Abstract
Natural killer (NK) cell function is critical for controlling initial tumor growth and determining chemosensitivity of the tumor. A synergistic relationship between rapamycin and cisplatin in uterine endometrial cancer (UEC) in vitro has been reported, but the mechanism and the combined therapeutic strategy for endometrial cancer (EC) are still unknown. We found a positive correlation between the level of IL-27 and the differentiated stage of UEC. The increase of IL-27 in uterine endometrial cancer cell (UECC) lines (Ishikawa, RL95-2 and KLE) led to a high cytotoxic activity of NK cells to UECC in the co-culture system. Exposure with rapamycin enhanced the cytotoxicity of NK cells by upregulating the expression of IL-27 in UECC and IL-27 receptors (IL-27Rs: WSX-1 and gp130) on NK cells and further restricted the growth of UEC in Ishikawa-xenografted nude mice. In addition, treatment with rapamycin resulted in an increased autophagy level of UECC, and IL-27 enhanced this ability of rapamycin. Cisplatin-mediated NK cells' cytotoxic activity and anti-UEC activation were independent of IL-27; however, the combination of rapamycin and cisplatin led to a higher cytotoxic activity of NK cells, smaller UEC volume and longer survival rate in vivo. These results suggest that rapamycin and cisplatin synergistically activate the cytotoxicity of NK cells and inhibit the progression of UEC in both an IL-27–dependent and –independent manner. This provides a scientific basis for potential rapamycin-cisplatin combined therapeutic strategies targeted to UEC, especially for the patients with low differentiated stage or abnormally low level of IL-27.
Introduction
The incidence of uterine endometrial cancer (UEC) as a common gynecological malignancy is increasing worldwide. The majority of women diagnosed with UEC have early-stage disease, which can often be cured with surgery alone. However, chemotherapeutic or hormonal treatment for women with advanced or recurrent disease has met with limited efficiency. There is an urgent need to find an additional agent with a low toxicity to increase the anti-UEC efficiency in cooperating with traditional hormonal and cytotoxic agents.
The inhibitors of the mammalian target of rapamycin (mTORs) (such as rapamycin and ridaforolimus) are known for potent antiproliferative and autophagy inducer properties [1] and are currently under evaluation in clinical trials for a broad range of cancers, including UEC [2], [3]. Rapamycin is a macrocyclic triene with immunoregulatory properties [4], [5], [6], for example, it stimulates the secretion of IL-12p70 and IL-27 by dendritic cells and further promotes allogeneic type 1 polarization modulated by natural killer (NK) cells [6]. Although a synergistic relationship exists between rapamycin and cisplatin in both the inhibition of cell growth and induction of apoptosis in UEC [7], the detailed mechanism for this process has remained elusive.
NK cells are critical for controlling initial tumor growth and determining chemosensitivity through the secretion of interferon-γ (IFN-γ) and other proinflammatory cytokines and a cytotoxic response [8], [9], [10]. NK cells have the potent ability of antitumor immunity in mice, and their depletion has been linked to metastatic spread [11]. In addition, the presence of tumor-infiltrating NK cells clinically correlates with good patient outcome in various human cancers [12], [13], [14]. However, the regulation mechanism of the function of NK cells in UEC is largely unclear.
Interleukin (IL)-27, a member of the IL-12 family of cytokines, is a heterodimeric cytokine that contains Epstein–Barr virus–induced gene 3 and a unique IL-12p35-like protein, IL-27p28, which signals through a receptor composed of IL-27Rα (also known as WSX-1 or TCCR) and gp130 (utilized by many cytokines, including IL-6) [15], [16]. IL-27 is mainly produced by antigen-presenting cells such as dendritic cells (DCs) and macrophages, and is involved in regulating the balance between protective and pathological immunity and promoting antitumor responses [17], [18]. Our group has found that IL-27 secreted by endometrial stromal cells and that macrophages promote the progression of endometriosis by inducing the differentiation of IL-10+T help 17 (Th17) cells [19]. However, the expression and role of IL-27 in UEC remain unknown.
Therefore, this study investigated the expression and effect of IL-27 in UEC progression, and the regulation relationship between rapamycin, IL-27, and NK cells in uterine endometrial cancer cells (UECCs) in vitro and in vivo and further clarifies the mechanism of rapamycin on immune regulation of the UEC microenvironment.
Materials and Methods
Antibodies
Anti-human IL-27 antibodies (Abs) and anti-human PCNA were purchased from Abcam (USA); Phycoerythrin (PE)-conjugated anti-human IL-27, PE-conjugated anti-human WSX-1, Allophycocyanin (APC)-conjugated anti-human gp130, Brilliant Violet 421 (BV421)–conjugated anti-human CD56, phycoerythrin-cyanine 7 (PE-Cy7)–conjugated anti-human CD16, fluorescein (FITC)-conjugated anti-human NKG2D, phycoerythrin-cyanine 7 (PE-Cy7)–conjugated anti-human NKp44, APC-conjugated anti-human NKp46, PE-conjugated anti-human NKp30, FITC-conjugated anti-human KIR2DL1, PE-conjugated anti-human KIR3DL1, APC-conjugated anti-human IFN-γ, PE-conjugated anti-human perforin, Brilliant Violet 421 (BV421)–conjugated anti-human Granzyme B, PE-conjugated anti-human Fas, APC-conjugated anti-human FasL, PE/Cy5.5-conjugated anti-human Ki-67, and PE-conjugated anti-human Bcl-xL were purchased from BD Biosciences (San Jose, CA).
Patients and Sample Collection
The protocol for this study was approved by the Human Research Ethics Committee of Obstetrics and Gynecology Hospital, Fudan University, and written informed consent was obtained from all participants. All the normal endometrial tissues, highly differentiated UEC, moderately differentiated UEC and poorly differentiated UEC tissues were obtained by laparoscopy from 45 patients (mean age 47.8 years; range 36–54 years) at the Obstetrics and Gynecology Hospital of Fudan University. All of the samples were confirmed histologically. Ten UEC patients had a highly differentiated degree of UEC, 10 UEC patients had a moderately differentiated degree of UEC, and 10 UEC patients had a poorly differentiated degree of the disease. Normal endometrium in the secretory phase of the cycle was obtained through hysterectomy from patients with leiomyoma (15 cases) as normal control samples. No patients took any medications or received hormonal therapy within 6 months prior to surgery.
In addition, the peripheral blood was collected from 72 healthy fertile women (mean age 28.7 years; range 23-37 years).
Cells Lines
The human endometrial carcinoma cell lines (Ishikawa, RL95-2, and KLE cells) were obtained from the cell bank of Chinese Academy of Science (Shanghai, China). Ishikawa was grown in RPMI-1640 medium (Gibco, USA) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT), 100 U/ml penicillin, and 100 mg/ml streptomycin. RL95-2 and KLE cells were grown in DMEM/F12 medium (Gibco, USA) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT), 100 U/ml penicillin, and 100 mg/ml streptomycin.
Immunohistochemistry
Paraffin sections (5 μM) of normal endometrium and UEC tissues from patients were dehydrated in graded ethanol and then incubated with hydrogen peroxide and 1% bovine serum albumin/TBS to block endogenous peroxidase. The samples were then incubated with mouse anti-human IL-27 (10 μg/ml, Abcam, USA) or mouse IgG isotype overnight at 4°C in a humid chamber. After washing three times with TBS, the sections were overlaid with peroxidase-conjugated goat anti-mouse IgG, and the reaction was developed with 3,3-diaminobenzidine and counterstained with hematoxylin.
Purification of NK Cells
Peripheral blood mononuclear cells (PBMCs) were isolated from healthy fertile women. Human NK cells were isolated from PBMCs using magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) for in vitro co-culture experiments and in vivo cell transfer.
Cell Co-Culture
We obtained the IL-27-overexpressed UECC (IL-27+) and control UECC (mock) through transfection with GV230-IL-27 plasmid and GV230-vector plasmid (GenePharma, Shanghai, China), and these UECCs were treated with or without rapamycin (100 nM, Sigma, USA) and/or cisplatin (10 μM, Sigma) for 48 hours. Cell supernatants were then discarded, and these cells were washed with phosphate-buffered solution (PBS) and co-cultured with NK cells from peripheral blood for 24 hours. After co-culture, these NK cells were collected for flow cytometry (FCM) analysis or co-cultured with fresh UECC cells for cytotoxicity trials.
Real-Time PCR (RT-PCR)
The efficiency of IL-27 overexpression in Ishikawa, RL95-2, and KLE cells was verified by RT-PCR according to the standard protocols. In addition, mock and IL-27+ Ishikawa cells were treated with rapamycin (100 nM, Sigma) for 48 hours, and then the transcriptional levels of BECN1, MAP1LC3B, MTOR, and SQSTM1 in these cells were analyzed by RT-PCR. The primer sequences were synthesized by Sangon Biotechnology Co., Ltd. (Shanghai, China). The primer sequences of these genes are described in Supplementary Table 1. The fold change in the gene expression of the above genes was calculated using the change in cycle threshold value method (ΔΔ Ct). All values obtained were normalized to the values obtained for glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
FCM
After co-culture, we collected and analyzed the expression of IL-27Rs (gp130 and WSX-1), CD16, NKG2D, NKp30, NKp44, NKp46, KIR2DL1, KIR3DL1, IFN-γ, perforin, and Granzyme B in CD56+NK cells by FCM according to the manufacturer's instructions. The samples were analyzed using a Beckman Cyan flow cytometer (Becton Dickinson, USA) and Cellquest software (Becton Dickinson). The statistical analysis was conducted using isotype-matched controls as references.
Cytotoxicity Trials of NK Cells to UECC [Lactate Dehydrogenase (LDH) Release-Based]
NK cells were first co-cultured with IL-27+UECC for 24 hours, and then the NK cells were collected and co-cultured with fresh UECC. The cytotoxicity of NK in response to fresh UECC (Ishikawa, RL95-2, and KLE) was analyzed using a lactate dehydrogenase (LDH) release assay. UECC (2500 to 5000 cells/well) were plated, and the next day, NK cells were added at various ratios (100:1, 10:1, 1:1, 1:3, and 1:10; target cells:effector cells) (all samples in triplicate). After 4 hours of co-culture, an aliquot of 50 μl media was used in LDH cytotoxic assay using the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega, G1780). The value of corrected experimental LDH release was calculated by subtracting the value of spontaneous LDH release from effector cells at corresponding dilutions. NK cytotoxicity was defined as %cytotoxicity = (experimental value − effector cells spontaneous control − target cells spontaneous control)/(target cell maximum control − target cells spontaneous control) × 100.
Enzyme-Linked Immunosorbent Assay (ELISA) for IL-27 Determination
UECC (Ishikawa, RL95-2, and KLE cells) (1 × 105 cells/well) were seeded in 24-well plates and treated with rapamycin (100 nM, Sigma, USA) for 48 hours, and the secretion level of IL-27 in the culture supernatant was analyzed by ELISA (Biolegend, USA).
In Vivo Experiments
Nude mice of 4 to 5 weeks of age were inoculated subcutaneously under the scruff on day 0 with 200 μl of 2 × 106 Ishikawa cells (IL-27+ on the right or NC on the left) (NC: Ishikawa cells were transfected with negative control lentivirus; IL-27over: Ishikawa was transfected with the lentiviruses expressing human IL-27). Rapamycin (2 mg/kg) and/or cisplatin (2 mg/kg) or PBS was intraperitoneally injected for 3 weeks after xenotransplantation. In addition, human NK cells from peripheral blood (5 × 105 cells/mouse) were adoptively transferred to Ishikawa-xenografted nude mice every 7 days for the first 2 weeks. Tumor growth was monitored by measuring the tumor volume every 2 days. Tumor volume was determined using the formula: volume (mm3) =1/2(length × width × height). After 30 days, mice were euthanized, and the tumor tissues were collected for analysis of immunohistochemistry (IHC) (proliferating cell nuclear antigen, or PCNA, expression), FCM, and transmission electron microscopy (TEM). For evaluation of survival rate, survive curves of all groups were recorded until 60 days after translation.
For FCM, the tumor tissues of nude mice were perfused thoroughly with cold PBS before cell collection, and then tissues were minced on ice and digested with an enzyme mix of Liberase and Dispase (Invitrogen). We then collected the cells and evaluated the expression of Ki-67, Fas, FasL, and Bcl-xL in CK7+UECC and the expression of IL-27Rs (gp130 and WSX-1), CD16, NKG2D, NKp30, NKp44, NKp46, KIR2DL1, KIR3DL1, IFN-γ, perforin, and Granzyme B in CD56+NK cells by flow cytometry.
To identify autophagosomes at the ultrastructural level, UEC tissues were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) for 45 minutes at 4°C, rinsed in cacodylate buffer, postfixed in 1% OsO4 in cacodylate buffer, dehydrated, and embedded in Eponate. Ultra-thin sections were briefly contrasted with uranyl acetate and photographed with a TEM (Hitachi 7100, Tokyo, Japan). The number of autophagosomes in UECC from UEC tissues was counted in 10 randomly chosen cells per sample. The autophagy grade was based on the average number of autophagic vacuoles and autolysosome/per cell visible by TEM [20].
Statistics
All values are shown as mean ± SEM. Data were analyzed with GraphPad Prism version 5 by t test or one-way ANOVA. A log-rank test was performed, and Kaplan-Meier survival curves were plotted. Differences were considered statistically significant at P < .05.
Results
IL-27 level Is Positively Correlated with the Differentiated Stage of UEC
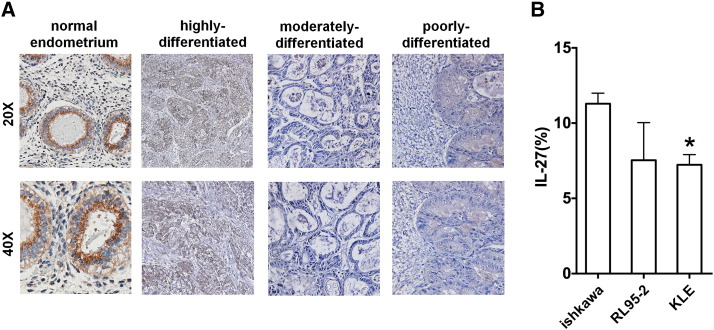
To investigate whether IL-27 expression is associated with the pathological stage of UEC, we first analyzed the expression of IL-27 in UEC tissues by IHC staining. As observed in Figure 1A, the staining of IL-27 in normal endometrium was stronger than that in UEC tissues. IL-27 level in UECC was gradually decreased with the decrease of differentiation degree of UEC (Figure 1A). Of note, IL-27 staining in moderately and poorly differentiated UEC was nearly undetected. Compared to Ishikawa and RL95-2 cells, KLE cells had a lower level of IL-27 (Figure 1B). This result indicates that there is a positive correlation between the expression of IL-27 and the degree of UEC differentiation.
Figure 1.
IL-27 level is positively correlated with the differentiated stage of UEC. (A) The expression of IL-27 in normal endometrium (n = 15) and UEC tissues (highly differentiated group = 10, moderately differentiated group = 10, poorly differentiated group = 10) by IHC staining. (B) The level of IL-27 in Ishikawa, RL95-2, and KLE cells was detected by FCM. Data are expressed as mean ± SEM. *P < .05.
IL-27 Improves the Cytotoxicity of NK Cells to UECC
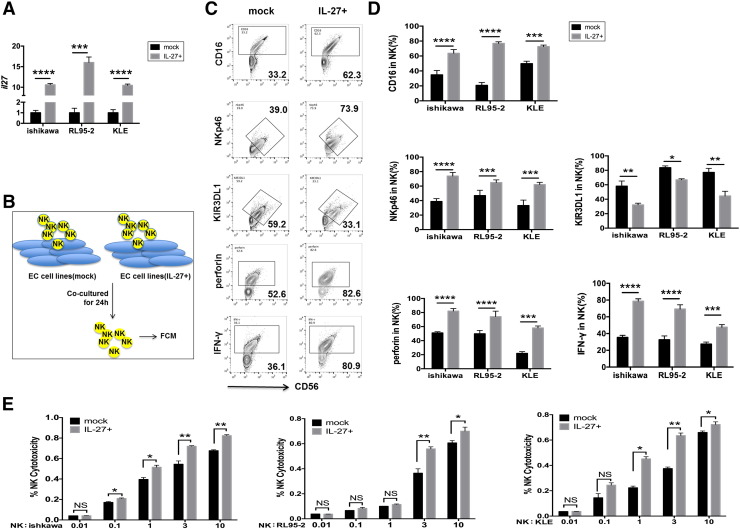
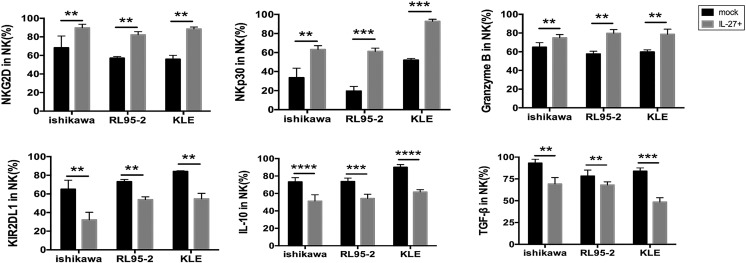
To investigate the potential effect of IL-27 secreted by UECC on NK cells function, we first constructed IL-27 overexpressed (IL-27over) Ishikawa, RL95-2, and KLE cells (Figure 2A) and further co-cultured them with NK cells from peripheral blood, respectively (Figure 2B). As shown, compared to control cells, IL-27over Ishikawa, RL95-2, and KLE cells led to the increase of CD16, NKp46, NKp30, NKG2D, perforin, IFN-γ, and Granzyme B and the decrease of killer cell immunoglobulin-like receptor (KIR) 3DL1, KIR2DL1, IL-10, and transforming growth factor (TGF)-β in co-cultured NK cells (Figure 3, C and D, Supplementary Figure 1). Subsequently, we collected these co-cultured NK cells and further co-cultured with fresh Ishikawa, RL95-2, or KLE cells in a different cell ratio of NK and UECC (1:100, 1:10, 1:1, 3:1, or 10:1). As presented in Figure 2E, NK co-cultured with IL-27overUECC had more powerful cytotoxic activity than the control UECC, especially with a cell ratio of NK and UECC of 3:1 or 10:1. Taken together, these data suggest that IL-27 secreted by UECC enhances the cytotoxic activity of NK cells to UECC.
Figure 2.
IL-27 improves the cytotoxicity of NK cells to UECC. (A) The transcription level of IL-27 in mock and IL-27-overexpressed (IL-27+) UECC (Ishikawa, RL95-2, and KLE cells) was measured by RT-PCT. (B-D) NK cells (n = 6) from peripheral blood were respectively co-cultured with mock or IL-27+ UECC (Ishikawa, RL95-2, and KLE cells) for 24 hours, and then the expression of KIR3DL1, NKp46, CD16, IFN-γ, and perforin in NK cells was analyzed by FCM. (E) After first being co-cultured with mock or IL-27+ UECC (Ishikawa, RL95-2, and KLE cells) for 48 hours, NK cells (n = 6) were then co-incubated with fresh UECC for the cytotoxicity assay at different E/T ratios (1:100, 1:10, 1:1, 3:1, or 10:1) for 3 hours. Mock: control UECC transfected with GV230-vector plasmid; IL-27+: UECC transfected with GV230-IL-27 plasmid. Data are expressed as the mean ± SEM. *P < .05, **P < .01, ***P < .001, and ****P < .0001. NS: no statistical difference.
Figure 3.
Rapamycin upregulates IL-27 production of UECC and IL-27R expression on NK cells. (A) After treatment with rapamycin (100 nM) for 48 hours, the secretion levels of IL-27 from UEC cells UECC (Ishikawa, RL95-2, and KLE cells) were evaluated by ELISA. (B, C) The expression of IL-27 in cytokeratin (CK)7+UECC of cancer lesions from Ishikawa-xenografted nude mice (n = 8 mice/group) after intraperitoneal injection of rapamycin (2 mg/kg) or PBS for 3 weeks was analyzed by FCM. (D-F) The mock or IL-27+ UECC (Ishikawa, RL95-2, and KLE cells) were prestimulated with or without rapamycin (100 nM) for 48 hours and co-cultured with NK cells for 24 hours, and then the IL-27 receptors (gp130 and WSX-1) in these NK cells were evaluated by FCM. (G-I) Nude mice of 4 to 5 weeks of age were inoculated subcutaneously under the scruff on day 0 with 200 μl of Ishikawa cells (2 × 106) (IL-27over Ishikawa cells on the right or NC Ishikawa cells on the left). After 7 days, Ishikawa-xenografted nude mice (n = 8 mice/group) were intraperitoneally injected rapamycin (2 mg/kg) or PBS for 3 weeks. Moreover, human NK cells from peripheral blood labeled with PKH67 were adoptively transferred to Ishikawa-xenografted nude mice every 7 days for the first 2 weeks. After 3 weeks, the tumors were collected for FCM, TEM and IHC. (H, I) FCM was used to analyze the IL-27 receptors on PKH-67–labeled NK cells in cancer lesions of Ishikawa-xenografted nude mice. NC: Ishikawa cells were transfected with negative control lentivirus; IL-27over: Ishikawa was transfected with the lentiviruses expressing human IL-27. Data are expressed as mean ± SEM. *P < .05, **P < .01, ***P < .001, and ****P < .0001.
Supplementary Figure 1.
IL-27 secreted by UECC improves the cytotoxicity of NK cells in vitro. The expression of KIR2DL1, NKG2D, NKp30, IL-10, Granzyme B, and TGF-β in NK cells (n = 6) treated as described in Figure 2B was analyzed by FCM. Mock: control UECC transfected with GV230-vector plasmid; IL-27+: UECC transfected with GV230-IL-27 plasmid. Data are expressed as the mean ± SEM. **P < .01, ***P < .001, and ****P < .0001. NS: no statistical difference.
Rapamycin Upregulates IL-27 Production of UECC and IL-27R Expression on NK cells
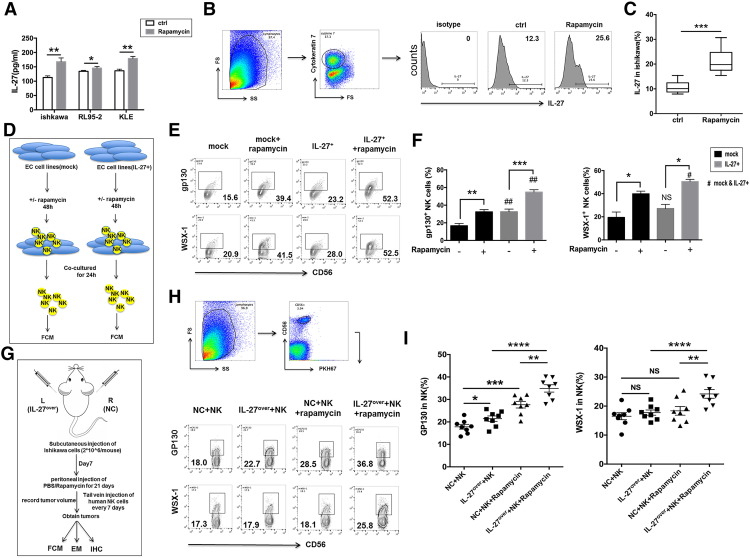
To investigate the role of rapamycin in NK cell function, we first analyzed the effect of rapamycin on the IL-27 and IL-27R expression. Treatment with rapamycin directly increased the secretion of IL-27 in Ishikawa, RL95-2, and KLE cells in vivo (Figure 3A) and the expression of IL-27 in cytokeratin (CK)7+UECC of cancer lesions from Ishikawa-xenografted nude mice (Figure 3, B and C). Additionally, control and IL-27over Ishikawa, RL95-2, and KLE cells were stimulated with rapamycin and then co-cultured with NK cells, respectively (Figure 3D). As shown, IL-27overUECC also increased gp130 but not WSX-1 expression on NK cells compared with control UECC (Figure 3, E and F). Rapamycin-pretreated UECC could upregulate the expression of gp130 and WSX-1 on NK cells and amplify the stimulatory effect of IL-27 on WSX-1 on NK cells in the co-culture system (Figure 3, E and F). Subsequently, Ishikawa-xenografted nude mice were studied, and the results of in vivo trials further echoed these phenomena (Figure 3, G-I).
The Increased Cytotoxicity of NK Cells to UECC Induced by Rapamycin Is Partly Dependent on IL-27
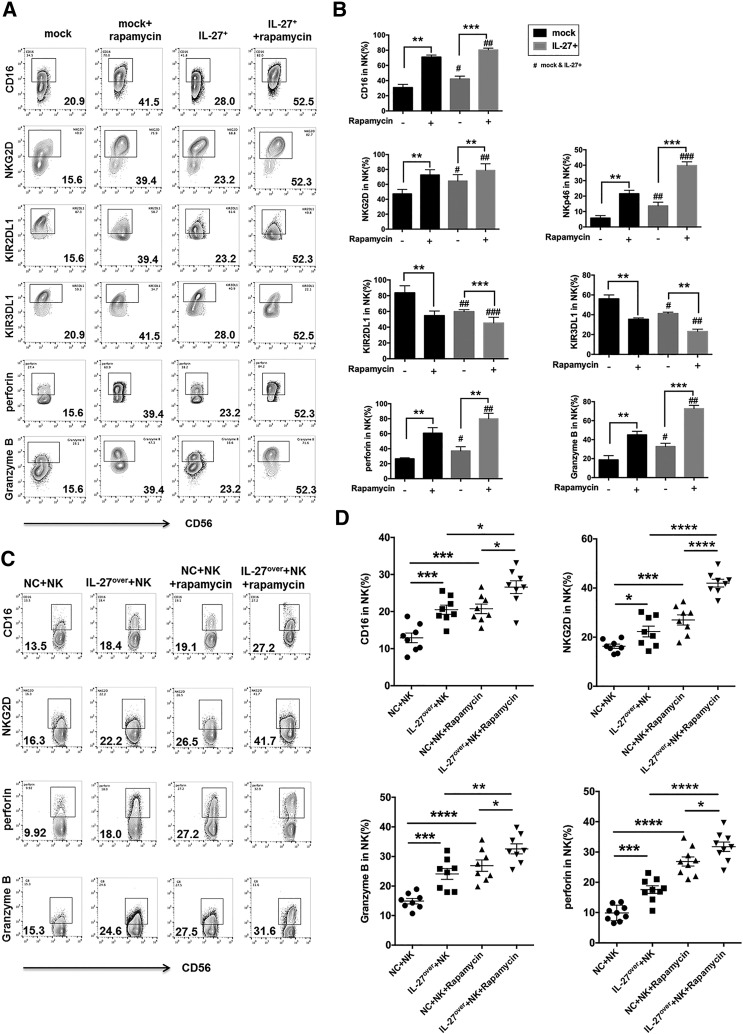
Next, we analyzed the effect of rapamycin on NK cell function and found that rapamycin-pretreated Ishikawa cells significantly elevated the expression of CD16, NKG2D, NKp46, perforin, and Granzyme B and downregulated the expression of KIR3DL1 and KIR2DL1 in co-cultured NK cells (Figure 4, A and B). In addition, these effects in the group of rapamycin pretreated IL-27over Ishikawa cells were further augmented (Figure 4, A and B). As shown, both rapamycin and IL-27 could enhance the cytotoxic activity of NK cells in vivo (Figure 4, C and D).
Figure 4.
The increased cytotoxicity of NK cells to UEC cells induced by rapamycin is partly dependent on IL-27. (A, B) After co-culture with or without rapamycin (100 nM)-pretreated mock or IL-27+ Ishikawa cells, the expression of KIR2DL1, KIR3DL1, NKG2D, CD16, NKp46, Granzyme B, and perforin in NK cells (n = 6) was analyzed by FCM. (C, D) The expression of KIR2DL1, KIR3DL1, NKG2D, CD16, NKp46, Granzyme B, and perforin in PKH-67 labeled NK cells was treated as described in Figure 3G. Data are expressed as mean ± SEM. *P < .05, **P < .01, ***P < .001, and ****P < .0001.
Rapamycin/IL-27 Restricts the Growth of UECC by Increasing Cytotoxicity of NK Cells
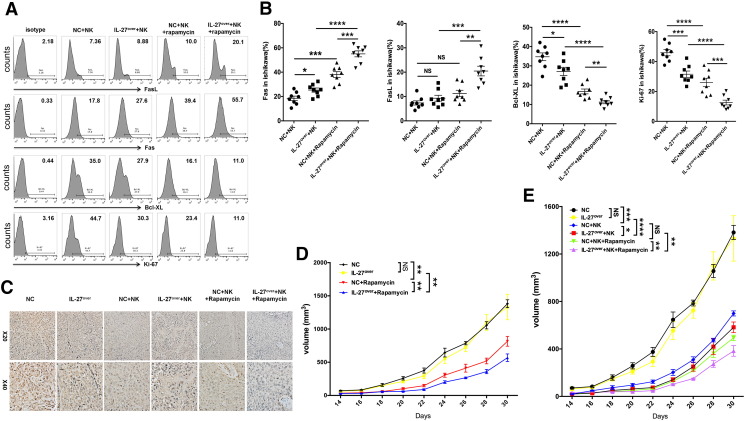
Subsequently, we investigated the effect of rapamycin and IL-27 on UEC. Along with the transfer of human peripheral NK cells, both overexpression of IL-27 and treatment with rapamycin led to the increase of Fas and B-cell lymphoma (Bcl)-xL and the decrease of Ki-67 and PCNA in UECC of cancer lesions from Ishikawa-xenografted nude mice, especially in the combination of rapamycin and IL-27 overexpression group (Figure 5, A-C). However, IL-27 overexpression did not influence the expression of PCNA (Figure 5C) and the tumor volume of UEC (Figure 5D) in the non–NK cells-transferred UEC mice model. Transferring with NK cells led to the decrease of PCNA expression (Figure 5C) and UEC volume (Figure 5, D and E), and the overexpression of IL-27 and treatment with rapamycin could amplify these effects mediated by NK cells (Figure 5, C-E).
Figure 5.
Rapamycin/IL-27 restricts the growth of UEC cells by increasing cytotoxicity of NK cells. (A, B) The expression of Fas, FasL, Bcl-XL, and Ki-67 in Ishikawa cells from the tumor of xenografted nude mice (n = 8 mice/group) as described in Figure 3G was analyzed by FCM. (C) IHC was used to evaluated the expression of PCNA in tumors of xenografted nude mice (n = 8 mice/group) as described in Figure 3G. (D, E) The xenografted nude mice (n = 8 mice/group) were described in Figure 3G, and the tumor volume was measured every other day. Data are expressed as mean ± SEM. *P < .05, **P < .01, ***P < .001, and ****P < .0001.
Taken together, these results suggest that rapamycin enhances cytotoxic activity of NK cells and further medicates anti-UEC activation and restricts the progression of UEC partly by upregulating the expression of IL-27 from UECC and the expression of IL-27R on NK cells.
IL-27 Improves Rapamycin-Induced Autophagy of UECC
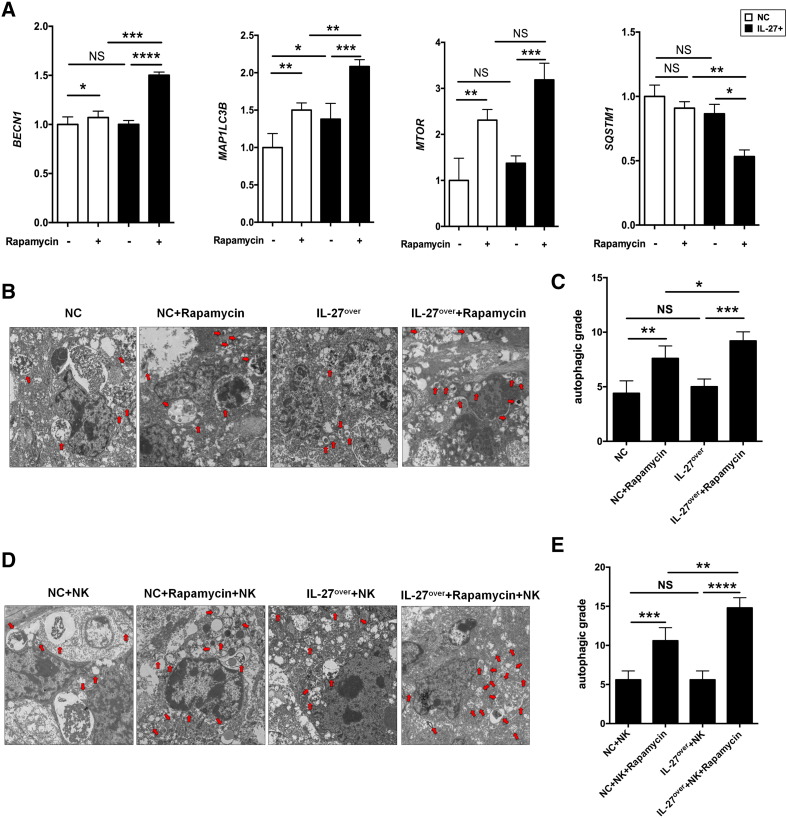
In view of the important role of rapamycin in promotion of cell autophagy [21], we investigated whether rapamycin regulated the levels of autophagy-related genes and autophagy in UEC and, if so, whether this effect was dependent on IL-27. As a “platform protein,” Beclin-1 in humans is encoded by the BECN1 gene and is essential in the induction process of autophagy [22], which provides a framework for other autophagy-related (Atg) proteins and class III phosphoinositide 3-kinase (Vps34), which initiate macroautophagic activity together. Microtubule-associated protein 1A/1B light chain 3B (hereafter referred to as LC3) is a protein that in humans is encoded by the MAP1LC3B gene. LC3 is the most widely used marker of autophagosomes [23]. mTOR is a kinase that in humans is encoded by the MTOR gene, which is a major repressor of autophagy [24], [25]. Sequestosome 1 is a protein encoded by the SQSTM1 gene. Also known as the ubiquitin-binding protein p62, the level of p62 is accumulated when autophagy is inhibited [26]. As expected, rapamycin resulted in the elevation of autophagy-related genes BECN, MAP1LC3B, and MTOR (Figure 6A) and autophagy levels (Figure 6, B and C) in cancer lesions from Ishikawa-xenografted nude mice; however, we had not observed the change of autophagy-related genes and autophagy levels after overexpression of IL-27 in UEC (Figure 6, A-C). Only the combination of IL-27 and rapamycin downregulated the transcription level of SQSTM1 (Figure 6A). Additionally, regardless of whether NK cells were present, IL-27 improved the regulation of rapamycin on BECN, MAP1LC3B, MTOR, and autophagy levels in UEC (Figure 6, A-E). These data suggest that IL-27 enhances rapamycin-mediated autophagy activation in UEC. However, the induction of autophagy by rapamycin is not necessarily dependent on IL-27.
Figure 6.
IL-27 improves rapamycin-induced autophagy of UECC. (A) The transcription level of BECN, MAP1LC3B, MTOR, and SQSTM1 in Ishikawa cells treated as described in Figure 4A was analyzed by RT-PCR. (B, C) TEM was used to analyze the autophagy levels of cancer lesions from Ishikawa (NC or IL-27over)-xenografted nude mice (n = 8 mice/group), which were peritoneally injected with or without rapamycin and (D, E) transferred with peripheral human NK cells. The autophagy grade was based on the average number of autophagic vacuoles and autolysosome/per cell visible by TEM. Data are expressed as mean ± SEM. *P < .05, **P < .01, ***P < .001, and ****P < .0001.
Rapamycin Combined with Cisplatin Exerts an Anti-UEC Effect by IL-27–Mediated NK Cell's Activation
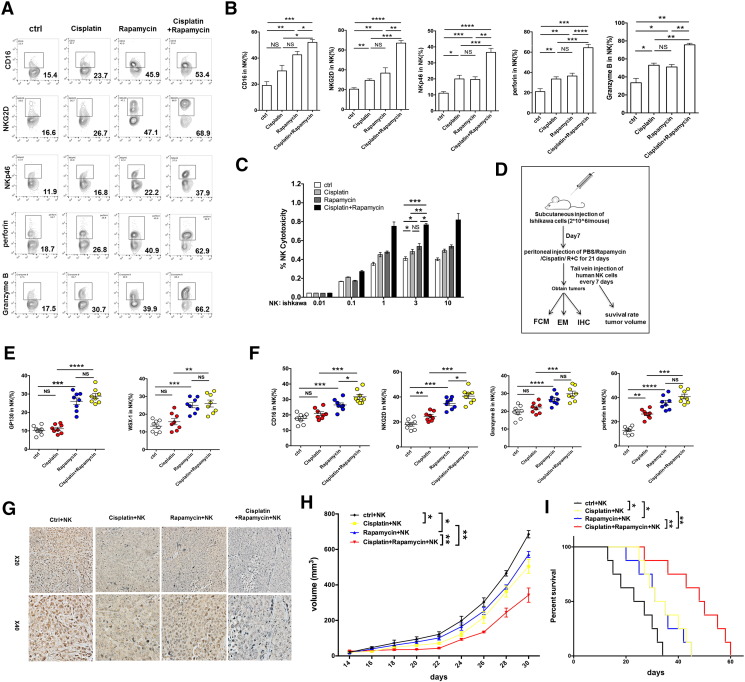
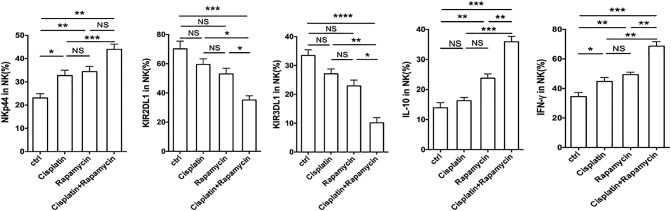
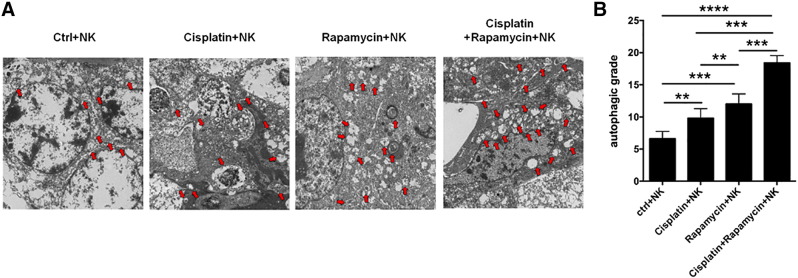
To further identify the potential synergistic anti-UEC effect of rapamycin/IL-27 and cisplatin, we collected Ishikawa cells after treatment with or without cisplatin and found that the level of IL-27 in these two groups of Ishikawa cells had no significant difference (data not shown). Similarly with rapamycin, cisplatin-pretreated Ishikawa cells upregulated the expression of NKG2D, NKp46, NKp44, Granzyme B, and IFN-γ in co-cultured NK cells (Figure 7, A and B; Supplementary Figure 2). Compared with rapamycin alone or cisplatin alone, the combination of rapamycin and cisplatin had a stronger regulation on NK cell function-related molecules (Figure 7, A and B; Supplementary Figure 2) and cytotoxic activity to UECC (Figure 7C). The results of trials in vivo (Figure 7D) showed that cisplatin also had no regulatory effect on IL-27R on NK cells, which is different with rapamycin (Figure 7E). In addition, we found that the combination of rapamycin and cisplatin led to the highest level of NK cells activation (Figure 7F) and UEC autophagy (Supplementary Figure 3), the lowest expression of PCNA in UEC (Figure 7G), the minimum of UEC volume (Figure 7H), and the longest survival rate of Ishikawa-xenografted nude mice (Figure 7I). These findings provide evidence of a synergistic anti-UEC effect between rapamycin and cisplatin; what is different is that the roles of the former and latter are independent and partly dependent on IL-27, respectively.
Figure 7.
Rapamycin combined with cisplatin exerts an anti-UEC effect by IL-27–mediated NK cell's activation. (A, B) After co-culture with rapamycin (100 nM) and/or cisplatin (10 μM)-pretreated Ishikawa cells, the expression of KIR2DL1, KIR3DL1, NKG2D, CD16, NKp46, Granzyme B, and perforin in NK cells (n = 6) was analyzed by FCM. (C) NK cells (n = 6) were treated as described Figure 7A and then co-cultured with fresh Ishikawa cells for the cytotoxicity assay at different E/T ratios (1:100, 1:10, 1:1, 3:1, or 10:1) for 3 hours. (D-I) Ishikawa-xenografted nude mice were intraperitoneally injected with rapamycin (2 mg/kg) and/or cisplatin (2 mg/kg) or PBS for 3 weeks. PKH67-labeled human peripheral NK cells were then adoptively transferred to these Ishikawa-xenografted nude mice (n = 8 mice/group) every 7 days for the first 2 weeks. After 3 weeks, the tumors were obtained for FCM (E, F) to analyze the expression of GP130, WSX-1, NKG2D, CD16, Granzyme B, and perforin in PKH-67–labeled NK cells, and IHC (G) to evaluate the expression of PCNA in cancer lesions. (H) In addition, the tumor volume was measured every other day. (I) Kaplan-Meier survival curves of these Ishikawa-xenografted nude mice. Data are expressed as mean ± SEM. *P < .05, **P < .01, **P < .01, ***P < .001, and ****P < .0001.
Supplementary Figure 2.
Rapamycin combined with cisplatin induces NK cell's activation in vitro. The expression of KIR2DL1, KIR3DL1, NKp44, IL-10, and IFN-γ in NK cells (n = 6) treated as described in Figure 7A was analyzed by FCM. Data are expressed as the mean ± SEM. *P < .05, **P < .01, ***P < .001, and ****P < .0001.
Supplementary Figure 3.
Rapamycin combined with cisplatin promotes the autophagy of UEC in vivo. TEM was used to analyze the autophagy levels of cancer lesions from Ishikawa (NC or IL-27over)-xenografted nude mice (n = 8 mice/group) treated as described in Figure 7D. Data are expressed as the mean ± SEM. **P < .01, ***P < .001, and ****P < .0001.
Discussion
Rapamycin acts as a specific inhibitor of mTOR, a serine/threonine kinase that appears to be downstream of the phosphoinositide 3-kinase/Akt signal pathway [27]. mTOR exists in two complexes, that is, mTOR complex 1 and 2 (mTORC1 and mTORC2). A complex of rapamycin and the FK506 binding protein 12 can bind to mTOR1 and inhibit its activity for controlling cell responses to environmental cues [28], [29], [30]. The signaling of mTOR modulates the activation of two major downstream components: eIF4E-binding protein 1 and p70S6K [31], [32]. mTOR plays a key role in regulating the transcription initiation of many genes that are related to the process of oncogenic transformation and cancer progression [33], [34]. Rapamycin is also a lipophilic macrolide antibiotic that was initially developed as a fungicide and immunosuppressant [35]. For example, rapamycin can inhibit myeloid DC differentiation, maturation, and function [6], [36]. However, the role and mechanism of rapamycin in immune regulation of the UEC microenvironment were not well understood. Here, we found that rapamycin could enhance the killing activation of NK cells to UEC possibly through upregulation of IL-27 expression.
The level of IL-27 was gradually decreased along with the decrease of the differentiated degree of UEC, suggesting that the level of IL-27 may be a good prognosis marker for UEC patients. Our and other studies have shown that the inflammatory environment also leads to the accumulation of IL-27 [19], [37]. TGF-β downregulates IL-27 production of monocytes [19]. In addition, estrogen can stimulate IL-27 secretion by monocytes [19]. Therefore, the low expression of IL-27 in KLE cells may be caused by the negative expression of estrogen receptor α [38]. The endocrine immune microenvironment of UEC should be involved in modulating IL-27 expression in UEC, and the detailed role and mechanism for these processes require further studies. Rapamycin not only upregulated the expression of IL-27 in UECC but also increased the expression of IL-27Rs (WSX-1 and gp130) on NK cells. Interestingly, overexpression of IL-27 also enhanced gp130 level of UECC through an unknown mechanism.
In our current study, IL-27 could not directly inhibit the proliferation, apoptosis, and growth of UEC. We found that IL-27 secreted by UECC could activate the cytotoxic activity of NK cells in vitro and in vivo; this effect should be attributed to the upregulation of CD16, NKG2D, NKp46, perforin, and Granzyme B and the downregulation of KIR3DL1 and KIR2DL1. This regulatory effect of IL-27 on NK cells partly echoed a previous report [37]. IL-27 has been shown to have antiproliferative activities that inhibit the growth and metastasis of murine melanoma, and the activation of signal transducers and activators of transcription-1 signaling is essential for the antiproliferative effects mediated by IL-27 [39]. However, apart from minor direct effects on the proliferation and survival of tumor cells, the major antitumor role of IL-27 had been reported to rely on antiangiogenic effects on surrounding endothelial cells and fibroblasts [18], [40], [41]. In addition, several studies have reported that IL-27 inhibits tumor growth and metastasis through CD8+ T cells, NK cells, and DCs [42], [43], [44]. Therefore, the regulatory effect of IL-27 on UEC is possibly dependent on other immune cells with a different molecular mechanism.
Autophagy has opposing, context-dependent roles in cancer, and interventions to both stimulate and inhibit autophagy have been proposed as cancer therapies [45]. Contrary to previous report [46], [47], cisplatin can induce the autophagy of UEC with the transfer of NK cells in vivo. Rapamycin also promoted UEC autophagy regardless of the presence of NK cells. Sharma et al. reported that IL-27 inhibited IFN-γ–induced autophagy in macrophages [48]. Here, we had not observed the regulation of IL-27 on UEC autophagy; however, IL-27 could amplify the induction of autophagy triggered by rapamycin. The specific mechanism needs further study. In addition to synergistic effects on autophagy induction, cisplatin and rapamycin also had a synergistic effect on activation of NK cells and suppression of UEC progression. These effects of cisplatin were independent on IL-27.
Collectively, based on our findings and other reports, it can be concluded that rapamycin can enhance the cytotoxic activity of NK cells and restrict the progression of UEC by upregulating IL-27 and IL-27Rs expression, and furthermore synergistically act with cisplatin as an anti-UEC. Moreover, rapamycin possibly modifies inflammatory conditions and/or promotes anti-UEC responses by IL-27–mediated immune regulation. Therefore, rapamycin should be proposed as a combination therapy for UEC for cooperating with traditional cytotoxic agents (e.g., cisplatin), especially in patients with abnormally low expression of IL-27 or poorly differentiated UEC.
The following are the supplementary data related to this article.
Sequences of Primers Used in the Paper
Disclosure of Potential Conflicts of Interest
The authors declare no financial or commercial conflict of financial interests.
Acknowledgements
We thank Dr. Yi-Qin Wang in the Department of Pathology, Hospital of Obstetrics and Gynecology, Fudan University, for help with histological analysis. This study was supported by the Major Research Program of National Natural Science Foundation of China (nos. 91542108, 81471513, 31671200, 81671460, 31600735, and 81601354), the Shanghai Rising-Star Program (16QA1400800), the Development Fund of Shanghai Talents (201557), the Oriented Project of Science and Technology Innovation from Key Lab. of Reproduction Regulation of NPFPC (CX2017-2), the Program for Zhuoxue of Fudan University, and the Shanghai Natural Science Foundation 17ZR1403200.
Contributor Information
Feng Xie, Email: ylxiefeng@163.com.
Ming-Qing Li, Email: mqli1311@126.com.
References
- 1.Kaur A, Sharma S. Mammalian target of rapamycin (mTOR) as a potential therapeutic target in various diseases. Inflammopharmacology. 2017;25:293–312. doi: 10.1007/s10787-017-0336-1. [DOI] [PubMed] [Google Scholar]
- 2.Oza AM, Pignata S, Poveda A, McCormack M, Clamp A, Schwartz B, Cheng J, Li X, Campbell K, Dodion P. Randomized phase II trial of ridaforolimus in advanced endometrial carcinoma. J Clin Oncol. 2015;33:3576–3582. doi: 10.1200/JCO.2014.58.8871. [DOI] [PubMed] [Google Scholar]
- 3.Tsoref D, Welch S, Lau S, Biagi J, Tonkin K, Martin LA, Ellard S, Ghatage P, Elit L, Mackay HJ. Phase II study of oral ridaforolimus in women with recurrent or metastatic endometrial cancer. Gynecol Oncol. 2014;135:184–189. doi: 10.1016/j.ygyno.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 4.Bunting MD, Varelias A, Souza-Fonseca-Guimaraes F, Schuster IS, Lineburg KE, Kuns RD, Fleming P, Locke KR, Huntington ND, Blazar BR. GVHD prevents NK-cell–dependent leukemia and virus-specific innate immunity. Blood. 2017;129:630–642. doi: 10.1182/blood-2016-08-734020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macedo C, Turnquist HR, Castillo-Rama M, Zahorchak AF, Shapiro R, Thomson AW, Metes D. Rapamycin augments human DC IL-12p70 and IL-27 secretion to promote allogeneic type 1 polarization modulated by NK cells. Am J Transplant. 2013;13:2322–2333. doi: 10.1111/ajt.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bae-Jump VL, Zhou C, Boggess JF, Gehrig PA. Synergistic effect of rapamycin and cisplatin in endometrial cancer cells. Cancer. 2009;115:3887–3896. doi: 10.1002/cncr.24431. [DOI] [PubMed] [Google Scholar]
- 8.Lanier LL. Evolutionary struggles between NK cells and viruses. Nat Rev Immunol. 2008;8:259–268. doi: 10.1038/nri2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soloski MJ. Recognition of tumor cells by the innate immune system. Curr Opin Immunol. 2001;13:154–162. doi: 10.1016/s0952-7915(00)00198-9. [DOI] [PubMed] [Google Scholar]
- 10.Maecker HL, Yun Z, Maecker HT, Giaccia AJ. Epigenetic changes in tumor Fas levels determine immune escape and response to therapy. Cancer Cell. 2002;2:139–148. doi: 10.1016/s1535-6108(02)00095-8. [DOI] [PubMed] [Google Scholar]
- 11.Zheng LM, Ojcius DM, Garaud F, Roth C, Maxwell E, Li Z, Rong H, Chen J, Wang XY, Catino JJ. Interleukin-10 inhibits tumor metastasis through an NK cell-dependent mechanism. J Exp Med. 1996;184:579–584. doi: 10.1084/jem.184.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coca S, Perez-Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C, Martos JA, Moreno M. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79:2320–2328. doi: 10.1002/(sici)1097-0142(19970615)79:12<2320::aid-cncr5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 13.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H, Aridome K, Hokita S, Aikou T. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88:577–583. [PubMed] [Google Scholar]
- 14.Villegas FR, Coca S, Villarrubia VG, Jiménez R, Chillón MJ, Jareño J, Zuil M, Callol L. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer. 2002;35:23–28. doi: 10.1016/s0169-5002(01)00292-6. [DOI] [PubMed] [Google Scholar]
- 15.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 16.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R, Kastelein RA. WSX-1and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 17.Hunter CA, Kastelein R. Interleukin-27: balancing protective and pathological immunity. Immunity. 2012;37:960–969. doi: 10.1016/j.immuni.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murugaiyan G, Saha B. IL-27 in tumor immunity and immunotherapy. Trends Mol Med. 2013;19:108–116. doi: 10.1016/j.molmed.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Chang KK, Liu LB, Jin LP, Zhang B, Mei J, Li H, Wei CY, Zhou WJ, Zhu XY, Shao J. IL-27 triggers IL-10 production in Th17 cells via a c-Maf/RORγt/Blimp-1 signal to promote the progression of endometriosis. Cell Death Dis. 2017;8:e2666. doi: 10.1038/cddis.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar D, Shankar S, Srivastava RK. Rottlerin-induced autophagy leads to the apoptosis in breast cancer stem cells: molecular mechanisms. Mol Cancer. 2013;12:171. doi: 10.1186/1476-4598-12-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallagher LE, Williamson LE, Chan EY. Advances in Autophagy Regulatory Mechanisms. Cell. 2016;5:24. doi: 10.3390/cells5020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsieh YC, Athar M, Chaudry IH. When apoptosis meets autophagy: deciding cell fate after trauma and sepsis. Trends Mol Med. 2009;15:129–138. doi: 10.1016/j.molmed.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H., Adams C.M., Adams P.D., Adeli K. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riffelmacher T, Richter FC, Simon AK. Autophagy dictates metabolism and differentiation of inflammatory immune cells. Autophagy. 2017 doi: 10.1080/15548627.2017.1362525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noda T, Fujita N, Yoshimori T. The late stages of autophagy: how does the end begin? Cell Death Differ. 2009;16:984–990. doi: 10.1038/cdd.2009.54. [DOI] [PubMed] [Google Scholar]
- 27.Mendez R, Myers MG, Jr., White MF, Rhoads RE. Stimulation of protein synthesis, eukaryotic translation initiation factor 4E phosphorylation, and PHAS-I phosphorylation by insulin requires insulin receptor substrate 1 and phosphatidylinositol 3-kinase. Mol Cell Biol. 1996;16:2857–2864. doi: 10.1128/mcb.16.6.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabers CJ, Martin MM, Brunn GJ, Williams JM, Dumont FJ, Wiederrecht G, Abraham RT. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J Biol Chem. 1995;270:815–822. doi: 10.1074/jbc.270.2.815. [DOI] [PubMed] [Google Scholar]
- 29.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol. 2012;12:325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown EJ, Beal PA, Keith CT, Chen J, Shin TB, Schreiber SL. Control of p70, s6 kinase by kinase activity of FRAP in vivo. Nature. 1995;377:441–446. doi: 10.1038/377441a0. [DOI] [PubMed] [Google Scholar]
- 32.Brunn GJ, Hudson CC, Sekulić A, Williams JM, Hosoi H, Houghton PJ, Lawrence JC, Jr., Abraham RT. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science. 1997;277:99–101. doi: 10.1126/science.277.5322.99. [DOI] [PubMed] [Google Scholar]
- 33.Agbunag C, Bar-Sagi D. Oncogenic K-ras drives cell cycle progression and phenotypic conversion of primary pancreatic duct epithelial cells. Cancer Res. 2004;64:5659–5663. doi: 10.1158/0008-5472.CAN-04-0807. [DOI] [PubMed] [Google Scholar]
- 34.Wislez M, Spencer ML, Izzo JG, Juroske DM, Balhara K, Cody DD, Price RE, Hittelman WN, Wistuba II, Kurie JM. Inhibition of mammalian target of rapamycin reverses alveolar epithelial neoplasia induced by oncogenic K-ras. Cancer Res. 2005;65:3226–3235. doi: 10.1158/0008-5472.CAN-04-4420. [DOI] [PubMed] [Google Scholar]
- 35.Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 1975;28:721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- 36.Hackstein H, Taner T, Logar AJ, Thomson AW. Rapamycin inhibits macropinocytosis and mannose receptor-mediated endocytosis by bone marrow-derived dendritic cells. Blood. 2002;100:1084–1087. doi: 10.1182/blood.v100.3.1084. [DOI] [PubMed] [Google Scholar]
- 37.Ziblat A, Domaica CI, Spallanzani RG, Iraolagoitia XL, Rossi LE, Avila DE, Torres NI, Fuertes MB, Zwirner NW. IL-27 stimulates human NK-cell effector functions and primes NK cells for IL-18 responsiveness. Eur J Immunol. 2015;45:192–202. doi: 10.1002/eji.201444699. [DOI] [PubMed] [Google Scholar]
- 38.Liu Z, Qi S, Zhao X, Li M, Ding S, Lu J, Zhang H. Metformin inhibits 17β-estradiol-induced epithelial-to-mesenchymal transition via βKlotho-related ERK1/2 signaling and AMPKα signaling in endometrial adenocarcinoma cells. Oncotarget. 2016;27:21315–21331. doi: 10.18632/oncotarget.7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshimoto T, Morishima N, Mizoguchi I, Shimizu M, Nagai H, Oniki S, Oka M, Nishigori C, Mizuguchi J. Antiproliferative activity of IL-27 on melanoma. J Immunol. 2008;180:6527–6535. doi: 10.4049/jimmunol.180.10.6527. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu M, Shimamura M, Owaki T, Asakawa M, Fujita K, Kudo M, Iwakura Y, Takeda Y, Luster AD, Mizuguchi J. Antiangiogenic and antitumor activities of IL-27. J Immunol. 2006;176:7317–7324. doi: 10.4049/jimmunol.176.12.7317. [DOI] [PubMed] [Google Scholar]
- 41.Zhang B, Xie F, Dong CL, Gu CJ, Cheng J, Wang Y, Xu XZ, Pu H, Wu YB, Qi XW. The cross talk between cervical carcinoma cells and vascular endothelial cells mediated by IL-27 restrains angiogenesis. Am J Reprod Immunol. 2017;78:e12706. doi: 10.1111/aji.12706. [DOI] [PubMed] [Google Scholar]
- 42.Hisada M, Kamiya S, Fujita K, Belladonna ML, Aoki T, Koyanagi Y, Mizuguchi J, Yoshimoto T. Potent antitumor activity of interleukin-27. Cancer Res. 2004;64:1152–1156. doi: 10.1158/0008-5472.can-03-2084. [DOI] [PubMed] [Google Scholar]
- 43.Salcedo R, Stauffer JK, Lincoln E, Back TC, Hixon JA, Hahn C, Shafer-Weaver K, Malyguine A, Kastelein R, Wigginton JM. IL-27 mediates complete regression of orthotopic primary and metastatic murine neuroblastoma tumors: role for CD8+ T cells. J Immunol. 2004;173:7170–7182. doi: 10.4049/jimmunol.173.12.7170. [DOI] [PubMed] [Google Scholar]
- 44.Shinozaki Y, Wang S, Miyazaki Y, Miyazaki K, Yamada H, Yoshikai Y, Hara H, Yoshida H. Tumor-specific cytotoxic T cell generation and dendritic cell function are differentially regulated by interleukin 27 during development of anti-tumor immunity. Int J Cancer. 2009;124:1372–1378. doi: 10.1002/ijc.24107. [DOI] [PubMed] [Google Scholar]
- 45.Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017 doi: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim M, Jung JY, Choi S, Lee H, Morales LD, Koh JT, Kim SH, Choi YD, Choi C, Slaga TJ. GFRA1 promotes cisplatin-induced chemoresistance in osteosarcoma by inducing autophagy. Autophagy. 2017;13:149–168. doi: 10.1080/15548627.2016.1239676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang D, Xu X, Dong Z. PRKCD/PKCδ contributes to nephrotoxicity during cisplatin chemotherapy by suppressing autophagy. Autophagy. 2017;13:631–632. doi: 10.1080/15548627.2016.1269990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma G, Dutta RK, Khan MA, Ishaq M, Sharma K, Malhotra H, Majumdar S. IL-27 inhibits IFN-γ induced autophagy by concomitant induction of JAK/PI3 K/Akt/mTOR cascade and up-regulation of Mcl-1 in Mycobacterium tuberculosis H37Rv infected macrophages. Int J Biochem Cell Biol. 2014;55:335–347. doi: 10.1016/j.biocel.2014.08.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequences of Primers Used in the Paper