Abstract
Intracellular calcium ([Ca2+]i) signaling is an essential universal secondary messenger in articular chondrocytes. However, little is known about its spatiotemporal features in the context of osteoarthritis (OA). Herein, by examining the cartilage samples collected from patients undergoing knee arthroscopic surgery, we investigated the spatiotemporal features of spontaneous [Ca2+]i signaling in in situ chondrocytes at different OA stages. Our data showed zonal dependent spontaneous [Ca2+]i signaling in healthy cartilage samples under 4 mM calcium environment. This signal was significantly attenuated in healthy cartilage samples but increased in early-degenerated cartilage when cultured in 0 mM calcium environment. No significant difference was found in [Ca2+]i intensity oscillation in chondrocytes located in middle zones among ICRS 1–3 samples under both 4 and 0 mM calcium environments. However, the correlation was found in deep zone chondrocytes incubated in 4 mM calcium environment. In addition, increased protein abundance of Cav3.3 T-type voltage dependent calcium channel and Nfatc2 activity were observed in early-degenerated cartilage samples. The present study exhibited OA severity dependent spatiotemporal features of spontaneous [Ca2+]i oscillations of in situ chondrocytes, which might reflect the zonal specific role of chondrocytes during OA progression and provide new insight in articular cartilage degradation during OA progression.
Introduction
Osteoarthritis (OA), which involves the dysfunction of adult articular cartilage, is the most common form of joint disease with manifestations of damaged articular cartilage, and may result in arthralgia, joint deformation, and limited mobility in patients. OA is the second leading cause of long-term disability in adults. Several risk factors of OA (e.g. genetics, age, and mechanical loading) have been identified1; however, the mechanisms of OA initiation and progression are yet to be understood. Chondrocytes, as the highly specialized, metabolically active cells, regulate the development, maintenance, and repair of extracellular matrix (ECM) in articular cartilage2. Unlike its low cellular activity and matrix turnover ability in healthy articular cartilage3,4, chondrocytes play a pivotal role in OA initiation and progression by upregulating expression of matrix metalloproteinases (MMP-1, MMP-14), aggrecanase (ADAMTS-5) and inflammatory cytokines (interleukin-1α/β, and tumor necrosis factor -α)4,5.
Intracellular calcium ([Ca2+]i) signaling is an essential universal secondary messenger that mediates the cellular metabolic activity in chondrocytes6–10. Based on its spatiotemporal parameters (amplitude, frequency, and duty cycle), encoded information is deciphered by various downstream transcription factors (e.g. nuclear factor of activated T cells (Nfat), nuclear factor-κB (NF-κB), c-Jun N-terminal kinase 1 (JNK1), myocyte enhancer factor-2 (MEF2), and cAMP response element-binding protein (CREB)), and ultimately leads to a range of metabolic and signaling processes11. Due to its unique role in extracellular-intracellular signal transduction, [Ca2+]i signaling has been widely studied in articular cartilage. It has been shown that chondrocytes not only respond to mechanical and chemical stimuli with [Ca2+]i signaling via modulating extracellular calcium influx and intracellular calcium release6–10, but also reflect its peri-cellular environment (e.g. osmotic stress, growth factors, and cytokines) with repetitive spontaneous [Ca2+]i signaling when statically cultured6,12, which plays a crucial role in regulating ECM metabolic activity12. With abnormal joint physiology such as increased mechanical loading and surrounding inflammatory activity, response of chondrocytes to their peri-cellular environment may be significantly altered, potentially contributing to the onset or progression of OA.
Articular cartilage is a highly organized tissue, its zonal differences in biochemical content and bioelectrical properties are directly associated with depth-dependent changes in mechanical properties13,14. Rolauffs and colleagues demonstrated the significantly increased cell density in the deep zone of synchronized primary metabolic chondrons of articular cartilage, compared with superficial zone15. Several studies16,17 confirmed the variety in cytokine sensitivity of chondrocytes located in different zones, indicating the distinctive role of chondrocytes located in different zones during the pathological process of OA. Previous studies investigating chondrocyte [Ca2+]i signaling were mainly focused on cells removed from their physiological environment, such as 2D cultures or chondrocytes/gel constructs6,18. These studies neglected the potential role of calcium channel activity linked to ECM, as well as the specialized peri-cellular matrix (PCM) that affected chondrocyte mechanics19.
In the present study, we aimed to investigate the spontaneous [Ca2+]i signaling of human articular chondrocytes located in its native PCM and ECM, and compared the zonal differences in [Ca2+]i signaling of in situ human chondrocytes. By comparing the spatiotemporal features of spontaneous [Ca2+]i signaling in in situ chondrocytes at different OA stages, the correlation between spontaneous [Ca2+]i signaling and OA severity was investigated. In addition, to address the possible mechanism of OA chondrocytes [Ca2+]i signaling alternation and downstream regulation of [Ca2+]i signaling, the protein level of T-type voltage-dependent calcium channels (VDCCs) and Nfatc2 activity were analyzed using total knee arthroplasty (TKA) samples.
Results
Histology evaluation
Based on the International Cartilage Research Society (ICRS) grading system, the severity of each collected human articular cartilage sample was verified by histomorphometry approaches. Later on, the spatiotemporal features of spontaneous [Ca2+]i signaling analyzed from each sample were classified into ICRS grades. Representative hematoxylin and eosin (H&E), alcian blue, and sirius red staining images for ICRS 0–3 grades were shown in Fig. 1. As expected, cartilage samples showed increased wear from top to bottom. As shown by alcian blue staining, gradually decreased proteoglycan concentration was most pronounced at superficial and deep zones in degenerated samples (ICRS 1–3). Sirius red staining suggested dramatically changed collagen composition from type П to type І at ICRS 1 samples, and absence of both types of collagen fibrils at late stage OA samples (ICRS 3).
Figure 1.
Histological feature of ICRS 1–3 grades articular cartilage samples collected from patients undergoing knee arthroscopic surgery. From top to bottom, the severity of collected articular cartilage samples was evaluated by hematoxylin and eosin (H&E), alcian blue, and sirius red, respectively (n = 3–4 for each ICRS grade).
Propagation of calcium signaling in middle and deep zones of articular cartilage samples
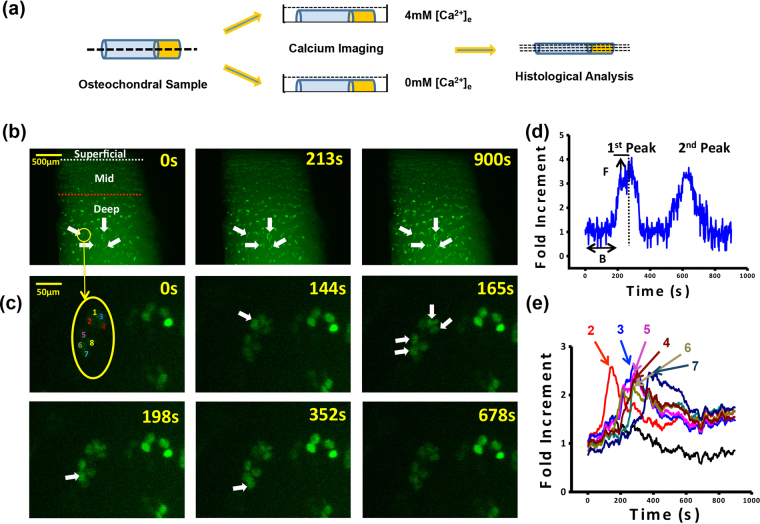
Spontaneous calcium signaling was observed in chondrocytes within both normal (ICRS 0) and OA (ICRS 1–3) articular cartilages without extraneous stimuli. Typical [Ca2+]i oscillations in ICRS 0 cartilage sample were shown in Fig. 2b. Propagation of [Ca2+]i oscillations were observed in a large portion of chondrons in middle and deep zones of both normal and OA cartilages. As shown in Fig. 2c and e, [Ca2+]i oscillations propagated from cell number 2 to neighboring cells (cell number 3, 4, 5 and 6), and from cell number 6 to cell number 7. The time lag of [Ca2+]i peaks between each propagation of cell 1–7 also suggested this phenomenon.
Figure 2.
Real-time calcium imaging of in situ human chondrocytes. (a) Study design, 2 mm in diameter cylindrical osteochondral samples at different ICRS grades were collected form femoral condyle during arthroscopy. Samples were cut into two half-cylinders and used for calcium imaging at 4 mM and 0 mM [Ca2+]e environments. To classify calcium imaging data, histology evaluation was performed on these samples afterward. (b) Typical [Ca2+]i image series in ICRS 0 articular cartilage sample and in (c) single lacuna of the same sample. (d) A typical [Ca2+]i intensity oscillations curve of a chondrocyte and the definitions of spatiotemporal parameters. (e) Typical [Ca2+]i intensity oscillations curve in the same lacuna shown in (c) (white arrows indicated cells displayed spontaneous [Ca2+]i signal).
Zonal difference of spontaneous calcium signaling in normal chondrocytes
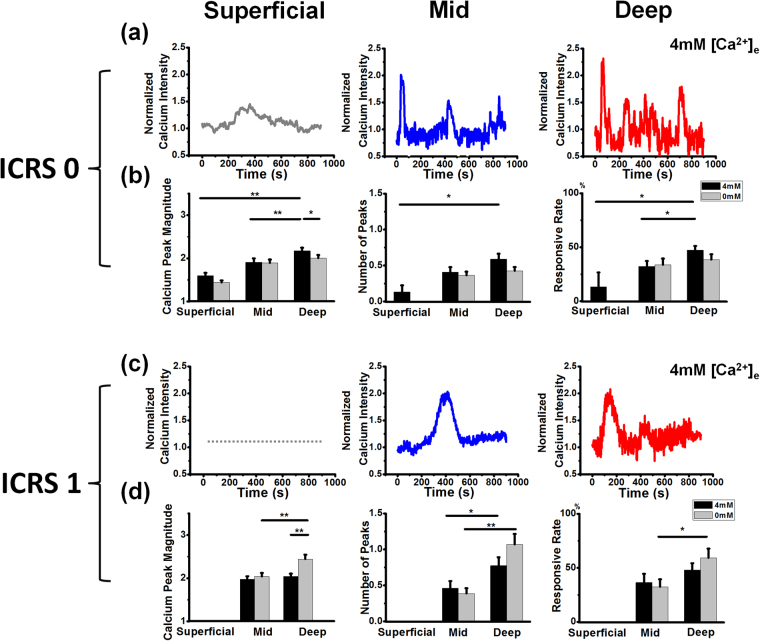
Spatiotemporal features of [Ca2+]i signaling in in situ chondrocytes were zonal, [Ca2+]e concentration, and ICRS grade dependent. Typical [Ca2+]i oscillations for both normal (ICRS 0) and early (ICRS 1) OA cartilages under 4 mM [Ca2+]e environment were shown in Fig. 3. In ICRS 0 samples, [Ca2+]i oscillations increased with the depth of cartilage (Fig. 3a). During the 15-min recording period, only a small portion of chondrocytes (13.33 ± 13.33%) located in the superficial zone displayed [Ca2+]i peaks larger than 2 fold increment (calcium peak magnitude: 1.60 ± 0.07) (Fig. 3b), while a significantly larger portion of chondrocytes (47.17 ± 4.01%, P < 0.05) located in single chondrons of deep zone demonstrated repetitive (0.60 ± 0.07 vs. 0.13 ± 0.09 peaks, P < 0.05) and stronger [Ca2+]i peaks (calcium peak magnitude: 2.17 ± 0.08, P < 0.01) during the same recording period (Fig. 3b). Compared with chondrocytes in deep zone, chondrocytes located in middle zone showed moderate [Ca2+]i oscillations: 32.25 ± 5.00% (P < 0.05) cells showed valid [Ca2+]i peak, with 0.41 ± 0.07 peaks (P > 0.05) and calcium peak magnitude of 1.91 ± 0.09 fold (P < 0.01) during the same recording period.
Figure 3.
Characterization of spatiotemporal features of spontaneous [Ca2+]i signaling in chondrocyte of health (ICSR 0) and early OA samples (ICRS 1). Representative [Ca2+]i oscillation curves of chondrocytes located in superficial, middle, and deep zones of (a) ICRS 0 and (c) ICRS 1 sample, spatiotemporal features (calcium peak magnitude, number of peaks, and responsive rate) of spontaneous [Ca2+]i signaling in chondrocyte located in different zones of (b) ICRS 0 and (d) ICRS 1 samples when cultured in either 0 mM or 4 mM [Ca2+]e environments. Results were expressed as mean ± SEM (for calcium peak magnitude and number of peaks, 15–102 cells were analyzed; for responsive rate, 4 individual samples were analyzed in superficial zones, and 10–20 chondrons were analyzed in middle and deep zones, * P < 0.05; ** P < 0.01).
In 0 mM [Ca2+]e environment, the difference in each parameter of [Ca2+]i oscillations between middle and deep zones was abolished (P > 0.05), due to decreased [Ca2+]i oscillations of chondrocytes in deep zone when cultured in 0 mM [Ca2+]e environment (calcium peak magnitude: 2.17 ± 0.08 vs. 2.00 ± 0.07, P < 0.05). Furthermore, [Ca2+]i oscillations in chondrocytes in the superficial zone were completely diminished when cartilage was incubated in 0 mM [Ca2+]e environment.
Altered spontaneous calcium signaling pattern in early OA chondrocytes
In early OA (ICRS 1) cartilage, zonal difference in [Ca2+]i oscillations of chondrocytes between middle and deep zones was attenuated under 4 mM [Ca2+]e environment (calcium peak magnitude: 1.98 ± 0.07 vs. 2.04 ± 0.07, P > 0.05, number of peaks: 0.46 ± 0.10 vs. 0.78 ± 0.12, P < 0.05, responsive rate: 36.50 ± 8.03% vs. 47.95 ± 6.41%, P > 0.05) (Fig. 3d). However, when ICRS 1 samples were incubated in 0 mM [Ca2+]e environment, chondrocytes in deep zone displayed enhanced [Ca2+]i oscillations compared to chondrocytes in middle zone (calcium peak magnitude: 2.44 ± 0.10 vs. 2.04 ± 0.08, P < 0.01, number of peaks: 1.07 ± 0.15 vs. 0.39 ± 0.07, P < 0.01, responsive rate: 59.29 ± 8.54% vs. 32.50 ± 6.97%, P < 0.05). Furthermore, compared with 4 mM [Ca2+]e culture, chondrocytes in ICRS 1 deep zone displayed stronger [Ca2+]i when incubated in 0 mM [Ca2+]e environment (calcium peak magnitude: 2.44 ± 0.10 vs. 2.04 ± 0.07, P < 0.01).
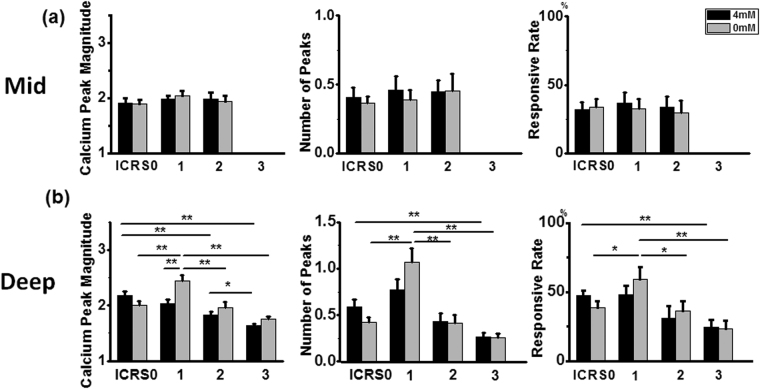
Comparison of spontaneous calcium signaling of chondrocytes in different ICRS grade samples
Comparison of spatiotemporal features of [Ca2+]i signaling was not performed in superficial zones since this region was only presented in ICRS 0 samples. No zonal or [Ca2+]e concentration-dependent difference was found in the middle zones of all ICRS grades (Fig. 4). In contrast, chondrocytes in deep zone showed significant variation in [Ca2+]i oscillations. The gradually decreased intensity of spontaneous calcium signaling in ICRS 0, 2, and 3 samples were observed. Compared with ICRS 0 samples, chondrocytes in ICRS 2 (calcium peak magnitude: 1.83 ± 0.06 vs. 2.17 ± 0.08, P < 0.01) and ICRS 3 (calcium peak magnitude: 1.64 ± 0.04 vs. 2.17 ± 0.08, P < 0.01, number of peaks: 0.26 ± 0.04 vs. 0.60 ± 0.07, P < 0.01, responsive rate: 24.65 ± 5.14% vs. 47.17 ± 4.01%, P < 0.01) samples demonstrated significantly weaker [Ca2+]i oscillations when incubated in 4 mM [Ca2+]e environment. Also, chondrocytes in ICRS 3 samples showed even weaker [Ca2+]i oscillations than that displayed by cells in ICRS 2 samples (calcium peak magnitude: 1.64 ± 0.04 vs. 1.83 ± 0.06, P < 0.05). However, this trend was not found in ICRS 1 samples. Chondrocytes located in the deep zone of ICRS 1 samples showed comparable [Ca2+]i oscillations of ICRS 0 chondrocytes when incubated in 4 mM [Ca2+]e environment (Fig. 4b, black bars).
Figure 4.
Comparison of spatiotemporal features of spontaneous [Ca2+]i signaling among ICRS 0–3 samples. (a) No significant difference was found in [Ca2+]i oscillation in chondrocytes located in middle zones under either 4 mM or 0 mM [Ca2+]e environment. (b) Decreased [Ca2+]i signaling intensity was found in deep zone chondrocytes of degenerated cartilage when incubated in 4 mM calcium environment. When samples were incubated in calcium 0 mM [Ca2+]e environment, ICRS 1 sample showed strongest [Ca2+]i oscillation. Results were expressed as mean ± SEM (for calcium peak magnitude and number of peaks, 46–107 cells were analyzed; for responsive rate, 10–20 chondrons were analyzed in middle and deep zones, * P < 0.05; ** P < 0.01).
In 0 mM [Ca2+]e environment, the difference in [Ca2+]i oscillations among ICRS grades was attenuated, except for ICRS 1 samples. Compared with other grades, chondrocytes located in deep zone of ICRS 1 samples showed the strongest [Ca2+]i oscillations (calcium peak magnitude: 2.44 ± 0.10, P < 0.01, number of peaks: 1.07 ± 0.15, P < 0.01, responsive rate: 59.29 ± 8.54%, P < 0.05 compared with ICRS 0 and 2, and <0.01 compared with ICRS3, respectively). Furthermore, compared with samples within each ICRS grade in 4 mM [Ca2+]e environment, no significant difference was found in each ICRS 0, 2, 3 samples; while in ICRS 1 samples, significantly stronger [Ca2+]i oscillations were found in 0 mM [Ca2+]e environment as described above.
Correlation between spontaneous calcium signaling and OA severity
To analyze the possible correlation between spontaneous calcium signaling and OA severity, Spearman’s rank correlation coefficient method was used to analyze the relationship of [Ca2+]i signaling parameters of ICRS 0–3 samples to ICRS grading system under 4 mM and 0 mM [Ca2+]e environment, respectively (Table 1). Results suggested that calcium peak magnitude (r = −0.33, P < 0.001) and responsive rate (r = −0.486, P < 0.001) were moderately correlated with ICRS grade when cartilage samples were incubated in 4 mM [Ca2+]e environment. In contrast, when cartilage samples were incubated in 0 mM [Ca2+]e environment, the correlation between parameters of [Ca2+]i signaling and ICRS grading system was very weak.
Table 1.
Spearman rank correlation coefficient (r) between the parameters of [Ca2+]i signaling in deep zones and ICRS grades.
| ICRS grade | Sample size | ||
|---|---|---|---|
| r | p | ||
| Calcium peak magnitude (4 mM) | −0.330 | <0.001 | 342 cells |
| Calcium peak magnitude (0 mM) | −0.124 | 0.023 | 331 cells |
| Number of peaks (4 mM) | −0.205 | <0.001 | 358 cells |
| Number of peaks (0 mM) | −0.151 | 0.006 | 330 cells |
| Responsive rate (4 mM) | −0.486 | <0.001 | 70 chondrons |
| Responsive rate (0 mM) | −0.219 | 0.093 | 65 chondrons |
Increased protein abundance of T-type VDCCs in early-degenerated articular cartilage
To explore the mechanism of unregulated [Ca2+]i signaling in ICRS 1 samples, non-degenerated (Normal) and early-degenerated (OA) regions of TKA cartilage samples were used for RT-qPCR and Western boltting analyses. Compared with non-degenerated cartilage, chondrocytes in early-degenerated cartilage had significantly higher level of MMP-1, MMP-13, and COL1A1; and lower level of SOX-9 and SOX-11 (Fig. 5a). Western boltting data suggested that chondrocytes located in both regions expressed T-type Cav3.1, Cav3.3, and α2σ1 proteins, but negatively expressed Cav3.2 protein. When compared with the non-degenerated region, significantly increased protein level of Cav3.3 was noticed in the early-degenerated region (Fig. 5b and c).
Figure 5.
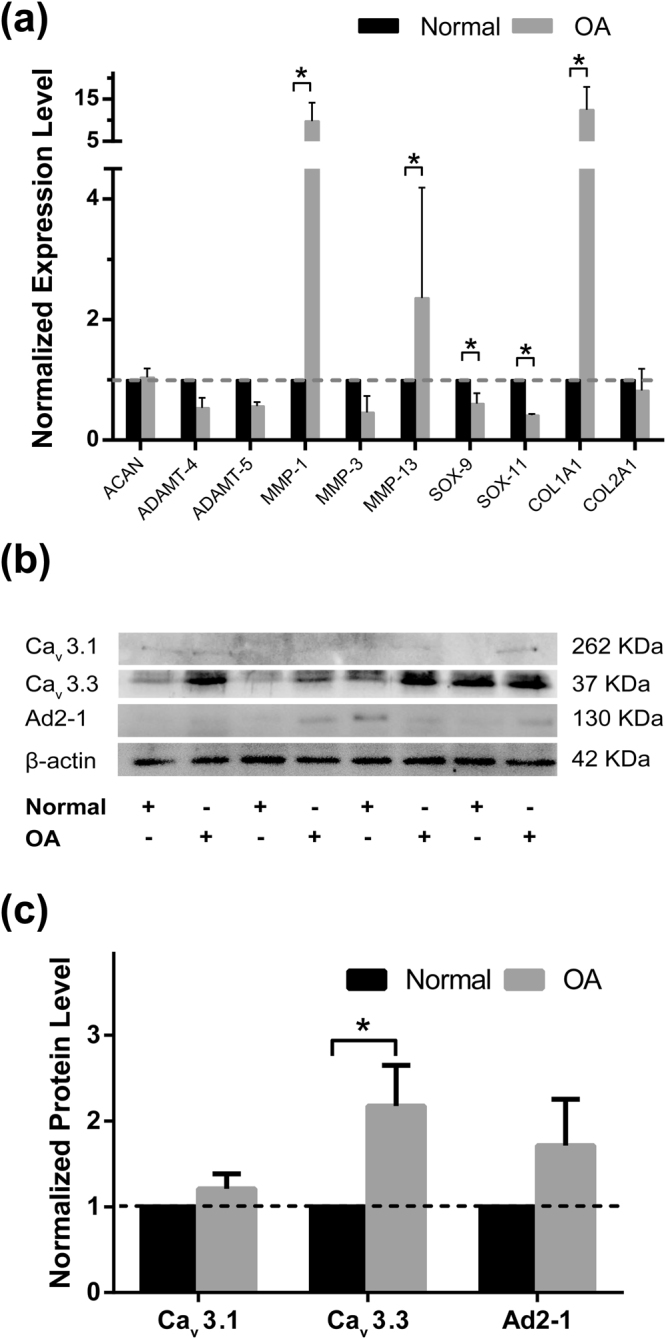
Expression level of chondrogenic genes and protein abundance of T-VDCCs in non-degenerated and early-degenerated articular cartilage samples. (a) Compared with non-degenerated cartilage (Normal, black bars), chondrocytes located in early-degenerated area (OA, grey bars) of TKA samples showed increased expression level of MMP-1, MMP-13, and COL1A1; and decreased SOX-9 and SOX-11 (* P < 0.05) (n = 6). (b) Representative Western blot detection of Cav3.1, Cav3.3, A2D-1, and β-actin (full-length blots/gels were presented in Supplementary Figure S1). (c) Compared with non-degenerated cartilage (Normal, black bars), significantly increased protein level of Cav3.3 was noticed in early-degenerated (OA, grey bars) regions (* P < 0.05) (n = 8).
Increased Nfatc2 activity in early-degenerated articular cartilage
To explore the role of unregulated [Ca2+]i signaling in OA progression, the activity of Nfatc2, downstream transcription factors of [Ca2+]i signaling was analyzed using RT-qPCR and Western boltting in TKA cartilage samples. Results indicated significantly up-regulated Nfatc2 expression (Fig. 6a), and increased protein level of de-phosphorylated Nfatc2 in chondrocytes located in early-degenerated regions (Fig. 6b and c).
Figure 6.
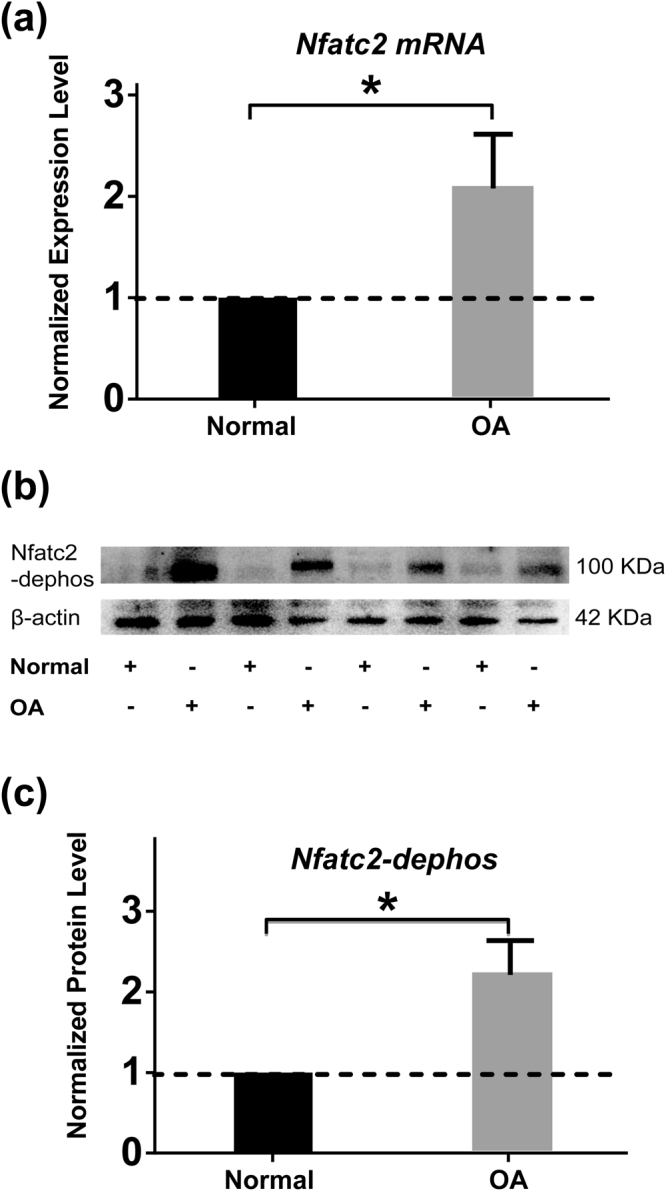
Expression level and activity of Nfactc2 in non-degenerated and early-degenerated articular cartilage sample. (a) Compared with non-degenerated cartilage (Normal, black bars), RT-qPCR data indicated that chondrocytes located in the early-degenerated area (OA, grey bars) of TKA sample showed increased expression level of Nfatc2 (* P < 0.05) (n = 6). (b) Representative Western blot detection of de-phosphorylated Nfatc2 (full-length blots/gels are presented in Supplementary Figure S1). (c) Compared with non-degenerated cartilage (Normal, black bars), Significantly increased dephosphorylated Nfatc2 was found in early-degenerated (OA, grey bars) regions (* P < 0.05) (n = 8).
Discussion
The objective of this study was to investigate the spatiotemporal features of spontaneous [Ca2+]i signaling in in situ chondrocytes of human healthy and OA articular cartilage, which might shed light on understanding the molecular mechanism of articular cartilage degradation in OA. Our data showed a significant difference of extracellular calcium dependent spontaneous [Ca2+]i signaling between zones in healthy cartilage, while this zonal difference was not observed in degenerated cartilage. In early-degenerated cartilage, abnormal [Ca2+]i activities in deep zone were seen when samples were cultured in both 0 mM and 4 mM [Ca2+]e environment. Increased Cav3.3 protein abundance and Nfatc2 activity were possibly associated with abnormal [Ca2+]i activities in early-degenerated cartilage. Also, the intensity of spontaneous [Ca2+]i signaling was found negatively correlated with OA severity. These data indicated that the zonal specific role of chondrocytes in maintaining ECM integrity in human healthy articular cartilages and reflected alternation of chondrocytes metabolic activity during OA progression.
It is recently discovered that both isolated and in situ animal chondrocytes can display repetitive [Ca2+]i signaling without any extracellular stimuli. Occasional spontaneous [Ca2+]i signaling in rabbit chondrocytes located in native ECM and 2D culture was first observed by Kono et al., in which spontaneous [Ca2+]i signaling did not propagate to adjacent cells unless mechanically stimulated6. This observation was later demonstrated by O’Conor et al., who showed that ~20% of 3D cultured porcine chondrocytes displayed spontaneous [Ca2+]i signaling7. More recently, Zhou et al. showed that ~45% of in situ bovine mature chondrocytes displayed spontaneous [Ca2+]i signaling, which had a significantly higher responsive rate than that of chondrocytes located in the middle zone of juvenile cartilage samples20. This phenomenon was validated in human chondrocytes in the present study, in which ~32.25% of chondrocytes displayed spontaneous [Ca2+]i signaling in the middle zone of healthy articular cartilage samples. However, the exact mechanism of spontaneous [Ca2+]i signaling in chondrocytes remains unclear. Similar to excitable cells such as neurons and myocytes21, mature chondrocytes are well equipped with rich complement of calcium channels such as voltage-dependent calcium channels (VDCCs)22, mechanical sensitive calcium channels (MSCCs)18, transient receptor potential vanilloid (TRPV)7, and Ca2+ release-activated Ca2+ (CRAC) channel23. These calcium channels synergistically regulate [Ca2+]i homeostasis by modulating extracellular calcium influx24 and intracellular calcium release from endoplasmic reticulum25. In the present study, total abolishment and significant attenuation of spontaneous [Ca2+]i signaling in chondrocytes located in superficial and deep zones were noticed when incubated in 0 mM [Ca2+]e environment. Since cells in these zones were located in the peripheral area of cartilage samples and more susceptible to extracellular changes of environmental calcium concentration than cells in the middle zones, our results suggested that similar to MSCs26, spontaneous [Ca2+]i signaling in chondrocytes was dependent on extracellular calcium influx. However, unlike the results presented by Kono et al., our observation suggested that [Ca2+]i signal propagation existed in most chondrons of both healthy and OA cartilage. This contradiction could be explained by variation in the area where the cartilage sample was examined. In our observation, propagation of [Ca2+]i signal was only observed in middle and deep zones, where 5–10 chondrocytes were closely located in one chondron. Since articular cartilage is an avascular and an eurogenic connective tissue, in which a chondrocyte is isolated by its native PCM and lacks direct cell-cell connection27; [Ca2+]i signaling at a distance12 is possibly through extracellular messengers such as nitric oxide (NO) and nucleotides and nucleosides, ATP, uridine triphosphate (UTP), or adenosine diphosphate (ADP)28. Propagated [Ca2+]i signal in in situ chondrocytes may serve as an efficient intercellular communication pathway6.
Similar to mechanically induced [Ca2+]i response, spontaneous [Ca2+]i signaling has been shown to regulate ECM maintenance in cartilage. Zhou et al. recently suggested that sustained spontaneous [Ca2+]i signaling enhanced mechanical properties of cartilage samples during long-term ex-vivo culture12. By analyzing the spatiotemporal features of spontaneous [Ca2+]i signaling in in situ chondrocytes of calf articular cartilage, they showed a high correlation between responsive rate and mechanical property of cultured explants. This observation was confirmed in the present study, in which we showed an increased responsive rate of spontaneous [Ca2+]i signaling from superficial to deep zone in normal cartilage. This phenomenon might reflect the distinct function of chondrocytes located in different zones revealed by microarray analysis29, which demonstrated that chondrocytes isolated from deep zones synthesized more proteoglycans than cells from the superficial zone30. In addition, Spearman’s rank correlation coefficient method also revealed that the responsive rate of spontaneous [Ca2+]i signaling was most correlated with the severity of cartilage degradation when incubated in 4 mM [Ca2+]e environment, suggesting that the spontaneous [Ca2+]i signaling could serve as a useful indicator of OA progression.
When spontaneous [Ca2+]i signaling of in situ chondrocytes was compared among different ICRS grade samples, no significant difference was seen in middle zone chondrocytes, indicating that chondrocytes in this zone were relatively inactive during the whole process of OA. In contrast, decreased intensity of [Ca2+]i signaling in deep zones of late-stage OA cartilages was found among grades. Decreased calcium channel activity caused by alternation in channel structure of OA chondrocytes could be one of the reasons22. Animal model using combination of orally administered monosodium iodoacetate (MIA) and treadmill over-loading showed decreased protein level of TRPV5, a voltage-independent cation channel in OA chondrocytes31. A recent study using OA animal model and human sample also revealed decreased TRPV6 expression in degraded cartilages32. In addition, by using RNA-seq analysis, Dunn et al. compared gene expression changes in human OA cartilage samples and found that α2/δ, a subunit of voltage-dependent calcium channels regulating channel properties and increasing the functional channel33, was significantly down-regulated34. By using the larger volume of cartilage sample acquired during total knee arthroplasty (TKA) surgery, we showed that chondrocytes in the early-degenerated area had significantly increased expression level of MMP-1, MMP-13, COL1A1; and decreased expression level of SOX-9 and SOX-11. In addition, increased protein abundance of Cav3.3, a α1 subunit of T-type VDCC in the early-degenerated area was noticed. The transiently active Cav3.3 T-type VDCC requires weak membrane depolarization for activation. Increased Cav3.3 protein abundance might decrease the membrane potential35 and pronounce [Ca2+]i signaling of chondrocytes in deep zone of early OA sample when few calcium irons were presented; and ultimately activated Nfatc2, a transcription factor has been shown to be crucial in chondrogenesis in tracheal cartilage36, and function regulation of adult chondrocytes37. Despite the fact that early OA was most pronounced in the superficial and middle zones38, our data suggested a unique role of deep zone chondrocytes in the initiation of OA. In early OA, the increased Nfatc2 activity might serve as a compensatory mechanism to withstand the established damage in articular cartilage. There is a growing consensus that OA is a whole-joint disease39. Increased turnover of subchondral bone seen in OA patients40 and animal models41 indicated an important role of subchondral bone-deep zone cartilage crosstalk during the onset of OA. Our previous studies using human OA samples and destabilization of medial meniscus (DMM) model showed decreased thickness in calcified cartilage zone (CCZ) at early stage40, accompanied by increased invading vessels density in this region41. These observations suggested elevated paracrine transport of inflammatory factors and cytokines released from subchondral bone could influence the calcium channel activities of deep zone chondrocytes, ultimately altering the ECM components such as proteoglycan concentration and composition of collagen observed in the present study.
The present study has several limitations. Firstly, although human biopsy samples at different OA stages represent the real progression of OA and retain the native PCM and ECM, they also suffer from disadvantages such as small sample volume, limited sample quantity, and restricted location for sample collection. In the current study, only a small volume of cartilage sample was acquired from each patient to prevent further damage to articular cartilage. To exclude the individual variation between patients, larger samples size and paired control sample representing non-degenerated cartilage from the same individual seems necessary for mechanism study. Secondly, the role of elevated spontaneous [Ca2+]i signaling in OA progression remains unclear. More stable sample source such as cartilage collected from the large-animal model is needed for performing [Ca2+]i dependent downstream pathway analysis.
Despite the limitations, the current study presented the direct evidence that in situ chondrocytes resided in both healthy and OA human articular cartilage displayed spontaneous [Ca2+]i signaling, and indicated that the spatiotemporal features of the [Ca2+]i oscillations were both zonal and OA severity dependent. While the exact mechanism needs further investigation, the changes of [Ca2+]i signaling might reflect the zonal specific role of chondrocytes during OA progression and provide new insight into molecular mechanism of articular cartilage degradation during OA progression. Also, our observation here also suggested that spontaneous [Ca2+]i signaling of in situ chondrocytes could serve as a potential biomarker for clinical diagnosis of early OA.
Methods
Cartilage explants
Articular cartilage samples for calcium imaging and histology evaluation were collected from patients receiving knee arthroscopy by one senior surgeon in Center for Joint Surgery, Southwest Hospital, Third Military Medical University, Chongqing, China. Informed consent was obtained from participant patients. During the surgical process, a single 2 mm in diameter cylindrical osteochondral sample was collected using a custom-designed biopsy needle that fits the arthroscopic system. For ICRS 0 group, healthy cartilage samples were collected in the non-load bearing area in knee joints with no sign of cartilage degeneration. For ICRS 1–3 groups, samples representing the most severe wear in each knee were collected from the femoral condyle before chondroplasty. The severity of cartilage degradation in their knee joints was evaluated during surgery based on ICRS Visual Assessment Scale, and confirmed by histomorphometry approaches afterward. Cartilage samples containing defects caused by trauma, tumor, or rheumatoid arthritis (RA) were excluded from this study. 3–4 cartilage samples for each ICRS grade were collected from 15 patients (Age: 42.69 ± 2.5 years; BMI: 23.55 ± 0.64; 5 males, 10 females; Table 2). This study was conducted in accordance with the declaration of Helsinki and with approval from the Ethics Committee of the Southwest Hospital of Third Military Medical University (Chongqing, China).
Table 2.
Demographic Data of Patientsa.
| ICRS 0 | ICRS 1 | ICRS 2 | ICRS 3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample set | Age | Gender | BMI | Sample set | Age | Gender | BMI | Sample set | Age | Gender | BMI | Sample set | Age | Gender | BMI |
| 1 | 28 | M | 25.5 | 1 | 46 | F | 22.6 | 1 | 42 | F | 27.1 | 1 | 53 | F | 28.6 |
| 2 | 38 | F | 20.9 | 2 | 44 | M | 22 | 2 | 49 | F | 24.8 | 2 | 37 | F | 24.2 |
| 3 | 24 | M | 22.5 | 3 | 50 | F | 23.8 | 3 | 45 | M | 23.4 | 3 | 44 | M | 23 |
| 4 | 50 | F | 18.5 | 4 | — | — | 4 | 51 | F | 23.4 | 4 | 40 | F | 22.9 | |
| Mean | 35 | — | 21.85 | 46.67 | 22.8 | 46.75 | 24.68 | 43.5 | 24.68 | ||||||
aValues were presented as mean ± SEM. No statistical differences were found for age and BMI among each group (P > 0.05).
Calcium imaging of in-situ chondrocytes
Harvested samples containing full-thickness cartilage and subchondral bone were immediately transferred to laboratory in PBS (Hyclone, Beijing, China). Samples were carefully cut into 2 half-cylinders using a scalpel. [Ca2+]i transients were monitored in in situ chondrocytes using Fluo-8 AM (AAT Bioquest, CA, USA) and fluorescence microscopy (IX71, Olympus, Tokyo, Japan). As shown in Fig. 2, collected samples were stained by 5 µM Fluo-8 AM at 37 °C for 30 min; excess dye was removed by washing with PBS for three times. Stained cartilage samples were then transferred into 96-well plates and kept in standard bath solution contained (in mM): NaCl, 144; NaH2PO4, 0.33; KCl, 4.0; MgCl2, 0.53; glucose, 5.5; HEPES, 5.0; CaCl2, 0 or 4 mM (the pH was adjusted to 7.4 with NaOH) at 37 °C for 15 min to remove any agitation during staining process26. Fluorescent images of chondrocytes were then recorded using 4 x objectives at room temperature for 15 min while the sample was undisturbed.
Image processing
To analyze the [Ca2+]i signaling, recorded image sequences were analyzed using Image J (Version 1.44p). The cartilage samples were separated into superficial, middle and deep zones in the image software according to chondrocyte/chondron size and arrangement. In detail, parallelly-arranged cells in cartilage surface were considered as superficial zone chondrocytes; cells in middle range of cartilage samples, and with the formation of medium-size chondrons in random arrangement were considered as middle zone chondrocytes; hypertrophy cells in deep range, and with the formation of large-size chondrons in vertical arrangement were considered as deep zone chondrocytes. For the superficial zone, 5–10 individual cells were randomly chosen and analyzed in each sample. For the middle and deep zone, cells in 5–10 randomly chosen chondrons were analyzed in each sample. Fluorescence intensity in individual cells was normalized with the mean background intensity obtained in three randomly chosen blank areas42. The calcium peak magnitude was reported as the mean fold increment of the peak intensity over the mean baseline fluorescence intensity in all tested chondrocytes. The responsive rate was determined by the number of cells whose calcium peak magnitude was over 2-fold of baseline divided by the number of total cells in the superficial zone or each chondron in middle and deep zone. For analyzing the number of peaks during the 15-min recording period, peaks with increment larger than two were counted as one peak, and the peak number was presented as the total number of peaks during the 15-min recording period.
Histology evaluation
For validating the severity of OA in acquired biopsies, the samples used for calcium imaging were fixed after calcium imaging in 4% paraformaldehyde at 4 °C overnight, followed by decalcification for 1 week with 10% EDTA before embedding in paraffin. Serial sections were cut at up to 5 µm thickness and stained with H&E, alcian blue, and sirius red respectively. Cartilage sections were then evaluated using ICRS grading system as previously reported43.
RNA extraction and RT-qPCR
Articular cartilage for RT-qPCR and Western boltting collected from TKA surgery with informed consent obtained from participant patients. Cartilage samples collected form non-degenerated and early-degenerated regions were processed into small fractions with a tissue homogenizer (Precellys 24, Bertin, MD, USA) at 4 °C. Total RNA was extracted using RNeasy® Mini Kit (Qiagen, Hilden, Germany). The purity of RNA was measured and quantitated on a Nanodrop-1000 spectrophotometer (Thermo Scientific, USA). l μg total RNA was used for cDNA synthesis. GAPDH was used as an internal reference, and target gene primers were presented in the supplementary information (see Supplementary information). RT-qPCR was performed in a reaction volume of 25 μl using QuantiTect SYBR Green PCR kit (Qiagen, Hilden, Germany). Assays were performed in triplicates, and the mRNA levels were normalized to GAPDH using the ∆∆CT method.
Western boltting
Protein fraction was prepared with RIPA Lysis Buffer (Beyotime, Beijing, China) using tissue homogenizer (Precellys 24, Bertin, MD, USA), and 20 μg/lane of proteins were subjected to SDS-PAGE (10%). The blots were incubated with anti-Cav3.1, Cav3.2, Cav3.3, A2D-1, and β-actin (Abcam, MA, USA), Nfatc2-dephos and Nfatc2-phos (Sigma, MO, USA) antibodies (1:100 dilution) and then incubated with anti-rabbit/mouse horseradish peroxidase-conjugated IgG (Sigma, MO, USA). GDS8000 detection system (UVP, USA) was used for the detection of the bound antibody.
Statistical analysis
Data were presented as mean ± SEM. For demographic data of patients, the statistical significance of age and BMI was determined by one-way ANOVA with Bonferroni’s post hoc test. For spatiotemporal features of spontaneous [Ca2+]i signaling, the statistical significance of the normalized fold increment, the number of peaks, and the responsive rate among all groups were determined by two-way ANOVA with Bonferroni’s post hoc test. Spearman’s rank correlation coefficient method was used to analyze the relationship between each parameter of [Ca2+]i signaling to ICRS grading system. For RT-qPCR and Western boltting data, the data from OA groups were compared with a hypothetical value of 1.0 (Normalized Normal control value) by using the student’s t-test. Statistical significance was defined with p < 0.05.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
This work was supported in part by grants from the National Natural Science Foundation of China (31130021, 11602046), The Foundation of Southwest Hospital (SWH2016JCYB-32).
Author Contributions
Authors’ roles: Study design: C.C. and L.Y. Study conduct: X.G. Data collection: W.X. and L.G. Data analysis: X.G., B.W. Data interpretation: F.W. and X.R. Drafting manuscript: X.G. Revising manuscript content: B.W. Approving final version of manuscript: all authors.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-17172-w.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Cheng Chen, Email: cclljjff@163.com.
Liu Yang, Email: jointsurgery@163.com.
References
- 1.Varady NH, Grodzinsky AJ. Osteoarthritis year in review 2015: mechanics. Osteoarthritis and cartilage. 2016;24:27–35. doi: 10.1016/j.joca.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pichler, K. et al. Expression of matrix metalloproteinases in human growth plate chondrocytes is enhanced at high levels of mechanical loading: A possible explanation for overuse injuries in children. Bone Joint J95-B, 568–573, doi:10.1302/0301-620X.95B4.30639 (2013). [DOI] [PubMed]
- 3.Buckwalter JA, Mankin HJ. Articular cartilage repair and transplantation. Arthritis and rheumatism. 1998;41:1331–1342. doi: 10.1002/1529-0131(199808)41:8<1331::AID-ART2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 4.Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis and rheumatism. 2001;44:585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 5.Akkiraju H, Nohe A. Role of Chondrocytes in Cartilage Formation, Progression of Osteoarthritis and Cartilage Regeneration. Journal of developmental biology. 2015;3:177–192. doi: 10.3390/jdb3040177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kono T, et al. Spontaneous oscillation and mechanically induced calcium waves in chondrocytes. Cell Biochem Funct. 2006;24:103–111. doi: 10.1002/cbf.1304. [DOI] [PubMed] [Google Scholar]
- 7.O’Conor CJ, Leddy HA, Benefield HC, Liedtke WB, Guilak F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc Natl Acad Sci USA. 2014;111:1316–1321. doi: 10.1073/pnas.1319569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanchez-Adams J, Leddy HA, McNulty AL, O’Conor CJ, Guilak F. The mechanobiology of articular cartilage: bearing the burden of osteoarthritis. Current rheumatology reports. 2014;16:451. doi: 10.1007/s11926-014-0451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doroudi M, Boyan BD, Schwartz Z. Rapid 1alpha,25(OH)(2)D (3) membrane-mediated activation of Ca(2)(+)/calmodulin-dependent protein kinase II in growth plate chondrocytes requires Pdia3, PLAA and caveolae. Connective tissue research. 2014;55(Suppl 1):125–128. doi: 10.3109/03008207.2014.923882. [DOI] [PubMed] [Google Scholar]
- 10.Rosa RG, et al. Clodronate exerts an anabolic effect on articular chondrocytes mediated through the purinergic receptor pathway. Osteoarthritis and cartilage. 2014;22:1327–1336. doi: 10.1016/j.joca.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smedler E, Uhlen P. Frequency decoding of calcium oscillations. Biochimica et biophysica acta. 2014;1840:964–969. doi: 10.1016/j.bbagen.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y, Park M, Cheung E, Wang L, Lu XL. The effect of chemically defined medium on spontaneous calcium signaling of in situ chondrocytes during long-term culture. Journal of biomechanics. 2015;48:990–996. doi: 10.1016/j.jbiomech.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen AC, Bae WC, Schinagl RM, Sah RL. Depth- and strain-dependent mechanical and electromechanical properties of full-thickness bovine articular cartilage in confined compression. Journal of biomechanics. 2001;34:1–12. doi: 10.1016/S0021-9290(00)00170-6. [DOI] [PubMed] [Google Scholar]
- 14.Klein TJ, Chaudhry M, Bae WC, Sah RL. Depth-dependent biomechanical and biochemical properties of fetal, newborn, and tissue-engineered articular cartilage. Journal of biomechanics. 2007;40:182–190. doi: 10.1016/j.jbiomech.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Rolauffs B, Williams JM, Grodzinsky AJ, Kuettner KE, Cole AA. Distinct horizontal patterns in the spatial organization of superficial zone chondrocytes of human joints. Journal of structural biology. 2008;162:335–344. doi: 10.1016/j.jsb.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darling EM, Hu JC, Athanasiou KA. Zonal and topographical differences in articular cartilage gene expression. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2004;22:1182–1187. doi: 10.1016/j.orthres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Lee DA, et al. Response of chondrocyte subpopulations cultured within unloaded and loaded agarose. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 1998;16:726–733. doi: 10.1002/jor.1100160615. [DOI] [PubMed] [Google Scholar]
- 18.Raizman I, De Croos JN, Pilliar R, Kandel RA. Calcium regulates cyclic compression-induced early changes in chondrocytes during in vitro cartilage tissue formation. Cell calcium. 2010;48:232–242. doi: 10.1016/j.ceca.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Han SK, Wouters W, Clark A, Herzog W. Mechanically induced calcium signaling in chondrocytes in situ. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2012;30:475–481. doi: 10.1002/jor.21536. [DOI] [PubMed] [Google Scholar]
- 20.Zhou, Y. et al. Effects of Osmolarity on the Spontaneous Calcium Signaling of In Situ Juvenile and Adult Articular Chondrocytes. Ann Biomed Eng, 10.1007/s10439-015-1406-4 (2015). [DOI] [PMC free article] [PubMed]
- 21.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 22.Matta C, Zakany R, Mobasheri A. Voltage-dependent calcium channels in chondrocytes: roles in health and disease. Current rheumatology reports. 2015;17:43. doi: 10.1007/s11926-015-0521-4. [DOI] [PubMed] [Google Scholar]
- 23.Inayama M, et al. Orai1-Orai2 complex is involved in store-operated calcium entry in chondrocyte cell lines. Cell calcium. 2015;57:337–347. doi: 10.1016/j.ceca.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Roberts SR, Knight MM, Lee DA, Bader DL. Mechanical compression influences intracellular Ca2+ signaling in chondrocytes seeded in agarose constructs. J Appl Physiol. 2001;90:1385–1391. doi: 10.1152/jappl.2001.90.4.1385. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez JC, Danks TA, Wilkins RJ. Mechanisms involved in the increase in intracellular calcium following hypotonic shock in bovine articular chondrocytes. Gen Physiol Biophys. 2003;22:487–500. [PubMed] [Google Scholar]
- 26.Kawano S, et al. Characterization of Ca(2+) signaling pathways in human mesenchymal stem cells. Cell calcium. 2002;32:165–174. doi: 10.1016/S0143416002001240. [DOI] [PubMed] [Google Scholar]
- 27.Wang F, et al. Histomorphometric analysis of adult articular calcified cartilage zone. Journal of structural biology. 2009;168:359–365. doi: 10.1016/j.jsb.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Elfervig MK, et al. ATP induces Ca(2+) signaling in human chondrons cultured in three-dimensional agarose films. Osteoarthritis and cartilage. 2001;9:518–526. doi: 10.1053/joca.2000.0435. [DOI] [PubMed] [Google Scholar]
- 29.Amanatullah DF, Yamane S, Reddi AH. Distinct patterns of gene expression in the superficial, middle and deep zones of bovine articular cartilage. J Tissue Eng Regen Med. 2014;8:505–514. doi: 10.1002/term.1543. [DOI] [PubMed] [Google Scholar]
- 30.Aydelotte MB, Greenhill RR, Kuettner KE. Differences between sub-populations of cultured bovine articular chondrocytes. II. Proteoglycan metabolism. Connective tissue research. 1988;18:223–234. doi: 10.3109/03008208809016809. [DOI] [PubMed] [Google Scholar]
- 31.Zhou X, Wang W, Miao J, Bai L. Expression and significance of transient receptor potential cation channel V5 in articular cartilage cells under exercise loads. Biomed Rep. 2014;2:813–817. doi: 10.3892/br.2014.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song T, et al. Regulation of Chondrocyte Functions by Transient Receptor Potential Cation Channel V6 in Osteoarthritis. J Cell Physiol. 2017 doi: 10.1002/jcp.25770. [DOI] [PubMed] [Google Scholar]
- 33.Wu J, et al. Structure of the voltage-gated calcium channel Cav1.1 at 3.6 A resolution. Nature. 2016;537:191–196. doi: 10.1038/nature19321. [DOI] [PubMed] [Google Scholar]
- 34.Dunn, S. L. et al. Gene expression changes in damaged osteoarthritic cartilage identify a signature of non-chondrogenic and mechanical responses. Osteoarthritis and cartilage, 10.1016/j.joca.2016.03.007 (2016). [DOI] [PMC free article] [PubMed]
- 35.Chevalier M, Lory P, Mironneau C, Macrez N, Quignard JF. T-type CaV3.3 calcium channels produce spontaneous low-threshold action potentials and intracellular calcium oscillations. The European journal of neuroscience. 2006;23:2321–2329. doi: 10.1111/j.1460-9568.2006.04761.x. [DOI] [PubMed] [Google Scholar]
- 36.Lin SS, et al. Cav3.2 T-type calcium channel is required for the NFAT-dependent Sox9 expression in tracheal cartilage. Proc Natl Acad Sci USA. 2014;111:E1990–1998. doi: 10.1073/pnas.1323112111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodova M, et al. Nfat1 regulates adult articular chondrocyte function through its age-dependent expression mediated by epigenetic histone methylation. J Bone Miner Res. 2011;26:1974–1986. doi: 10.1002/jbmr.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tchetina EV. Developmental mechanisms in articular cartilage degradation in osteoarthritis. Arthritis. 2011;2011:683970. doi: 10.1155/2011/683970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis and rheumatism. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deng B, et al. Quantitative study on morphology of calcified cartilage zone in OARSI 0 approximately 4 cartilage from osteoarthritic knees. Current research in translational medicine. 2016;64:149–154. doi: 10.1016/j.retram.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Pan J, et al. Elevated cross-talk between subchondral bone and cartilage in osteoarthritic joints. Bone. 2012;51:212–217. doi: 10.1016/j.bone.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gong, X.-y. et al. Inserted rest period resensitizes MC3T3-E1 cells to fluid shear stress in a time-dependent manner via F-actin-regulated mechanosensitive channel(s). Bioscience, Biotechnology, and Biochemistry, 10.1080/09168451.2014.895657. [DOI] [PubMed]
- 43.Mainil-Varlet P, et al. Histological assessment of cartilage repair: a report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS) The Journal of bone and joint surgery. American volume. 2003;85-A(Suppl 2):45–57. doi: 10.2106/00004623-200300002-00007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.