Abstract
Previously, we reported that MyoD, a master gene for myogenic cells, could efficiently convert primary skin fibroblasts into myoblasts and myotubes, thereby effecting direct reprogramming. In this study, we further demonstrated that MyoD-expressing primary fibroblasts displayed rapid movement in culture, with a movement velocity that was significantly faster, almost four times, than mouse primary myoblasts. MyoD-transduced cells obtained the characteristics of Ca2 + release and electrically-stimulated contraction, which was comparable to C2C12 myotubes, suggesting that the essential features of muscle were observed in the transduced cells. Furthermore, the ability to fuse to the host myoblasts means that gene transfer from MyoD-transduced cells to host muscle cells could be obtained by cell fusion. In comparison with the iPS method (indirect reprogramming), our transduction method has a low risk for tumorigenesis and carcinogenesis because the starting cells are fibroblasts and the transduced cells are myoblasts, both normal and mortal cells. Accordingly, MyoD transduction of human skin fibroblasts using the adenoviral vector is a simple, inexpensive and promising candidate as a new cell transplantation therapy for patients with muscular disorders.
Keywords: MyoD, Adenovirus, Direct reprograming, Contraction, Migration, Fusion
Highlights
-
•
Adenoviral MyoD vector transduced fibroblasts directly to myoblasts.
-
•
Myoblast cells were well differentiated into functional muscle cells.
-
•
Direct reprogramming is cost-effective and safe compared to iPS method.
-
•
Cell fusion and high motility were observed in MyoD-transduced cells.
-
•
Transduced cells are candidates for cell transplantation in muscle disorders.
1. Introduction
Duchenne muscular dystrophies (DMD) is X-linked recessive disorder caused by loss of function with a mutation in the dystrophin gene. Although one of fundamental treatments for DMD is muscle transplantation, a clinically significant transplantation requires plenty of muscle cells from the donor, which do not have high proliferation potency in culture. As a solution, we chose skin fibroblast, which is known to be easily obtained and have a high proliferation potency especially in young child. In addition, direct reprogramming from skin fibroblasts to muscle cells is previously reported [1].
For many hereditary and refractory diseases, treatment strategies using iPS cell reprogramming have been explored worldwide. iPS cells have the potential to grow indefinitely, which is beneficial for obtaining sufficient amounts of cells for use in clinical treatment. However, the drawback of immortalization is the possibility of inducing carcinogenesis in culture and in the body. In 1987, cellular reprogramming was utilized in an attempt to generate myoblast cells from fibroblasts using the MyoD gene [2]; a strategy now called “direct reprogramming” as compared to “indirect reprogramming” through the use of iPS cells. The advantages (pluripotency and permanent cell growth) and disadvantages (possibility of carcinogenicity) of the iPS reprogramming method are two sides of the same coin.
Similarly, gene transfer using viral vectors can be broadly classified into two methods, transient and stable expression, each of which has advantages and disadvantages. Adenovirus vectors have the advantage of achieving high titers and consequently a high percentage of transduction and reprogramming. In our previous report, transient expression of the MyoD gene enabled myogenesis from skin fibroblasts. We found that transferring the MyoD gene into skin fibroblasts using an adenoviral vector (Ad.CAGMyoD) generated myoblast morphology and gene expression, and the development of myotubes in culture [1].
In this study, we examined whether Ad.CAGMyoD-induced myogenesis produced functional characteristics of muscle cells (contraction and intracellular Ca2 + release) in vitro. Furthermore, the directly reprogrammed cells were tested for motility and the capability to fuse with host myoblasts, which are vital factors in transplantation as gene transfer to host muscle cells from donor cells is desirable.
2. Materials and methods
2.1. Cell culture
Human primary fibroblast cells were obtained from Cell Systems Corporation (Kirkland, WA, USA). Primary mouse myoblasts were isolated from the hind limbs of 6-week-old C57BL/10J mice. Animal experiments were carried out according to the guidelines of the Laboratory Protocol for Animal Handling, Sojo University Faculty of Pharmaceutical Sciences. The C2C12 mouse myoblast cell line was obtained from ATCC (Manassas, VA, USA). All cell types were grown in high glucose Dulbecco's modified Eagles medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin-streptomycin, and 1% GlutaMax supplement (Invitrogen, Tokyo, Japan) at 37 °C under humidified 5% (v/v) CO2.
2.2. Adenoviral vectors
We constructed five adenoviral vectors. Among these, the Ad.CAGMyoD and control Ad.CAGEGFP vectors were previously described [1], [3]. The Ad.CAG-MyoD-ires-EGFP (Ad.CAGMiG) vector was generated from Ad.CAGMyoD by adding an internal ribosome entry site (IRES) linked to the enhanced green fluorescence protein (EGFP) reporter gene. The Ad.CAG-MyoD-ires-Cherry (Ad.CAGMiC) and Ad.CAGCherry vectors were generated from Ad.CAGMiG and Ad.CAGEGFP by replacing EGFP with the red fluorescent protein Cherry. The organization of the adenovirus vectors is depicted in Fig. S1. The expression units were inserted into the E1 region of E1-E3-deleted human adenovirus type 5. Each vector was amplified in HEK-293 cells (ATCC) and was purified using double CsCl gradient ultra-centrifugation [4]. The biological titer for each adenoviral vector is shown in Table 1A.
Table 1A.
Table of Adenoviral vectors.
| Adenovirus vector | Titer |
|---|---|
| Ad.CAG-MyoD | 1.0 × 109 PFU/ml |
| Ad.CAG-MyoD-ires-EGFP | 1.0 × 109 PFU/ml |
| Ad.CAG-EGFP | 1.0 × 109 PFU/ml |
| Ad.CAG-MyoD-ires-Cherry | 2.5 × 109 PFU/ml |
| Ad.CAG-Cherry | 1.0 × 109 PFU/ml |
2.3. Adenoviral transduction
Human fibroblasts were seeded at a density of 7 × 105 cells/well in 4-well plates or 2 × 105 cells/well in 12-well plates coated with matrigel before adenoviral transduction. Cells were transduced with adenoviral vectors at multiplicities of infection (MOI) of 10–30 for 1 h in 2% FBS-DMEM (Dulbecco's Modified Eagle's Medium supplemented with 2% fetal bovine serum and 1% penicillin-streptomycin). The medium containing the virus vector was removed and the cells were washed twice with fresh 10% FBS-DMEM. Subsequently, the cells were cultured for 2 days in 10% FBS-DMEM. To induce differentiation, the culture medium was replaced with differentiation medium (2% FBS-DMEM) (day0 in this experiment).
2.4. RNA isolation and reverse transcription
Total RNA was isolated using Nucleo Spin RNA® (Takara, Tokyo, Japan) according to the manufacturer's instructions at day-2 (no virus vector), 0, 3, 5, 8, 12 after replacing the differentiation medium. Reverse transcription was performed using a PrimeScript® RT reagent Kit (Perfect Real Time) (Takara). PCR was performed with Takara Ex Taq® (Takara). RT-PCR conditions and primers for each gene are shown in Table 1B.
Table 1B.
Sequences of primers used for mMyoD, hMyoD, hCK-M, hMyogenin, hMHC, hDystrophin, hMyomaker.
| Gene name | Number cycle | Ann. temp. | Sequence |
|---|---|---|---|
| hβactin | 30 | 55 | CTCTTCCAGCCTTCCTTCCT |
| CACCTTCACCGTTCCAGTTT | |||
| mMyoD | 30 | 55 | CTTCTATGACCCGTGTTTCGAC |
| CTGGGTTCCCTGTTCTGTGT | |||
| hMyoD | 30 | 61 | CACTCCGGTCCCAAATGTAG |
| TTCCCTGTAGCACCACACAC | |||
| hCK-M | 30 | 55 | ACATGGCCAAGGTACTGACC |
| TGATGGGGTCAAAGAGTTCC | |||
| hMyogenin | 30 | 64 | TAAGGTGTGTAAGGGAAGTCG |
| CCACAGACACATCTTCCACTGT | |||
| hMHC | 30 | 61 | CTGCTGAAGGAGAGGGAGCT |
| TGATTAGCTGGTCACACCTT | |||
| hDystrophin | 30 | 61 | GATGCACGAATGGATGACAC |
| TGTGCTACAGGTGGAGCTTG | |||
| hMyomaker | 30 | 61 | GAAGGAGAAGAAGGGCCTGT |
| CCTTCTTGTTGACCTTGGGC |
2.5. Ca2 + measurement
Calcium imaging was carried out using Fluo4-AM special packaging® (Dojindo, Kumamoto, Japan) according to the manufacturer's protocol. To measure intracellular Ca2 +, cells were loaded with Fluo4-AM at 37 °C for 1 h, washed, and changed to recording medium (Fluo4-AM special packaging® kit) for cellular analysis. For each culture, one of the following reagents was added 10 s after the start of observation: adenosine triphosphate (ATP) (MP Biomedicals, Tokyo, Japan), or the chlorophenol derivatives 4-chloro-3-ethylphenol (4-CEP) (Sigma-Aldrich, Tokyo, Japan) or 4-chloro-m-cresol (4-CmC) (Sigma-Aldrich). Fluorescence intensity was calculated using a BZ-X Analyzer (Keyence, Osaka, Japan). A total of 15 individual cells from the same dish were studied and maximal peak amplitudes were measured. Ca2 + response data were expressed as mean ± S.E.M. The student's t-test was used to determine significant differences between groups. The significance level was set at P < 0.05.
2.6. Electrical stimulation
The MyoD-transduced fibroblasts at day 14 after the myogenic-induction and C2C12 myotubes were electrically stimulated as previously described [5], [6]. The medium was replaced with 3 ml of fresh medium before electrical stimulation using the C-dish system (ION Optix, MA, USA) with an electric stimulator (Uchida Denshi, Hachioji, Japan). The electric pulse used was 150 V for 3 ms with a 997 ms interval. Cellular contraction was evaluated as the change in distance between two points on individual cells using a motion analyzer (BZ-X Analyzer).
2.7. Time-lapse imaging
Primary mouse myoblasts or adenoviral transduced human skin fibroblast cells were seeded at 5 × 105 cells/well of 35-mm collagen 1-coated Petri dishes and cultivated in DMEM containing 2% FBS. The culture dish was secured on a dish holder on the stage and covered with a heated quartz glass lid that allowed for long-term imaging without condensation. Humidified air with 5% CO2 was supplied to the chamber through a flexible pipe. Morphological changes in cells were recorded using a fluorescent Keyence BZ-8000 microscope time-lapse photo system. Individual Z-stacks (1 μm thick) were captured every 10 min over 2–5 days. Cellular velocity was calculated using BZ-H1M software (Keyence) by tracking the paths of individual cells. For each isolate, 15 individual cells chosen at random from the same dish were analyzed. The student's t-test was used to determine significant differences between groups. The significance level was set at P < 0.05. Motion pictures were created from time-lapse images at 10 frames per second.
2.8. Cell fusion assay
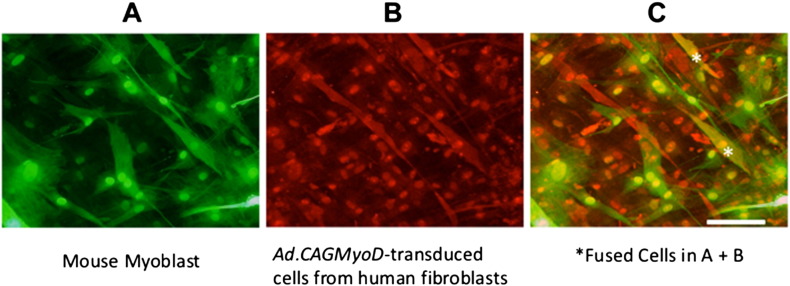
Ad.CAGEGFP-transduced mouse primary myoblasts and Ad.CAGMyoD-transduced human primary fibroblasts were co-seeded at 1 × 105 for each cell type in 24-well tissue culture plates containing collagen 1-coated 9-mm square glass coverslips. Cell fusion was assayed at 7 days in co-culture. Dysferlin was assayed using fluorescence immunocytochemistry following labeling with a monoclonal mouse anti-human dysferlin antibody (NCL-Hamlet-2, 1:200; Leica Microsystems, Newcastle Upon Tyne, UK) as the primary antibody (incubated for 1 h at room temperature) and Alexa 588-conjugated-anti-mouse IgG secondary antibody (incubated for 1 h at 37 °C). This anti-human dysferlin antibody we used reacts with human, rabbit, hamster, pig and dog muscle, but not with mouse, rat or chicken. Green fluorescence indicated mouse myoblasts, red indicated transduced human fibroblasts, while orange indicated fused cells (Fig. 5).
Fig. 5.
Immunofluorescence analyses of co-cultures of Ad.CAGMyoD-transduced human primary fibroblasts with mouse primary myoblasts. A. Positive EGFP fluorescence indicates myotubes from mouse primary myoblasts. B. Red myotubes indicate expression of human dysferlin (a muscle specific marker) in the transduced myotubes derived from human primary fibroblasts. C. Merged image of A + B. *Double-positive cells (orange color) represent fused cells. Scale bar = 100 μm.
3. Results
3.1. MyoD-transduced human fibroblasts express muscle-specific markers
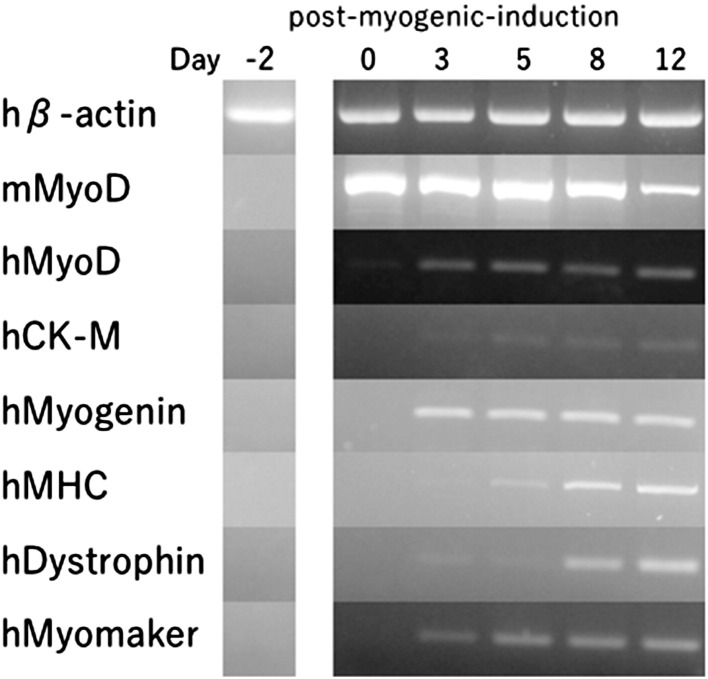
In order to validate muscle differentiation, muscle-specific gene expression determined using RT-PCR was performed at day-2, 0, 3, 5, 8, 12 after the myogenic-induction with Ad.CAGMyoD (Fig. 1). In this assay, it should be noted that mouse MyoD (mMyoD) expression derived from the adenoviral vector provokes endogenous human muscle-specific genes, including human MyoD (hMyoD) gene. Other studied genes were hCK-M (human creatine kinase muscle isoform) [7], hMyogenin [8], [9], hMHC [10], hDystrophin [11], [12], [13], hMyomaker (a membrane activator of myoblast fusion) [14], and the control gene, human β-actin (hβ-actin). The gene expression patterns shown in Fig. 1 are standard profiles for muscle differentiation [6], [15].
Fig. 1.
Time course of myogenic marker expression in Ad.CAGMyoD-transduced human fibroblasts. RT-PCR analyses of myogenic marker genes at day-2, 0, 3, 5, 8, 12 post-myogenic-induction are shown.
3.2. MyoD-transduced cellular contraction
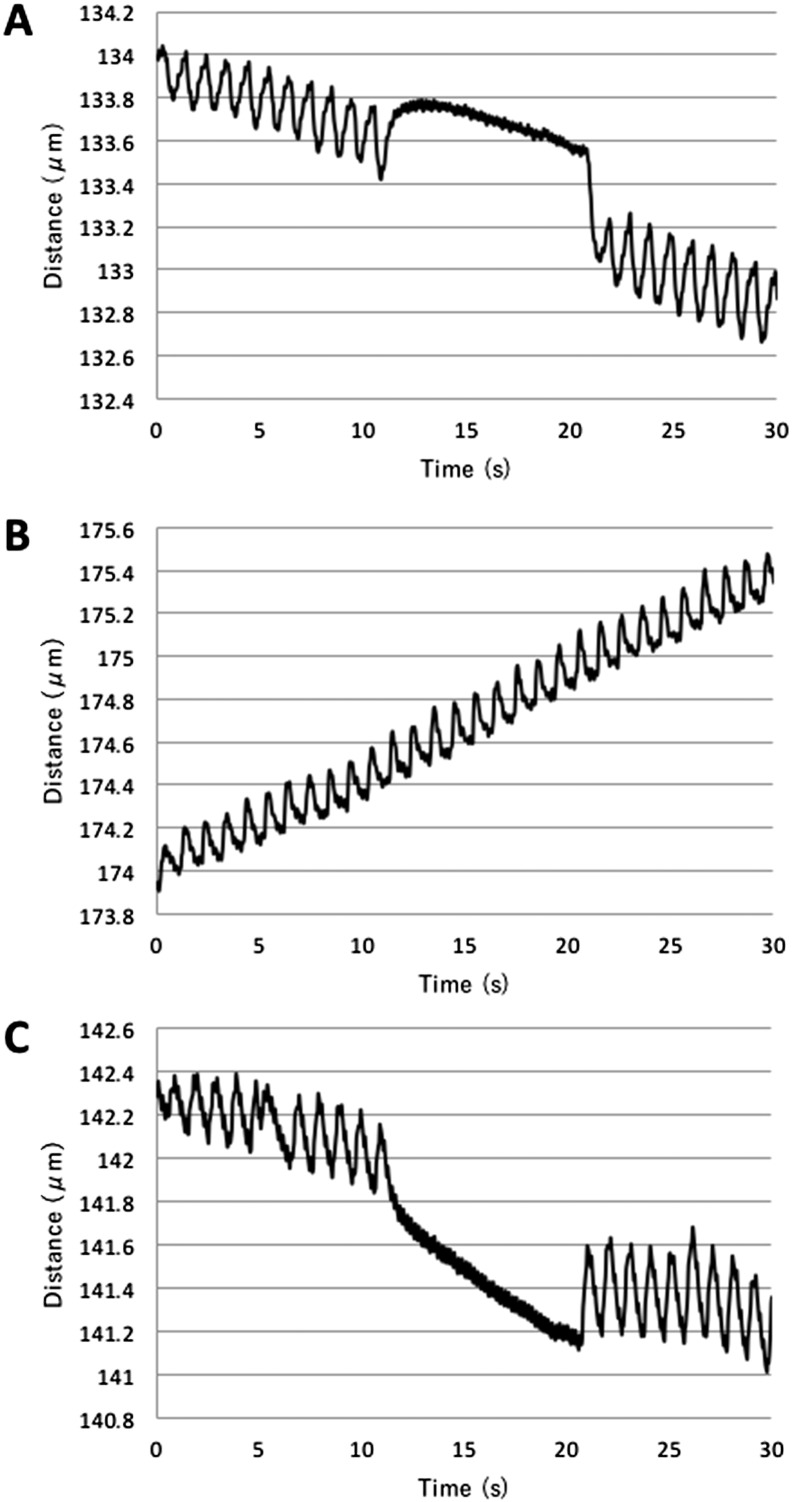
Analysis of cellular contraction was performed according to the procedure described by Manabe et al. [5], [6]. When C2C12 myoblasts were nearly confluent, the growth medium was changed to differentiation medium containing 2% FBS-DMEM (day 0). C2C12 cells gradually formed myotubes and, after 5 days, a few C2C12 cells were observed to stretch in response to electric pulses (150 V, 3 ms, 997 ms interval; Fig. 2A; Movie 1A). Ad.CAGMiC infected fibroblasts started contracting from day 12, similar to C2C12 cells (Fig. 2B; Movie 1B). From 10 to 20 s after the start of electrical stimulation, electrical stimulation was discontinued and simultaneously muscle contraction ceased. Furthermore, the observed cellular contractions were synchronized with the rhythm of electrical stimulation (Fig. 2C). Ad.CAGCherry (no MyoD) infected fibroblasts and non-infection fibroblasts showed no response to electrical stimulation. (data not shown).
Fig. 2.
Contraction of MyoD-transduced human fibroblasts. Contraction was assessed by measuring the change in distance between two points on individual cells. The cells were stimulated with electric pulses of 150 V for 3 ms with 997 ms intervals (1 pulse/s). The trend of the curve represents slight changes in the position on the dish during electric stimulation. A. C2C12 at day 5 in differentiation culture medium. Electrical stimulation was stopped between 10 and 20 s. B. Day 14 of Ad.CAGMiC (MOI 30) transduced human fibroblasts with continuous pulse stimulation. C. Day 14 of Ad.CAGMiC (MOI 30) transduced human fibroblasts. Electrical stimulation was stopped between 10 and 20 s.
3.3. MyoD-transduced cells release Ca2 +
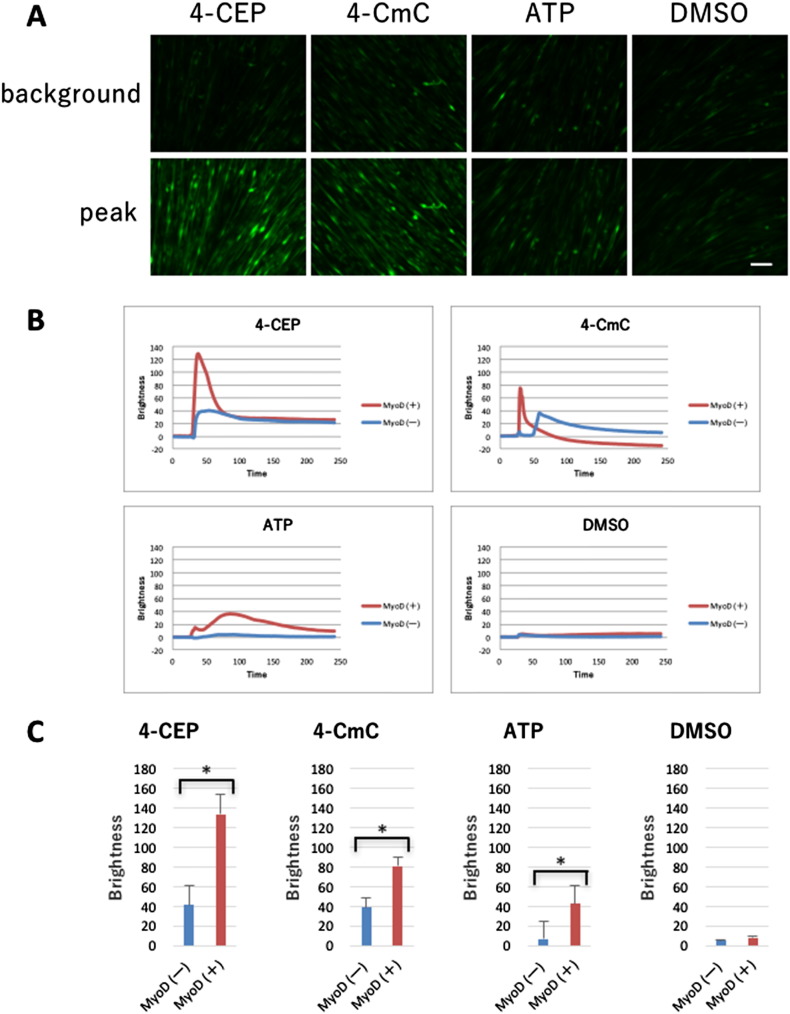
To assess intracellular Ca2 + release, we studied the responses to chlorophenol derivatives and ATP. Ad.CAGMiC-infected human fibroblasts at day 14 or normal human fibroblasts were examined in the absence and presence of the ryanodine receptor stimulants 4-CEP or 4-CmC [16]. Extracellular ATP stimulates P2Y receptors to activate phospholipase C, thereby promoting the production of IP3 and the consequent stimulation of the IP3 receptor [17]. The effects of 500 μM 4-CEP, 500 μM 4-CmC and 100 μM ATP are shown in Fig. 3. Fig. 3A shows the minimum (pre-drug) and maximum responses in MyoD-transduced fibroblasts. Fig. 3B shows the time-course of the Ca2 + release signals. Following the addition of 4-CEP, 4-CmC and ATP, the MyoD-transduced cells (MyoD (+)) released more Ca2 + than normal human fibroblasts (MyoD (−)). 4-CEP, 4-CmC, and ATP produced significantly more Ca2 + release in MyoD (+) cells compared to MyoD (−) fibroblasts at their maximum (P < 0.05) (Fig. 3C). Along with the transduction from fibroblast to myoblast, a significant increase of Ca2 + signaling was observed as myoblasts previously reported [17], [18], [19], which suggests the presence of sarcoplasmic reticulum in the transduced cells.
Fig. 3.
Intracellular Ca2 + measurements. Intracellular Ca2 + release was evaluated by monitoring Fluo4 fluorescence. A. Background (before stimulation) fluorescence is shown in the upper panels and peak (after stimulation) fluorescence is shown in the lower panels. Scale bar = 100 μm. B. Time course of Ca2 + release responses. The data represent the average of 15 individual cells from the same dish. MyoD (+): MyoD-transduced fibroblasts, MyoD (−): normal fibroblasts. C. The difference between the peak and background intensity of fluorescence. n = 15. MyoD (+): MyoD-transduced fibroblasts, MyoD (−): normal fibroblasts.
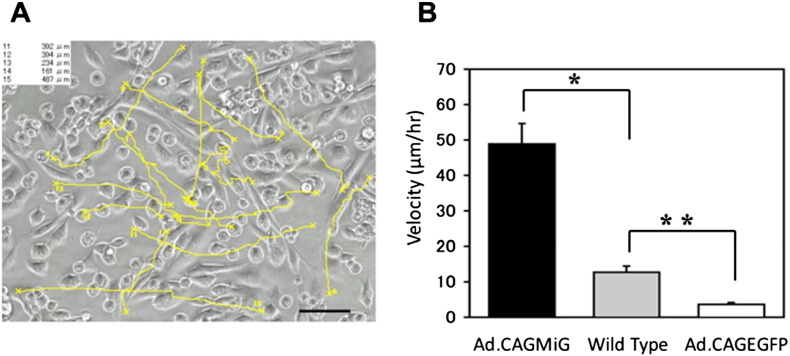
3.4. Cellular mobility of Ad.CAGMiG-transduced fibroblasts is superior to that of wild-type myoblasts
To compare the behavior of Ad.CAGMiG-transduced cells to that of wild-type myoblasts, we analyzed the movement of cells using a time-lapse imaging system. The paths of individual cells were tracked and it was found that Ad.CAGMiG-transduced fibroblasts migrated further than wild-type myoblasts. The average velocity of Ad.CAGMiG-transduced cells was approximately four-fold greater than wild-type myoblasts (Fig. 4). MyoD-transduced fibroblasts also exhibited altered morphology. At the end of the observation period, the cells displayed a spindle shape similar to that of a myotube (Movie 2). No migration was observed with Ad.CAGEGFP-transduced fibroblasts. Thus, the superior migratory ability of the Ad.CAGMiG-transduced cells appears to be conferred by the exogenous expression of MyoD, but not by adenoviral-mediated expression of EGFP.
Fig. 4.
Analysis of cell migration of Ad.CAGMiG-transduced cells using time-lapse microscopy. A. Migratory path of Ad.CAGMiG-transduced primary human skin fibroblasts is analyzed using time-lapse microscopy. Fifteen individual cells were marked at random and individually tracked as all shown (yellow lines). The total migration distances of cells #11–15 are indicated in the upper left corner. Scale bar = 100 μm. B. Average velocity (μm/h) of 15 individual cells Ad.CAGMiG- and Ad.CAGEGFP-transduced primary human skin fibroblasts and non-transduced mouse myoblasts (wild type) calculated using BZ-H1M software (Keyence). The velocity of Ad.CAGMiG-transduced cells was significantly higher compared to the other cells. Data are expressed as the mean ± SEM; n = 15; *P < 0.001 **P < 0.001.
3.5. Ad.CAGMyoD-transduced human fibroblasts fuse with mouse myoblasts and supply human dysferlin to hybrid myotubes
We investigated whether the myogenic cells derived from Ad.CAGMyoD-transduced cells are able to restore muscle by examining the transfer of human dysferlin from Ad.CAGMyoD-transduced human fibroblasts to co-cultured Ad.CAGEGFP-transduced mouse myoblasts. Specific detection of human dysferlin in cells was assayed using the NCL-Hamlet-2 anti-human dysferlin antibody [20]. Dysferlin is linked to skeletal muscle repair that is expressed early in myotube formation. Anti-human dysferlin positivity means that the cells were in early myotube formation and were, in part, from human cells. Ad.CAGEGFP-transduced mouse myoblasts were confirmed by the presence of green fluorescence (Fig. 5A). Seven days after co-culture, dysferlin protein was expressed in some myotubes (spindle-shaped cells), indicating that dysferlin expression was derived from Ad.CAGMyoD-transduced human primary skin fibroblasts (Fig. 5B). The merged image (Fig. 5C) shows single positive cells (green or red) and double positive cells (orange). This indicates that Ad.CAGMyoD-transduced cells fuse with mouse myoblasts. Therefore, Ad.CAGMyoD transduction can confer the ability to fuse and supply human dysferlin to mouse myotubes.
4. Discussion
When considering cell transplantation for the treatment of diseases, it is important to realize that numerous factors may affect the efficiency of reconstitution.
The reprogramming of differentiated cells was first realized in 1987 as the transduction of fibroblast cells into myoblast cells using the MyoD gene [2]. Subsequently, ES cells have been differentiated into many cell types such as liver, cardiomyocyte, muscle, and neuron. Thereafter, researchers aimed to identify methods for creating pluripotent cells similar to ES cells. Accordingly, iPS cells were found to be pluripotent and capable of differentiating into many cell types, similar to ES cells. For muscle, human ES (hES) cells and human iPS (hiPS) cells are utilized with an adenovirus expressing MyoD [3]. Currently, iPS reprogramming methods for affected organs has been frequently studied. Obtaining differentiated cells via iPS from other differentiated cells (e.g., fibroblasts) is known as “indirect reprogramming”, while directly changing one differentiated cell type to another type of differentiated cell is called “direct reprogramming”.
The advantages of “indirect reprogramming” are 1) abundant growth of iPS cells, and 2) many cell types of tissues/organs can be differentiated from iPS cells. However, these advantages can also be considered disadvantages, due to 1) the possibility of carcinogenesis in vitro and in vivo, and 2) contamination with different cell types in the target cell/tissue. If so, when we considered a reprogramming strategy for muscle diseases, direct reprogramming was thought to be beneficial due its safety and simplicity/low cost. Skin fibroblasts are abundant and grow well in culture, especially at a young age. Since establishing primary skin fibroblast cultures from patients is simple and extremely cost effective, skin may provide a rich source of fibroblasts for transduction. Moreover, using the patient's own skin fibroblasts modified to express a causal protein would decrease the risk of immunological rejection [21].
Reprogramming methods utilizing viral vectors have advantages and disadvantages in the transient or stable expression of transmitting genes. The transformation of cells does not necessarily require stable expression of genes in direct reprogramming.
In this study, we confirmed that very high levels of exogenous mouse MyoD expression obtained with our adenoviral system resulted in positivity for several muscle differentiation markers (hMyoD, hCK-M, hMyogenin, hMHC, hDystrophin, hMyomaker) [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]. Since these genes are not expressed in primary fibroblasts, the gene expression observed following viral infection was induced by mouse MyoD expression (Fig. 1). During the process of degeneration and regeneration of skeletal muscle, extensive migration of myogenic cells is important in muscle precursor cell (MPC) transplantation. However, implanted mouse myoblasts migrate less than 0.1 mm (the approximate diameter of two myofibers) [22]. In our system, we observed that MyoD-transduced skin fibroblasts showed high migration during the period of transformation from fibroblasts to myoblast cells in vitro.
Since intracellular Ca2 + release and cellular contraction are characteristics of muscle cells [23], we examined intracellular Ca2 + levels and the responses to electrical stimulation. As shown in Fig. 3A, substantial Ca2 + release was observed in the presence of the ryanodine receptor agonists 4-CEP and 4-CmC. In addition, indirect stimulation of the IP3 receptor with ATP also elevated intracellular Ca2 + levels. Myoblast cells from a pluripotent mesenchymal precursor cell line (C2C12) can be contracted with an electrical stimulus (Fig. 2A, Movie 1A); similarly, Ad.CAGMiC (MOI 30) transduced human fibroblasts exhibited electrically evoked contractions (Fig. 2B and C, Movie 1B). Ultimately, in vitro transduction of human primary fibroblasts with Ad.CAGMyoD replicates the characteristics of skeletal muscle.
Because skeletal muscle is the largest tissue in the human body, it has an exceptionally large number of cells and functions with tendons to bind bone. Thus, if donor cells were needed to replace all of the host muscle cells, a huge amount of tissue culture would be required. With genetic muscle diseases such as Duchenne muscular dystrophy (DMD), groups of donor cells could be used as vectors to deliver genes to host cells, which would require the donor cells to live inside the host muscle fasciculus, fusing and working with the host muscle cells in syncytium formation. Therefore, we focused on the motility and fusion of host and donor cells, which could deliver intact (corrected) gene(s) into host cells.
First, we monitored cellular motility by tracking the migration paths of individual cells. We found that the Ad.CAGMiG cells were significantly faster in culture than wild type mouse myoblasts, which is a novel observation, and is possibly due to the transformation from fibroblasts to myoblasts. In vivo experiment using preceding cardiotoxin injection into muscle and subsequent injection of the muscle cells including transformed myoblast may have uncertainty of location and depth of the injections possibly resulting in wide range of cell spreading area. Therefore, initially we employed in vitro cellular motility experiment to study the abilities per se of the cells. Phenomenally, at least, Ad.CAGMiG cells have an excellent ability of cell motility in culture. If in vitro motility of the cells represents in vivo cellular motility, Ad.CAGMiG cells have an advantage over other methods on effective repair of muscles. Although, this time, we did not study the relationship between expression of the genes (under-mentioned) and cell motility, previous studies have suggested that transmembrane proteins such as Mannose receptor and CD44 influence myogenic cell motility [24], [25]. Factors for cellular motility should be studied further.
Second, cellular fusion was examined using a co-culture system consisting of Ad.CAGEGFP-transduced mouse primary myoblasts (green) and Ad.CAGMyoD-transduced human primary fibroblasts (red), which were detected by staining human dysferlin with NCL-Hamlet-2 anti-human dysferlin antibody [20]. Double positive cells were detected, indicating the in vitro fusion of mouse myoblasts (recipient) and transduced cells (donor of human dysferlin). In support of this, Myomaker is an essential gene for cell fusion [14] and was also expressed in MyoD-transduced skin fibroblast (Fig. 1). Furthermore, dysferlin is a skeletal muscle protein. Therefore, this expression also indicate MyoD-transduced fibroblasts converted myotube.
As mentioned above, MyoD-transduced skin fibroblasts represent a distinguished candidate therapeutic tool that is similar to both hES and hiPS cells [3]; moreover, MyoD-transduced skin fibroblasts have safety advantages. Our group is now working on an in vivo transplantation study involving the transplantation of mouse skin fibroblasts into muscle fibers as a therapeutic model of self-transplantation. Eventually, mdx skin carrying a corrected dystrophin gene (using a lentiviral vector with mini-dystrophin) will be transduced by Ad.CAGMyoD and transplanted into mdx mice as a model of cell therapy for DMD patients.
The following are the supplementary data related to this article.
Contraction of cells. Contraction was assessed by measuring changes in distance between two points on individual cells. The cells were stimulated with electric pulses of 150 V for 3 ms with 997 ms intervals. A. C2C12 at day 5 in differentiation culture medium (Quick Time; 56.2 MB). B. Ad.CAGMiC-transduced skin fibroblasts (Quick Time; 51.1 MB).
Motility of Ad.CAGMiG-transduced skin fibroblasts cultured in 2%FBS-DMEM. The movie shows the migratory behavior of Ad.CAGMiG-transduced skin fibroblast cells. Images were captured every 10 min. This sequence consists of 360 frames, corresponding to 60 h (Quick Time; 9 MB).
Construction of adenovirus vectors. A. Ad.CAGMyoD. B. Ad.CAGMyoDiresEGFP. C. Ad.CAGEGFP. D. Ad.CAGMyoDiresCherry. E. Ad.CAGCherry.
Acknowledgements
We would like to thank Dr. E. Kimura, Dr. Y. Manabe, and Dr. I. Nonaka for their valuable scientific discussion. We also thank members of the Gene Technology Center in Kumamoto University for their technical assistance and Z. Kono for movie editing. This work was supported by a Research Grant (20B-13) for Nervous and Mental Disorders from the Ministry of Health, Labour and Welfare.
References
- 1.Fujii I., Matsukura M., Ikezawa M. Adenoviral mediated MyoD gene transfer into fibroblasts: myogenic disease diagnosis. Brain and Development. 2006;28:420–425. doi: 10.1016/j.braindev.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Davis R.L., Weintraub H., Lassar A.B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- 3.Goudenege S., Lebel C., Huot N.B. Myoblasts derived from normal hESCs and dystrophic hiPSCs efficiently fuse with existing muscle fibers following transplantation. Mol. Ther. 2012;20(11):2153–2167. doi: 10.1038/mt.2012.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanegae Y., Makimura M., Saito I. A simple and efficient method for purification of infectious recombinant adenovirus. Jpn. J. Med. Sci. Biol. 1994;47(3):157–166. doi: 10.7883/yoken1952.47.157. [DOI] [PubMed] [Google Scholar]
- 5.Manabe Y., Miyatake S., Takagi M. Characterization of an acute muscle contraction model using cultured C2C12 myotubes. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0052592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka A., Woltjen K., Miyake K. Efficient and reproducible myogenic differentiation from human iPS cells: prospects for modeling Miyoshi Myopathy in vitro. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0061540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tai P.W., Fisher-Aylor K.I., Himeda C.L. Differentiation and fiber type-specific activity of a muscle creatine kinase intronic enhancer. Skelet. Muscle. 2011;1:25. doi: 10.1186/2044-5040-1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trapecar M., Kelc R., Gradisnik L. Myogenic progenitors and imaging single-cell flow analysis: a model to study commitment of adult muscle stem cells. J. Muscle Res. Cell Motil. 2014;35(5–6):249–257. doi: 10.1007/s10974-014-9398-5. [DOI] [PubMed] [Google Scholar]
- 9.Kuang S., Rudnicki M.A. The emerging biology of satellite cells and their therapeutic potential. Trends Mol. Med. 2008;14(2):82–91. doi: 10.1016/j.molmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Miller J.B. Myogenic programs of mouse muscle cell lines: expression of myosin heavy chain isoforms, MyoD1, and myogenin. J. Cell Biol. 1990;111(3):1149–1159. doi: 10.1083/jcb.111.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman E.P., Brown R.H., Jr., Kunkel L.M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 12.Blake D.J., Weir A., Newey S.E. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol. Rev. 2002;82:291–329. doi: 10.1152/physrev.00028.2001. [DOI] [PubMed] [Google Scholar]
- 13.James M.E., Kevin P.C. Dystrophin and membrane skeleton. Curr. Opin. Cell Biol. 1993;5:82–87. doi: 10.1016/s0955-0674(05)80012-2. [DOI] [PubMed] [Google Scholar]
- 14.Millay D.P., O'Rourke J.R., Sutherland L.B. Myomaker is a membrane activator of myoblast fusion and muscle formation. Nature. 2013;499(7458):301–305. doi: 10.1038/nature12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fakhfakh R., Lee S.J., Tremblay J.P. Administration of a soluble activin type IIB receptor promotes the transplantation of human myoblasts in dystrophic mice. Cell Transplant. 2012;21(7):1419–1430. doi: 10.3727/096368911X627480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng B., Chen G.L., Daskoulidou N. The ryanodine receptor agonist 4-chloro-3-ethylphenol blocks ORAI store-operated channels. Br. J. Pharmacol. 2014;171(5):1250–1259. doi: 10.1111/bph.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buvinic S., Almarza G., Bustamante M. ATP released by electrical stimuli elicits calcium transients and gene expression in skeletal muscle. J. Biol. Chem. 2009;284(50):34490–34505. doi: 10.1074/jbc.M109.057315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobson A.R., Moe S.T., Allen P.D. Structural determinants of 4-chloro-m-cresol required for activation of ryanodine receptor type 1. Mol. Pharmacol. 2006;70(1):259–266. doi: 10.1124/mol.106.022491. [DOI] [PubMed] [Google Scholar]
- 19.Westerblad H., Andrade F.H., Islam M.S. Effects of ryanodine receptor agonist 4-chloro-m-cresol on myoplasmic free Ca2 + concentration and force of contraction in mouse skeletal muscle. Cell Calcium. 1998;24(2):105–115. doi: 10.1016/s0143-4160(98)90078-1. [DOI] [PubMed] [Google Scholar]
- 20.Leriche-Guerin K., Anderson L.V.B., Wrogemann K. Dysferlin expression after normal myoblast transplantation in SCID and in SJL mice. Neuromuscul. Disord. 2002;12:167–173. doi: 10.1016/s0960-8966(01)00254-1. [DOI] [PubMed] [Google Scholar]
- 21.Peault B., Rudnicki M., Torrente Y. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol. Ther. 2007;15:867–877. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- 22.Benabdallah B.F., Bouchentouf M., Rousseau J. Inhibiting myostatin with follistatin improves the success of myoblast transplantation in dystrophic mice. Cell Transplant. 2008;17:337–350. doi: 10.3727/096368908784153913. [DOI] [PubMed] [Google Scholar]
- 23.Wakabayashi T. Mechanism of the calcium-regulation of muscle contraction—in pursuit of its structural basis. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2015;91(7):321–350. doi: 10.2183/pjab.91.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mylona E., Jones K.A., Mills S.T. CD44 regulates myoblast migration and differentiation. J. Cell. Physiol. 2006;209(2):314–321. doi: 10.1002/jcp.20724. [DOI] [PubMed] [Google Scholar]
- 25.Jansen K.M., Pavlath G.K. Mannose receptor regulates myoblast motility and muscle growth. J. Cell Biol. 2006;174(3):403–413. doi: 10.1083/jcb.200601102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Contraction of cells. Contraction was assessed by measuring changes in distance between two points on individual cells. The cells were stimulated with electric pulses of 150 V for 3 ms with 997 ms intervals. A. C2C12 at day 5 in differentiation culture medium (Quick Time; 56.2 MB). B. Ad.CAGMiC-transduced skin fibroblasts (Quick Time; 51.1 MB).
Motility of Ad.CAGMiG-transduced skin fibroblasts cultured in 2%FBS-DMEM. The movie shows the migratory behavior of Ad.CAGMiG-transduced skin fibroblast cells. Images were captured every 10 min. This sequence consists of 360 frames, corresponding to 60 h (Quick Time; 9 MB).
Construction of adenovirus vectors. A. Ad.CAGMyoD. B. Ad.CAGMyoDiresEGFP. C. Ad.CAGEGFP. D. Ad.CAGMyoDiresCherry. E. Ad.CAGCherry.