Abstract
Background
Caffeine is a widely consumed psychoactive stimulant and is of epidemiological interest. Major sources of caffeine are challenging to standardize, and the use of biomarkers has been proposed as an alternative means of assessing intake.
Objective
We described urine caffeine and caffeine metabolite concentrations (n = 2466) and excretion rates (n = 2261) in the U.S. population ≥6 y by age, sex, race-ethnicity and caffeine intake (from foods, beverages and dietary supplements).
Methods
We measured caffeine and 14 of its metabolites in spot urine samples from the cross- sectional NHANES 2009–2010 by use of liquid chromatography-tandem mass spectrometry.
Results
Caffeine and its metabolites were detectable in the urine of most persons, generally at concentrations ≥1 μmol/L. Median concentrations (95% CI) ranged from 0.560 (0.497–0.620) μmol/L to 58.6 (48.6–67.2) μmol/L; median excretion rates from 0.423 (0.385–0.468) nmol/min to 46.0 (40.7–50.2) nmol/min. Urine concentrations and excretion rates for 9 analytes (caffeine, theophylline, paraxanthine, 1-methylxanthine, 1-methyluric acid, 1,3-dimethyluric acid, 1,7- dimethyluric acid, 1,3,7-trimethyluric acid, and 5-acetylamino-6-amino-3-methyluracil) had moderate correlations with caffeine intake (Spearman |r| 0.55–0.68, P <0.0001); the remaining analytes had low correlations (|r| 0.15–0.33, P <0.0001). We observed larger differences in geometric mean concentrations and excretion rates between the highest vs. lowest quartiles of caffeine intake for these 9 compounds compared to the rest. Consistent with dietary caffeine intake, we observed that urine concentrations and excretion rates for most compounds were significantly (P <0.05) higher in males vs. females, non-Hispanic whites vs. Hispanics and non- Hispanic blacks, and highest in persons aged 40–59 y.
Conclusion
Excretion of caffeine and its metabolites in urine was common in the U.S. population. Based on the observed associations between spot urine concentrations or excretion rates with caffeine intake, several of these compounds show promise as potential biomarkers of caffeine intake.
Keywords: NHANES, urine, caffeine, metabolites, dietary intake, biomarkers, phenotyping, LC-MS/MS
Introduction
Caffeine is the most widely consumed psychoactive substance in the world. In 2009–2010, average dietary caffeine consumption in U.S. adults was >200 mg/day. Approximately 35% dietary caffeine was consumed away from home, with 43% consumed outside of any defined meal (1). Although caffeine intake in U.S. adults and children has remained relatively unchanged from 1999 to 2010, some changes in sources have been observed. In children and adolescents, the relative dietary contribution of caffeine from sodas has decreased (62% to 38%) whereas coffee has increased (from 10% to 24%) and “energy” drinks have emerged as a conspicuous source (non-existent to 6%) (2). Caffeine continues to be a dietary compound of public health interest. Recent meta-analyses have examined the association of caffeine intake with diseases and conditions such as breast cancer (3) and atrial fibrillation (4), and associations with positive health outcomes have been observed in some cases, such as with Parkinson’s disease (5). Caffeine consumption is generally considered to be safe; however, fatalities due to apparent caffeine intoxication (6), as well as associations of adverse health events with caffeinated “energy” drinks (7) have been reported.
Assessment of caffeine exposure in epidemiological studies has been done almost exclusively using dietary intake data. Accurately establishing the caffeine content of dietary sources can be challenging (8) and efforts to improve the quality of dietary intake data methods are ongoing (9). The possibility of using caffeine and caffeine metabolites as biomarkers of intake has been explored in a limited number of settings. Crews et al. (10) examined the relationship of caffeine dose to the concentration of caffeine and several caffeine metabolites in 24-hour urine samples in 8 subjects of known cytochrome P450 1A2 (CYP1A2) phenotype. Grosso et al. (11) studied the relationship between urine (n = 98–263) and umbilical cord blood (n = 455–1609) concentrations of caffeine, paraxanthine, theophylline and theobromine, and self-reported caffeine intake in pregnant women. Klebanoff et al. (12) looked at serum caffeine and paraxanthine concentrations in relation to caffeine intake in 239 pregnant women. In addition to their use as intake biomarkers, urine caffeine and its metabolites have been used for phenotyping CYP1A2 (13) and N-acetyltransferase-2 (14) activity in population-based settings.
To the best of our knowledge, no representative population based studies of biologic levels of caffeine and caffeine metabolites and their relationship with caffeine intake exist. The objective of our study was to describe urine concentrations and excretion rates of caffeine and its metabolites in the U.S. population ≥6 y, by age, sex, race-ethnicity and caffeine intake. The NHANES 2009–2010 data provide the first nationally representative description of urine caffeine and caffeine metabolite concentrations and excretion rates measured in spot urine samples, and a preliminary evaluation of their potential use as biomarkers of caffeine intake.
Subjects and Methods
Study design and subjects
The NHANES is a cross-sectional survey on the health and nutritional status of the civilian non-institutionalized U.S. population conducted by the CDC National Center for Health Statistics (NCHS) (15). The survey makes use of a stratified, multistage, probability sample designed to represent the U.S. population on the basis of age, sex, and race-ethnicity. Demographic participant characteristics, dietary supplement use, and health-related data are obtained during a home interview; a physical examination, the collection of blood and urine samples, and other assessments such as dietary intake, are performed in a mobile examination center (MEC). All respondents gave their informed consent, and the NHANES protocol was reviewed and approved by the NCHS Research Ethics Review Board. Interview and examination response rates for each survey period are publically available (16).
Urine collection and storage
Spot urine specimens were collected from all NHANES participants from the 2009–2010 survey cycle ≥6 y of age who were able to provide a specimen. Each participant was instructed to provide a urine sample as soon as possible upon entry to the MEC, and to completely empty their bladder when providing the sample. The date, time, and volume of urine collection were recorded. Urine specimens were then allocated into aliquots for the various urine specimen assays to be performed; for caffeine and caffeine metabolites, a 1-mL aliquot of urine in a 2-mL polypropylene cryovial was created from a random 1/3 subset of NHANES participants (n = 2831) (17). Samples were frozen immediately after processing and shipped on dry ice to the CDC on a weekly basis. Samples were stored at ≤−70°C until analysis (18).
Laboratory methods
Urine samples were analyzed for caffeine and caffeine metabolites by use of liquid chromatography-tandem mass spectrometry (LC-MS/MS) with electrospray ionization (19). Urine concentrations were reported for the following compounds: caffeine (1,3,7-trimethylxanthine, 137X); paraxanthine (1,7-dimethylxanthine, 17X); theobromine (3,7-dimethylxanthine, 37X); theophylline (1,3-dimethylxanthine, 13X); 1,3,7-trimethyluric acid (137U); 1,3-dimethyluric acid (13U); 1,7-dimethyluric acid (17U); 1-methyluric acid (1U); 1-methylxanthine (1X); 3,7-dimethyluric acid (37U); 3-methyluric acid (3U); 3-methylxanthine (3X); 7-methyluric acid (7U); 7-methylxanthine (7X); 5-acetylamino-6-amino-3-methyluracil (AAMU). AAMU is the decomposition product of the relatively unstable caffeine metabolite 5-acetylamino-6-formylamino-3-methyluracil (AFMU). Samples were treated such that all AFMU was converted to the more stable AAMU. Limits of detection (LOD) in urine were as follows: theophylline, theobromine, 1U, 13U, 17U, 37U, 137U, 1X, 3X, 7X, 13X, 37X: 0.05 μmol/L; caffeine, paraxanthine, AAMU, 3U, 7U: 0.1 μmol/L (19). Results were reported for caffeine and caffeine metabolites in 2714 participants (17). All reported results satisfied the requirements of a multi-rule quality control system (20) using 3 QC pool concentrations for each analyte. Coefficients of variation for the study were ≤5% at analyte concentrations ≥1 μmol/L (21).
Study variables
Spot urine concentrations (μmol/L) and excretion rates (nmol/min) of caffeine and caffeine metabolites were used as dependent variables in our analyses. The inclusion of urine flow rate data in the NHANES 2009–2010, calculated by dividing the total volume of the urine sample collected in the MEC by the total time between the previous urine sample collection and the urine sample collected in the MEC, permitted the calculation of urine excretion rates. Excretion rates were calculated as the product of the spot urine concentration and the urine flow rate (22).
Independent variables consisted of general demographic variables (age, sex, race-ethnicity) and caffeine intake. Demographic variables used in the study were categorized as follows: sex (male, female); age (6–11 y, 12–19 y, 20–39 y, 40–59 y, ≥60 y); race-ethnicity [Hispanic (sum of Mexican-American and other Hispanic), non-Hispanic black (NHB), non-Hispanic white (NHW)]. Caffeine intake was calculated as the sum of intake from foods and beverages (23), and non-prescription and prescription dietary supplements, as well as non-prescription antacids that contain calcium and/or magnesium (24) from the 24-h dietary recall interview (Day 1) conducted at the MEC as it preceded the MEC urine collection. Caffeine intake was used as both a continuous and categorical (quartiles) variable.
Analytic sample
All MEC-examined NHANES 2009–2010 participants ≥6 y with caffeine or caffeine metabolite measurements were eligible for inclusion in our study. Individuals who reported using in the past 30 d any prescription medications known to inhibit or induce any of the enzyme systems involved in caffeine metabolism (n = 246) or were uncertain about their use of prescription medications (n = 2) were excluded because of potential spurious effects on analyte concentrations and excretion rates (Supplemental Table 1). We used pairwise deletion for missing values in a particular analysis and verified using Little’s test [25] that these deletions would not introduce any biases. Based on these criteria urine concentration data were available for 2466 participants and excretion rate data were available for 2261 participants. (Supplemental Table 2).
Statistical methods
Statistical analyses were performed using SAS (version 9, SAS Institute Inc., Cary, NC) and SUDAAN (version 9.2, RTI, Research Triangle Park, NC) software. MEC examination weights were used to account for differential nonresponse or non-coverage and to adjust for oversampling. Urine concentration and excretion rate distributions were highly right-skewed; consequentially, geometric means were used instead of arithmetic means. Bivariate associations for categorical variables were described by presenting geometric means and selected percentiles with 95% CIs. Geometric means were compared across categories by use of Wald F tests which assess whether at least one of the means across the categories is significantly different from the others, or pairwise t-tests. Spearman correlation was used to describe bivariate association between urine concentration or excretion rate and caffeine intake. We did not perform any adjustments for multiple comparisons as the primary objective of our study was descriptive and not to test a particular hypothesis.
Results
Detection by use of LC-MS/MS
We found that the detection rates for urine caffeine and caffeine metabolites in the NHANES 2009–2010 cycle ranged from 80.2% to 100% (Supplemental Table 3). One-third of the compounds measured were detected in either 100% (1U, 1X) or ≥99% (3X, 7X, 37X) of study samples. The lowest detection rates among analytes included caffeine (91%), 137U (88%) and 3U (80%).
Total population
We calculated geometric mean, median and central 95% reference interval (i.e. 2.5th and 97.5th percentiles) concentrations for caffeine and its metabolites in spot urine samples (Table 1; excretion rate data is presented in Supplemental Table 4). Caffeine and its metabolites were present in the urine of most persons at concentrations ≥1 μmol/L for a majority of analytes. The highest geometric mean and median spot urine concentrations and excretion rates were observed for 1U, and the lowest concentrations and excretion rates were observed for 3U. Geometric mean spot urine concentrations (95% CI) across all analytes ranged from 0.539 (0.492–0.590) μmol/L to 54.1 (48.7–60.0) μmol/L, and median concentrations ranged from 0.560 (0.497–0.620) μmol/L to 58.6 (48.6–67.2) μmol/L. Analytes in descending order of concentration central tendency (median) were as follows: 1U > 7X > AAMU > 3X > 1X > 17U > theobromine > 7U > paraxanthine > 13U > caffeine > theophylline > 137U > 37U > 3U. Geometric mean excretion rates across all analytes ranged from 0.390 (0.357–0.426) nmol/min to 39.4 (35.8–43.4) nmol/min, and median excretion rates ranged from 0.423 (0.385–0.468) nmol/min to 46.0 (40.7–50.2) nmol/min. With the exception of AAMU and 7X, the descending order of excretion rate central tendency was identical to that observed with concentration (1U > AAMU > 7X > 3X > 1X > 17U > theobromine > 7U > paraxanthine > 13U > caffeine > theophylline > 137U > 37U > 3U).
Table 1.
Spot urine caffeine and caffeine metabolite concentrations in U.S. persons aged ≥6 y, NHANES 2009–20101
| Analyte | Geometric mean (95% CI) | Median (95% CI) | Central 95% reference interval2 | |
|---|---|---|---|---|
|
| ||||
| 2.5th percentile (95% CI) | 97.5th percentile (95% CI) | |||
|
| ||||
| μmol/L | ||||
| Trimethylxanthines | ||||
| Caffeine (137X) | 2.48 (2.18–2.81) | 3.39 (2.90–4.22) | <0.1 | 33.8 (31.4–38.1) |
| Trimethyluric acids | ||||
| 137U | 1.05 (.924–1.19) | 1.42 (1.20–1.69) | <0.05 | 16.2 (13.6–19.6) |
| Dimethylxanthines | ||||
| Theophylline (13X) | 1.20 (1.09–1.32) | 1.63 (1.46–1.87) | <0.05 | 11.4 (10.3–13.3) |
| Paraxanthine (17X) | 10.2 (9.06–11.5) | 15.2 (12.8–17.5) | <0.1 | 105 (94.3–119) |
| Theobromine (37X) | 17.4 (16.1–18.9) | 20.3 (18.5–22.4) | 0.406 (0.237–0.669) | 186 (162–223) |
| Dimethyluric acids | ||||
| 13U | 4.78 (4.26–5.37) | 6.42 (5.68–7.12) | <0.05 | 62.9 (50.8–73.2) |
| 17U | 16.5 (14.5–18.9) | 24.8 (21.2–28.8) | 0.066 (<0.05–0.130) | 224 (195–279) |
| 37U | 1.10 (1.01–1.19) | 1.24 (1.13–1.37) | <0.05 | 13.1 (12.2–16.5) |
| Methylxanthines | ||||
| 1X | 23.5 (21.1–26.1) | 27.6 (24.0–32.3) | 0.986 (0.848–1.05) | 276 (264–300) |
| 3X | 26.7 (24.2–29.4) | 30.9 (27.5–35.0) | 0.693 (0.407–0.997) | 305 (273–372) |
| 7X | 43.8 (39.6–48.4) | 51.3 (44.0–57.2) | 1.13 (.670–1.45) | 546 (484–621) |
| Methyluric acids | ||||
| 1U | 54.1 (48.7–60.0) | 58.6 (48.6–67.2)) | 4.58 (4.08–5.13) | 508 (471–703) |
| 3U | 0.539 (0.492–0.590) | 0.560 (0.497–0.620) | <0.1 | 6.60 (5.93–8.01) |
| 7U | 13.5 (12.2–15.0) | 15.5 (13.3–17.7) | 0.368 (0.282–0.435) | 182 (166–210) |
| Uracils | ||||
| AAMU | 37.1 (32.6–42.3) | 49.9 (42.5–56.2) | 0.339 (0.078–0.537) | 539 (462–727) |
n = 2466. Individuals who reported using any prescription medications known to inhibit or induce cytochrome P450, N-acetyltransferase 2 or xanthine oxidase activity in the past 30 d were excluded. 137U, 1,3,7-trimethyluric acid; 13U, 1,3-dimethyluric acid; 17U, 1,7-dimethyluric acid; 1U, 1-methyluric acid; 1X, 1-methylxanthine; 37U, 3,7-dimethyluric acid; 37X, 3,7-dimethylxanthine; 3U, 3-methyluric acid; 3X, 3-methylxanthine; 7U, 7-methyluric acid; 7X, 7-methylxanthine; AAMU, 5-acetylamino-6-amino-3-methyluracil.
In cases where the 2.5th percentile was less than the limit of detection (< LOD), the LOD value is presented.
Demographic variables
We determined geometric mean spot urine concentrations of caffeine and caffeine metabolites stratified by sex and race-ethnicity (Table 2; excretion rate data appear in Supplemental Table 5) and by age categories (Table 3; excretion data appear in Supplemental Table 6). Higher geometric mean concentrations were observed in men vs. women for 1X, 1U (Wald F P <0.01), paraxanthine, and AAMU (Wald F P <0.05). Geometric mean excretion rates were higher in men vs. women for 1X, 1U, 13U, AAMU (Wald F P <0.0001), paraxanthine (Wald F P <0.001), theophylline, 17U, and 7X (Wald F P <0.01). No statistically significant cases were observed where urine concentrations or excretion rates were higher in women vs. men. Geometric mean concentrations and excretion rates for all compounds were higher in NHW vs. Hispanics and NHB (1.6–3.4× and 1.3–2.6×, respectively; Wald F P ≤ 0.0001 in all cases). Geometric mean concentrations were highest in persons aged 40–59 y and lowest in persons aged 6–11 y for caffeine, paraxanthine, theophylline, 1X, 1U, 13U, 17U, 137U, and AAMU. Geometric mean excretion rates showed the same behavior with age for the same analytes plus 3X, 3U, 7U and 37U.
Table 2.
Spot urine caffeine and caffeine metabolite concentrations by sex and race-ethnicity for U.S. persons aged ≥6 y, NHANES 2009–20101
| Analyte | Sex | Race-ethnicity | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Male (n = 1215) | Female (n = 1251) | Wald-F P value | NHW (n = 1042) | NHB (n = 460) | All Hispanics (n = 826) | Wald-F P value | |
|
| |||||||
| μmol/L | μmol/L | μmol/L | μmol/L | μmol/L | |||
| Trimethylxanthines | |||||||
| Caffeine (137X) | 2.44 (2.13–2.78) | 2.52 (2.13–2.98) | 0.67 | 3.03 (2.58–3.56) | 1.27 (1.02–1.58) | 1.88 (1.58–2.24) | <0.0001 |
| Trimethyluric acids | |||||||
| 137U | 1.07 (.921–1.24) | 1.03 (.873–1.21) | 0.67 | 1.27 (1.09–1.50) | .621 (.497–.774) | .779 (.655–.927) | <0.0001 |
| Dimethylxanthines | |||||||
| Theophylline (13X) | 1.21 (1.08–1.36) | 1.18 (1.05–1.33) | 0.69 | 1.40 (1.24–1.59) | .672 (.553–.816) | 1.04 (.900–1.20) | <0.0001 |
| Paraxanthine (17X) | 11.1 (9.74–12.6) | 9.41 (8.13–10.9) | 0.0228 | 11.8 (10.1–13.8) | 5.81 (4.51–7.49) | 8.65 (7.55–9.90) | <0.0001 |
| Theobromine (37X) | 16.8 (15.1–18.6) | 18.1 (16.0–20.4) | 0.34 | 21.0 (19.0–23.1) | 10.9 (9.35–12.8) | 12.8 (11.3–14.5) | <0.0001 |
| Dimethyluric acids | |||||||
| 13U | 5.16 (4.48–5.94) | 4.44 (3.85–5.12) | 0.06 | 6.14 (5.38–7.01) | 2.57 (2.02–3.28) | 3.26 (2.80–3.79) | <0.0001 |
| 17U | 16.8 (14.4–19.7) | 16.3 (13.9–19.0) | 0.67 | 21.4 (18.4–24.8) | 8.24 (6.15–11.0) | 11.5 (9.65–13.6) | <0.0001 |
| 37U | 1.11 (1.02–1.22) | 1.08 (.949–1.23) | 0.63 | 1.38 (1.24–1.53) | .747 (.660–.846) | .680 (.587–.789) | <0.0001 |
| Methylxanthines | |||||||
| 1X | 26.7 (23.9–29.9) | 20.7 (17.7–24.1) | 0.0048 | 29.1 (25.9–32.6) | 15.8 (12.7–19.7) | 15.3 (13.7–17.1) | <0.0001 |
| 3X | 25.6 (22.8–28.7) | 27.9 (24.4–31.8) | 0.25 | 35.9 (32.5–39.8) | 15.4 (12.8–18.4) | 15.2 (13.4–17.2) | <0.0001 |
| 7X | 45.6 (40.9–50.8) | 42.1 (36.5–48.6) | 0.29 | 57.1 (51.4–63.4) | 29.1 (24.5–34.6) | 24.2 (21.3–27.5) | <0.0001 |
| Methyluric acids | |||||||
| 1U | 61.0 (54.2–68.6) | 48.1 (42.0–55.1) | 0.0022 | 65.8 (58.4–74.0) | 40.8 (33.6–49.5) | 34.5 (31.5–37.9) | <0.0001 |
| 3U | 0.533 (0.477–0.595) | 0.544 (0.482–0.615) | 0.76 | 0.731 (0.654–0.816) | 0.319 (0.270–0.377) | 0.297 (0.260–0.340) | <0.0001 |
| 7U | 13.6 (12.1–15.3) | 13.4 (11.6–15.5) | 0.86 | 18.2 (16.3–20.3) | 8.28 (6.86–10.0) | 7.10 (6.21–8.11) | <0.0001 |
| Uracils | |||||||
| AAMU | 41.4 (35.2–48.8) | 33.3 (28.6–38.9) | 0.02 | 47.1 (40.5–54.8) | 19.1 (14.1–25.9) | 25.3 (21.3–30.2) | <0.0001 |
Values are geometric means (95% CIs). Individuals who reported using any prescription medications known to inhibit or induce cytochrome P450, N-acetyltransferase 2 or xanthine oxidase activity in the past 30 d were excluded. 137U, 1,3,7-trimethyluric acid; 13U, 1,3-dimethyluric acid; 17U, 1,7-dimethyluric acid; 1U, 1-methyluric acid; 1X, 1-methylxanthine; 37U, 3,7-dimethyluric acid; 37X, 3,7-dimethylxanthine; 3U, 3-methyluric acid; 3X, 3-methylxanthine; 7U, 7-methyluric acid; 7X, 7-methylxanthine; AAMU, 5-acetylamino-6-amino-3-methyluracil.
Table 3.
Spot urine caffeine and caffeine metabolite concentrations by age for U.S. persons aged ≥6 y, NHANES 2009–20101
| Analyte | 6–11 y (n = 377) | 12–19 y (n = 385) | 20–39 y (n = 586) | 40–59 y (n = 600) | ≥60 y (n = 518) | Wald F P value |
|---|---|---|---|---|---|---|
|
| ||||||
| μmol/L | μmol/L | μmol/L | μmol/L | μmol/L | ||
| Trimethylxanthines | ||||||
| Caffeine (137X) | 0.464 (0.386–0.559) | 0.945 (0.731–1.22) | 2.74 (2.46–3.06) | 4.51 (3.60–5.65) | 3.89 (3.24–4.66) | <0.0001 |
| Trimethyluric acids | ||||||
| 137U | 0.229 (0.189–0.278) | 0.518 (0.406–0.663) | 1.18 (1.03–1.36) | 1.69 (1.36–2.11) | 1.46 (1.18–1.81) | <0.0001 |
| Dimethylxanthines | ||||||
| Theophylline (13X) | 0.292 (0.252–0.339) | 0.596 (0.489–0.727) | 1.38 (1.22–1.56) | 1.96 (1.65–2.33) | 1.49 (1.25–1.76) | <0.0001 |
| Paraxanthine (17X) | 2.52 (2.05–3.09) | 5.34 (4.02–7.10) | 12.0 (10.4–13.8) | 16.9 (13.7–20.9) | 11.5 (9.45–14.1) | <0.0001 |
| Theobromine (37X) | 21.1 (16.6–26.8) | 21.1 (16.2–27.6) | 15.3 (13.5–17.4) | 19.0 (16.7–21.7) | 14.6 (12.3–17.3) | 0.05 |
| Dimethyluric acids | ||||||
| 13U | 1.08 (.913–1.29) | 2.44 (1.95–3.05) | 5.29 (4.52–6.20) | 7.68 (6.38–9.23) | 6.71 (5.44–8.26) | <0.0001 |
| 17U | 3.04 (2.44–3.79) | 7.91 (5.98–10.5) | 17.9 (15.0–21.3) | 28.6 (22.9–35.6) | 25.2 (20.4–31.1) | <0.0001 |
| 37U | 1.25 (1.03–1.51) | 1.43 (1.10–1.85) | 0.986 (0.862–1.13) | 1.13 (0.983–1.31) | 0.956 (0.796–1.15) | 0.05 |
| Methylxanthines | ||||||
| 1X | 8.06 (6.95–9.35) | 15.0 (12.2–18.3) | 27.3 (23.5–31.7) | 34.2 (28.8–40.6) | 24.0 (19.7–29.2) | <0.0001 |
| 3X | 28.1 (21.7–36.5) | 30.6 (24.0–39.1) | 22.6 (19.6–26.1) | 29.2 (25.2–33.8) | 27.0 (23.1–31.7) | 0.13 |
| 7X | 57.4 (44.8–73.6) | 58.8 (45.3–76.3) | 38.4 (33.1–44.4) | 44.8 (39.1–51.3) | 36.6 (30.5–43.9) | 0.0165 |
| Methyluric acids | ||||||
| 1U | 20.9 (18.7–23.5) | 36.7 (31.1–43.3) | 56.8 (49.7–65.0) | 72.0 (61.6–84.2) | 68.7 (57.3–82.3) | <0.0001 |
| 3U | 0.524 (0.437–0.629) | 0.544 (0.431–0.687) | 0.435 (0.384–0.494) | 0.581 (0.498–0.677) | 0.691 (0.595–0.802) | <0.0001 |
| 7U | 17.7 (14.0–22.2) | 16.6 (12.9–21.4) | 9.99 (8.69–11.5) | 14.3 (12.3–16.7) | 15.3 (12.5–18.6) | 0.0008 |
| Uracils | ||||||
| AAMU | 8.49 (6.83–10.6) | 20.2 (15.2–26.8) | 40.0 (33.4–47.9) | 60.7 (49.8–74.1) | 50.0 (40.6–61.5) | <0.0001 |
Values are geometric means (95% CIs). Individuals who reported using any prescription medications known to inhibit or induce cytochrome P450, N-acetyltransferase 2 or xanthine oxidase activity in the past 30 d were excluded. 137U, 1,3,7-trimethyluric acid; 13U, 1,3-dimethyluric acid; 17U, 1,7-dimethyluric acid; 1U, 1-methyluric acid; 1X, 1-methylxanthine; 37U, 3,7-dimethyluric acid; 37X, 3,7-dimethylxanthine; 3U, 3-methyluric acid; 3X, 3-methylxanthine; 7U, 7-methyluric acid; 7X, 7-methylxanthine; AAMU, 5-acetylamino-6-amino-3-methyluracil; NHB, non-Hispanic black; NHW, non-Hispanic white.
Caffeine intake
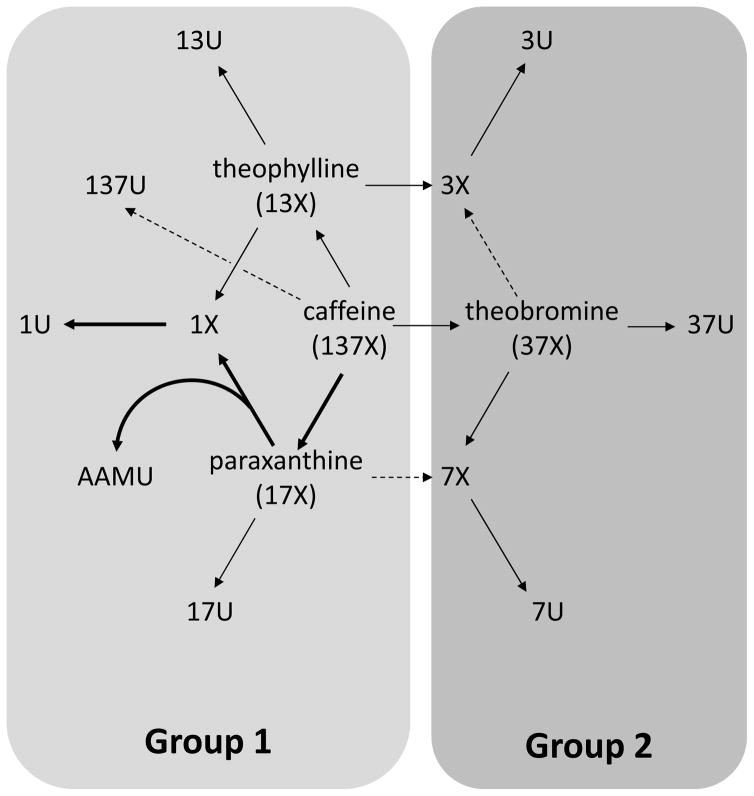
We calculated Spearman correlations of spot urine caffeine and caffeine metabolite concentration with caffeine intake, and geometric mean concentrations by intake quartiles (Table 4; excretion rate data appear in Supplemental Table 7). We found that dietary intake was a significant correlate of both concentration and excretion rate. Spot urine concentrations were weakly to moderately correlated with caffeine intake (Spearman |r| 0.15–0.63, P <0.0001). The overall range of correlations observed with excretion rate was similar (|r| 0.24–0.68 P <0.0001) and the individual correlation coefficients were also similar (difference <0.10) to those noted with urine concentrations. We observed that the correlations appeared to fall into two distinct ranges (illustrated in Figure 1 in relation to caffeine metabolism). The group of compounds in the higher range (identified herein as “Group 1” (0.55 ≤ |r| ≤ 0.68), consisted of caffeine, three of its metabolites (paraxanthine, theophylline, and 137U), and 5 subsequent metabolites of paraxanthine and/or theophylline (17U, AAMU, 1X, 1U, and 13U). The group of compounds in the lower range (identified herein as “Group 2”, 0.15 ≤ |r| ≤ 0.33) consisted of theobromine, 37U, and the remaining 3- and 7-monomethyl xanthines and uric acids (3X, 7X, 3U, 7U).
Table 4.
Associations between spot urine caffeine and caffeine metabolite concentrations and 24-h dietary caffeine intake from foods and dietary supplements in U.S. persons aged ≥6 y, NHANES 2009–20101,2
| Analyte | Spearman correlation3 | Caffeine intake quartile4 | |||
|---|---|---|---|---|---|
|
| |||||
| Q1 [0–8] (n = 720) | Q2 [9–84] (n = 656) | Q3 [85–213] (n = 560) | Q4 [214–3020] (n = 434) | ||
|
| |||||
| |r| | μmol/L | ||||
| Trimethylxanthines | |||||
| Caffeine (137X) | 0.59 | 0.539 (0.445–0.653) | 1.84 (1.64–2.06) | 4.62 (4.11–5.19) | 7.88 (7.13–8.71) |
| Trimethyluric acids | |||||
| 137U | 0.55 | 0.244 (0.209–0.286) | 0.814 (0.716–0.925) | 1.92 (1.68–2.20) | 3.05 (2.64–3.52) |
| Dimethylxanthines | |||||
| Theophylline (13X) | 0.63 | 0.285 (0.248–0.328) | 0.987 (0.881–1.10) | 2.13 (1.94–2.35) | 3.30 (2.95–3.70) |
| Paraxanthine (17X) | 0.61 | 2.02 (1.67–2.43) | 8.77 (7.61–10.1) | 19.1 (17.1–21.2) | 30.5 (26.4–35.2) |
| Theobromine (37X) | 0.15 | 10.5 (8.56–12.9) | 19.1 (16.5–22.0) | 19.2 (17.1–21.5) | 23.8 (21.0–27.0) |
| Dimethyluric acids | |||||
| 13U | 0.62 | 1.01 (.847–1.20) | 4.01 (3.44–4.68) | 8.81 (7.91–9.81) | 14.1 (12.3–16.1) |
| 17U | 0.60 | 2.78 (2.28–3.40) | 14.7 (12.5–17.3) | 32.8 (29.3–36.8) | 53.5 (45.8–62.5) |
| 37U | 0.15 | 0.750 (0.639–0.880) | 1.14 (.967–1.35) | 1.16 (1.03–1.30) | 1.46 (1.25–1.70) |
| Methylxanthines | |||||
| 1X | 0.59 | 6.38 (5.57–7.30) | 19.1 (16.7–21.9) | 37.6 (34.3–41.3) | 63.6 (54.0–74.9) |
| 3X | 0.21 | 14.7 (12.0–18.1) | 28.6 (24.1–34.0) | 30.4 (27.6–33.5) | 39.5 (35.0–44.6) |
| 7X | 0.18 | 26.1 (21.1–32.3) | 46.5 (38.7–55.9) | 47.3 (42.9–52.2) | 63.2 (54.8–72.9) |
| Methyluric acids | |||||
| 1U | 0.58 | 20.0 (18.0–22.1) | 42.6 (37.1–48.8) | 77.7 (70.6–85.5) | 126 (107–147) |
| 3U | 0.25 | 0.355 (0.309–0.409) | 0.504 (0.422–0.603) | 0.594 (0.535–0.660) | 0.798 (0.700–0.911) |
| 7U | 0.19 | 8.05 (6.62–9.78) | 14.2 (11.5–17.4) | 14.5 (12.9–16.3) | 20.0 (17.1–23.5) |
| Uracils | |||||
| AAMU | 0.61 | 6.89 (5.61–8.45) | 32.9 (27.6–39.2) | 67.3 (59.1–76.6) | 118 (101–138) |
Values are geometric means (95% CIs). Individuals who reported using any prescription medications known to inhibit or induce cytochrome P450, N-acetyltransferase 2 or xanthine oxidase activity in the past 30 d were excluded. 137U, 1,3,7-trimethyluric acid; 13U, 1,3-dimethyluric acid; 17U, 1,7-dimethyluric acid; 1U, 1-methyluric acid; 1X, 1-methylxanthine; 37U, 3,7-dimethyluric acid; 37X, 3,7-dimethylxanthine; 3U, 3-methyluric acid; 3X, 3-methylxanthine; 7U, 7-methyluric acid; 7X, 7-methylxanthine; AAMU, 5-acetylamino-6-amino-3-methyluracil.
P values for pairwise t-tests among intake quartiles appear in Supplemental Table 8
Spearman correlation P <0.0001 in all cases.
Total 24-h caffeine intake from diet and dietary supplements in mg/d.
Figure 1.
Relationship of caffeine metabolism in humans to correlations observed between spot urine caffeine and caffeine metabolite concentrations and excretion rates with 24-h caffeine intake from foods and dietary supplements in U.S. persons aged ≥6 y, NHANES 2009–2010. “Group 1” consists of compounds for which Spearman correlations of 0.55 ≤ |r| ≤ 0.68 with intake were observed; “Group 2” consists of compounds for which Spearman correlations of 0.15 ≤ |r| ≤ 0.33 with intake were observed (Table 4, Supplemental Table 7). 137U, 1,3,7-trimethyluric acid; 13U, 1,3-dimethyluric acid; 17U, 1,7-dimethyluric acid; 1U, 1-methyluric acid; 1X, 1-methylxanthine; 37U, 3,7-dimethyluric acid; 3U, 3-methyluric acid; 3X, 3-methylxanthine; 7U, 7-methyluric acid; 7X, 7-methylxanthine; AAMU, 5-acetylamino-6-amino-3-methyluracil.
We found that geometric mean concentrations and excretion rates increased with increasing quartiles of caffeine intake for all compounds (trend P <0.0001). For the Group 1 compounds, strong trends were observed for both geometric mean concentrations and excretion rates with increasing quartiles of caffeine intake, and all differences among quartiles were significant (pairwise t-test P < 0.0001) (Supplemental Table 8). For these compounds we observed a large difference between the highest quartile of intake vs. the lowest for geometric mean concentrations (6–19×) and excretion rates (9–27×). By comparison, for the Group 2 compounds we observed a smaller difference between the highest vs. lowest quartiles of caffeine intake for geometric mean concentrations (2–3×) and excretion rates (3–4×). Differences in geometric mean concentrations between the second and third intake quartiles for these compounds were non-significant (pairwise t-test P = 0.10–0.95) and differences among mean excretion rates ranged from significant to non-significant (pairwise t-test P = 0.0013–0.08).
Correlation among analytes
We calculated Spearman correlations among spot urine concentrations and excretion rates of caffeine and caffeine metabolites (Supplemental Tables 9 and 10, respectively). A high degree of correlation was observed among analytes for both concentration and excretion rate. Approximately 40% of the possible correlations had Spearman |r| ≥0.8, 25% of which were ≥0.9. In the case of excretion rate, nearly half of the correlations had |r| ≥0.8, 30% of which were ≥0.9. All inter-analyte correlations ≥0.8 occurred within either Group 1 or Group 2; we did not observe a single correlation of ≥0.8 between a Group 1 and Group 2 compound with either urine concentration or excretion rate.
Discussion
This study presents urine concentrations and excretion rates for caffeine and caffeine metabolites in the U.S. population aged ≥6 y (NHANES 2009–2010) in the context of age, sex, race- ethnicity, and caffeine intake. Based on their correlations with caffeine intake, we identified 2 apparent groups of biomarkers: Group 1 with moderate and Group 2 with weak correlations (Figure 1). The Group 1 compounds had among the highest detection rates, concentrations, and excretion rates of all compounds examined in our study, and exhibited patterns that were generally consistent with caffeine intake when stratified by age, sex and race-ethnicity. These favorable characteristics support their consideration as potential biomarkers of caffeine intake.
Although spot urine samples do not represent quantitative recovery and inter-individual differences in metabolism exist, the high detection rates, concentrations, and excretion rates we observed for the Group 1 paraxanthine metabolites (1U, 1X and AAMU) seem consistent with what is known regarding caffeine metabolism. Paraxanthine is the main pathway for caffeine metabolism in humans (72%) and is rarely encountered as a dietary compound while theobromine (20%) and theophylline (8%) are less prominent products of caffeine metabolism (26). Paraxanthine metabolites may therefore deserve special consideration as potential biomarkers of caffeine intake.
Most caffeine is expected to be recovered in the urine as 1U (26.5%), followed by 1X (19%), AFMU (16%), 7X (7.5%), paraxanthine (6.5%), 17U (6%) and 3X (3%) (26). The production and clearance of AFMU (measured as AAMU in our analyses) behaves in tandem with 1U and 1X due to the believed existence of a common precursor (27). The relatively high concentrations and excretion rates we observed for 3X and 7X, however, suggest that contributions from dietary theophylline and theobromine are also present. Both theophylline and theobromine are recovered in the urine as 3X (14%, 21.5%, respectively) and theobromine also contributes to the appearance of 7X (36%) (26). Our finding of a near ubiquitous presence of caffeine metabolites in the urine, particularly those produced via paraxanthine metabolism (1U, AAMU, 1X, 17U, 17X) appears to be consistent with a high prevalence of caffeine consumption in the U.S. population, with 87% of the U.S. population (28) and 73% of children (2) consuming caffeine on a given day. Interestingly, our detection of AAMU and 17U at the 2.5th percentile implies that caffeine intake may be under-reported, since AAMU and 17U are produced exclusively via paraxanthine and dietary exposure to paraxanthine is virtually nonexistent (26).
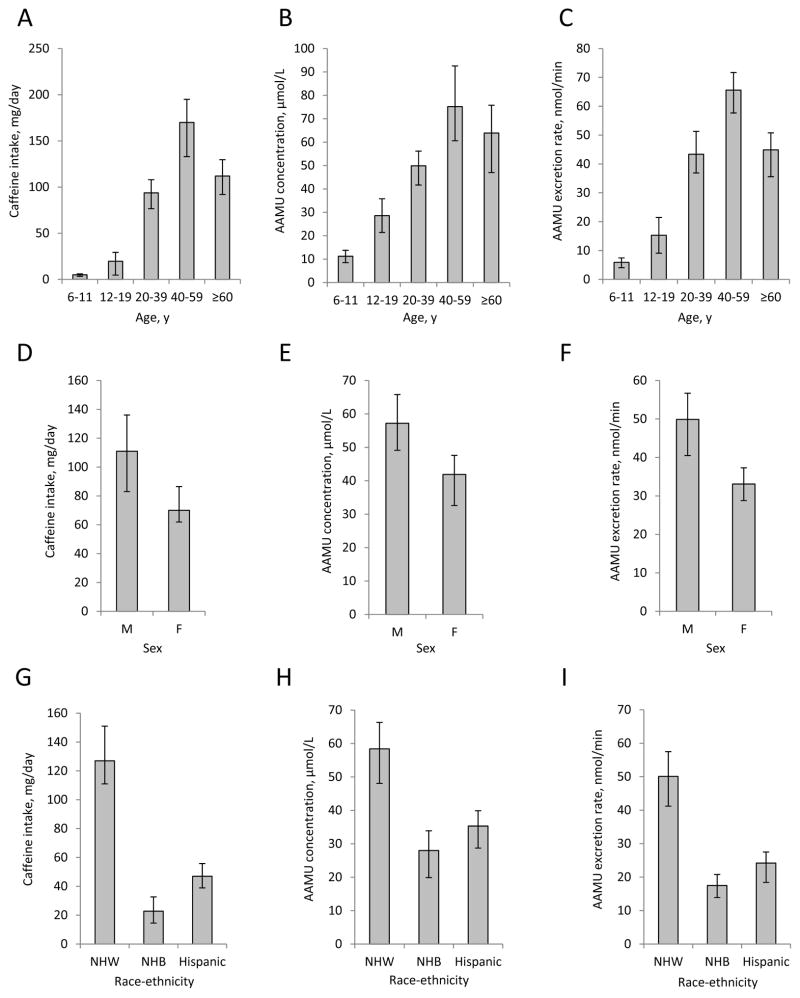
Caffeine intake in the U.S. tends to follow an inverted U-shape age pattern with intake highest in persons aged 40–59 (1), higher in males vs. females (1,25), and higher in NHW vs. NHB and Hispanics/Mexican-Americans (1). We observed many cases where compound concentrations and excretion rates showed patterns with age, sex and race-ethnicity analogous to those seen with intake (illustrated in Figure 2, using AAMU as an example). We found that these similarities occurred more frequently in compounds most correlated with dietary intake (Group 1) and with excretion rate data. The association of age, sex, and race-ethnicity with caffeine metabolism is not straightforward; however, there is some evidence suggesting that these demographic factors may have limited influence on biomarker behavior. Age has been shown in males to have no significant effect on peak plasma concentration, time to reach peak concentration, or half-life of caffeine metabolites (29,30), although urine excretion of 17U, 1U and 7U was statistically higher in older subjects (31). No sex-based differences in caffeine metabolism have been observed based on urine metabolites and metabolite ratios (32), although CYP1A2 activity, which affects multiple stages of caffeine metabolism, has been shown to be higher in men vs. women (33).
Figure 2.
Comparison of 24-h caffeine intake from diet and supplements, spot urine 5-acetylamino-6-amino-3-methyluracil (AAMU) concentrations, and urine AAMU excretion rates s in U.S. persons aged ≥6 y, NHANES 2009–2010, stratified by demographic variables. Values are medians ± 95% CI. (A) caffeine intake, (B) AAMU concentration, and (C) AAMU excretion rate stratified by age. (D) caffeine intake, (E) AAMU concentration, and (F) AAMU excretion rate stratified by sex. (G) caffeine intake, (H) AAMU concentration, and (I) AAMU excretion rate stratified by race-ethnicity. M, male; F, female; NHW, non-Hispanic white; NHB, non-Hispanic black. Sample sizes (n) for spot urine concentration and excretion rate data appear in Supplemental Table 2. Intake data sample sizes were as follows: Age: 6–11 y, n = 358; 12–19 y, n = 381; 20–39 y, n = 575; 40–59 y, n = 589; ≥60 y, n = 505; Sex: male, n = 1178; female, n = 1230; Race-ethnicity: non-Hispanic white, n = 1028; non-Hispanic black, n = 447; all Hispanic, n = 807.
Our findings appear to be consistent with other urine biomarker studies (10,11). Crews et al. (10) performed a caffeine dosing study with 8 volunteers of known CYP1A2 phenotypes in which 24-h urines were collected and analyzed for caffeine, paraxanthine, 1X, 17U, and AFMU. Correlations with caffeine dose (r, not specified but presumed to be Pearson correlation) were 0.76–0.93 for all subjects, and 0.77–0.95 by CYP1A2 phenotype. In our study, excretion rates for paraxanthine, 17U and 1X showed similar promise as intake biomarkers based on their high detection frequency (>95%), correlation with intake (|r| = 0.62–0.66) and concordant patterns with demographic variables. Although not directly comparable, the higher correlations observed by Crews et al. are likely a result of their controlled experimental design in which 24-hour urines were collected, and diet, caffeine dosing, and urine collection timing were regulated, whereas the NHANES is limited to self-reported dietary intake data on individuals consuming their normal diets, and a spot urine sample collected at varying times relative to caffeine consumption. AFMU was dismissed as a potential biomarker by Crews et al. due to compound stability and analysis issues, and 1U was not evaluated as a biomarker candidate; however, in our study the excretion rates for these two compounds also showed demonstrably high detection frequency (> 97%), correlation with intake (|r| 0.65–0.67), and consistent demographic trends. In another study, Grosso et al. (11) et al. studied the correlation of spot urine caffeine, paraxanthine, theophylline, and theobromine concentrations with both average and 24-h caffeine intake in pregnant women (n = 98–263) at various times throughout pregnancy. They observed correlations in pregnant women similar to what we found in a representative sample of the U.S. population. Spearman correlations for urine caffeine, paraxanthine and theophylline were 0.50–0.66 with average intake and 0.46–0.71 with 24-h intake depending on the analyte and timeframe (P <0.0001); all correlations with urine theobromine were <0.2 and most often non-significant (P >0.05).
We believe that our study has 2 key strengths: it is the first to describe urine caffeine and caffeine metabolite concentrations and excretion rates in a diverse, representative population subset in relation to age, sex, and race-ethnicity; and it is the first to examine the relationship of urine caffeine metabolites with caffeine intake to identify potential intake biomarkers using spot urine samples from a representative population subset. We do acknowledge, however, that there are limitations to our study and that while our findings are promising further work is needed. Cross-sectional data has many sources of variability that are absent from more controlled study designs such as the dosing study performed by Crews et al. (10). We did not account for physiologic (e.g., fasting time, kidney and liver function) and lifestyle (e.g., exercise, smoking status, alcohol use) variables that may affect caffeine pharmacokinetics. Future study of these variables to gain an understanding of their association with potential caffeine biomarkers would be prudent. The caffeine intake data we used from the NHANES also has its limitations. Although dietary intake data was collected during an interview by highly trained personnel using a well-validated dietary recall instrument (USDA Automated Multi-Pass Method) (34), the presence of inaccuracies—underestimations in particular—is possible due to challenges in identifying and accurately establishing the caffeine content of dietary sources or errors made by survey respondents during the interview. We included caffeine intake from dietary supplements in our analyses; however, caffeine intake from sources such as prescription and non-prescription medications was not available in the NHANES. We were able to identify groups of compounds with similar characteristics in relation to caffeine intake and rationalize their existence based on an understanding of caffeine metabolism; however, the existence of these apparent groups was based entirely on perception, and a more objective study using statistical approaches such as cluster analyses is warranted. Nonetheless, even with these limitations considered, we believe our study provides a unique population based perspective on urine caffeine and caffeine metabolite concentrations and excretion rates and serves as a valuable step towards identifying and validating compounds that could serve as biomarkers of caffeine intake.
Supplementary Material
Acknowledgments
The authors acknowledge contributions from the following laboratory members: Patrick W. Simon and Donna J. LaVoie (CDC National Center for Environmental Health). MER designed the overall research project in consultation with all coauthors; CIP directly oversaw all laboratory analyses; MRS performed all statistical analyses; MER wrote the initial manuscript draft, incorporating feedback from all authors, and has primary responsibility for content. All authors have read and approved the final manuscript.
Footnotes
No specific sources of financial support. The findings and conclusions in this report are those of the authors and do not necessarily represent the official views or positions of the Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry or the Department of Health and Human Services.
Author disclosures: ME Rybak, MR Sternberg, C Pao, N Ahluwalia, CM Pfeiffer: no conflicts of interest
Supplemental Tables 1–10 are available as Online Supporting Material with the online posting of this paper at http://jn.nutrition.org.
Abbreviations used: 137U, 1,3,7-trimethyluric acid; 137X, caffeine (1,3,7-trimethylxanthine); 13U, 1,3-dimethyluric acid; 13X, 1,3-dimethylxanthine; 17U, 1,7-dimethyluric acid; 17X, 1,7-dimethylxanthine; 1U, 1-methyluric acid; 1X, 1-methylxanthine; 37U, 3,7-dimethyluric acid; 37X, 3,7-dimethylxanthine; 3U, 3-methyluric acid; 3X, 3-methylxanthine; 7U, 7-methyluric acid; 7X, 7-methylxanthine; AAMU, 5-acetylamino-6-amino-3-methyluracil; AFMU, 5-acetylamino-6-formylamino-3-methyluracil; LC, liquid chromatography; LOD, limit of detection; MS/MS, tandem mass spectrometry; NHB, non-Hispanic black; NHW, non-Hispanic white.
References
- 1.US Department of Agriculture, Agricultural Research Service. What We Eat In America, Data Tables, 2009–2010 [Internet] Washington: US Department of Agriculture; 2012. [cited 2014 May 5]. Available from: http://www.ars.usda.gov/SP2UserFiles/Place/12355000/pdf/0910/tables_1-40_2009-2010.pdf. [Google Scholar]
- 2.Branum AM, Rossen LM, Schoendorf KC. Trends in caffeine intake among US children and adolescents. Pediatrics. 2014;133:386–93. doi: 10.1542/peds.2013-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang W, Wu Y, Jiang X. Coffee and caffeine intake and breast cancer risk: An updated dose–response meta-analysis of 37 published studies. Gynecol Oncol. 2013;129:620–9. doi: 10.1016/j.ygyno.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Caldeira D, Martins C, Alves LB, Pereira H, Ferreira JJ, Costa J. Caffeine does not increase the risk of atrial fibrillation: a systematic review and meta-analysis of observational studies. Heart. 2013;99:1383–9. doi: 10.1136/heartjnl-2013-303950. [DOI] [PubMed] [Google Scholar]
- 5.Palacios N, Gao X, McCullough ML, Schwarzchild MA, Shah R, Gapstur S, Ascherio A. Caffeine and risk of Parkinson’s disease in a large cohort of men and women. Movement Disord. 2012;27:1276–82. doi: 10.1002/mds.25076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerjee P, Ali Z, Levine B, Fowler DR. Fatal caffeine intoxication: a series of eight cases from 1999 to 2009. J Forensic Sci. 2014;59:865–8. doi: 10.1111/1556-4029.12387. [DOI] [PubMed] [Google Scholar]
- 7.US Food and Drug Administration, Center for Food Safety and Applied Nutrition. CFSAN Adverse Event Reporting System, Voluntary and Mandatory Reports on 5-Hour Energy, Monster Energy, and Rockstar Energy Drink, January 1, 2004, through October 23, 2012 [Internet] Silver Spring: US Food and Drug Administration; 2013. [cited 2014 May 5]. Available from: http://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofFoods/CFSAN/CFSANFOIAElectronicReadingRoom/UCM328270.pdf. [Google Scholar]
- 8.Bracken MB, Triche E, Grosso L, Hellenbrand K, Belanger K, Leaderer BP. Heterogeneity in assessing self-reports of caffeine exposure: implications for studies of health effects. Epidemiol. 2002;13:165–71. doi: 10.1097/00001648-200203000-00011. doi:0.1097/00001648-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Falomir Z, Arregui M, Madueño F, Corella D, Coltell Ó. Automation of food questionnaires in medical studies: a state-of-the-art review and future prospects. Comput Biol Med. 2012;42:964–74. doi: 10.1016/j.compbiomed.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Crews HM, Olivier L, Wilson LA. Urinary biomarkers for assessing dietary exposure to caffeine. Food Addit Contam. 2001;18:1075–87. doi: 10.1080/02652030110056630. [DOI] [PubMed] [Google Scholar]
- 11.Grosso LM, Triche E, Benowitz NL, Bracken MB. Prenatal caffeine assessment: fetal and maternal biomarkers or self-reported intake? Ann Epidemiol. 2008;18:172–8. doi: 10.1016/j.annepidem.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klebanoff MA, Levine RJ, Dersimonian R, Clemens JD, Wilkins DG. Serum caffeine and paraxanthine as markers for reported caffeine intake in pregnancy. Ann Epidemiol. 1998;8:107–11. doi: 10.1016/s1047-2797(97)00125-7. [DOI] [PubMed] [Google Scholar]
- 13.Nordmark A, Lundgren S, Cnattingius S, Rane A. Dietary caffeine as a probe agent for assessment of cytochrome P4501A2 activity in random urine samples. Br J Clin Pharmacol. 1999;7:397–402. doi: 10.1046/j.1365-2125.1999.00918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jetter A, Kinzig-Schippers M, Illauer M, Hermann R, Erb K, Borlak J, Wolf H, Smight G, Cascorbi I, Sorgel F, Fuhr U. Phenotyping of N-acetyltransferase type 2 by caffeine from uncontrolled dietary exposure. Eur J Clin Pharmacol. 2004;60:17–21. doi: 10.1007/s00228-003-0718-8. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Examination Survey Homepage [Internet] Atlanta: US Centers for Disease Control and Prevention; 2014. [cited 2014 May 5]. Available from: http://www.cdc.gov/nchs/nhanes.htm. [Google Scholar]
- 16.Centers for Disease Control and Prevention, National Center for Health Statistics. NHANES–Response Rates and CPS Totals [Internet] Atlanta: US Centers for Disease Control and Prevention; 2014. [cited 2014 May 5]. Available from: http://www.cdc.gov/nchs/nhanes/response_rates_CPS.htm. [Google Scholar]
- 17.Centers for Disease Control and Prevention, National Center for Health Statistics. NHANES 2009–2010: Urinary Caffeine and Caffeine Metabolites Data Documentation, Codebook, and Frequencies [Internet] Atlanta: US Centers for Disease Control and Prevention; 2014. [cited 2014 May 5]. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2009-2010/CAFE_F.htm. [Google Scholar]
- 18.Centers for Disease Control and Prevention, National Center for Health Statistics. NHANES 2009–2010 Laboratory Procedures Manual [Internet] Atlanta: US Centers for Disease Control and prevention; 2009. [cited 2014 November 13]. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/lab.pdf. [Google Scholar]
- 19.Rybak ME, Pao C-I, Pfeiffer CM. Determination of urine caffeine and its metabolites by use of high-performance liquid chromatography-tandem mass spectrometry: estimating dietary caffeine exposure and metabolic phenotyping in population studies. Anal Bioanal Chem. 2014;406:771–84. doi: 10.1007/s00216-013-7506-9. [DOI] [PubMed] [Google Scholar]
- 20.Caudill SP, Schleicher RL, Pirkle JL. Multi-rule quality control for the age-related eye disease study. Stat Med. 2008;27:4094–106. doi: 10.1002/sim.3222. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention, National Center for Health Statistics. Laboratory Procedure Manual, Urine Caffeine and Caffeine Metabolites [Internet] Atlanta: US Centers for Disease Control and Prevention; 2014. [cited 2014 May 5]. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/CAFE_F_met.pdf. [Google Scholar]
- 22.Centers for Disease Control and Prevention, National Center for Health Statistics. NHANES 2009–2010: Urine Flow Rate Data Documentation, Codebook, and Frequencies [Internet] Atlanta: US Centers for Disease Control and Prevention; 2014. [cited 2014 May 5]. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2009-2010/UCFLOW_F.htm. [Google Scholar]
- 23.Centers for Disease Control and Prevention, National Center for Health Statistics. NHANES 2009–2010: Dietary Interview: Total Nutrient Intakes–First Day Version current 5 May 2014 [Internet] Atlanta: US Centers for Disease Control and Prevention; 2014. [cited 2014 May 5]. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2009-2010/DR1TOT_F.htm. [Google Scholar]
- 24.Centers for Disease Control and Prevention, National Center for Health Statistics. NHANES 2009–2010: Dietary Supplement Use 24-Hour: Individual Dietary Supplements–First Day [Internet] Atlanta: US Centers for Disease Control and Prevention; 2014. [cited 2014 May 5]. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2009-2010/DS1IDS_F.htm. [Google Scholar]
- 25.Little RJA. A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc. 1988;83:1198–1202. [Google Scholar]
- 26.Arnaud MJ. Pharmacokinetics and metabolism of natural methylxanthines in animal and man. Handb Exp Pharmacol. 2011;200:33–91. doi: 10.1007/978-3-642-13443-2_3. [DOI] [PubMed] [Google Scholar]
- 27.Yesair DW, Branfman AR, Callahan MM. Human disposition and some biochemical aspects of methylxanthines. Prog Clin Biol Res. 1984;158:215–33. [PubMed] [Google Scholar]
- 28.Frary CD, Johnson RK, Wang MQ. Food sources and intakes of caffeine in the diets of persons in the United States. J Am Diet Assoc. 2004;105:110–3. doi: 10.1016/j.jada.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 29.Blanchard J, Sawers SJA. Comparative pharmacokinetics of caffeine in young and elderly men. J Pharmacokinet Biopharm. 1983;11:109–26. doi: 10.1007/BF01061844. [DOI] [PubMed] [Google Scholar]
- 30.Blanchard J, Sawers SJ. Relationship between urine flow rate and renal clearance of caffeine in man. J Clin Pharmacol. 1983;23:134–8. doi: 10.1002/j.1552-4604.1983.tb02716.x. [DOI] [PubMed] [Google Scholar]
- 31.Blanchard J, Sawers SJA, Jonkman JHG, Tang-Liu D-S. Comparison of the urinary metabolite profile of caffeine in young and elderly males. Br J Clin Pharmacol. 1985;19:225–32. doi: 10.1111/j.1365-2125.1985.tb02635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grant DM, Tang BK, Kalow W. Variability in caffeine metabolism. Clin Pharmacol Ther. 1983;33:591–602. doi: 10.1038/clpt.1983.80. [DOI] [PubMed] [Google Scholar]
- 33.Landi MT, Sinha R, Lang NP, Kadlubar FF. Human cytochrome P4501A2. IARC Sci Publ. 1999;148:173–95. [PubMed] [Google Scholar]
- 34.Blanton CA, Moshfegh AJ, Baer DJ, Kretsch MJ. The USDA Automated Multiple-Pass Method accurately estimates group total energy and nutrient intake. J Nutr. 2006;136:2594–9. doi: 10.1093/jn/136.10.2594. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.