Abstract
Transient receptor potential canonical 3/6/7 (TRPC3/6/7) are highly homologous receptor-operated non-selective cation channels. Despite their physiological significance, very few selective and potent agonists are available for functional examination of these channels. Using a cell-based high throughput screening approach, a lead compound with the pyrazolopyrimidine skeleton was identified as a TRPC6 agonist. Synthetic schemes for the lead and its analogues were established, and structural–activity relationship studies were carried out. A series of potent and direct agonists of TRPC3/6/ 7 channels were identified, and among them, 4m–4p have a potency order of TRPC3 > C7 > C6, with 4n being the most potent with an EC50 of <20 nM on TRPC3. Importantly, these compounds exhibited no stimulatory activity on related TRP channels. The potent and selective compounds described here should be suitable for evaluation of the roles of TRPC channels in the physiology and pathogenesis of diseases, including glomerulosclerosis and cancer.
Graphical Abstract
INTRODUCTION
Transient receptor potential canonical (TRPC) channels constitute a group of receptor-operated calcium-permeable nonselective cation channels of the TRP superfamily. The seven mammalian TRPC members can be further divided into four subgroups (TRPC1, TRPC2, TRPC4/5, and TRPC3/6/7) based on their amino acid sequences and functional similarities.1 Native TRPC channels may be formed of either homo- or heterotetramers from the same subgroup or from different subgroups; they also interact with a variety of other proteins, which exert modulatory roles on their assembly, trafficking, and/or function.2 So far, the majority of studies on TRPC channels have focused on their roles in mediating Ca2+ and Na+ influx after the stimulation of phospholipase C (PLC) either by G protein-coupled receptors that signal through the Gq/11 subgroup of heterotrimeric G proteins or by receptor tyrosine kinases.3 These activities cause membrane depolarization and a sustained increase in intracellular Ca2+ concentration ([Ca2+]i), which are important for a plethora of cellular functions in many physiological systems.4 On the other hand, a few studies also implicated TRPC channel expression and function in intracellular membranes,5 suggesting that very diverse cellular activities may be regulated by these channels.
Intensive investigations have been instrumental in defining the physiological roles of TRPC channels in different tissues and organs, including excitation–contraction coupling in smooth muscles,6,7 protein filtration in the kidney,8,9 synaptic formation and transmission,10,11 myofibroblast transdifferentiation and wound healing,12 regulation of vascular tone, and cell growth and proliferation.13–15 Particularly, TRPC6 has been implicated in the physiological regulation of glomerular hemodynamics in kidney. Genetic mutations of TRPC6, including both gain-of-function and loss-of-function ones, have been linked to glomerular dysfunction in the kidney, where the channel is expressed in podocytes and involved in the regulation of slit diaphragm for protein filtration.8,9,16 Elevated expression of TRPC6 was found in glomeruli from patients with proteinuria, which is a common feature of kidney dysfunction of glomerular origin and is by itself a risk factor for both renal and extrarenal diseases.17 Additionally, TRPC6 and its closely related TRPC3, are both suggested to be involved in the development of cardiac hypertrophy and hypertension.14,18–20 The roles for TRPC3/6 in cancer have also been suggested.21,22 However, the exact roles played by TRPC channels in kidney and cardiovascular functions and the underlying mechanisms concerning how they contribute to these functions and/or pathogenesis have proven difficult to elucidate due to the lack of specific compounds that modulate these channels.
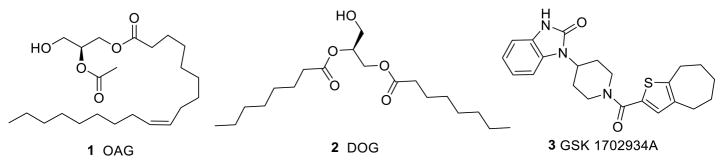
TRPC3, TRPC6, and TRPC7 are closely related, sharing 65–78% sequence identity, and form channels that are generally activated downstream from receptors that activate PLC. These channels are also directly activated by diacylglycerols (DAG), one of the products of phosphatidylinositol 4,5-bisphosphate (PIP2) hydrolysis by PLC.23,24 Analogues of DAG, such as 1-oleoyl-2-acetyl-sn-glycerol (1) (OAG) and 1,2-dioctanoyl-sn-glycerol (2) (DOG) have been widely used to activate TRPC3 and TRPC6 channels (Figure 1). However, not only are these stimulators nonspecific for TRPC3/6/7 channels, but also they are highly hydrophobic and unstable in the aqueous solution. Recently, small molecular probes, including both natural products and synthetic compounds, have been reported for TRPC channels. For example, a novel synthetic agonist for TRPC3/6 named GSK1702934A (3)25 was used at 1 μM to study cardiac contractility and arrhythmogenesis in heart.26 A few small-molecule compounds including Pyr3 and analogues,27 cationic, N′-substituted 1-benzylpiperidines,7 anilino-thiazole,28 norges-timate,29 4-({(1R,2R)-2-[(3R)-3-aminopiperidin-1-yl]-2,3-dihy-dro-1H-inden-1-yl}oxy)-3-chlorobenzonitrile,30 and larixyl acetate31 have been identified as inhibitors of TRPC3/6/7 channels. Englerin A,32 riluzole,33 clemizole hydrochloride,34 4-methyl-2-(1-piperidinyl)-quinoline (ML204),35 and 2-amino-benzimidazole derivatives36 have been described as TRPC4 and/or TRPC5 agonists or antagonists. Often, these probes cross-react within the subgroup of TRPC3/6/7 or TRPC4/5 but not between the two subgroups.
Figure 1.
Examples of reported TRPC3/6/7 agonists.
While selectivity remains lacking for most compounds, identification of their pharmacophores through systematic structure–activity approaches can be expected to lead to development of subtype-selective TRPC agonists or antagonists and may perhaps lead to development of novel drug therapies such as for the treatment of kidney and cardiovascular diseases or cancer. For such a reason, we have carried out a high-throughput screening (HTS) effort to identify small-molecule probes of TRPC6 and related channels as a starting point for lead optimization and target validation. Here, we report the identification of a novel pyrazolopyrimidine-based TRPC6 lead agonist, its resynthesis, and the structure–activity relationship (SAR) analysis of the initial set of its analogues on TRPC3, TRPC6, and TRPC7. With minor modifications, we obtained an agonist with relatively strong selectivity for TRPC3, exhibiting apparent affinity at the low nanomolar range.
RESULTS AND DISCUSSION
Chemistry
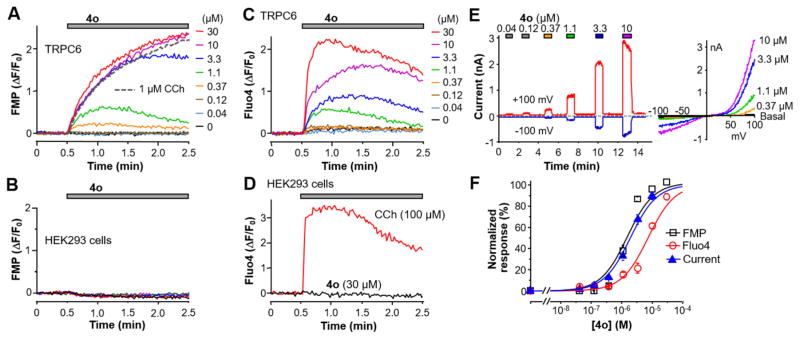
The scarcity of TRPC3/6/7 probes has severely hampered the characterization of these channels in their assembly, function, and pathophysiological roles. To functionally screen for TRPC3/6/7 small molecular probes, we created a stable HEK293 cell line that coexpressed mouse TRPC6 and the Gq/11-PLCβ coupled M5 muscarinic receptor (TRPC6 cells). These cells responded to the muscarinic agonist, carbachol (CCh), with a strong sustained membrane depolarization, which can be detected by the FLIPR membrane potential (FMP) dye as an increase in fluorescence (Figure 2A, gray dashed line). Using this cell-based assay, we screened 305000 compounds from the Molecular Libraries Small Molecule Repository (MLSMR), supported by the Molecular Libraries Probe Production Centers Network (MLPCN) and identified an activator with the pyrazolopyrimidine skeleton (PubChem CID: 5308428, Table 1, compound 4o) as the primary hit. As shown in Figure 2A, the original lead compound elicited membrane potential depolarization in TRPC6 cells in a concentration-dependent manner but not in the parental HEK293 cells (Figure 2B). It also evoked [Ca2+]i rise in the TRPC6 cells (Figure 2C) but not parental HEK293 cells (Figure 2D). We then confirmed that the compound concentration dependently elicited half maximal stimulation (EC50) were estimated to be 1.4 ± 0.1 μM by the FMP assay (n = 6), 2.5 ± 0.2 μM (at +80 mV) by whole-cell recordings (n = 5–7), and 7.2 ± 0.5 μM by the Ca2+ assay (n = 12) (Figure 2F).
Figure 2.
Lead compound 4o identified from HTS activated TRPC6 expressed in HEK293 cells. (A) Representative traces of membrane potential changes evoked by carbachol (CCh, 1 μM, gray dashed line) or varying concentrations of compound 4o obtained from the compound library (colored lines) in the TRPC6 cells. The fluorescence increases indicate depolarization. (B) Compound 4o did not induce membrane depolarization in parental HEK293 cells. (C) Representative traces of Fluo4 fluorescence changes, indicative of [Ca2+]i increase, evoked by varying concentrations of compound 4o in the TRPC6 cells. (D) CCh, but not compound 4o, evoked [Ca2+]i increase in the parental HEK293 cells. (E) Representative traces of whole-cell currents at +100 mV (red trace) and –100 mV (blue trace) elicited by varying concentrations of compound 4o in a TRPC6 cell. The bath contained 2 mM Ca2+, and the pipet solution had 400 nM free Ca2+ buffered by BAPTA. Voltage ramps from +100 mV to –100 mV were applied every second. Current–voltage (I–V) relationships obtained from the voltage ramps for peak currents elicited by selected compound concentrations are shown at right. These I–V curves are typical to TRPC6 currents evoked through receptor stimulation.37 (F) Concentration response curves for compound 4o activation of TRPC6, as determined by the membrane potential assay (FMP), Ca2+ assay (Fluo4) and electrophysiology recording shown in A, C, and E, respectively. For fluorescence assays, area under the curve, and for whole-cell recordings, peak current changes at +80 mV were used for quantification. Solid lines represent fits by the Hill equation, which yielded the EC50 values described in the text.
Table 1.
Structures of Compounds 4a–4p and Their Effects on TRPC6 and TRPC4
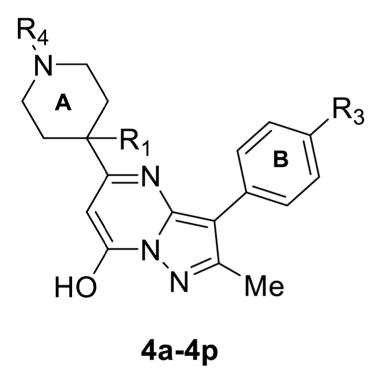
| |||||
|---|---|---|---|---|---|
| Compound | R1 | R3 | R4 | TRPC6 EC50(μM)a | TRPC4β+MOR IC50(μM)b |
| 4a | H | F |
|
12.3±1.2 | ~10 |
| 4b | Me | F |
|
NA | NA |
| 4c | Me | F |
|
NA | NA |
| 4d | Me | F |
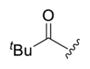
|
NA | NA |
| 4e | Me | NO2 |
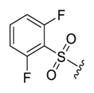
|
NA | NA |
| 4f | Me | NO2 |
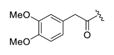
|
NA | NA |
| 4g | Me | NO2 | CO2Et | ~8c | NA |
| 4h | Me | F | Boc | NA | NA |
| 4i | H | NO2 | CO2Et | ~2c | NA |
| 4j | H | F |

|
28.8±8.3 | ~35 |
| 4k | H | F |

|
NA | NA |
| 41 | H | Cl | Boc | NA | NA |
| 4m | H | Cl | Cbz | 7.79±0.05 | NA |
| 4n | H | CF3 | CO2Et | 1.39±0.01 | NA |
| 4o | H | F | CO2Et | 4.66±0.03 | NA |
| 4p | H | F | Cbz | 3.91±0.02 | NA |
EC50 determined based on Ca2+ assay.
IC50 against that activated by 1 μM DAMGO, estimated based on one experiment with triplicates;
Estimated value because of the self-fluorescence of the compound.
NA: No activity.
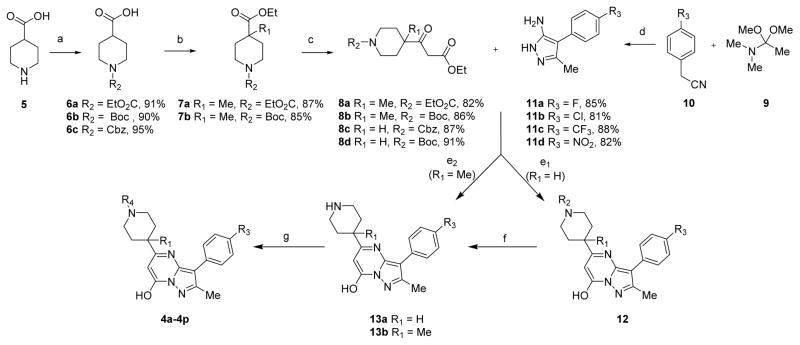
To further evaluate this compound and the SAR on TRPC6 activation, we first synthesized compounds 4a–4p (Scheme 1, Table 1). Starting from the commercially available piperidine-4-carboxylic acid 5, our initial attempt to introduce a methyl group or alkyl group into the R1 position did not work until a published protocol38 was adapted to protect the carboxyl group before adding methyl at position R1. Using this strategy, compounds 6a–6c were prepared with high yields of 90–95% by N-acylation with ethyl chloroformate, di-tert-butyl dicarbonate, or benzyl chloroformate groups at position R2, respectively (Scheme 1). deprotonation of piperidine of 6a and 6b with 1.2 equiv of n-butyllithium followed by reaction with methyl iodide afforded amides 7a and 7b in 87% and 85% yields, respectively. The ethyl ester portion of 7a and 7b was converted to 3-oxopropanoate group using a modified Masamune procedure,39 which furnished β-keto esters 8a and 8b in 82% and 86% yields, respectively. Compounds 8c and 8d were obtained with high yields from 6c and 6b, respectively, using a similar procedure. Condensation of nitrile intermediate 10 with 1,1-dimethoxy-N,N-dimethylethan-1-amine (9) using dichloromethane as the solvent under microwave irradiation (MWI), followed by the cyclization reaction with hydrazine hydrochloride in EtOH, generated the 5-aminopyrazoles 11a–d in excellent yields (81–88%) over two steps. We then carried out a cyclization study of compounds 8 and 11 using a variety of acids, such as HCl, trifluoroacetic acid (TFA), and H2SO4, in order to test the viability of our design. Unfortunately, only a trace amount of product 12 was formed in TFA at reflux for 18 h. However, mixing 8a–8c with 11a–11d in acetic acid in a microwave reactor at 120 °C overnight produced the desired products 12 in good yields. Deprotection of the Boc group of compounds 12 (R2 = Boc) with TFA/dichloromethane (DCM) at 0 °C resulted in the formation of compounds 13 in high yield. Finally, amidation of compounds 13 gave rise to compounds 4 (4a–4p, Table 1) successfully (Scheme 1).
Scheme 1. Synthetic Route for Compound 4a and its Derivativesa.
aReagents and conditions: (a) R1O2CCl, NaOH, H2O, rt, 5 h, or (Boc)2O, K2CO3, THF/H2O, rt, 7 h; (b) (i) ClCO2Et, NEt3, rt, 12 h, (ii) n-BuLi, i-Pr2NH, THF, –78 °C, 1 h, MeI, THF, 5 h; (c) (i) 2 M NaOH, (ii) CDI, THF, 4 h, KO2CCH2CO2Et, MgCl2, DMAP, THF/CH3CN, overnight; (d) (i) DCM, microwave, 100 °C, (ii) N2H4·HCl, H2O, EtOH, 80 °C, 8 h; (e1) AcOH, reflux, 120 °C, overnight; (e2) EtOH, TFA, reflux, 85 °C, 48 h; (f) TFA, DCM, 0 °C, 2 h; (g) DMF, HOBt, HBTU, DIPEA, rt, overnight.
Biological Evaluation
The effect of the newly synthesized compounds (Table 1) on TRPC6 channel function was evaluated using the fluorescence Ca2+ assay. As the original lead, the resynthesized compound 4o (R1 = H, R3 = F, R4 = CO2Et) evoked [Ca2+]i rise in the TRPC6 cells in a concentration dependent manner, with a mean EC50 value of 4.66 ± 0.03 μM (Figure 2C,E, Table 1, entry 15), demonstrating a comparable activity of the resynthesized compound on TRPC6 as the original lead. Compound 4o was also tested on HEK293 cells that coexpressed μ opioid receptor (MOR) and TRPC4β,35,36 which belongs to a different subgroup of TRPC channels than TRPC3/6/7. Upon stimulation by a μ agonist, (D-Ala, N-MePhe, Gly-ol]-enkephalin (DAMGO, 1 μM), these cells show a robust increase in [Ca2+]i.36 However, compound 4o did not elicit a Ca2+ response in the MOR/TRPC4β cells, nor did it alter the DMAGO-evoked response, demonstrating its selectivity for TRPC6 over TRPC4.
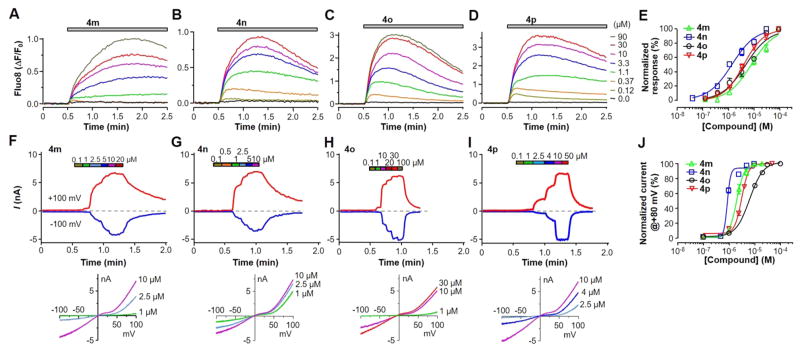
We next investigated SAR between substituents in R1 and R4 of ring A and R3 of ring B using the Ca2+ assay on the TRPC6 cells (Table 1). For most compounds, the MOR/TRPC4β cells were used as a control for subtype selectivity. First, the hydrogen is required in R1, as the substitution by a methyl group rendered all compounds (4b–4h) inactive at stimulating TRPC6 (Table 1, entries 2–8), except for 4g, which, however, showed high fluorescence by the compound itself (not shown). This could be due in part to the ability of methyl at position R1 to bias a particular A ring conformer. Second, the substitution of F at R3 by Cl moderately increased the EC50 (4m, Figure 3A,E, Table 1, entry 13), whereas that by NO2 or CF3 decreased the EC50 by ~60–70% to ≤2 μM (4i and 4n, Figure 3B,E, Table 1, entries 9 and 14), suggesting a possible position for further modifications to improve the agonist potency. However, like 4g, the introduction of NO2 at R3 rendered the compound (4i) to become self-fluorescent (not shown). Third, substitutions at R4 of the A ring revealed that the carbamate group, such as ethyl carbamate, is optimal. To identify suitable carbamate group replacements for R4, we used 6-bromo-nicotine (4a), ethyl carbamoyl (4j), tert-butyloxycarbonyl (Boc, 4l), and carboxybenzyl (Cbz, 4m, 4p). Intriguingly, compound 4l did not exhibit stimulatory activity on TRPC6, likely because of the steric hindrance blockade by the Boc group introduced at the R4 position. The benzyl carbamate or Cbz group in R4 (4p) displayed comparable stimulatory activity on TRPC6 as the corresponding ethyl carbamate (4o), giving an EC50 value of 3.91 ± 0.02 μM (Figure 3D,E, Table 1, entry 16).
Figure 3.
Compounds 4m–4p activated TRPC6 expressed in HEK293 cells. (A–E) Compounds 4m–4p were synthesized as described in the text and tested in the Ca2+ assay using Fluo8-loaded TRPC6 cells. (A–D) Representative traces of [Ca2+]i rise, as indicated by the Fluo8 fluorescence increase, evoked by varying concentrations of 4m (A), 4n (B), 4o (C), or 4p (D). (E) Summary data for (A–D) and the fits by the Hill equation. (F–J) Compounds 4m–4p were tested on TRPC6 cells in whole-cell recordings. The bath contained 0.1 mM Ca2+, and the pipet solution had 400 nM free Ca2+ buffered by BAPTA. (F–I) Representative current traces at +100 mV (red traces) and –100 mV (blue traces) in response to consecutive applications of increasing concentrations of 4m (F), 4n (G), 4o (H), or 4p (I) (upper panels) and I–V curves for selected compound concentrations (lower panels). (J) Summary data for (F–I) and the fits by the Hill equation.
Functional Characterization of 4m–4p
To validate the agonistic action of pyrazolopyrimidine compounds on TRPC6 channels, we focused on the last four compounds listed in Table 1 (4m–4p), which evoked robust [Ca2+]i increases in TRPC6-expressing cells in the Ca2+ assay with EC50 values <8 μM (Figure 3A–E) and no self-fluorescence. In whole-cell voltage clamp recordings, we used a low Ca2+ bath solution (0.1 mM Ca2+) in order to minimize current rundown. Under these conditions, increasing concentrations (0.1–100 μM) of the test compound were applied consecutively, and in each case the compound evoked stepwise current increases in individual cells at both negative and positive potentials (Figure 3F–I). Noticeably, the outward currents at positive potentials developed earlier at low compound concentrations than the inward currents at negative potentials, indicative of a shift of voltage dependence as the compound concentration increased. Typically, the currents did not desensitize even at highest concentrations (20–100 μM) used. On the basis of the currents at +80 mV, the EC50 values for monovalent cation currents of TRPC6 evoked by 4m–4p were determined to be 2.05 ± 0.02, 0.89 ± 0.01, 6.28 ± 0.02, and 3.24 ± 0.01 μM (n = 6–8), respectively (Figure 3J). These values are comparable to that obtained from the Ca2+ assay (Table 1).
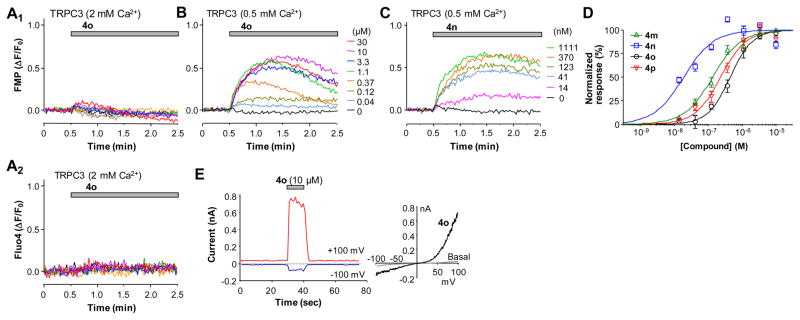
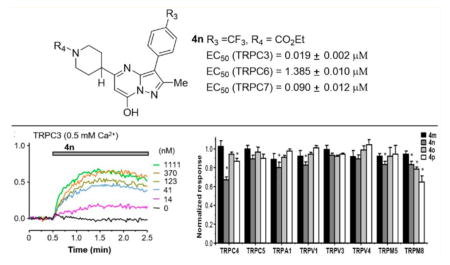
We then examined whether the compounds directly activate closely related TRPC3 and TRPC7 channels. Although our initial attempts using the FMP and Ca2+ assays did not reveal obvious activation of human TRPC3 stably expressed in HEK293 cells by compound 4o (Figure 4A1,A2), lowering the Ca2+ concentration in the extracellular solution from the standard 2 mM to 0.5 mM allowed 4o to evoke membrane depolarization in these cells in a concentration-dependent manner (Figure 4B). These results suggest that the activation of TRPC3 by 4o is sensitive to inhibition by extracellular Ca2+, consistent with the previous finding that physiological concentrations of extracellular Ca2+ suppressed receptor-operated TRPC3 currents.40 Supporting the FMP assay data, 4o also evoked whole-cell currents in HEK293 cells that expressed human TRPC3 (Figure 4E). Using the FMP assay with 0.5 mM extracellular Ca2+, we determined the EC50 values of compounds 4m–4p on stimulating TRPC3 to be 138 ± 23, 19.2 ± 2.3, 450 ± 87, and 236 ± 47 nM (Figure 4B–D, n = 6 for each), respectively. Among the four analogues, 4n really stands out, exhibiting >7-fold increase in the potency on TRPC3 as compared to other compounds and >70-fold increase as compared to the same compound on TRPC6.
Figure 4.
Compounds 4m–4p activated TRPC3 expressed in HEK293 cells when extracellular solution contained 0.5 mM Ca2+. (A) Compound 4o failed to elicit membrane depolarization (A1) or [Ca2+]i rise (A2) in TRPC3 cells in normal extracellular solution that contained 2 mM Ca2+. (B,C) Representative traces of membrane potential depolarization induced by varying concentrations of compounds 4o (B) and 4n (C) in TRPC3 cells in an extracellular solution that contained 0.5 mM Ca2+. (D) Summary data for membrane depolarization induced by compounds 4m–4p in TRPC3 cells bathed in the 0.5 mM Ca2+ solution. The data points were fitted by the Hill equation. (E) Compound 4o (10 μM) elicited TRPC3 currents in the bath that contained 0.5 mM Ca2+. The I–V curves under basal and 4o-stimulated conditions are shown at right.
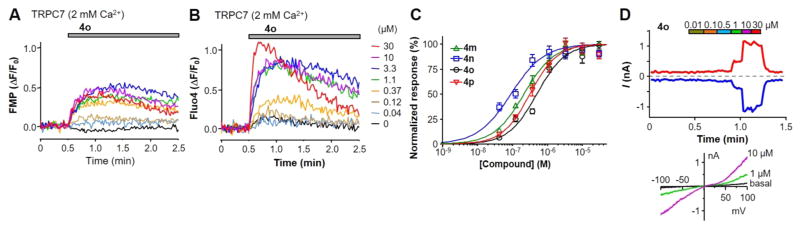
Among the three members of the TRPC3/6/7 subgroup, the sequence of TRPC7 is more similar to TRPC3 than to TRPC6. Interestingly, compound 4o was able to elicit membrane depolarization and [Ca2+]i elevation in HEK293 cells that stably expressed human TRPC7 in the normal extracellular solution that contained 2 mM Ca2+ (Figure 5A,B), although the extents of the response, even when they reached the maximum, appeared to be markedly lower than that seen in the TRPC6 cells. Previously, it was shown that extracellular Ca2+ potentiated TRPC6 but inhibited TRPC7 currents.41 Thus, the lower response of TRPC7 to 4o than TRPC6 reflected at least in part a block by the 2 mM Ca2+ included in the extracellular solution, although such a block may be much weaker than TRPC3, which was almost completely inhibited by the same concentration of Ca2+ (see above and Figure 4A). In whole-cell voltage clamp recordings, 4o elicited concentration dependent current increases in the TRPC7 cells (Figure 5D). Different from TRPC6, the currents showed some desensitization in the high 4o concentrations (10 and 30 μM) at both negative and positive potentials even though the low Ca2+ (0.1 mM) bath solution was used. Nonetheless, a stepwise increase in the TRPC7 currents was still detected at <10 μM 4o. On the basis of the whole-cell recordings performed in the low Ca2+ bath solution, 4o had an estimated EC50 of 1.13 ± 0.03 μM (n = 6) on TRPC7. This value is only slightly higher than that obtained from the Ca2+ assay. On the basis of the Ca2+ assay, the EC50 values of 4m–4p were estimated to be 213 ± 36, 90 ± 12, 497 ± 91, and 314 ± 59 nM (Figure 5C, n = 6 for each), respectively. For each of the compounds, the EC50 value for TRPC7 is in between those for TRPC3 and TRPC6 (Table 2).
Figure 5.
Compounds 4m–4p activated TRPC7 expressed in HEK293 cells. (A,B) Representative traces of membrane potential depolarization (A) and [Ca2+]i increase (B) induced by varying concentrations of compounds 4o in TRPC7 cells in normal extracellular solution that contained 2 mM Ca2+. (C) Summary data for [Ca2+]i rise induced by compounds 4m–4p in TRPC7 cells bathed in the 2 mM Ca2+ solution, as exemplified in (B). The data points were fitted by the Hill equation. (D) Activation of TRPC7 currents by increasing concentrations of compound 4o in the bath solution that contained 0.1 mM Ca2+. The I–V curves under basal and selected concentrations of 4o are shown below.
Table 2.
EC50 Values (μM) for Compounds 4m–4p to TRPC3/6/7 Channels
| compd | TRPC3a | TRPC7b | TRPC6b | TRPC6c |
|---|---|---|---|---|
| 4m | 0.138 ± 0.023 | 0.213 ± 0.036 | 7.79 ± 0.05 | 2.05 ± 0.02 |
| 4n | 0.019 ± 0.002 | 0.090 ± 0.012 | 1.39 ± 0.01 | 0.89 ± 0.01 |
| 4o | 0.450 ± 0.087 | 0.497 ± 0.091 | 4.66 ± 0.03 | 6.28 ± 0.02 |
| 4p | 0.236 ± 0.047 | 0.314 ± 0.059 | 3.91 ± 0.02 | 3.24 ± 0.01 |
Cells were loaded with the FLIPR membrane potential (FMP) blue dye; the [Ca2+] in the bath was 0.5 mM.
Cells were loaded with Fluo8-AM; the [Ca2+] in the bath was 2 mM.
Determined based on currents at +80 mV by whole-cell patch clamp recordings using a bath solution that contained 0.1 mM Ca2+ and a peptide solution that contained 400 nM free Ca2+ buffered with BAPTA.
The above data clearly demonstrate the agonistic activity of the pyrazolopyrimidine compounds 4m–4p on the TRPC3/6/ 7 subgroup of TRPC channels. To verify the selectivity, we tested the effects of 4m–4p on a number of related TRP channels, including TRPC4, TRPC5, TRPA1, TRPV1, TRPV3, TRPV4, TRPM5, and TRPM8, using either the FMP or Ca2+ assay. All compounds were applied at 33 μM when tested for stimulatory activity, which revealed no obvious effect (data not shown). Then the channels were stimulated with their corresponding agonists in the absence or presence of 22 μM 4m–4p. For most channels, the compounds did not significantly alter the agonist-evoked responses, except for TRPC4, where 4n and 4p suppressed the MOR agonist DAMGO-evoked membrane depolarization by ~33% and 13%, respectively, and for TRPM8, where 4n, 4o, and 4p suppressed the menthol-evoked [Ca2+]i increase by ~17%, 22%, and 35%, respectively (Figure 6). A small (<20%) inhibition by 4n was also detected for TRPA1, TRPV1, and TRPM5 (Figure 6).
Figure 6.
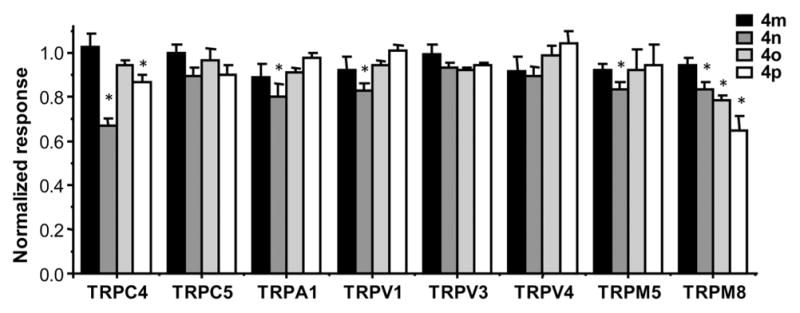
Compounds 4m–4p had little or no effect on related TRP channels. Stable HEK293 cell lines expressing MOR/TRPC4β, TRPC5, TRPA1, TRPV1, TRPV3, TRPV4, TRPM5, and TRPM8 were seeded in wells of 96-well plates and loaded with either FMP dye (TRPC4, C5, and M5) or Fluo8-AM (TRPA1, V1, V3, V4, and M8). Changes in membrane potential or [Ca2+]i were read in a microplate reader, while compound 4m, 4n, 4o, or 4p (33 μM) was applied for 2 min before the corresponding agonist known to activate the channel (C4, 1 μM DAMGO; C5, 100 μM CCh; A1, 100 μM flufenamic acid; V1, 1 μM capsaicin; V3, 200 μM 2-APB; V4, 30 μM RN-1747; M5, 100 μM CCh; M8, 100 μM menthol) was added, and measurement continued for 2.5 min. After the agonist addition, the test compound was diluted to 22 μM. Shown are summary data for the agonist-evoked response (area under the curve) in the presence of the test compound normalized to that in the absence of the test compound. n = 4–10 measurements. * p < 0.05 compared to no test compound by Student’s t test.
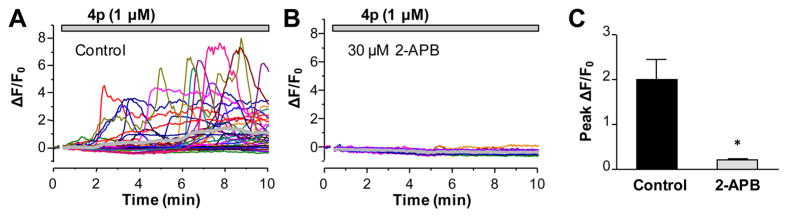
Finally, we evaluated the ability of compound 4p to elicit [Ca2+]i rise in rat primary glomerular mesangial cells, which endogenously express functional TRPC3/6 channels.42,43 4p (1 μM) evoked marked increases in [Ca2+]i, as revealed by the elevation in Fluo-4 fluorescence (Figure 7A). These activities were abolished by 2-aminoethoxydiphenyl borate (2-APB, 30 μM), a nonspecific TRP modulator that inhibits TRPC channels.7,37
Figure 7.
Compound 4p induced Ca2+ transients in rat glomerular mesangial cells. (A) Representative traces of Fluo4-loaded mesangial cells exposed to 1 μM compound 4p. Each trace represents a single cell, and the thick gray line is the average of all 36 cells in the same coverslip. (B) Similar to (A), but the cells were pretreated for 2 min with 30 μM 2-APB, which was also present throughout the period when compound 4p was applied. Each trace represents a single cell, and the thick gray line is the average of all 30 cells in the same coverslip. (C) Summary of four experiments quantified by the mean peak fluorescence changes induced by compound 4p for the individual experiments. * p < 0.05 compared to no 2-APB control by Student’s t test.
CONCLUSIONS
Pharmacological tool compounds play a vital role in drug development and functional characterization of TRPC channels. Using HTS and SAR studies, we have identified a series of potent and direct agonists of TRPC3/6/7 channels. Four of them, compounds 4m–4p, were further characterized and shown a potency order of TRPC3 > C7 > C6, with 4n being the most potent with an EC50 value of <20 nM on TRPC3. Importantly, these compounds exhibited no stimulatory activity on related TRPC channels, including the subfamily members TRPC4 and C5. Although not directly tested, it is unlikely that compounds 4m–4p would activate other Ca2+ permeable channels endogenously expressed in HEK293 cells such as inositol 1,4,5-trisphosphate receptors and Orai1 or inhibit the sarco/endoplasmic reticulum Ca2+-ATPase as they did not elicit detectable [Ca2+]i in parental HKE293 cells and a number of stable cell lines. A few compounds exhibited weak inhibition on TRPC4 and TRPM8 at high concentrations. Therefore, they are quite selective for the TRPC3/6/7 subgroup of TRPCs. It remains to be determined if the compounds affect ryanodine receptors or voltage-gated Ca2+ channels, which are mainly present in excitable cells. Nevertheless, given the current general lack of good pharmacological agonists for the TRPC channels and our demonstration that they activated native TRPC3/6 channels in rat glomerular mesangial cells, the compounds described here should find excellent use in TRPC channel research. Our results may lead to more potent and specific agonists or antagonists of targeting TRPC channels and facilitate the development of related disease therapeutics.
EXPERIMENTAL SECTION
General Methods
Unless noted otherwise, all reagents and solvents were purchased from commercial suppliers and used without further purification. Melting points were determined on a Yuhua X-5 melting point apparatus. All reactions were performed under an argon atmosphere unless otherwise specified. Reaction progress was monitored using analytical thin-layer chromatography (TLC). The purification of all compounds was processed by silica gel column chromatography. 1H and 13C NMR spectra were recorded on a Bruker AV400 spectrometer (400 MHz, 1H NMR; 101 MHz, 13C NMR) at room temperature (rt). NMR spectra were calibrated to the solvent signals of CDCl3 (δ 7.26 and 77.16 ppm), CD3OD (δ 3.31 and 49.00 ppm), or DMSO-d6 (δ 2.50 and 39.52 ppm). The chemical shifts are provided in ppm and the coupling constants in Hz. The following abbreviations for multiplicities are used: s, singlet; d, doublet; dd, double doublet; ddd, triple doublet; t, triplet; dt, double triplet; q, quadruplet; m, multiplet; and br, broad. High-resolution MS was carried out with a Thermo LTQ XL Orbitrap instrument. The yield of all compounds for biological testing was determined by HPLC analysis, confirming >95% purity.
General Procedure for the Synthesis of 4a, 4c, 4d, 4f, 4i, 4j, and 4k
To a solution of 13 (1 equiv) in DMF were added appropriate carboxylic acid (1.2 equiv), HOBT (3 equiv), and HBTU (3 equiv). Diisopropyl ethyl amine (9 equiv) was added 15 min later. The reaction was stirred for 5–24 h, quenched with water, diluted with EtOAc, and then washed with water. The organic layer was dried over anhydrous MgSO4 and the solvent evaporated under reduced pressure. The residue was purified by silica gel column chromatography (CH2Cl2:MeOH = 100:1–40:1) to obtain the title compound.
General Procedure44 for the Synthesis of 4b, 4e, 4g, and 4h
To a solution of 13 (1 equiv) in CH2Cl2 was added appropriate acyl chloride or anhydride. Et3N (1.2 equiv) was added, and the reaction was stirred at rt for 4–8 h. After removing the solvent, the title compound was purified by silica gel column chromatography (CH2Cl2:MeOH = 100:1–40:1).
General Procedure for the Synthesis of 4l–4p
Compound 4l–4p were synthesized according to the general procedure for the synthesis of 12 (see later).
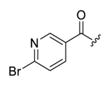
(6-Bromopyridin-3-yl)(4-(3-(4-fluorophenyl)-7-hydroxy-2-methyl-pyrazolo[1,5-a]pyrimidin-5-yl)piperidin-1-yl) methanone (4a).44
The title compound was isolated as a white solid in 83% yield. 1H NMR (400 MHz, DMSO-d6) δ 11.70 (s, 1H), 8.48 (d, J = 2.3 Hz, 1H), 7.84 (dd, J = 8.2, 2.2 Hz, 1H), 7.75 (d, J = 8.1 Hz, 1H), 7.47–7.44 (m, 2H), 7.30 (t, J = 8.8 Hz, 2H), 5.67 (s, 1H), 4.61 (d, J = 10.8 Hz, 1H), 3.62 (d, J = 11.1 Hz, 1H), 3.13 (s, 1H), 2.91 (t, J = 11.4 Hz, 1H), 2.78 (s, 1H), 2.25 (s, 3H), 1.98 (d, J = 5.2 Hz, 1H), 1.79 (s, 1H), 1.69 (d, J = 9.0 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 165.7, 162.4, 160.0, 156.2, 150.0, 148.4, 142.1, 138.0, 131.7, 131.6, 131.5, 128.1, 115.6, 115.4, 102.2, 92.8, 47.3, 41.7, 30.6, 13.0. HRMS (ESI) calcd for C24H22BrFN5O2 [M + H]+ 510.0941, found 510.0960.
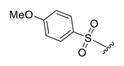
3-(4-Fluorophenyl)-5-(1-((4-methoxyphenyl)sulfonyl)-4-methyl-piperidin-4-yl)-2-methylpyrazolo[1,5-a]pyrimidin-7-ol (4b).44
The title compound was isolated as a white solid in 60% yield; mp decomposition. 1H NMR (400 MHz, DMSO-d6) δ 10.99 (s, 1H), 7.68 (d, J = 8.6 Hz, 2H), 7.38 (m, 2H), 7.27 (dd, J = 8.7, 6.7 Hz, 2H), 7.12 (d, J = 8.5 Hz, 2H), 5.62 (s, 1H), 3.82 (s, 3H), 2.97 (d, J = 10.8 Hz, 4H), 2.19 (m, 5H), 1.77 (d, J = 4.5 Hz, 2H), 1.17 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 162.6, 158.1, 155.8, 142.8, 131.7, 131.7, 129.6, 127.4, 127.4, 127.3, 115.4, 115.2, 114.5, 93.7, 55.7, 42.2, 36.5, 33.6, 24.7, 13.0. HRMS (ESI) calcd for C26H28FN4O4S [M + H]+ 511.1815, found 511.1803
1-(4-(3-(4-Fluorophenyl)-7-hydroxy-2-methylpyrazolo[1,5-a]-pyrimidin-5-yl)-4-methylpiperidin-1-yl)propan-1-one (4c).44
The title compound was isolated as a gray solid in 73% yield; mp decomposition. 1H NMR (400 MHz, CD3OD) δ 7.45 (s, 2H), 7.21 (s, 2H), 5.36–5.33 (m, 1H), 4.65 (s, 2H), 3.60 (s, 2H), 2.43–2.39 (m, 2H), 2.32 (s, 3H), 2.04 (s, 2H), 1.76 (s, 2H), 1.39 (s, 3H), 1.11 (t, J = 7.5 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 171.2, 162.5, 160.1, 158.9, 156.1, 150.7, 138.9, 131.8, 127.5, 115.5, 115.3, 102.6, 93.6, 41.1, 37.4, 34.8, 34.1, 25.6, 24.3, 13.1, 9.5. HRMS (ESI) calcd for C22H26FN4O2 [M + H]+ 397.2040, found 397.2053.
1-(4-(3-(4-Fluorophenyl)-7-hydroxy-2-methylpyrazolo[1,5-a]-pyrimidin-5-yl)-4-methylpiperidin-1-yl)-2,2-dimethyl-propan-1-one (4d).44
The title compound was isolated as a yellow solid in 55% yield; mp 203–206 °C. 1H NMR (400 MHz, CD3OD) δ 7.42 (s, 2H), 7.20 (s, 2H), 5.87 (s, 0.5H), 5.34 (s, 0.5H), 4.65 (s, 1H), 3.71 (s, 3H), 2.30 (s, 3H), 2.10 (s, 2H), 1.78 (d, J = 12.7 Hz, 2H), 1.40 (s, 3H), 1.27 (s, 9H). HRMS (ESI) calcd for C24H30FN4O2 [M + H]+ 425.2353, found 425.2351.
5-(1-((2,6-Difluorophenyl)sulfonyl)-4-methylpiperidin-4-yl)-2-methyl-3-(4-nitrophenyl)pyrazolo[1,5-a]pyrimidin-7-ol (4e).44
The title compound was isolated as an orange solid in 67% yield; mp 224–227 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.74 (s, 1H), 8.23 (d, J = 8.9 Hz, 3H), 7.65–7.57 (m, 2H), 7.22 (t, J = 9.1 Hz, 2H), 5.74 (d, J = 28.9 Hz, 1H), 2.96 (t, J = 9.7 Hz, 2H), 2.56 (s, 2H), 2.41–2.34 (m, 3H), 1.70 (s, 2H), 1.23 (s, 2H), 1.18 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 169.6, 160.0, 157.5, 145.1, 143.7, 140.7, 137.8, 135.9, 128.4, 123.7, 113.8, 113.8, 113.5, 113.5, 112.1, 112.1, 93.2, 45.7, 42.9, 35.2, 22.7, 10.8, 9.0. HRMS (ESI) calcd for C25H24F2N5O5S [M + H]+ 544.1466, found 544.1461.
2-(3,4-Dimethoxyphenyl)-1-(4-(7-hydroxy-2-methyl-3-(4-nitro-phenyl)pyrazolo[1,5-a]pyrimidin-5-yl)-4-methyl-piperidin-1-yl)-ethan-1-one (4f).44
The title compound was isolated as an orange solid in 58% yield; mp 113–116 o C. 1H NMR (400 MHz, DMSO-d6) δ 11.95 (s, 1H), 8.20 (s, 4H), 6.82–6.80 (m, 2H), 6.71 (d, J = 8.1 Hz, 1H), 5.85 (d, J = 8.1 Hz, 1H), 3.78 (d, J = 6.5 Hz, 1H), 3.66 (d, J = 5.7 Hz, 6H), 3.60 (s, 2H), 3.30 (d, J = 10.5 Hz, 2H), 3.17 (d, J = 9.5 Hz, 1H), 2.54 (s, 2H), 2.16 (s, 2H), 1.45 (d, J = 39.3 Hz, 2H), 1.21 (s, 1H), 1.18 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 168.8, 158.0, 149.3, 148.5, 147.3, 129.7, 128.4, 123.7, 120.7, 112.7, 111.8, 55.5, 55.4, 43.0, 40.1, 38.6, 36.3, 35.7, 22.1, 14.0. HRMS (ESI) calcd for C29H32N5O6 [M + H]+ 546.2353, found 546.2322.
Ethyl 4-(7-Hydroxy-2-methyl-3-(4-nitrophenyl) pyrazolo[1,5-a]-pyrimidin-5-yl)-4-methylpiperidine-1-carboxylate (4g).44
The title compound was isolated as an orange solid in 76% yield; mp 174–178 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.20 (s, 4H), 5.73 (s, 1H), 3.97 (q, J = 6.9 Hz, 2H), 3.57 (d, J = 12.4 Hz, 2H), 3.16 (s, 2H), 2.54 (s, 3H), 2.19 (s, 2H), 1.51 (s, 2H), 1.18 (s, 3H), 1.13 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 154.7, 123.7, 60.5, 40.8, 35.7, 28.8, 14.7. HRMS (ESI) calcd for C22H26N5O5 [M + H]+ 440.1934, found 440.1931.
tert-Butyl 4-(3-(4-Fluorophenyl)-7-hydroxy-2-methylpyrazolo-[1,5-a]pyrimidin-5-yl)-4-methylpiperidine-1-carboxylate (4h).44
The title compound was isolated as a white solid in 62% yield; mp decomposition. 1H NMR (400 MHz, CDCl3) δ 8.92 (s, 1H), 7.21 (dd, J = 8.0, 5.5 Hz, 2H), 7.04 (t, J = 8.5 Hz, 2H), 5.75 (s, 1H), 3.60–3.55 (m, 2H), 3.42–3.37 (m, 2H), 2.26 (s, 3H), 2.02–1.98 (m, 2H), 1.73 (s, 2H), 1.44 (s, 9H), 1.41 (s, 3H). 13C NMR (101 MHz, CDCl3) δ163.1, 160.7, 157.7, 157.0, 154.8, 151.7, 138.2, 130.7, 130.6, 127.1, 116.3, 116.1, 105.3, 103.1, 95.1, 80.2, 37.4, 28.5, 25.4, 13.1, 1.2, 0.13. HRMS (ESI) calcd for C24H30FN4O3 [M + H]+ 441.2302, found 441.2310.
Ethyl 4-(7-Hydroxy-2-methyl-3-(4-nitrophenyl)pyrazolo[1,5-a]-pyrimidin-5-yl)piperidine-1-carboxylate (4i).44
The title compound was isolated as a yellow solid in 61% yield; mp decomposition. 1H NMR (400 MHz, CDCl3) δ 11.37 (s, 1H), 8.05 (d, J = 8.7 Hz, 2H), 7.45 (d, J = 8.6 Hz, 2H), 5.65 (s, 1H), 4.30 (d, J = 10.9 Hz, 2H), 4.12 (q, J = 7.1 Hz, 2H), 2.95 (t, J = 12.1 Hz, 1H), 2.80 (s, 2H), 2.31 (s, 3H), 2.06–2.04 (m, 2H), 1.67 (ddd, J = 25.3, 12.7, 4.3 Hz, 2H), 1.27 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 157.9, 157.3, 155.4, 151.0, 145.9, 139.3, 138.4, 130.1, 123.3, 102.6, 94.1, 61.7, 43.9, 40.3, 30.7, 14.8, 13.2. HRMS (ESI) calcd for C21H24N5O5 [M + H]+ 426.1777, found 426.1771.
1-(4-(3-(4-Fluorophenyl)-7-hydroxy-2-methylpyrazolo[1,5-a]-pyrimidin-5-yl)piperidin-1-yl)propan-1-one (4j).44
The title compound was isolated as a white solid in 85% yield; mp 164–166 °C. 1H NMR (400 MHz, CD3OD) δ 7.51 (s, 2H), 7.17 (s, 2H), 5.80 (s, 1H), 4.67 (d, J = 15.8 Hz, 2H), 4.08 (d, J = 11.4 Hz, 1H), 3.16 (t, J = 12.2 Hz, 1H), 2.87 (s, 1H), 2.67 (d, J = 11.0 Hz, 1H), 2.45 (d, J = 7.0 Hz, 2H), 2.32 (s, 3H), 1.99 (t, J = 15.4 Hz, 2H), 1.75–1.63 (m, 2H), 1.14 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, CD3OD) δ 174.7, 164.8, 162.3, 159.2, 153.0, 140.3, 133.0, 132.9, 127.9, 116.6, 116.4, 104.7, 93.9, 46.6, 42.8, 41.1, 32.3, 31.9, 27.3, 12.9, 10.0. HRMS (ESI) calcd for C21H24FN4O2 [M + H]+ 383.1883, found 383.1876.
1-(4-(3-(4-Fluorophenyl)-7-hydroxy-2-methylpyrazolo[1,5-a]-pyrimidin-5-yl)piperidin-1-yl)-2,2-dimethylpropan-1-one (4k).44
The title compound was isolated as a white solid in 85% yield; mp 267–270 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.71 (s, 1H), 7.43 (dd, J = 8.5, 5.6 Hz, 2H), 7.30 (t, J = 8.8 Hz, 2H), 5.59 (s, 1H), 4.41 (d, J = 13.3 Hz, 2H), 2.90–2.84 (m, 1H), 2.75 (dd, J = 20.8, 8.0 Hz, 2H), 2.23 (s, 3H), 1.89 (d, J = 12.2 Hz, 2H), 1.50 (qd, J = 12.5, 3.3 Hz, 2H), 1.19 (s, 9H). 13C NMR (101 MHz, DMSO-d6) δ 175.1, 162.6, 160.2, 157.3, 156.3, 150.3, 138.8, 131.8, 131.7, 127.2, 127.1, 115.8, 115.5, 102.3, 92.8, 44.8, 38.3, 30.9, 30.8, 28.3, 13.1. HRMS (ESI) calcd for C23H28FN4O2 [M + H]+ 411.2196, found 411.2170.
tert-Butyl 4-(3-(4-Chlorophenyl)-7-hydroxy-2-methylpyrazolo-[1,5-a]pyrimidin-5-yl)piperidine-1-carboxylate (4l).44
The title compound was isolated as a white solid in 82% yield; mp 119–123 °C. 1H NMR (400 MHz, CDCl3) δ 11.35 (s, 1H), 7.17 (d, J = 8.2 Hz, 2H), 7.09 (d, J = 8.1 Hz, 2H), 5.51 (s, 1H), 4.22 (s, 2H), 3.07 (t, J = 11.7 Hz, 1H), 2.74 (s, 2H), 2.16 (s, 3H), 2.09 (d, J = 12.0 Hz, 2H), 1.62 (dt, J = 12.2, 8.7 Hz, 2H), 1.45 (s, 9H). 13C NMR (101 MHz, DMSO-d6) δ 157.6, 156.5, 154.2, 150.4, 139.1, 132.0, 131.8, 130.1, 129.1, 102.4, 93.3, 79.2, 30.7, 28.5, 13.4. HRMS (ESI) calcd for C23H28ClN4O3 [M + H]+ 443.1850, found 443.1832.
Benzyl 4-(3-(4-Chlorophenyl)-7-hydroxy-2-methylpyrazolo[1,5-a]pyrimidin-5-yl)piperidine-1-carboxylate (4m).44
The title compound was isolated as a white solid in 85% yield; mp 236–239 °C. 1H NMR (400 MHz, CD3OD) δ 7.69–7.66 (m, 2H), 7.39–7.31 (m, 7H), 5.68 (s, 1H), 5.15 (s, 2H), 4.27 (d, J = 13.3 Hz, 2H), 2.94 (s, 2H), 2.70–2.67 (m, 1H), 2.48 (s, 3H), 1.93 (d, J = 9.3 Hz, 2H), 1.71 (tt, J = 12.5, 6.3 Hz, 2H). 13C NMR (101 MHz, CD3OD) δ 168.0, 161.4, 156.9, 150.5, 150.2, 138.2, 134.6, 131.4, 131.1, 129.5, 129.1, 129.0, 128.8, 105.0, 91.2, 68.2, 45.5, 45.2, 32.7, 14.5. HRMS (ESI) calcd for C26H26ClN4O3 [M + H]+ 477.1693, found 477.1691.
Ethyl 4-(7-Hydroxy-2-methyl-3-(4-(trifluoromethyl)phenyl)-pyrazolo[1,5-a]pyrimidin-5-yl)piperidine-1-carboxylate (4n).44
The title compound was isolated as a white solid in 78% yield; mp 258–261 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.74 (s, 1H), 7.85–7.81 (m, 2H), 7.68–7.66 (m, 2H), 5.64 (s, 1H), 4.06 (dt, J = 14.1, 9.2 Hz, 4H), 2.78 (t, J = 11.8 Hz, 3H), 2.30 (s, 3H), 1.85 (d, J = 12.4 Hz, 2H), 1.56 (tt, J = 12.4, 6.3 Hz, 2H), 1.18 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 157.2, 156.0, 154.5, 150.0, 139.0, 135.3, 130.2, 125.5, 125.4, 101.9, 93.3, 60.7, 43.4, 30.2, 14.6, 13.1. HRMS (ESI) calcd for C22H24F3N4O3 [M + H]+ 449.1801, found 449.1792.
Ethyl 4-(3-(4-Fluorophenyl)-7-hydroxy-2-methylpyrazolo[1,5-a]-pyrimidin-5-yl)piperidine-1-carboxylate (4o).44
The title compound was isolated as a white solid in 87% yield; mp 241–244 °C. 1H NMR (400 MHz, CDCl3) δ 12.12 (s, 1H), 7.14 (dd, J = 8.5, 5.4 Hz, 2H), 6.74 (t, J = 8.7 Hz, 2H), 5.48 (s, 1H), 4.26 (s, 2H), 4.09 (q, J = 7.1 Hz, 3H), 3.20 (t, J = 11.9 Hz, 1H), 2.78 (s, 2H), 2.12 (d, J = 9.7 Hz, 6H), 1.64 (dt, J = 12.1, 8.7 Hz, 3H), 1.24 (t, J = 7.1 Hz, 4H). 13C NMR (101 MHz, CDCl3) δ 160.3, 158.0, 157.7, 155.6, 150.9, 139.0, 131.2, 126.8, 115.0, 114.8, 103.17, 92.9, 61.6, 43.9, 39.8, 31.0, 14.8, 12.8. HRMS (ESI) calcd for C21H24FN4O3 [M + H]+ 399.1832, found 399.1813.
Benzyl 4-(3-(4-Fluorophenyl)-7-hydroxy-2-methylpyrazolo[1,5-a]pyrimidin-5-yl)piperidine-1-carboxylate (4p).44
The title compound was isolated as a white solid in 82% yield; mp 206–209 °C. 1H NMR (400 MHz, DMSO-d6) δ 7.79 (dd, J = 8.5, 5.8 Hz, 2H), 7.28 (d, J = 4.3 Hz, 4H), 7.23 (dd, J = 8.4, 4.0 Hz, 1H), 7.09 (t, J = 8.9 Hz, 2H), 5.34 (s, 1H), 5.01 (s, 2H), 4.04 (t, J = 12.0 Hz, 2H), 3.09 (d, J = 4.9 Hz, 1H), 2.82 (s, 2H), 2.37 (s, 3H), 1.74 (d, J = 11.9 Hz, 2H), 1.52 (qd, J = 12.6, 3.8 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 165.5, 158.6, 158.2, 154.6, 149.0, 147.5, 137.2, 131.7, 128.6, 128.6, 128.5, 127.9, 127.6, 114.8, 114.6, 102.1, 90.3, 66.2, 48.7, 44.0, 43.5, 15.2. HRMS (ESI) calcd for C26H26FN4O3 [M + H]+ 461.1989, found 461.1984.
General Procedure for the Synthesis of 6a–6c
To a solution of 5 (1 equiv) in water were added NaOH (6a, 6c) (1.2 equiv) or K2CO3 (6b) (1.2 equiv), appropriate acyl chloride (6a, 6c) (1.2 equiv), or (Boc)2O (6b) (1.2 equiv) dissolved in THF. The mixture was stirred at rt for 5–7 h. The solvent was removed under reduced pressure, and the aqueous phase was adjusted to pH 2 with 1 M HCl. The sample was then extracted with CH2Cl2 and dried over anhydrous MgSO4. The solvent was evaporated under reduced pressure. The residue was used in the next step without further purification.
1-(tert-Butoxycarbonyl)piperidine-4-carboxylic Acid (6a).45
The title compound was isolated as a white solid in 90% yield. 1H NMR (400 MHz, CDCl3) δ 4.01 (s, 2H), 2.85 (t, J = 11.7 Hz, 2H), 2.48 (tt, J = 10.9, 3.9 Hz, 1H), 1.90 (d, J = 10.9 Hz, 2H), 1.68–1.58 (m, 2H), 1.45 (s, 9H).
1-(Ethoxycarbonyl)piperidine-4-carboxylic Acid (6b).46
The title compound was isolated as a white solid in 91% yield. 1H NMR (400 MHz, CDCl3) δ 11.01 (s, 1H), 4.14–4.05 (m, 4H), 2.90 (t, J = 10.8 Hz, 2H), 2.49 (tt, J = 10.9, 3.8 Hz, 1H), 1.91 (d, J = 11.9 Hz, 2H), 1.64 (qd, J = 11.5, 4.1 Hz, 2H), 1.24 (t, J = 7.1 Hz, 3H).
1-((Benzyloxy)carbonyl)piperidine-4-carboxylic Acid (6c).47
The title compound was isolated as a white solid in 95% yield. 1H NMR (400 MHz, CDCl3) δ 10.72 (s, 1H), 7.39–7.29 (m, 5H), 5.13 (s, 2H), 4.10 (s, 2H), 2.95 (s, 2H), 2.51 (tt, J = 10.7, 3.8 Hz, 1H), 1.93 (s, 2H), 1.67 (d, J = 10.3 Hz, 2H).
General Procedure for the Synthesis of 7a–7b
To a solution of diisopropyl amine (6.73 mL, 48 mmol) in THF (145 mL) was added n-BuLi (2 M in hexane, 24 mL, 48 mmol) at 0 °C. The reaction was stopped to –78 °C 15 min later. Compound 6 (40 mmol) in THF (60 mL) was added to the above solution and stirred for 1 h. Iodomethane (3.75 mL, 60 mmol) in THF (60 mL) was then added. The reaction was warmed to rt and stirred overnight, quenched with saturated NH4Cl aqueous solution at 0 °C, and the solvent removed. The sample was extracted with EtOAc (3 × 100 mL). The combined organic layer was washed with brine (100 mL), dried over anhydrous MgSO4, and solvent evaporated under reduced pressure. The residue was purified by silica gel column chromatography (petroleum ether/ EtOAc, 150:1–50:1) to obtain the title compound.
Diethyl 4-Methylpiperidine-1,4-dicarboxylate (7a).46
The title compound was isolated as a light-yellow oil in 87% yield. 1H NMR (400 MHz, CDCl3) δ 4.13–4.04 (m, 4H), 3.79 (s, 2H), 2.98 (t, J = 11.4 Hz, 2H), 2.03 (d, J = 13.5 Hz, 2H), 1.35–1.28 (m, 2H), 1.21 (td, J = 7.1, 5.5 Hz, 6H), 1.16 (s, 3H).
1-(tert-Butyl) 4-Ethyl 4-Methylpiperidine-1,4-dicarboxylate (7b).48
The title compound was isolated as a light-yellow oil in 85% yield. 1H NMR (400 MHz, CDCl3) δ 4.17–4.09 (m, 2H), 3.76–3.71 (m, 2H), 2.96 (t, J = 11.5 Hz, 2H), 2.04 (d, J = 13.1 Hz, 2H), 1.43 (s, 9H), 1.37–1.30 (m, 2H), 1.24 (t, J = 7.1 Hz, 3H), 1.18 (s, 3H).
General Procedure for the Synthesis of 8a–8d
To a solution of compound 7 (40 mmol) in THF (10 mL) was added 5 M NaOH (40 mL, 200 mmol) at 0 °C, and the resulting solution was then reacted at 70 °C overnight. After removing the solvent, the sample was diluted with water and extracted with EtOAc (3 × 15 mL). The resulting aqueous phase was adjusted to pH 3 with 1 M HCl and extracted with CH2Cl2 for three times, which was then combined with the organic phase and dried over anhydrous MgSO4. The solvent was removed under reduced pressure. The corresponding carboxylic acid was obtained and used directly in the next step without further purification. Then, to a round-bottom flask with the above carboxylic acid (1 equiv) dissolved in THF was added N,N′-carbonyldiimidazole (1.3 equiv) at 0 °C. The resulting solution was stirred at rt for 4 h. To another solution of potassium 3-ethoxy-3-oxopropanoate (1 equiv) in MeCN:THF (1:2) were added MgCl2 (2 equiv) and 4-dimethylaminopyridine (DMAP, 0.1 equiv) at 0 °C. The resulting solution was stirred at rt for 5 h. The former solution was then added to the latter solution at 0 °C. The resulting mixture was stirred at rt overnight. After removing the solvent and adjusting pH to 4 with 1 M HCl at 0 °C, the product was extracted with EtOAc for three times. The combined organic layer was washed with 1 M HCl, 1 M NaOH, and brine separately, dried over anhydrous MgSO4, and the solvent removed under reduced pressure to obtain 8 as a yellow oil, which was used directly in the next step without further purification.
Ethyl 4-(3-Ethoxy-3-oxo)propanoyl)-4-methylpiperidine-1-carboxylate (8a).46
Yield of 82%. 1H NMR (400 MHz, CDCl3) δ 4.21–4.15 (m, 2H), 4.10 (q, 2H), 3.62 (s, 2H), 3.51 (s, 2H), 3.35–3.20 (m, 2H), 1.97 (m, 2H), 1.44 (d, J = 3.7 Hz, 2H), 1.26 (tt, J = 10.5, 7.1 Hz, 6H), 1.18 (s, 3H).
tert-Butyl 4-(3-Ethoxy-3-oxopropanoyl)-4-methylpiperidine-1-carboxylate (8b).46
Yield of 86%. 1H NMR (400 MHz, CDCl3) δ 4.17 (qd, J = 7.1, 1.3 Hz, 2H), 3.53–3.46 (m, 4H), 3.29–3.16 (m, 2H), 1.96–1.91 (m, 2H), 1.42 (s, 11H), 1.29–1.23 (m, 3H), 1.16 (s, 3H).
Benzyl 4-(3-Ethoxy-3-oxopropanoyl)piperidine-1-carboxylate (8c).46
Yield of 87%. 1H NMR (400 MHz, CDCl3) δ 7.39–7.36 (m, 5H), 5.14 (s, 2H), 4.21 (q, J = 7.1 Hz, 4H), 3.51 (s, 2H), 2.89 (d, J = 10.8 Hz, 2H), 2.67 (tt, J = 11.2, 3.7 Hz, 1H), 1.88 (s, 2H), 1.59 (m, 2H), 1.30 (td, J = 7.1, 5.6 Hz, 3H).
tert-Butyl 4-(3-Ethoxy-3-oxopropanoyl) piperidine-1-carboxylate (8d).44
Yield of 91%. 1H NMR (400 MHz, CDCl3) δ 4.12 (dd, J = 14.1, 7.0 Hz, 2H), 4.04 (s, 2H), 3.43 (s, 2H), 2.72 (t, J = 11.4 Hz, 2H), 2.59–2.54 (m, 1H), 1.77 (t, J = 13.6 Hz, 2H), 1.52–1.43 (m, 2H), 1.38 (s, 9H), 1.22–1.19 (m, 3H).
General Procedure for the Synthesis of 11a–11d
To a sealing tube was added 9 (14.4 mL, 100 mmol), 10 (14.6 mL, 120 mmol), dry CH2Cl2 (10 mL) under N2 atmosphere. The mixture was heated at 100 °C for 24 h. After cooling to rt, the resulting solution was added to a solution of hydrazine hydrochloride (10.3 g, 150 mmol) in EtOH (20 mL) and H2O (20 mL) in a 250 mL round-bottom flask. The mixture was then heated at 80 °C for 12 h. The resulting solution was concentrated and neutralized with saturated solution of NaHCO3 to pH 9. The mixture was extracted with EtOAc (3 × 100 mL). The combined organic layer was washed with brine (100 mL), dried over anhydrous MgSO4, and the solvent removed under reduced pressure. The product was recrystallized with EtOAc to obtain the title compound.
4-(4-Fluorophenyl)-3-methyl-1H-pyrazol-5-amine (11a).44
The title compound was isolated as a white solid in 85% yield. 1H NMR (400 MHz, CDCl3) δ 7.28–7.24 (m, 2H), 7.07 (t, J = 8.7 Hz, 2H), 2.22 (s, 3H).
4-(4-Chlorophenyl)-3-methyl-1H-pyrazol-5-amine (11b).44
The title compound was isolated as a white solid in 81% yield. 1H NMR (400 MHz, CDCl3) δ 7.35 (d, J = 8.4 Hz, 2H), 7.25 (d, J = 8.2 Hz, 2H), 2.24 (s, 3H).
3-Methyl-4-(4-(trifluoromethyl) phenyl)-1H-pyrazol-5-amine (11c).44
The title compound was isolated as a yellow solid in 88% yield. 1H NMR (400 MHz, CDCl3) δ 7.65 (d, J = 8.1 Hz, 2H), 7.47 (d, J = 8.0 Hz, 2H), 2.31 (d, J = 5.5 Hz, 3H).
3-Methyl-4-(4-nitrophenyl)-1H-pyrazol-5-amine (11d).44
The title compound was isolated as a yellow solid in 82% yield. 1H NMR (400 MHz, DMSO-d6) δ 11.76 (s, 1H), 8.21–8.19 (m, 2H), 7.63 (d, J = 8.7 Hz, 2H), 4.95 (s, 2H), 2.26 (s, 3H).
General Procedure for the Synthesis of 12
To a solution of 8 (1 equiv) in AcOH was added 11 (1.1 equiv), and the resulting solution was refluxed overnight. After removing the solvent and adjusting pH of the aqueous phase to 8 with a saturated NaHCO3 solution, the product was extracted with CH2Cl2 and dried over anhydrous MgSO4. The solvent was evaporated under reduced pressure, and the residue was purified by silica gel column chromatography (CH2Cl2/MeOH, 100:1–40:1) to obtain compound 12.
General Procedure for the Synthesis of 13a
To a solution of 12 (R2 = Boc) in CH2Cl2 (3 V/V) was added TFA (V/V) and the mixture stirred at 0 °C for 2 h, After removing the solvent and adjusting pH of the aqueous phase to 8 with a saturated NaHCO3 solution, the resulting precipitation was filtrated, washed with water, and dried over vacuum. 13a was obtained and used in the next step without further purification.
General Procedure for the Synthesis of 13b
To a solution of 8 (1 equiv) in EtOH:TFA = (50 mL:10 mL) was added 11a (1.2 equiv). The resulting solution was stirred at 85 °C, when another 6.5, 8.5, and 25 mL of TFA was added after 10, 20, and 30 h separately. The reaction was quenched with water at 48 h. After removing the solvent and adjusting pH of the aqueous phase to 8 with a saturated NaHCO3 solution, the product was filtered, the residue washed with water, CH3OH, and CH2Cl2 separately, and then dried over vacuum to obtain 13b as a white solid, which was used directly in the next step without further purification.
Cell Lines and Cell Culture
HEK293 cells were grown in DMEM (high glucose) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 units/mL penicillin, and 100 μg/mL streptomycin at 37 °C, 5% CO2. Stable cell lines that express human TRPC3, mouse TRPC4, mouse TRPC6, mouse TRPV3, or mouse TRPM8 were established as described previously and maintained in the medium supplemented with G418 (400 μg/mL; Invitrogen).49 For those that coexpressed MOR or M5 muscarinic receptor, 100 μg/mL hygromycin B (Calbiochem) was also included in the culture medium. Stable cell lines that inducibly express human TRPC5, TRPC7, TRPA1, TRPV1, TRPV4, or TRPM5 were established as described previously and maintained in the medium supplemented with 100 μg/mL hygromycin B and 5 μg/mL blasticidin.50 The expression of TRP channels was induced by the addition of 0.1 μM doxycycline to the culture medium 24 h prior to the assay.
The use of animals was approved by the Animal Experimentation Ethics Committee of Anhui Medical University and in accordance with the guidelines for ethical conduct in the care and use of animals. Glomerular mesangial cells were prepared according to previous report.43,51 Briefly, glomeruli were isolated from male Sprague–Dawley rats (260–280 g) using the graded sieving technique. Glomeruli were collected by centrifugation and digested by collagenase (2 mg/mL, Sigma-Aldrich) for 45 min at 37 °C. Following the wash-off of the enzymes, mesangial cells were cultured in RPMI 1640 medium supplemented with 17% FBS, 100 units/mL penicillin G, and 100 μg/mL streptomycin at 37 °C in a 5% CO2 humidified incubator.
Fluorescence Ca2+ and Membrane Potential Assays
Cells were seeded in wells of 96-well plates precoated with polyornithine at ~100000 cells/well. Cells were loaded with Fluo4-AM (Invitrogen) or Fluo8-AM (TEFLabs) to monitor intracellular Ca2+ changes or loaded with the FLIPR Membrane Potential dye (FMP, Molecular Devices) to monitor membrane potential changes with the use of a FlexStation microplate reader (Molecular Devices). The detailed protocols for the FlexStation FMP and Ca2+ assays have been described previously.37,49,52 Briefly, the extracellular solution for all FlexStation assays contained (in mM): 140 NaCl, 2 CaCl2, 1 MgCl2, 10 glucose, and 10 HEPES, pH adjusted to 7.4 with NaOH, except for TRPC3 when the 2 CaCl2 was replaced with 0.5 CaCl2. Probenecid (2 mM) was included in all Ca2+ assays except for TRPV1. Assays were run at 32 °C.
[Ca2+]i measurement in rat mesangial cells was carried out as described previously.53 Briefly, glomerular mesangial cells were seeded on glass coverslips and allowed to grow overnight. In the next day, the cells were loaded with 10 μM Fluo4-AM in the presence of 0.02% pluronic acid F-127 at 37 °C for 1 h in the dark in 1 mM Ca2+ saline containing (in mM): 140 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 glucose, and 10 HEPES, pH adjusted to 7.4 with NaOH. After washing, the fluorescent signal was measured using a Leica TCS SP5 laser scanning confocal imaging system. The excitation and emission wavelength were 488 and 515 nm, respectively.
For all fluorescence measurement, changes in the fluorescence intensity were displayed as a ratio of fluorescence change to the fluorescence before the application of stimulating compounds (ΔF/F0).
Electrophysiology Recordings
HEK293 cells stably expressing TRPC channels were seeded in 35 mm dishes at least 5 h before whole-cell patch clam recordings were performed. Recording pipettes were pulled from micropipette glass (Sutter Instrument) to 2–5 MΩ when filled with a pipet solution containing (in mM): 110 CsCl, 10 HEPES, 10 BAPTA, 1 MgCl2, 4.77 CaCl2, pH adjusted to 7.2 with HCl (400 nM free Ca2+) and placed in the bath solution containing (in mM): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, and 10 HEPES, pH 7.4. Individual cells were voltage-clamped in the whole-cell mode using either an EPC9 or EPC10 (HEKA Instruments Inc., Bellmore, NY) amplifier. Voltage commands were made from the PatchMaster program (version 2.60; HEKA), and currents were recorded at 5 kHz. Voltage ramps of 100 ms to –100 mV after a brief (20 ms) step to +100 mV from holding potential of 0 mV were applied every 1 s. Cells were continuously perfused with the bath solution through a gravity-driven multichannel system with the desired channel opening placed about 50 μm away from the cell being recorded. For some recordings, CaCl2 in the bath solution was reduced to 0.5 or 0.1 mM as indicated in the figure legends. Drugs were diluted in the bath solution with the desired Ca2+ concentration and applied to the cell through perfusion.
Data Analysis
All data were analyzed and plotted using Origin 7.5 (OriginLab) and Graphpad prism (version 5.01). Summary data are presented as the mean ± SEM. Statistical comparisons were made using Student’s t test. A p value of <0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This work was partially supported by grants from NSFC (81573383, 21390402), NSFHP (2014CFB704), IS&TCPC (2015DFA30440, 2014cFB30020), the Applied Basic Research Program of Wuhan Municipal Bureau of Science & Technology, the Applied Basic Research Programs of Scientific and Technologic Council of Suzhou (SYG201521), Natural Science Foundation of Jiangsu Province (BK20160387), Beijing Nova Plan Z (131107000413063), the Fundamental Research Funds for the Central Universities and U.S. National Institutes of Health (NS056942, NS092377, U54 MH084691). We thank Dr. Corey Hopkins for the initial evaluation of the lead compounds.
ABBREVIATIONS USED
- [Ca2+]i
intracellular Ca2+ concentration
- Cbz
carboxybenzyl
- CCh
carbachol
- DAG
diacylglycerols
- DAMGO
[D-Ala2, N-MePhe4,Gly-ol]-enkephalin
- DCM
dichloromethane
- EC50
half-maximal stimulation
- FBS
fetal bovine serum
- FMP
FLIPR membrane potential
- HTS
high-throughput screening
- MOR
μ opioid receptor
- PLC
phospholipase C
- PIP2
phosphatidylinositol 4,5-bisphosphate
- SAR
structure–activity relationship
- TFA
trifluoroacetic acid
- TRPA
transient receptor potential ankyrin
- TRPC
transient receptor potential canonical
- TRPM
transient receptor potential melatatin
- TRPV
transient receptor potential vanilloid
Footnotes
Author Contributions
Chunrong Qu, Mingmin Ding, and Yingmin Zhu contributed equally to this work. X. Hong, M. X. Zhu, M. Li, B. Shen, Z. Cao, H. Wang, and H. R. Luo designed the research; C. Qu, M. Ding, Y. Zhu, Y. Lu, J. Zhu, J. Du, M. Miller, J. Tian, J. Xu, M. Wen, M. Wu, and J. Wu performed experiments; X. Hong, M. X. Zhu, H. Wang, C. Qu, M. Ding, Y. Lu, Y. Xiao, O. B. McManus, and J. Wang performed data analyses; M. Ding and AGA Er-Bu performed HPLC analyses; X. Hong, M. X. Zhu, H. Wang, C. Qu, M. Ding, Y. Lu, and H. R. Luo wrote the paper.
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jmed-chem.7b00304.
1H NMR spectral information for compounds 6a–6c, 7a–7b, 8a–8d, 11a–11c; 1H NMR and 13C NMR spectral information for compounds 4a–4p; HPLC results and HPLC traces information for compounds 4a–4p (PDF)
Molecular formula strings (CSV)
References
- 1.Putney JW. Physiological mechanisms of TRPC activation. Pfluegers Arch. 2005;451:29–34. doi: 10.1007/s00424-005-1416-4. [DOI] [PubMed] [Google Scholar]
- 2.Kiselyov K, Lee KP, Yuan JP, Muallem S. Methods to study TRPC channel regulation by interacting proteins. In: Zhu MX, editor. TRP Channels. CRC Press; Boca Raton, FL: 2011. pp. 21–43. [PubMed] [Google Scholar]
- 3.Sun Y, Sukumaran P, Bandyopadhyay BC, Singh BB. Physiological function and characterization of TRPCs in neurons. Cells. 2014;3:455–475. doi: 10.3390/cells3020455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu J, Gao Z, Shen B, Zhu MX. Canonical transient receptor potential 4 and its small molecule modulators. Sci China: Life Sci. 2015;58:39–47. doi: 10.1007/s11427-014-4772-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng S, Li H, Tai Y, Huang J, Su Y, Abramowitz J, Zhu MX, Birnbaumer L, Wang Y. Canonical transient receptor potential 3 channels regulate mitochondrial calcium uptake. Proc Natl Acad Sci U S A. 2013;110:11011–11016. doi: 10.1073/pnas.1309531110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsvilovskyy VV, Zholos AV, Aberle T, Philipp SE, Dietrich A, Zhu MX, Birnbaumer L, Freichel M, Flockerzi V. Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology. 2009;137:1415–1424. doi: 10.1053/j.gastro.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urban N, Hill K, Wang L, Kuebler WM, Schaefer M. Novel pharmacological TRPC inhibitors block hypoxia-induced vasoconstriction. Cell Calcium. 2012;51:194–206. doi: 10.1016/j.ceca.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, Pericak-Vance MA, Howell DN, Vance JM, Rosenberg PB. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801–1804. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- 9.Reiser J, Polu KR, Möller CC, Kenlan P, Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C, McGee M, Sugimoto H, Brown D, Kalluri R, Mundel P, Smith PL, Clapham DE, Pollak MR. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005;37:739–744. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou J, Du W, Zhou K, Tai Y, Yao H, Jia Y, Ding Y, Wang Y. Critical role of TRPC6 channels in the formation of excitatory synapses. Nat Neurosci. 2008;11:741–743. doi: 10.1038/nn.2127. [DOI] [PubMed] [Google Scholar]
- 11.Hartmann J, Dragicevic E, Adelsberger H, Henning HA, Sumser M, Abramowitz J, Blum R, Dietrich A, Freichel M, Flockerzi V, Birnbaumer L, Konnerth A. TRPC3 channels are required for synaptic transmission and motor coordination. Neuron. 2008;59:392–398. doi: 10.1016/j.neuron.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis J, Burr AR, Davis GF, Birnbaumer L, Molkentin JD. A TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev Cell. 2012;23:705–715. doi: 10.1016/j.devcel.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dietrich A, Gudermann T. TRPC6: Physiological function and pathophysiological relevance. In: Nilius B, Flockerzi VB, editors. Mammalian Transient Receptor Potential (TRP) Cation Channels; Handbook of Experimental Pharmacology. Vol. 222. Springer-Verlag; Berlin, Heidelberg: 2014. pp. 157–187. [DOI] [PubMed] [Google Scholar]
- 14.Seo K, Rainer PP, Shalkey Hahn V, Lee D, Jo S, Andersen A, Liu T, Xu X, Willette RN, Lepore JJ, Marino JP, Jr, Birnbaumer L, Schnackenberg CG, Kass DA. Combined TRPC3 and TRPC6 blockade by selective small-molecule or genetic deletion inhibits pathological cardiac hypertrophy. Proc Natl Acad Sci U S A. 2014;111:1551–1556. doi: 10.1073/pnas.1308963111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Lu R, Yang J, Li H, He Z, Jing N, Wang X, Wang Y. TRPC6 specifically interacts with APP to inhibit its cleavage by γ-secretase and reduce Aβ production. Nat Commun. 2015;6:8876. doi: 10.1038/ncomms9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riehle M, Büscher AK, Gohlke BO, Kaßmann M, Kolatsi-Joannou M, Bräsen JH, Nagel M, Becker JU, Winyard P, Hoyer PF, Preissner R, Krautwurst D, Gollasch M, Weber S, Harteneck C. TRPC6 G757D Loss-of-function mutation associates with FSGS. J Am Soc Nephrol. 2016;27:2771–2783. doi: 10.1681/ASN.2015030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zandi-Nejad K, Eddy AA, Glassock RJ, Brenner BM. Why is proteinuria an ominous biomarker of progressive kidney disease? Kidney Int. 2004;92:S76–S89. doi: 10.1111/j.1523-1755.2004.09220.x. [DOI] [PubMed] [Google Scholar]
- 18.Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res. 2004;95:496–505. doi: 10.1161/01.RES.0000138952.16382.ad. [DOI] [PubMed] [Google Scholar]
- 19.Onohara N, Nishida M, Inoue R, Kobayashi H, Sumimoto H, Sato Y, Mori Y, Nagao T, Kurose H. TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J. 2006;25:5305–5316. doi: 10.1038/sj.emboj.7601417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan JX. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci U S A. 2004;101:13861–13866. doi: 10.1073/pnas.0405908101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang SL, Cao Q, Zhou KC, Feng YJ, Wang YZ. Transient receptor potential channel C3 contributes to the progression of human ovarian cancer. Oncogene. 2009;28:1320–1328. doi: 10.1038/onc.2008.475. [DOI] [PubMed] [Google Scholar]
- 22.Ding X, He Z, Shi Y, Wang Q, Wang Y. Targeting TRPC6 channels in oesophageal carcinoma growth. Expert Opin Ther Targets. 2010;14:513–527. doi: 10.1517/14728221003733602. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 24.Okada T, Inoue R, Yamazaki K, Maeda A, Kurosaki T, Yamakuni T, Tanaka I, Shimizu S, Ikenaka K, Imoto K, Mori Y. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. Ca2+-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. J Biol Chem. 1999;274:27359–27370. doi: 10.1074/jbc.274.39.27359. [DOI] [PubMed] [Google Scholar]
- 25.Xu X, Lozinskaya I, Costell M, Lin Z, Ball JA, Bernard R, Behm DJ, Marino JP, Schnackenberg CG. Characterization of small molecule TRPC3 and TRPC6 agonist and antagonists. Biophys J. 2013;104:454a. [Google Scholar]
- 26.Doleschal B, Primessnig U, Wölkart G, Wolf S, Schernthaner M, Lichtenegger M, Glasnov TN, Kappe CO, Mayer B, Antoons G, Heinzel F, Poteser M, Groschner K. TRPC3 contributes to regulation of cardiac contractility and arrhythmogenesis by dynamic interaction with NCX1. Cardiovasc Res. 2015;106:163–173. doi: 10.1093/cvr/cvv022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schleifer H, Doleschal B, Lichtenegger M, Oppenrieder R, Derler I, Frischauf I, Glasnov TN, Kappe CO, Romanin C, Groschner K. Novel pyrazole compounds for pharmacological discrimination between receptor-operated and store-operated Ca2+ entry pathways. Br J Pharmacol. 2012;167:1712–1722. doi: 10.1111/j.1476-5381.2012.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Washburn DG, Holt DA, Dodson J, McAtee JJ, Terrell LR, Barton L, Manns S, Waszkiewicz A, Pritchard C, Gillie DJ, Morrow DM, Davenport EA, Lozinskaya IM, Guss J, Basilla JB, Negron LK, Klein M, Willette RN, Fries RE, Jensen TC, Xu X, Schnackenberg CG, Marino JP., Jr The discovery of potent blockers of the canonical transient receptor channels TRPC3 and TRPC6 based on an anilino-thiazole pharmacophore. Bioorg Med Chem Lett. 2013;23:4979–4984. doi: 10.1016/j.bmcl.2013.06.047. [DOI] [PubMed] [Google Scholar]
- 29.Miehe S, Kleemann HW, Struebing C. Use of norgestimate as a selective inhibitor of TRPC3, TRPC6 and TRPC7 ion channels. 8,455,469. US Patent. 2013 Jun 4;
- 30.Maier T, Follmann M, Hessler G, Kleemann HW, Hachtel S, Fuchs B, Weissmann N, Linz W, Schmidt T, Lohn M, Schroeter K, Wang L, Rutten H, Strubing C. Discovery and pharmacological characterization of a novel potent inhibitor of diacylglycerol-sensitive TRPC cation channels. Br J Pharmacol. 2015;172:3650–3660. doi: 10.1111/bph.13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urban N, Wang L, Kwiek S, Rademann J, Kuebler WM, Schaefer M. Identification and Validation of Larixyl Acetate as a Potent TRPC6 Inhibitor. Mol Pharmacol. 2016;89:197–213. doi: 10.1124/mol.115.100792. [DOI] [PubMed] [Google Scholar]
- 32.Akbulut Y, Gaunt HJ, Muraki K, Ludlow MJ, Amer MS, Bruns A, Vasudev NS, Radtke L, Willot M, Hahn S, Seitz T, Ziegler S, Christmann M, Beech DJ, Waldmann H. (–)-Englerin A is a potent selective activator of TRPC4 and TRPC5 calcium channels. Angew Chem Int Ed. 2015;54:3787–3791. doi: 10.1002/anie.201411511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richter JM, Schaefer M, Hill K. Riluzole activates TRPC5 channels independently of PLC activity. Br J Pharmacol. 2014;171:158–170. doi: 10.1111/bph.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richter JM, Schaefer M, Hill K. Clemizole hydrochloride is a novel and potent inhibitor of transient receptor potential channel TRPC5. Mol Pharmacol. 2014;86:514–521. doi: 10.1124/mol.114.093229. [DOI] [PubMed] [Google Scholar]
- 35.Miller M, Shi J, Zhu Y, Kustov M, Tian JB, Stevens A, Wu M, Xu J, Long S, Yang P, Zholos AV, Salovich JM, Weaver CD, Hopkins CR, Lindsley CW, McManus O, Li M, Zhu MX. Identification of ML204, a novel potent antagonist that selectively modulates native TRPC4/C5 ion channels. J Biol Chem. 2011;286:33436–33446. doi: 10.1074/jbc.M111.274167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu Y, Lu Y, Qu C, Miller M, Tian J, Thakur DP, Zhu J, Deng Z, Hu X, Wu M, McManus OB, Li M, Hong X, Zhu MX, Luo HR. Identification and optimization of 2-amino-benzimidazole derivatives as novel inhibitors of TRPC4 and TRPC5 channels. Br J Pharmacol. 2015;172:3495–3509. doi: 10.1111/bph.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu HZ, Gu Q, Wang C, Colton CK, Tang J, Kinoshita-Kawada M, Lee LY, Wood JD, Zhu MX. 2-amino-ethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J Biol Chem. 2004;279:35741–35748. doi: 10.1074/jbc.M404164200. [DOI] [PubMed] [Google Scholar]
- 38.Harada H, Yamazaki H, Toyotomi Y, Tateishi H, Mine Y, Yoshida N, Kato S. Novel N-1-(1-Substituted 4-piperidinylmethyl)-4-piperidinyl]benzamides as potent colonic prokinetic agents. Bioorg Med Chem Lett. 2002;12:967–970. doi: 10.1016/s0960-894x(02)00060-4. [DOI] [PubMed] [Google Scholar]
- 39.Brooks DW, Lu LDL, Masamune S. C-Acylation under virtually neutral conditions. Angew Chem, Int Ed Engl. 1979;18:72–74. [Google Scholar]
- 40.Zhu MX, Tang J. TRPC channel interactions with calmodulin and IP3 receptors. Novartis Found Symp. 2004;258:44–58. [PubMed] [Google Scholar]
- 41.Shi J, Mori E, Mori Y, Mori M, Li J, Ito Y, Inoue R. Multiple regulation by calcium of murine homologues of transient receptor potential proteins TRPC6 and TRPC7 expressed in HEK293 cells. J Physiol. 2004;561:415–432. doi: 10.1113/jphysiol.2004.075051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sours S, Du J, Chu S, Ding M, Zhou XJ, Ma R. Expression of canonical transient receptor potential (TRPC) proteins in human glomerular mesangial cells. Am J Physiol Renal Physiol. 2006;290:1507–1515. doi: 10.1152/ajprenal.00268.2005. [DOI] [PubMed] [Google Scholar]
- 43.Shen B, Kwan HY, Ma X, Wong CO, Du J, Huang Y, Yao X. cAMP activates TRPC6 channels via the phosphatidylinositol 3-kinase (PI3K)-protein kinase B (PKB)-mitogen-activated protein kinase kinase (MEK)-ERK1/2 signaling pathway. J Biol Chem. 2011;286:19439–19445. doi: 10.1074/jbc.M110.210294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong X, Wang H, Zhu X, Ding M, Lv G, Zhang J, Wen M, Qu C, Zhu J, Hu X. Preparation of pyrazolo[1,5-a]pyrimidine derivatives as antitumor agents. CN 104292233A. Faming Zhuanli Shenqing. 2015 Jan 21;
- 45.Attardo G, Breining T, Courchesne M, Lamothe S, Lavallee JF, Nguyen D, Rej R, St-Denis Y, Wang W, Xu Y, Barbeau F, Lebeau E, Kraus J. Antineoplastic heteronapthoquinones. 5,736,523. US Patent. 1998 Apr 7;
- 46.Luo H, Hong X, Zhu X, Zhu J, Wu G, Deng Z, Zhu Y, Lu Y, Deng K, Qu C. Preparation of pyrazolopyrimidine compounds for treatment of glomerular diseases or myocardial hypertrophy. CN 103694242A. Faming Zhuanli Shenqing. 2014 Apr 2;
- 47.Brown GR, Hollinshead DM, Stokes ES, Waterson D, Clarke DS, Foubister AJ, Glossop SC, McTaggart F, Mirrlees DJ, Smith GJ, Wood R. A novel series of 4-piperidinopyridine and 4-piperidinopyrimidine inhibitors of 2, 3-oxidosqualene cyclase–lanosterol synthase. J Med Chem. 2000;43:4964–4972. doi: 10.1021/jm000139k. [DOI] [PubMed] [Google Scholar]
- 48.Davies D, Jones G, Markwell R, Pearson N. Preparation of N-(1,5-naphthyridin-4-yl)piperidine-4-carboxamide derivatives as antibacterial agents. 2003010138A2. PCT Int Appl WO. 2003 Feb 6;
- 49.Miller M, Wu M, Xu J, Weaver D, Li M, Zhu MX. High-throughput screening of TRPC channel ligands using cell-based assays. In: Zhu MX, editor. TRP Channels. CRC Press; Boca Raton, FL: 2011. pp. 1–20. [PubMed] [Google Scholar]
- 50.Hu H, Tian J, Zhu Y, Wang C, Xiao R, Herz JM, Wood JD, Zhu MX. Activation of TRPA1 channels by fenamate nonsteroidal anti-inflammatory drugs. Pfluegers Arch. 2010;459:579–592. doi: 10.1007/s00424-009-0749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X, Chen X, Wu D, Liu W, Wang J, Feng Z, Cai G, Fu B, Hong Q, Du J. Downregulation of connexin 43 expression by high glucose induces senescence in glomerular mesangial cells. J Am Soc Nephrol. 2006;17:1532–1542. doi: 10.1681/ASN.2005070776. [DOI] [PubMed] [Google Scholar]
- 52.Otsuguro K, Tang J, Tang Y, Xiao R, Freichel M, Tsvilovskyy V, Ito S, Flockerzi V, Zhu MX, Zholos AV. Isoform-specific inhibition of TRPC4 channel by phosphatidylinositol 4, 5-bisphosphate. J Biol Chem. 2008;283:10026–10036. doi: 10.1074/jbc.M707306200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen B, Cheng KT, Leung YK, Kwok YC, Kwan HY, Wong CO, Chen ZY, Huang Y, Yao X. Epinephrine-induced Ca2+ influx in vascular endothelial cells is mediated by CNGA2 channels. J Mol Cell Cardiol. 2008;45:437–445. doi: 10.1016/j.yjmcc.2008.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.