Abstract
Background:
Electronic health records (EHRs) are commonplace in industrialized countries. Many hospitals are granting their patients access to their medical information through online patient portals. In this report, we describe a retrospective analysis of patient access to diagnostic test results released through the patient portal (MyChart; Epic, Inc.) at a state academic medical center.
Methods:
We analyzed 6 months of data for anatomic pathology, clinical laboratory, and radiology test results to evaluate variations in results release (automated vs. manual) and subsequent patient access to the institutional patient portal. During this period, diagnostic test results were released for all patient encounters including inpatient units, outpatient clinics, and the emergency department.
Results:
Manual results release by providers before automated release time occurred most commonly in the outpatient setting. The highest rates of access of diagnostic test results occurred for outpatients (about 30% overall view rate), females (two times or more compared to males in nearly every age bracket), and 20–45-year-old. Access rates of diagnostic tests in the emergency department or inpatient units were <10% across all populations. Access of diagnostic test results was very low for 12–17-year-old, likely influenced by institutional policies limiting parental proxy access within this pediatric age range. Approximately 20% of outpatient laboratory results were viewed by patients within 8 h of release from the EHR to the patient portal and 10% within 2 h of release.
Conclusions:
Patient accessing of diagnostic test results were generally higher for females, outpatients, and 20–45-year-old. Approximately, 20% of outpatient results were viewed quickly by patients after release to the EHR.
Keywords: Clinical laboratory information system, electronic health records, medical informatics, patient portals, personal health records, routine diagnostic tests
INTRODUCTION
Electronic health records (EHRs) are commonplace in industrialized countries.[1,2,3] In the United States, a combination of incentives and penalties has spurred institutions to replace paper records with EHRs.[2,3,4,5,6] Many hospitals are granting patients access to their medical information through online patient portals such as MyChart (Epic, Inc.)[7,8,9] and My HealtheVet (Department of Veterans Affairs).[10,11] Depending on institutional policies, patient portals can allow patients access to a wide variety of personal health information such as clinical notes, laboratory results, radiology reports, immunization records, and medication history.[8,12,13,14,15,16,17]
The release of diagnostic test results is a common feature of patient portals, although patterns of test result release can vary substantially.[18] Based on the recent emergence of the concept and the associated technology, policies for patient access are not well standardized across institutions and lag times for results to appear in patient portals vary considerably.[19] There are both ethical and safety considerations with release of diagnostic test results, especially those that are abnormal, complicated to interpret, and/or convey unfavorable news for the patient.[18,20] Thus, the process is a balance between allowing health-care providers control over interpretation and communication of results while trying to minimize the patient stress associated with the uncertainty of waiting for diagnostic test results.[21]
In the present study at an academic medical center, we focused on the release of diagnostic test results by providers and the subsequent patient access patterns of clinical laboratory, anatomic pathology, and radiology results within the patient portal. We hypothesized that there would be differences in the time to result release to the online patient portals based on whether they were auto-released or manually released by the provider and that encounter type, gender, and age differences would influence access of diagnostic test results through the patient portal.
METHODS
Institutional details
The study was conducted at the University of Iowa Hospitals and Clinics (UIHC), a 761-bed tertiary/quaternary care academic medical center located in Iowa City, Iowa, USA. UIHC includes pediatric and adult inpatient units, multiple intensive care units, an emergency department with Level I trauma capability and outpatient services. Outpatient clinics are located at the main medical campus, at a multispecialty facility located three miles away, and various primary care and specialty clinics scattered throughout the local region. The data in the study were collected as part of a retrospective study approved by the University of Iowa Institutional Review Board (protocol #201608720) covering the period from January 1, 2016, to June 30, 2016. This study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
Informatics
The EHR throughout the retrospective study period was Epic (Epic Systems, Inc., Madison, Wisconsin, USA) which has been in place since May 2009. The patient portal access through MyChart was first established in 2010, with primary organizational oversight provided by the Health Information Management subcommittee, a multidisciplinary group that meets monthly with representation from all clinical departments, hospital administration, and information technology. An additional group, the Epic Core Team, meets weekly on various practical issues of the EHR, including patient-portal related issues, and reports to the Health Information Management subcommittee. In response to identified issues and an attempt to improve the utilization of the patient portal, an additional group (MyChart Results Release Working Group) was initiated in 2016 and meets monthly to work specifically on the patient portal. The Health Information Management subcommittee ultimately reports to a Hospital Advisory Committee (which includes all clinical Department Executive Officers) that provides final oversight and approval for policies. Pathology has representation at each of these governance groups.
The laboratory information system for both anatomic pathology and clinical pathology was Epic Beaker.[22,23,24] Radiology reports are accessible through the EHR. As described in our previous studies, Epic Reporting Workbench (RWB) was used to retrieve patient demographics, laboratory results, and radiology reports,[25,26] incorporating additional parameters related to the patient portal. In particular, RWB captured dates and times of when laboratory or radiology results were filed to patients’ EHR, when they were released to the patient portal, the method of release (manually by provider vs. automated), when the patients accessed the results through the patient portal, and when patients activated their patient portal accounts (if they were activated at all). Results were then grouped into test-based subcategories. Finally, the encounters were divided into three major categories based on the location of the patient when the test was ordered, i.e., inpatient unit, outpatient clinic (including laboratory-only encounters at outpatient phlebotomy sites), or emergency department. The total data set included 1,585,749 results including 1,524,961 clinical laboratory tests, 34,142 anatomic pathology reports (included 5,855 dermatopathology reports), and 26,646 radiology reports. There were a total of 59,388 unique patients in the study period.
Patient portal access
Patients have the option to sign up for patient portal access, but it is not required. In the clinical settings, instructions for setting up access to the patient portal are provided with the after visit summary sheet. There were also policies that allowed for caretakers or legal guardians to have proxy access to patient portal accounts of adult dependent patients. Parents and legal guardians could obtain proxy access to child patient portal account in an unrestricted manner through age 11. Proxy privileges by parents and guardians were restricted for children 12–17-year-old, with limited access to information such as immunization records. For the purposes of analysis in this study, proxy access of results was also counted in terms of diagnostic test result viewing.
Patient portal policies
During the study period, diagnostic tests were divided into three main categories with regard to the automatic release of results into the patient portal: never release, one business day, and four business day [Table 1]. Only a small number of tests were never released at all to the patient portal; tests in this category do not populate the patient portal either by automated release or by the manual release by provider. Human immunodeficiency virus (HIV) screening and confirmation testing were the most frequently ordered tests in this category. Positive HIV test results cannot release to a patient portal under state of Iowa law unless direct counseling of the patient (by phone or in person) can be documented. Due to the technical complications of meeting this requirement with existing EHR and patient portal functionality, all HIV screening and confirmatory results were blocked from release to the patient portal. Huntington gene analysis was another example of a test within the never release test category.
Table 1.
Patient portal auto-release categories in the electronic health record
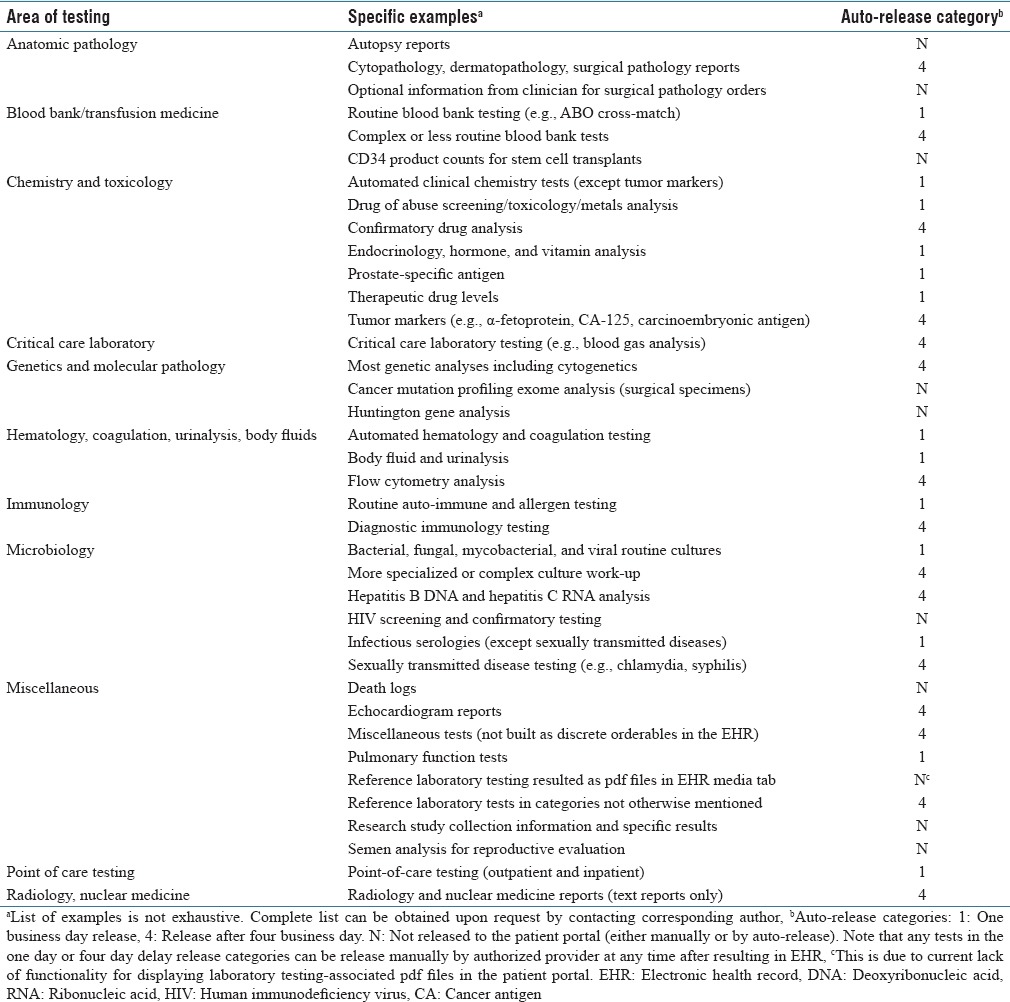
During the retrospective period of analysis (January 1, 2016-June 30, 2016), for the one business day automatic release category, the schedule was to release these to the patient portal the next business day at 3:00 AM after the result first appeared in the EHR. Thus, a test appearing in the EHR on a Tuesday would auto-release Wednesday at 3:00 AM (assuming Wednesday was not a holiday). For a test appearing in the EHR on Friday or Saturday or Sunday, the auto-release would be Monday at 3:00 AM (assuming that Monday was not a holiday). The time of day had been chosen to release the results during “off hours” to decrease the informatics burden on the system. The logic was similar for four business day release except these would be auto-released on the fourth business day after the result first appeared in the EHR (additional delay would occur if holidays intervened). This method of auto-release was changed in September 2016 (discussed further in Results and Discussion).
Many of the common blood bank/transfusion medicine, chemistry, hematology, immunology, microbiology, and point-of-care tests were one business day release [Table 1]. Exceptions included blood gas analysis, sexual transmitted disease testing, drug of abuse confirmatory analysis, and tumor markers, which were in the 4 day business day release category. Other clinical laboratory tests (e.g., genetic, molecular pathology tests) along with anatomic pathology and radiology reports were generally also in the 4 day release category.
Any of the tests in the one business day or four business day auto-release categories could be released to the patient portal manually by provider at any time. The manual route was most straightforward for tests ordered in the outpatient setting since these routed to the EHR in-basket of the ordering provider who could then immediately release result to the patient portal with a single mouse click. In the inpatient and emergency department settings, diagnostic test results do not get sent to provider inbaskets to avoid the overload of information in these settings that have heavy volumes of test ordering. To manually release inpatient and emergency department test results to the patient portal, providers would need to first navigate to a patient portal result release activity window. This requires a minimum of three extra steps, compared to manual release in the outpatient setting, and is not required by providers. Consequently, inpatient and emergency department results are less commonly released manually to the patient portal.
Analysis
Retrospective data were analyzed to determine how fast results were released to patients’ online portal after they had been resulted and filed into their EHR. Furthermore, the data were divided into three categories based on the location of the patient when the test was ordered: Outpatient, inpatient, and emergency department. For the purposes of comparison, diagnostic tests were divided into six categories of tests performed in-house at UIHC: Clinical chemistry (includes automated infectious disease serologies, endocrinology, and toxicology), hematology (includes coagulation), microbiology/molecular pathology, anatomic pathology, diagnostic X-ray (an example of simpler imaging), and diagnostic magnetic resonance imaging (MRI; an example of more complex imaging). The clinical chemistry and hematology testing include a high percentage that is performed quickly on receipt in the laboratory as opposed to being run in batches. The other test categories would have much more variable time for final result/report to appear in the EHR.
RESULTS
Patterns of diagnostic test results release and patient portal access in the outpatient setting
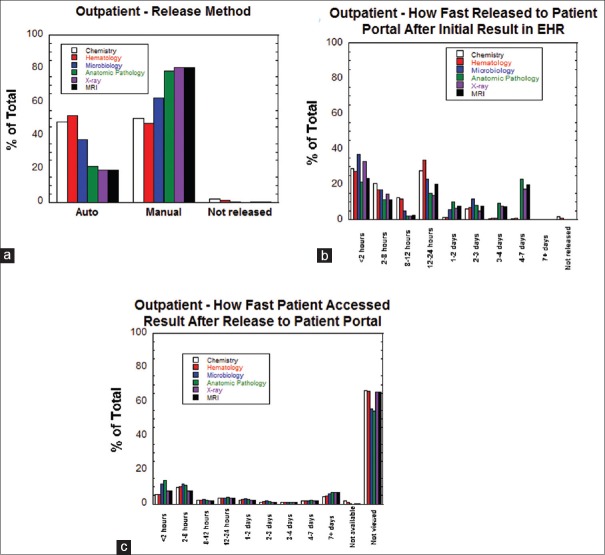
Figure 1a shows the route of release for outpatient testing into the patient portal. There were approximately equal ratios of auto-release and manual release for chemistry and hematology testing. For the other categories of testing, manual release was more common than auto-release. Figure 1b shows a breakdown of how fast test results were transmitted to the patient portal after first appearing in the EHR. Across all categories, between approximately 30%–55% of results were released within 12 h of first appearing within the EHR. The peaks in Figure 1b in the 1–2 days and 2–3 days categories are influenced by the one business day auto-release patterns for some tests in combination with manual release that may have occurred during that period. For anatomic pathology, X-ray, and MRI results, the peak at 4–7 days mainly reflects the four business day auto-release for these categories of results. Figure 1c illustrates how fast patients accessed results after result was released to the patient portal. For all six categories of testing, approximately 20% of patients viewed results within 8 h of release and between 5%–15% viewed within 2 h of release. Approximately 5%–10% of patients viewed results 7 or more days after release to the patient portal. Overall, approximately 70% of outpatient results were never viewed in the patient portal, due to the patient either not having an active patient portal account or having an account but not accessing the result (discussed in further detail in the “Influence of Age and Gender on Patient Access of Diagnostic Test Results” section). Overall, 72,672 of 241,801 results (30.1%) from outpatient encounters were reviewed by patients on the patient portal.
Figure 1.
Release patterns for outpatient results. (a) Method of release (auto-release, manual release, not released) for outpatient results sorted by category of testing. (b) How fast outpatient result was released to the patient portal after the first appearance in the electronic health record sorted by category of testing.(c) How fast patients accessed outpatient results (or did not access at all) after the result first appeared in the patient portal sorted by category of testing
Patterns of diagnostic test results release and patient portal access in the inpatient setting
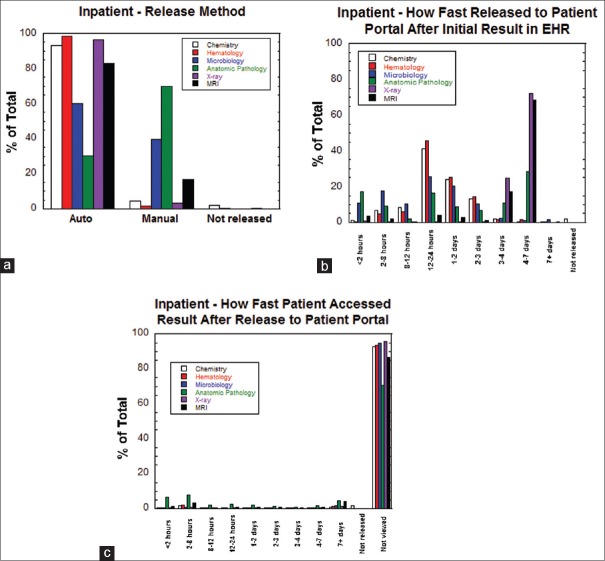
Figure 2a shows that diagnostic test results originating from inpatient unit encounters were predominantly auto-released (as opposed to manually released) for all categories of testing except anatomic pathology. Figure 2b shows a breakdown of how fast inpatient unit test results were transmitted to the patient portal after first appearing in the EHR. The overall pattern in Figure 2b is heavily influenced by auto-release patterns, with peaks at 1–2 days and 2–3 days (for tests with one business day auto-release) and 4–7 days (for tests with four business day auto-release). Interestingly, anatomic pathology and microbiology test results had the highest frequency of release to the patient portal within 2 h following appearance in the EHR. Figure 2c shows that a high percentage of inpatient test results across all categories were not viewed in the patient portal by patients, with multiple categories showing >90% of results not being viewed. Anatomic pathology tests have the highest frequency of viewing (approximately 30%) of diagnostic tests in the inpatient setting. Overall, only 25,385 of 455,833 results (5.6%) from inpatient encounters were reviewed by patients on the patient portal.
Figure 2.
Release patterns for inpatient results. (a) Method of release (auto-release, manual release, not released) for inpatient results sorted by category of testing. (b) How fast inpatient result was released to the patient portal after first appearance in the electronic health record sorted by category of testing. (c) How fast patients accessed inpatient results (or did not access at all) after the result first appeared in the patient portal sorted by category of testing
Patterns of diagnostic test results release and patient portal access in the emergency department
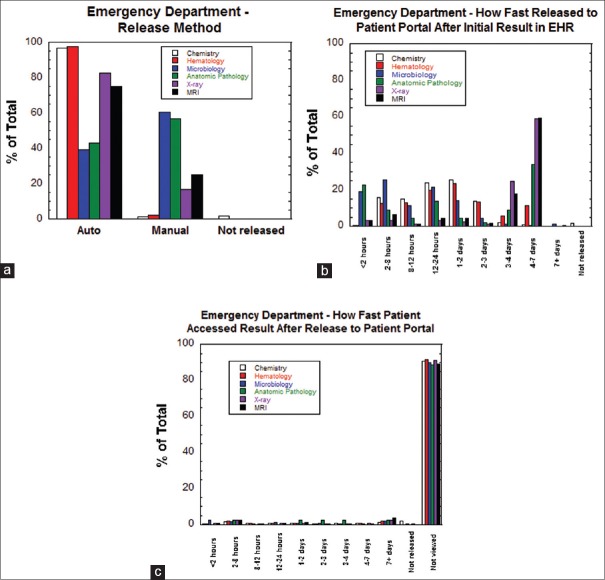
The patterns of release of tests ordered within the emergency department to the patient portal were very similar to those for inpatient tests described above. Figure 3a shows a high percentage of auto-release compared to manual release for all categories except anatomic pathology and microbiology results. The overall pattern in Figure 3b of transmission of results from the EHR to the patient portal is influenced by auto-release patterns. Similar to the inpatient setting, anatomic pathology and microbiology test results had the highest frequency of release to the patient portal within 2 h following appearance in the EHR. For diagnostic tests originating from the emergency department, Figure 3c shows that patient access of results was uniformly low (<10%) across all testing categories. Overall, 53,560 of 888,115 results (6.0%) from emergency department encounters were reviewed by patients on the patient portal.
Figure 3.
Release patterns for emergency department results. (a) Method of release (auto-release, manual release, not released) for emergency department results sorted by category of testing. (b) How fast emergency department result was released to the patient portal after first appearance in the electronic health record sorted by category of testing. (c) How fast patients accessed emergency department results (or did not access at all) after the result first appeared in the patient portal sorted by category of testing
Influence of age and gender on patient access of diagnostic test results
Data were analyzed to examine the demographics of the patient portal account activation (i.e., having set up an active user account) for the patient population that had diagnostic testing performed during the study period. The overall patient portal activation rate for the population that had diagnostic tests performed was 44.2%. Figure 4 shows the percentage of patients within gender and age categories that had set up and activated their patient portal account. For most age categories, females had active patient portal accounts more frequently than males. The highest rates of patient portal activation were for females 11 years or younger and 26–50-year-old. For both males and females, the percent of patients with active patient portal accounts was low for ages >70-year-old.
Figure 4.
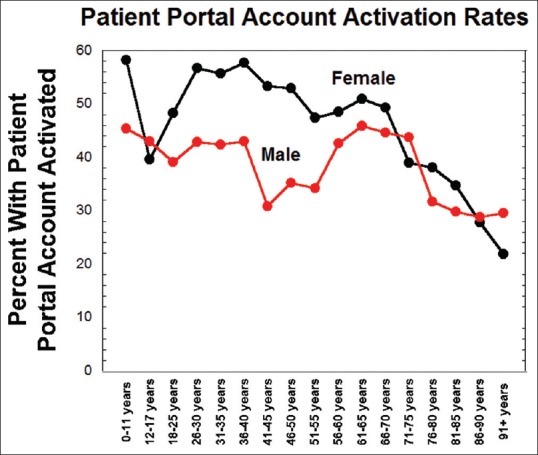
Patient portal activation rates broken down by gender and age categories
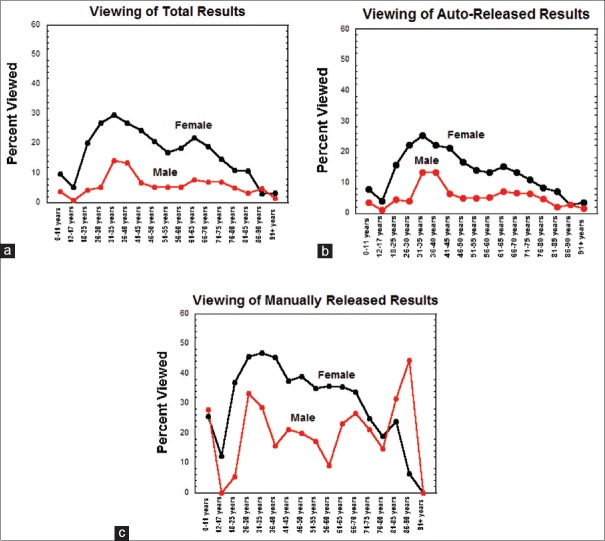
Figure 5a shows the overall trends for diagnostic test result viewing in the patient portal. Females generally viewed results at a higher percentage than men (double or more in nearly every age category), with the only exception being the 86–90-year-old category that had low viewing rates for both males and females. Viewing rates of diagnostic test results that were auto-released [Figure 5b] were generally lower than those that were manually released [Figure 5c].
Figure 5.
Viewing of patient portal results broken down by gender and age categories. (a) Overall viewing rates for results for all diagnostic tests. (b) Viewing rates for auto-released diagnostic test results. (c) Viewing rates for manually released diagnostic test results
The trends in Figure 5 for viewing of diagnostic test results in the patient portal roughly parallel the trends for having an active patient portal account [Figure 4]. The most obvious difference between the two trends is that the high rates of patient portal account activation in children contrast with low percentage of viewing diagnostic test results. In general, females 18–70-year-old had viewing rates of diagnostic test results in the patient portal of at least 20%, with a peak of approximately 30% in the 31–35-year-old range. In contrast, males accessed test results in their patient portal at 15% or lower throughout all age ranges, with the peak also in the 31–35-year-old range. Both males and females had a secondary, smaller peak of patient portal viewing in the 61–65-year-old range. Males between the ages of 12 and 17 years had the lowest percentage of viewing their results in patient portal (<1%) even though approximately 40% of patients in this age range had an active patient portal account.
Examples of patient concerns
While we found that the patient portal was overall a positive feature for patients and families, release of diagnostic test results through portals can have unintended consequences. The true extent of this occurrence is hard to measure, but we have encountered anecdotal cases which resulted in psychological patient harm. Several cases involved inadvertent manual release of sensitive results before a provider could discuss the results with the patient. Two examples are as follows. In one case, a woman had cytogenetic testing performed. The provider reviewed the results and clicked “Reviewed/To MyChart” in the EHR, resulting in the patient receiving an immediate E-mail notification of results within the patient portal. The patient read the abnormal report findings before any communication and counseling by the provider. The patient reported emotional stress from this notification. A second similar case involved a patient who was undergoing staging for known prostate cancer, with a bone scan showing extensive bone metastasis and thus unfavorable prognosis. Similar to the first case, the provider clicked “Reviewed/To MyChart” before any communication with the patient. The patient's wife reviewed the abnormal report findings, and both patient and wife reported emotional distress. These types of cases led to a Patient Safety Alert E-mail communication to all providers within our medical center. In addition, EHR chart review functionality was adjusted to more prominently display the timing of patient portal release. A “Reviewed” button was also added as a more visible means of reviewing/acknowledging results in the EHR in-basket without manually releasing to the patient portal.
We also encountered multiple cases where auto-release of test results caused issues. A common scenario occurred with “laboratory only” encounters for diagnostic testing that had been originally placed (sometimes months earlier) as future orders. In this situation, the patient may get testing performed when the ordering provider is unavailable to review results. This proves especially challenging when the laboratory workup may be very extensive (e.g., evaluation of potential organ transplant recipient) and uncover previously unknown disease such as sexually transmitted infections. An illustrative example occurred with thyroglobulin tumor marker testing, when a common assay used for antithyroglobulin antibodies was temporarily unavailable in the United States, leading to change to an alternative assay with different reference range and reporting limit.[27] Multiple patients with a history of thyroid cancer, who were being monitored following thyroid ablation, received results by the patient portal by auto-release and mistakenly thought their cancer had returned due to the higher numeric values for antithyroglobulin antibodies on the new assay compared to previous testing using the now unavailable assay (even though both sets of results were within reference range). Several of these cases involved situations where the original ordering provider was on vacation when the testing occurred as a laboratory only visit.
Finally, there were some issues with the original setting of auto-release of results at 3:00 AM, a time chosen to facilitate the mass release of results to the patient portal at a time of low network system activity. One practical challenge arose with samples from outlying clinics that did not arrive to the clinical laboratory until late in the evening. The original patient portal auto-release settings for most common laboratory tests (which encompassed the retrospective period of analysis for the present study) were auto-release next business day; however, this meant that results could transmit to the patient portal only hours after appearing in the EHR and almost certainly before providers working day shift would have a chance to review results and, if appropriate, contact the patient directly. This led to complaints from providers that patients were seeing results too soon for the provider to review them. This led to a change in policy in September 2016 to delay all auto-release an additional full business day. There were also sporadic complaints from patients that E-mail notifications at 3:00 AM were disruptive (e.g., if their mobile devices had audio alert for incoming E-mails).
DISCUSSION
EHR patient portals represent an option to increase patient access to their medical information, providing an alternative to slower or more labor-intensive methods such as regular mail, in-person visits, or telephone calls.[7,8,9,10,11,12,13,14,15,16,17] Access to diagnostic test results is one of the most popular features of EHR patient portals.[18,20] Understanding patterns of EHR patient portal use can be helpful to healthcare providers, hospital administrators, information technology personnel, and patients.
Regulatory initiatives are also influencing EHR patient portals in the United States. The Centers for Medicare and Medicaid Services (CMS) Meaningful Use requirements incentivized patient portal usage through a Patient Electronic Access Objective.[28] To meet the 2014 requirements of the CMS EHR Incentive Program, Stage 1 Requirements included that 50% of all patients must be provided timely online access to their health information subject to provider's discretion to withhold certain information. Access was defined by CMS as “when the patient possesses all the necessary information to review, download, or transmit their health information.”[29] Stage 2 Requirements added that 5% of patients must actually engage in the process of reviewing their information online.[30] For both Stage 1 and Stage 2 measures, the results release must be available within four business days which is a requirement that informed the timing of our institutional process.[29,30] Stage 3 Requirements include specific objectives related to patient electronic access to health information and patient-specific education.[31] For example, objective 5 of the Eligible Hospital and Critical Access Hospital (CAH) Medicaid EHR Incentive Program dictates that “For more than 80 percent of all unique patients discharged from the eligible hospital or CAH inpatient or emergency department: The patient (or the patient authorized representative) is provided timely access to view online, download, and transmit his or her health information; and The provider ensures the patient's health information is available for the patient (or patient authorized representative) to access using any application of their choice that is configured to meet the technical specifications of the application programming interface in the provider's certified electronic health record technology.”[32] There are some exclusions to these requirements, for example, hospitals situated in geographic regions with low access to broadband Internet.[31,32]
In the present study at an academic medical center in the United States, trends in accessing diagnostic test results in the patient portal were examined. Several general trends were readily apparent in the data. First, test results from outpatient encounters were consistently accessed at higher rates than results originating from inpatient units or emergency department visits. Second, the speed of patient access of a result after release from the EHR to the patient portal shows considerable variability. Approximate 20% of outpatient test results are viewed within 8 h of release to the patient portal, and nearly 10% are viewed within 2 h of release. This presents challenges for providers in that some patients may view results before the provider has had a chance to review the test results in detail or, alternatively, before other results are available. At the other extreme, between 5% and 10% of outpatient results are viewed 7 or more days after release to the patient portal. Third, females accessed test results in the patient portal at higher rates compared to males throughout nearly all age ranges, reaching a peak of approximately 30% in females compared to approximately 15% in males.
Viewing of test results was especially low for those 12–17-year-old, particularly for boys in this age range (<1% of results viewed). The institutional policy was to limit parental proxy access in this age range to foster patient privacy, especially for issues such as pregnancy and sexual transmitted diseases testing. Interestingly, the low percentage of diagnostic test results viewed by the preteen and adolescent pediatric population contrasted with fairly high rates of having an active patient portal account (some of which were created when the patient was <12-year-old and parents had full proxy access). The patient population >70-year-old had low rates of having an active patient portal account and also low rates of viewing of diagnostic test results in the patient portal. These results indicate there is opportunity for both increasing patient portal account activation and further promoting use of its full functionality.
The data in this report were reviewed by multiple subcommittees and working groups at our institution that had oversight of the patient portal, including representation by some of the authors of the present study. Several broad opportunities for improvement and education were noted. First, the variable use of the patient portal across different patient demographics can guide marketing and other efforts to increase both activation and use of the patient portal functionality in specific patient populations. Second, the variability in release patterns for results in the patient portal (manual vs. auto-release, next day vs. 4 day, etc.) can present confusion for both patients and providers. Patients receiving care from different providers may get manually released results via the patient portal quickly from one provider while waiting for auto-release of tests ordered by another provider. In response to these concerns, provider education was developed at our institution, including adding a column notifying the provider of the planned release date, but universal understanding of the concept remains challenging in a teaching hospital with regular turnover of personnel.
Third, there were some issues with the auto-release of results at 3:00 AM, a time chosen to facilitate mass release of results to the patient portal at a time of low network system activity. As mentioned in the results, one practical challenge arose with samples that did not arrive to the clinical laboratory until late in the evening. This led to the current policy entailing an additional full business day delay between appearance in the EHR and transmission to the patient portal.
Fourth, while the patient portal may reduce time for patients to access diagnostic test results, such a portal can also have unintended consequences. Inadvertent manual release of sensitive results or auto-release of tests before being reviewed by providers and discussed with the patient can create significant patient anxiety, as discussed above. The true extent of this occurrence is hard to measure.
Fifth, the results of the study also spurred efforts at our institution to facilitate the conditional release of specific tests based on results. There was a particular focus on HIV results. In the state of Iowa, positive HIV results cannot be released to patients through a patient portal without prior counseling; however, there are no restrictions on release of negative results. Such a strategy could also be adopted for other “sensitive” results such as other sexually transmitted diseases (e.g., chlamydia, gonorrhea, syphilis) or tumor markers whereby normal or negative results are released more quickly than abnormal results. Potential downsides include that patients may be able to infer positive results due to slower release to the patient portal and that, for some quantitative tests, even a result within the reference range might be unfavorable news in some contexts (e.g., tumor marker that has increased from the baseline but is still within reference range. The concept of “normal” or “negative” can also be very challenging if conditional reporting were to be applied to complex anatomic pathology and radiology reports.
Finally, the subgroup of patients who access results in the patient portal very quickly may present some challenges. One of the main challenges expressed during institutional subcommittee meetings were that anatomic pathology and imaging results can be available in the patient portal before key therapeutic decisions are made. Examples include results being available in the patient portal before tumor board meetings or cases where a series of laboratory tests result at different time points, leading to patients seeing partial results before the entire diagnostic workup is completed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The implementation and maintenance of an electronic health record patient portal are a major undertaking and requires the combined effort of many people from different teams. The authors would like to thank all the other individuals involved in implementation, validation, policy revisions, compliance/regulatory review, help support, training, and other key tasks in this project. The authors would also like to acknowledge the instrumental feedback of patients in providing feedback on the patient portal.
Footnotes
Available FREE in open access from: http://www.jpathinformatics.org/text.asp?2017/8/1/45/219121
REFERENCES
- 1.Adler-Milstein J, DesRoches CM, Kralovec P, Foster G, Worzala C, Charles D, et al. Electronic health record adoption in US hospitals: Progress continues, but challenges persist. Health Aff (Millwood) 2015;34:2174–80. doi: 10.1377/hlthaff.2015.0992. [DOI] [PubMed] [Google Scholar]
- 2.Adler-Milstein J, Everson J, Lee SY. Sequencing of EHR adoption among US hospitals and the impact of meaningful use. J Am Med Inform Assoc. 2014;21:984–91. doi: 10.1136/amiajnl-2014-002708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mennemeyer ST, Menachemi N, Rahurkar S, Ford EW. Impact of the HITECH act on physicians’ adoption of electronic health records. J Am Med Inform Assoc. 2016;23:375–9. doi: 10.1093/jamia/ocv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DesRoches CM, Charles D, Furukawa MF, Joshi MS, Kralovec P, Mostashari F, et al. Adoption of electronic health records grows rapidly, but fewer than half of US hospitals had at least a basic system in 2012. Health Aff (Millwood) 2013;32:1478–85. doi: 10.1377/hlthaff.2013.0308. [DOI] [PubMed] [Google Scholar]
- 5.Heisey-Grove D, Danehy LN, Consolazio M, Lynch K, Mostashari F. A national study of challenges to electronic health record adoption and meaningful use. Med Care. 2014;52:144–8. doi: 10.1097/MLR.0000000000000038. [DOI] [PubMed] [Google Scholar]
- 6.Kern LM, Edwards A, Kaushal R. HITEC Investigators. The meaningful use of electronic health records and health care utilization. Am J Med Qual. 2016;31:301–7. doi: 10.1177/1062860615572439. [DOI] [PubMed] [Google Scholar]
- 7.Curtis J, Cheng S, Rose K, Tsai O. Promoting adoption, usability, and research for personal health records in Canada: The MyChart experience. Healthc Manage Forum. 2011;24:149–54. doi: 10.1016/j.hcmf.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Miller H, Vandenbosch B, Ivanov D, Black P. Determinants of personal health record use: A large population study at Cleveland Clinic. J Healthc Inf Manag. 2007;21:44–8. [PubMed] [Google Scholar]
- 9.Serrato CA, Retecki S, Schmidt DE. MyChart – A new mode of care delivery: 2005 personal health link research report. Perm J. 2007;11:14–20. doi: 10.7812/tpp/07-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charters KG, Nazi K. Personal health record evaluation: My HealtheVet and RE-AIM. AMIA Annu Symp Proc. 2007;1:899. [PubMed] [Google Scholar]
- 11.Nazi KM, Woods SS. MyHealtheVet PHR: A description of users and patient portal use. AMIA Annu Symp Proc. 2008;1:1182. [PubMed] [Google Scholar]
- 12.Alpert JM, Krist AH, Aycock RA, Kreps GL. Applying multiple methods to comprehensively evaluate a patient portal's effectiveness to convey information to patients. J Med Internet Res. 2016;18:e112. doi: 10.2196/jmir.5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffin A, Skinner A, Thornhill J, Weinberger M. Patient portals: Who uses them? What features do they use? And do they reduce hospital readmissions? Appl Clin Inform. 2016;7:489–501. doi: 10.4338/ACI-2016-01-RA-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nahm ES, Sagherian K, Zhu S. Use of patient portals in older adults: A comparison of three samples. Stud Health Technol Inform. 2016;225:354–8. [PubMed] [Google Scholar]
- 15.Pillemer F, Price RA, Paone S, Martich GD, Albert S, Haidari L, et al. Direct release of test results to patients increases patient engagement and utilization of care. PLoS One. 2016;11:e0154743. doi: 10.1371/journal.pone.0154743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thistlethwaite J. Patient portals: Furthering the reality of patient partnership. Aust Fam Physician. 2015;44:524–5. [PubMed] [Google Scholar]
- 17.Wallace LS, Angier H, Huguet N, Gaudino JA, Krist A, Dearing M, et al. Patterns of electronic portal use among vulnerable patients in a nationwide practice-based research network: From the OCHIN Practice-Based Research Network (PBRN) J Am Board Fam Med. 2016;29:592–603. doi: 10.3122/jabfm.2016.05.160046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis KA, Smith LB. Ethical considerations about EHR-mediated results disclosure and pathology information presented via patient portals. AMA J Ethics. 2016;18:826–32. doi: 10.1001/journalofethics.2016.18.8.pfor1-1608. [DOI] [PubMed] [Google Scholar]
- 19.Collins SA, Vawdrey DK, Kukafka R, Kuperman GJ. Policies for patient access to clinical data via PHRs: Current state and recommendations. J Am Med Inform Assoc. 2011;18(Suppl 1):i2–7. doi: 10.1136/amiajnl-2011-000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choudhry A, Hong J, Chong K, Jiang B, Hartman R, Chu E, et al. Patients’ preferences for biopsy result notification in an era of electronic messaging methods. JAMA Dermatol. 2015;151:513–21. doi: 10.1001/jamadermatol.2014.5634. [DOI] [PubMed] [Google Scholar]
- 21.Lang EV, Berbaum KS, Lutgendorf SK. Large-core breast biopsy: Abnormal salivary cortisol profiles associated with uncertainty of diagnosis. Radiology. 2009;250:631–7. doi: 10.1148/radiol.2503081087. [DOI] [PubMed] [Google Scholar]
- 22.Blau JL, Wilford JD, Dane SK, Karandikar NJ, Fuller ES, Jacobsmeier DJ, et al. Implementation of Epic Beaker Anatomic Pathology at an academic medical center. J Pathol Inform. 2016;7:7. doi: 10.4103/jpi.jpi_31_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krasowski MD, Davis SR, Drees D, Morris C, Kulhavy J, Crone C, et al. Autoverification in a core clinical chemistry laboratory at an academic medical center. J Pathol Inform. 2014;5:13. doi: 10.4103/2153-3539.129450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krasowski MD, Wilford JD, Howard W, Dane SK, Davis SR, Karandikar NJ, et al. Implementation of Epic Beaker Clinical Pathology at an academic medical center. J Pathol Inform. 2016;7:7. doi: 10.4103/2153-3539.175798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krasowski MD, Chudzik D, Dolezal A, Steussy B, Gailey MP, Koch B, et al. Promoting improved utilization of laboratory testing through changes in an electronic medical record: Experience at an academic medical center. BMC Med Inform Decis Mak. 2015;15:11. doi: 10.1186/s12911-015-0137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson LS, Davis SR, Humble RM, Kulhavy J, Aman DR, Krasowski MD, et al. Impact of add-on laboratory testing at an academic medical center: A five year retrospective study. BMC Clin Pathol. 2015;15:11. doi: 10.1186/s12907-015-0011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Algeciras-Schimnich A, Lasho MA, Ness KM, Cheryk LA, Grebe SK. The Roche Elecsys and Siemens-Centaur thyroglobulin autoantibody assays show comparable clinical performance to the recently unavailable beckman-coulter access thyroglobulin autoantibody assay in identifying samples with potentially false-low thyroglobulin measurements due to thyroglobulin autoantibody interference. Thyroid. 2011;21:813–4. doi: 10.1089/thy.2011.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Center for Medicare & Medicaid Services. Patient Electronic Access Tipsheet. 2014. [Last accessed on: 2017 Oct 24]. Available from: https://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/Downloads/PatientElecAccTipsheet_06182014.pdf .
- 29.Center for Medicare & Medicaid Services. Eligible Professional Meaningful Use Core Measures. Measure 11 of 13. Stage 1 (2014 Definition) [Last accessed on: 2017 Oct 24]. Available from: https://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/Downloads/11_Patient_Electronic_Access.pdf .
- 30.Center for Medicare & Medicaid Services. Eligible Professional Meaningful Use Core Measures. Measure 7 of 17. Stage 2. 2014. [Last accessed on: 2017 Oct 24]. Available from: https://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/downloads/Stage2_EPCore_7_PatientElectronicAccess.pdf .
- 31.Center for Medicare & Medicaid Services. Medicaid EHR Incentive Program Stage 3 Patient Electronic Access to Health Information. 2016. [Last accessed on: 2017 Oct 24]. Available from: https://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/Downloads/MedicaidStage3_PatientElectronicAccessTipsheet.pdf .
- 32.Center for Medicare & Medicaid Services. Eligible Hospital and Critical Access Hospital Medicaid EHR Incentive Program Stage 3 Objectives and Measures. 2017. [Last accessed on: 2017 Oct 24]. Available from: https://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/Downloads/MedicaidEHStage3_Obj5.pdf .