). Moribund animals were defined as those reaching a score of 18 or higher at any time and were immediately euthanized by cardiac puncture under anesthesia. The primary outcome was survival to 48 hours; all surviving animals were euthanized. Blood and lung tissue were obtained from all euthanized animals for analysis. Similar to previous studies (18, 23), no antibiotics or vasopressors were used in this model.
Figure 2.
Fluid resuscitation algorithm. Fluid therapy was delivered as an hourly bolus during the 6-hour resuscitation period (t=22h to t=28h). Point-of-care lactate level was checked every two hours (at t=22, 24, and 26 h). At t=24h and t=26h, the next two resuscitation bolus volumes were adjusted based on the lactate clearance from the previous two hours.
Sham animals
To obtain the appropriate controls for our laboratory studies, 5 animals underwent a sham procedure (negative controls). In sham animals, laparotomy was performed at t=0h with cecal manipulation only (no ligation or puncture). At t=22h, sham animals underwent femoral artery and vein catheterization under anesthesia. From t=22h to t=28h, they were kept under anesthesia by nose cone, and no fluids were administered. Animals were awakened from anesthesia at t=28h and euthanized at t=48 hours.
Blood sample analysis
Blood samples were drawn at t=22h (pre-resuscitation), t=28h (post resuscitation), and at time of euthanasia for FFP, NS, and sham animals. We obtained 2 ml of arterial blood for pre and post-resuscitation samples, of which 150μL were analyzed immediately on a pHOx Ultra Critical Care Analyzer (Nova Biomedical) for blood gas, electrolytes, and hematocrit. The remainder was centrifuged at 3200g for 10 minutes, and the plasma was saved for later analysis (stored at −80°C). To negate the impact of anemia, red blood cells were resuspended in 0.5 ml FFP (FFP group) or 1.5 ml NS (NS and sham groups) and auto-transfused back into the animal. These NS and FFP volumes were included in the total resuscitation volume administered. Terminal blood samples were collected at time of animal euthanasia by cardiac puncture and analyzed as above.
Plasma from pre and post-resuscitation blood samples was analyzed by enzyme-linked immunosorbent assay (ELISA) for the following: syndecan-1 (SEB966Ra, Cloud-Clone Corp., Houston, TX), epinephrine and norepinephrine (BA E-5400, LDN, Nordhorn, Germany), interleukin (IL)-6 (R6000B, R&D Systems, Minneapolis, MN), and IL-10 (R1000, R&D Systems).
Lung wet-to-dry weights
Rat lungs were obtained at time of animal euthanasia. The wet-to-dry weight ratio was calculated using the weight of one lobe immediately following animal euthanasia compared to its weight after desiccation in a vacuum oven at −25mmHg and 65°C for 72 hours as described previously (24).
Statistical analysis
Data are expressed as means ± standard deviation or proportions as appropriate. Pre-resuscitation (t=22 h) vital signs and laboratory parameters were analyzed by one-way analysis of variance (ANOVA) for FFP, NS, and sham groups with significant results further analyzed by Tukey’s post-hoc test. FFP versus NS intra-resuscitation and post-resuscitation parameters were analyzed by ANOVA with repeated measures (at each time point, only data from surviving animals were analyzed), and statistical significance was determined by a significant time by treatment interaction. The primary endpoint of 48-hour survival was analyzed by Fisher’s exact test. Survival curves were also analyzed by Kaplan-Meier statistics with log-rank test. Terminal blood samples were analyzed by one-way ANOVA with Tukey’s post-hoc test. Wet-to-dry lung weights were analyzed by Student’s t test. We used Stata version 14.1 (Stata Corporation, College Station, TX) for all calculations. A two-tailed significance level of α=0.05 was used for all statistical tests.
We estimated that a sample size of 64 animals (32 in each group) was needed to detect a 30% absolute increase in 48-hour survival (from 10% to 40%) given α level of 0.05 and power of 0.80. However, in order to limit animal suffering and resource utilization, we conducted interim analyses after every 4 animals starting at n=20. The experiment was stopped when a significant improvement in 48-hour survival was detected in the FFP group after 28 animals (14 per treatment group) underwent the experimental protocol.
Results
Pre-resuscitation parameters (t=22h)
Twenty-eight animals (14 per treatment group) underwent the CLP protocol, and an additional 5 animals underwent a sham procedure. Pre-CLP (t=0 h) weights were not different between groups (FFP, 389 ± 28 g; NS, 389 ± 29 g; sham, 387 ± 7 g). Hemodynamic and laboratory parameters at t=22 hours after CLP or sham procedure are summarized in Table 1. As expected after CLP, FFP and NS animals had significant physiologic derangements compared to sham animals including weight loss, tachycardia, hyponatremia, hyperkalemia, as well as elevations in BUN, and hematocrit. On post-hoc tests, there were no differences in pre-resuscitation parameters between FFP and NS animals.
Table 1.
Pre-resuscitation parameters (t=22h)
| Variable | Sham (n=5) | FFP (n=14) | NS (n=14) | p-value* |
|---|---|---|---|---|
| Weight (g) | 388 ± 7 | 383 ± 30 | 381 ± 33 | 0.95 |
| ΔWeight (g)† | 0 ± 1 | −8 ± 6 | −6 ± 6 | 0.03 |
| MAP (mmHg) | 99 ± 16 | 94 ± 15 | 94 ± 16 | 0.75 |
| Heart rate (bpm) | 336 ± 40 | 428 ± 30 | 405 ± 24 | <0.01 |
| Temperature (°C) | 36.5 ± 0.5 | 35.7 ± 1.2 | 35.7 ± 0.7 | 0.12 |
| Sodium (mEq/L) | 144 ± 1 | 138 ± 4 | 137 ± 4 | <0.01 |
| Potassium (mEq/L) | 3.8 ± 0.3 | 4.7 ± 0.8 | 5.1 ± 0.7 | <0.01 |
| Chloride (mEq/L) | 97 ± 1 | 97 ± 3 | 97 ± 6 | 0.95 |
| Bicarbonate (mEq/L) | 21 ± 4 | 24 ± 5 | 23 ± 4 | 0.40 |
| BUN (mg/dL) | 14 ± 1 | 44 ± 14 | 51 ± 17 | <0.01 |
| Creatinine (mg/dL) | 0.7 ± 0.1 | 1.0 ± 0.7 | 1.2 ± 0.6 | 0.16 |
| Lactate (mEq/L) | 0.6 ± 0.3 | 3.4 ± 1.1 | 3.4 ± 0.9 | <0.01 |
| Hematocrit | 41 ± 2 | 46 ± 3 | 47 ± 4 | 0.02 |
| pH | 7.28 ± 0.03 | 7.32 ± 0.10 | 7.27 ± 0.13 | 0.37 |
| PCO2 (mmHg) | 46 ± 9 | 47 ± 10 | 51 ± 16 | 0.61 |
| PO2 (mmHg)‡ | 395 ± 43 | 413 ± 73 | 390 ± 87 | 0.72 |
| PO2 to FiO2 ratio | 403 ± 44 | 421 ± 75 | 398 ± 89 | 0.72 |
| Base excess (mEq/L) | −3.9 ± 3.5 | −1.8 ± 5.5 | −4.1 ± 5.3 | 0.47 |
One-way analysis of variance for 3 groups. For all p<0.05, there were no differences between FFP and NS groups on post-hoc tests.
Pre-resuscitation (t=22h) minus pre-CLP (t=0h) weight
On 98% FiO2
MAP, mean arterial pressure; BUN, blood urea nitrogen; IL, interleukin; FFP, fresh frozen plasma; NS, normal saline; CLP, cecal ligation and puncture.
Intra-resuscitation parameters: hemodynamics, lactate, and fluid volumes (t=22h to t=28h)
Intra-resuscitation MAP, heart rate, temperature, and lactate levels during the course of resuscitation (t=22 to t=28 hours) are summarized in Figure 3. Of note, 13 of 14 NS animals survived to t=24h, and 11 of 14 NS animals survived to t=26 and t=28 h. All 14 FFP animals survived the 6-hour resuscitation period. At each time point, only data from surviving animals were analyzed.
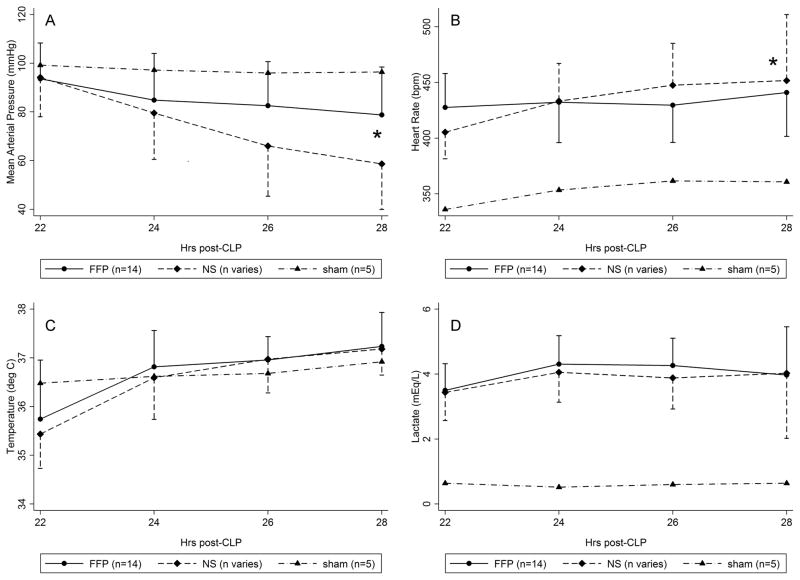
Figure 3A–D.
Intra-resuscitation vital signs and lactate level. Curves for sham animals provided for reference, and error bars for sham animals were omitted for clarity. The FFP curves summarize data for all animals (n=14/14). The NS curves summarize data for 14/14 animals at t=22h, 13/14 animals at t=24h, and 11/14 animals at t=26 to 28h due to animal death during resuscitation. FFP animals had significantly higher MAP (A) and lower heart rate (B) than NS animals, but the temperature (C) and lactate (D) curves were not statistically different. *, p<0.05 for time by treatment interaction on ANOVA with repeated measures of FFP vs NS. NS, normal saline; FFP, fresh frozen plasma; ANOVA, analysis of variance.
The mean arterial pressure (MAP) was higher in FFP animals compared to NS animals, although the MAP decreased in both groups over time (Figure 3a). Overall, FFP animals had decreased heart rate compared to NS animals (Figure 3b). The temperature and lactate curves in surviving animals were similar, however (Figure 3c–d). The lactate level was never ≤2 mmol/L in any FFP or NS animal at any point during resuscitation, and fluid was never withheld. The proportion of lactate clearances <20% was high and not different between groups (93% vs 92%), and fluid bolus volumes increased over time for both groups.
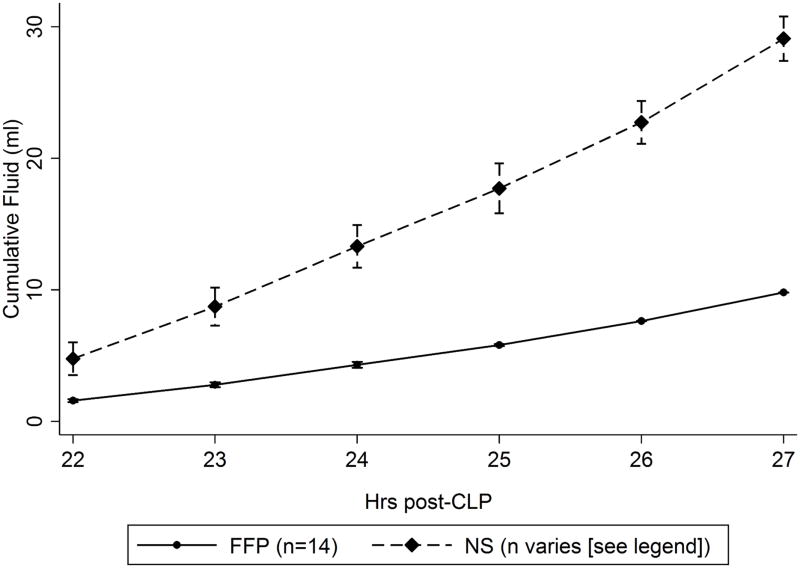
As expected, FFP animals received significantly less fluid volume than NS animals (Figure 4). Because not all NS animals survived to receive all fluid boluses, the discrepancy in total uncensored infused volumes is smaller than expected (9.8 ± 2.7 ml FFP versus 23.5 ± 6.7 ml NS). Fluid calculated as volume per hour of resuscitation is near the expected 1:3 ratio (1.6 ± 0.4 ml/hr FFP versus 4.7 ± 0.5 ml/hr NS).
Figure 4.
Cumulative resuscitation volumes. The FFP curve summarizes data for all animals (n=14/14). The NS curves summarizes data for 14/14 animals at t=22 to 23h, 13/14 animals at t=24h, 12/14 animals at t=25, and 11/14 animals at t=26–28h due to animal death during resuscitation.
Post-resuscitation parameters (t=28h)
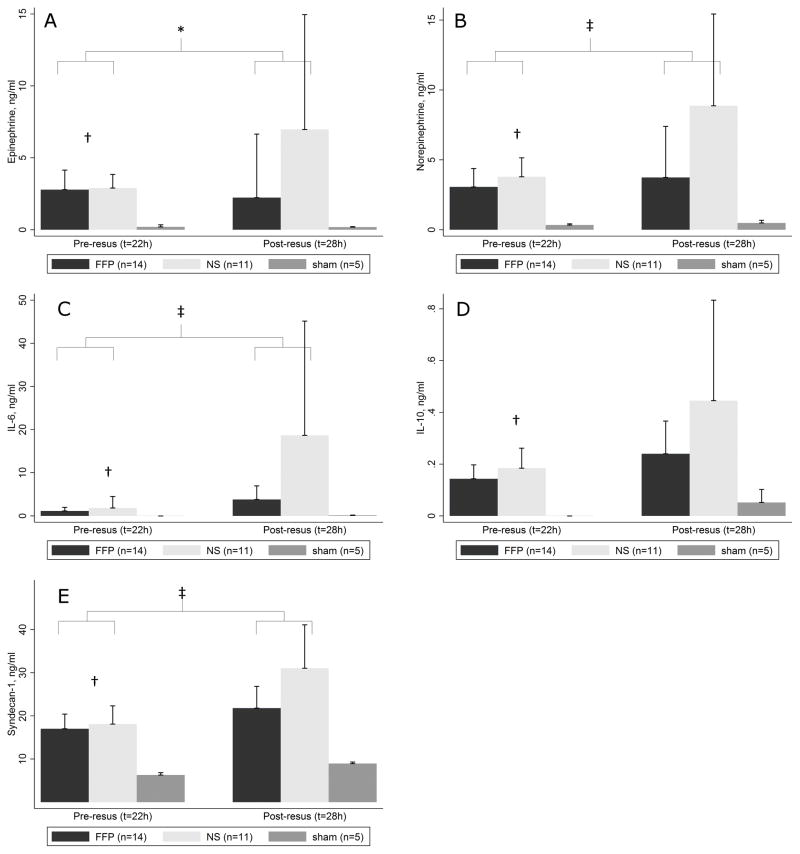
All 14 FFP animals (100%) compared to 11 NS animals (78%) survived to the end of resuscitation (t=28h) (p=0.22). Immediate post-resuscitation laboratory parameters from all 14 FFP animals and 11 NS animals which survived the resuscitation period are summarized in Table 2. NS animals had significantly higher weight of 6 g due to excess 13.7 ml fluid administered, were hyperkalemic, acidotic, and hypoxic compared to FFP animals, and were borderline more hypercarbic. FFP significantly attenuated the rise in epinephrine, norepinephrine, IL-6, and syndecan-1, but not IL-10, compared to NS (Figure 5). Of note, results of one-way ANOVA for pre-resuscitation epinephrine, norepinephrine, IL-6, IL-10, and syndecan-1 were unchanged when data from 3 NS animals which died during resuscitation were included. Although the distribution of catecholamines, IL-6, IL-10, and syndecan-1 levels were skewed, ANOVA is robust against the normality assumption25 and was therefore considered an appropriate test for these data.
Table 2.
Post-resuscitation parameters (t=28h) for animals surviving to end of resuscitation.
| Variable | FFP (n=14) | NS (n=11) | p-value* |
|---|---|---|---|
| Weight (g) | 389 ± 25 | 395 ± 32 | <0.01 |
| MAP (mmHg) | 79 ± 20 | 59 ± 19 | <0.01 |
| Heart rate (bpm) | 441 ± 39 | 452 ± 59 | 0.04 |
| Temperature (°C) | 37.2 ± 0.7 | 37.2 ± 0.5 | 0.93 |
| Sodium (mEq/L) | 143 ± 3 | 144 ± 5 | 0.35 |
| Potassium (mEq/L) | 5.6 ± 2.3 | 8.9 ± 3.4 | 0.01 |
| Chloride (mEq/L) | 93 ± 5 | 96 ± 5 | 0.29 |
| Bicarbonate (mEq/L) | 24 ± 6 | 22 ± 5 | 0.58 |
| BUN (mg/dL) | 68 ± 22 | 78 ± 22 | 0.20 |
| Creatinine (mg/dL) | 1.6 ± 0.7 | 1.9 ± 0.5 | 0.69 |
| Lactate (mEq/L) | 4.0 ± 1.5 | 4.0 ± 2.0 | 0.90 |
| Hematocrit | 36 ± 3 | 35 ± 3 | 0.18 |
| pH | 7.20 ± 0.17 | 6.97 ± 0.16 | 0.02 |
| PCO2 (mmHg) | 66 ± 37 | 98 ± 28 | 0.06 |
| PO2 (mmHg)† | 350 ± 121 | 149 ± 117 | <0.01 |
| PO2 to FiO2 ratio | 353 ± 122 | 151 ± 119 | <0.01 |
| Base excess (mEq/L) | −4.1 ± 6.8 | −9.0 ± 8.4 | 0.16 |
Analysis of variance with repeated measures for two groups (treatment by time interaction)
on 99% FiO2
MAP, mean arterial pressure; BUN, blood urea nitrogen; IL, interleukin; FFP, fresh frozen plasma; NS, normal saline.
Figure 5A–E.
Pre-resuscitation (t=22 h) and post-resuscitation (t=28 h) ELISA results for FFP (n=14/14) versus NS (n=11/14) versus sham (n=5/5). Data from 3 NS animals which died during resuscitation were excluded. Compared to NS, FFP attenuated the post-resuscitation levels of adrenaline (A), noradrenaline (B), IL-6 (C), and syndecan-1 (E), but not IL-10 (D). *, p<0.05 for time by treatment interaction on ANOVA with repeated measures of FFP vs NS; †, p<0.05 for FFP + NS vs sham on ANOVA. ‡, p<0.01 for time by treatment interaction on ANOVA with repeated measures of FFP vs NS. NS, normal saline; FFP, fresh frozen plasma; ANOVA, analysis of variance; IL-6, interleukin-6; IL-10, interleukin-10.
Survival, terminal parameters, and pulmonary wet-to-dry weight ratio
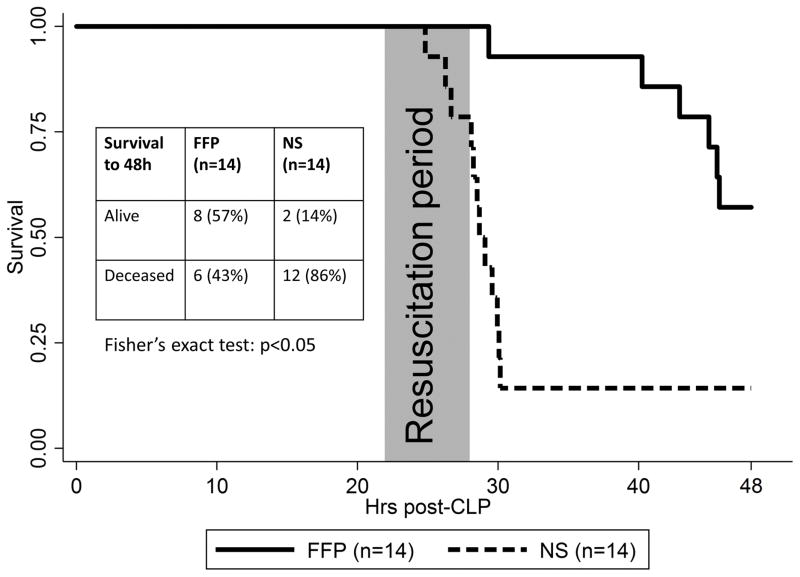
In the FFP group, all animals survived the resuscitation period, and 6 animals died during the subsequent observation period, leaving 8 animals alive at 48 hours (57%). Conversely, 3 NS animals died during the resuscitation period, and an additional 9 died during the observation period, leaving only 2 (14%) animals alive at 48 hours. FFP significantly improved 48-hour survival compared to NS on Fisher’s exact test (p=0.04). The Kaplan-Meier survival curve is given in Figure 6; on log-rank test, FFP treatment resulted in significantly improved survival compared to NS treatment (p<0.001). Assessment scores were decreasing in all surviving animals (lower scores are better), and there were no differences in score at 48 hours between FFP and NS survivors (12.4 ± 1.4 versus 13.0 ± 1.4, p=0.59).
Figure 6.
Kaplan-Meier survival curve for FFP (n=14/14) versus NS (n=14/14) animals (log rank p<0.001). The primary outcome is summarized as an inset table. FFP animals had significantly improved 48-hour survival compared to NS (p<0.05).
Analysis of terminal blood samples collected during euthanasia of moribund animals or at study conclusion are summarized in Table 3. FFP and NS samples are subdivided by 48-hour survival. Two FFP animals died before a terminal blood sample could be obtained and were excluded from this analysis. On ANOVA, there were significant between-group differences in potassium, renal function (BUN and creatinine), and blood gas (pH, PCO2, and base excess). On Tukey’s post-hoc test, there were no significant differences between surviving FFP versus surviving NS groups or between decreased FFP versus decreased NS groups. The only significant pairwise comparisons were between living and deceased groups.
Table 3.
Parameters at time of euthanasia of moribund animals or at study conclusion.
| Variable | FFP (n=14) | NS (n=14) | p-value† | ||
|---|---|---|---|---|---|
| Survived to 48h (n=8, 57%) | Died before 48h (n=4, 29%)* | Survived to 48h (n=2, 14%) | Died before 48h (n=12, 86%) | ||
| Weight (g) | 363 ± 20 | 361 ± 42 | 349 ± 20 | 399 ± 33 | 0.05 |
| Sodium (mEq/L) | 144 ± 2 | 147 ± 4 | 142 ± 6 | 145 ± 3 | 0.28 |
| Potassium (mEq/L) | 3.8 ± 0.4 | 7.6 ± 3.7 | 4.8 ± 1.5 | 9.9 ± 3.4 | <0.01 |
| Chloride (mEq/L) | 97 ± 2 | 94 ± 3 | 99 ± 6 | 96 ± 4 | 0.35 |
| Bicarbonate (mEq/L) | 23 ± 6 | 21 ± 2 | 24 ± 5 | 20 ± 3 | 0.57 |
| BUN (mg/dL) | 27 ± 18 | 94 ± 7 | 22 ± 2 | 84 ± 16 | <0.01 |
| Creatinine (mg/dL) | 0.8 ± 0.4 | 2.0 ± 0.6 | 1.2 ± 0.1 | 2.0 ± 0.5 | <0.01 |
| Lactate (mEq/L) | 2.0 ± 0.5 | 5.3 ± 2.3 | 2.6 ± 0.1 | 4.9 ± 2.1 | <0.01 |
| Hematocrit | 35 ± 3 | 35 ± 5 | 30 ± 1 | 36 ± 4 | 0.22 |
| pH‡ | 7.27 ± 0.03 | 6.9 ± 0.22 | 7.24 ± 0.21 | 6.77 ± 0.13 | <0.01 |
| PCO2 (mmHg)‡ | 49 ± 13 | 101 ± 50 | 55 ± 16 | 145 ± 43 | <0.01 |
| PO2 (mmHg)‡ | 80 ± 76 | 41 ± 20 | 100 ± 113 | 36 ± 17 | 0.17 |
| Base excess (mEq/L)‡ | −4.5 ± 5.6 | −11.8 ± 4.8 | −3.8 F± 8.3 | −14.0 ± 3.9 | <0.01 |
In two FFP animals, a terminal blood sample was not obtained prior to animal death.
One-way analysis of variance for four groups. For all p<0.05, post-hoc tests demonstrated differences only between surviving and deceased groups.
Mixed venous blood gas from cardiac puncture under 99% FiO2
FFP animals had significantly reduced pulmonary wet-to-dry weight ratios compared to NS (5.28 ± 0.02 vs 5.94 ± 0.87, p=0.01).
Discussion
We randomized adult male Sprague-Dawley rats to resuscitation with pooled rat FFP versus NS after induction of sepsis by CLP. To our knowledge, this is the first investigation of the use of plasma as a primary resuscitative fluid in the setting of sepsis. In this proof-of-concept study, our primary endpoint was survival to 48 hours after CLP. Our model included ligation of 35% of the distal cecum, which resulted in a highly lethal septic burden, consistent with a previous study (18). At the pre-resuscitation time point (t=22 h), FFP and NS animals were profoundly septic compared to shams. During the course of resuscitation, FFP animals had higher MAP and lower heart rate compared to NS animals, although lactate levels were not different between groups. Specifically, lactate levels remained relatively constant despite increasing volumes of fluid boluses in both groups. FFP resuscitation resulted in substantial improvement in the primary outcome of survival to 48 hours after CLP (57% vs 14%, p<0.05) with concomitant attenuation in markers of inflammation (IL-6), catecholamine levels (epinephrine and norepinephrine), and endothelial injury (syndecan-1). Over the course of the resuscitation period, both FFP and NS animals developed a mixed respiratory and metabolic acidosis, which was more severe in the NS group. At the end of resuscitation, NS animals were more acidotic, hypoxic, and marginally more hypercarbic compared to FFP animals. Animals which died during or shortly after the resuscitation period appeared to succumb to acidosis and/or hyperkalemia. FFP animals had better pulmonary function as demonstrated by improved gas exchange and decreased pulmonary edema on wet-to-dry weight ratios compared to NS animals.
In the last ten years, plasma has replaced crystalloids and artificial colloids as a resuscitative fluid for trauma patients with hemorrhagic shock (9, 10, 11). This transition has led to reductions in mortality (10, 11) as well as several inflammatory and edema-mediated complications including abdominal compartment syndrome, acute kidney injury, and acute respiratory distress syndrome (ARDS) (12, 13). Much of the focus of plasma has been on the replacement of volume and clotting factors, but plasma’s effects on trauma-induced endothelial damage and inflammation likely contributes to the observed benefits (26). In a rat model of hemorrhagic shock, resuscitation with plasma – but not crystalloids – resulted in partial restoration of the endothelial glycocalyx layer (EGL) and reversal of hemorrhage-induced endothelial injury (15,16). In a porcine model of concurrent traumatic brain injury (TBI) and hemorrhagic shock, resuscitation with plasma – but not crystalloids or artificial colloids – reduced the expansion of secondary brain injury (27). We have also recently shown in a retrospective study of 656 isolated TBI patients that early plasma therapy was associated with improved survival to hospital discharge in patients with multifocal intracranial hemorrhage (28). The underlying mechanism and exact protein moieties involved are currently unknown but likely involves repair of the endothelium and is an area of intense investigation.
Treatment of endothelial dysfunction may be of therapeutic value in a variety of conditions where endothelial injury is present. Several acute and chronic inflammatory conditions, such as trauma, sepsis, ischemia, viral infections such as Dengue virus, and diabetes all result in damage to the vascular endothelium. Many of these insults are characterized by loss of the endothelial glycocalyx layer (EGL) (6,7,8), a layer of proteoglycans (syndecan-1, heparan sulfate, chondroitin sulfate, hyaluronic acid) 0.8 – 2.0 microns thick which project into the blood vessel lumen from the endothelium. Revision of the outdated Starling model has established the EGL as the primary determinant of vascular permeability (8). Loss of the EGL (“shedding”) leads to extravascular leakage of protein and fluid (resulting in edema) and can be quantified as a concomitant increase in circulating EGL components, particularly syndencan-1.6 (14), In a secondary analysis of a randomized trial of 1,200 critically ill (predominantly septic) adults, Johansen et al found that increasing syndecan-1 levels significantly predicted mortality as well as hepatic and renal failure (29). Similar associations have been observed in other critically ill patient populations including after severe trauma (30), acute myocardial infarction (31), and out of hospital cardiac arrest (32). Endothelial activation is mediated at least in part by elevated catecholamine concentrations, and evidence suggests that increasing catecholamine activity becomes maladaptive, resulting in endothelial injury (33), vascular permeability, and organ dysfunction in several states of critical illness including sepsis (29). In our current study, we found moderate correlations in post-resuscitation (t=28 h) values between syndecan-1 and epinephrine (Pearson’s r: 0.66, p<0.001) and syndecan-1 and norepinephrine (Pearson’s r: 0.74, p<0.001). Furthermore, we found that FFP attenuated the increase in norepinephrine and actually decreased epinephrine levels during the course of resuscitation, which supports the hypothesis of catecholamine-mediated endothelial activation and injury in the setting of sepsis. However, these observed associations do not establish causality, nor do they clarify the biochemical mechanism.
An additional method by which plasma conferred a survival benefit in this study was likely its capacity for buffering acid. In our study, FFP significantly blunted the mixed respiratory and metabolic acidosis, which also resulted in a less severe hyperkalemia. It has long been recognized that the abundance of negatively charged proteins in plasma, as well as the sodium citrate (34) used to collect donor blood (as was used in this study), makes plasma an excellent buffer for acidosis (35). This is in contrast to NS, which is known to induce a non-anion gap metabolic acidosis.
This study has several limitations. Bias is possible given the unblinded design, specifically during the resuscitation and monitoring periods. During the monitoring period, we used prespecified criteria to facilitate early identification of moribund animals to minimize animal suffering (Supplement). Although the investigator assessing for these criteria was not blinded to treatment assignment, we believe that the observation period was of sufficient duration where animals with sustained physiologic derangement after resuscitation would not have survived to the primary endpoint. At 48 hours, surviving animals had decreasing assessment scores, indicating that they were recovering and suggesting that they may have survived long-term. To minimize animal suffering and reduce resource utilization, we conducted several interim analyses of the primary outcome, which increases the likelihood of a type I error. Although there were no statistically significant differences in pre-resuscitation parameters between FFP and NS animals, NS animals had a tendency to have more deranged pre-resuscitation markers (such as norepinephrine), and our sample size may have been underpowered to detect these differences. However, our analysis method of using repeated measures ANOVA takes into account the pre-resuscitation measurements. Additionally, we purposefully designed this CLP model with high lethality in order to maximize the mortality difference between the FFP and NS groups. Given the nature of the chi-squared distribution, we targeted 90% mortality for the control arm in order to increase statistical power. For these reasons, we did not administer antibiotics or vasopressors. Even though balanced crystalloids such as Plasma-lyte have been associated with improved outcomes in septic and other critically ill patients compared to unbalanced fluids such as NS (4), we used NS as the control fluid in order to maximize the expected mortality reduction in the FFP group. Balanced crystalloids are advantageous in that they do not induce a metabolic acidosis. Given that NS likely exacerbated the sepsis-induced mixed respiratory and metabolic acidosis, it is possible that the mortality difference would have been reduced if a balanced crystalloid was used instead of NS. Nevertheless, NS is frequently the fluid of choice in septic patients (3, 4, 36). Furthermore, a balanced crystalloid would not be expected to treat endothelial injury in the same manner as FFP, nor does it have the same buffering capacity for acidosis. Our resuscitation protocol was driven by lactate clearance, but lactate levels remained elevated in both groups throughout the resuscitation period despite increasing fluid volumes. The mean total fluid volumes in this study were 25.2 ml/kg of FFP and 60.2 ml/kg of NS over 6 hours, translating to 1,764 and 4,214 ml respectively for a 70 kg adult, which is comparable to fluid volumes reported in several randomized controlled trials of fluid resuscitation in septic patients such as CRISTAL (36). In addition to impaired perfusion, lactate levels may also have remained elevated due to catecholamine-mediated aerobic glycolysis (37). We designed the experiment to administer three times the volume of NS as FFP, and the NS group may have had improved survival if a different volume was used. Finally, since three NS animals died prior to the end of resuscitation, we analyzed post-resuscitation samples in only eleven NS animals. However, since the three animals with early mortality were expected to have greatly deranged catecholamine, IL-6, IL-10, and syndecan-1 levels, exclusion of these samples likely made it more difficult to demonstrate statistically significant differences.
In conclusion, FFP resuscitation increased 48-hour survival, improved pulmonary function and decreased pulmonary edema, and attenuated markers for inflammation, endothelial injury, and catecholamines compared to NS resuscitation in this clinically-relevant and highly lethal rat CLP sepsis model. Potential use of plasma as a primary resuscitation fluid for the treatment of sepsis warrants further investigation.
Supplementary Material
Acknowledgments
We are grateful to Lisa Baer for technical support in the animal laboratory.
Footnotes
This study was accepted for poster presentation at the 40th Annual Conference on Shock, June 3-6, 2017 at Ft. Lauderdale, FL.
Author contributions: Research inception and design by RC, JBH, and CEW. Animal protocol performed by RC. Analysis of blood and tissue samples performed by RC and SP. All authors participated in data synthesis and interpretation as well as manuscript preparation and critical appraisal.
Disclosures: RC is supported by a T32 fellowship (grant no. 5T32GM008792) from NIGMS. Authors report no conflicts of interest.
References
- 1.Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, Rubenfeld G, Kahn JM, Shankar-Hari M, Singer M, et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):762–74. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 3.Chang R, Holcomb JB. Choice of Fluid Therapy in the Initial Management of Sepsis, Severe Sepsis, and Septic Shock. Shock. 2016;46(1):17–26. doi: 10.1097/SHK.0000000000000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raghunathan K, Bonavia A, Nathanson BH, Beadles CA, Shaw AD, Brookhart MA, Miller TE, Lindenauer PK. Association between Initial Fluid Choice and Subsequent In-hospital Mortality during the Resuscitation of Adults with Septic Shock. Anesthesiology. 2015;123(6):1385–93. doi: 10.1097/ALN.0000000000000861. [DOI] [PubMed] [Google Scholar]
- 5.Shaw AD, Bagshaw SM, Goldstein SL, Scherer LA, Duan M, Schermer CR, Kellum JA. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma-Lyte. Ann Surg. 2012;255(5):821–829. doi: 10.1097/SLA.0b013e31825074f5. [DOI] [PubMed] [Google Scholar]
- 6.Chignalia AZ, Yetimakman F, Christiaans SC, Unal S, Bayrakci B, Wagener BM, Russell RT, Kerby JD, Pittet JF, Dull RO. The glycocalyx and trauma: A review. Shock. 2016;45(4):338–48. doi: 10.1097/SHK.0000000000000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ince C, Mayeux PR, Nguyen T, Gomez H, Kellum JA, Ospina-Tascón GA, Hernandez G, Murray P, De Backer D. ADQI XIV Workgroup: The endothelium in sepsis. Shock. 2016;45(3):259–70. doi: 10.1097/SHK.0000000000000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodcock TE, Woodcock TM. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. Br J Anaesth. 2012;108(3):384–94. doi: 10.1093/bja/aer515. [DOI] [PubMed] [Google Scholar]
- 9.Chang R, Holcomb JB. Optimal fluid therapy for traumatic hemorrhagic shock. Crit Care Clin. 2017;33(1):15–36. doi: 10.1016/j.ccc.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, Alarcon LH, Bai Y, Brasel KJ, Bulger EM, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148(2):127–36. doi: 10.1001/2013.jamasurg.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–82. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson BR, Cotton BA, Pritts TA, Branson R, Holcomb JB, Muskat P, Fox EE, Wade CE, del Junco DJ, Bulger EM, et al. Application of the Berlin definition in PROMMTT patients: the impact of resuscitation on the incidence of hypoxemia. J Trauma Acute Care Surg. 2013;75(1 Suppl 1):S61–7. doi: 10.1097/TA.0b013e31828fa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balogh Z, McKinley BA, Cocanour CS, Kozar RA, Valdivia A, Sailors RM, Moore FA. Supranormal trauma resuscitation causes more cases of abdominal compartment syndrome. Arch Surg. 2013;138(6):637–42. doi: 10.1001/archsurg.138.6.637. [DOI] [PubMed] [Google Scholar]
- 14.Rahbar E, Cardenas JC, Baimukanova G, Usadi B, Bruhn R, Pati S, Ostrowski SR, Johansson PI, Holcomb JB, Wade CE. Endothelial glycocalyx shedding and vascular permeability in severely injured trauma patients. J Transl Med. 2015;13:117. doi: 10.1186/s12967-015-0481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng Z, Pati S, Potter D, Brown R, Holcomb JB, Grill R, Wataha K, Park PW, Xue H, Kozar RA. Fresh frozen plasma lessens pulmonary endothelial inflammation and hyperpermeability after hemorrhagic shock and is associated with loss of syndecan 1. Shock. 2013;40(3):195–202. doi: 10.1097/SHK.0b013e31829f91fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kozar RA, Peng Z, Zhang R, Holcomb JB, Pati S, Park P, Ko TC, Paredes A. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg. 2011;112(6):1289–95. doi: 10.1213/ANE.0b013e318210385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torres LN, Sondeen JL, Ji L, Dubick MA, Torres Filho I. Evaluation of resuscitation fluids on endothelial glycocalyx, venular blood flow, and coagulation function after hemorrhagic shock in rats. J Trauma Acute Care Surg. 2013;75(5):759–66. doi: 10.1097/TA.0b013e3182a92514. [DOI] [PubMed] [Google Scholar]
- 18.Singleton KD, Wischmeyer PE. Distance of cecum ligated influences mortality, tumor necrosis factor-alpha and interleukin-6 expression following cecal ligation and puncture in the rat. Eur Surg Res. 2003;35(6):486–91. doi: 10.1159/000073387. [DOI] [PubMed] [Google Scholar]
- 19.Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW, 3rd, Bland KI, Chaudry IH. Cecal ligation and puncture. Shock. 2005;24(Suppl 1):52–7. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 20.Cardenas JC, Cap AP, Swartz MD, Huby Mdel P, Baer LA, Matijevic N, Cotton BA, Holcomb JB, Wade CE. Plasma resuscitation promotes coagulation homeostasis following shock-induced hypercoagulability. Shock. 2016;45(2):166–173. doi: 10.1097/SHK.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 21.Jansen TC, van Bommel J, Schoonderbeek FJ, Sleeswijk Visser SJ, van der Klooster JM, Lima AP, Willemsen SP, Bakker J the LACTATE study group. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182:752–761. doi: 10.1164/rccm.200912-1918OC. [DOI] [PubMed] [Google Scholar]
- 22.Lilley E, Armstrong R, Clark N, Gray P, Hawkins P, Mason K, López-Salesansky N, Stark AK, Jackson SK, Thiemermann C, Nandi M. Refinement of animal models of sepsis and septic shock. Shock. 2015;43(4):304–16. doi: 10.1097/SHK.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda A, Furukawa K, Suzuki H, Matsutani T, Tajiri T, Chaudry IH. Dehydroepiandrosterone modulates toll-like receptor expression on splenic macrophages of mice after severe polymicrobial sepsis. Shock. 2005;24(4):364–9. doi: 10.1097/01.shk.0000180624.36811.97. [DOI] [PubMed] [Google Scholar]
- 24.Wagner EM, Karagulova G, Jenkins J, Bishai J, McClintock J. Changes in lung permeability after chronic pulmonary artery obstruction. J Appl Physiol. 2006;100(4):1224–9. doi: 10.1152/japplphysiol.01060.2005. [DOI] [PubMed] [Google Scholar]
- 25.Stevens JP. Intermediate statistics: A modern approach. 3. London: Routledge; 2007. p. 57. [Google Scholar]
- 26.Watson JJ, Pati S, Schreiber MA. Plasma Transfusion: History, Current Realities, and Novel Improvements. Shock. 2016;46(5):468–479. doi: 10.1097/SHK.0000000000000663. [DOI] [PubMed] [Google Scholar]
- 27.Jin G, DeMoya MA, Duggan M, Knightly T, Mejaddam AY, Hwabejire J, Lu J, Smith WM, Kasotakis G, Velmahos GC, et al. Traumatic brain injury and hemorrhagic shock: evaluation of different resuscitation strategies in a large animal model of combined insults. Shock. 2012;38(1):49–56. doi: 10.1097/SHK.0b013e3182574778. [DOI] [PubMed] [Google Scholar]
- 28.Chang R, Folkerson LE, Sloan D, Tomasek JS, Kitagawa RS, Choi HA, Wade CE, Holcomb JB. Early plasma transfusion is associated with improved survival after isolated traumatic brain injury in patients with multifocal intracranial hemorrhage. Surgery. 2017;161(2):538–545. doi: 10.1016/j.surg.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansen ME, Johansson PI, Ostrowski SR, Bestle MH, Hein L, Jensen AL, Søe-Jensen P, Andersen MH, Steensen M, Mohr T, et al. Profound endothelial damage predicts impending organ failure and death in sepsis. Semin Thromb Hemost. 2015;41(1):16–25. doi: 10.1055/s-0034-1398377. [DOI] [PubMed] [Google Scholar]
- 30.Johansson PI, Henriksen HH, Stensballe J, Gybel-Brask M, Cardenas JC, Baer LA, Cotton BA, Holcomb JB, Wade CE, Ostrowski SR. Traumatic endotheliopathy: A prospective observational study of 424 severely injured patients. Ann Surg. 2017;265(3):597–603. doi: 10.1097/SLA.0000000000001751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ostrowski SR, Pedersen SH, Jensen JS, Mogelvang R, Johansson PI. Acute myocardial infarction is associated with endothelial glycocalyx and cell damage and a parallel increase in circulating catecholamines. Crit Care. 2013;17(1):R32. doi: 10.1186/cc12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bro-Jeppesen J, Johansson PI, Hassager C, Wanscher M, Ostrowski SR, Bjerre M, Kjaergaard J. Endothelial activation/injury and associations with severity of post-cardiac arrest syndrome and mortality after out-of-hospital cardiac arrest. Resuscitation. 2016;107:71–9. doi: 10.1016/j.resuscitation.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Makhmudov RM, Mamedov YaD, Dolgov VV, Repin VS. Catecholamine-mediated injury to endothelium in rabbit perfused aorta: a quantitative analysis by scanning electron microscopy. Cor Vasa. 1985;27:456–463. [PubMed] [Google Scholar]
- 34.Collins JA, Simmons RL, James PM, Bredenberg CE, Anderson RW, Heisterkamp CA. Acid-base status of seriously wounded combat casualties. II. Resuscitation with stored blood. Ann Surg. 1971;173(1):6–18. doi: 10.1097/00000658-197101000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Traverso LW, Hollenbach SJ, Bolin RB, Langford MJ, DeGuzman LR. Fluid resuscitation after an otherwise fatal hemorrhage: II. Colloid solutions. J Trauma. 1986;26(2):176–82. doi: 10.1097/00005373-198602000-00015. [DOI] [PubMed] [Google Scholar]
- 36.Annane D, Siami S, Jaber S, Martin C, Elatrous S, Declère AD, Preiser JC, Outin H, Troché G, Charpentier C, et al. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA. 2013;310(17):1809–17. doi: 10.1001/jama.2013.280502. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Alvarez M, Marik P, Bellomo R. Sepsis-associated hyperlactemia. Crit Care. 2014;18(5):503. doi: 10.1186/s13054-014-0503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.