Summary
Since the development of vaccinia virus as a vaccine vector in 1984, the utility of numerous viruses in vaccination strategies has been explored. In recent years, key improvements to existing vectors such as those based on adenovirus have led to significant improvements in immunogenicity and efficacy. Furthermore, exciting new vectors that exploit viruses such as cytomegalovirus (CMV) and vesicular stomatitis virus (VSV) have emerged. Herein, we summarize these recent developments in viral vector technologies, focusing on novel vectors based on CMV, VSV, measles and modified adenovirus. We discuss the potential utility of these exciting approaches in eliciting protection against infectious diseases.
Keywords: memory, T cell, vaccination, viral
Introduction
Recombinant viral vectors represent promising vaccine platforms due to their ability to express heterologous antigens and induce antigen‐specific cellular immune responses in addition to robust antibody titres, without the need for exogenous adjuvants. Vaccinia virus was the first virus to be developed as a vaccine vector,1 and numerous others have since been explored as delivery vehicles for foreign immunogens. Here, we discuss a selection of novel vaccine vectors (Table 1) that have entered clinical trials recently and/or are forerunners for licensure. As a number of other viral vectors have already been comprehensively reviewed,2, 3, 4, 5, 6, 7 we will not discuss them further.
Table 1.
Viral vaccine vectors discussed in this review and their characteristics
| Vector | Type of virus | Cargo capacity (kb) | Predominant immune response | Clinical development stage |
|---|---|---|---|---|
| Cytomegalovirus | DNA | >6 | CD4+, CD8+ and antibodies | Phase I |
| Novel adenoviruses | DNA | 7 | CD8+ and antibodies | Phase II |
| Vesicular stomatitis virus | ‐ssRNA | 6 | Antibodies and some CD4+, CD8+ | Phase III |
| Measles virus | ‐ssRNA | >6 | CD4+ and antibodies | Phase I |
Cytomegalovirus
Human cytomegalovirus (HCMV) is a β‐herpesvirus with a large (236 kbp) DNA genome that establishes life‐long, usually asymptomatic, infection in healthy individuals. Significant interest in harnessing HCMV in vaccine vector development has stemmed from the observation that HCMV induces unusually large T‐cell responses (reviewed elsewhere8). Natural infection with HCMV elicits broad T‐cell responses directed to a vast array of antigens, with HCMV‐specific responses comprising ~10% of the entire CD4+ and CD8+ T‐cell memory compartments.9 Although HCMV employs numerous immune evasion strategies to avoid control by the host immune system (reviewed in refs 10, 11), HCMV‐specific T‐cell responses are long‐lived, with particularly high frequencies in elderly individuals.12 Human CMV‐specific T cells also maintain functionality during virus chronicity and readily produce multiple effector molecules (e.g. interferon‐γ, tumour necrosis factor‐α) upon stimulation13, 14 and control virus replication in vitro.15 Furthermore, experiments in the murine CMV (mCMV) model that recapitulates the accumulation of highly functional CMV‐specific T cells over time16, 17, 18 demonstrate that CMV infection triggers seeding of tissue‐resident memory T cells in peripheral, including mucosal, tissues.19, 20 Hence, although HCMV also induces substantial antibody responses upon infection,21 this virus represents a particularly exciting tool for inducing potent T‐cell immunity.
Human CMV exhibits several other properties attractive for viral vectors. CMV‐based vectors can be engineered to express multiple exogenous immunogens.22, 23 Human CMV also super‐infects HCMV‐immune hosts.24 The immunogenicity of CMV‐based vaccines was first investigated using recombinant mCMV‐based constructs. These induced accumulation of T cells reactive to peptides derived from heterologous antigens that, importantly, conferred protection from heterologous (vaccinia) viral challenge.25 Subsequently, induction of protective pathogen‐specific T‐cell responses by mCMV‐based vectors has been demonstrated in Ebola virus, herpes simplex virus and Mycobacterium tuberculosis challenge models,26, 27, 28, 29 although non‐specific induction of natural killer cell responses partially contributes to early anti‐mycobacterial activity of mCMV.28
Broad interest in HCMV‐based vaccines was triggered by encouraging data from rhesus CMV (RhCMV) ‐based vaccines engineered to express antigens from simian immunodeficiency virus (SIV). Using vectors expressing SIV Gag, Env and a Rev‐Tat‐Nef fusion protein, Picker and colleagues demonstrated vaccine‐induced protection from mucosal SIV challenge that was associated with the development of effector memory T‐cell responses.22, 23 Whereas RhCMV‐induced protection did not preclude SIV spread from mucosal sites of entry, progressive clearance of SIV in ~ 50% macaques was reported, providing evidence that CMV‐based vaccines may be exploited to induce protective anti‐HIV immunity.30, 31
Analysis of RhCMV vaccine‐induced T‐cell immunity revealed broad CD8+ T‐cell responses with an altered epitope hierarchy to responses induced by natural SIV infection. Intriguingly, RhCMV‐induced CD8+ T cells were restricted by MHC class II30 and HLA‐E32 rather than through classical MHC‐Ia restriction. This unique induction of CD8+ T‐cell responses was attributed to deletion of the Rh157.5/4 genes within the fibroblast‐adapted RhCMV vector.32 Rh157.5/4 are orthologues of the HCMV UL128/UL130 genes that encode components of the viral pentameric complex that is required for viral entry into non‐fibroblast cells.33 How deletion of these genes leads to the induction of unique T‐cell responses is unclear. Interestingly, in a phase I trial in humans using chimeric HCMV that lacked the pentameric complex, vaccine‐induced CD8+ T‐cell responses exhibited classical MHC restriction.34 This may indicate key biological differences between RhCMV and HCMV, or may reflect the incomplete understanding of how RhCMV vectors elicit their unusual responses. It will be important to define the mechanisms that underpin the induction of these unusual T‐cell responses, and to identify which responses are critical for protection, to generate human CMV vectors that are equally effective.
Cytomegalovirus‐based vectors are clearly exciting. However, HCMV is pathogenic, and attenuated vectors are necessary for translation into humans. In mice, temperature‐sensitive mCMV fails to induce robust virus‐specific CD8+ T‐cell memory,35 eliminating this strategy from exploration for vector attenuation. Encouragingly, however, spread‐deficient mCMV vectors lacking the surface glycoprotein L36 or the virion protein M9437 induce robust T‐cell immunity. Indeed, glycoprotein L‐deficient (ΔgL) mCMV induces circulating effector memory‐like CD8+ T‐cell responses, although the degree to which different mCMV‐specific responses are induced varies substantially.36 Whether variation reflects the differential dependence of mCMV‐specific CD8+ T cells on CD4+ T‐cell help38, 39, 40 is unclear. Importantly, we observed that gL deficiency substantially impairs the seeding of multiple epitope‐specific CD8+ T‐cell responses within peripheral tissues, and ΔgL mCMV‐induced CD8+ T cells exhibit sub‐optimal recall responsiveness (I.R. Humphreys, unpublished observation). Hence, a greater understanding of how to safely induce potent T‐cell responses using CMV‐based vectors will inform future strategies.
Studying CMV‐induced T‐cell immunity may also inform alternative vector‐based vaccine strategies. Experiments with mCMV have suggested that antigen expression rather than peptide‐intrinsic properties influence mCMV‐induced T‐cell expansions.29 Furthermore, C‐terminal localization of peptide in viral proteins greatly increases peptide availability for proteosomal processing and subsequent accumulation of protective peptide‐specific T‐cell memory.41 Interestingly, adenovirus‐based vectors engineered to express peptide mini‐genes can induce effector memory T‐cell accumulations indicative of CMV‐induced T‐cell immunity.42, 43 Hence, studies of CMV‐induced T‐cell responses may inform the development of alternative viral vector systems capable of inducing robust effector memory T‐cell responses.
Enhanced adenoviral vectors
Soon after their pioneering development as gene therapy vectors in the early 1990s, adenoviruses were also explored as vaccine vectors44 and have been used in numerous clinical vaccine trials since 2003.45 Hence, they are not considered novel vectors per se. However, in the past few years, several groups have made innovative improvements to adenoviral vectors that are worth exploring here because they are already, or have the potential to become, clinically relevant. The various enhancements broadly address two challenges: (i) overcoming pre‐existing anti‐vector immunity and (ii) enhancing vaccine‐induced antigen‐specific immunogenicity.
Adenoviruses are non‐enveloped icosahedral viruses with 30–40 kb linear DNA genomes, which can be genetically manipulated without difficulty. The antigen expression cassette is most often inserted into the E1 genomic locus, rendering the virus replication‐deficient. Vectors in which non‐essential E3 genes are also deleted can accommodate expression cassettes up to a size of 7 kb. Until recently, replication‐deficient AdHu5 was the most widely used adenovirus vector in vaccine development, because of its ability to elicit exceptionally strong CD8+ T‐cell and antibody responses, and the ability to generate high titres of virus during manufacturing. However, pre‐existing immunity against this vector, specifically neutralizing antibody titre, was shown to correlate with a reduction in antigen‐specific immunogenicity in clinical trials.46, 47 Many groups consequently explored adenoviruses with lower seroprevalence in the human population, such as different human adenovirus serotypes or simian adenoviruses. Interestingly, different serotypes were found to elicit different immunogenicity profiles in mice with respect to phenotype, function and longevity of the cellular immune response.48 For example, the human adenoviral vectors Ad26 and Ad35 induced enhanced memory CD8+ T cells and more polyfunctional CD8+ T cells compared with Ad5.48 These two alternative vectors (Ad26 and Ad35) have also been evaluated in clinical trials, with variable outcomes.49, 50, 51, 52 For example, Ad26 and Ad35 vectors containing the HIV‐1 env antigen were used in heterologous prime‐boost combinations in a Phase I trial.53 The authors found that the Ad26‐Ad35 prime‐boost elicited significantly higher antibody titres than the Ad35‐Ad26 regimen, but T‐cell responses were modest overall. Ad26 was also used as a priming vector in Ebola clinical trials, where together with a Modified Vaccinia virus Ankara (MVA) boost, vaccination was able to elicit a strong and durable antibody response to the Ebola virus antigen.54, 55 Ad35 has additionally been evaluated in several other HIV vaccine trials,51, 56, 57, 58 where it was demonstrated to be safe and immunogenic. Furthermore, in a tuberculosis vaccine trial it was found to be safe in both infants and HIV+/− adults. However, it only elicited a cellular immune response upon repeated high‐dose vaccinations.49
In addition to human adenoviruses, many simian adenovirus‐based vaccine vectors have been tested preclinically, and five have advanced to clinical studies to date,59, 60, 61 with promising results. A prominent example is ChAd3‐EBOZ, a chimpanzee adenoviral vector encoding the Ebola Zaire glycoprotein, which was evaluated in Phase I and II clinical trials in response to the recent Ebola epidemic.62, 63, 64 This vector was assessed with and without an MVA booster dose, and was found to elicit strong antibody and T‐cell responses, which could be increased in magnitude and durability by an MVA boost. Another chimpanzee adenoviral vector, ChAd63, has also been evaluated in several clinical trials (malaria,65, 66, 67 leishmaniasis68) with results showing excellent safety and immunogenicity, even in infants and children.
Improving transgene immunogenicity is also a significant focus of ongoing studies. One strategy to enhance the immune response against exogenous antigen is to increase immunogen production from the vaccine vector. This is difficult to achieve with replication‐deficient (E1‐deleted) adenovirus vectors, as antigen expression is restricted to the single copy of the vector genome present in the infected target cell. Increased antigen expression could be achieved by enabling the vector to self‐amplify in one additional round of genome replication after cell entry (so‐called single‐cycle adenoviruses), or by using replication‐competent adenoviruses. The former approach has been explored through deletion of a structural gene (pIIIa) from a replication‐competent adenovirus69 that renders the virus unable to spread. The subsequent virus expresses early viral genes and replicates its genome, producing ~ 30 to 100‐fold more copies of the antigen expression cassette than replication‐deficient vectors. Impressively, a single‐cycle adenovirus encoding influenza A haemagglutinin showed a significantly higher antibody induction than its replication‐deficient equivalent, even at a 10‐fold lower dose.70, 71 One drawback of this method, however, is the requirement for a trans‐complementing cell line (in this case expressing pIIIa) for the production of such a single‐cycle adenovirus, which may represent a bottleneck for clinical development.
To fully exploit the advantages of a self‐amplifying vaccine, several groups have also examined the use of replication‐competent adenovirus vectors, which were administered by varying mucosal routes in permissive species (mice are not permissive for human or simian adenoviruses.) Unfortunately, results with regard to induction of humoral immunity were mixed72, 73, 74 or disappointing.75, 76, 77 One beneficial feature of replication‐deficient adenoviral vectors is the fact that the transgene is typically immuno‐dominant by default, as the lack of adenoviral gene expression precludes immune competition. It is therefore likely that replication‐competent vectors can only be effective vaccines if the transgene remains immuno‐competent while competing with numerous other viral gene products; achieving this has been challenging (reviewed in ref. 78). Furthermore, replicating adenoviral vectors carry higher safety risks because of their ability to cause systemic infection in certain susceptible populations.79 However, although they are indeed not suitable for use in the severely immunocompromised, a live (oral) adenovirus vaccine used to protect against respiratory disease caused by Ad4 and Ad7 has nevertheless long been used in the United States army, with a very good safety profile.80
In addition to exploiting vector amplification to increase antigen expression, several other methods have been described that aim to enhance the immunogenicity of adenoviral vectors. One of these approaches, antigen capsid incorporation, has been particularly successful in preclinical studies (reviewed in ref. 81). Here, antigenic epitopes are incorporated into viral structural proteins in such a way that they are exposed on the virus surface and are therefore able to elicit robust antibody responses. Epitopes from a variety of different pathogens have been tested (e.g. HIV‐1,82 influenza A,83 Plasmodium falciparum 84). One group, for example, demonstrated that a multivalent HIV‐1 vector based on AdHu5 elicited antibody responses to an externally presented HIV‐1 B‐cell epitope, in addition to cellular response to the virally encoded HIV‐1 gag antigen.85 One clear disadvantage of this method is that only short heterologous sequences can be inserted into viral structural genes without affecting virus assembly or stability. As an exception, the adenoviral capsid protein pIX can accommodate C‐terminal fusions with larger antigens. However, these fusions can have a destabilizing effect on the virion.86 Overall, considering the large numbers of publications reporting encouraging results, it is surprising that (to our knowledge) the capsid‐display approach has not yet been evaluated in the clinic.
Vesicular stomatitis virus
The use of vesicular stomatitis virus (VSV) as a vaccine vector was pioneered by Rose and colleagues in the late 1990s,87 and the vector has since been employed in numerous preclinical studies. However, due to the challenges encountered in developing a sufficiently attenuated, safe VSV backbone, a first‐in‐human evaluation of an recombinant VSV (rVSV) vaccine did not take place until 2011.88 This was soon followed by clinical trials of an rVSV‐vectored Ebola vaccine (2014), which was the most advanced vaccine candidate in the recent Ebola virus epidemic in West Africa. Buoyed by this success, the VSV vector has been the subject of much interest by numerous investigators.
Vesicular stomatitis virus is an enveloped bullet‐shaped virus that belongs to the Rhabdovirus family and contains an 11‐kb negative‐sense RNA genome. Apart from its ability to induce robust cellular and humoral immunity against encoded transgenes, its high titre growth in validated cell lines (e.g. Vero) and the lack of a DNA intermediate during viral replication add to its attractiveness as a vaccine vector. However, owing to its negative‐sense RNA genome, rescue of recombinant virus from plasmid DNA is more challenging than rescue of DNA viruses, as it involves co‐transfection of five plasmids into a permissive cell line.89 The cargo capacity of rVSV vectors was found to be at least 4·5 kb,90 and its genomic structure conveniently allows insertion of transgenes at multiple sites, which will result in transgene expression at varying levels. Unlike adenoviral vectors (which are typically replication‐deficient), most VSV vaccine vectors are replication‐competent, albeit attenuated. Attenuation represents an important safety feature, as wild‐type VSV is neurovirulent upon intracranial inoculation.91 Attenuation of VSV can be achieved in several ways; the most prominent example combines down‐regulation of N protein expression with truncation of the VSV‐G cytoplasmic tail, resulting in the attenuated vector rVSVN4CT1, which has been approved for clinical studies.92
In the past 20 years, VSV vaccine vectors have been demonstrated to induce robust cellular and humoral immune responses in numerous preclinical studies, leading to protection in many animal models of pathogen challenge, frequently after a single vector administration (reviewed in ref. 93). Durability of a protective immune response (up to 1 year, so far) has also been demonstrated.94 Since 2011, rVSV vectors have been evaluated in four completed or ongoing clinical trials for HIV‐1,88, 95, 96, 97 and in Phase I, II and III trials for Ebola (reviewed in ref. 98). Of note, the Phase III trial that took place during the 2014/15 outbreak in Guinea showed promising efficacy in a ring‐vaccination strategy.99 Analysis of immunogenicity in these studies revealed a robust induction of neutralizing antibodies and a modest CD8+ response for all participants in the Ebola virus clinical trials,100 whereas the only report of an HIV‐1 trial was more disappointing, with modest CD4+ levels in two‐thirds of participants and low antigen‐specific antibody levels in one‐third of participants.88 Significant differences existed in the vector backbones used in these trials: rVSV‐HIV‐1 vectors were attenuated by genetic engineering as mentioned above (containing the rVSVN4CT1 backbone) and the HIV‐1 antigen coding sequence was placed in position 1 of the genome. In contrast, the rVSV‐EBOV clinical vector was simply based on the cell culture adapted VSV Indiana strain lacking VSV‐G (rVSVΔG), with the Ebola glycoprotein (EBOV‐GP) placed in position 4. In the absence of the native glycoprotein, EBOV‐GP acted as the vaccine antigen in addition to the viral entry protein. As the VSV glycoprotein is the main determinant of viral tropism, safety considerations for the rVSV‐EBOV vector are different from those for the rVSV‐HIV vectors. In initial Phase I trials, replication of the rVSV‐EBOV vector was detected in synovial fluid and skin lesions, most probably due to EBOV‐GP‐specific tissue tropism.101
In an effort to address safety concerns, more attenuated, second‐generation rVSV‐EBOV vectors were recently developed.102 Attenuation was achieved by employing the rVSVN4CT1 backbone, and reduced virus growth was demonstrated in cell culture.102 The vaccine was still protective in a non‐human primate Ebola virus challenge model after a single administration and, reassuringly, vaccination resulted in a 10‐fold to 50‐fold reduction in vaccine‐associated viraemia in the blood compared with first‐generation vectors in previous studies. Unfortunately, this study did not include a direct comparison with first‐generation rVSV‐ZEBOV. However, a Phase I trial of this vaccine (rVSVN4CT1‐EBOVGP1) was recently completed,103 and so insight into the safety profile of this vector will soon be available. In addition, GemEvac‐Combi, an Ebola virus vaccine containing an rVSV expressing the Ebola glycoprotein, has also been developed.104 This vaccine was evaluated for safety and immunogenicity in a 2015 Phase I trial and subsequently licensed by the Ministry of Health of the Russian Federation. However, it is difficult to assess the potential impact of this vaccine, considering the paucity of published preclinical data and the lack of information regarding vector construction. A Phase 4 study of this vaccine involving 2000 volunteers in Russia and Guinea is planned for 2017–2019.105
In parallel with ongoing clinical studies, VSV vectors have been modified to further improve utility to create multivalent vectors. Mire et al. generated trivalent rVSV encoding glycoproteins from Zaire Ebola virus, Sudan Ebola virus and Marburg virus, and demonstrated protection against all three virus strains in a guinea pig challenge model after a single immunization, even though antibody responses to each antigen differed in magnitude.106 Encouragingly, vectors containing ~ 6 kb transgenic cargo were generated, suggesting that multiple or large inserts can be incorporated into rVSV vectors. Another recent valuable observation regarding rVSV vector development is their ability to provide protection even after exposure to the pathogen. For example, full protection was shown when rhesus monkeys were vaccinated with an rVSV‐MARV vector 30 min after receiving a lethal dose of Marburg virus,107 and five of six animals were still protected when vaccinated 24 hr after challenge.108 T‐cell depletion had no impact on vaccine‐induced protection,109 suggesting that rapid induction of antibodies may underlie vaccine efficacy. Taken together, both preclinical and clinical studies suggest that the strengths of the VSV vector lie in its attenuated replicative capacity and its ability to elicit high and durable antibody levels to surface‐displayed antigens; characteristics that make it a promising vaccine vector for emerging or outbreak‐prone viral diseases.
Measles virus
Despite its pathogenicity, the development of measles virus (MV) as a vaccine vector was initiated in the late 1990s. This was based on the large success of the live attenuated measles vaccine itself. Measles vaccines were developed in the early 1960s by cell culture adaptation of wild‐type virus isolates, leading to attenuation through an accumulation of mutations. The most attenuated strains, still used today, have excellent safety profiles while still inducing extremely durable, protective antibody‐ and T‐cell‐mediated immunity in 95% of recipients after a single vaccination.110, 111, 112 Interestingly, T‐cell‐mediated responses to MV are predominantly of the CD4+ phenotype,113 unlike the CD8+ dominated response to adenoviral vectors,114 which may have important implications when considering these vector platforms for vaccine development.
A member of the Paramyxovirus family, MV is an enveloped spherical virus and contains a 16‐kb negative‐sense RNA genome. The development of reverse genetics tools and rescue of MV from cDNA in 1995115 accelerated both basic MV virology research and exploration of MV as a vaccine vector. Studies demonstrated a cargo capacity in excess of 6 kb and an excellent induction of humoral and cellular immune responses against encoded transgenes.116 In addition, MV was easily adaptable to large‐scale bio‐manufacture at low production cost. Recombinant MVs expressing one or more genes from heterologous pathogens have now been used in numerous preclinical vaccine studies (reviewed in ref. 117). One group, for example, generated an rMV expressing HIV‐1 Gag, RT and Nef as a fusion protein (MV1‐F4) and assessed immunogenicity of the vector in prime or prime‐boost regimens in cynomolgus macaques.118 Vaccination induced robust antigen‐specific CD4+ and modest CD8+ T‐cell responses, and high levels of antibody reactive to exogenous antigens and MV‐encoded proteins that were further amplified after boosting.118
During preclinical development, concerns arose regarding the impact of pre‐existing immunity against the MV vector, as the live attenuated measles vaccine is part of routine childhood immunization programmes in many countries. However, higher doses or alternative administration routes of the vector can overcome existing anti‐measles antibody levels in mice.119 Of note, in this study pre‐existing immunity was artificially modelled using intravenous administration of anti‐measles antibodies. However, another study that examined previous exposure to attenuated measles vaccine found no influence of existing anti‐MV immunity on transgene immunogenicity in mice or macaques.120 Another important consideration for the possible paediatric use of MV vectors is the requirement for the vector to retain vaccine competence against MV itself. This was demonstrated in a macaque model using an MV‐based hepatitis B vaccine candidate.121
After almost two decades of preclinical development, MV vaccine vectors have recently been advanced into clinical trials, with two Phase I studies completed (HIV‐1,122 Chikungunya virus (CHIKV)123) and two Phase II CHIKV trials ongoing or planned in Europe and Puerto Rico, respectively.124, 125 In the Phase I CHIKV study, volunteers received escalating priming doses followed by a booster dose of an rMV encoding the structural genes (C, E3, E2, 6K and E1) of CHIKV, a mosquito‐borne alphavirus of the tropics and sub‐tropics that is threatening to become a global public health burden. Protective immunity against CHIKV is antibody‐mediated in a mouse model.126 Encouragingly, seroconversion was demonstrated for 90% of participants in the high‐dose group after one immunization, and for all participants after the second vaccination. In addition, immunogenicity was not affected by pre‐existing anti‐measles immunity, an important finding that will hopefully be confirmed in larger ongoing studies.
Conclusions and future perspective
Viral vectors hold much promise for vaccine vector development to counter infectious diseases. Significant advances have been made regarding the production of immunogenic vectors that can be used in individuals with previous immunity to the viruses on which vectors are based. More detailed understanding of which immune responses are preferentially induced by vectors and how they are triggered will inform decisions as to which vectors are most relevant for vaccination against a specific infectious disease (Fig. 1). Safety considerations remain a significant challenge in the development of certain viral vectors. This is relevant not only for vaccination against infectious diseases, but also for the potential exploitation of virus‐based vectors in cancer vaccination strategies where individuals are often immune compromised. Hence, understanding better how to balance safety and immunogenicity will have broad implications for the management of infectious diseases and beyond.
Figure 1.
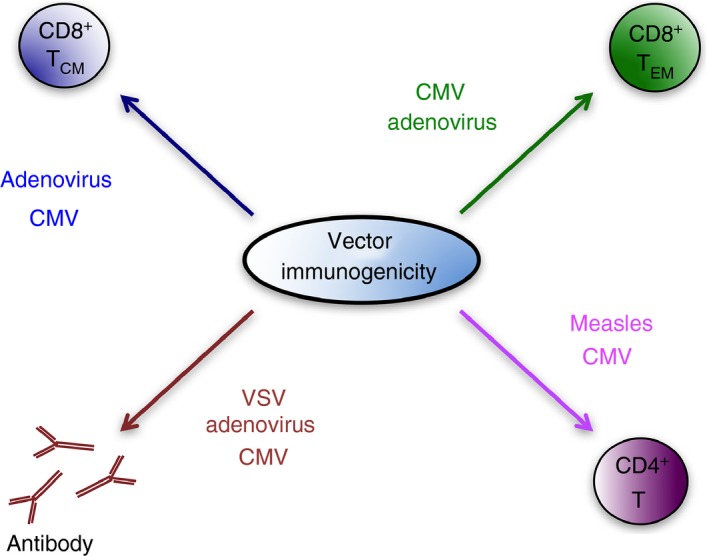
Viral vector‐induced immune responses. Schematic of relative induction of adaptive immune responses by different viral‐based vaccine vectors. Text size represents relative induction of adaptive immunity. CMV, cytomegalovirus; VSV, vesicular stomatitis virus.
Disclosures
The authors declare no competing interests.
Acknowledgements
The authors wish to thank Richard Stanton and Teresa Lambe for critical reading of the manuscript. Ian Humphreys is supported by a Wellcome Trust Senior Research Fellowship in Basic Biomedical Sciences.
Contributor Information
Ian R. Humphreys, Email: humphreysir@cf.ac.uk.
Sarah Sebastian, Email: sarah.sebastian@ndm.ox.ac.uk.
References
- 1. Moss B, Smith GL, Gerin JL, Purcell RH. Live recombinant vaccinia virus protects chimpanzees against hepatitis B. Nature 1984; 311:67–9. [DOI] [PubMed] [Google Scholar]
- 2. Ertl HC. Viral vectors as vaccine carriers. Curr Opin Virol 2016; 21:1–8. [DOI] [PubMed] [Google Scholar]
- 3. Dhanwani R, Ly H. Arenaviral vaccine vectors to combat infectious diseases. Oncotarget 2016; 7:44875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonaldo MC, Sequeira PC, Galler R. The yellow fever 17D virus as a platform for new live attenuated vaccines. Hum Vaccin Immunother 2014; 10:1256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lundstrom K. Alphavirus‐based vaccines. Viruses 2014; 6:2392–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hu B, Tai A, Wang P. Immunization delivered by lentiviral vectors for cancer and infectious diseases. Immunol Rev 2011; 239:45–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Volz A, Sutter G. Modified vaccinia virus Ankara: history, value in basic research, and current perspectives for vaccine development. Adv Virus Res 2017; 97:187–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klenerman P, Oxenius A. T cell responses to cytomegalovirus. Nat Rev Immunol 2016; 16:367–77. [DOI] [PubMed] [Google Scholar]
- 9. Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F et al Broadly targeted human cytomegalovirus‐specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med 2005; 202:673–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilkinson GW, Tomasec P, Stanton RJ, Armstrong M, Prod'homme V, Aicheler R et al Modulation of natural killer cells by human cytomegalovirus. J Clin Virol 2008; 41:206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jackson SE, Mason GM, Wills MR. Human cytomegalovirus immunity and immune evasion. Virus Res 2011; 157:151–60. [DOI] [PubMed] [Google Scholar]
- 12. Khan N, Shariff N, Cobbold M, Bruton R, Ainsworth JA, Sinclair AJ et al Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol 2002; 169:1984–92. [DOI] [PubMed] [Google Scholar]
- 13. Jackson SE, Sedikides GX, Mason GM, Okecha G, Wills MR. Human cytomegalovirus (HCMV)‐specific CD4+ T cells are polyfunctional and can respond to HCMV‐infected dendritic cells in vitro . J Virol 2017; 91:e02128–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lachmann R, Bajwa M, Vita S, Smith H, Cheek E, Akbar A et al Polyfunctional T cells accumulate in large human cytomegalovirus‐specific T cell responses. J Virol 2012; 86:1001–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jackson SE, Mason GM, Okecha G, Sissons JG, Wills MR. Diverse specificities, phenotypes, and antiviral activities of cytomegalovirus‐specific CD8+ T cells. J Virol 2014; 88:10894–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karrer U, Sierro S, Wagner M, Oxenius A, Hengel H, Koszinowski UH et al Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J Immunol 2003; 170:2022–9. [DOI] [PubMed] [Google Scholar]
- 17. Holtappels R, Pahl‐Seibert MF, Thomas D, Reddehase MJ. Enrichment of immediate‐early 1 (m123/pp89) peptide‐specific CD8 T cells in a pulmonary CD62Llo memory‐effector cell pool during latent murine cytomegalovirus infection of the lungs. J Virol 2000; 74:11495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sierro S, Rothkopf R, Klenerman P. Evolution of diverse antiviral CD8+ T cell populations after murine cytomegalovirus infection. Eur J Immunol 2005; 35:1113–23. [DOI] [PubMed] [Google Scholar]
- 19. Thom JT, Weber TC, Walton SM, Torti N, Oxenius A. The salivary gland acts as a sink for tissue‐resident memory CD8+ T cells, facilitating protection from local cytomegalovirus infection. Cell Rep 2015; 13:1125–36. [DOI] [PubMed] [Google Scholar]
- 20. Smith CJ, Caldeira‐Dantas S, Turula H, Snyder CM. Murine CMV infection induces the continuous production of mucosal resident T cells. Cell Rep 2015; 13:1137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gerna G, Lilleri D, Fornara C, Bruno F, Gabanti E, Cane I et al Differential kinetics of human cytomegalovirus load and antibody responses in primary infection of the immunocompetent and immunocompromised host. J Gen Virol 2015; 96:360–9. [DOI] [PubMed] [Google Scholar]
- 22. Hansen SG, Vieville C, Whizin N, Coyne‐Johnson L, Siess DC, Drummond DD et al Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med 2009; 15:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne‐Johnson L et al Profound early control of highly pathogenic SIV by an effector memory T‐cell vaccine. Nature 2011; 473:523–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N Engl J Med 2001; 344:1366–71. [DOI] [PubMed] [Google Scholar]
- 25. Karrer U, Wagner M, Sierro S, Oxenius A, Hengel H, Dumrese T et al Expansion of protective CD8+ T‐cell responses driven by recombinant cytomegaloviruses. J Virol 2004; 78:2255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsuda Y, Parkins CJ, Caposio P, Feldmann F, Botto S, Ball S et al A cytomegalovirus‐based vaccine provides long‐lasting protection against lethal Ebola virus challenge after a single dose. Vaccine 2015; 33:2261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsuda Y, Caposio P, Parkins CJ, Botto S, Messaoudi I, Cicin‐Sain L et al A replicating cytomegalovirus‐based vaccine encoding a single Ebola virus nucleoprotein CTL epitope confers protection against Ebola virus. PLoS Negl Trop Dis 2011; 5:e1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beverley PC, Ruzsics Z, Hey A, Hutchings C, Boos S, Bolinger B et al A novel murine cytomegalovirus vaccine vector protects against Mycobacterium tuberculosis . J Immunol 2014; 193:2306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dekhtiarenko I, Jarvis MA, Ruzsics Z, Cicin‐Sain L. The context of gene expression defines the immunodominance hierarchy of cytomegalovirus antigens. J Immunol 2013; 190:3399–409. [DOI] [PubMed] [Google Scholar]
- 30. Hansen SG, Sacha JB, Hughes CM, Ford JC, Burwitz BJ, Scholz I et al Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science 2013; 340:1237874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hansen SG, Piatak M Jr, Ventura AB, Hughes CM, Gilbride RM, Ford JC et al Immune clearance of highly pathogenic SIV infection. Nature 2013; 502:100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hansen SG, Wu HL, Burwitz BJ, Hughes CM, Hammond KB, Ventura AB et al Broadly targeted CD8+ T cell responses restricted by major histocompatibility complex E. Science 2016; 351:714–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lilja AE, Shenk T. Efficient replication of rhesus cytomegalovirus variants in multiple rhesus and human cell types. Proc Natl Acad Sci USA 2008; 105:19950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Murray SE, Nesterenko PA, Vanarsdall AL, Munks MW, Smart SM, Veziroglu EM et al Fibroblast‐adapted human CMV vaccines elicit predominantly conventional CD8 T cell responses in humans. J Exp Med 2017; 214:1889–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Beswick M, Pachnio A, Al‐Ali A, Sweet C, Moss PA. An attenuated temperature‐sensitive strain of cytomegalovirus (tsm5) establishes immunity without development of CD8+ T cell memory inflation. J Med Virol 2013; 85:1968–74. [DOI] [PubMed] [Google Scholar]
- 36. Snyder CM, Cho KS, Bonnett EL, Allan JE, Hill AB. Sustained CD8+ T cell memory inflation after infection with a single‐cycle cytomegalovirus. PLoS Pathog 2011; 7:e1002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mohr CA, Arapovic J, Muhlbach H, Panzer M, Weyn A, Dolken L et al A spread‐deficient cytomegalovirus for assessment of first‐target cells in vaccination. J Virol 2010; 84:7730–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Humphreys IR, Loewendorf A, de Trez C, Schneider K, Benedict CA, Munks MW et al OX40 costimulation promotes persistence of cytomegalovirus‐specific CD8 T cells: a CD4‐dependent mechanism. J Immunol 2007; 179:2195–202. [DOI] [PubMed] [Google Scholar]
- 39. Snyder CM, Loewendorf A, Bonnett EL, Croft M, Benedict CA, Hill AB. CD4+ T cell help has an epitope‐dependent impact on CD8+ T cell memory inflation during murine cytomegalovirus infection. J Immunol 2009; 183:3932–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Walton SM, Torti N, Mandaric S, Oxenius A. T‐cell help permits memory CD8+ T‐cell inflation during cytomegalovirus latency. Eur J Immunol 2011; 41:2248–59. [DOI] [PubMed] [Google Scholar]
- 41. Dekhtiarenko I, Ratts RB, Blatnik R, Lee LN, Fischer S, Borkner L et al Peptide processing is critical for T‐cell memory inflation and may be optimized to improve immune protection by CMV‐based vaccine vectors. PLoS Pathog 2016; 12:e1006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bolinger B, Sims S, O'Hara G, de Lara C, Tchilian E, Firner S et al A new model for CD8+ T cell memory inflation based upon a recombinant adenoviral vector. J Immunol 2013; 190:4162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Colston JM, Bolinger B, Cottingham MG, Gilbert S, Klenerman P. Modification of antigen impacts on memory quality after adenovirus vaccination. J Immunol 2016; 196:3354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tatsis N, Ertl HC. Adenoviruses as vaccine vectors. Mol Ther 2004; 10:616–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Priddy FH, Brown D, Kublin J, Monahan K, Wright DP, Lalezari J et al Safety and immunogenicity of a replication‐incompetent adenovirus type 5 HIV‐1 clade B gag/pol/nef vaccine in healthy adults. Clin Infect Dis 2008; 46:1769–81. [DOI] [PubMed] [Google Scholar]
- 46. Zak DE, Andersen‐Nissen E, Peterson ER, Sato A, Hamilton MK, Borgerding J et al Merck Ad5/HIV induces broad innate immune activation that predicts CD8+ T‐cell responses but is attenuated by preexisting Ad5 immunity. Proc Natl Acad Sci USA 2012; 109:E3503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pine SO, Kublin JG, Hammer SM, Borgerding J, Huang Y, Casimiro DR et al Pre‐existing adenovirus immunity modifies a complex mixed Th1 and Th2 cytokine response to an Ad5/HIV‐1 vaccine candidate in humans. PLoS ONE 2011; 6:e18526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tan WG, Jin HT, West EE, Penaloza‐MacMaster P, Wieland A, Zilliox MJ et al Comparative analysis of simian immunodeficiency virus gag‐specific effector and memory CD8+ T cells induced by different adenovirus vectors. J Virol 2013; 87:1359–72.23175355 [Google Scholar]
- 49. Tameris M, Hokey DA, Nduba V, Sacarlal J, Laher F, Kiringa G et al A double‐blind, randomised, placebo‐controlled, dose‐finding trial of the novel tuberculosis vaccine AERAS‐402, an adenovirus‐vectored fusion protein, in healthy, BCG‐vaccinated infants. Vaccine 2015; 33:2944–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ouedraogo A, Tiono AB, Kargougou D, Yaro JB, Ouedraogo E, Kabore Y et al A phase 1b randomized, controlled, double‐blinded dosage‐escalation trial to evaluate the safety, reactogenicity and immunogenicity of an adenovirus type 35 based circumsporozoite malaria vaccine in Burkinabe healthy adults 18 to 45 years of age. PLoS ONE 2013; 8:e78679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Keefer MC, Gilmour J, Hayes P, Gill D, Kopycinski J, Cheeseman H et al A phase I double blind, placebo‐controlled, randomized study of a multigenic HIV‐1 adenovirus subtype 35 vector vaccine in healthy uninfected adults. PLoS ONE 2012; 7:e41936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Baden LR, Walsh SR, Seaman MS, Tucker RP, Krause KH, Patel A et al First‐in‐human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV‐1 Env vaccine (IPCAVD 001). J Infect Dis 2013; 207:240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Baden LR, Karita E, Mutua G, Bekker LG, Gray G, Page‐Shipp L et al Assessment of the safety and immunogenicity of 2 novel vaccine platforms for HIV‐1 prevention: a randomized trial. Ann Intern Med 2016; 164:313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Milligan ID, Gibani MM, Sewell R, Clutterbuck EA, Campbell D, Plested E et al Safety and immunogenicity of novel adenovirus type 26‐ and modified vaccinia Ankara‐vectored Ebola vaccines: a randomized clinical trial. JAMA 2016; 315:1610–23. [DOI] [PubMed] [Google Scholar]
- 55. Shukarev G, Callendret B, Luhn K, Douoguih M, Consortium E . A two‐dose heterologous prime‐boost vaccine regimen eliciting sustained immune responses to Ebola Zaire could support a preventive strategy for future outbreaks. Hum Vaccin Immunother 2017; 13:266–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fuchs JD, Bart PA, Frahm N, Morgan C, Gilbert PB, Kochar N et al Safety and immunogenicity of a recombinant adenovirus serotype 35‐vectored HIV‐1 vaccine in adenovirus serotype 5 seronegative and seropositive individuals. J AIDS Clin Res 2015; 6:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nyombayire J, Anzala O, Gazzard B, Karita E, Bergin P, Hayes P et al First‐in‐human evaluation of the safety and immunogenicity of an intranasally administered replication‐competent Sendai virus‐vectored HIV type 1 gag vaccine: induction of potent T‐cell or antibody responses in prime‐boost regimens. J Infect Dis 2017; 215:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mpendo J, Mutua G, Nyombayire J, Ingabire R, Nanvubya A, Anzala O et al A phase I double blind, placebo‐controlled, randomized study of the safety and immunogenicity of electroporated HIV DNA with or without Interleukin 12 in prime‐boost combinations with an Ad35 HIV vaccine in healthy HIV‐seronegative African adults. PLoS ONE 2015; 10:e0134287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Colloca S, Barnes E, Folgori A, Ammendola V, Capone S, Cirillo A et al Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci Transl Med 2012; 4:115ra2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Morris SJ, Sebastian S, Spencer AJ, Gilbert SC. Simian adenoviruses as vaccine vectors. Future Virol. 2016; 11:649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. A study to determine the safety and immunogenicity of a candidate MAP vaccine ChAdOx2 HAV in healthy adult volunteers. ClinicalTrials.gov/NCT03027193.
- 62. Ewer K, Rampling T, Venkatraman N, Bowyer G, Wright D, Lambe T et al A monovalent Chimpanzee adenovirus Ebola vaccine boosted with MVA. N Engl J Med 2016; 374:1635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ledgerwood JE, DeZure AD, Stanley DA, Coates EE, Novik L, Enama ME et al Chimpanzee adenovirus vector Ebola vaccine. N Engl J Med 2017; 376:928–38. [DOI] [PubMed] [Google Scholar]
- 64. Tapia MD, Sow SO, Lyke KE, Haidara FC, Diallo F, Doumbia M et al Use of ChAd3‐EBO‐Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA‐BN‐Filo: a phase 1, single‐blind, randomised trial, a phase 1b, open‐label and double‐blind, dose‐escalation trial, and a nested, randomised, double‐blind, placebo‐controlled trial. Lancet Infect Dis 2016; 16:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. O'Hara GA, Duncan CJ, Ewer KJ, Collins KA, Elias SC, Halstead FD et al Clinical assessment of a recombinant simian adenovirus ChAd63: a potent new vaccine vector. J Infect Dis 2012; 205:772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mensah VA, Gueye A, Ndiaye M, Edwards NJ, Wright D, Anagnostou NA et al Safety, immunogenicity and efficacy of prime‐boost vaccination with ChAd63 and MVA encoding ME‐TRAP against Plasmodium falciparum infection in adults in Senegal. PLoS ONE 2016; 11:e0167951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Afolabi MO, Tiono AB, Adetifa UJ, Yaro JB, Drammeh A, Nebie I et al Safety and immunogenicity of ChAd63 and MVA ME‐TRAP in West African children and infants. Mol Ther 2016; 24:1470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Osman M, Mistry A, Keding A, Gabe R, Cook E, Forrester S et al A third generation vaccine for human visceral leishmaniasis and post Kala Azar dermal leishmaniasis: first‐in‐human trial of ChAd63‐KH. PLoS Negl Trop Dis 2017; 11:e0005527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Crosby CM, Barry MA. IIIa deleted adenovirus as a single‐cycle genome replicating vector. Virology 2014; 462–463:158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Crosby CM, Matchett WE, Anguiano‐Zarate SS, Parks CA, Weaver EA, Pease LR et al Replicating single‐cycle adenovirus vectors generate amplified influenza vaccine responses. J Virol 2017; 91:e00720–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Crosby CM, Nehete P, Sastry KJ, Barry MA. Amplified and persistent immune responses generated by single‐cycle replicating adenovirus vaccines. J Virol 2015; 89:669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Karen KA, Deal C, Adams RJ, Nielsen C, Ward C, Espinosa DA et al A replicating adenovirus capsid display recombinant elicits antibodies against Plasmodium falciparum sporozoites in Aotus nancymaae monkeys. Infect Immun 2015; 83:268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Thomas MA, Song R, Demberg T, Vargas‐Inchaustegui DA, Venzon D, Robert‐Guroff M. Effects of the deletion of early region 4 (E4) open reading frame 1 (orf1), orf1‐2, orf1‐3 and orf1‐4 on virus–host cell interaction, transgene expression, and immunogenicity of replicating adenovirus HIV vaccine vectors. PLoS ONE 2013; 8:e76344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Thomas MA, Nyanhete T, Tuero I, Venzon D, Robert‐Guroff M. Beyond oncolytics: E1B55K‐deleted adenovirus as a vaccine delivery vector. PLoS ONE 2016; 11:e0158505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Berg MG, Adams RJ, Gambhira R, Siracusa MC, Scott AL, Roden RB et al Immune responses in macaques to a prototype recombinant adenovirus live oral human papillomavirus 16 vaccine. Clin Vaccine Immunol 2014; 21:1224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gurwith M, Lock M, Taylor EM, Ishioka G, Alexander J, Mayall T et al Safety and immunogenicity of an oral, replicating adenovirus serotype 4 vector vaccine for H5N1 influenza: a randomised, double‐blind, placebo‐controlled, phase 1 study. Lancet Infect Dis 2013; 13:238–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Buge SL, Richardson E, Alipanah S, Markham P, Cheng S, Kalyan N et al An adenovirus‐simian immunodeficiency virus env vaccine elicits humoral, cellular, and mucosal immune responses in rhesus macaques and decreases viral burden following vaginal challenge. J Virol 1997; 71:8531–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fougeroux C, Holst PJ. Future prospects for the development of cost‐effective adenovirus vaccines. Int J Mol Sci 2017; 18:E686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Munoz FM, Piedra PA, Demmler GJ. Disseminated adenovirus disease in immunocompromised and immunocompetent children. Clin Infect Dis 1998; 27:1194–200. [DOI] [PubMed] [Google Scholar]
- 80. Kuschner RA, Russell KL, Abuja M, Bauer KM, Faix DJ, Hait H et al A phase 3, randomized, double‐blind, placebo‐controlled study of the safety and efficacy of the live, oral adenovirus type 4 and type 7 vaccine, in U.S. military recruits. Vaccine 2013; 31:2963–71. [DOI] [PubMed] [Google Scholar]
- 81. Matthews QL. Capsid‐incorporation of antigens into adenovirus capsid proteins for a vaccine approach. Mol Pharm 2011; 8:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gu L, Li ZC, Krendelchtchikov A, Krendelchtchikova V, Wu H, Matthews QL. Using multivalent adenoviral vectors for HIV vaccination. PLoS ONE 2013; 8:e60347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tang X, Yang Y, Xia X, Zhang C, Yang X, Song Y et al Recombinant adenoviruses displaying matrix 2 ectodomain epitopes on their fiber proteins as universal influenza vaccines. J Virol 2017; 91:e02462–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Palma C, Vendetti S, Cassone A. Role of 4‐1BB receptor in the control played by CD8+ T cells on IFN‐γ production by Mycobacterium tuberculosis antigen‐specific CD4+ T cells. PLoS ONE 2010; 5:e11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Matthews QL, Fatima A, Tang Y, Perry BA, Tsuruta Y, Komarova S et al HIV antigen incorporation within adenovirus hexon hypervariable 2 for a novel HIV vaccine approach. PLoS ONE 2010; 5:e11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Salisch NC, Vujadinovic M, van der Helm E, Spek D, Vorthoren L, Serroyen J et al Antigen capsid‐display on human adenovirus 35 via pIX fusion is a potent vaccine platform. PLoS ONE 2017; 12:e0174728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Roberts A, Kretzschmar E, Perkins AS, Forman J, Price R, Buonocore L et al Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J Virol 1998; 72:4704–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fuchs JD, Frank I, Elizaga ML, Allen M, Frahm N, Kochar N et al First‐in‐human evaluation of the safety and immunogenicity of a recombinant vesicular stomatitis virus human immunodeficiency virus‐1 gag vaccine (HVTN 090). Open Forum Infect Dis 2015; 2:ofv082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Witko SE, Kotash CS, Nowak RM, Johnson JE, Boutilier LA, Melville KJ et al An efficient helper‐virus‐free method for rescue of recombinant paramyxoviruses and rhadoviruses from a cell line suitable for vaccine development. J Virol Methods 2006; 135:91–101. [DOI] [PubMed] [Google Scholar]
- 90. Haglund K, Forman J, Krausslich HG, Rose JK. Expression of human immunodeficiency virus type 1 Gag protein precursor and envelope proteins from a vesicular stomatitis virus recombinant: high‐level production of virus‐like particles containing HIV envelope. Virology 2000; 268:112–21. [DOI] [PubMed] [Google Scholar]
- 91. Johnson JE, Nasar F, Coleman JW, Price RE, Javadian A, Draper K et al Neurovirulence properties of recombinant vesicular stomatitis virus vectors in non‐human primates. Virology 2007; 360:36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cooper D, Wright KJ, Calderon PC, Guo M, Nasar F, Johnson JE et al Attenuation of recombinant vesicular stomatitis virus‐human immunodeficiency virus type 1 vaccine vectors by gene translocations and g gene truncation reduces neurovirulence and enhances immunogenicity in mice. J Virol 2008; 82:207–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rose JK, Clarke DK. Rhabdoviruses as vaccine vectors: from initial development to clinical trials In: Pattnaik AK, Whitt MA, eds. Biology and Pathogenesis of Rhabdo‐ and Filoviruses. Singapore: World Scientific, 2005:199–230. [Google Scholar]
- 94. Mire CE, Geisbert JB, Agans KN, Satterfield BA, Versteeg KM, Fritz EA et al Durability of a vesicular stomatitis virus‐based Marburg virus vaccine in nonhuman primates. PLoS ONE 2014; 9:e94355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Evaluating the safety of and immune response to HIV‐MAG DNA vaccine with or without plasmid IL‐12 adjuvant delivered intramuscularly via electroporation followed by VSV‐gag HIV vaccine boost in healthy, HIV‐uninfected adults. ClinicalTrials.gov/NCT01578889.
- 96. Therapeutic vaccine for HIV. ClinicalTrials.gov/NCT01859325.
- 97. Evaluating the safety, tolerability, and immunogenicity of a prime‐boost regimen of HIV‐1 Nef/Tat/Vif, Env pDNA vaccine delivered intramuscularly with electroporation and HIV‐1 rVSV envC vaccine in healthy, HIV‐uninfected adults. ClinicalTrials.gov/NCT02654080.
- 98. Mire CE, Geisbert TW, Feldmann H, Marzi A. Ebola virus vaccines – reality or fiction? Expert Rev Vaccines 2016; 15:1421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Henao‐Restrepo AM, Camacho A, Longini IM, Watson CH, Edmunds WJ, Egger M et al Efficacy and effectiveness of an rVSV‐vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open‐label, cluster‐randomised trial (Ebola Ca Suffit!). Lancet 2017; 389:505–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Dahlke C, Kasonta R, Lunemann S, Krahling V, Zinser ME, Biedenkopf N et al Dose‐dependent T‐cell dynamics and cytokine cascade following rVSV‐ZEBOV immunization. EBioMedicine 2017; 19:107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Huttner A, Dayer JA, Yerly S, Combescure C, Auderset F, Desmeules J et al The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double‐blind, placebo‐controlled phase 1/2 trial. Lancet Infect Dis 2015; 15:1156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mire CE, Matassov D, Geisbert JB, Latham TE, Agans KN, Xu R et al Single‐dose attenuated Vesiculovax vaccines protect primates against Ebola Makona virus. Nature 2015; 520:688–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Trial to evaluate safety and immunogenicity of an ebola zaire vaccine in healthy adults. ClinicalTrials.gov/NCT02718469.
- 104. Dolzhikova IV, Zubkova OV, Tukhvatulin AI, Dzharullaeva AS, Tukhvatulina NM, Shcheblyakov DV et al Safety and immunogenicity of GamEvac‐Combi, a heterologous VSV‐ and Ad5‐vectored Ebola vaccine: an open phase I/II trial in healthy adults in Russia. Hum Vaccin Immunother 2017; 13:613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. International Multicenter Study of the Immunogenicity of Medicinal Product GamEvac‐Combi. ClinicalTrials.gov/NCT03072030.
- 106. Mire CE, Geisbert JB, Versteeg KM, Mamaeva N, Agans KN, Geisbert TW et al A single‐vector, single‐injection trivalent filovirus vaccine: proof of concept study in outbred guinea pigs. J Infect Dis 2015; 212(Suppl 2):S384–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Daddario‐DiCaprio KM, Geisbert TW, Stroher U, Geisbert JB, Grolla A, Fritz EA et al Postexposure protection against Marburg haemorrhagic fever with recombinant vesicular stomatitis virus vectors in non‐human primates: an efficacy assessment. Lancet 2006; 367:1399–404. [DOI] [PubMed] [Google Scholar]
- 108. Geisbert TW, Hensley LE, Geisbert JB, Leung A, Johnson JC, Grolla A et al Postexposure treatment of Marburg virus infection. Emerg Infect Dis 2010; 16:1119–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Marzi A, Engelmann F, Feldmann F, Haberthur K, Shupert WL, Brining D et al Antibodies are necessary for rVSV/ZEBOV‐GP‐mediated protection against lethal Ebola virus challenge in nonhuman primates. Proc Natl Acad Sci USA 2013; 110:1893–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Jacobson RM, Ovsyannikova IG, Vierkant RA, Pankratz VS, Poland GA. Independence of measles‐specific humoral and cellular immune responses to vaccination. Hum Immunol 2012; 73:474–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Dai B, Chen ZH, Liu QC, Wu T, Guo CY, Wang XZ et al Duration of immunity following immunization with live measles vaccine: 15 years of observation in Zhejiang Province, China. Bull World Health Organ 1991; 69:415–23. [PMC free article] [PubMed] [Google Scholar]
- 112. Ovsyannikova IG, Dhiman N, Jacobson RM, Vierkant RA, Poland GA. Frequency of measles virus‐specific CD4+ and CD8+ T cells in subjects seronegative or highly seropositive for measles vaccine. Clin Diagn Lab Immunol 2003; 10:411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Howe RC, Dhiman N, Ovsyannikova IG, Poland GA. Induction of CD4 T cell proliferation and in vitro Th1‐like cytokine responses to measles virus. Clin Exp Immunol 2005; 140:333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Tatsis N, Fitzgerald JC, Reyes‐Sandoval A, Harris‐McCoy KC, Hensley SE, Zhou D et al Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines. Blood 2007; 110:1916–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Radecke F, Spielhofer P, Schneider H, Kaelin K, Huber M, Dotsch C et al Rescue of measles viruses from cloned DNA. EMBO J 1995; 14:5773–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zuniga A, Wang Z, Liniger M, Hangartner L, Caballero M, Pavlovic J et al Attenuated measles virus as a vaccine vector. Vaccine 2007; 25:2974–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Naim HY. Measles virus. Hum Vaccin Immunother 2015; 11:21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Stebbings R, Li B, Lorin C, Koutsoukos M, Fevrier M, Mee ET et al Immunogenicity of a recombinant measles HIV‐1 subtype C vaccine. Vaccine 2013; 31:6079–86. [DOI] [PubMed] [Google Scholar]
- 119. Knuchel MC, Marty RR, Morin TN, Ilter O, Zuniga A, Naim HY. Relevance of a pre‐existing measles immunity prior immunization with a recombinant measles virus vector. Hum Vaccin Immunother 2013; 9:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Lorin C, Mollet L, Delebecque F, Combredet C, Hurtrel B, Charneau P et al A single injection of recombinant measles virus vaccines expressing human immunodeficiency virus (HIV) type 1 clade B envelope glycoproteins induces neutralizing antibodies and cellular immune responses to HIV. J Virol 2004; 78:146–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. del Valle JR, Devaux P, Hodge G, Wegner NJ, McChesney MB, Cattaneo R. A vectored measles virus induces hepatitis B surface antigen antibodies while protecting macaques against measles virus challenge. J Virol 2007; 81:10597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Study to evaluate the dosage and safety of two intramuscular injections of an investigational Clade B HIV Vaccine. ClinicalTrials.gov/NCT01320176.
- 123. Ramsauer K, Schwameis M, Firbas C, Mullner M, Putnak RJ, Thomas SJ et al Immunogenicity, safety, and tolerability of a recombinant measles‐virus‐based Chikungunya vaccine: a randomised, double‐blind, placebo‐controlled, active‐comparator, first‐in‐man trial. Lancet Infect Dis 2015; 15:519–27. [DOI] [PubMed] [Google Scholar]
- 124. Phase II study to evaluate safety and immunogenicity of a Chikungunya vaccine. ClinicalTrials.gov/NCT02861586.
- 125. Study of a live attenuated Chikungunya vaccine in a previously epidemic area. ClinicalTrials.gov/NCT03101111.
- 126. Fric J, Bertin‐Maghit S, Wang CI, Nardin A, Warter L. Use of human monoclonal antibodies to treat Chikungunya virus infection. J Infect Dis 2013; 207:319–22. [DOI] [PubMed] [Google Scholar]


