Lymphoma is a hematological malignancy that involves T or B cells. Diffuse large B-cell lymphoma (DLBCL) accounts for ~40% of cases of non-Hodgkin’s lymphoma (NHL) and is a highly aggressive and heterogeneous subtype of NHL.1 The pathogenesis of DLBCL remains unclear. DLBCL is also closely related to inflammatory factors and immune cells in the tumor microenvironment (TME), and alterations of the cytokine network in the TME may activate oncogenes or inactivate tumor suppressor genes.2, 3, 4 In the TME, a variety of immune cells and cytokines have crucial roles in the pathogenesis of tumors.
T helper (Th) 17 cells are a type of CD4+ effector T cell that secrete interleukin (IL)-17. IL-17 exerts a variety of biological effects by binding to the IL-17 receptor (IL-17R), which is widely expressed on the surface of numerous cells, including tumor cells. IL-17 has important roles in promoting inflammation, exhibits anti-infection effects and is involved in autoimmune diseases and graft-versus-host disease in hematopoietic stem cell transplantation. In addition, IL-17 is a critical cytokine in TME. Th17 cells have a role in tumor cells by secreting cytokines (such as IL-17 and IL-21 among others) in the TME, which is different from cytotoxic T cells killing tumor cells directly. However, the role of IL-17 in tumorigenesis in DLBCL or other tumors remains elusive. Furthermore, the antitumor versus pro-tumor effects of IL-17 remain controversial.5, 6 For example, IL-17 has a pro-tumor role by inhibiting tumor cell apoptosis, promoting tumor cell proliferation and promoting tumor angiogenesis, metastasis and invasion.6 In contrast, IL-17 exhibits antitumor effects by recruiting CD4+ T, CD8+ T and dendritic cells, and enhancing the activity of natural killer and cytotoxic lymphocyte cells.6 The role of IL-17 in tumorigenesis is still thought to depend on the immune context and tumor type. The mechanisms underlying the effect of IL-17 on activating signaling pathways involved in DLBCL tumorigenesis remain to be elucidated.5
Radiotherapy and immunochemotherapy (containing rituximab) currently represent the two major treatments for DLBCL. However, irradiation and rituximab resistance limit the efficacy of these treatments in clinical practice. Rituximab combined with cyclophosphamide, Adriamycin, vincristine and prednisone (R-CHOP) is currently the standard first-line regimen for DLBCL, resulting in complete remission in ~80% of patients. However, the widespread clinical use of rituximab has increased rituximab resistance in DLBCL patients. A previous report revealed that 30% of DLBCL cases were resistant to rituximab or rituximab-based chemotherapy regimens.7 Resistance to radiotherapy is also a common phenomenon. The underlying mechanisms of resistance to radiation and rituximab have not been completely elucidated. The relationship between resistance to these two types of therapy and IL-17 will be discussed in the following sections.
Studies focusing on clinical irradiation biology have suggested that certain biological factors affect the sensitivity of tumor cells to radiotherapy. Changes in the TME are among the major mechanisms of tumor cell resistance to radiation injury.8 IL-17A has an important role in DLBCL tumorigenesis. We recently explored the distributions of immune cells and cytokine profiles in extranodal tumor lesions and their adjacent benign tissues in DLBCL patients. Th17 cell percentages and IL-17 levels were significantly lower in DLBCL tumor tissues than in benign tissues.4 Similarly, Yang et al.9 reported that Th17 cells and IL-17A levels were significantly reduced in the TME of NHL patients. Liu et al. demonstrated that the frequency of Th17 cells and the level of IL-17A in the peripheral blood were markedly lower in DLBCL patients than in healthy individuals. In addition, the frequency of circulating Th17 cells increases in relapsed DLBCL patients.10 Published data revealed that in addition to tumor cells, immune cells in DLBCL lesions and their microvascular distribution affect the efficacy of the R-CHOP regimen.2 Ferretti et al.11 verified that IL-17A promotes the growth of human germinal center-derived NHL, including DLBCL. We found that increased expression of interferon (IFN) regulation factor 8 (IRF8) in tumor cells inhibits the generation of Th17 cells, and high IRF8 expression in tumor cells predicts unfavorable survival for DLBCL patients.4 Therefore, IL-17 may be a pro-tumor factor in DLBCL and may affect resistance to radiotherapy and rituximab.
On the basis of these studies, we investigated the involvement of IL-17 in radiation resistance in DLBCL. Immune cells in the TME have an important role in the pathogenesis of DLBCL. In the steady state, forkhead box P3 (Foxp3) antagonizes RAR-related orphan receptor gamma (RORγt) in CD4+ T cells and inhibits IL-17 production. However, under certain conditions, regulatory T (Treg) cells can produce IL-17. IL-17+Foxp3+ Treg cells represent the intermediate stage of Treg and Th17 cells. In our study, to clarify the role of Treg cells in IL-17 secretion induced by irradiation, we isolated CD4+ CD25+ Treg cells from peripheral blood mononuclear cells and co-cultured them with transforming growth factor-β and IL-2. Our results demonstrated that B-cell lymphoma Karpas1106 (k1106) cells secrete increased levels of IL-6 in response to radiotherapy. IL-6 subsequently stimulates Treg cells to secrete IL-17, thereby inhibiting the expression of the tumor suppressor gene p53 and inhibiting irradiation-induced apoptosis of tumor cells. In conclusion, we demonstrated that IL-17 induces radiotherapy resistance in B-cell lymphoma.12
IL-6 is produced by a variety of cells, including monocytes, fibroblasts, epithelial cells and hematological tumor cells. Our data were consistent with previous data demonstrating that naive k1106 cells produce detectable IL-6, which is upregulated after irradiation. The production of IL-17 is abolished in the presence of anti-IL-6 antibodies. Our results indicated that irradiated k1106 cells induce the production of IL-17 from Treg cells.12 Previous studies demonstrated that anti-IL-6 does not reverse resistance to radiation and chemotherapy in lymphoma cells, suggesting that resistance is not directly mediated by IL-6 and could potentially involve other cytokines or other signaling pathways. Radiotherapy kills DLBCL cells mainly through apoptosis. The p53 protein is a tumor suppressor that induces tumor cell apoptosis. Our previous study provided an explanation for this finding.12
The serine/threonine protein kinase tumor progression locus 2 (TPL2), which belongs to the mitogen-activated protein kinase (MAPK) family, is widely distributed in tumor cells. The ‘double-sided effect’ of TPL2 depends on the different upstream and downstream signals in the TME or on the tumor type. Mounting data indicate that TPL2 is closely related to cytokines released from inflammatory cells.13 For instance, recent studies confirmed that IL-17 has a critical role in the oncogenesis of colon, cervical and breast cancers.13 IL-17 activates the MEK/ERK or JNK/c-Jun pathways related to TPL2 upstream signals, promoting c-fos or c-jun transcriptional activity. This activity induces AP-1-dependent transcription and ultimately promotes tumor formation.14 The downstream signaling pathways of IL-17/IL-17R may include TPL2/MAPK/ERK, PI3K/AKT and STATs. However, whether activation of the IL-17R/TPL2 signaling pathway affects DLBCL tumor growth requires further clarification.
In the following two paragraphs, we will discuss on how rituximab promotes IL-17 secretion and thereby induces rituximab resistance. The antitumor effects of rituximab are mediated by three mechanisms: induction of tumor cell apoptosis; complement-dependent cytotoxicity; and antibody-dependent cytotoxicity. Published data indicate that rituximab may affect Th17 cells and IL-17 level differently in different diseases. Rituximab inhibits the differentiation of Th17 cells in autoimmune diseases, including rheumatoid arthritis, antineutrophil cytoplasmic antibodies-associated refractory vasculitis and Sjogren syndrome, and this effect is associated with treatment efficacy. By contrast, recent experiments in mice inoculated with EL4-huCD20 lymphoma cells expressing human CD20 antigen suggested that anti-CD20mb promotes Th1 cell differentiation and prevents pro-tumor Treg cell expansion in mice through the IFN-γ/IL-12 axis.15 Yang et al.9 reported the presence of reduced Th17 cell levels in lymph node lesions of B-cell NHL patients. Compared with normal B lymphocytes, malignant B-cell lymphoma cells express high levels of costimulatory molecules CD70, CD80 or CD86, which are involved in Treg cell generation via CD27/CD27-CD70 or CD86/CD28-CD80 connections, which cause an imbalance in Th17/Treg cells.
On the basis of our previous study,12 we hypothesized that given the differences in the expression of costimulatory molecules in normal and malignant B cells, rituximab may induce Treg cells to secrete IL-17 upon the elimination of malignant B cells. Previous studies demonstrated that IL-17 promotes tumor angiogenesis11 and the activity of the IL-17/IL-17R/p53 signaling pathway.12 Other cytokines in addition to IL-17 may be involved in DLBCL tumor formation. Namely, rituximab and radiation therapy may alter cytokine expression profiles with the exception of IL-6 and IL-17. We hypothesized that rituximab promotes Foxp3+Treg cell-mediated secretion of IL-17, which subsequently induces tumor growth in DLBCL patients. Tumor formation may thus be stimulated by the IL-17R/TPL2/MAPK signaling pathway.13 Our latest unpublished data confirmed that rituximab promoted Th17 cells and IL-17+Foxp3+ Treg cells to secrete IL-17, which subsequently promoted rituximab resistance. This finding could explain why some DLBCL patients do not respond to the R-CHOP regimen and frequently relapse. Therefore, rituximab could be a ‘double-edged sword’ in the treatment of DLBCL.
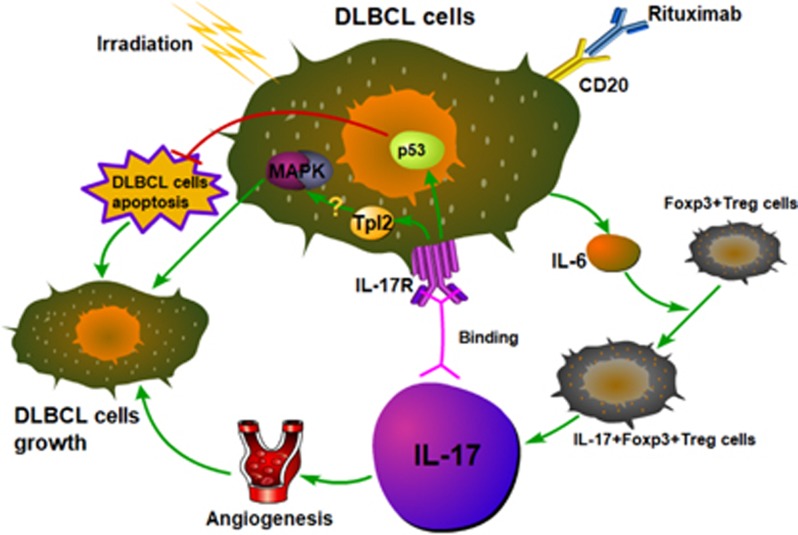
In summary, we demonstrated that both irradiation and rituximab promote IL-17 production in the TME of DLBCL, thereby increasing IL-17-induced irradiation and rituximab resistance mediated by angiogenesis, apoptosis and the activation of IL-17/IL-17R or other signaling pathways (Figure 1). In the future, in addition to IL-6 and IL-17, we plan to explore other proteomes or cytokines in the TME of DLBCL that are responsible for DLBCL pathogenesis and treatment resistance. To further investigate the mechanisms underlying IL-17-induced irradiation or rituximab resistance, we will investigate alterations in the gene expression profiles of DLBCL cells treated with irradiation or rituximab using gene chips or next-generation sequencing to identify functional genes to support our existing results and provide further insight into the mechanism by which IL-17 promotes DLBCL development. We will then validate these findings in animal models. These efforts will provide a new strategy to prevent and overcome radiation and rituximab resistance in DLBCL patients and may reveal the molecular mechanisms of irradiation and rituximab resistance in DLBCL.
Figure 1.
Increased IL-17 induced by rituximab or irradiation promotes the growth of DLBCL cells. Anti-CD20 monoclonal antibody (rituximab) or irradiation promotes DLBCL cells to secrete IL-6, thereby inducing the secretion of IL-17 by Foxp3+Treg cells. This group of cells is named IL-17+Foxp3+Treg cells, which is an intermediate cells group between Th17 and Treg cells. The mechanisms of IL-17 promoting the growth of DLBCL cells are as follows: (1) IL-17 promotes the formation of tumor angiogenesis. (2) IL-17 promotes the expression of tumor apoptotic suppressor p53. These two mechanisms are confirmed. (3) IL-17 may trigger the pathway of TPL2/MAPK by binding to IL-17R. DLBCL, diffuse large B-cell lymphoma; Foxp3, forkhead box P3; IL-17, interleukin-17; IL-6, interleukin-6; MAPK, mitogen-activated protein kinase; Th17, T helper 17; TPL2, tumor progression locus 2; Treg, regulatory T.
Acknowledgments
This work was supported by the Guangzhou Planned Project of Science and Technology, China (nos. 201707010279 and 201704020105).
Footnotes
The authors declare no conflict of interest.
References
- Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood 2015; 125: 22–32. [DOI] [PubMed] [Google Scholar]
- Gomez-Gelvez JC, Salama ME, Perkins SL, Leavitt M, Inamdar KV. Prognostic impact of tumor microenvironment in diffuse large B-cell lymphoma uniformly treated with R-CHOP chemotherapy. Am J Clin Pathol 2016; 145: 514–523. [DOI] [PubMed] [Google Scholar]
- Fridman W, Pagès F, Sautè-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12: 298–306. [DOI] [PubMed] [Google Scholar]
- Zhong W, Xu X, Zhu Z, Du Q, Du H, Yang L et al. Increased expression of IRF8 in tumor cells inhibits the generation of Th17 cells and predicts unfavorable survival of diffuse large B cell lymphoma patients. Oncotarget 2017; 8: 49757–49772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemdan NY. Anti-cancer versus cancer-promoting effects of the interleukin-17-producing T helper cells. Immunol Lett 2013; 149: 123–133. [DOI] [PubMed] [Google Scholar]
- Qian X, Chen H, Wu X, Hu L, Huang Q, Jin Y. Interleukin-17 acts as double-edged sword in anti-tumor immunity and tumorigenesis. Cytokine 2017; 89: 34–44. [DOI] [PubMed] [Google Scholar]
- Rezvani AR, Maloney DG. Rituximab resistance. Best Pract Res Clin Haematol 2011; 24: 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doss M, Kolb H, Walsh J, Mocharla V, Fan H, Chaudhary A et al. Biodistribution and radiation dosimetry of 18F-CP-18, a potential apoptosis imaging agent, as determined from PET/CT scans in healthy volunteers. J Nucl Med 2013; 54: 2087–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. Malignant B cells skew the balance of regulatory T cells and TH17 cells in B-cell non-Hodgkin's lymphoma. Cancer Res 2009; 69: 5522–5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Yu S, Liu Y, Yin C, Ye J, Liu Z et al. Aberrant circulating Th17 cells in patients with B-cell non-Hodgkin's lymphoma. PLoS One 2016; 11: e0148044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti E, Di Carlo E, Ognio E, Guarnotta C, Bertoni F, Corcione A et al. Interleukin-17A promotes the growth of human germinal center derived non-Hodgkin B cell lymphoma. Oncoimmunology 2015; 4: e1030560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Xu X, Zhong W, Du Q, Yu B, Xiong H. IL-17 induces radiation resistance of B lymphoma cells by suppressing p53 expression and thereby inhibiting irradiation-triggered apoptosis. Cell Mol Immunol 2015; 12: 366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Choi H, Joo K, Nam D. Tumor progression locus 2 (Tpl2) kinase as a novel therapeutic target for cancer: double-sided effects of Tpl2 on cancer. Int J Mol Sci 2015; 16: 4471–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G, Khanal P, Lim S, Yun H, Ahn S, Ki S et al. Interleukin-17 induces AP-1 activity and cellular transformation via upregulation of tumor progression locus 2 activity. Carcinogenesis 2013; 34: 341–350. [DOI] [PubMed] [Google Scholar]
- Deligne C, Metidji A, Fridman W, Teillaud J. Anti-CD20 therapy induces a memory Th1 response through the IFN-γ/IL-12 axis and prevents protumor regulatory T-cell expansion in mice. Leukemia 2015; 29: 947–957. [DOI] [PubMed] [Google Scholar]