Abstract
Objectives
This study is the largest to date examining executive function and adaptive skills in females with autism spectrum disorder (ASD). Its primary aim was to utilize parent ratings of real-world executive functioning and adaptive behavior to better understand whether females with ASD differ from males with ASD in these areas of everyday functioning.
Methods
We compared 79 females with ASD to 158 males with ASD (ages 7-18) who were statistically matched on age, IQ, and level of ADHD or ASD traits. All participants were assessed using the Behavior Rating Inventory of Executive Function (BRIEF) and a subset (56 females and 130 males) also received the Vineland Adaptive Behavior Scales (VABS).
Results
Females were rated by parents as having greater problems with executive function on the BRIEF. Parents also rated females as exhibiting more difficulties than males on the Daily Living Skills domain of the VABS. There was a correlation between increased global EF difficulty and decreased adaptive ability in both males and females.
Conclusions
Our results indicate relative weaknesses for females compared to males diagnosed with ASD on executive function and daily living skills. These differences occur in the absence of sex differences in our sample in age, IQ, clinician ratings of core ASD symptomatology, parent ratings of ADHD symptoms, and parent-reported social and communication adaptive skills on the VABS. These findings indicate specific liabilities in real world EF and daily living skills for females with ASD and have important implications for targeting their treatments.
Keywords: Autism Spectrum, Sex Differences, Executive Function, Adaptive Functioning
Introduction
Sources commonly cite a 4 to 1 ratio of males to females diagnosed with an autism spectrum disorder (ASD) (Rivet & Matson, 2011), and this is confirmed by the most recent data from Centers for Disease Control, which estimate the overall prevalence ratio to be 4.5 males to 1 female (Christensen et al., 2016). This discrepancy in diagnosis increases in those without co-occurring intellectual disability (ID), and decreases to less than 2 to 1 among individuals with moderate to severe ID (Fombonne, 1999; Werling & Geschwind, 2013; Yeargin-Allsopp et al., 2003), indicating that the overall diagnostic discrepancy is driven primarily by those without ID. Females also consistently receive ASD diagnoses later, on average, than their male counterparts (Begeer et al., 2013).
Sex differences in the prevalence and age of diagnosis of autism have been attributed to inherent discrepancies in genetic make-up or environmental demands. Sexual dimorphism may well make up part of the explanation, as biological differences between males and females, in both the typically developing (Baron-Cohen, Knickmeyer, & Belmonte, 2005) and ASD populations (Robinson, Lichtenstein, Anckarsäter, Happé, & Ronald, 2013) have been found. Evidence has also been presented for a ‘female protective effect’ (Robinson et al., 2013), whereby females with ASD are protected against some of the symptoms of ASD, and could therefore require more genetic (Levy et al., 2011) or environmental “hits” to bring them to an ASD diagnosis, consistent with patterns seen in other genetic and developmental disorders. Biologically-based differences in prevalence rates could be enhanced by environmental factors, such as parents typically interacting differently with male and female infants, whereby mothers respond preferentially to female infants (Johnson, Caskey, Rand, Tucker, & Vohr, 2014).
It is in many ways premature, however, to study the mechanism underlying sex differences in ASD, because we do not yet have adequate data to determine the diagnostic presentation of ASD in females (Lai, Lombardo, Auyeung, Chakrabarti, & Baron-Cohen, 2015). Our current diagnostic criteria for ASD have been defined mainly in boys. Gold standard diagnostic measures, such as the Autism Diagnostic Observation Schedule (ADOS) and Autism Diagnostic Interview (ADI), were developed with a predominately male ASD population (Lord et al., 2012; Lord, Rutter, & Le Couteur, 1994). Therefore, it must be recognized that any terminology used in this paper (and more broadly) related to “autistic traits” most accurately describes the autistic traits appearing in males and could be exclusive of other groups such as adults, females, and transgender and gender nonconforming individuals.
The reliance of our understanding of autism on predominantly male groups can lead to a self perpetuating cycle whereby autism traits may be missed in females, especially those who are younger and do not have ID, further contributing to an ascertainment bias and a paucity of information on the presentation of ASD in females. Clinicians may be assessing females in areas that do not entirely relate to the everyday difficulties they are having, and are more appropriate for males. Regarding diagnostic measures in general, even when males and females present with equally high levels of ASD traits, there is evidence that females are less likely to receive a diagnosis (Dworzynski, Ronald, Bolton, & Happé, 2012). Better communication skills and stronger social motivation may mean that females do better than males at “masking” their social understanding deficits (Dworzynski et al., 2012; Gould & Ashton-Smith, 2011) for the short time frame of a diagnostic interview. This could be particulary true of females without ID, explaining the jump in the male:female discrepancy moving from the ID to the non-ID population. Ascertainment bias against identifying females with autism may also occur long before the application of diagnostic procedures. Similar to the typically developing population (Achenbach, Howell, Quay, & Conners, 1991), there is evidence that males with ASD more frequently show externalizing behaviors, such as hyperactivity and aggression, that may in turn trigger referrals to a clinic, leading males to receive evaluations more commonly (Kopp & Gillberg, 1992; Rivet & Matson, 2011).
One tool for combatting the cycle of limited ascertainiment and male dominated diagnostic criteria is the characterization of females and males on continuous measures of features strongly associated with ASD, such as executive function and adaptive abilities (American Psychiatric Association, 2013) which remove sex bias with either sex-specific normative data or norms with equal representation of males and females. Because they require informants to report on behavior over a previous number of weeks, they capture a broader sample of behavior than a diagnostic interview, such as the ADOS, does. Additionally, prior studies have demonstrated that quality of life in individuals with ASD is more strongly associated with factors such as adaptive behavior and daily executive functioning, making these variables more relevant to understanding the lived experience of individuals with ASD (Kuhlthau et al., 2010). Comparison of females and males with ASD using these tools may help to delineate true phenotypic differences between males and females that we are currently identifying as falling on the autism spectrum.
Executive Function (EF) is an important area of focus in the ASD population (Kenworthy, Black, Harrison, Della Rosa, & Wallace, 2009; Kenworthy, Yerys, Anthony, & Wallace, 2008). Problems with EF, flexibility in particular, are common in those with ASD, and this area presents as a promising target for intervention. These difficulties have been linked to key outcomes, such as decreased adaptive ability (Pugliese et al., 2015), co-occurring psychopathology (Lawson et al., 2015; Wallace et al., 2016), decreased job success (Hume, Loftin, & Lantz, 2009), and poorer quality of life (Bishop-Fitzpatrick et al., 2016). There has been a limited amount of research conducted on EF sex differences in ASD, mostly utilizing lab-based tasks, and the results have been mixed. In individuals with no ID nor significant differences in FSIQ between groups, it has been found that females with ASD have decreased response inhibition, measured through a stop task (Lemon, Gargaro, Enticott, & Rinehart, 2011). In contrast, no differences in sensitivity to signals nor response inhibition was found between sexes on a Go/No-Go task (Lai et al., 2012); this finding was in the context of a female sample with significantly lower social and communication difficulites on the ADOS than males. Poorer cognitive flexibility, as measured by greater perseveration errors on the Wisconsin card sorting task (Memari et al., 2013), has been reported in females as compared to males with ASD. Another study found that females with ASD showed better switching ability than males with ASD, however, as assessed by the Trail Making Task B-A (Bölte, Duketis, Poustka, & Holtmann, 2011). The lack of consensus in this small literature on sex differences in people with ASD on EF measures warrants further exploration in this area. Furthermore, no research has been conducted on how EF-related behaviors may differ between the sexes in real-world settings, outside of the lab.
In terms of adaptive ability, it is historically known that there is a more balanced male:female ratio of ASD in low IQ populations (Lord & Schopler, 1985), which was recently corroborated in an epidemiological study of adults with ASD (Brugha et al., 2016). IQ is also known to be commensurate with adaptive ability in typically developing populations (Sparrow, Cicchetti, & Balla, 2005), but not in ASD populations without ID, where adaptive ability is generally worse than predicted based on IQ (Kanne et al., 2010; Lee & Park, 2007). Based on the limited existing evidence, it might be expected that any differences in adaptive ability between males and females with ASD would occur alongside IQ differences. While it has been found that females with ASD have more limited adaptive behavior skills than males with ASD, this effect was fully mediated by lower general IQ for females with ASD (Frazier, Georgiades, Bishop, & Hardan, 2014). There has been limited research on sex differences in adaptive abilities among individuals with ASD without ID, making it impossible to draw any conclusions within that population. Furthermore, while EF-related behaviors have been observed to predict adaptive ability in the ASD population (Pugliese et al., 2015), it remains unclear whether the relationship between these two important areas of concern differs by sex in ASD.
The current study compares parent-reported EF and adaptive ability between well-matched males and females with ASD, primarily without co-occurring ID, and examines the relationship between these two domains of everyday behavior in males and females separately. Due to the heterogeneity of IQ and comorbid presentation of ADHD in ASD (Leyfer et al., 2006), the two groups are also compared on IQ scores and presence of ADHD symptoms. Given the equivocal nature of the limited past research comparing males and females with ASD in areas of EF, this study is exploratory in that regard. However, acknowledging the connection between adaptive ability and IQ, in this IQ-matched and largely non-ID sample, it is hypothesized that males and females with ASD will demonstrate comparable adaptive skill deficits. If sex differences in EF are apparent, it is also possible that different associations between EF and adaptive ability might emerge when examining the sexes separately.
Methods
Procedures
This project used archival data and was conducted in compliance with standards established by the institution’s IRB including procedures for informed consent. Participants were evaluated for clinical or research purposes in a hospital setting. Analyses were conducted using IBM SPSS Statistics, Version 22. All participants were assessed using the Behavior Rating Inventory of Executive Function (BRIEF; Gioia, Isquith, Guy, & Kenworthy, 2000) and a subset of 56 females and 130 males were assessed using the Vineland Adaptive Behavior Scales, first or second edition (VABS, VABS-II (Sparrow, Balla, & Cicchetti, 1984; Sparrow, Cicchetti, & Balla, 2005).
Participants
A cohort of 79 females (mean age=12.17, SD=2.74; mean IQ=106.68, SD=20.37) and 158 males (mean age=12.65, SD=2.43; mean IQ=106.74, SD=18.95), were included in this study. Age ranged from 7-18 years and Full-Scale IQ ranged from 62-149, with no outliers. All participants met DSM criteria for an autism spectrum disorder based on clinical judgment of a research reliable and clinically experienced ASD expert using the Autism Diagnostic Interview (ADI) or Autism Diagnostic Interview–Revised (ADI-R; Le Couteur et al., 1989; Lord et al., 1994) and/or the first or second edition of the Autism Diagnostic Observation Schedule (ADOS, ADOS-2; Lord et al., 2000, 2012).
In addition to meeting criteria for ASD, female participants were included in the analyses if they had scores for all subscales on the BRIEF, and an FSIQ score. After identifying all females who met these criteria, males were selected from the total dataset of 226 males in a 2:1 male:female ratio using the FUZZY case-control matching function, without replacement, within IBM SPSS (Version 22). This function takes two datasets, one typically larger than the other, and allows for matching of groups based on multiple factors within a range of tolerance. It prioritizes selecting participants who are the closest matches. In this case, each female was matched to two male participants within 4 years of age and 14 IQ points. Males and females did not differ significantly in ADOS and Attention Deficit Hyperactivity Disorder (ADHD) symptom ratings for the subset of the group that had each (see Table 1). In addition, there was a second subset of 56 males and 29 females with normed ADHD ratings (T scores) based on parent report on the ADHD rating scale. These groups did not have significantly different ratings.
Table 1.
Demographic and diagnostic characteristics for male and female participants with autism spectrum disorder.
| Measure | Females | Males | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Statsa | |||||||
|
| |||||||
| Matched with tolerance | M | SD | n | M | SD | n | |
| Age | 12.17 | 2.74 | 79 | 12.65 | 2.43 | 158 | p=.17 |
| Full Scale IQ | 106.68 | 20.37 | 79 | 106.74 | 18.95 | 158 | p=.98 |
|
| |||||||
| Other Measures | Mb | SD | n | Mb | SD | n | |
|
| |||||||
| ADOS Social + Comm Total | 11.71 | 4.33 | 58 | 11.82 | 5.00 | 63 | p=.45 |
| ADOS Restricted Interests | 2.29 | 1.71 | 58 | 1.98 | 1.65 | 62 | p=.32 |
| ADOS-2 Social Affect Total | 11.36 | 3.20 | 11 | 11.30 | 4.21 | 50 | p=.53 |
| ADOS-2 RRB Total | 3.91 | 2.02 | 11 | 3.36 | 1.89 | 50 | p=.64 |
|
| |||||||
| ADI-R Social | 16.67 | 6.65 | 69 | 17.92 | 5.75 | 146 | p=.50 |
| ADI-R Verbal Communication | 13.99 | 5.04 | 69 | 14.98 | 4.45 | 146 | p=.25 |
| ADI-R RRBI Total | 5.13 | 2.87 | 69 | 5.72 | 2.69 | 146 | p=.39 |
|
| |||||||
| ADHD Inattentive (yes:no)c | 24:17 | 41 | 49:58 | 107 | p=.17 | ||
| ADHD Hyperactive (yes:no) | 12:29 | 41 | 27:80 | 107 | p=.62 | ||
|
| |||||||
| Race | 79 | 158 | p=.66 | ||||
|
| |||||||
| Unknown (not in chi sq.) | 10 | 4 | |||||
| Asian | 3 | 10 | |||||
| African American | 10 | 15 | |||||
| White | 52 | 117 | |||||
| Other | 4 | 12 | |||||
|
| |||||||
| Mother’s Level of Ed. | 55 | 158 | p=.84 | ||||
|
| |||||||
| Graduate | 24 | 68 | |||||
| College | 21 | 64 | |||||
| Partial College/technical | 9 | 18 | |||||
| High School | 1 | 6 | |||||
| Partial High School | 0 | 1 | |||||
| Junior High School | 0 | 1 | |||||
Note: ADOS: Autism Diagnostic Observation Schedule, Comm: Communication, RRB: Restricted and Repetitive Behavior; ADI-R: Autism Diagnostic Interview-Revised, RRBI: Repetitive, Restricted Behaviors and Interests; ADHD: Attention Deficit Hyperactivity Disorder; Ed: Education
Independent sample t-tests were conducted for Age and FSIQ comparisons. Pearson Chi-Square analyses were conducted for ADOS, ADI-R, ADHD, Race, and Mother’s Education comparisons.
Means and standard deviations are reported here for informational purposes. The frequencies of each unique score, compared between males and females, were used for the chi-square analysis.
Yes:no indicates ratio of meeting criteria for ADHD (inattentive or hyperactive):not meeting criteria. Six or more symptoms on the 18-item scale are necessary to meet criteria.
Measures
Behavior Rating Inventory of Executive Function, Parent Form (BRIEF; Gioia et al., 2000). The BRIEF assesses behavioral manifestation of Executive Function (EF) difficulties in children through parent questionnaire. Scores are divided into two main indices, the Behavioral Regulation Index (BRI), and the Metacognition Index (MCI). The BRI is further divided into three scales (Initiate, Emotional control, Shift) and the MCI is divided into five scales (Inhibit, Organize/plan, Organization of materials, Working memory, Monitor). Higher scores indicate poorer EF, with T-scores above 65 indicating clinically significant ratings. The BRIEF has good reliability, and convergent and discriminant validity (Gioia et al., 2000). It is important to note that BRIEF scores have sex-specific norms.
The Vineland Adaptive Behavior Scales, First and Second Editions (VABS, VABS-II; Sparrow et al., 1984, 2005). The VABS is a standardized, structured parent/caregiver interview of adaptive skills. The current study used the Communication, Daily Living, and Socialization domain standard scores. The VABS has demonstrated strong reliability and validity (Sparrow et al., 2005). Importantly, the VABS has an approximately equal representation of males and females in its normative sample (though it is not normed separately for males and females), and analyses in the normative process showed no evidence of sex bias (Sparrow et al., 2005).
Diagnostic and Statistical Manual of Mental Disorders-IV Attention Deficit Hyperactivity Disorder Rating Scale-Parent Edition (DSM-IV; ADHD rating scale). The ADHD Rating Scale (DuPaul, Power, Anastopoulos, & Reid, 1998) assesses severity in inattention and hyperactivity/impulsivity symptoms. This 18-question scale yields two domains: inattention and hyperactivity/impulsivity. For each question, parents use a 0–3 scale to rate the participant. A higher score indicates more symptom severity, and a score of 2 or 3 is considered a significant symptom; six or more significant symptoms in either the inattention or hyperactivity/impulsivity domains meet criteria for an ADHD diagnosis. Just as with the BRIEF, the ADHD Rating Scale is normed separately for males and females.
Child and Adolescent Symptom Inventory-4R (CASI-4R; Gadow & Sprafkin, 2005). This is a parent-rated questionnaire of youth’s symptoms that combines the symptom modules of the Child Symptom Inventory-4 and the Adolescent Symptom Inventory-4. Individual items bear one-to one correspondence with DSM-IV symptoms and are rated on a scale from 0 (never) to 3 (very often). Symptom counts (not normed) from the ADHD subscale of the CASI-4R were used in this study.
Data Analysis
Data Inspection and Demographic Analyses
In the fully matched data set, scores were examined to ensure there were no outliers using stem and leaf plots. Summary scores for ADOS modules 2, 3, and 4 were consolidated to create overall Communication + Social Totals and Stereotyped Behavior scores. ADOS-2 modules 2, 3, and 4 were similarly combined to create overall Social Affect and Restricted and Repetitive Behavior (RRB) Total scores. ADI and ADI-R scores were also combined to create overall Social, Communication, and RRB Total scores.
For those participants that had them available, ADHD symptom ratings from the Child and Adolescent Symptom Inventory (CASI-4R) and ADHD rating scale were consolidated and given a dichotomous, “yes” or “no”, value indicating whether each participant met or did not meet criteria for each type: Inattentive and Hyperactive. For both the Inattentive and Hyperactive Types, the proportion of males and females who met criteria for each were compared using a chi square test to ensure equal parent-rated presentation of ADHD symptoms across groups.
T-tests and chi-square analyses (as appropriate) were also conducted to evaluate for equal distributions of IQ, age, ADOS/ADI scores, race, and maternal education across the male and female samples. Given unequal sample size in the 2 groups, dependent variables of interest (BRIEF, Vineland) were evaluated with Levene’s test of homogeneity of variances with non-significant results (ps = .08-.89). Regardless, results reported below were confirmed with Welch and Brown Forsythe tests.
Executive Function and Adaptive Behavior Analyses
Two mixed-model ANOVAs were conducted to compare sex differences across the eight scales of the BRIEF and three domains of the VABS-I/II and, when appropriate, post-hoc independent t-tests were used to assess differences on specific scales. The first was a Sex (male, female) by BRIEF scale (eight scales) ANOVA and the second was a Sex (male, female) by VABS-I/II Domain (three domains) ANOVA.
Correlational Analyses
For each of the male and female groups, partial correlations, accounting for the influence of age and FSIQ, were computed to assess the relationship between the BRIEF and each of the three VABS-I/II Domains: Communication, Daily Living Skills (DLS), and Socialization.
Results
Demographic Analyses
Independent samples t-tests between males with ASD and females with ASD on age and FSIQ and chi-square analyses of ADOS/ADOS-2 and ADI/ADI-R indicated there were no significant differences between males and females on any of these factors. Chi-square analyses of proportions of males and females exhibiting ADHD Inattentive Type showed no significant differences. Similarly, the proportions were not significantly different for ADHD Hyperactive Type. For those participants for whom it was available, maternal level of education (n=55 females, 158 males) and racial demographic data (n=69 females, 158 males) were analyzed using chi-square analyses, which showed no significant differences in either area (see Table 1). The subset of males and females with VABS-I/II scores also did not differ from each other statistically on age, FSIQ, ADOS scores, ADI scores, ADHD symptom ratings, maternal level of education, and racial demographic data.
Executive Function
A mixed-model ANOVA resulted in a main effect of sex (F=5.03; p=.03, ηp2=.02), whereby parents rated females as exhibiting greater overall EF problems than males (see Table 2). This difference occurred despite no significant sex-based differences in terms of ASD and ADHD symptomatology. There was no significant interaction between sex and BRIEF subscale. Females’ mean scores also surpassed the level of clinically significant problems (T score>65) on 5/8 scales: Inhibit, Shift, Working Memory, Plan/Organization, and Monitor, while males’ mean scores passed this level on only the Shift scale (see Figure 1).
Table 2.
Executive function and adaptive behavior ability scores for male and female participants with autism spectrum disorder.
| Scale | Females | Males | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| M | SD | n | M | SD | n | p | |
| BRIEFa Global Executive Composite | 69.27 | 11.56 | 78 | 66.54 | 10.05 | 158 | |
| BRIEF Behavior Regulation Index | 67.96 | 11.69 | 78 | 66.09 | 10.98 | 158 | |
| BRIEF Metacognition Index | 68.14 | 11.84 | 78 | 64.89 | 10.16 | 158 | |
|
| |||||||
| Vineland Adaptive Behavior Domains | 76.43 | 13.14 | 54 | 80.92 | 12.38 | 115 | .03 |
| VABSb Communication | 84.40 | 15.57 | 57 | 84.87 | 13.60 | 130 | .84 |
| VABS Daily Living Skills | 77.79 | 15.68 | 57 | 85.08 | 15.01 | 130 | .03 |
| VABS Socialization | 74.41 | 12.96 | 56 | 77.98 | 13.35 | 130 | .09 |
Note: BRIEF scores are reported as T scores (M=50, SD=10) and VABS-I/II scores are reported as standard scores (M=100, SD=15). P values are not included for the BRIEF because post-hoc tests were not warranted given the lack of an interaction effect between BRIEF subscale and sex in the ANOVA conducted.
Behavior Rating Inventory of Executive Function
Vineland Adaptive Behavior Scale
Figure 1.
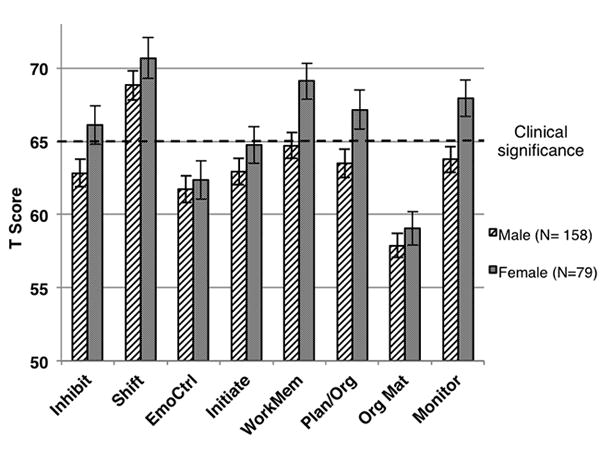
The profile of scores on the Behavior Rating Inventory of Executive Function for male and female participants with autism spectrum disorder. T scores above 65 are indicative of clinically significant problems.
Adaptive Behavior
This mixed-model ANOVA yielded a main effect of sex across the three domains of adaptive behavior (F=3.89; p=.05, ηp2=.02). There was also an interaction between VABS-I/II domain and sex (F=5.15; p<.01, ηp2=.03). Post-hoc t-tests revealed significantly greater impairments (i.e., lower standard scores) for females with ASD in the domain of DLS (p<.01). In the adaptive behavior domains of Communication and Socialization, there were no significant differences in parent reports of males and females, however there was a trend level difference in the socialization domain, whereby females show slightly lower social adaptive ability (p=.09). See Table 2.
Correlational Analyses
For both males and females, there were significant correlations, controlling for the effects of age and FSIQ, between the BRIEF Global Executive Composite (GEC) score and the VABS-I/II domains of DLS and Socialization (see Table 3). The GEC score, rather than the eight individual subscales, from the BRIEF was used because there was no interaction effect in the BRIEF ANOVA, but rather only a main effect, indicating differences at the composite level. Also use of the GEC score limits the issue of multiple comparisons during this analysis. Higher BRIEF GEC scores (i.e., more impaired EFs) were associated with decreased VABS-I/II DLS and Socialization abilities. The male group also showed a significant correlation between BRIEF GEC score and VABS-I/II Communication, whereby more EF impairment was associated with less communication ability. For females, this correlation was not significant. Comparing the magnitude of these correlations using an r to z transformation revealed that the size of the correlations between BRIEF GEC and VABS-I/II domains was not significantly different between males and females. There was, however, a trend towards a stronger relationship between DLS and BRIEF GEC in females than males with ASD (z diff=1.9, p=.06).
Table 3.
Partial correlations (accounting for age and IQ) between the Behavior Rating Inventory of Executive Function Global Executive Composite score and domain scores from the Vineland Adaptive Behavior Scales for males and females with autism spectrum disorder.
| Vineland Domain | Global Executive Composite | |
|---|---|---|
| Males | Females | |
| Communication | -.30** | -.14 |
| Daily Living Skills | -.18* | -.46** |
| Socialization | -.34** | -.42** |
p<.05;
p<.01
For all correlations, male n=130, female n=55.
Confirmatory Analyses
The above results of analyses conducted in the 2:1 FUZZY matched dataset were confirmed in the full sample, including the same 79 females, but comparing them to all 226 males. This larger sample showed no significant differences in IQ between males and females (p=.25), however, there were differences in age (p<.01), with a mean female age of 12.2 years and a mean male age of 13.1 years. Autism symptoms as ascertained on the ADOS remained equivalent between the sexes in this broader sample, although females showed fewer parent-reported past symptoms as ascertained on the ADI. Controlling for age, we repeated the BRIEF subscale by sex comparison. Mauchly’s test of sphericity was significant, indicating that the assumption of sphericity had been violated, thus we interpreted with the Greenhouse-Geisser, resulting in a main effect of sex (F=4.04; p=.045), with no interaction. Similar to the findings from the 2:1 matched sample, parents rated females as showing greater EF problems than males. In the subset of those with a VABS-I/II (n=56 females, 176 males), the Sex by Domain comparison, while controlling for age, yielded a main effect of sex across the three domains of adaptive behavior (F=4.12; p=.04) and an interaction between VABS-I/II domain and sex (F=6.07; p<.01). This pattern of differences is the same as that found in the 2:1 matched sample.
Discussion
This study presents a novel exploration of sex differences in a large cohort of individuals with ASD, a majority without ID, on continuous measures of everyday executive function and adaptive ability. In this sample of individuals with ASD, females show greater executive function problems than males and markedly worse adaptive daily living skills. Importantly, these differences occur on measures shown not to have sex bias and in the context of equivalent ratings for males and females on clinician-administered measures of ASD symptomatology (ADOS/ADOS-2 and ADI-R), parent-reported ADHD levels, and FSIQ.
These findings are consistent with previous research indicating diminished EF performance on laboratory tasks in females with ASD (Lemon et al., 2011; Memari et al., 2013), but broaden the implications to difficulties these females have in the real world, as reported by parents. Greater EF problems in females occur in the context of similar levels of parent reported ADHD symptoms between the sexes, which is important given previous work showing that the presence of ADHD symptoms is associated with increased EF problems in people with ASD (Corbett, Constantine, Hendren, Rocke, & Ozonoff, 2009; Yerys, Kenworthy, Jankowski, Strang, & Wallace, 2013).
Regarding adaptive behavior skills, we found the expected one to two standard deviation gap between IQ and adaptive ability in both males and females that has been typically described in studies of individuals with ASD without ID (e.g., Kanne et al., 2010; Pugliese, et al 2015). In contrast to our prediction of equivalent adaptive skills across the sexes, however, we found better parent reported daily living skills in males than females. Socialization and communication skills were not significantly different. While previous reports have linked reported female specific adaptive behavior deficits to discrepancies in cognitive level (Lord, Schopler, & Revicki, 1982; Frazier et al., 2014), this explanation does not apply to our sample because the male and female groups were matched for FSIQ. There is potentially corroborating evidence in one previous study, which found particular weakness for adolescent females in daily living skills within the Simons Simplex Collection (Howe et al., 2015), but this difference did not survive corrections for false discovery rate.
Thus, in the context of similar IQ level, ASD symptoms and social/communication skills, the females in this study had greater problems with parent reported daily living as well as EF skills. If supported in future studies, this raises the possibility of a concentration of female specific phenotypic differences outside of social communication domains. Such a pattern could reflect biological/genetic differences or previously described sex differences in child rearing (Johnson et al., 2014), which emphasize social and communication skills for females and thus may provide more intensive support for girls to develop these skills (Bornstein, Giusti, Leach, & Venuti, 2005). Since ASD is diagnosed later in females, they may be missing out on specific interventions that would target their ASD related weaknesses more broadly. It is also possible that these biological or environmental differences in upbringing could lead to the commonly ventured idea of a female “masking” effect. Multiple self-advocates have discussed females being better able to blend into social and communicative situations (Rosa, 2016), such as during an evaluation with a clinician. It could be in other areas of functioning, such as EF and DLS, and particularly on real-world measures where behavior is assessed over long time periods, that the problems females are experiencing become apparent.
In terms of sex differences in the relationship between parent reported EF problems and adaptive behavior, our exploratory analyses reveal a generally consistent pattern across the sexes of a negative relationship between EF problems and adaptive abilities. For males in this study, the relationship between EF problems and adaptive behavior domains was consistent with that reported in a large predominantly male sample (male n=301, female n=53; Pugliese et al., 2015) in that all three domains of the Vineland were correlated with EF problems (although the correlation with daily living skills in this study is small). For females in this study, however, the pattern is different. The correlation between the Communication domain and EF problems was not significant. It must be noted that this finding is weakened by that fact that the difference in the size of the correlation between communication skills and EF problems in males and females was not significant. Nonetheless, the indication that communication skills in females may be artificially dissociated from EF problems, could be related to advanced use of scripting, as reported by some self-advocates (Baggs, 2007; Mead, 2016). Reports from female self-advocates indicating that they use verbal scripts to mask communication weaknesses, in combination with our findings of greater difficulty with EF and daily living skills for females, indicates that the possible dissociation of communication from EF skills in females merits future research. Direct investigation of the use and utility of scripting to people with ASD without ID is needed to explore this possibility. Furthermore, without longitudinal data collection in larger samples of males and females with and without ASD, we are limited to speculation regarding causality in the relationships we have observed and the uniqueness to ASD of sex differences in these relationships.
It is necessary to recognize the limitations of this mainly exploratory study. The measures of both EF and adaptive ability are based on parent report and lack the additional input of other adults in the participants’ lives, the participants themselves, and reliable lab-based tasks. Although this study does not provide indications of the etiology of these differences (e.g., whether parental bias based on sex-specific expectations for these skills drove sex differences), it is notable that the differences in adaptive behavior and EF in this study were found on measures that are either sex normed or derived from a balanced male:female standardization sample. It is important to recognize the possibility that utilizing norms based on typically developing youth could mask ASD-specific sex differences. However, if anything, these findings are conservative and constrained by the the sex-based norms of the BRIEF in which the normative sample of TD females show either fewer or equivalent problems when compared to TD males across age. Nonetheless, a crucial future step will be the investigation of a larger sample of males and females with ASD, which would allow for the exploration of the psychometrics of these scales in males and females with ASD specifically. Ascertainment bias should also be considered in interpreting these findings. First, because of our mostly clinically referred sample, it is possible that those females who were referred were also those with more noticeable problems in everyday life. Second, all participants in this study were included based on meeting ASD criteria on a gold standard diagnostic measure, either the ADOS/ADOS-2 or ADI/ADI-R. Among those females who presented to the clinic, sex bias in these diagnostic measures could mean that only the females showing the most “ASD traits” (as determined based on a majority male characterization sample) met criteria and ended up being included in this study. Therefore, our findings are not necessarily representative of the ASD population overall. This ascertainment problem is not unique to our study. Addressing this problem is a critical goal for future research on sex differences in ASD. Failing that, research which intends to explore sex differences may only capture at differences between one sex and a subset of another, rather than comparable populations. Additional future goals for our research include population based studies that use diagnostic measures with sensitivity across all gender identities, thereby allowing for representation of females, transgender and gender nonconforming individuals. Like others in the field, we have conceptualized individuals in a binary way (male vs. female) when in fact there could be many more profiles, or possibly no difference in profile, as we include those who are transgender and gender nonconforming. Given high rates of gender variance in people with autism (Strang et al, 2015), it is important to include these under-represented groups in future research.
Given the present findings and input from self-advocates about the propensity for females to compensate for social and communication deficits, increased clinical and research inquiry is needed into distinctive cognitive and behavioral phenotypes in females with ASD. This study also makes clear the importance of evaluating functioning outside of ASD-specific symptoms, into related domains that have major impacts on quality of life and overall daily functioning. Also, paying particular attention to continuous measures, including others in addition to the BRIEF and VABS-I/II, as a support to the clinical interaction could be of strong value for getting the complete real-world picture of a female’s situation. While the focus on females with ASD has substantially increased in recent years, there is still much more research needed, especially towards determining whether there is a specific profile for females with ASD and which particular strengths/difficulties parents and clinicians might look to observe. A greater awareness around the female phenotype can begin to diminish the number of females with ASD without ID that slip under the radar.
Acknowledgments
Grant Sponsor: NIMH Intramural Research Program, NIH; Grant number: 1-ZIA- MH002920
Grant Sponsor: NIH; Grant number: P30HD040677
Grant Sponsor: Isadore Bertha Gudelsky Family Foundation
Footnotes
ClinicalTrials.gov Number: NCT01031407
Conflicts of Interest: Lauren Kenworthy receives financial compensation for use of the BRIEF.
References
- Achenbach TM, Howell CT, Quay HC, Conners CK. National survey of problems and competencies among four- to sixteen-year-olds: parents’ reports for normative and clinical samples. Monographs of the Society for Research in Child Development. 1991;56(3):1–131. [PubMed] [Google Scholar]
- American Psychiatric Association. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) 5. Washington, D.C: American Psychiatric Association; 2013. [Google Scholar]
- Baggs M. The naked mechanisms of echolalia. 2007 Apr 5; Retrieved from https://ballastexistenz.wordpress.com/2007/04/05/the-naked-mechanisms-of-echolalia/
- Baron-Cohen S, Knickmeyer R, Belmonte M. Sex differences in the brain: implications for explaining autism. Science. 2005 doi: 10.1126/science.1115455. Retrieved from http://www.sciencemag.org/content/310/5749/819.short. [DOI] [PubMed]
- Begeer S, Mandell D, Wijnker-Holmes B, Venderbosch S, Rem D, Stekelenburg F, Koot HM. Sex differences in the timing of identification among children and adults with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2013;43(5):1151–6. doi: 10.1007/s10803-012-1656-z. https://doi.org/10.1007/s10803-012-1656-z. [DOI] [PubMed] [Google Scholar]
- Bishop-Fitzpatrick L, Hong J, Smith LE, Makuch RA, Greenberg JS, Mailick MR. Characterizing Objective Quality of Life and Normative Outcomes in Adults with Autism Spectrum Disorder: An Exploratory Latent Class Analysis. Journal of Autism and Developmental Disorders. 2016:1–13. doi: 10.1007/s10803-016-2816-3. https://doi.org/10.1007/s10803-016-2816-3. [DOI] [PMC free article] [PubMed]
- Bölte S, Duketis E, Poustka F, Holtmann M. Sex differences in cognitive domains and their clinical correlates in higher-functioning autism spectrum disorders. Autism. 2011;15(4):497–511. doi: 10.1177/1362361310391116. https://doi.org/10.1177/1362361310391116. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, Giusti Z, Leach DB, Venuti P. Maternal reports of adaptive behaviours in young children: urban–rural and gender comparisons in Italy and United States. Infant and Child Development. 2005;14(4):403–424. https://doi.org/10.1002/icd.414. [Google Scholar]
- Brugha TS, Spiers N, Bankart J, Cooper S-A, McManus S, Scott FJ, Tyrer F. Epidemiology of autism in adults across age groups and ability levels. The British Journal of Psychiatry. 2016 doi: 10.1192/bjp.bp.115.174649. bjp.bp.115.174649 https://doi.org/10.1192/bjp.bp.115.174649. [DOI] [PubMed]
- Christensen DL, Baio J, Braun KVN, Bilder D, Charles J, Constantino JN, Yeargin-Allsopp M. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Surveillance Summaries. 2016;65(3):1–23. doi: 10.15585/mmwr.ss6503a1. https://doi.org/10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Constantine LJ, Hendren R, Rocke D, Ozonoff S. Examining executive functioning in children with autism spectrum disorder, attention deficit hyperactivity disorder and typical development. Psychiatry Research. 2009;166(2-3):210–22. doi: 10.1016/j.psychres.2008.02.005. https://doi.org/10.1016/j.psychres.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale-IV: Checklists, norms, and clinical interpretation. Guilford Press; New York: 1998. [Google Scholar]
- Dworzynski K, Ronald A, Bolton P, Happé F. How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(8):788–97. doi: 10.1016/j.jaac.2012.05.018. https://doi.org/10.1016/j.jaac.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Fombonne E. The epidemiology of autism: a review. Psychological Medicine. 1999;29(04):769–786. doi: 10.1017/s0033291799008508. https://doi.org/null. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Georgiades S, Bishop SL, Hardan AY. Behavioral and cognitive characteristics of females and males with autism in the Simons Simplex Collection. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53(3):329–40.e1. doi: 10.1016/j.jaac.2013.12.004. https://doi.org/10.1016/j.jaac.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow KD, Sprafkin J. Child and Adolescent Symptom Inventory-4R (CASI-4R) Stony Brook, NY: Checkmate Plus; 2005. [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior Rating Inventory of Executive Function. Lutz, FL: Psychological Assessment Resources, Inc; 2000. [Google Scholar]
- Gioia GA, Isquith PK, Kenworthy L, Barton RM. Profiles of everyday executive function in acquired and developmental disorders. Child Neuropsychology: A Journal on Normal and Abnormal Development in Childhood and Adolescence. 2002;8(2):121–137. doi: 10.1076/chin.8.2.121.8727. https://doi.org/10.1076/chin.8.2.121.8727. [DOI] [PubMed] [Google Scholar]
- Gould J, Ashton-Smith J. Missed diagnosis or misdiagnosis? Girls and women on the autism spectrum. Good Autism Practice (GAP) 2011;12(1):34–41. [Google Scholar]
- Howe YJ, O’Rourke JA, Yatchmink Y, Viscidi EW, Jones RN, Morrow EM. Female Autism Phenotypes Investigated at Different Levels of Language and Developmental Abilities. Journal of Autism and Developmental Disorders. 2015;45(11):3537–3549. doi: 10.1007/s10803-015-2501-y. https://doi.org/10.1007/s10803-015-2501-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume K, Loftin R, Lantz J. Increasing Independence in Autism Spectrum Disorders: A Review of Three Focused Interventions. Journal of Autism and Developmental Disorders. 2009;39(9):1329–1338. doi: 10.1007/s10803-009-0751-2. [DOI] [PubMed] [Google Scholar]
- Johnson K, Caskey M, Rand K, Tucker R, Vohr B. Gender Differences in Adult-Infant Communication in the First Months of Life. Pediatrics. 2014;134(6):e1603–e1610. doi: 10.1542/peds.2013-4289. https://doi.org/10.1542/peds.2013-4289. [DOI] [PubMed] [Google Scholar]
- Kanne SM, Gerber AJ, Quirmbach LM, Sparrow SS, Cicchetti DV, Saulnier CA. The Role of Adaptive Behavior in Autism Spectrum Disorders: Implications for Functional Outcome. Journal of Autism and Developmental Disorders. 2010;41(8):1007–1018. doi: 10.1007/s10803-010-1126-4. https://doi.org/10.1007/s10803-010-1126-4. [DOI] [PubMed] [Google Scholar]
- Kenworthy L, Black DO, Harrison B, Della Rosa A, Wallace GL. Are executive control functions related to autism symptoms in high-functioning children? Child Neuropsychology: A Journal on Normal and Abnormal Development in Childhood and Adolescence. 2009;15(5):425–440. doi: 10.1080/09297040802646983. https://doi.org/10.1080/09297040802646983. [DOI] [PubMed] [Google Scholar]
- Kenworthy L, Yerys BE, Anthony LG, Wallace GL. Understanding executive control in autism spectrum disorders in the lab and in the real world. Neuropsychology Review. 2008;18(4):320–338. doi: 10.1007/s11065-008-9077-7. https://doi.org/10.1007/s11065-008-9077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp S, Gillberg C. Girls with social deficits and learning problems: Autism, atypical Asperger syndrome or a variant of these conditions. European Child & Adolescent Psychiatry. 1992;1(2):89–99. doi: 10.1007/BF02091791. https://doi.org/10.1007/BF02091791. [DOI] [PubMed] [Google Scholar]
- Kuhlthau K, Orlich F, Hall TA, Sikora D, Kovacs EA, Delahaye J, Clemons TE. Health-Related Quality of Life in children with autism spectrum disorders: results from the autism treatment network. Journal of Autism and Developmental Disorders. 2010;40(6):721–729. doi: 10.1007/s10803-009-0921-2. https://doi.org/10.1007/s10803-009-0921-2. [DOI] [PubMed] [Google Scholar]
- Lai MC, Lombardo MV, Auyeung B, Chakrabarti B, Baron-Cohen S. Sex/Gender Differences and Autism: Setting the Scene for Future Research. Journal of the American Academy of Child & Adolescent Psychiatry. 2015;54(1):11–24. doi: 10.1016/j.jaac.2014.10.003. https://doi.org/10.1016/j.jaac.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M-C, Lombardo MV, Ruigrok ANV, Chakrabarti B, Wheelwright SJ, Auyeung B, Baron-Cohen S. Cognition in Males and Females with Autism: Similarities and Differences. PLoS ONE. 2012;7(10) doi: 10.1371/journal.pone.0047198. https://doi.org/10.1371/journal.pone.0047198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson RA, Papadakis AA, Higginson CI, Barnett JE, Wills MC, Strang JF, Kenworthy L. Everyday executive function impairments predict comorbid psychopathology in autism spectrum and attention deficit hyperactivity disorders. Neuropsychology. 2015;29(3):445–453. doi: 10.1037/neu0000145. https://doi.org/10.1037/neu0000145. [DOI] [PubMed] [Google Scholar]
- Le Couteur A, Rutter M, Lord C, Rios P, Robertson S, Holdgrafer M, McLennan J. Autism diagnostic interview: A standardized investigator-based instrument. Journal of Autism and Developmental Disorders. 1989;19(3):363–387. doi: 10.1007/BF02212936. https://doi.org/10.1007/BF02212936. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Park HR. An Integrated Literature Review on the Adaptive Behavior of Individuals With Asperger Syndrome. Remedial and Special Education. 2007;28(3):132–139. https://doi.org/10.1177/07419325070280030201. [Google Scholar]
- Lemon JM, Gargaro B, Enticott PG, Rinehart NJ. Executive functioning in autism spectrum disorders: a gender comparison of response inhibition. Journal of Autism and Developmental Disorders. 2011;41(3):352–6. doi: 10.1007/s10803-010-1039-2. https://doi.org/10.1007/s10803-010-1039-2. [DOI] [PubMed] [Google Scholar]
- Levy D, Ronemus M, Yamrom B, Lee Y, Leotta A, Kendall J, Wigler M. Rare De Novo and Transmitted Copy-Number Variation in Autistic Spectrum Disorders. Neuron. 2011;70(5):886–897. doi: 10.1016/j.neuron.2011.05.015. https://doi.org/10.1016/j.neuron.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, Lainhart JE. Comorbid psychiatric disorders in children with autism: interview development and rates of disorders. Journal of Autism and Developmental Disorders. 2006;36(7):849–861. doi: 10.1007/s10803-006-0123-0. https://doi.org/10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop SL. ADOS-2: Autism Diagnostic Observation Schedule, Second Edition. 2. Torrance, CA: Western Psychological Services; 2012. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic Interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Schopler E. Differences in sex ratios in autism as a function of measured intelligence. Journal of Autism and Developmental Disorders. 1985;15(2):185–193. doi: 10.1007/BF01531604. [DOI] [PubMed] [Google Scholar]
- Lord C, Schopler E, Revicki D. Sex differences in autism. Journal of Autism and Developmental Disorders. 1982;12(4):317–330. doi: 10.1007/BF01538320. [DOI] [PubMed] [Google Scholar]
- Mead You call me expressive and miss the struggle it takes. 2016 Jul 14; Retrieved from https://kpagination.wordpress.com/2016/07/13/you-call-me-expressive-and-miss-the-struggle-it-takes/
- Memari AH, Ziaee V, Shayestehfar M, Ghanouni P, Mansournia MA, Moshayedi P. Cognitive flexibility impairments in children with autism spectrum disorders: Links to age, gender and child outcomes. Research in Developmental Disabilities. 2013;34(10):3218–3225. doi: 10.1016/j.ridd.2013.06.033. https://doi.org/10.1016/j.ridd.2013.06.033. [DOI] [PubMed] [Google Scholar]
- Pugliese CE, Anthony L, Strang JF, Dudley K, Wallace GL, Kenworthy L. Increasing Adaptive Behavior Skill Deficits From Childhood to Adolescence in Autism Spectrum Disorder: Role of Executive Function. Journal of Autism and Developmental Disorders. 2015;45:1579–1587. doi: 10.1007/s10803-014-2309-1. https://doi.org/http://dx.doi.org/10.1007/s10803-014-2309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivet TT, Matson JL. Review of gender differences in core symptomatology in autism spectrum disorders. Research in Autism Spectrum Disorders. 2011;5(3):957–976. https://doi.org/10.1016/j.rasd.2010.12.003. [Google Scholar]
- Robinson EB, Lichtenstein P, Anckarsäter H, Happé F, Ronald A. Examining and interpreting the female protective effect against autistic behavior. Proceedings of the National Academy of Sciences. 2013;110(13):5258–5262. doi: 10.1073/pnas.1211070110. https://doi.org/10.1073/pnas.1211070110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa SDR. THINKING PERSON’S GUIDE TO AUTISM: How Can We All Do Better By Our Autistic Girls? 2016 Apr; Retrieved from http://www.thinkingautismguide.com/2016/04/how-can-we-all-do-better-by-our.html.
- Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales: Survey Form Manual. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- Sparrow SS, Cicchetti D, Balla DA. Vineland Adaptive Behavior Scales--2nd Edition manual. Minneapolis, MN: NCS Pearson, Inc; 2005a. [Google Scholar]
- Sparrow SS, Cicchetti D, Balla DA. Vineland Adaptive Behavior Scales--2nd Edition manual. Minneapolis, MN: NCS Pearson, Inc; 2005b. [Google Scholar]
- Wallace GL, Kenworthy L, Pugliese CE, Popal HS, White EI, Brodsky E, Martin A. Real-World Executive Functions in Adults with Autism Spectrum Disorder: Profiles of Impairment and Associations with Adaptive Functioning and Co-morbid Anxiety and Depression. Journal of Autism and Developmental Disorders. 2016;46(3):1071–1083. doi: 10.1007/s10803-015-2655-7. https://doi.org/10.1007/s10803-015-2655-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werling DM, Geschwind DH. Sex differences in autism spectrum disorders. Current Opinion in Neurology. 2013;26(2):146–53. doi: 10.1097/WCO.0b013e32835ee548. https://doi.org/10.1097/WCO.0b013e32835ee548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C. PRevalence of autism in a us metropolitan area. JAMA. 2003;289(1):49–55. doi: 10.1001/jama.289.1.49. https://doi.org/10.1001/jama.289.1.49. [DOI] [PubMed] [Google Scholar]
- Yerys BE, Kenworthy L, Jankowski KF, Strang J, Wallace GL. Separate components of Emotional Go/No-Go performance relate to autism versus attention symptoms in children with autism. Neuropsychology. 2013;27(5):537–545. doi: 10.1037/a0033615. https://doi.org/10.1037/a0033615. [DOI] [PMC free article] [PubMed] [Google Scholar]


