Abstract
The phloem plays a central role in transporting resources and signalling molecules from fully expanded leaves to provide precursors for, and to direct development of, heterotrophic organs located throughout the plant body. We review recent advances in understanding mechanisms regulating loading and unloading of resources into, and from, the phloem network; highlight unresolved questions regarding the physiological significance of the vast array of proteins and RNAs found in phloem saps; and evaluate proposed structure/function relationships considered to account for bulk flow of sap, sustained at high rates and over long distances, through the transport phloem.
Keywords: Phloem transport, signalling, plants, water
Introduction
Successful functioning of land plants depends upon specialized transport systems. With some exceptions such as remobilized reduced carbon in winter-deciduous trees during the winter-spring transition, xylem conducts water and essential mineral elements from roots to transpiring leaves. Phloem transports water, mineral elements, amino nitrogen compounds, and sugars (resources), together with signalling molecules, from fully expanded leaves (sources) to meet the nutrient requirements of heterotrophic growth or storage organs (sinks) and to direct their development, respectively. Thus, phloem transport is at the heart of plant growth and development and, as such, is a primary factor determining crop yield potential. (For recent reviews, see Braun et al. 1, Ham and Lucas 2, and Yadav et al. 3.)
Phloem transport occurs as a pressure-driven bulk flow through a longitudinally arrayed subset of transport-specialized cells termed sieve elements (SEs). During their development, SEs undergo partial autophagy that leaves a parietal enucleate cytoplasm enclosed by a plasma membrane (PM). (For more details of recent progress in understanding phloem development, see reviews by Rodriguez-Villalon 4 and de Rybel et al. 5.) In contrast to SEs, their adjoining companion cells (CCs) contain a dense cytoplasm and are connected to their corresponding SE through an extensive network of plasmodesmata (PD) to form a metabolic and genetic unit, the sieve element-companion cell complex (SECCC). Loading SECCCs in the collection phloem of source leaves generates an osmotically derived hydrostatic pressure potential that drives bulk flow through the SE arrays of the transport phloem to reach the release phloem where resources and signals are unloaded to enter pathways leading to plant growth or storage 6, 7.
In this commentary, we review new insights into key aspects of phloem transport and highlight unresolved questions that need to be addressed to gain a fuller understanding of phloem transport.
Phloem loading
Sugar uptake into the phloem is a principal contributor to establishing source-sink hydrostatic pressure differentials driving phloem transport 6, 7. Different phloem loading types have been identified, and abundant insight into phloem loading mechanisms has become available. However, two key aspects remain largely unexplored: the ecology and regulation of phloem loading.
Three major types of phloem loading
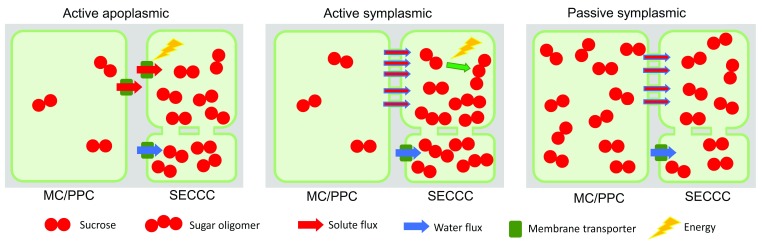
The three main types of phloem loading currently recognized are (i) active apoplasmic, (ii) active symplasmic, and (iii) passive symplasmic ( Figure 1). The mechanism of active apoplasmic loading ( Figure 1) is well understood with proton-coupled sucrose transporters (SUTs), also referred to as SUCs, concentrating sucrose into SECCCs 8– 10 released from surrounding cells by sucrose uniporters, termed sugars will eventually be exported transporters (SWEETs) 11, 12. In contrast, the function of the two symplasmic loading types has been established only recently.
Figure 1. Major phloem loading types.
In active apoplasmic loading, the sieve element-companion cell complex (SECCC) is symplasmically isolated. Sucrose produced in the mesophyll cells (MCs) diffuses into phloem parenchyma cells (PPCs), where it is released into the apoplasm by efflux carriers (SWEETs) before being taken up into the SECCC by plasma membrane-localized sucrose transporters. In active symplasmic loading, sucrose can diffuse or convect into the companion cell (CC) through the abundant plasmodesmata (PD). Sucrose in the CC is converted to sugar oligomers, which are hindered from diffusing back into the phloem parenchyma but instead enter the SE through the larger PD at the SE-CC interface. In passive symplasmic loading, sucrose can diffuse or convect along the whole phloem loading pathway from mesophyll cells to SEs following the sugar concentration gradient. Aquaporins facilitate the osmotic uptake of water from the phloem apoplasm into SEs in all loading types. In symplasmic loaders, water also enters the SECCC through PD. SWEET, sugar will eventually be exported transporters.
A remaining major question for active symplasmic loading is how PD enable steric filtering to allow sucrose movement into CCs while preventing the synthesized sugar oligomers from leaking back to the mesophyll cells ( Figure 1). No method exists to resolve PD canal sub-structure to the degree required to answer this question, and current insights rest solely on mathematical modelling. For instance, Liesche and Schulz 13 showed that a PD structure that is highly restrictive to solute movement, with cytosolic channels of 0.65 nm in diameter compared with diameters of 2.5 nm in PD between leaf mesophyll cells 14, could enable steric sugar filtering while accommodating observed phloem loading rates. However, this extreme PD anatomy might not be necessary as differences in intracellular sugar concentrations along the loading pathway from mesophyll to phloem could be sufficient to drive a combination of diffusion and mass flow of molecules (convective flow) into SECCCs. As a consequence, diffusion of sugar oligomers out of the SECCC, against the direction of the convective flow, would be limited 15, 16.
New evidence regarding passive symplasmic loading ( Figure 1) was provided by the finding that sucrose export from poplar leaves was not compromised by blocking apoplasmic loading of sucrose 17. This was achieved through expressing a cell wall invertase to cleave sucrose into hexoses, thereby making the sugar unavailable for uptake by SUTs. Furthermore, microfluidic experiments showed that passive phloem loading is feasible with convective flow through PD to the SECCCs 18. However, it is unclear whether this concept is universally applicable across all species exhibiting features of passive symplasmic loading. For example, in several designated passive loaders, higher sugar concentrations were detected in phloem compared with mesophyll cells 19, 20. In addition to methodological controversies surrounding measurements of intracellular sugar concentrations, a switch between loading types or even their concomitant operation could explain the conflicting conclusions 21.
Parallel and sequential operation of different phloem loading types
Coexistence of phloem loading types has become apparent for many plant species 22. For instance, two anatomical types of CCs have been detected in minor veins in 35% of 320 Asteridae species exhibiting PD characteristics consistent with active symplasmic or apoplasmic loading 23 as well as other species of various growth forms 24, 25. Moreover, switching from active symplasmic to apoplasmic phloem loading, putatively by modifying PD conductance in the same CC, has been described for virus-infected melon plants 26. A switch between loading types in the same CC is thought to be impossible without adaptation of the relevant PD 21, 27. Consequently, elucidating this potential mechanism of switching between loading types is a major task for future research on phloem loading.
In addition to switching between loading types, concomitant operation of passive and active loading by the same CC has been proposed 20 and shown to be theoretically possible though attenuated by a low efficiency 21. Passive loading could function as a fallback mechanism as demonstrated by continued growth, at a reduced rate, of plants in which active loading is blocked through introduction of mutations that render either the relevant SUT or enzymes involved in sucrose oligomerization non-functional 22, 28, 29.
Ecology of phloem loading types
No satisfactory answer has been provided to account for why there are different phloem loading types. Slewinski et al. 22 theorized that some plants load certain molecules symplasmically in addition to those transported across the PM of SECCCs, but no candidates have been identified so far. Analysis of growth form concluded that herbaceous plants generally are not passive symplasmic loaders 30. Presumably, this is because active loading allows the plant to keep carbon content low in leaves, which provides a substantial, positive effect on growth rate 31. However, this does not mean that the slower-growing trees are all passive loaders. Indeed, half of the families dominated by woody plant species exhibit an active loading mechanism 21, 30. Evolutionary and distribution analyses similarly do not provide a clear explanation for why certain families use a particular phloem loading type 30. A closer examination of a plant’s ecology might reveal ecophysiological adaptations that different loading types offer. For example, a link between loading type and a plant’s adaptive environmental response has been demonstrated in a comparative study of minor vein structure developed under different temperature and light conditions 32.
Regulation of phloem loading
While sugar export rates are a function of sugar availability and sink demand 33, 34 as well as phloem loading capacity 35, 36, the underlying molecular mechanisms are poorly understood. SUT activities are expected to be principal regulators of sugar export in apoplasmic loaders 6 as demonstrated by SUT overexpression 37. In this context, transcriptional SUT regulation by phytohormones and corresponding changes in sugar export rates were observed in potato 38. Several studies suggest that post-translational regulation of SUTs contributes to determining export rates (for example, Sakr et al. 39). How post-translational regulation of SUTs occurs in vivo is unclear but could involve differential intracellular localization 40, dimerization 41, and protein-protein interactions 42. However, other factors should be considered. For instance, simultaneous upregulated expression of SUTs and SWEETs, in response to water deficit, increased phloem loading 43. Furthermore, constitutive overexpression of a proton-pumping pyrophosphatase in Arabidopsis thaliana leaf CCs increased phloem loading possibly by enhancing the proton motive force driving sucrose flux through SECCC localized SUTs 44, 45. Regulation of sucrose exchange between cytosol and vacuole of cells in the pre-phloem pathway influences phloem loading rates even in apoplasmic loaders 46, 47. So far not demonstrated is the short-term regulation of phloem loading through modification of PD permeability, which would be especially relevant in symplasmic loaders 21. After elucidation of the molecular mechanisms regulating phloem loading, the important question will be how these are linked to sink demand 48.
Phloem transport
This section combines a description of the transport phloem, especially how its anatomy facilitates sap flow, with a more general discussion on the mechanism of phloem transport and the molecules transported within.
The mechanism of phloem transport in trees
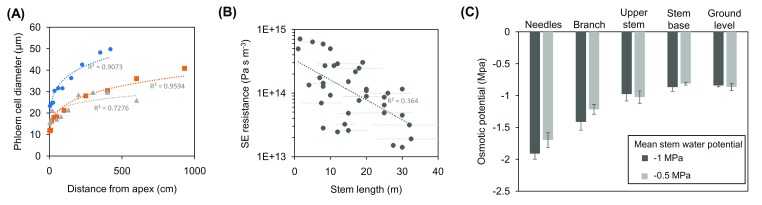
Osmotically driven pressure flow has been widely accepted as the mechanism of phloem transport in herbaceous plants 49, 50. However, in regard to trees, where distances between source and sink can extend up to 100 m, there are doubts about whether a hydrostatic pressure potential sufficient to drive flow could be generated 51, 52. A variety of approaches have been employed to answer this question. Simple theoretical models of Münch-type pressure flow agree with measurements of stem diameter variations 53 or SE anatomy 54, 55. Importantly, the main prediction of pressure flow, that SE conductivity scales with transport distance 56, could be confirmed. The scaling of SE conductivity with tree height was shown within a single tree ( Figure 2A; 57, 58), within a species 59, and across species ( Figure 2B; 55, 60), confirming that resistance decreases to accommodate mass flow in larger trees. Furthermore, it was recently shown in mature, field-grown Scots pine trees that there is an osmotic pressure gradient along the phloem pathway from leaves to the stem base ( Figure 2C; 61). The osmotic pressure gradient, supported by gravity, was calculated to be large enough to overcome the xylem water pressure potential and establish a phloem turgor pressure gradient that drives mass flow according to the Münch mechanism at all times across the diel cycle 61. Taken together, these results confirm that Münch-type pressure flow works even in the tallest angiosperm and gymnosperm trees, although transport speed might be up to 10 times lower than in herbaceous plants 62. However, this is in agreement with equally lower rates of photosynthesis and growth 63.
Figure 2. Scaling of sieve element (SE) conductivity and osmotic pressure potential enable Münch-type pressure flow in trees.
( A) Axial widening of phloem cells along the stem of individual trees of Norway spruce (blue), European ash (orange), and bitter willow (gray). Average values of cell diameter contain up to 30% non-SE cells. Axial widening of SEs toward the bottom of a tree translates to decreased hydraulic resistance, and the scaling relationship was found to enable optimal xylem-phloem water exchange that is critical to drive flow in tall trees. ( B) SE resistance of 44 different tree species with sampled individuals grown to their typical maximum height. Negative scaling of resistance with transport distance illustrates the anatomical optimization of SEs for phloem transport in trees. ( C) Measurements of osmolality indicated the presence of an osmotic pressure gradient along the phloem of 18 m high Scots pine trees, which changed in accordance with the xylem water potential. The differences in osmotic potential were calculated to be large enough to overcome the xylem water pressure potential and establish a phloem turgor pressure gradient that drives mass flow according to the Münch mechanism across the diel cycle 56. Frames ( A– C) are based on data from Petit and Crivellaro 57, Liesche et al. 60, and Paljakka et al. 61, respectively.
Open questions regarding the functional anatomy of the phloem
Despite major progress in determining how phloem anatomy facilitates osmotically driven pressure flow, not all questions regarding its functional anatomy have been resolved. For example, sieve plates forming the axial connection between adjoining SEs have been assumed to evolve toward a simple form associated with lower hydraulic resistance—a paradigm overturned by an anatomical analysis of 447 species 60. Rather, the distinct organizational patterns of sieve plates might relate to general vascular anatomy (for example, SE length or xylem cell anatomy) instead of differences in conductivity 60. Another aspect that is relevant for phloem transport is the symplasmic coupling along the transport path. Even in apoplasmically loading herbaceous plants, unloading along the stem can switch between apoplasmic and symplasmic unloading pathways, depending upon the source/sink ratio, with high ratios favouring symplasmic unloading 64. In many tree species, especially conifers, the presence of PD connecting the SECCC with surrounding cells 65 indicates symplasmic coupling, presumably to efficiently supply the cambium with the large amounts of sugars needed for wood formation. The question is in how far symplasmic coupling along the path compromises whole-plant phloem transport. A recent theoretical analysis showed that unloading along the transport path considerably influences flow if it is not balanced by reloading 66. The most likely explanation is that unloading is symplasmic only in case of high sugar concentrations in the ground tissue, which then could also have a function in homeostatically maintaining SE turgor 67.
Another central issue is the relationship between fibrous SE protein (p-protein) agglomerations, considered to occlude sieve pores to prevent sap loss in the event of damage 68. Careful preparation of material for electron microscope examination reveals unobstructed SE lumens bounded by a parietal cytoplasm. However, Froelich et al. 69 showed that in A. thaliana phloem, the p-protein, AtSEOR1, forms a meshwork at the margins and clots in the lumen of intact SEs and their presence does not impede longitudinal flow. Together with discrepancies between theoretical flow speeds calculated according to SE conductivity and observed flow speeds 70, these observations have led to the conclusion that phloem transport in herbaceous plants and grasses is not limited by SE conductivity but only by sink strength 71.
Phloem sap composition
Knowledge of phloem sap composition is relevant to reach a quantitative understanding of resource allocation and inter-organ signalling. Sugar concentrations are typically around 20%, but the sap contains other molecules (for example, amino nitrogen compounds, proteins, and RNAs) and mineral ions 72. Determining the precise abundance of the different sap components remains one of the biggest challenges in phloem research as all methods available so far are prone to artifacts 73– 75. Recently, laser-capture microdissection was used to uncover the link between seasonal differences in phloem metabolites and phloem formation in Norway spruce. However, since sampling could not be restricted to SEs, results were not representative of phloem sap 76. In the future, insight on relative sap composition might be gained from using rapid freezing and nanoscale secondary ion mass spectrometry (NanoSIMS), which enables semi-quantitative imaging of ions as well as isotope-labeled nitrogen and carbon compounds within SEs 77. Gaining a complete picture of the spatiotemporal dynamics of phloem sap composition will be instrumental in refining multi-compartmental metabolic models describing source-to-sink metabolite flows (for example, Zakhartsev et al. 78).
Controversy persists surrounding the function of phloem-mobile proteins and RNA 79. Recently, a meta-analysis comparing proteomes of xylem, phloem, and leaf apoplasmic saps highlighted phloem sap as being the most enriched in signalling proteins of which 13 were conserved across experiments and that included the ubiquitous phloem-mobile protein FLOWERING LOCUS T 80. Grafting studies suggest phloem mobility of a multitude of additional proteins 81. However, without further validation of their signalling function, many of these proteins could have entered the translocation stream “by accident” rather than by a control mechanism located at PD interconnecting CCs with SEs 82. Of the proteins identified in phloem sap by proteomics, those associated with redox regulation and plant defense consistently have been found across species 80, suggesting that many phloem proteins function in the response to biotic and abiotic stress. Prominent examples of phloem-mobile RNAs include the transcription factors BEL5, which influences potato tuber induction 83, and the Cucurbit NACP regulating apical meristem development 84. Recently 2,006 A. thaliana phloem-mobile mRNAs were identified 85 with a conserved tRNA-derived sequence conferring mobility 86. However, since most of the mRNAs are expressed in mesophyll cells, it is unclear how they could enter the phloem stream in the apoplasmic loader A. thaliana. In addition to those many phloem-mobile proteins and mRNAs, the role of microRNAs 87 and lipids 88 as systemic signals needs to be further explored.
Phloem unloading
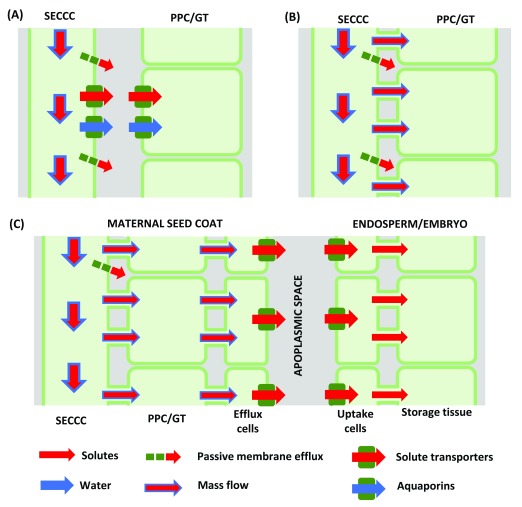
Phloem unloading describes the movement of phloem sap constituents from SECCC lumens (SECCC unloading) and their subsequent cell-to-cell transport to final destinations in non-SECCC vascular or ground tissues. Cellular pathways of phloem unloading are apoplasmic ( Figure 3A) or symplasmic with or without an intervening apoplasmic step located in the post-SECCC pathway ( Figure 3B, C; 89). As outlined below, phloem unloading mechanisms are only partially resolved.
Figure 3. Cellular pathways and mechanisms of phloem unloading.
( A) Apoplasmic sieve element-companion cell complex (SECCC) unloading of sucrose mediated by sucrose transporter reversal and/or possible by sugars will eventually be exported transporters (SWEETs) with energy coupled transporters retrieving sugars from the sink apoplasm while accompanying water transport across the plasma membranes facilitated by aquaporins. ( B) Symplasmic phloem unloading by bulk flow. ( C) Symplasmic phloem unloading by bulk flow with an intervening apoplasmic step in the post-phloem pathway of developing seeds. Sucrose release to the seed apoplasm from maternal seed coats is mediated by SWEETs, sucrose facilitators and possibly a yet-to-be-cloned sucrose/proton antiporter. SWEETs and sucrose transporters recover released sucrose from the seed apoplasm into the endosperm/embryo. Membrane transport of water facilitated by aquaporins. GT, ground tissue; PPC, phloem parenchyma cell.
Apoplasmic phloem unloading mechanisms
Possible mechanisms contributing to efflux across any cell PM, including SECCC PMs, are passive diffusion, transporter-mediated facilitated diffusion, and energy-coupled movement. Passive diffusive fluxes of molecules and ions are the product of their membrane permeability coefficients and trans-membrane gradients in chemical potential for non-electrolytes or electrochemical potential for electrolytes 90. To illustrate the magnitude that these passive fluxes could reach for apoplasmic SECCC unloading of sucrose along the entire phloem pathway from source to sink, a plausible maximum of 1 M for the SECCC trans-PM sucrose concentration difference and a membrane permeability coefficient of 10 −10 m s −1 91 predict a sucrose efflux of 1 × 10 −7 mol m −2 s −1. This matches the maximal membrane flux for loading sucrose into SECCCs (2.3 × 10 −7 mol m −2 s −1; Giaquinta 92). Thus, it is imperative to experimentally determine rates of diffusive leak from SECCCs and include this component in theoretical models of phloem transport.
For facilitated membrane efflux of sugars by uniporters, SWEETs 11, 12 have been detected in transport phloem parenchyma cells 93, but whether these function in efflux to, or retrieval from, the phloem apoplasm remains to be determined. However, sucrose/proton symporters occur in SECCCs of root and stem transport phloem (for example, 94, 95). Depolarized membrane potentials (−55 mV) of root SECCCs predict that sucrose/proton symport could reverse to an efflux mode driven by a trans-membrane sucrose concentration difference of 85.5 mM (for more information, see Carpaneto et al. 94). In contrast, symporter reversal in stem phloem is highly unlikely as their SE/CC membrane potential of −110 mV 96 would require a sucrose concentration difference of 7,200 mM, a concentration difference that far exceeds physiological limits.
During sugar accumulation in fleshy fruits, phloem unloading follows an apoplasmic route 64, 97– 100. Whether the sucrose leak from fruit SECCCs is augmented by reversal of sucrose/proton symporters 101, 102 cannot be evaluated, as their membrane potentials are unknown. However, pharmacological studies suggest that sucrose efflux from vascular bundles of apple fruit and grape berries is mediated by energy-coupled carriers 97, 103. Resolving the identity (or identities) of the putative effluxer (or effluxers) is central to acquiring a full understanding of phloem unloading in fleshy fruits. What is clearer is that monosaccharide/proton symporters retrieve inverted disaccharides from the fruit apoplasm into storage parenchyma cells 104– 107 and, together with cell wall invertases, function to co-regulate phloem unloading 106, 108, 109.
In all cases, unloading of osmotic solutes must be accompanied by a proportionate loss of phloem water to maintain water potential equilibrium. The exit of water likely occurs through aquaporins localized in PMs of the unloading SEs ( Figure 3A and, for example, 110, 111).
Symplasmic unloading mechanisms with or without an intervening apoplasmic step located in the post-SECCC pathway
In recent years, it is becoming increasingly clear that a major component of symplasmic unloading from SECCCs is contributed by bulk flow. For instance, experimental manipulations of hydrostatic pressure gradients in root tips and stems cause changes in phloem unloading rates consistent with bulk flow (reviewed by Patrick 112). In developing wheat seeds, hydrostatic pressure differences of up to 1.0 MPa between SEs and vascular parenchyma cells account for observed PD volume flow rates 113. In contrast, modelling flows through large-diameter funnel-shaped PD, interconnecting SEs, and adjacent pericycle cells in A. thaliana root tips predicted that hydrostatic pressure differences of only 0.05 to 0.2 MPa were required 114. Whether these model-based hydrostatic pressure differences can be reconciled with measured hydrostatic or osmotic pressures of 1.3 and 0.6 MPa, respectively, between SEs and surrounding cells in barley and maize root tips 115, 116 awaits determination.
For the apoplasmic step in the mandatory phloem-unloading pathway of developing seeds ( Figure 3B), sucrose release from maternal seed coats occurs by a combination of facilitated diffusion and sucrose/proton antiport while retrieval by embryo/endosperm is mediated by sucrose symporters 117 or SWEETs 118 or both. Cloned sucrose facilitators (SUFs) from grain legume seed coats exhibited transport properties consistent with those found for facilitated diffusion of sucrose in native membranes of seed coats and were expressed in cells considered responsible for sucrose efflux 119, 120. In developing A. thaliana seeds, SWEETs are positioned to provide a cascade of sucrose transport from the outer (SWEET15) and inner (SWEET12) integuments and uptake into the filial tissues (SWEET11 and SWEET15 121) while SWEET11 facilitates release from maternal tissues of developing rice seeds 122. Consistent with relative contributions of facilitated diffusion to sucrose effluxed from grain legume seed coats 117, seed weights, but not number, were depressed by 50% in the A. thaliana triple-mutant sweet11, 12 and 15, and by 65% in the rice sweet11 mutant 121, 122. The challenge now is to identify the gene or (genes) encoding the membrane protein (or proteins) responsible for energy-coupled sucrose/proton antiport from maternal seed tissues 123 that may also operate in fleshy fruit (see previous section). Membrane transport of solutes is matched by water movement through aquaporins located in PMs of maternal and filial tissues of developing seeds ( Figure 3C and, for example, 124, 125).
Conclusions and future directions
Throughout the text, we have highlighted a series of unresolved questions of phloem transport biology required to move the understanding of phloem loading, axial transport, and unloading forward. Their resolution will inform profitable approaches to address the key question of how the components of phloem transport are integrated into a functional whole 71 and how phloem transport mechanistically intermeshes with photosynthesis and sink demand for resources 63, 71.
Abbreviations
CC, companion cell; PD, plasmodesmata; PM, plasma membrane; SE, sieve element; SECCC, sieve element-companion cell complex; SUT, sucrose transporter; SWEET, sugars will eventually be exported transporters
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
William W Adams, Department of Ecology and Evolutionary Biology, University of Colorado, Boulder, USA
Brian Ayre, Department of Biological Sciences, University of North Texas, Texas, USA
Robert Turgeon, Plant Biology, Cornell University, New York, USA
Friedrich Kragler, Department of Intercellular Macromolecular Transport, Max Planck Institute of Molecular Plant Physiology, Potsdam, Germany
Antia Rodriguez-Villalon, Department of Biology, Swiss Federal Institute of Technology (ETH-Z), Zurich, Switzerland
Funding Statement
Published work authored by JWP was funded by the Australian Research Council.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 5 approved]
References
- 1. Braun DM, Wang L, Ruan YL: Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signalling to enhance crop yield and food security. J Exp Bot. 2014;65(7):1713–35. 10.1093/jxb/ert416 [DOI] [PubMed] [Google Scholar]
- 2. Ham BK, Lucas WJ: The angiosperm phloem sieve tube system: a role in mediating traits important to modern agriculture. J Exp Bot. 2014;65(7):1799–816. 10.1093/jxb/ert417 [DOI] [PubMed] [Google Scholar]
- 3. Yadav UP, Ayre BG, Bush DR: Transgenic approaches to altering carbon and nitrogen partitioning in whole plants: assessing the potential to improve crop yields and nutritional quality. Front Plant Sci. 2015;6:275. 10.3389/fpls.2015.00275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rodriguez-Villalon A: Wiring a plant: genetic networks for phloem formation in Arabidopsis thaliana roots. New Phytol. 2016;210(1):45–50. 10.1111/nph.13527 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 5. De Rybel B, Mähönen AP, Helariutta Y, et al. : Plant vascular development: from early specification to differentiation. Nat Rev Mol Cell Biol. 2016;17(1):30–40. 10.1038/nrm.2015.6 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. Ayre BG: Membrane-transport systems for sucrose in relation to whole-plant carbon partitioning. Mol Plant. 2011;4(3):377–94. 10.1093/mp/ssr014 [DOI] [PubMed] [Google Scholar]
- 7. Ainsworth EA, Bush DR: Carbohydrate export from the leaf: a highly regulated process and target to enhance photosynthesis and productivity. Plant Physiol. 2011;155(1):64–9. 10.1104/pp.110.167684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Riesmeier JW, Willmitzer L, Frommer WB: Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J. 1992;11(13):4705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Riesmeier JW, Willmitzer L, Frommer WB: Evidence for an essential role of the sucrose transporter in phloem loading and assimilate partitioning. EMBO J. 1994;13(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gahrtz M, Stolz J, Sauer N: A phloem-specific sucrose-H + symporter from Plantago major L. supports the model of apoplastic phloem loading. Plant J. 1994;6(5):697–706. 10.1046/j.1365-313X.1994.6050697.x [DOI] [PubMed] [Google Scholar]
- 11. Chen LQ: SWEET sugar transporters for phloem transport and pathogen nutrition. New Phytol. 2014;201(4):1150–5. 10.1111/nph.12445 [DOI] [PubMed] [Google Scholar]
- 12. Chen LQ, Qu XQ, Hou BH, et al. : Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science. 2012;335(6065):207–11. 10.1126/science.1213351 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. Liesche J, Schulz A: Modeling the parameters for plasmodesmal sugar filtering in active symplasmic phloem loaders. Front Plant Sci. 2013;4:207. 10.3389/fpls.2013.00207 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Ding B, Turgeon R, Parthasarathy MV: Substructure of freeze-substituted plasmodesmata. Protoplasma. 1992;169(1–2):28–41. 10.1007/BF01343367 [DOI] [Google Scholar]
- 15. Münch E: Die Stoffbewegungen in der Pflanze. Protoplasma. 1932;15(1):488–9. 10.1007/BF01610295 [DOI] [Google Scholar]
- 16. Comtet J, Turgeon R, Stroock AD: Phloem loading through plasmodesmata: a biophysical analysis. Plant Physiol. 2017;175(2):904–15. 10.1104/pp.16.01041 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Zhang C, Han L, Slewinski TL, et al. : Symplastic phloem loading in poplar. Plant Physiol. 2014;166(1):306–13. 10.1104/pp.114.245845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Comtet J, Jensen KH, Turgeon R, et al. : Passive phloem loading and long-distance transport in a synthetic tree-on-a-chip. Nat Plants. 2017;3:17032. 10.1038/nplants.2017.32 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Nadwodnik J, Lohaus G: Subcellular concentrations of sugar alcohols and sugars in relation to phloem translocation in Plantago major, Plantago maritima, Prunus persica, and Apium graveolens. Planta. 2008;227(5):1079–89. 10.1007/s00425-007-0682-0 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Oner-Sieben S, Lohaus G: Apoplastic and symplastic phloem loading in Quercus robur and Fraxinus excelsior. J Exp Bot. 2014;65(7):1905–16. 10.1093/jxb/eru066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liesche J: Sucrose transporters and plasmodesmal regulation in passive phloem loading. J Integr Plant Biol. 2017;59(5):311–21. 10.1111/jipb.12548 [DOI] [PubMed] [Google Scholar]
- 22. Slewinski TL, Zhang C, Turgeon R: Structural and functional heterogeneity in phloem loading and transport. Front Plant Sci. 2013;4:244. 10.3389/fpls.2013.00244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Batashev DR, Pakhomova MV, Razumovskaya AV, et al. : Cytology of the minor-vein phloem in 320 species from the subclass Asteridae suggests a high diversity of phloem-loading modes. Front Plant Sci. 2013;4:312. 10.3389/fpls.2013.00312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Öner-Sieben S, Rappl C, Sauer N, et al. : Characterization, localization, and seasonal changes of the sucrose transporter FeSUT1 in the phloem of Fraxinus excelsior. J Exp Bot. 2015;66(15):4807–19. 10.1093/jxb/erv255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Bel AJ, van Kesteren WJ, Papenhuijzen C: Ultrastructural indications for coexistence of symplastic and apoplastic phloem loading in Commelina benghalensis leaves: Differences in ontogenic development, spatial arrangement and symplastic connections of the two sieve tubes in the minor vein. Planta. 1988;176(2):159–72. 10.1007/BF00392441 [DOI] [PubMed] [Google Scholar]
- 26. Gil L, Yaron I, Shalitin D, et al. : Sucrose transporter plays a role in phloem loading in CMV-infected melon plants that are defined as symplastic loaders. Plant J. 2011;66(2):366–74. 10.1111/j.1365-313X.2011.04498.x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 27. Hannah MA, Zuther E, Buchel K, et al. : Transport and metabolism of raffinose family oligosaccharides in transgenic potato. J Exp Bot. 2006;57(14):3801–11. 10.1093/jxb/erl152 [DOI] [PubMed] [Google Scholar]
- 28. McCaskill A, Turgeon R: Phloem loading in Verbascum phoeniceum L. depends on the synthesis of raffinose-family oligosaccharides. Proc Natl Acad Sci U S A. 2007;104(49):19619–24. 10.1073/pnas.0707368104 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Srivastava AC, Ganesan S, Ismail IO, et al. : Functional characterization of the Arabidopsis AtSUC2 Sucrose/H + symporter by tissue-specific complementation reveals an essential role in phloem loading but not in long-distance transport. Plant Physiol. 2008;148(1):200–11. 10.1104/pp.108.124776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Davidson A, Keller F, Turgeon R: Phloem loading, plant growth form, and climate. Protoplasma. 2011;248(1):153–63. 10.1007/s00709-010-0240-7 [DOI] [PubMed] [Google Scholar]
- 31. Turgeon R: The role of phloem loading reconsidered. Plant Physiol. 2010;152(4):1817–23. 10.1104/pp.110.153023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Adams WW, 3rd, Cohu CM, Muller O, et al. : Foliar phloem infrastructure in support of photosynthesis. Front Plant Sci. 2013;4:194. 10.3389/fpls.2013.00194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jiao J, Grodzinski B: The effect of leaf temperature and photorespiratory conditions on export of sugars during steady-state photosynthesis in Salvia splendens. Plant Physiol. 1996;111(1):169–78. 10.1104/pp.111.1.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grodzinski B, Jiao J, Leonardos ED: Estimating photosynthesis and concurrent export rates in C 3 and C 4 species at ambient and elevated CO 21,2. Plant Physiol. 1998;117(1):207–15. 10.1104/pp.117.1.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Amiard V, Mueh KE, Demmig-Adams B, et al. : Anatomical and photosynthetic acclimation to the light environment in species with differing mechanisms of phloem loading. Proc Natl Acad Sci U S A. 2005;102(36):12968–73. 10.1073/pnas.0503784102 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Muller O, Cohu CM, Stewart JJ, et al. : Association between photosynthesis and contrasting features of minor veins in leaves of summer annuals loading phloem via symplastic versus apoplastic routes. Physiol Plant. 2014;152(1):174–83. 10.1111/ppl.12155 [DOI] [PubMed] [Google Scholar]
- 37. Wang L, Lu Q, Wen X, et al. : Enhanced sucrose loading improves rice yield by increasing grain size. Plant Physiol. 2015;169(4):2848–62. 10.1104/pp.15.01170 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Chincinska IA, Liesche J, Krügel U, et al. : Sucrose transporter StSUT4 from potato affects flowering, tuberization, and shade avoidance response. Plant Physiol. 2008;146(2):515–28. 10.1104/pp.107.112334 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Sakr S, Noubahni M, Bourbouloux A, et al. : Cutting, ageing and expression of plant membrane transporters. Biochim Biophys Acta. 1997;1330(2):207–16. 10.1016/S0005-2736(97)00169-7 [DOI] [PubMed] [Google Scholar]
- 40. Liesche J, He HX, Grimm B, et al. : Recycling of Solanum sucrose transporters expressed in yeast, tobacco, and in mature phloem sieve elements. Mol Plant. 2010;3(6):1064–74. 10.1093/mp/ssq059 [DOI] [PubMed] [Google Scholar]
- 41. Krügel U, Veenhoff LM, Langbein J, et al. : Transport and sorting of the solanum tuberosum sucrose transporter SUT1 is affected by posttranslational modification. Plant Cell. 2008;20(9):2497–513. 10.1105/tpc.108.058271 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Krügel U, Kühn C: Post-translational regulation of sucrose transporters by direct protein-protein interactions. Front Plant Sci. 2013;4:237. 10.3389/fpls.2013.00237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Durand M, Porcheron B, Hennion N, et al. : Water deficit enhances C export to the roots in Arabidopsis thaliana plants with contribution of sucrose transporters in both shoot and roots. Plant Physiol. 2016;170(3):1460–79. 10.1104/pp.15.01926 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Khadilkar AS, Yadav UP, Salazar C, et al. : Constitutive and companion cell-specific overexpression of AVP1, encoding a proton-pumping pyrophosphatase, enhances biomass accumulation, phloem loading, and long-distance transport. Plant Physiol. 2016;170(1):401–14. 10.1104/pp.15.01409 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Pizzio GA, Paez-Valencia J, Khadilkar AS, et al. : Arabidopsis type I proton-pumping pyrophosphatase expresses strongly in phloem, where it is required for pyrophosphate metabolism and photosynthate partitioning. Plant Physiol. 2015;167(4):1541–53. 10.1104/pp.114.254342 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Leach KA, Tran TM, Slewinski TL, et al. : Sucrose transporter2 contributes to maize growth, development, and crop yield. J Integr Plant Biol. 2017;59(6):390–408. 10.1111/jipb.12527 [DOI] [PubMed] [Google Scholar]
- 47. Wingenter K, Schulz A, Wormit A, et al. : Increased activity of the vacuolar monosaccharide transporter TMT1 alters cellular sugar partitioning, sugar signaling, and seed yield in Arabidopsis. Plant Physiol. 2010;154(2):665–77. 10.1104/pp.110.162040 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Griffiths CA, Paul MJ, Foyer CH: Metabolite transport and associated sugar signalling systems underpinning source/sink interactions. Biochim Biophys Acta. 2016;1857(10):1715–25. 10.1016/j.bbabio.2016.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Crafts AS, Crisp CE: Phloem transport in plants. Bioscience. 1972;22:236. [Google Scholar]
- 50. Knoblauch M, Knoblauch J, Mullendore DL, et al. : Testing the Münch hypothesis of long distance phloem transport in plants. eLife. 2016;5: pii: e15341. 10.7554/eLife.15341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Knoblauch M, Peters WS: Münch, morphology, microfluidics - our structural problem with the phloem. Plant Cell Environ. 2010;33(9):1439–52. 10.1111/j.1365-3040.2010.02177.x [DOI] [PubMed] [Google Scholar]
- 52. de Schepper V, de Swaef T, Bauweraerts I, et al. : Phloem transport: a review of mechanisms and controls. J Exp Bot. 2013;64(16):4839–50. 10.1093/jxb/ert302 [DOI] [PubMed] [Google Scholar]
- 53. de Schepper V, Steppe K: Development and verification of a water and sugar transport model using measured stem diameter variations. J Exp Bot. 2010;61(8):2083–99. 10.1093/jxb/erq018 [DOI] [PubMed] [Google Scholar]
- 54. Jensen KH, Liesche J, Bohr T, et al. : Universality of phloem transport in seed plants. Plant Cell Environ. 2012;35(6):1065–76. 10.1111/j.1365-3040.2011.02472.x [DOI] [PubMed] [Google Scholar]
- 55. Hölttä T, Kurppa M, Nikinmaa E: Scaling of xylem and phloem transport capacity and resource usage with tree size. Front Plant Sci. 2013;4:496. 10.3389/fpls.2013.00496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mencuccini M, Hölttä T: The significance of phloem transport for the speed with which canopy photosynthesis and belowground respiration are linked. New Phytol. 2010;185(1):189–203. 10.1111/j.1469-8137.2009.03050.x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Petit G, Crivellaro A: Comparative axial widening of phloem and xylem conduits in small woody plants. Trees. 2014;28(3):915–21. 10.1007/s00468-014-1006-1 [DOI] [Google Scholar]
- 58. Jyske T, Hölttä T: Comparison of phloem and xylem hydraulic architecture in Picea abies stems. New Phytol. 2015;205(1):102–15. 10.1111/nph.12973 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Woodruff DR: The impacts of water stress on phloem transport in Douglas-fir trees. Tree Physiol. 2014;34(1):5–14. 10.1093/treephys/tpt106 [DOI] [PubMed] [Google Scholar]
- 60. Liesche J, Pace MR, Xu Q, et al. : Height-related scaling of phloem anatomy and the evolution of sieve element end wall types in woody plants. New Phytol. 2017;214(1):245–56. 10.1111/nph.14360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Paljakka T, Jyske T, Lintunen A, et al. : Gradients and dynamics of inner bark and needle osmotic potentials in Scots pine ( Pinus sylvestris L.) and Norway spruce ( Picea abies L. Karst). Plant Cell Environ. 2017;40(10):2160–73. 10.1111/pce.13017 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 62. Liesche J, Windt C, Bohr T, et al. : Slower phloem transport in gymnosperm trees can be attributed to higher sieve element resistance. Tree Physiol. 2015;35(4):376–86. 10.1093/treephys/tpv020 [DOI] [PubMed] [Google Scholar]
- 63. Demmig-Adams B, Stewart JJ, Adams WW, Jr: Environmental regulation of intrinsic photosynthetic capacity: an integrated view. Curr Opin Plant Biol. 2017;37:34–41. 10.1016/j.pbi.2017.03.008 [DOI] [PubMed] [Google Scholar]
- 64. Patrick JW, Offler CE: Post-sieve element transport of photoassimilates in sink regions. J Exp Bot. 1996;47 Spec No:1165–77. 10.1093/jxb/47.Special_Issue.1165 [DOI] [PubMed] [Google Scholar]
- 65. den Outer RW: Histological investigations of the secondary phloem of gymnosperms.Meded Landbouwhogeschool Wageningen. H. Veenman & Zonen N.V.: Wageningen.1967;67–7: 1-119. Reference Source [Google Scholar]
- 66. Minchin PEH, Lacointe A: Consequences of phloem pathway unloading/reloading on equilibrium flows between source and sink: A modelling approach. Functional Plant Biol. 2017;44:507–514. 10.1071/FP16354 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Gould N, Minchin PEH, Thorpe MR: Direct measurements of sieve element hydrostatic pressure reveal strong regulation after pathway blockage. Functional Plant Biol. 2004;31:987–993. 10.1071/FP04058 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 68. Knoblauch M, Froelich DR, Pickard WF, et al. : SEORious business: structural proteins in sieve tubes and their involvement in sieve element occlusion. J Exp Bot. 2014;65(7):1879–93. 10.1093/jxb/eru071 [DOI] [PubMed] [Google Scholar]
- 69. Froelich DR, Mullendore DL, Jensen KH, et al. : Phloem ultrastructure and pressure flow: Sieve-Element-Occlusion-Related agglomerations do not affect translocation. Plant Cell. 2011;23(12):4428–45. 10.1105/tpc.111.093179 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. Mullendore DL, Windt CW, van As H, et al. : Sieve tube geometry in relation to phloem flow. Plant Cell. 2010;22(3):579–93. 10.1105/tpc.109.070094 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Patrick JW, Botha FC, Birch RG: Metabolic engineering of sugars and simple sugar derivatives in plants. Plant Biotechnol J. 2013;11(2):142–56. 10.1111/pbi.12002 [DOI] [PubMed] [Google Scholar]
- 72. Turgeon R, Wolf S: Phloem transport: cellular pathways and molecular trafficking. Annu Rev Plant Biol. 2009;60:207–21. 10.1146/annurev.arplant.043008.092045 [DOI] [PubMed] [Google Scholar]
- 73. Will T, Tjallingii WF, Thönnessen A, et al. : Molecular sabotage of plant defense by aphid saliva. Proc Natl Acad Sci U S A. 2007;104(25):10536–41. 10.1073/pnas.0703535104 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 74. Liu DD, Chao WM, Turgeon R: Transport of sucrose, not hexose, in the phloem. J Exp Bot. 2012;63(11):4315–20. 10.1093/jxb/ers127 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Dinant S, Kehr J: Sampling and analysis of phloem sap. Methods Mol Biol. 2013;953:185–94. 10.1007/978-1-62703-152-3_12 [DOI] [PubMed] [Google Scholar]
- 76. Jyske TM, Suuronen JP, Pranovich AV, et al. : Seasonal variation in formation, structure, and chemical properties of phloem in Picea abies as studied by novel microtechniques. Planta. 2015;242(3):613–29. 10.1007/s00425-015-2347-8 [DOI] [PubMed] [Google Scholar]
- 77. Kaiser C, Kilburn MR, Clode PL, et al. : Exploring the transfer of recent plant photosynthates to soil microbes: mycorrhizal pathway vs direct root exudation. New Phytol. 2015;205(4):1537–51. 10.1111/nph.13138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zakhartsev M, Medvedeva I, Orlov Y, et al. : Metabolic model of central carbon and energy metabolisms of growing Arabidopsis thaliana in relation to sucrose translocation. BMC Plant Biol. 2016;16(1):262. 10.1186/s12870-016-0868-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schulz A: Long-distance trafficking: lost in transit or stopped at the gate? Plant Cell. 2017;29(3):426–30. 10.1105/tpc.16.00895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rodríguez-Celma J, Ceballos-Laita L, Grusak MA, et al. : Plant fluid proteomics: Delving into the xylem sap, phloem sap and apoplastic fluid proteomes. Biochim Biophys Acta. 2016;1864(8):991–1002. 10.1016/j.bbapap.2016.03.014 [DOI] [PubMed] [Google Scholar]
- 81. Guan D, Yan B, Thieme C, et al. : PlaMoM: a comprehensive database compiles plant mobile macromolecules. Nucleic Acids Res. 2017;45(D1):D1021–D1028. 10.1093/nar/gkw988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Paultre DS, Gustin MP, Molnar A, et al. : Lost in transit: long-distance trafficking and phloem unloading of protein signals in Arabidopsis homografts. Plant Cell. 2016;28(9):2016–2025, pii: tpc.00249.2016. 10.1105/tpc.16.00249 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 83. Banerjee AK, Chatterjee M, Yu Y, et al. : Dynamics of a mobile RNA of potato involved in a long-distance signaling pathway. Plant Cell. 2006;18(12):3443–57. 10.1105/tpc.106.042473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ruiz-Medrano R, Xoconostle-Cázares B, Lucas WJ: Phloem long-distance transport of CmNACP mRNA: implications for supracellular regulation in plants. Development. 1999;126(20):4405–19. [DOI] [PubMed] [Google Scholar]
- 85. Thieme CJ, Rojas-Triana M, Stecyk E, et al. : Endogenous Arabidopsis messenger RNAs transported to distant tissues. Nat Plants. 2015;1(4):15025. 10.1038/nplants.2015.25 [DOI] [PubMed] [Google Scholar]
- 86. Zhang W, Thieme CJ, Kollwig G, et al. : tRNA-related sequences trigger systemic mRNA transport in plants. Plant Cell. 2016;28(6):1237–49. 10.1105/tpc.15.01056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Huen AK, Rodriguez-Medina C, Ho AYY, et al. : Long-distance movement of phosphate starvation-responsive microRNAs in Arabidopsis. Plant Biol (Stuttg). 2017;19(4):643–9. 10.1111/plb.12568 [DOI] [PubMed] [Google Scholar]
- 88. Barbaglia AM, Hoffmann-Benning S: Long-distance lipid signaling and its role in plant development and stress response. Subcell Biochem. 2016;86:339–61. 10.1007/978-3-319-25979-6_14 [DOI] [PubMed] [Google Scholar]
- 89. Patrick JW: PHLOEM UNLOADING: Sieve element unloading and post-sieve element transport. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:191–222. 10.1146/annurev.arplant.48.1.191 [DOI] [PubMed] [Google Scholar]
- 90. Patrick JW, Tyeramn SD, van Bel AJE: Long-distance transport. In Biochemistry & Molecular Biology of Plants, eds Buchanan BB, Gruissem W, Jones RL, John Wiley and Sons, Oxford UK,2015;658–710. Reference Source [Google Scholar]
- 91. Cram WJ: Mannitol transport and suitability as an osmoticum in root cells. Physiol Plant. 1984;61(3):396–404. 10.1111/j.1399-3054.1984.tb06346.x [DOI] [Google Scholar]
- 92. Giaquinta RT: Phloem loading of sucrose. Annu Rev Plant Physiol. 1983;34:347–87. 10.1146/annurev.pp.34.060183.002023 [DOI] [Google Scholar]
- 93. Le Hir R, Spinner L, Klemens PA, et al. : Disruption of the sugar transporters AtSWEET11 and AtSWEET12 affects vascular development and freezing tolerance in Arabidopsis. Mol Plant. 2015;8(11):1687–90. 10.1016/j.molp.2015.08.007 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 94. Carpaneto A, Geiger D, Bamberg E, et al. : Phloem-localized, proton-coupled sucrose carrier ZmSUT1 mediates sucrose efflux under the control of the sucrose gradient and the proton motive force. J Biol Chem. 2005;280(22):21437–43. 10.1074/jbc.M501785200 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 95. Milne RJ, Perroux JM, Rae AL, et al. : Sucrose transporter localization and function in phloem unloading in developing stems. Plant Physiol. 2017;173(2):1330–41. 10.1104/pp.16.01594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hafke JB, Höll S, Kühn C, et al. : Electrophysiological approach to determine kinetic parameters of sucrose uptake by single sieve elements or phloem parenchyma cells in intact Vicia faba plants. Front Plant Sci. 2013;4:274. 10.3389/fpls.2013.00274 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 97. Zhang LY, Peng YB, Pelleschi-Travier S, et al. : Evidence for apoplasmic phloem unloading in developing apple fruit. Plant Physiol. 2004;135(1):574–86. 10.1104/pp.103.036632 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 98. Zhang XY, Wang XL, Wang XF, et al. : A shift of Phloem unloading from symplasmic to apoplasmic pathway is involved in developmental onset of ripening in grape berry. Plant Physiol. 2006;142(1):220–32. 10.1104/pp.106.081430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hu L, Sun H, Li R, et al. : Phloem unloading follows an extensive apoplasmic pathway in cucumber ( Cucumis sativus L.) fruit from anthesis to marketable maturing stage. Plant Cell Environ. 2011;34(11):1835–48. 10.1111/j.1365-3040.2011.02380.x [DOI] [PubMed] [Google Scholar]
- 100. Chen C, Yuan Y, Zhang C, et al. : Sucrose phloem unloading follows an apoplastic pathway with high sucrose synthase in Actinidia fruit. Plant Sci. 2017;255:40–50. 10.1016/j.plantsci.2016.11.011 [DOI] [PubMed] [Google Scholar]
- 101. Hackel A, Schauer N, Carrari F, et al. : Sucrose transporter LeSUT1 and LeSUT2 inhibition affects tomato fruit development in different ways. Plant J. 2006;45(2):180–92. 10.1111/j.1365-313X.2005.02572.x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 102. Zanon L, Falchi R, Santi S, et al. : Sucrose transport and phloem unloading in peach fruit: potential role of two transporters localized in different cell types. Physiol Plant. 2015;154(2):179–93. 10.1111/ppl.12304 [DOI] [PubMed] [Google Scholar]
- 103. Wang ZP, Deloire A, Carbonneau A, et al. : An in vivo experimental system to study sugar phloem unloading in ripening grape berries during water deficiency stress. Ann Bot. 2003;92(4):523–8. 10.1093/aob/mcg159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ruan YL, Patrick JW, Brady C: Protoplast hexose carrier activity is a determinate of genotypic difference in hexose storage in tomato fruit. Plant Cell Environ. 1997;20(3):341–9. 10.1046/j.1365-3040.1997.d01-73.x [DOI] [Google Scholar]
- 105. Hayes MA, Davies C, Dry IB: Isolation, functional characterization, and expression analysis of grapevine ( Vitis vinifera L.) hexose transporters: differential roles in sink and source tissues. J Exp Bot. 2007;58(8):1985–97. 10.1093/jxb/erm061 [DOI] [PubMed] [Google Scholar]
- 106. McCurdy DW, Dibley S, Cahyanegara R, et al. : Functional characterization and RNAi-mediated suppression reveals roles for hexose transporters in sugar accumulation by tomato fruit. Mol Plant. 2010;3(6):1049–63. 10.1093/mp/ssq050 [DOI] [PubMed] [Google Scholar]
- 107. Cheng JT, Li X, Yao FZ, et al. : Functional characterization and expression analysis of cucumber ( Cucumis sativus L.) hexose transporters, involving carbohydrate partitioning and phloem unloading in sink tissues. Plant Sci. 2015;237:46–56. 10.1016/j.plantsci.2015.05.006 [DOI] [PubMed] [Google Scholar]
- 108. Jin Y, Ni DA, Ruan YL: Posttranslational elevation of cell wall invertase activity by silencing its inhibitor in tomato delays leaf senescence and increases seed weight and fruit hexose level. Plant Cell. 2009;21(7):2072–89. 10.1105/tpc.108.063719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zanor MI, Osorio S, Nunes-Nesi A, et al. : RNA interference of LIN5 in tomato confirms its role in controlling Brix content, uncovers the influence of sugars on the levels of fruit hormones, and demonstrates the importance of sucrose cleavage for normal fruit development and fertility. Plant Physiol. 2009;150(3):1204–18. 10.1104/pp.109.136598 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 110. Shi J, Wang J, Li R, et al. : Expression patterns of genes encoding plasma membrane aquaporins during fruit development in cucumber ( Cucumis sativus L.). Plant Physiol Biochem. 2015;96:329–36. 10.1016/j.plaphy.2015.08.018 [DOI] [PubMed] [Google Scholar]
- 111. Stanfield RC, Hacke UG, Laur J: Are phloem sieve tubes leaky conduits supported by numerous aquaporins? Am J Bot. 2017;104(5):719–32. 10.3732/ajb.1600422 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 112. Patrick JW: Does Don Fisher's high-pressure manifold model account for phloem transport and resource partitioning? Front Plant Sci. 2013;4:184. 10.3389/fpls.2013.00184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Fisher DB, Cash-Clark CE: Gradients in water potential and turgor pressure along the translocation pathway during grain filling in normally watered and water-stressed wheat plants. Plant Physiol. 2000;123(1):139–48. 10.1104/pp.123.1.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ross-Elliott TJ, Jensen KH, Haaning KS, et al. : Phloem unloading in Arabidopsis roots is convective and regulated by the phloem-pole pericycle. eLife. 2017;6: pii: e24125. 10.7554/eLife.24125 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 115. Gould N, Thorpe MR, Minchin PEH, et al. : Solute is imported to elongating root cells of barley as a pressure driven-flow of solution. Functional Plant Biol. 2004;31(4):391–397. 10.1071/FP03231 [DOI] [PubMed] [Google Scholar]
- 116. Warmbrodt RD: Solute concentrations in the phloem and apex of the root of Zea mays. Am J Bot. 1987;74(3):394–402. 10.2307/2443815 [DOI] [Google Scholar]
- 117. Zhang W, Zhou Y, Dibley KE, et al. : Review: Nutrient loading of developing seeds. Functional Plant Biol. 2007;34(4):314–331. 10.1071/FP06271 [DOI] [PubMed] [Google Scholar]
- 118. Sosso D, Luo D, Li QB, et al. : Seed filling in domesticated maize and rice depends on SWEET-mediated hexose transport. Nat Genet. 2015;47(12):1489–93. 10.1038/ng.3422 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 119. Ritchie RJ, Fieuw-Makaroff S, Patrick JW: Sugar retrieval by coats of developing seeds of Phaseolus vulgaris L. and Vicia faba L. Plant Cell Physiol. 2003;44(2):163–72. 10.1093/pcp/pcg022 [DOI] [PubMed] [Google Scholar]
- 120. Zhou Y, Qu H, Dibley KE, et al. : A suite of sucrose transporters expressed in coats of developing legume seeds includes novel pH-independent facilitators. Plant J. 2007;49(4):750–64. 10.1111/j.1365-313X.2006.03000.x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 121. Chen LQ, Lin IW, Qu X, et al. : A cascade of sequentially expressed sucrose transporters in the seed coat and endosperm provides nutrition for the Arabidopsis embryo. Plant Cell. 2015;27(3):607–19. 10.1105/tpc.114.134585 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 122. Ma L, Zhang D, Miao Q, et al. : Essential role of sugar transporter OsSWEET11 during the early stage of rice grain filling. Plant Cell Physiol. 2017;58(5):863–73. 10.1093/pcp/pcx040 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 123. Walker NA, Patrick JW, Zhang W, et al. : Efflux of photosynthate and acid from developing seed coats of Phaseolus vulgaris L: A chemiosmotic analysis of pump-driven efflux. J Exp Bot. 1995;46(5):539–49. 10.1093/jxb/46.5.539 [DOI] [Google Scholar]
- 124. Zhou Y, Setz N, Niemietz C, et al. : Aquaporins and unloading of phloem-imported water in coats of developing bean seeds. Plant Cell Environ. 2007;30(12):1566–77. 10.1111/j.1365-3040.2007.01732.x [DOI] [PubMed] [Google Scholar]
- 125. Hayashi H, Ishikawa-Sakurai J, Murai-Hatano M, et al. : Aquaporins in developing rice grains. Biosci Biotechnol Biochem. 2015;79(9):1422–9. 10.1080/09168451.2015.1032882 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation