Abstract
In the United States, colorectal cancer (CRC) is the second-leading cause of cancer-related deaths. The most effective strategy for prevention of CRC is to screen for and remove precancerous polyps. There are various ways to screen for CRC. In the United States, the most common method is colonoscopy. Polyps are classified primarily through pathology. Size is the primary risk factor for malignancy. In general, the bigger the polyp, the greater the risk for malignancy. There are 3 basic options for removal: standard polypectomy; advanced resection techniques, known as endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD); and surgical removal. In the past 10 years, the use of surgical removal has significantly decreased. Noninvasive, nonmalignant polyps can be removed endoscopically. EMR and ESD are very effective and achieve similar clinical outcomes. Both procedures begin with a submucosal injection. The submucosal lift is one of the most significant advances that have been made in polypectomy. Traditionally, the approach to achieving a submucosal lift has relied on the use of a saline solution. Saline is inexpensive and widely available, but it dissipates quickly. Various viscous agents have been added to saline to maintain mucosal lifting throughout the procedure. Although most are effective, they are used off-label. The only solution approved by the US Food and Drug Administration for the submucosal lift procedure is Eleview. In a clinical trial, Eleview decreased the mean total injected volume and the mean total injected volume per lesion as compared with saline. Other advances in polypectomy techniques include the use of cold snare polypectomy and high-definition colonoscopes. The quality of the colonoscopy can also be improved if a physician knows and monitors his or her adenoma detection rate.
Introduction
Colorectal cancer (CRC) is one of the more commonly diagnosed malignancies in the United States. Both the incidence rate and mortality rate have steadily declined throughout much of the previous 20 years.1 More than 1,317,000 patients were living with either colon or rectal cancer in 2014. In 2017, an estimated 135,430 new cases of CRC are expected to be diagnosed, making it the fourth most common cancer diagnosis overall. It is the second-leading cause of cancer-related mortality (after lung/bronchus cancer), and will lead to an estimated 50,260 deaths in 2017. Overall, an American has a 4.3% lifetime risk of being diagnosed with either colon cancer or rectal cancer (based on data from 2012-2014).1
CRC is more common in men than women. The age-adjusted incidence was 46.0 per 100,000 persons for men and 35.1 per 100,000 persons for women in the Surveillance, Epidemiology, and End Results database from 2010 to 2014.1 The incidence is also affected by race. African Americans have the highest incidence in both men (56.4 per 100,000 persons) and women (43.2 per 100,000 persons). The lowest incidence is found in Hispanics (40.0 cases per 100,000 persons in men and 29.1 cases per 100,000 persons in women) and in Asian/Pacific Islanders (40.2 cases per 100,000 persons in men and 28.8 cases per 100,000 persons in women).1
Age is another important factor. The median age at the diagnosis of CRC is 67 years.1 Most patients (67.6%) are diagnosed between the ages of 55 and 84 years. Nearly 80% of patients are diagnosed at ages 45 years or older. Starting at age 45, the percent of new cases increases with each decade of life, until it peaks at 24.2% between ages 65 and 74 years.1
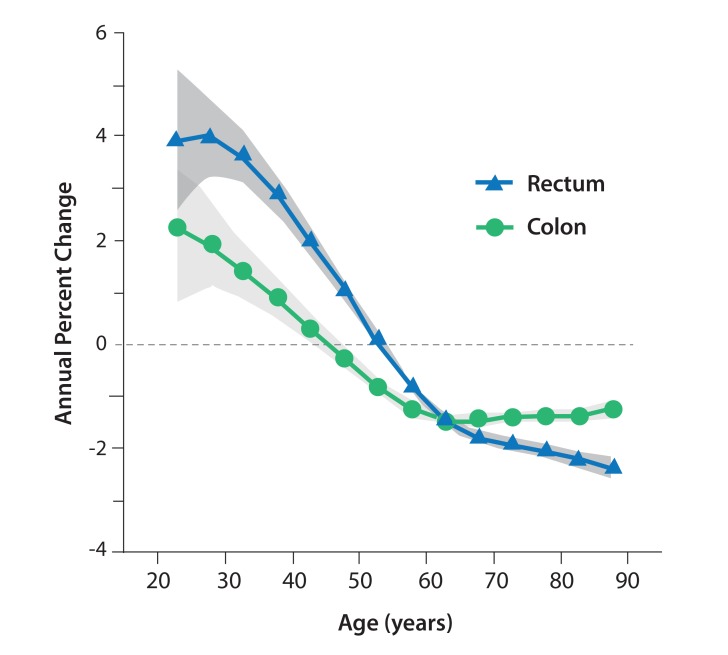
The rate of CRC has recently increased among patients younger than 50 years (Figure 1).2 This finding is significant because screening for CRC typically begins at age 50 years. A 2017 study found that the annual incidence rate of colon cancer has increased by 1.0% to 2.4% since the mid-1980s among adults ages 20 to 39 years. For adults ages 40 to 54 years, the annual incidence rate of colon cancer has increased by 0.5% to 1.3% since the mid-1990s.2 The annual incidence rates of rectal cancer increased at an even greater magnitude, 3.2%, from 1974 to 2013 among adults ages 20 to 29 years. These increases, however, should be considered within the context of the overall low number of colon and rectal cancer cases in younger individuals.
Figure 1.
Annual percent changes in the incidences of colon and rectal cancer according to age. The 95% CIs are indicated by the shaded bands. Adapted from Siegel RL et al. J Natl Cancer Inst. 2017;109(8).2
The reasons behind these rising incidence rates are unknown. One possibility is the obesity epidemic in the United States and throughout much of the Western world.3 Obesity is a major risk factor for many cancers, including CRC.4 Another possible explanation is the current screening strategy, which omits younger patients in the general population. It may be necessary for screening to begin at an earlier age, either in all people or just those in high-risk groups. For example, among African Americans, not only is the incidence of CRC higher, but disease onset is earlier.5 Current screening guidelines have already been changed to reflect this observation, and now recommend that African Americans begin to undergo CRC screening at age 45 years instead of age 50.6,7
Based on data from the years 2007 to 2013, the 5-year relative survival rate for patients with CRC was 64.9%.1 It is clear that patient prognosis is significantly affected by the disease stage at the time of diagnosis, and that the more advanced the disease, the worse the survival. For example, patients with localized disease (confined to the primary site) have a higher 5-year relative survival rate (89.9%) and a very good prognosis. However, when the disease has spread to local lymph nodes (known as regional disease), the 5-year relative survival rate decreases to 71.3%. In patients whose disease is already metastatic at diagnosis (with distant disease spread), the 5-year relative survival rate drops precipitously, to just 13.9%.1
Prevention of Colon Cancer
The main goals for prevention are twofold: first, to prevent the development of colon cancer via the removal of precancerous polyps and lesions; and second, to allow for early detection of the disease at a localized stage or when regional spread is minimal. Disease stage at diagnosis strongly impacts patient prognosis and survival.1 There is a significant effort to diagnose patients at an earlier stage, when the cancer is more treatable and the prognosis is better.
The most effective strategy for prevention of CRC is screening to detect precancerous polyps and remove them. There are various ways to screen for CRC. In the United States, the most common method is colonoscopy.8 Other approved methodologies include the fecal occult blood test, the DNA stool test, virtual colonoscopy, and sigmoidoscopy. Even barium enemas may be useful, although their use is not widespread.
The Role of Screening Colonoscopy
Large studies in the United States, Canada, and Europe have demonstrated that colonoscopy is very effective.9-11 Updated data from the National Polyp Study showed that colonoscopies reduce the risk of CRC by approximately 50%.12
The rates of screening have been steadily increasing over the years.13 Approximately two-thirds of the older population undergoes some form of screening for CRC.13 Given the important impact of CRC screening on survival, there has been a major focus on increasing screening among the eligible population. The National Colorectal Cancer roundtable has a public relations campaign, known as “80 by ‘18,” which reflects the goal of achieving an 80% rate of screening among eligible patients by 2018.14 To achieve this goal, physicians and other healthcare providers must consider how to encourage patients to undergo colonoscopy. One of the most important ways is to emphasize the benefits. Among all of the cancer prevention strategies (eg, mammography, Pap smears, prostate screening, lung cancer screening), colonoscopy is probably the most effective. However, many people are still averse to the procedure, primarily because of the bowel preparation. Some people find this requirement embarrassing, difficult, and/or uncomfortable. In reality, bowel preparation formulations have become much easier to use. There are now low-volume bowel preps and so-called split-dose bowel preps.15,16 The dietary restrictions required for the day before a colonoscopy have also become less strict. In the past, patients had to adhere to a clear, liquids-only diet. There is now good evidence that low-residue foods, such as milkshakes and smoothies, are fine. Lastly, sedation has greatly improved, so that colonoscopy is now essentially a painless procedure.
Together, all of these factors should encourage people to undergo screening colonoscopy. For most people who are not at increased risk for CRC, the guidelines recommend a colonoscopy every 10 years. In comparison to other routine medical examinations, screening colonoscopies are far less frequent.
Polyp Classification
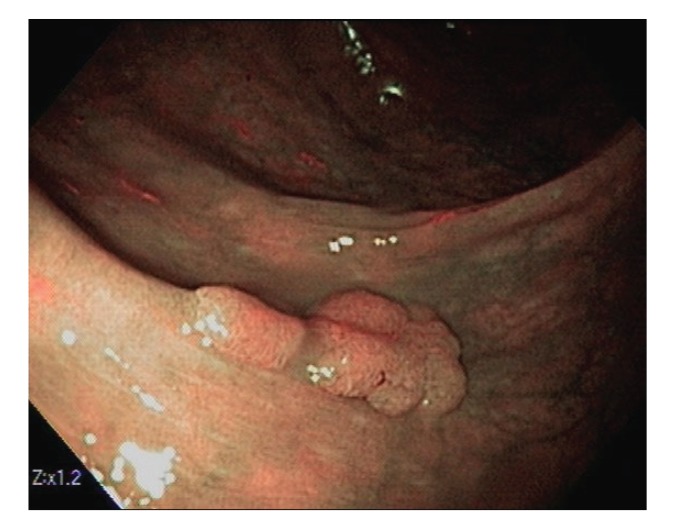
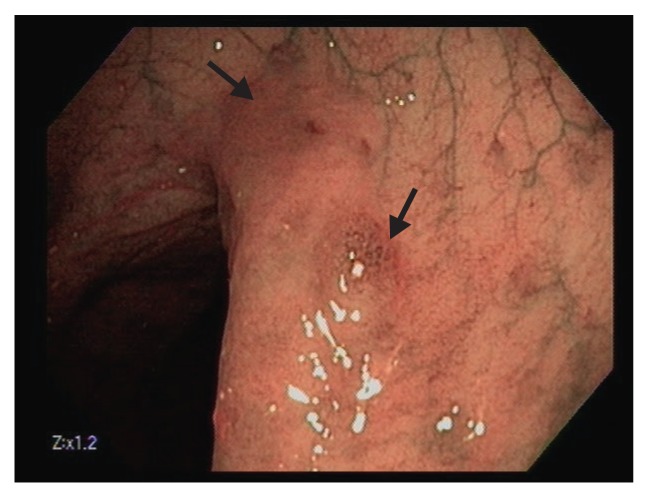
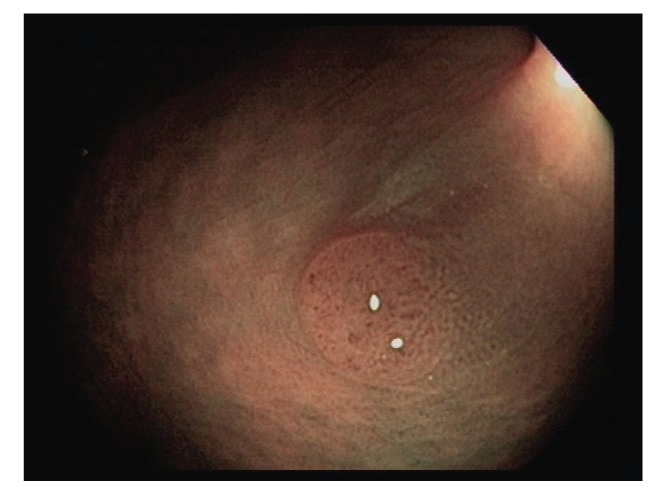
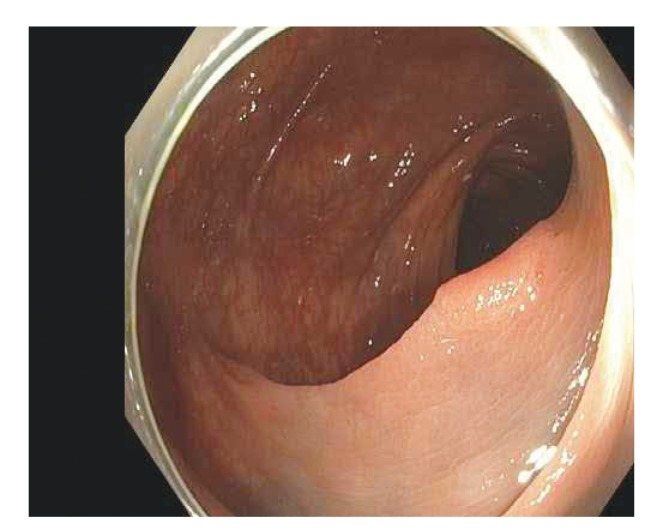
Polyps are classified primarily through pathology. Completely benign polyps are referred to as hyperplastic, especially when they are small and located in the rectosigmoid colon. There are several types of premalignant polyps, most commonly adenomas and sessile serrated polyps (Figures 2-4). Polyps can also be classified based on their shape, using the Paris classification system (Table 1).17 Pedunculated polyps are shaped like mushrooms. Sessile polyps are shaped like mounds. As mentioned, there are also flat polyps, which are the most difficult to see.
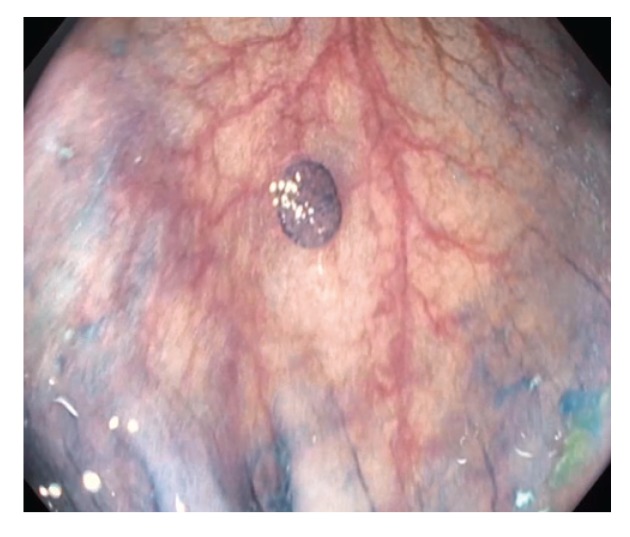
Figure 2.
This 1.2-cm lateral polyp (Paris class IIa) was removed by standard hot snare. The histology was a tubular adenoma.
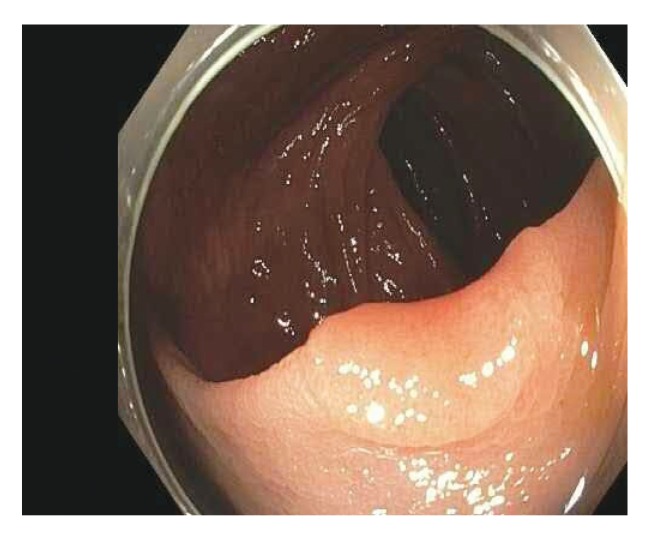
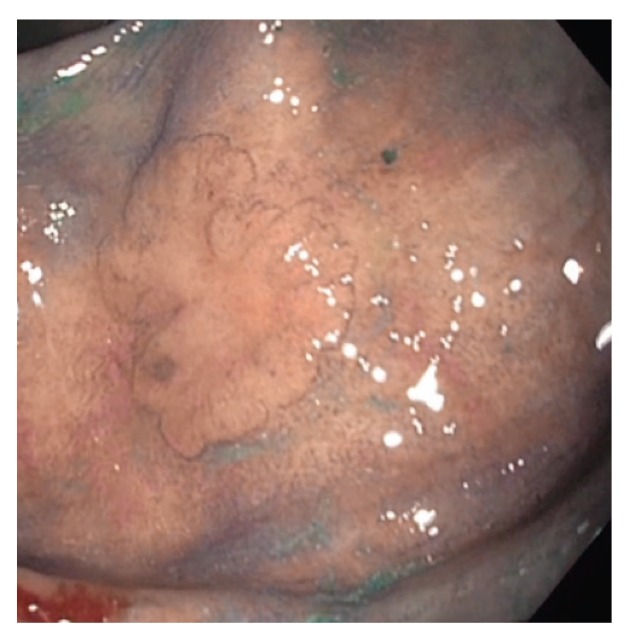
Figure 4.
This 2-cm, flat serrated polyp (Paris class IIb/Is) has a focal sessile region of dysplasia in the right center. Note the broad, flat area left of the lesion with slight erythema and loss of vascular markings.
Table 1.
The Paris Classification
| Endoscopic Appearance | Paris Class | Description |
|---|---|---|
| Protruded lesions | Ip | Pedunculated polyps |
| Ips | Subpedunculated polyps | |
| Is | Sessile polyps | |
| Flat elevated lesions | IIa | Flat elevation of the mucosa |
| IIa/IIc | Flat elevation with central depression | |
| Flat lesions | IIb | Flat mucosal change |
| IIc | Mucosal depression | |
| IIc/IIa | Mucosal depression with raised edge |
Adapted from Endoscopic Classification Review Group. Endoscopy. 2005;37(6):570-578.17
Figure 3.
This 6-mm sessile polyp was removed (not shown) by cold snare polypectomy. The histology was tubular adenoma.
A minority of polyps become malignant. Size is the primary risk factor for malignancy. In general, the bigger the polyp, the greater the risk for malignancy.18 Once the polyp exceeds 1 cm (or 0.5 in), the risk rises significantly. When a polyp is removed and examined pathologically, certain microscopic features can show an increased risk for malignancy. Villous polyps, which have microscopic fingerlike projections, have a higher risk.18 Polyps that exhibit high-grade dysplasia are more likely to become malignant over time (if not removed).19 Shape is another risk factor, with the highest likelihood for malignancy found in polyps that are flat, particularly those with a central depression.20
Polypectomy
Typically, all polyps that are encountered should be removed. (The only exceptions are polyps that are readily identifiable as completely benign/hyperplastic; these polyps are very small and located in the lower part of the bowel or the rectum.) There are 3 basic options for removal: standard polypectomy; advanced resection techniques, known as endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD); and surgical removal. In the past 10 years, the use of surgical removal has significantly decreased. All noninvasive, nonmalignant polyps can be removed endoscopically. Even very superficial stage 1A cancers can be removed endoscopically, without surgical removal of the bowel.
The choice among these techniques is based on the size and shape of the polyp. Most small polyps (<1 to 2 cm) are removed with snare polypectomy.21 Polyps that are larger than 2 cm but that lack the appearance of an invasive cancer are removed by EMR (Figure 5). When there is suspicion of early invasive cancer (stage T1A), and the polyp is larger than 1 to 2 cm (the limit of what can be removed en bloc by EMR), ESD is the most appropriate technique to allow en bloc removal. Typically, these polyps are flat with a central depression. Surgical removal is generally required if there is evidence of a deeply invasive cancer, such as an ulcerated appearance or bleeding. In these cases, the polyp is biopsied to assess for cancer, and the patient is referred to a surgeon for removal.
Figure 5.
En bloc removal with endoscopic mucosal resection.
The Submucosal Lift
EMR and ESD are very effective and achieve similar clinical outcomes. Both procedures begin with a submucosal injection. The submucosal lift is one of the most significant advances that have been made in polypectomy. The major goals of the submucosal lift are twofold. The first goal is to vertically elevate the polyp so that a snare can grab onto the tissue. It is difficult to grab a flat, nonraised surface. The second goal is to increase the safety cushion by creating a greater margin between the surface (the mucosa to be resected) and the deepest layers (the muscle layer and serosa to be protected). These goals are accomplished by expanding the submucosal layer, which is the layer between the mucosa and the muscle.
Traditionally, the approach to achieving a submucosal lift has relied on the use of a simple saline solution.22 Saline is inexpensive and widely available. A disadvantage, however, is that saline dissipates very quickly. It provides a 1- or 2-minute span to perform the resection. Another disadvantage of saline is that it is clear, and not easily visible.
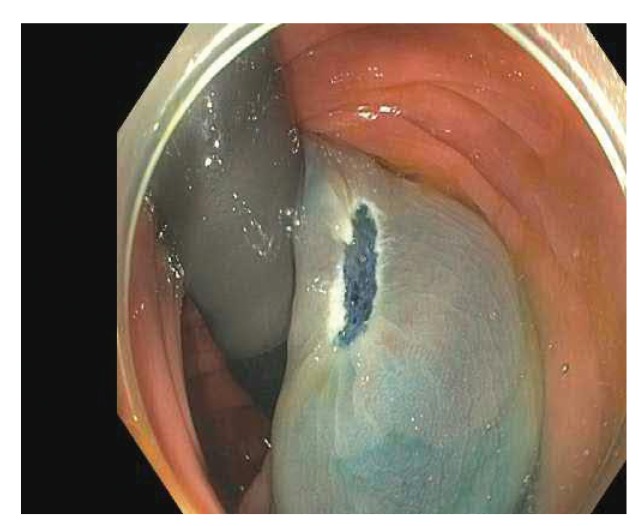
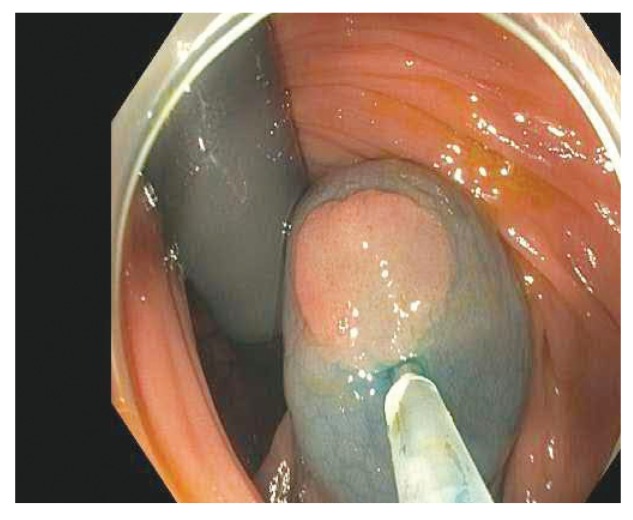
Newer methods for the submucosal lift procedure use a combination of a blue dye, such as methylene blue or indigo carmine, and a viscous agent to maintain the vertical elevation (Figures 6-8). The major advantage of using a blue dye is that it clearly distinguishes what has been lifted from what has not been lifted. Other agents that can be effective include hydroxyethyl and hydroxypropyl methylcellulose. There has recently been some difficultly in obtaining methylene blue and indigo carmine in the United States owing to various supply problems.
Figure 6.
A 1-cm flat lesion in the ascending colon with a slight central depression.
Figure 8.
The same lesion after en bloc resection, with clear lateral margins.
Figure 7.
The same lesion after a submucosal lift using a viscous agent with methylene blue.
The only solution approved by the US Food and Drug Administration for the submucosal lift procedure is Eleview. Eleview consists of a premixed solution of methylene blue; water for injection; medium-chain triglycerides; the bulking/cushioning agent poloxamer 188; polyoxyl-15-hydroxystearate; and sodium chloride. Eleview was recently evaluated in a large clinical trial. The interim results were presented by Rex and colleagues at the 2017 Digestive Disease Week.23 This randomized, double-blind study compared Eleview vs saline solution for use with EMR for removal of colonic polyps larger than 2 cm. A total of 226 patients were enrolled on-study by April 21, 2017, and 211 were included in the primary analysis. The study excluded patients with lesions that showed macroscopic or pit pattern features suggestive of invasive cancer, those who had undergone previous endoscopic attempts at resection, and those with coagulation disorders.23
The study had 3 primary efficacy endpoints: total injected volume needed to complete the EMR procedure, total injected volume per lesion size, and time to resect the lesion completely. The mean total injected volume needed to complete the EMR procedure was 16.1 mL (±9.8 mL) with Eleview vs 31.6 mL (±32.1 mL) with saline, a significant difference (P<.001; Table 2). The mean total injected volume per lesion size was also less for Eleview, at 0.53 mL/mm (±0.32 mL), vs 0.92 mL/mm (±0.65 mL) for saline (P<.001; Table 3). The time to complete the lesion resection was 19.15 minutes (±16.80 minutes) with Eleview vs 29.70 minutes (±69.18 minutes) with saline, although this difference did not reach statistical significance (P=.326).
Table 2.
Total Injected Volume Used to Complete the EMR Procedure in a Trial of Eleview vs Saline
| Endpoint | Statistics | Eleview (n=102) | Saline (n=109) |
|---|---|---|---|
| Total injected volume to complete the EMR procedure (mL) | Mean (±SD) | 16.1 (±9.8) | 31.6 (±32.1) |
| Range | 3.0-41.0 | 4.0-248.0 | |
| P value | <.001 |
EMR, endoscopic mucosal resection; SD, standard deviation.
Data from Rex D et al. DDW abstract 689. Gastrointest Endosc. 2017;85(5 suppl).23
Table 3.
Total Injected Volume Per Lesion Size in a Trial of Eleview vs Saline
| Endpoint | Statistics | Eleview (n=102) | Saline (n=109) |
|---|---|---|---|
| Total injected volume per lesion size (mL/mm) | Mean (±SD) | 0.53 (±0.32) | 0.92 (±0.65) |
| Range | 0.09-1.75 | 0.20-4.96 | |
| P value | <.001 |
SD, standard deviation.
Data from Rex D et al. DDW abstract 689. Gastrointest Endosc. 2017;85(5 suppl).23
The trial had several secondary efficacy endpoints. The mean number of resected pieces was 5.70 (±6.0) with Eleview vs 6.47 (±5.0) with saline (P=.052). The mean Sydney Resection Quotient, which measures the mean size of each snared tissue specimen, was 10.3 (±8.1) with Eleview vs 8.0 (±5.7) with saline (P=.044). Rates of en bloc resection were 18.6% for Eleview compared with 11.0% for saline, a nonsignificant difference (P=.125).
The rate of complications was similar with Eleview vs saline (15.0% vs 15.2%). The risk for bleeding complications was not significantly increased with Eleview. When surveyed, most endoscopists ranked the use of Eleview and the reference comparator as either very easy, easy, or neutral (80.3% vs 78.9%).23
There are a few general points to keep in mind when using EMR injections. The primary method to achieve a good injection is to properly place the needle into the superficial submucosa. The needle is usually inserted at a shallow angle relative to the wall of the bowel. The fluid is slowly injected as the needle enters through the mucosa into the submucosa. Once it enters the submucosal space, a rapid elevation in the tissue can be visualized. When the tissue starts to rise, the needle is lifted toward the center of the lumen of the bowel to increase the vertical elevation of the injection and allow the liquid to stay within the superficial submucosa. This is done by deflecting the endoscope while the needle is embedded in the submucosal space. Generally, enough fluid is injected to provide a 5-mm to 10-mm rim around the polyp to obtain a complete lift. The entire polyp does not need to be lifted and snared with a single injection. Typically, polyps up to 2 to 3 cm can be removed with 1 injection and 1 snare. Larger polyps can be removed in pieces of approximately 2 cm, repeating the steps of injection and removal as needed.
EMR vs ESD
EMR is a highly effective method for removal of most flat and laterally spreading polyps larger than 2 cm, as the vast majority of these polyps are noninvasive or only superficially invasive in the mucosa. ESD is a more complex technique that was originally developed in Japan. It is now performed worldwide, including in the United States. Like EMR, ESD begins with an injection to create a fluid cushion under the polyp. But then, instead of using a wire loop snare, a small needle knife is used to make a precise incision around the perimeter of the polyp and then to dissect underneath the submucosal space. The advantage to ESD is that the entire polyp can be removed as a single piece, regardless of size.
ESD has a theoretical benefit in the case of an invasive cancer because the entire lesion can be removed as a single piece, which allows precise deep and lateral margin assessment pathologically. ESD is also associated with a slightly lower rate of recurrence,24,25 although any clinical benefit to this improvement is unknown. A disadvantage to ESD is that the procedure takes approximately 2 to 3 hours to perform, compared with 20 to 30 minutes for EMR. ESD is also associated with greater risks, particularly perforation. Fortunately, nearly all of these perforations can be closed with endoscopic clips. It is rare that a perforation requires surgery.
Cold Snare Polypectomy
An interesting development in polypectomy techniques is the use of cold snare polypectomy. Typically, smaller polyps have been removed with either biopsy forceps or a snare with an electrosurgical current to burn through the polyp, but the limitations of these techniques became clear over time. The biopsy forceps were inadequate for the removal of nearly all polyps, with the exception of those that were very small (<2-3 mm). The use of an electrosurgical current increases the risk of complications, including both bleeding and pain after the procedure. A number of studies have now shown that cold snare polypectomy is preferred to these other methods.21,26,27 Cold snare polypectomy increases the resection rate and reduces the complication rate. It is also less expensive and more convenient to use because it does not require additional devices to apply electrosurgical currents through the snare. Recent studies suggest that cold snare methods in combination with submucosal lifting can be used to remove even larger polyps.28,29 This procedure is termed “cold-snare EMR.”
Challenges in Polypectomy
The biggest challenge in the removal of large, flat polyps comes from a lack of awareness and access to high-quality EMR services, particularly in Western countries. Many physicians still refer patients with large, flat lesions for surgery because they may be unaware of the many expert centers throughout the United States where EMR and ESD are routinely performed. There is a significant learning curve for EMR. An endoscopist must perform approximately 150 EMR procedures before becoming adept.30 For this reason, it is generally recommended that EMR procedures be performed at high-volume centers or referral centers. A major goal is to increase national awareness of the widespread availability of these centers. Many of them are listed at: http://www.sease.com/polyp/emr.html.31
There are several other challenges in EMR and ESD. An important issue concerns the first steps taken when a polyp is found during the index colonoscopy. Procedures such as biopsy, use of a cautery or hot snare, and tattooing can cause scarring of the tissue underneath the polyp, thereby complicating subsequent definitive removal. As a result, when fluid is injected for EMR or ESD, the polyp does not lift up very well. During the index colonoscopy, it is good practice for the physician to place a tattoo mark 2 to 3 cm away from the lesion, preferably on the opposite side of the colon. If there is a concern for cancer, it is acceptable to take 1 or 2 small biopsies. In most cases, however, a biopsy is not necessary because the polyp will subsequently be removed and examined by a pathologist.
Another challenge concerns the removal of small polyps, especially those that are flat. The CARE study (Complete Adenoma Resection) examined the rate of incomplete resection by assessing for the presence of neoplastic tissue in postpolypectomy biopsies.32 Among 346 polyps (from 269 patients) that were removed by 11 gastroenterologists, 10.1% were incompletely resected. The rate of incomplete resection rose as the size of the neoplastic polyp increased, at 17.3% for large lesions (10 to 20 mm) vs 6.8% for small lesions (5 to 9 mm). Sessile serrated polyps were notably more difficult to remove in their entirety compared with adenomas; the rate of incomplete resection was 31.0% vs 7.2%, respectively. Unfortunately, large, sessile serrated polyps are most likely to progress to cancer. The best way to avoid the incomplete removal of a lesion is to perform EMR.
Improving the Adenoma Detection Rate
Recent attention has focused on variability in the quality of colonoscopy among different physicians. Screening colonoscopy may fail to identify a patient with CRC for several reasons. The major cause is missed polyps, particularly sessile serrated polyps, which tend to be flat. Another cause is rapidly growing polyps that develop during the 5-to-10-year interval between colonoscopies. In addition, a resection procedure can leave behind some of the polyp. It can be difficult to visualize a polyp’s exact dimensions and edges, particularly when it is flat. Throughout the past decade, efforts have been made to increase the adenoma detection rate (ADR), especially for polyps that are difficult to detect. These efforts have focused on 2 areas: better technologies and better techniques. The introduction of better technologies has had a modest benefit. For example, high-definition scopes can increase the detection rate by approximately 3% to 4%. Also helpful are new devices that can be attached to the end of the colonoscope to flatten the twists, turns, and folds of the colon and thereby improve visibility as the scope is pulled back. Wide-angle scopes show some promise, although the data are mixed.33 Some newer scopes have rear-viewing cameras in addition to the traditional front-viewing cameras, doubling the viewing area from the colonoscope.34
The other major interest has been in refining aspects of the technique. The colon should be well-distended with gas; carbon dioxide is preferable because it is more comfortable for the patient.35 An endoscopist must be trained to find the subtle flat polyps that are difficult to see and to look carefully behind all the folds and flexures of the colon. Optimal bowel preparation is key.
The EQUIP studies (Endoscopic Quality Improvement Project) aimed to find ways to improve the ADR, even among experienced endoscopists. A series of EQUIP studies from the past 5 years demonstrated that it is possible to significantly improve the ADR with a quality-training program.36,37 Importantly, this improvement was durable over time.
Another technique that may improve the ADR is chromoendoscopy, in which the bowel is stained with a blue dye.38,39 The blue dye highlights the surface patterns of the colon and increases the contrast between the polyp tissue and the normal colon wall. Traditionally, this effect has been achieved by spraying blue dye onto the colon wall during the colonoscopy.
There have been advances in the availability of a delayed-release,40 tablet form of methylene blue, which is taken with the bowel preparation the evening before the colonoscopy. The colon will be stained before the physician performs the procedure, eliminating the need to use additional dyes (Figures 9-11). A tablet form of methylene blue was recently evaluated in a clinical trial.41 The results are not yet published, but preliminary data suggested that it will significantly increase the ADR.
Figure 9.
This flat lesion (Paris class IIb) in the right colon is highlighted by chromoendoscopy.
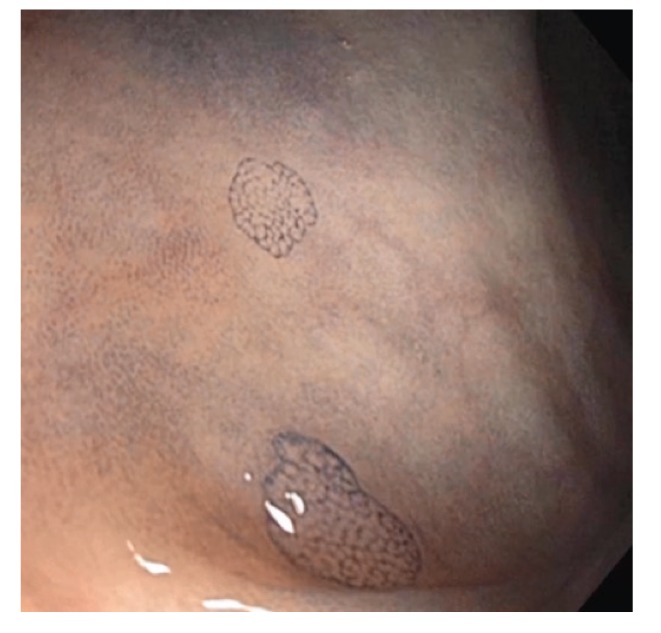
Figure 11.
This 3-mm sessile polyp (Paris class Is) in the sigmoid colon demonstrates avid uptake of methylene blue. The blue dye highlights the overall lesion and also provides pit pattern staining to differentiate polyp subtypes.
Figure 10.
Two diminutive flat lesions are highlighted with methylene blue chromoendoscopy. Note the avid uptake of methylene blue by the polyp in contrast to the minimal uptake by the surrounding normal mucosa.
Importantly, the quality of the colonoscopy can also be improved if a physician knows and monitors his or her own ADR.42 If a physician has this information, he or she is likely to make changes to improve the rate, particularly if there is significant room for improvement. Benchmarks for the ADR have been published by the American Society for Gastrointestinal Endoscopy. The current benchmark for a high-quality examination is an ADR of at least 25%.43
Conclusion
The current approaches to colon cancer prevention are effective, but there is room for improvement. The most important step for an individual to take is to undergo screening by one of the accepted screening methods, chiefly colonoscopy. For physicians, the primary ways to improve the quality of colonoscopy include thorough bowel preparation, high-quality inspection of the colon, methods to improve the ADR, and techniques to ensure that once polyps are found, they are completely removed. Virtually all polyps of any size, as long as they are not invasive cancers, can be removed endoscopically without surgery.
New Strategies to Improve Polypectomy During Colonoscopy
CME Post-Test: Circle the correct answer for each question below.
1. An American has a ___ lifetime risk of being diagnosed with either colon cancer or rectal cancer.
a. 1.9%
b. 2.6%
c. 3.5%
d. 4.3%
2. Recently, the rate of colorectal cancer has increased among:
a. People with diabetes
b. People with esophageal cancer
c. People older than 80 years
d. People younger than 50 years
3. Data from the National Polyp Study showed that colonoscopies reduce the risk of colorectal cancer by approximately ____.
a. 30%
b. 40%
c. 50%
d. 60%
4. Which polypectomy technique has become less common in the past 10 years?
a. Cold snare polypectomy
b. Endoscopic mucosal resection
c. Endoscopic submucosal dissection
d. Surgery
5. Most small polyps (<1 to 2 cm) are removed with:
a. Snare polypectomy
b. Endoscopic mucosal resection
c. Endoscopic submucosal dissection
d. Surgery
6. Which is the better technique to remove polyps that are larger than 2 cm but that lack the appearance of an invasive cancer?
a. Endoscopic mucosal resection
b. Endoscopic submucosal dissection
7. Which procedure can be used to remove the entire polyp as a single piece, regardless of size?
a. Endoscopic mucosal resection
b. Endoscopic submucosal dissection
8. In a clinical trial of solutions used for a submucosal lift, the mean total injected volume needed to complete the endoscopic mucosal resection procedure was:
a. 15.0 mL with Eleview vs 24.9 mL with saline
b. 18.3 mL with Eleview vs 27.7 mL with saline
c. 16.1 mL with Eleview vs 31.6 mL with saline
d. 20.8 mL with Eleview vs 41.6 mL with saline
9. When is surgical removal of a polyp required?
a. When the patient is older than 75 years
b. When the polyp is pedunculated
c. When the polyp has an ulcerated appearance or is bleeding
d. When the polyp is located in the rectosigmoid colon
10. During the index colonoscopy, a tattoo to mark the presence of a polyp should be made:
a. In the center of the lesion
b. Under the lesion
c. 1 cm to the right of the lesion
d. 2 to 3 cm away from the lesion, preferably on the opposite side of the colon
Evaluation Form: New Strategies to Improve Polypectomy During Colonoscopy
If you wish to receive acknowledgment for completing this activity, please complete the post-test by selecting the best answer to each question, complete this evaluation verification of participation, and fax to: (303) 790-4876. You may also complete the post-test online at www.cmeuniversity.com. On the navigation menu, click on “Find Post-tests by Course” and search by project ID 12730. Upon successfully registering/logging in, completing the post-test and evaluation, your certificate will be made available immediately.
1. What degree best describes you?
□ MD/DO
□ PA/PA-C
□ NP
□ RN
□ PharmD/RPh
□ PhD
□ Other, please specify:
2. What is your area of specialization?
□ Gastroenterology
□ Oncology, Medical
□ Other Clinical
3. Which of the following best describes your primary practice setting?
□ Solo Practice
□ Group Practice
□ Government
□ University/teaching system
□ Community Hospital
□ HMO/managed care
□ Non-profit/community
□ I do not actively practice
□ Other, please specify:
4. How long have you been practicing medicine?
□ More than 20 years
□ 11-20 years
□ 5-10 years
□ 1-5 years
□ Less than 1 year
□ I do not directly provide care
5. Approximately how many patients do you see each week?
□ Less than 50
□ 50-99
□ 100-149
□ 150-199
□ 200+
□ I do not directly provide care
6. How many patients do you currently see each week who are eligible to undergo colonoscopy screening for colorectal cancer?
□ Fewer than 5
□ 6-15
□ 16-25
□ 26-35
□ 36-45
□ 46-55
□ 56 or more
□ I do not directly provide care
7. Rate how well the activity supported your achievement of these learning objectives:
Explain the importance of colonoscopy for the prevention of colon cancer
□ Strongly Agree
□ Agree
□ Neutral
□ Disagree
□ Strongly Disagree
Review the histopathologic and morphologic characteristics of polyps
□ Strongly Agree
□ Agree
□ Neutral
□ Disagree
□ Strongly Disagree
Apply the optimal resection techniques for certain clinical scenarios
□ Strongly Agree
□ Agree
□ Neutral
□ Disagree
□ Strongly Disagree
Describe newer strategies to improve polypectomy and avoid incomplete resection
□ Strongly Agree
□ Agree
□ Neutral
□ Disagree
□ Strongly Disagree
Discuss the benefits and limitations associated with the different subcutaneous injection agents used to achieve a submucosal lift
□ Strongly Agree
□ Agree
□ Neutral
□ Disagree
□ Strongly Disagree
8. Rate how well the activity achieved the following:
The faculty were effective in presenting the material
□ Strongly Agree
□ Agree
□ Neutral
□ Disagree
□ Strongly Disagree
The content was evidence based
□ Strongly Agree
□ Agree
□ Neutral
□ Disagree
□ Strongly Disagree
The educational material provided useful information for my practice
□ Strongly Agree
□ Agree
□ Neutral
□ Disagree
□ Strongly Disagree
The activity enhanced my current knowledge base
□ Strongly Agree
□ Agree
□ Neutral
□ Disagree
□ Strongly Disagree
The activity provided appropriate and effective opportunities for active learning (e.g., case studies, discussion, Q&A, etc.)
□ Strongly Agree
□ Agree
□ Neutral
□ Disagree
□ Strongly Disagree
The opportunities provided to assess my own learning were appropriate (e.g., questions before, during or after the activity)
□ Strongly Agree
□ Agree
□ Neutral
□ Disagree
□ Strongly Disagree
9. Based upon your participation in this activity, do you intend to change your practice behavior? (choose only one of the following options)
□ I do plan to implement changes in my practice based on the information presented
□ My current practice has been reinforced by the information presented
□ I need more information before I will change my practice
10. Thinking about how your participation in this activity will influence your patient care, how many of your patients are likely to benefit?
Please use a number (for example, 250):
11. If you plan to change your practice behavior, what type of changes do you plan to implement? (check all that apply)
□ Apply latest guidelines
□ Choice of treatment/management approach
□ Change in pharmaceutical therapy
□ Change in current practice for referral
□ Change in nonpharmaceutical therapy
□ Change in differential diagnosis
□ Change in diagnostic testing
□ Other, please specify:
12. How confident are you that you will be able to make your intended changes?
□ Very confident
□ Somewhat confident
□ Unsure
□ Not very confident
13. Which of the following do you anticipate will be the primary barrier to implementing these changes?
□ Formulary restrictions
□ Insurance/financial issues
□ Time constraints
□ Lack of multidisciplinary support
□ System constraints
□ Treatment-related adverse events
□ Patient adherence/compliance
□ Other, please specify:
14. Was the content of this activity fair, balanced, objective and free of bias?
□ Yes
□ No, please explain:
15. Please list any clinical issues/problems within your scope of practice you would like to see addressed in future educational activities:
Request for Credit (*required fields)
Name*
Degree*
Organization
Specialty*
City, State, ZIP*
Telephone Fax
E-mail*
Signature*
Date*
For Physicians Only:
I certify my actual time spent to complete this educational activity to be:
□ I participated in the entire activity and claim 1.25 credits.
□ I participated in only part of the activity and claim ____ credits.
Post-Test Answer Key
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|
Project ID: 12730
Biography

References
- 1.National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: colon and rectum cancer. [Accessed August 9, 2017]. https://seer.cancer.gov/statfacts/html/colorect.html
- 2.Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109(8) doi: 10.1093/jnci/djw322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frezza EE, Wachtel MS, Chiriva-Internati M. Influence of obesity on the risk of developing colon cancer. Gut. 2006;55(2):285–291. doi: 10.1136/gut.2005.073163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams R, White P, Nieto J, Vieira D, Francois F, Hamilton F. Colorectal cancer in African Americans: an update. Clin Transl Gastroenterol. 2016;7(7):e185. doi: 10.1038/ctg.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. US Preventive Services Task Force. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315(23):2564–2575. doi: 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- 7.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM American College of Gastroenterology. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104(3):739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 8.Hewett DG, Kahi CJ, Rex DK. Does colonoscopy work? J Natl Compr Canc Netw. 2010;8(1):67–76. doi: 10.6004/jnccn.2010.0004. [DOI] [PubMed] [Google Scholar]
- 9.Doubeni CA, Corley DA, Quinn VP, et al. Effectiveness of screening colonoscopy in reducing the risk of death from right and left colon cancer: a large community-based study. Gut. 2016 doi: 10.1136/gutjnl-2016-312712. gutjnl-2016-312712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh H, Turner D, Xue L, Targownik LE, Bernstein CN. Risk of developing colorectal cancer following a negative colonoscopy examination: evidence for a 10-year interval between colonoscopies. JAMA. 2006;295(20):2366–2373. doi: 10.1001/jama.295.20.2366. [DOI] [PubMed] [Google Scholar]
- 11.Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154(1):22–30. doi: 10.7326/0003-4819-154-1-201101040-00004. [DOI] [PubMed] [Google Scholar]
- 12.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366(8):687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Cancer Society. Atlanta, Georgia: 2017. [Accessed August 15, 2017]. Cancer Facts and Figures: 2017.https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html [Google Scholar]
- 14.Meester RG, Doubeni CA, Zauber AG, et al. Public health impact of achieving 80% colorectal cancer screening rates in the United States by 2018. Cancer. 2015;121(13):2281–2285. doi: 10.1002/cncr.29336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siddiki HA, Fath AR, Ravi S, Ramirez FC, Crowell MD, Gurudu S. Comparison of ADR and SSP detection rate (SSP-DR) between small volume split dose preparation (SDP) and large volume SDP [DDW abstract 553] Gastrointest Endosc. 2017;85(5 suppl) [Google Scholar]
- 16.Horton N, Garber A, Hasson H, Lopez R, Burke CA. Impact of single vs. split dose low volume bowel preparations on bowel movement kinetics, patient inconvenience, and polyp detection: a prospective trial. Am J Gastroenterol. 2016;111(9):1330–1337. doi: 10.1038/ajg.2016.273. [DOI] [PubMed] [Google Scholar]
- 17.Endoscopic Classification Review Group. Update on the Paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37(6):570–578. doi: 10.1055/s-2005-861352. [DOI] [PubMed] [Google Scholar]
- 18.O’Brien MJ, Winawer SJ, Zauber AG, et al. The National Polyp Study. Patient and polyp characteristics associated with high-grade dysplasia in colorectal adenomas. Gastroenterology. 1990;98(2):371–379. [PubMed] [Google Scholar]
- 19.Lansdown M, Quirke P, Dixon MF, Axon AT, Johnston D. High grade dysplasia of the gastric mucosa: a marker for gastric carcinoma. Gut. 1990;31(9):977–983. doi: 10.1136/gut.31.9.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soetikno RM, Kaltenbach T, Rouse RV, et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA. 2008;299(9):1027–1035. doi: 10.1001/jama.299.9.1027. [DOI] [PubMed] [Google Scholar]
- 21.Repici A, Hassan C, Vitetta E, et al. Safety of cold polypectomy for <10mm polyps at colonoscopy: a prospective multicenter study. Endoscopy. 2012;44(1):27–31. doi: 10.1055/s-0031-1291387. [DOI] [PubMed] [Google Scholar]
- 22.Uraoka T, Saito Y, Yamamoto K, Fujii T. Submucosal injection solution for gastrointestinal tract endoscopic mucosal resection and endoscopic submucosal dissection. Drug Des Devel Ther. 2009;2:131–138. doi: 10.2147/dddt.s3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rex D, Wallace MB, Sharma P, Lollo G, Maselli R, Repici A. A randomized, double-blind trial of a new injectable solution (Sic 8000) for endoscopic resection of colonic polyps larger than 2 cm: an interim report [DDW abstract 689] Gastrointest Endosc. 2017;85(5 suppl) [Google Scholar]
- 24.Holmes I, Friedland S. Endoscopic mucosal resection versus endoscopic submucosal dissection for large polyps: a western colonoscopist’s view. Clin Endosc. 2016;49(5):454–456. doi: 10.5946/ce.2016.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moss A, Williams SJ, Hourigan LF, et al. Long-term adenoma recurrence following wide-field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: results and risk factors in 1000 cases from the Australian Colonic EMR (ACE) study. Gut. 2015;64(1):57–65. doi: 10.1136/gutjnl-2013-305516. [DOI] [PubMed] [Google Scholar]
- 26.Piraka C, Saeed A, Waljee AK, Pillai A, Stidham R, Elmunzer BJ. Cold snare polypectomy for nonpedunculated colon polyps greater than 1 cm. Endosc Int Open. 2017;5(3):E184–E189. doi: 10.1055/s-0043-101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tutticci NJ, Hewett DG. Cold piecemeal endoscopic mucosal resection (EMR) for large sessile serrated colonic polyps [DDW abstract 693] Gastrointest Endosc. 2017;85(5 suppl) [Google Scholar]
- 28.Choksi N, Elmunzer BJ, Stidham RW, Shuster D, Piraka C. Cold snare piecemeal resection of colonic and duodenal polyps ≥1 cm. Endosc Int Open. 2015;3(5):E508–E513. doi: 10.1055/s-0034-1392214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicholas NJ, Hewett DG. Cold piecemeal endoscopic mucosal resection (EMR) for large sessile serrated colonic polyps [DDW ASGE abstract 693] Gastrointest Endosc. 2017;85(5 suppl) [Google Scholar]
- 30.Bhurwal A, Bartel MJ, Heckman MG, et al. Endoscopic mucosal resection: learning curve for large nonpolypoid colorectal neoplasia. Gastrointest Endosc. 2016;84(6):959–968.e7. doi: 10.1016/j.gie.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 31.Sease J Removing a large flat colon polyp by EMR without surgery. [Accessed September 5, 2017]. http://www.sease.com/polyp/emr.html Updated June 8, 2017.
- 32.Pohl H, Srivastava A, Bensen SP, et al. Incomplete polyp resection during colonoscopy—results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144(1):74–80.e1. doi: 10.1053/j.gastro.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 33.Adler A, Aminalai A, Aschenbeck J, et al. Latest generation, wide-angle, high-definition colonoscopes increase adenoma detection rate. Clin Gastroenterol Hepatol. 2012;10(2):155–159. doi: 10.1016/j.cgh.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 34.Leufkens AM, DeMarco DC, Rastogi A, et al. Third Eye Retroscope Randomized Clinical Evaluation [TERRACE] Study Group. Effect of a retrograde-viewing device on adenoma detection rate during colonoscopy: the TERRACE study. Gastrointest Endosc. 2011;73(3):480–489. doi: 10.1016/j.gie.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Geyer M, Guller U, Beglinger C. Carbon dioxide insufflation in routine colonoscopy is safe and more comfortable: results of a randomized controlled double-blinded trial. Diagn Ther Endosc. 2011;2011:378906. doi: 10.1155/2011/378906. published online June 15, 2011. doi:10.1155/2011/378906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coe SG, Crook JE, Diehl NN, Wallace MB. An endoscopic quality improvement program improves detection of colorectal adenomas. Am J Gastroenterol. 2013;108(2):219–226. doi: 10.1038/ajg.2012.417. [DOI] [PubMed] [Google Scholar]
- 37.Ussui V, Coe S, Rizk C, Crook JE, Diehl NN, Wallace MB. Stability of increased adenoma detection at colonoscopy. Follow-up of an endoscopic quality improvement program-EQUIP-II. Am J Gastroenterol. 2015;110(4):489–496. doi: 10.1038/ajg.2014.314. [DOI] [PubMed] [Google Scholar]
- 38.Trivedi PJ, Braden B. Indications, stains and techniques in chromoendoscopy. QJM. 2013;106(2):117–131. doi: 10.1093/qjmed/hcs186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hashimoto K, Higaki S, Nishiahi M, Fujiwara K, Gondo T, Sakaida I. Does chromoendoscopy improve the colonoscopic adenoma detection rate? Hepatogastroenterology. 2010;57(104):1399–1404. [PubMed] [Google Scholar]
- 40.Repici A, Di Stefano AF, Radicioni MM, Jas V, Moro L, Danese S. Methylene blue MMX tablets for chromoendoscopy. Safety tolerability and bioavailability in healthy volunteers. Contemp Clin Trials. 2012;33(2):260–267. doi: 10.1016/j.cct.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 41.ClinicalTrials.gov. The safety and efficacy of methylene blue MMX® modified release tablets administered to subjects undergoing screening or surveillance colonoscopy. [Accessed September 12, 2017]. https://clinicaltrials.gov/ct2/show/NCT01694966 Identifier: NCT01694966.
- 42.Lin OS, Kozarek RA, Arai A, et al. The effect of periodic monitoring and feedback on screening colonoscopy withdrawal times, polyp detection rates, and patient satisfaction scores. Gastrointest Endosc. 2010;71(7):1253–1259. doi: 10.1016/j.gie.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 43.Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonosopy. Gastrointest Endosc. 2015;81(1):31–53. doi: 10.1016/j.gie.2014.07.058. [DOI] [PubMed] [Google Scholar]