Summary
Background
Tendinopathies are conditions characterized by activity-induced pain, local tenderness and swelling for which a gold standard treatment is not established yet. Hyaluronic Acid (HA) is a key-molecule in several cellular activities and it is normally present in the extra-cellular matrix of tendons and ligaments. Amongst its properties, HA injections may reduce pain and determine disease-modifying effects. This study is an investigator-initiated open-label trial conducted to investigate the efficacy and safety of HA (500–730 kDa) peritendinous injections on pain reduction in patients affected by lateral elbow, Achilles or patellar tendinopathy.
Methods
A total of 71 tendons (34 with Achilles tendinopathy, 26 with lateral elbow tendinopathy, 11 with patellar tendinopathy) of 62 patients with painful tendinopathy were treated with a cycle of ultrasound-guided peritendinous injections one injection per week for three consecutive weeks. Efficacy assessments included changes in pain intensity measured by Visual Analogue Scale (VAS) at follow-up evaluations were performed 7 (V2), 14 (V3) and 56 days aften first treatment. An Ultrasound (US) assessment was also performed to evaluate changes in tendon thickness and neovascularization. Adverse events were recorded for safety analysis throughout the study. All results were analyzed with descriptive statistics appropriate to the nature of the variables.
Results
Significant reduction in VAS (p<0.001) from baseline was observed in Achilles (−6.16 ± 0.45 cm), patellar (−6.16 ± 0.72 cm) and lateral elbow (−5.33 ± 0.43 cm) tendinopathies. The sagittal thickness decreased significantly from baseline at each endpoint (V3 day 14 and V4 day 56) in each type of tendinopathy analyzed (p<0.05). Neovascularization decreased for each tendons at V3 and V4, except for patellar tendon at V3 V1 (p=0.125). Nevertheless, reduction at V4 compared to baseline remained significant (p=0.016).
Conclusions
US-guided HA (500–730 kDa) peritendinous injections determine significant pain relief and reduction in tendon thickness and neovascularization in US evaluations. The effect of HA did not show differences regarding the site of affected tendon. The treatment proved to be safe and very well tolerated.
Level of evidence
4.
Keywords: achilles, colour power doppler, hyaluronic acid, injection, patellar, tendinopathies, tennis elbow
Introduction
Tendinopathies are multifactorial clinical conditions, which affect millions of people, producing disability that may last for several months1. Even mechanical overloading is considered as the main risk factor, other intrinsic and extrinsic factors may contribute to the pathogenesis2, 3. In particular, poor vascularity, underload, age, gender, as well as genetic, endocrine and metabolic factors may play a central role4–6.
Over the last decade, various models have been proposed to explain the intrinsic pathogenic mechanism of tendinopathies, which determine the failed healing response. Chronic stages of this condition are characterized by increased tenocyte apoptosis, disarrangement of collagen fibres with decreased of type I collagen and altered type I collagen/type III collagen ratio and neoangiogenesis3.
Regardless of the cause or the type of tendon, clinical findings are quite uniform: patients refer pain at the affected tendon site, which sometimes arises during intense muscular effort or gradually if the effort is repeated and continuous; with time and continued activity, however, the pain may get worse and limit sport performance7.
In addition to pain, physical examination may reveal local tenderness, swelling and reduced articular range of motion8.
US is a valid tool to confirm the diagnosis, showing tendon thickening, focal hypo-echogenic areas with altered grey scale pattern and neovascularization detected at Power Doppler with sensitivity and specifity up to 90%9–12.
Although several options are available to manage tendinopathies, there is currently lack of consensus on the gold standard treatment. Eccentric exercise may be beneficial to ameliorate tendon structure13, while the role of oral and local NSAIDs is controversial in the short-term and could lead to several side effects in the long-term14.
Corticosteroid injections are widely used in clinical practice. Recent findings demonstrate that corticoid injection are worse than most conservative interventions in the long-term15 they could weak tendon structure enhancing the risk of tendon rupture16. Several studies have shown that peritendinous application of hyaluronic acid (HA) is an effective therapeutic option for the treatment of chronic tendinopathy17, 18.
Hyaluronic acid (HA) is one of the main components of synovial fluid, which is produced in the normal tendon sheath and it is a main component of tendon extracellular matrix19. In vitro models suggest that HA may increase tenocyte viability and collagen I production and deposition, with positive collagen I/collagen III ratio in a dose-dependent manner20. Its viscoelastic properties permit to reduce the surface friction of tendons increasing the gliding ability. It has been demonstrated that the application of HA, together with the formation of a network of the cells on the tendon surface, results in the “gliding effect”21, 22.
The aim of this prospective, open label, single-centre study was to evaluate the efficacy and safety of three US-guided peritendineous injections of 2 mL of medium weight HA (500–730 kDa) at an interval of one week on pain relief in patients affected by Achilles and patellar or lateral elbow tendinopathies.
Methods
Study design
This was an investigator-initiated, prospective, open-label, single-centre clinical study that evaluated the efficacy of HA US-guided peritendineous injections on pain reduction at rest and on movement in patients with painful tendinopathies.
The study was approved by the local Ethical Committee and was conducted ethically according to international standards23. Written informed consent was obtained from each patient before the inclusion.
Patients
All patients were enrolled over a 5-year period (2009 – 2013). Male or female patients aged ≥18 years in the symptomatic phase of chronic Achilles, patellar or lateral elbow tendinopathies (characterized by the presence of at last one among: tendon swelling, pain on palpation, pressure and/or prehension, painful limited range of motion) for ≥6 weeks and a pain score of at least 50 mm on VAS, confirmed by US evaluation, non-responders to conventional therapies for more than one month, were eligible.
Patients with recent tendon surgery, pregnant or breast-feeding and patients with diabetes (glycated haemoglobin > 6.7) were excluded from the study. Patients were also excluded if had been treated with systemic or topical steroids less than 1 month prior to inclusion or with NSAIDs therapies less than 10 days before inclusion. During the study, the use of any pain medication or physical therapy were not allowed. Demographic characteristics of patients and the presence of concomitant hypothyroidism, diabetes and/or hypercholesterolemia were collected.
Study treatment
Sixty-two evaluable subjects were included (mean age 46.26) for a total of 71 treated tendons, classified as lateral elbow, Achilles or patellar tendinopathies. Patients received 3 ultrasound-guided injection of 2mL HA (500–730 kDa, Hyalgan®, Fidia Farmaceutici SpA, Abano Terme, Italy) once a week for 3 consecutive weeks.
Each injection was administered with a 25 Gauge needle approximately oriented 45° between the sagittal and coronal planes. Ultrasound imaging of treated tendons allowed examination of tendon morphology, detecting the areas of tendon degeneration and tendon thickness. Neovascularization was assessed using Power Doppler imaging. The procedure was performed with a high-frequency (5–18 MHz) linear probe (MyLab25 Gold, Esaote SpA, Italy) and to be sure of the reproducibility of the assessment, the osteotendinous junction was chosen as a reference in probe positioning.
Description of ultrasound guided injection scheme
To treat patellar tendon, 2 mL of HA were injected distally the lower pole of the patella with the needle oriented in cranio-caudal direction at an angle of 45° to the Hoffa’s body.
For the Achilles tendon, 2 mL of HA were injected about 1 cm from the calcaneal insertion site and proceeding proximally with the needle at an angle of 45° in the direction of Kager’s triangle.
For the epicondyle, 2 mL of HA were injected distal to the bone insertion site in the forearm direction, between the extensor carpi radialis brevis and the finger extensor digitorum communis tendon.
Efficacy and Safety assessments
The primary endpoint was pain intensity changes from baseline (V1), assessed by VAS (0–10 cm) in which 0 represents ‘no pain’ and 10 represents ‘extreme pain’ at each study timepoint (V2 day 7, V3 day 14 and V4 day 56). Secondary variables were changes in tendon thickness measured by US and in Power Doppler signal of the target tendon (severity was classified as: 0= “No vascularization”, 1= “Mild”, 2= “Moderate” and 3= “Severe”), according to Hoksrund et al.24 at V3 and V4. Moreover, a clinical evaluation of tendinopathy based on redness, warmth, swelling, tenderness, crepitus during movement and peritendinous effusion was performed at any study visit. Adverse events related and not related to the study drug were assessed for safety. Table I summarises demographic characteristics of sample.
Table I.
Demographic characteristics of sample.
| Age | N | 62 |
|---|---|---|
| Mean (SD) | 46.26 (12.53) | |
| Median (Min, Max) | 46.00 (16.71) | |
| Gender | Male | 47 (75.81%) |
| Female | 15 (24.19%) |
Statistical Analysis
Statistical analysis was performed using SAS® software release for Windows® (SAS Institute, Inc, Cary, North Carolina, USA). There was no control group and a statistical calculation of sample size was not performed. The population size was enough to guarantee a 5% significance level. All statistical tests were 2-sided.
To assess changes in pain and tendon characteristics at each endpoint, both the Student’s t test and the Wilcoxon Signed Rank Test were used, considering significant p-values ≤0.05. Finally, for each endpoint, an ANCOVA model was constructed to estimate the mean of the differences between the final and the initial assessments, adjusting for the following independent variables present at baseline: sex, age, type of tendon treated, number of infiltrations performed and number of risk factors.
Safety population included all patients receiving at least 1 injection while Efficacy included all patients receiving at least 1 injection with at least 1 post-baseline value. Safety and Efficacy Populations were comparable.
Results
Baseline characteristics
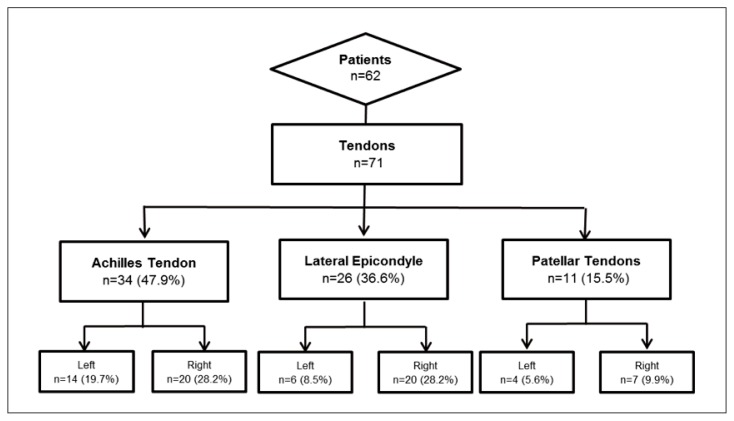
Sixty-two patients were included in the database, and 71 tendons were treated: 47.9% Achilles tendons (14 left and 20 right), 36.6% lateral epicondyles (6 left and 20 right) and 15.5% patellar tendons (4 left and 7 right) (Fig. 1). Of the 62 patients included in the study, 47 were males (75.8%) and 15 females (24.2%). The mean age was 46.3 years (±12.5) (Tab. I).
Figure 1.
Subject disposition.
The main risk factor present in the study population was hypercholesterolemia (16.1%) followed by hypothyroidism (9.7%). Diabetes was absent in 98.4% of the patients (Fig. 2).
Figure 2.
Distribution of diabetes, hypercholesterolemia and hypothyroidism on study population.
Clinical outcomes
Pain decreased significantly from baseline at each endpoint (V2 day 17, V3 day 14 and V4 day 56) in each type of tendinopathy analysed (p<0.001) (Fig. 3). Specifically, in patients affected by Achilles tendinopathy, the mean VAS value was 7.92 cm (± 1.44) and it progressively decreased at V2 (7.59 cm ±0.97; p<0.001), V3 (3.91 cm±2.08; p<0.001) and V4 (2.02 cm±2.10; p<0.001). Similarly, in patients affected by patellar tendinopathy the mean VAS value was (7.59 cm ± 0.97) and it progressively decreased at V2 (5.32 cm ±1.60; p<0.001), V3 (3.95 cm±1.64; p<0.001) and V4 (2.50 cm±2.16; p<0.001). In lateral elbow tendinopathy, the mean VAS value was 8.19 cm (± 0.79) and it progressively decreased at V2 (5.25 cm±1.80; p<0.001), V3 (3.39 cm±1.91; p<0.001) and V4 (1.74cm ±2.17; p<0.001)
Figure 3.
Mean pain intensity on VAS at each evaluation, by site of treated tendon. Legend: V1 Baseline; V2 Day 7; V3 Day 14; V4 Day 56.
Results from the ANCOVA model, after adjustment by sex, age, type of treated tendon, number of injections and number of risk factors, showed that the reduction in pain intensity was similar for each tendon and that there were no significant differences between types of treated tendon (p>0.05) (Tab. II).
Table II.
Result from ANCOVA model in which the reduction in pain intensity was similar for each site and that there were no significant differences between types of treated tendon (p>0.05).
| Type of treated tendon | Mean (SD) | Lower 95% |
CL Upper 95% |
CL P-value |
|---|---|---|---|---|
| Achilles Tendon | −6.16 (0.45) | −7.05 | −5.26 | 0.326 |
| Lateral Epicondyle | −5.33 (0.43) | −6.20 | −4.46 | 0.994 |
| Patellar Tendon | −6.16 (0.72) | −7.59 | −4.73 | _ |
Ultrasonographic examination
The sagittal thickness decreased significantly from baseline at each endpoint (V3 and V4) in each type of tendinopathy analysed (p<0.05). At baseline, mean sagittal thickness of Achilles tendon was 9.41mm (±2.38) at baseline, while it was 8.44 mm (±2.59) at V3 and 8.31 mm (±2.28) at V4. Mean sagittal thickness of patellar tendon was 7.09 mm at baseline (±1.65), while it was 6.55 mm (±1.36) at V3 and 6.16 mm (±1.58) at V4. For lateral elbow tendon, mean sagittal thickness was 6.20 mm (±0.78) at baseline, while it was 5.80 mm (±0.94) at V3 and 5.32 mm (±0.87) at V4 (Fig. 4).
Figure 4.
Mean sagittal thickness on US at each evaluation by site of treated tendon. Legend: V1 Baseline; V3 Day 14; V4 Day 56.
Regarding vascularity measured by Power Doppler, it was observed a significant decrease for each tendon at V3 and V4, except for patellar tendon from V3 to V1 (p=0.125). Nevertheless, reduction at V4 compared to baseline remained significant (p=0.016). (Fig. 5)
Figure 5.
Mean vascularization on US at each evaluation by site of treated tendon. Legend: V1 Baseline; V3 Day 14; V4 Day 56.
Safety
No serious adverse events were recorded. Two patients reported transient adverse events during the study, one mild endometriosis and one mild episode of lumbosciatic syndrome, neither of them related to the study medication. The peritendinous injections of HA were well tolerated by all patients.
Discussion
Although tendinopathies are a common complaint, accounting for 30% of all musculoskeletal consultations with a general practitioner, a gold standard treatment has not been identified yet25.
Hyaluronic acid has already been shown to be safe and effective in treating osteoarthritis26, 27, while few studies have examined its effectiveness in tendon disorders.
The main objective of this study was to evaluate the efficacy and safety of HA injections of LMW-HA on pain reduction at rest and on movement in patients with painful tennis elbow, Achilles and patellar tendinopathies.
Kumai et al.28 found that a single injection of a high molecular weight (HMW) HA (2700 kDa), determined a prompt decrease in pain in 62 patients affected by Achilles, patellar and tennis elbow insertional tendinopathies in the short-term (1 week after injection). Similarly, these study results indicate that symptom relief is achieved immediately after the first injection. Furthermore, in our study, we observed that pain continue to decrease until the last follow-up (56 days). This may indicate that a cycle of 3 injections of 500–730 kDa HA may determine long-term decrease in pain intensity in patients affected by chronic tendinopathies non-responders to conventional therapies
In accordance with our findings, Frizziero et al.29, found that 3 injections of 500–730 kDa compared to low-energy extracorpored shock-ware treatment (ESWT) provide prompt clinical improvement that last until 3 months of follow-up in rotator cuff tendinopathy.
Similar results were obtained also in mid-portion Achilles tendinopathy, in which 2 injections of a combination HA and mannitol were found to be superior to ESWT30. The Authors found 51.7% of participants in the HA group and 42.3% in the ESWT group were free of neovascularization within the tendon at Power Doppler US evaluation30. Our findings confirm the significant reduction in neovascularization detected at Power Doppler at V3 and V4 compared to baseline, except for V3 in patients treated for patellar tendinopathy, while reduction in V4 was significant.
Furthermore, it was observed a significant reduction in tendon thickness at V3 and V4 compared to the baseline that were similar and not dependent on the tendon.
The amelioration in US parameters could be partially explained by reduction in friction and peritendinous adhesions due to the lubrificant action of HA. These results may also indicate a possible “structure-modifying effect” here of HA (500–730kDa) on tendon, which need to be confirmed by further studies. The present study has the following 4 main limitations: the lack of a control group and randomization, the short-term follow-up and the lack of functional outcomes specific for each tendon considered in this study. However, HA resulted efficient in short-term pain reduction and safe, without relevant adverse effects related to the molecule in the treatment of tendon pathology.
Conclusions
US-guided peritendinous injections of hyaluronic acid (500–730 kDa) in Achilles and patellar tendinopathy and lateral epicondylitis resulted in significant pain relief, decrease in tendon thickness and neovascularization without differences regarding the affected tendon object of this study.
These clinical findings and the absence of adverse events, may confirm that hyaluronic acid represent a safe and effective option in the managements of painful tendinopathies.
Footnotes
Conflict of interest
This study was an investigator initiated study carried out in accordance with internationally recognized standards, such as the Declaration of Helsinki and the ICH’s Guideline for Good Clinical Practice. The Authors declare that they have no conflict of commercial interest. Nicola Giordan is an employee of Fidia Farmaceutici S.p.A. All Authors state, however, that Fidia Farmaceutici S.p.A. did not solicit this research project or protocols with investigators or institutions. Fidia Farmaceutici S.p.A. was not responsible for the initiation of the study and did not participate in the scientific design of the trial, in the creation of the protocol, management of the study, data analysis, reporting and in the decision to submit the manuscript for publication.
References
- 1.Maffulli N, Longo UG, Loppini M. Current treatment options for tendinopathy. Expert Opin Pharmaco Ther. 2010;11:2177–2186. doi: 10.1517/14656566.2010.495715. [DOI] [PubMed] [Google Scholar]
- 2.Cook JL, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load induced tendinopathy. Br J Sports Med. 2009;43:409–416. doi: 10.1136/bjsm.2008.051193. [DOI] [PubMed] [Google Scholar]
- 3.Del Buono A, Battery L, Denaro V, Maccauro G, Maffulli N. Tendinopathy and inflammation: some truths. Int J Immunopathol Pharmacol. 2011 Jan-Mar;24(1 Suppl 2):45–50. doi: 10.1177/03946320110241S209. [DOI] [PubMed] [Google Scholar]
- 4.Frizziero A, Salamanna F, Della Bella E, Vittadini F, Gasparre G, Nicoli Aldini N, Masiero S, Fini M. The Role of Detraining in Tendon Mechanobiology. Front Aging Neurosci. 2016 Feb 29;8:43. doi: 10.3389/fnagi.2016.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliva F, Piccirilli E, Berardi AC, Frizziero A, Tarantino U, Maffulli N. Hormones and tendinopathies: the current evidence. Adv Exp Med Biol. 2016;920:133–138. doi: 10.1093/bmb/ldv054. [DOI] [PubMed] [Google Scholar]
- 6.Frizziero A, Vittadini F, Gasparre G, Masiero S. Impact of oestrogen deficiency and aging on tendon: concise review. Muscles Ligaments Tendons J. 2014 Nov 17;4(3):324–328. [PMC free article] [PubMed] [Google Scholar]
- 7.Ackermann PW, Renström P. Tendinopathy in sport. Sports Health. 2012 May;4(3):193–201. doi: 10.1177/1941738112440957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abate M, Silbernagel KG, Siljeholm C, Di Iorio A, De Amicis D, Salini V, Werner S, Paganelli R. Pathogenesis of tendinopathies: inflammation or degeneration? Arthritis Res Ther. 2009;11(3):235. doi: 10.1186/ar2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paavola M, Kannus P, Jarvinen TA, Khan K, Jozsa L, Jarvinen M. Achilles tendinopathy. J Bone Joint Surg Am. 2002;84-A:2062–2076. doi: 10.2106/00004623-200211000-00024. [DOI] [PubMed] [Google Scholar]
- 10.Gisslen K, Alfredson H. Neovascularisation and pain in jumper’s knee: a prospective clinical and sonographic study in elite junior volleyball players. Br J Sports Med. 2005;39:423–428. doi: 10.1136/bjsm.2004.013342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohberg L, Lorentzon R, Alfredson H. Neovascularisation in Achilles tendons with painful tendinosis but not in normal tendons: an ultrasonographic investigation. Knee Surg Sports Traumatol Arthrosc. 2001;9:233–238. doi: 10.1007/s001670000189. [DOI] [PubMed] [Google Scholar]
- 12.Knobloch K, Kraemer R, Lichtenberg A, Jagodzinski M, Gossling T, Richter M, et al. Achilles tendon and paratendon microcirculation in midportion and insertional tendinopathy in athletes. Am J Sports Med. 2006;34:92–97. doi: 10.1177/0363546505278705. [DOI] [PubMed] [Google Scholar]
- 13.Frizziero A, Vittadini F, Fusco A, Giombini A, Masiero S. Efficacy of eccentric exercise in lower limb tendinopathies in athletes. J Sports Med Phys Fitness. 2016 Nov;56(11):1352–1358. [PubMed] [Google Scholar]
- 14.Kaux JF, Forthomme B, Goff CL, Crielaard JM, Croisier JL. Current opinions on tendinopathy. J Sports Sci Med. 2011 Jun 1;10(2):238–253. [PMC free article] [PubMed] [Google Scholar]
- 15.Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010 Nov 20;376(9754):1751–1767. doi: 10.1016/S0140-6736(10)61160-9. [DOI] [PubMed] [Google Scholar]
- 16.Smidt N, van der Windt DA, Assendelft WJ, Deville WL, Korthals-de Bos IB, Bouter LM. Corticosteroid injections, physical therapy or a wait and see policy for lateral epicondylitis: A randomized controlled trial. Lancet. 2002;359:657–662. doi: 10.1016/S0140-6736(02)07811-X. [DOI] [PubMed] [Google Scholar]
- 17.Meloni F, Milia F, Cavazzuti M, et al. Clinical evaluation of sodium hyaluronate in the treatment of patients with supraspinatus tendinosis under echographic guide: experimental study of periarticular injections. Eur J Radiol. 2008;68:170–173. doi: 10.1016/j.ejrad.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Petrella RJ, Cogliano A, Decaria J, Mohamed N, Lee R. Management of tennis elbow with sodium hyaluronate periarticular injections. Sports Medicine, Arthroscopy, Rehabilitation, Therapy & Technology. 2010;2:1–6. doi: 10.1186/1758-2555-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abate M, Schiavone C, Salini V. The use of hyaluronic acid after tendon surgery and in tendinopathies. Biomed Res Int. 2014;2014:783632. doi: 10.1155/2014/783632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiig M, Abrahmsson SO, Lundborg G. Effects of hyaluronan on cell proliferation and collagen synthesis: a study of rabbit flexor tendons in vitro. J Hand Surg (Am) 1996 Jul;21(4):599–604. doi: 10.1016/S0363-5023(96)80010-4. [DOI] [PubMed] [Google Scholar]
- 21.Kolodzinskyi MN, Zhao C, Sun YL, An KN, Thoreson AR, Amadio PC, et al. The effects of hylan g–f 20 surface modification on gliding of extrasynovial canine tendon grafts in vitro. J Hand Surg Am. 2013 Feb;38(2):231–236. doi: 10.1016/j.jhsa.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akasaka T, Nishida J, Araki S, Shimamura T, Amadio PC, An KN. Hyaluronic acid diminishes the resistance to excursion after flexor tendon repair: an in vitro biomechanical study. J Biomech. 2005;38:503–507. doi: 10.1016/j.jbiomech.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 23.Padulo J, Oliva F, Frizziero A, Maffulli N. Muscles, Ligaments and Tendons Journal - Basic principles and recommendations in clinical and field science research: 2016 update. MLTJ. 2016;6(1):1–5. doi: 10.11138/mltj/2016.6.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoksrud A, Ohberg L, Alfredson H, Bahr R. Color Doppler ultrasound findings in patellar tendinopathy (jumper’s knee) Am J Sports Med. 2008 Sep;36(9):1813–1820. doi: 10.1177/036354650831989. [DOI] [PubMed] [Google Scholar]
- 25.Akasaka T, Nishida J, Araki S, Shimamura T, Amadio PC, An KN. Hyaluronic acid diminishes the resistance to excursion after flexor tendon repair: an in vitro biomechanical study. J Biomech. 2005;38:503–507. doi: 10.1016/j.jbiomech.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 26.Monticone M, Frizziero A, Rovere G, Vittadini F, Uliano D, LA Bruna S, Gatto R, Nava C, Leggero V, Masiero S. Hyaluronic acid intra-articular injection and exercise therapy: effects on pain and disability in subjects affected by lower limb joints osteoarthritis. A systematic review by the Italian Society of Physical and Rehabilitation Medicine (SIMFER) Eur J Phys Rehabil Med. 2016 Jun;52(3):389–399. Epub 2015 Sep 10. [PubMed] [Google Scholar]
- 27.Bruyère O, Cooper C, Pelletier JP, Maheu E, Rannou F, Branco J, Luisa Brandi M, Kanis JA, Altman RD, Hochberg MC, Martel-Pelletier J, Reginster JY. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis-From evidence-based medicine to the real-life setting. Semin Arthritis Rheum. 2016 Feb;45(4 Suppl):S3–11. doi: 10.1016/j.semarthrit.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Kumai T, Muneta T, Tsuchiya A, Shiraishi M, Ishizaki Y, Sugimoto K, Samoto N, Isomoto S, Tanaka Y, Takakura Y. The short-term effect after a single injection of high-molecularweight hyaluronic acid in patients with enthesopathies (lateral epicondylitis, patellar tendinopathy, insertional Achilles tendinopathy, and plantar fasciitis): a preliminary study. J Orthop Sci. 2014 Jul;19(4):603–611. doi: 10.1007/s00776-014-0579-2. [DOI] [PubMed] [Google Scholar]
- 29.Frizziero A, Vittadini F, Barazzuol M, Gasparre G, Finotti P, Meneghini A, Maffulli N, Masiero S. Extracorporeal shockwaves therapy versus hyaluronic acid injection for the treatment of painful non-calcific rotator cuff tendinopathies: preliminary results. J Sports Med Phys Fitness. 2016 Apr 12; doi: 10.23736/S0022-4707.16.06408-2. [DOI] [PubMed] [Google Scholar]
- 30.Lynen N, De Vroey T, Spiegel I, Van Ongeval F, Hendrickx NJ, Stassijns G. Comparison of Peritendinous Hyaluronan Injections Versus Extracorporeal Shock Wave Therapy in the Treatment of Painful Achilles’ Tendinopathy: A Randomized Clinical Efficacy and Safety Study. Arch Phys Med Rehabil. 2017 Jan;98(1):64–71. doi: 10.1016/j.apmr.2016.08.470. [DOI] [PubMed] [Google Scholar]